Abstract
Because other coronaviruses enter the cells by binding to dipeptidyl‐peptidase‐4 (DPP‐4), it has been speculated that DPP‐4 inhibitors (DPP‐4is) may exert an activity against severe acute respiratory syndrome coronavirus 2. In the absence of clinical trial results, we analysed epidemiological data to support or discard such a hypothesis. We retrieved information on exposure to DPP‐4is among patients with type 2 diabetes (T2D) hospitalized for COVID‐19 at an outbreak hospital in Italy. As a reference, we retrieved information on exposure to DPP‐4is among matched patients with T2D in the same region. Of 403 hospitalized COVID‐19 patients, 85 had T2D. The rate of exposure to DPP‐4is was similar between T2D patients with COVID‐19 (10.6%) and 14 857 matched patients in the region (8.8%), or 793 matched patients in the local outpatient clinic (15.4%), 8284 matched patients hospitalized for other reasons (8.5%), and when comparing 71 patients hospitalized for COVID‐19 pneumonia (11.3%) with 351 matched patients with pneumonia of another aetiology (10.3%). T2D patients with COVID‐19 who were on DPP‐4is had a similar disease outcome as those who were not. In summary, we found no evidence that DPP‐4is might affect hospitalization for COVID‐19.
Keywords: antidiabetic drug, database research, DPP‐4 inhibitor, pharmacoepidemiology
1. INTRODUCTION
The coronaviruses causing 2002 severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome used dipeptidyl peptidase‐4 (DPP‐4) to enter the cells and initiate infection. 1 , 2 The molecular studies that are available do not support that SARS coronavirus 2 (SARS‐CoV‐2) enters the cells via DPP‐4, 3 but structural models predict that SARS‐CoV‐2 spike protein can interact with membrane‐bound DPP‐4 (CD26). 4 CD26 is expressed on several cell types, including lymphocytes, where it interacts with adenosine deaminase to regulate immune responses. 5 CD26/DPP‐4 also exists as a soluble form but the interplay between membrane and soluble DPP‐4 in regulating the internalization of coronaviruses is complex and not entirely understood. 6 Because DPP‐4 physiologically cleaves incretin hormones, DPP‐4 inhibitors (DPP‐4is) are used pharmacologically to prolong incretin half‐life, potentiate meal‐induced insulin secretion and to treat type 2 diabetes (T2D). 7 DPP‐4 has many other substrates, including cytokines and chemokines that regulate innate immunity. 8 During the early phases of clinical development, concerns emerged that DPP‐4is could increase the risk of respiratory tract infections, but these signs were not confirmed in larger trials. 9
Notably, T2D negatively affects the outcome of COVID‐19, 10 for which there are limited treatment options. Based on prior evidence regarding the role of DPP‐4 on coronavirus infection, it has been speculated that DPP‐4is could exert a protective effect against COVID‐19. 11 This could be especially relevant for patients with T2D, who display elevated DPP‐4 activity. 12 While waiting for the results of ongoing trials (e.g. NCT04341935), we used observational data from a SARS‐CoV‐2 outbreak area in north‐east Italy to explore whether DPP‐4is might be protective against COVID‐19.
2. METHODS
2.1. Study design
This was a retrospective study on anonymized patients’ data collected from electronic medical records. The protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and, in compliance with national regulation on retrospective observational studies, it was notified to the local ethical committee (no. 23554) and the need for patients’ informed consent was waived.
