Abstract
This retrospective study was designed to explore whether neutrophil to lymphocyte ratio (NLR) is a prognostic factor in patients with coronavirus disease 2019 (COVID‐19). A cohort of patients with COVID‐19 admitted to the Tongren Hospital of Wuhan University from 11 January 2020 to 3 March 2020 was retrospectively analyzed. Patients with hematologic malignancy were excluded. The NLR was calculated by dividing the neutrophil count by the lymphocyte count. NLR values were measured at the time of admission. The primary outcome was all‐cause in‐hospital mortality. A multivariate logistic analysis was performed. A total of 1004 patients with COVID‐19 were included in this study. The mortality rate was 4.0% (40 cases). The median age of nonsurvivors (68 years) was significantly older than survivors (62 years). Male sex was more predominant in nonsurvival group (27; 67.5%) than in the survival group (466; 48.3%). NLR value of nonsurvival group (median: 49.06; interquartile range [IQR]: 25.71‐69.70) was higher than that of survival group (median: 4.11; IQR: 2.44‐8.12; P < .001). In multivariate logistic regression analysis, after adjusting for confounding factors, NLR more than 11.75 was significantly correlated with all‐cause in‐hospital mortality (odds ratio = 44.351; 95% confidence interval = 4.627‐425.088). These results suggest that the NLR at hospital admission is associated with in‐hospital mortality among patients with COVID‐19. Therefore, the NLR appears to be a significant prognostic biomarker of outcomes in critically ill patients with COVID‐19. However, further investigation is needed to validate this relationship with data collected prospectively.
Keywords: lymphopenia, neutrophils, pneumonia, prognostic, SARS‐CoV‐2
Highlights
NLR is a significant prognostic biomarker of outcomes in critically ill patients with COVID‐19.
COVID‐19 is more likely to infected those elder men with chronic comorbidities.
NLR may also help in the early identification of older patients at higher risk of COVID‐19.
Close monitoring and timely intervention are needed for elderly patients with COVID‐19.
1. INTRODUCTION
Since December 2019, a cluster of acute respiratory illness, now known as novel coronavirus‐infected pneumonia (NCIP), and has spread throughout China and now to 195 other countries and territories. 1 , 2 , 3 , 4 On 7 January 2020, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; previously known as 2019‐nCoV), was identified in samples of bronchoalveolar lavage fluid from a patient in Wuhan and was confirmed as the cause of the NCIP. 5 Studies have shown that the disease caused by SARS‐CoV‐2, recently named as coronavirus disease 2019 (COVID‐19) by World Health Organization (WHO), could induce symptoms including fever, dry cough, dyspnea, fatigue, and lymphopenia in infected patients. In more severe cases, infections causing viral pneumonia may lead to severe acute respiratory syndrome (SARS) and even death. 2 , 3 , 6 Most of the initial cases of COVID‐19, the disease caused by SARS‐CoV‐2, were all related to Huanan Seafood Wholesale Market, which sells aquatic products, live poultries, and wild animals. 2 The first batch of cases identified later showed no exposure or even no relation to Huanan Wholesale Market, and the human‐to‐human transmission was confirmed; moreover, nosocomial infections were reported in some health care workers. 3 , 7 The number of fatalities due to COVID‐19 is escalating. However, at this time, there are no specific vaccines or treatments for COVID‐19. Most therapy consists of empirical antibiotics, antiviral therapy (oseltamivir), and systemic corticosteroids, which may have a limited effect on a fatal outcome. Evidence is now accumulating that the condition of some patients with COVID‐19 deteriorates rapidly. As of 20 May 2020, WHO's online COVID‐19 situation dashboard reveals there have been 4 801 202 confirmed cases of COVID‐19, including 318 935 deaths. Thus, identifying the prognostic predictors of mortality in patients with COVID‐19 might be useful for assessing the disease severity and making optimal treatment decisions. The neutrophil to lymphocyte ratio (NLR) has been suggested as a simple marker of the systemic inflammatory response in critical care patients. 8 , 9 It has also been reported as an independent prognostic factor for noninfectious diseases, such as acute myocardial infarction, stroke, and several types of cancers. 10 , 11 , 12 In addition, NLR has been shown to be an independent indicator of both short‐term and long‐term mortality in critically ill patients. 13 Therefore, the NLR is a systemic inflammatory marker and a potential predictor of clinical risk and outcome in many diseases. To our knowledge, the utility of NLR to predict mortality in patients with COVID‐19 has not been studied yet. Based on the previous studies, we hypothesized that NLR might relate to the disease severity and mortality in patients with COVID‐19. In the current study, we sought to investigate whether NLR is a prognostic factor in patients with COVID‐19.
2. METHODS
2.1. Study design and participants
All adult (age >18 years old) patients who were diagnosed with COVID‐19 according to WHO interim guidance were screened, and those who died or were discharged between 11 January 2020 and 3 March 2020 were included in our study. Data were collected from patients who were cured and discharged, or who died without a cure. Confirmation of COVID‐19 enrolled in this study was diagnosed according to the Guidance for Corona Virus Disease 2019 (6th edition) released by the National Health Commission of China. 14 This study complied to the ethical guidelines of the Declaration of Helsinki. The study was performed in accordance with the Research Ethics Commission of Wuhan Third Hospital & Tongren Hospital of Wuhan University (KY‐2020‐010) and the requirement for informed consent was waived by the Ethics Commission.
2.2. RT‐PCR assay for COVID‐19
Throat swab samples were collected for extracting 2019‐nCoV RNA from patients suspected of having the 2019‐nCoV infection. The reverse transcription‐polymerase chain reaction assay was performed according to the manufacturer's protocol using a 2019‐nCoV nucleic acid detection kit (Shanghai Bio‐germ Medical Biotechnology). Two target genes, including open reading frame 1ab (ORF1ab) and nucleocapsid protein (N), were amplified and tested. The primers used were as follows: Target 1 (ORF1ab): forward primer, CCCTGTGGGTTTTACA‐CTTAA; reverse primer, ACGATTGTGCATCAGCTGA; and the probe, 5′‐FAM‐CCGTCTGCGGTATGTGGAA‐AGGTTATGG‐BHQ1‐3′. Target 2 (N): forward primer, GGGGAACTTCTCCTGCTAGAAT; reverse primer, CAGACATTTTGCTCTCAAGCTG; and the probe, 5′‐FAM‐TTGCTGCTGC‐TTGACAGATT‐TAMRA‐3′. These diagnostic criteria are based on the recommendations of the National Institute for Viral Disease Control and Prevention (China). Routine blood examinations were complete blood count, coagulation profile, serum biochemical tests (including renal and liver function, creatine kinase, lactate dehydrogenase, and electrolytes and myocardial enzymes. Chest radiographs or computed tomography scan were also done for all inpatients. 15
2.3. Inclusion and exclusion criteria
All patients age more than 18 years, and having neutrophil and lymphocyte counts results within 24 hours after admission, were included in this study. Patients who missed the neutrophil and lymphocyte records or with chronic hematological disorder were excluded.
2.4. Data collection
The electronic medical records of the patients were reviewed by a trained research team of physicians in Wuhan Third Hospital & Tongren Hospital of Wuhan University. Epidemiological, clinical, laboratory, treatment, and outcome data were analyzed. Patient data included demographics, medical history, exposure history, comorbidities, symptoms, signs, laboratory findings, and treatment (antiviral, antibiotic, and corticosteroid therapies, and respiratory support). All data were checked by two physicians (HC and JS) and a third researcher (SN) adjudicated any difference in interpretation between the two primary reviewers.