2.2. Data collection
We retrieved data on patients with T2D hospitalized for COVID‐19 from February to April 2020 at the University Hospital of Padova, located at the centre of one of the first SARS‐CoV‐2 outbreaks in north‐east Italy. We screened the records of all patients admitted to hospital with a confirmed SARS‐CoV‐2 infection. The presence of T2D was ascertained based on self‐reported history, prior electronic medical records indicative of diabetes and ongoing therapy with glucose‐lowering medications. Patients with a diagnosis of type 1 diabetes were excluded. For all patients with T2D, we collected the following information: demographics (age, sex), concomitant risk factors (smoking, hypertension, dyslipidaemia), co‐morbidities (chronic obstructive pulmonary disease and history of cancer), complications (cardiovascular disease and microangiopathy), presence or absence of COVID‐19‐related pneumonia, and ongoing therapies before hospitalization. First, we calculated the proportion of DPP‐4i users among patients with T2D prior to hospitalization for COVID‐19. Then, to evaluate whether such prevalence was lower than expected, and therefore suggestive of a possible protective effect of DPP‐4is against COVID‐19, we retrieved data on patients with T2D from the administrative repository of the Veneto region. This database contains all healthcare contacts involving ~5 million inhabitants, complemented by the regional Health Information Exchange system. 13 Patients with T2D and COVID‐19 were matched with patients in the regional administrative database by means of propensity score matching (PSM). Variables used to compute propensity scores (PS) were: age, sex, prevalence of hypertension, dyslipidaemia, history of cardiovascular disease, chronic kidney disease, chronic pulmonary disease, cancer (past or current), use of insulin, angiotensin‐converting enzyme (ACE) inhibitors, calcium channel blockers, beta‐blockers, platelet aggregation inhibitors, statins and anticoagulants. Each diabetic patient with COVID‐19 was matched with 200 control individuals with T2D at the last observation in 2018, or with 100 controls with T2D hospitalized for any other reasons in 2018, or with five patients with 2TD hospitalized for pneumonia in 2018. For hospitalized patients, a DPP‐4i user was defined as a patient with a refill for DPP‐4is before hospitalization, to avoid including patients who initiated DPP‐4is during their hospital stay or at discharge.
As a secondary analysis, we retrieved the expected proportion of DPP‐4i users among patients with T2D from the database of the diabetes outpatient clinic at the same university hospital. Patients with COVID‐19 were matched 1:10 with diabetic controls, and the following variables were used to compute PS: age, sex, HbA1c, weight, prevalence of hypertension, dyslipidaemia, history of cardiovascular disease, atrial fibrillation, chronic kidney disease, microangiopathy, use of insulin, ACE inhibitors, calcium channel blockers, beta‐blockers, platelet aggregation inhibitors, statins and anticoagulants.
2.3. Statistical analysis
Continuous variables are shown as mean and standard deviation, while categorical variables are shown as percentages. Continuous variables were compared between the two groups using two‐tail Student's t test or Mann–Whitney's U test as appropriate, whereas categorical variables were compared using the chi‐square test. PSM was performed with a caliper of 0.3 for non‐COVID‐19 controls, 0.91 for non‐COVID pneumonia controls and 0.25 for the clinical database, using the nearest neighbour method without replacement. To evaluate the balance between matched groups, an absolute standardized mean difference <0.10 was considered indicative of a good match, even when, because of a large sample size, the P‐value of the comparison was <.05. The proportion of patients exposed to DPP‐4is was calculated with 95% CI and compared between the various groups using the chi‐square test and odds ratios (OR) are presented. Statistical analyses were conducted with a significance threshold of P < .05. Calculations were performed in SAS version 9.4 (TS1M4), R statistical package and scipy python library.
3. RESULTS
3.1. Characteristics of diabetic patients with COVID‐19
Figure S1 shows the study flowchart. Of 403 patients hospitalized for COVID‐19, 85 had pre‐existing T2D, nine of whom were on treatment with DPP‐4is, equal to a proportion of 10.6% (95% CI 5.7%‐18.9%). The clinical characteristics of the study patients are shown in Table 1 . On average, patients were aged 70.3 years, 64.7% were male, with an average diabetes duration of 10.4 years. The majority had hypertension (69.4%), while only 27.1% had a history of cardiovascular disease and 15.3% had chronic kidney disease. Except for the concomitant use of metformin and angiotensin receptor blockers, no significant difference was noted between patients with T2D who were on DPP‐4is compared with those who were not. None of these differences were statistically significant if adjusted for multiple testing. There was no significant difference in the rate of intensive care unit admission or death between the two groups.
TABLE 1.
Clinical characteristics of COVID‐19 patients with type 2 diabetes, divided based on the prior use of dipeptidyl‐peptidase‐4 inhibitors (DPP‐4is)
| Not on DPP‐4is | On DPP‐4is | ||||
|---|---|---|---|---|---|
| No. available | Value | No. available | Value | P | |
| Demographics | |||||
| Age, years | 76 | 70.1 (13.3) | 9 | 72.2 (12.8) | .647 |
| Sex male, % | 76 | 63.2 | 9 | 77.8 | .392 |
| Fasting plasma glucose, mmol/L | 55 | 10.7 (4.8) | 9 | 12.7 (6.1) | .277 |
| HbA1c, mmol/mol | 50 | 59.1 (23.1) | 6 | 58.8 (12.4) | .976 |
| Concomitant risk factors | |||||
| Hypertension, % | 76 | 67.1 | 9 | 88.9 | .184 |
| SBP, mm hg | 74 | 136.1 (22.7) | 9 | 136.6 (16.3) | .955 |
| DBP, mm hg | 74 | 74.6 (11.4) | 9 | 72.3 (11.5) | .573 |
| Current smoking, % | 37 | 2.7 | 4 | 0.0 | .977 |
| Dyslipidaemia, % | 76 | 43.4 | 9 | 22.2 | .227 |
| Body weight, % | 33 | 83.1 (10.6) | 5 | 81.5 (8.5) | .749 |
| Co‐morbidities | |||||
| Cardiovascular disease, % | 73 | 26.3 | 9 | 33.3 | .713 |
| Atrial fibrillation, % | 73 | 9.2 | 9 | 11.1 | .886 |
| CKD, % | 76 | 15.8 | 9 | 11.1 | .716 |
| Serum creatinine, umol/L | 76 | 89.2 (40.3) | 9 | 92.3 (29.1) | .825 |
| COPD, % | 73 | 9.2 | 9 | 0.0 | .337 |
| Cancer, % | 72 | 18.4 | 9 | 22.2 | .846 |
| Microvascular disease, % | 57 | 18.4 | 8 | 11.1 | .456 |
| COVID‐19 pneumonia, % | 68 | 82.9 | 8 | 88.9 | .434 |
| Diabetes therapy | |||||
| Basal insulin, % | 76 | 27.6 | 9 | 0.0 | .071 |
| Bolus insulin, % | 76 | 25.0 | 9 | 0.0 | .091 |
| Metformin, % | 76 | 48.7 | 9 | 88.9 | .022 |
| Sulphonylurea, % | 76 | 13.2 | 9 | 22.2 | .466 |
| Pioglitazone, % | 76 | 3.9 | 9 | 0.0 | .550 |
| Acarbose, % | 76 | 1.3 | 9 | 0.0 | .733 |
| GLP‐1 receptor agonists, % | 76 | 7.9 | 9 | 0.0 | .388 |
| SGLT‐2 inhibitors, % | 76 | 2.6 | 9 | 0.0 | .627 |
| Other therapies | |||||
| ACE inhibitors, % | 76 | 25.0 | 9 | 22.2 | .857 |
| ARBs, % | 76 | 15.8 | 9 | 44.4 | .038 |
| CCB, % | 76 | 21.1 | 9 | 0.0 | .130 |
| Beta‐blockers, % | 76 | 36.8 | 9 | 44.4 | .661 |
| Antiplatelet agents, % | 76 | 28.9 | 9 | 33.3 | .788 |
| Statins, % | 76 | 39.5 | 9 | 22.2 | .318 |
| Warfarin, % | 76 | 3.9 | 9 | 0.0 | .550 |
| Outcomes | |||||
| ICU admittance, % | 73 | 19.2 | 9 | 33.3 | .329 |
| Semi‐intensive care, % | 74 | 32.4 | 9 | 44.4 | .478 |
| Death, % | 72 | 13.9 | 9 | 11.1 | .821 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blockers; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GLP‐1, glucagon‐like peptide‐1; ICU, intensive care unit; SBP, systolic blood pressure; SGLT‐2, sodium‐glucose co‐transporter‐2.