2.5. Statistical analysis
Baseline characteristics were presented as median with interquartile range (IQR) for continuous variables and as number with percentage for categorical variables. Comparisons between continuous variables were analyzed by the Student t and Mann‐Whitney U test based on variable distribution, and categorical variables were analyzed by the χ 2 test. The data were tested to the Kolmogorov‐Smirnov normality test for homogeneity of variance. To assess the relationship between the NLR and in‐hospital mortality, we did the receiver operating characteristics (ROC) curve and reported area under the ROC curve. Next, we grouped the patients based on the cut‐off value of NLR during hospitalization. In addition, to adjust potential confounding with variables, we did a multivariate regression analysis. The following variables were considered for multivariable adjustment: age, gender, smoking status, laboratory data, comorbidities, and intensive care unit (ICU) length of stay. The results were reported as hazard ratio and 95% confidence interval (95% CI). All statistical processes were performed with the Statistical Package for the Social Sciences 20.0 (SPSS Inc, Chicago, IL). A two‐sided P value less than .05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
From 11 January 2020 to 3 March 2020, a total of 1004 patients diagnosed with COVID‐19 were admitted to Wuhan Third Hospital & Tongren Hospital of Wuhan University. Of these, 40 of these patients had died of COVID‐19. The median age of nonsurvivors was 68 (IQR: 58‐79) years, which was significantly older than survivors (62 [50‐70] years); male sex was more predominant in nonsurvivors (27; 67.5%) than in survivors (466; 48.3%). In the laboratory examination, the levels of white blood cell (WBC) count, neutrophil count, serum creatine kinase, creatine kinase isozyme, lactate dehydrogenase, blood urea nitrogen, serum creatinine, hydroxybutyrate‐dehydrogenase, cTnI, D‐dimer, NT‐proBNP, alanine aminotransferase, aspartate aminotransferase, bilirubin, and hs‐CRP were higher in nonsurvivors, but levels of platelets, lymphocyte, albumin, total cholesterol, high‐density lipoprotein, low‐density lipoprotein levels were lower (all P < .05). In addition, the number of comorbid diseases (diseases diagnosed at admission or related to previous medical history) in nonsurvivors was significantly higher than that in survivors, especially renal insufficiency, respiratory failure, and cerebrovascular disease. One important finding is that the median NLR value was 49.06 (range: 25.71‐69.70) in nonsurvivors and 4.11 (range: 2.44‐8.12) in survivors, with a large difference between the two groups (P < .001). The baseline characteristics, medical history, and major laboratory findings of the two groups were shown in Table 1.
Table 1.
Baseline characteristics of survivors and nonsurvivors
| Variables | Survivors (N = 964) | Nonsurvivors (N = 40) | P value |
|---|---|---|---|
| Age, y | 62 (50‐70) | 68 (58‐79) | .007 |
| Male, n (%) | 466 (48.3) | 27 (67.5) | .018 |
| Smoking, n (%) | 440 (45.6) | 22 (55) | .245 |
| Hemoglobin, g/dL | 128 (118‐138)* | 133 (123‐146) | .069 |
| WBC, 10 × 9/L | 6.05 (4.70‐7.80) | 14.85 (11.73‐18.68) | <.001 |
| Platelet, 10 × 9/L | 180 (139‐228)* | 119 (82‐186) | <.001 |
| Albumin, g/L | 35.3 (31.2‐39.3) | 26.8 (24.9‐31.5) | <.001 |
| Neutrophil, 10 × 9/L | 4.196 (3.000‐6.150) | 13.305 (10.550‐17.593) | <.001 |
| Lymphocyte, 10 × 9/L | 0.97 (0.65‐1.41) | 0.33 (0.16‐0.47) | <.001 |
| NLR | 4.11 (2.44‐8.12) | 49.06 (25.71‐69.70) | <.001 |
| High sensitivity CRP, ng/mL | 18.89 (3.68‐66.91)* | 208.46 (117.47‐284.78) | <.001 |
| Creatinine, mol/L | 69.25 (56.60‐89.08) | 143.95 (91.20‐355.70) | <.001 |
| CK, IU/L | 75.00 (48.00‐139.75) | 274.00 (107.50‐643) | <.001 |
| CK‐MB, IU/L | 11 (8‐16) | 28.00 (14.25‐43.75) | <.001 |
| LDH, IU/L | 227 (177‐317)* | 568.50 (352.00‐823.25) | <.001 |
| cTnI, ng/L | 0.002 (0.000‐0.011)* | 0.478 (0.106‐1.205) | <.001 |
| HBDH, U/L | 193 (153‐264)* | 435 (250‐662) | <.001 |
| NT‐proBNP pmol/L | 29.53 (12.75‐70.96)* | 251.56 (102.27‐471.65) | <.001 |
| Blood urea nitrogen, mmol/L | 4.9 (3.9‐6.8)* | 21.6 (6.5‐36.9) | <.001 |
| ALT, U/L | 33 (20‐61) | 51 (23‐96) | .048 |
| AST, U/L | 31 (22‐49) | 79 (38‐133) | <.001 |
| D‐Dimer, ng/mL | 0.73 (0.35‐2.16)* | 6.12 (3.40‐21.27) | <.001 |
| Triglyceride, mmol/L | 1.54 (1.11‐2.26) | 1.85 (1.29‐2.63) | .124 |
| Total bilirubin, μmol/L | 10.4 (7.7‐13.7)* | 15.6 (7.6‐22.7) | .01 |
| Total cholesterol, mmol/L | 4.34 (3.72‐5.09)* | 3.70 (3.03‐4.37) | <.001 |
| HDL, mmol/L | 0.88 (0.72‐1.06)* | 0.63 (0.50‐0.89) | <.001 |
| LDL, mmol/L | 2.69 (2.23‐3.23)* | 2.20 (1.68‐2.78) | <.001 |
| ICU history | 40 (4.1) | 26 (65) | <.001 |
| ICU length of stay | 14 (9‐17) | 10.5 (5.25‐17.75) | .052 |
| Diabetes | 97 (10.1) | 10 (25) | .003 |
| Hypertension | 215 (22.3) | 20 (50) | <.001 |
| CHD | 65 (6.7) | 10 (25) | <.001 |
| Hyperlipemia | 19 (2) | 1 (2.5) | .814 |
| COPD | 8 (0.8) | 0 (0) | .563 |
| Other lung diseases | 126 (13.1) | 21 (52.5) | <.001 |
| Respiratory failure | 48 (5) | 25 (62.5) | <.001 |
| Acute renal failure | 3 (0.3) | 0 (0) | .724 |
| Other kidney diseases | 91 (9.4) | 8 (20) | .028 |
| Livercirrhosis | 8 (0.8) | 0 (0) | .563 |
| Other liver diseases | 68 (7.1) | 7 (17.5) | .014 |
| Digestive system disease | 19 (2) | 2 (5) | .19 |
| Tumour | 11 (1.1) | 1 (2.5) | .438 |
| Cerebrovascular disease | 21 (2.2) | 9 (22.5) | <.001 |
Note: Data are presented as median with IQR for continuous variables and as number with percentage for categorical variables.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, serum creatine kinase; CK‐MB, creatine kinase‐MB; COPD, chronic obstructive pulmonary disease; HBDH, hydroxybutyrate‐dehydrogenase; HDL, high‐density lipoprotein; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; LDL, low‐density lipoprotein; NLR, neutrophil to lymphocyte ratio; WBC, white blood cell.
P < .05.
3.2. Association between NLR and in‐hospital mortality
Nonparametric ROC analysis showed that the cut‐off value of the ROC curve as the optimal threshold for in‐hospital mortality was 11.75, that is, when NLR was more than 11.75, the model had the strongest predictive ability, with an area under the curve of 0.945% and 95% CI of 0.917 to 0.973 (Figure 1), sensitivity of 97.5%, specificity of 78.1%, and Yoden index of 0.756. We further divided patients into two groups using NLR greater than or less than or equal to 11.75 as the criterion to compare the clinical characteristics of patients with a high NLR and those with a low NLR (Table 2).
Figure 1.
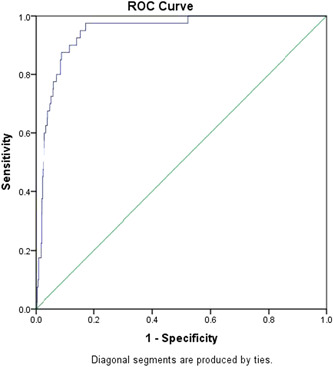
Receiver operating characteristic (ROC) curves for in‐hospital mortality
Table 2.