Data are expressed as mean (SD) or as a percentage, where appropriate.
3.2. Proportion of DPP‐4i users among COVID‐19 cases and controls
The group of 85 T2D patients with COVID‐19 was matched with different groups of diabetic patients without COVID‐19 to compare the proportions of DPP‐4i users (Figure 1). Matching was always successful. In the administrative database (Table S1 ), the proportion of DPP‐4i users was 8.8% (95% CI 8.3%‐9.3%) among all patients with T2D, and 8.6% (95% CI 8.0%‐9.2%) among patients with T2D hospitalized for other reasons. In both cases, the proportion of DPP‐4i users was not significantly different from the proportion of DPP‐4i users among COVID‐19 patients (OR 1.23; P = .693 and OR = 1.27; P = .635, respectively). The proportion of DPP‐4i users among patients with COVID‐19 pneumonia was 11.3% (95% CI 5.8%‐20.7%) and 10.3% (95% CI 7.5%‐13.9%) among patients with 2TD hospitalized for pneumonia of another aetiology (OR 1.11. P = .967).
FIGURE 1.
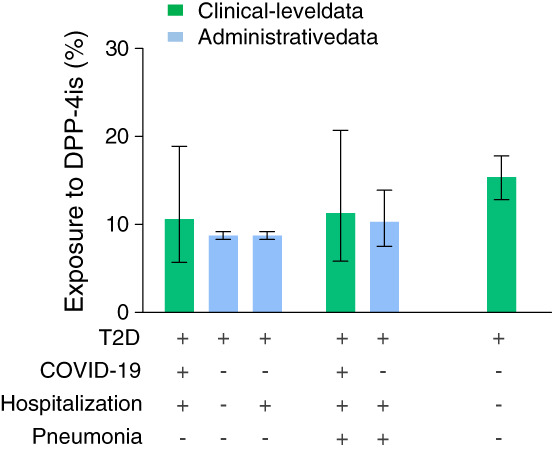
Proportion of dipeptidyl‐peptidase‐4 inhibitor (DPP‐4i) users among COVID‐19 patients with diabetes and control groups. The combination of clinical conditions in the various groups are illustrated by the table (+ for presence; − for absence). T2D, type 2 diabetes
In the secondary analysis performed on the database of the local diabetes outpatient clinic (Table S2), the expected proportion of DPP‐4i users was 15.4% (95% CI 12.8%‐17.8%) and was again non‐significantly different from the proportion of DPP‐4i users among COVID‐19 patients (OR 0.65; P = .250).
4. DISCUSSION
The current study does not support the hypothesis that DPP‐4is might be protective against COVID‐19. To explore the role of DPP‐4is in COVID‐19, we should ideally perform a cohort study to record incident cases of COVID‐19 among people exposed to DPP‐4is versus those who were unexposed. However, such a strategy would require ascertaining infection status in the entire population of individuals with T2D, which would be highly inefficient. We therefore conducted a case‐control study and compared DPP‐4i exposure among COVID‐19 patients with T2D with the expected rate of DPP‐4i exposure among similar patients without COVID‐19. A protective effect against COVID‐19 should result in a lower proportion of DPP‐4i users among COVID‐19 compared with control patients. We found that the exposure rate to DPP‐4is among patients with T2D who were infected with SARS‐CoV‐2 prior to hospitalization for COVID‐19 was superimposable to that among matched patients in the same region or to those in the local outpatient clinic. Similar rates of DPP‐4i exposure were also observed when patients hospitalized for COVID‐19 were compared with patients hospitalized for other reasons, and when comparing patients with COVID‐19 pneumonia with those with pneumonia because of other aetiologies. Although small, the subgroup of diabetic COVID‐19 patients on prior DPP‐4i therapy displayed no difference in disease outcome, further making an effect of DPP‐4i against COVID‐19 improbable.