Clinical characteristics between high NLR and low NLR
| Variables | Low NLR ≤11.75 | High NLR >11.75 | P value |
|---|---|---|---|
| NLR | 3.30 (2.22‐5.44) | 22.0 (14.49‐44.80) | <.001 |
| Number of death | 1 (0.1) | 39 (19) | <.001 |
| ICU history | 11 (1.4) | 55 (26.8) | <.001 |
| Length of stay | 14 (8‐16) | 15 (9‐23) | <.001 |
| Age, y | 62 (49‐69) | 63 (54‐73) | .001 |
| Male, n (%) | 370 (46.3) | 123 (60.0) | <.001 |
| Smoking, n (%) | 347 (43.4) | 115 (56.1) | .001 |
| Hemoglobin, g/dL | 128 (118‐137)* | 132 (118‐144)* | .049 |
| WBC, 10 × 9/L | 5.6 (4.4‐6.9) | 10.8 (7.8‐14.8) | <.001 |
| Platelet, 10 × 9/L | 187 (148‐237)* | 145 (107‐188) | <.001 |
| Albumin, g/L | 36.4 (32.7‐39.8) | 28.8 (26.9‐32.5) | <.001 |
| Neutrophil, 10 × 9/L | 3.75 (2.83‐5.94) | 10.49 (7.27‐13.52) | <.001 |
| Lymphocyte, 10 × 9/L | 1.09 (0.83‐1.51) | 0.42 (0.29‐0.55) | <.001 |
| High sensitivity CRP, ng/mL | 12.7 (2.8‐44.4)* | 116.6 (64.2‐200.0) | <.001 |
| Creatinine, mol/L | 67.2 (55.6‐83.2) | 90.6 (68.8‐177.1) | <.001 |
| CK, IU/L | 67 (46‐118) | 178 (74‐433) | <.001 |
| CK‐MB, IU/L | 11 (8‐15) | 19 (11‐31) | <.001 |
| LDH, IU/L | 214 (170‐278)* | 401 (274‐620) | <.001 |
| cTnI, ng/L | 0.002 (0.000‐0.006)* | 0.022 (0.004‐0.227)* | <.001 |
| HBDH, U/L | 181 (147‐234)* | 327 (225‐495) | <.001 |
| NT‐proBNP pmol/L | 25.88 (12.26‐52.34)* | 102.47 (30.94‐265.24)* | <.001 |
| Blood urea nitrogen, mmol/L | 4.6 (3.7‐5.9)* | 9.1 (6.0‐20.4) | <.001 |
| ALT, U/L | 30 (19‐52) | 54 (28‐114) | <.001 |
| AST, U/L | 29 (21‐43) | 59 (33‐101) | <.001 |
| D‐Dimer, ng/mL | 0.64 (0.32‐1.35)* | 3.76 (0.83‐6.44)* | <.001 |
| Triglyceride, mmol/L | 1.52 (1.09‐2.22) | 1.70 (1.26‐2.45) | .004 |
| Total bilirubin, μmol/L | 9.7 (7.4‐12.6)* | 14 (10.3‐21.1) | <.001 |
| Total cholesterol, mmol/L | 4.30 (3.70‐5.04)* | 4.28 (3.65‐5.11) | .645 |
| HDL, mmol/L | 0.89 (0.74‐1.09)* | 0.79 (0.61‐0.92) | <.001 |
| LDL, mmol/L | 2.68 (2.21‐3.19)* | 2.66 (2.17‐3.38) | .96 |
| Diabetes | 78 (9.8) | 29 (14.1) | .07 |
| Hypertension | 174 (21.8) | 61 (29.8) | .016 |
| CHD | 43 (5.4) | 32 (15.6) | <.001 |
| Hyperlipemia | 18 (2.3) | 2 (1.0) | .243 |
| COPD | 7 (0.9) | 1 (0.5) | .577 |
| Other lung diseases | 87 (10.9) | 60 (29.3) | <.001 |
| Respiratory failure | 18 (2.3) | 55 (26.8) | <.001 |
| Acute renal failure | 1 (0.1) | 2 (1) | .047 |
| Other kidney diseases | 68 (8.5) | 31 (15.1) | .005 |
| Livercirrhosis | 6 (0.8) | 2 (1.0) | .747 |
| Other liver diseases | 46 (5.8) | 29 (14.1) | <.001 |
| Digestive system disease | 15 (1.9) | 6 (2.9) | .349 |
| Tumour | 8 (1.0) | 4 (2.0) | .264 |
| Cerebrovascular disease | 15 (1.9) | 15 (7.3) | <.001 |
Note: Data are presented as median with IQR for continuous variables and as number with percentage for categorical variables.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, serum creatine kinase; CK‐MB, creatine kinase‐MB; COPD, chronic obstructive pulmonary disease; HBDH, hydroxybutyrate‐dehydrogenase; HDL, high‐density lipoprotein; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; LDL, low‐density lipoprotein; NLR, neutrophil to lymphocyte ratio; WBC, white blood cell.
P < .05.
3.3. Risk factors for in‐hospital mortality
After multivariate logistic regression analysis, high‐NLR remained independently associated with in‐hospital death. In addition, we found that higher levels of hs‐CRP, NT‐proBNP, and blood urea nitrogen, as well as history of hypertension, respiratory failure, digestive system diseases, and cerebrovascular diseases, were also independently associated with in‐hospital mortality (Table 3).
Table 3.
Multivariate regression analysis of predictors of in‐hospital mortality
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| NLR >11.75 | 44.351 | 4.627‐425.088 | .001 |
| High sensitivity CRP, ng/mL | 1.009 | 1.004‐1.015 | .001 |
| NT‐proBNP pmol/L | 1.001 | 1.000‐1.002 | .054 |
| Blood urea nitrogen, mmol/L | 1.015 | 0.991‐1.039 | .218 |
| Hypertension | 2.606 | 0.988‐6.870 | .053 |
| Respiratory failure | 4.207 | 1.626‐10.881 | .003 |
| Digestive system disease | 39.463 | 3.851‐404.357 | .002 |
| Cerebrovascular disease | 13.534 | 3.162‐57.940 | <.001 |
Abbreviations: CI, confidence interval; NLR, neutrophil to lymphocyte ratio; OR, odds ratio.
4. DISCUSSION
In this study, we found there was an association between NLR and the clinical outcomes in patients with COVID‐19. Following SARS‐CoV‐2 infection, most of the patients presented lymphopenia and elevated levels of infection‐related biomarkers, more interestingly, a higher number of neutrophils and a lower number of lymphocytes, that is, the increase of NLR, were found in nonsurvivors with COVID‐19 compared to survivors. NLR has been shown to be a simple, stand‐alone method for evaluating systemic inflammation. Neutrophils play a central role in the innate immune response, resulting in multiple organ failure and lethality of severely injured patients. In contrast, lymphocytes play a major role in the inflammatory response. Therefore, a high NLR indicates an imbalance of the inflammatory response and may be a surrogate marker of disease severity in infectious diseases, such as sepsis and bacteremia. 16 Based on the ROC curve analysis, the NLR showed the significant performance to predict in‐hospital mortality. The cut‐off level for the NLR was 11.75 (Figure 1). Our study supports that a high NLR is a strong predictor for in‐hospital mortality in patients with COVID‐19. NLR is a convenient marker of systemic inflammation and infection, outperforms the predictive ability of absolute leukocyte, lymphocyte or neutrophil counts, and has been demonstrated to be a useful biomarker for predicting bacterial infection, included pneumonia. 9 , 13 , 17 Furthermore, NLR is shown to predict outcomes in patients with cardiovascular disease 18 as well as solid tumor malignancy. 19 In addition, higher NLR has been reported to be associated with worse prognosis in patients with acute pancreatitis and was identified as a significant predictor of ICU admission and a longer length of stay. 20 Increasing clinical data suggest that the NLR might be a powerful predictive and prognostic indicator for severe COVID‐19. 6 , 21 , 22 Consistent with previous findings that several patients infected with SARS‐CoV‐2 had a high neutrophil count and a low lymphocyte count during the severe phase, 6 the increased mortality rate in patients with NLR found in our study, indicated a serious disturbance in the internal environment and potential critical condition in severe infected cases. 23 In general, increased NLR was common among patients with COVID‐19, which become more pronounced in severe cases. On the basis of these, we suggested that COVID‐19 might damage lymphocytes, and the immune system was impaired during the course of the disease. Consistent with the findings from Chen et al, 24 we observed that the overall fatality rate for confirmed COVID‐19 cases was higher in male than in female patients, with the risk of death rising with age for both sexes. Furthermore, we observed that 92.5% of patients had at least one underlying disorder (ie, renal insufficiency, respiratory failure, and cerebrovascular disease), and a higher percentage of hypertension and cardiovascular disease in nonsurvivors than survivors, in consistent with those reports. 2 , 6 These findings suggested that COVID‐19 is more likely to infected those elder men with chronic comorbidities due to weaker immune functions. Older patients often present with atypical signs and symptoms and have multiple comorbid conditions, which often leads to delayed diagnosis and treatment and even death. Indeed, these patients are at higher risk of COVID‐19, hospital admission, ICU admission, and in‐hospital death than are young adults. 16 , 25 Physicians may find it time‐consuming and difficult to completely assess older patients. However, NLR is a biomarker that integrates two WBC subtypes representing two inversely and related immune pathways. It represents an easily accessible objective parameter, can be readily calculated from differential WBC counts, more stable for measurement than the individual WBC counts, and less affected by conditions that could change the individual cell counts. 26 The NLR is beneficial for clinicians to inform their understanding of the severity of COVID‐19. A high calculated NLR may, therefore, aid in determining outcome risk, need for close clinical monitoring, and urgent effective treatment. Samaras et al 27 proposed a targeted approach for high‐risk patients. 25 Our results may also help in the early identification of older patients at higher risk of COVID‐19.