We acknowledge that our estimate of DPP‐4i exposure before COVID‐19 was calculated for patients admitted to a university hospital, and therefore not necessarily representative of patients admitted to other hospitals. Exposure estimates in the control groups were obtained from much larger populations and matching abated statistically significant or clinically meaningful differences between cases and controls. It should, however, be noted that control patients with conditions other than COVID‐19 could have a higher prevalence of some co‐morbidities leading to hospital admission, which were unaccounted for in matching. Finally, control patients attending the local diabetes clinic, although matched with cases for the geographic area, may not be representative of the general diabetes population.
Our study provides no insight into whether ongoing therapy with DPP‐4is might impede SARS‐CoV‐2 from entering the cells, thus preventing patients with T2D from becoming infected. Patients admitted for COVID‐19 are not necessarily representative of all SARS‐CoV‐2‐infected people or those who developed severe forms of the disease. However, if DPP‐4is prevented infection by SARS‐CoV‐2, this would result in a lower prevalence of DPP‐4i users among hospitalized COVID‐19 patients.
Among other limitations, we acknowledge that calculation of the expected rate of exposure to DPP‐4is among control patients was based on data collected in 2018, is affected by ascertainment bias and by the variable degree of matching that could be achieved. In addition, we calculated that the study was powered to detect a difference in the rate of DPP‐4i users that was 1.8‐fold lower among COVID‐19 than among control patients. Therefore, we cannot rule out a smaller effect.
In conclusion, this study does not provide evidence for either a reduction or an increase in the risk of hospitalization for COVID‐19 patients with T2D. Nonetheless, DPP‐4is exhibit an optimal safety profile even in fragile populations, such as older people. 14 Treating people with T2D affected by COVID‐19 can be challenging because of the need to withdraw metformin resulting from hypoxia, and the concomitant use of drugs that can cause hyperglycaemia (glucocorticoids) or hypoglycaemia (hydroxychloroquine). 15 Thus, DPP‐4is remain a valid therapeutic option for the management of patients with T2D and symptomatic COVID‐19.
CONFLICT OF INTEREST
GPF received lecture fees or grant support from Abbott, AstraZeneca, Boehringer, Lilly, Merck‐Sharp‐Dome, Mundipharma, Novartis, Novo Nordisk, Sanofi and Servier. MLM received lecture fees or grant support from Amryt Pharma and Servier. BMB received lecture or advisory board fees from AstraZeneca, Eli Lilly, Boehringer Ingelheim, Novo Nordisk and Novartis. PF received lecture or consultancy fees from AstraZeneca, Boehringer, Lilly and Mundipharma. RV has received grants from AstraZeneca and personal fees from AstraZeneca, Novo Nordisk, Eli Lilly, Novartis and Gilead. AA received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier and Takeda. EL, SP, ES, GV, DF, ST, GC, GS, BdC, LT, AMC and AV have nothing to disclose.
AUTHOR CONTRIBUTIONS
Study design: GPF, MLM, EL, PF, RR and AA. Data collection and analysis: GPF, MLM, EL, BMB, SP, ES, GV, DF, ST, GC, GS, BdC, LT, AMC, AV, PF, RV and AA. Manuscript writing: GPF, MLM and EL. Manuscript revision: PF, RV and AA. All authors approved the final version of the manuscript. GPF is the guarantor of this work.
DATA AVAILABILITY
Original data are available from the corresponding author upon reasonable request.
Supporting information
Figure S1 Study flowchart. T2D, type 2 diabetes. COVID‐19, coronavirus disease 2019. DPP‐4i, dipeptidyl‐peptidase 4 inhibitors.
Table S1. Matching COVID‐19 patients in the administrative database. Patients with diabetes and COVID‐19 were matched 1:200 with patients with diabetes in the Region, or 1:100 with patients hospitalized for other causes. Patients with COVID‐19 pneumonia were matched 1:5 with patients with pneumonia of other origin. p‐values refer to the comparison with COVID‐19 patients (1) or COVID‐19 pneumonia patients (2).