Our results show a significant increase in mortality with increasing quartile of baseline NLR in patients with COVID‐19. The main reason for this difference may be due to an insufficient number of patients with low NLR in our study. Another reason is that part of patients in our study included had a diagnosis of sepsis in patients with COVID‐19, which may suggest a more severe inflammation in patients in our study.
Systemic inflammation is associated with the development and progression of COVID‐19, recent studies have shown increased levels of inflammatory biomarkers were associated with poor outcomes in COVID‐19. 21 NLR may serve as an additional inflammatory biomarker mainly because it reflects the systemic inflammatory and stress responses with neutrophils rising and lymphocytes apoptosis. 28 , 29 A study of patients with trauma and systemic inflammatory response syndrome showed lymphopenia was associated with high mortality, the lymphocytes were important in the regulation of an appropriate inflammatory response and low circulating lymphocytes may perpetuate a harmful inflammatory status. 30 High NLR in patients is associated with high levels of inflammation. de Jager et al 31 showed that NLR predicts bacteremia was better than conventional inflammation markers like C‐reactive protein, WBC count, and neutrophil count. A recent study showed that NLR, as well as other inflammatory factors like lymphopenia and plasma calprotectin, are independent predictors of increased all‐cause mortality in moderate to severe chronic obstructive pulmonary disease in stable phase and not in treatment with systemic glucocorticoids. 32 Thus, we supposed increased NLR values could predict the poor outcome in COVID‐19 patients on the evidence of the association between NLR‐related inflammation and disease severity.
This study has some important limitations to consider. First, it was a single‐center and retrospective observational study, subject selection bias could not be avoided and the causal relationship between NLR and mortality could not be drawn. Therefore, our findings need to be confirmed in a prospective multicenter study. Second, our primary outcome was all‐cause in‐hospital mortality, so we could not analyze the cause of specific mortalities. The exact mechanisms underlying the association between NLR and mortalities in elderly individuals are not certain. Third, NLR value is influenced by comorbidities and medications that affect the neutrophil and lymphocyte count. Forth, systemic inflammation may cause mortality, but further studies are needed to identify the exact mechanisms. We only analyzed NLR at admission; however, there may be a specific pattern in serial NLR examination. If there is an association between serial NLR and mortality, then this association could have more specificity in predicting mortality. As previously discussed, our study only shows an association, so there is a limitation of the clinical implications. Thus, more studies are needed to identify the associations between NLR and cause‐specific mortality in elderly individuals.
In summary, we found high NLR was associated with poor outcome in critically ill patients with COVID‐19. NLR was an independent risk factor for predicting all‐cause in‐hospital mortality in COVID‐19 patients. Thus, the NLR at admission may represent a surrogate marker of disease severity. However, we need more external validation to use the NLR in the clinical decision‐making process.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTION
XY and FL conceived the project. Data collection and analyses were performed by FL, FZ, SF, YD, HW, RC, ZY, YL, JS, LZ, JZ, CG, HC, WG, XH, and YZ. The manuscript was written by XY and WZ, and revised by DL and SN. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (No. 81871088), and the Applied Basic Research Program of Wuhan Municipal Bureau of Science and Technology (No. 2019020701011473) to XY, in part by the Hubei Province Health and Family Planning Scientific Research Project (No. WJ2019M006), Wuhan Municipal Population and Family Planning Commission Foundation (No. WX16C03) to DL, and in part by the Hubei Province Natural Science Fund (No. 2018CFC880) to JL.