Table S2. Matching COVID‐19 patients in the outpatient clinic database. Patients with diabetes and COVID‐19 were matched 1:10 with patients with diabetes followed at the local specialist diabetes outpatient clinic. CKD, chronic kidney disease. CVD, cardiovascular disease ACEi, angiotensin converting enzyme inhibitors. ARBs, angiotensin receptor blockers. CCB, calcium channel blockers. APT, anti‐platelet therapies.
Fadini GP, Morieri ML, Longato E, et al. Exposure to dipeptidyl‐peptidase‐4 inhibitors and COVID‐19 among people with type 2 diabetes: A case‐control study. Diabetes Obes Metab. 2020;22:1946–1950. 10.1111/dom.14097
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14097.
Funding information Università degli Studi di Padova
REFERENCES
- 1. Yuan Y, Qi J, Peng R, et al. Molecular basis of binding between middle east respiratory syndrome coronavirus and CD26 from seven bat species. J Virol. 2020;94:e01387‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature. 2013;495:251‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vankadari N, Wilce JA. Emerging WuHan (COVID‐19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morimoto C, Schlossman SF. The structure and function of CD26 in the T‐cell immune response. Immunol Rev. 1998;161:55‐70. [DOI] [PubMed] [Google Scholar]
- 6. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011. 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153‐165. [DOI] [PubMed] [Google Scholar]
- 8. Fadini GP, Avogaro A. Cardiovascular effects of DPP‐4 inhibition: beyond GLP‐1. Vascul Pharmacol. 2011;55:10‐16. [DOI] [PubMed] [Google Scholar]
- 9. Goossen K, Graber S. Longer term safety of dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes Obes Metab. 2012;14:1061‐1072. [DOI] [PubMed] [Google Scholar]
- 10. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest. 2020;43(6):867‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iacobellis G. COVID‐19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fadini GP, Albiero M, Menegazzo L, de Kreutzenberg SV, Avogaro A. The increased dipeptidyl peptidase‐4 activity is not counteracted by optimized glucose control in type 2 diabetes, but is lower in metformin‐treated patients. Diabetes Obes Metab. 2012;14:518‐522. [DOI] [PubMed] [Google Scholar]
- 13. Regulation Concerning the Health Information Exchange [Regolamento in materia di fascicolo sanitario elettronico]. 2019. http://wwwgazzettaufficialeit/eli/id/2015/11/11/15G00192/sg.
- 14. Schott G, Martinez YV, Ediriweera de Silva RE, et al. Effectiveness and safety of dipeptidyl peptidase 4 inhibitors in the management of type 2 diabetes in older adults: a systematic review and development of recommendations to reduce inappropriate prescribing. BMC Geriatr. 2017;17:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol. 2020;8(6):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study flowchart. T2D, type 2 diabetes. COVID‐19, coronavirus disease 2019. DPP‐4i, dipeptidyl‐peptidase 4 inhibitors.
Table S1. Matching COVID‐19 patients in the administrative database. Patients with diabetes and COVID‐19 were matched 1:200 with patients with diabetes in the Region, or 1:100 with patients hospitalized for other causes. Patients with COVID‐19 pneumonia were matched 1:5 with patients with pneumonia of other origin. p‐values refer to the comparison with COVID‐19 patients (1) or COVID‐19 pneumonia patients (2).
Table S2. Matching COVID‐19 patients in the outpatient clinic database. Patients with diabetes and COVID‐19 were matched 1:10 with patients with diabetes followed at the local specialist diabetes outpatient clinic. CKD, chronic kidney disease. CVD, cardiovascular disease ACEi, angiotensin converting enzyme inhibitors. ARBs, angiotensin receptor blockers. CCB, calcium channel blockers. APT, anti‐platelet therapies.
Data Availability Statement
Original data are available from the corresponding author upon reasonable request.