Yan X, Li F, Wang X, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross‐sectional study. J Med Virol. 2020;92:2573–2581. 10.1002/jmv.26061
Xisheng Yan and Fen Li contributed equally to this work.
Contributor Information
Wen Zheng, Email: zhengwen12@mails.jlu.edu.cn.
Shaoping Nie, Email: spnie@126.com.
Dongsheng Li, Email: dongshengli196809@163.com.
REFERENCES
- 1. Muhareb R, Giacaman R. Tracking COVID‐19 responsibly. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zahorec R. Ratio of neutrophil to lymphocyte counts‐‐rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5‐14. [PubMed] [Google Scholar]
- 9. Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S. Prognostic significance of neutrophil‐to‐lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016:8191254‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayça B, Akın F, Celik O, et al. Neutrophil to lymphocyte ratio is related to stent thrombosis and high mortality in patients with acute myocardial infarction. Angiology. 2015;66(6):545‐552. [DOI] [PubMed] [Google Scholar]
- 11. Zuin M, Rigatelli G, Picariello C, et al. Correlation and prognostic role of neutrophil to lymphocyte ratio and SYNTAX score in patients with acute myocardial infarction treated with percutaneous coronary intervention: a six‐year experience. Cardiovasc Revasc Med. 2017;18(8):565‐571. [DOI] [PubMed] [Google Scholar]
- 12. Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta‐analysis. Clinics (Sao Paulo). 2015;70(7):524‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curbelo J, Luquero Bueno S, Galván‐Román JM, et al. Inflammation biomarkers in blood as mortality predictors in community‐acquired pneumonia admitted patients: importance of comparison with neutrophil count percentage or neutrophil‐lymphocyte ratio. PLoS One. 2017;12(3):e0173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. New coronavirus pneumonia prevention and control program (6th ed) (in Chinese). 2020. http://www.nhc.gov.cn/jkj/s3577/202003/4856d5b0458141fa9f376853224d41d7.shtml
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song H, Kim HJ, Park KN, Kim SH, Oh SH, Youn CS. Neutrophil to lymphocyte ratio is associated with in‐hospital mortality in older adults admitted to the emergency department. Am J Emerg Med. 2020;S0735‐6757(20):30044‐30049. [DOI] [PubMed] [Google Scholar]
- 17. Berhane M, Melku M, Amsalu A, Enawgaw B, Getaneh Z, Asrie F. The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community‐acquired pneumonia: a cross‐sectional study at Ayder and Mekelle Hospitals, Ethiopia. Clin Lab. 2019;65(4). [DOI] [PubMed] [Google Scholar]
- 18. Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 19. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation‐based neutrophil‐lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218‐230. [DOI] [PubMed] [Google Scholar]
- 20. Azab B, Jaglall N, Atallah JP, et al. Neutrophil‐lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11(4):445‐452. [DOI] [PubMed] [Google Scholar]
- 21. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu M. SARS immunity and vaccination. Cell Mol Immunol. 2004;1(3):193‐198. [PubMed] [Google Scholar]
- 23. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. MedRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 24. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39(3):238‐247. [DOI] [PubMed] [Google Scholar]
- 26. Kounis NG, Soufras GD, Tsigkas G, Hahalis G. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. 2015;21(2):139‐143. [DOI] [PubMed] [Google Scholar]
- 27. Samaras N, Chevalley T, Samaras D, Gold G. Older patients in the emergency department: a review. Ann Emerg Med. 2010;56(3):261‐269. [DOI] [PubMed] [Google Scholar]
- 28. Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157‐169. [DOI] [PubMed] [Google Scholar]
- 29. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heffernan DS, Monaghan SF, Thakkar RK, Machan JT, Cioffi WG, Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Jager CP, van Wijk PT, Mathoera RB, de Jongh‐Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil‐lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14(5):R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sorensen AK, Holmgaard DB, Mygind LH, Johansen J, Pedersen C. Neutrophil‐to‐lymphocyte ratio, calprotectin and YKL‐40 in patients with chronic obstructive pulmonary disease: correlations and 5‐year mortality—a cohort study. J Inflamm (Lond). 2015;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]


