To the Editor:
Hyperglycaemia, even in people without previous diabetes, has often been reported in the complicated coronavirus disease 2019 (COVID‐19). 1 , 2
Hyperglycaemia in COVID‐19 is a strong predictor of worsening the prognosis and increasing the possibility of death. 1 , 2 Therefore, understanding the process behind this evidence is of great interest.
It is known that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) may infect endocrine pancreas cells via their expression of angiotensin‐converting enzyme‐2 (ACE‐2) receptors. 3 It is possible that the pancreatic damage caused by the virus, and the resultant impairment in beta‐cell insulin secretion, may worsen pre‐existing diabetes or determine the appearance of hyperglycaemia in non‐diabetes. 3 Interestingly, the 2003 SARS, which was caused by another coronavirus closely related to COVID‐19, also produced a transient impairment of pancreatic islet cell function. 4 Cytokines, including IL‐6 and TNF‐alpha, have been found to be elevated in patients with severe COVID‐19. 5 Inflammation generates insulin resistance. 6 Therefore, it is possible that during COVID‐19, because of the huge production of cytokines, insulin resistance may be exacerbated or de novo‐induced. This phenomenon may also contribute to the appearance of hyperglycaemia. Interestingly, cytokines may, in turn, also affect beta‐cell function, contributing to a further decrease in insulin secretion. 6 Therefore, the generation of a vicious circle may be possible: SARS‐CoV‐2 infection, through both a decrease in insulin secretion and the appearance/worsening of insulin resistance, may induce hyperglycaemia, which, in turn, may further damage beta‐cells and worsen insulin resistance (Figure 1).
FIGURE 1.
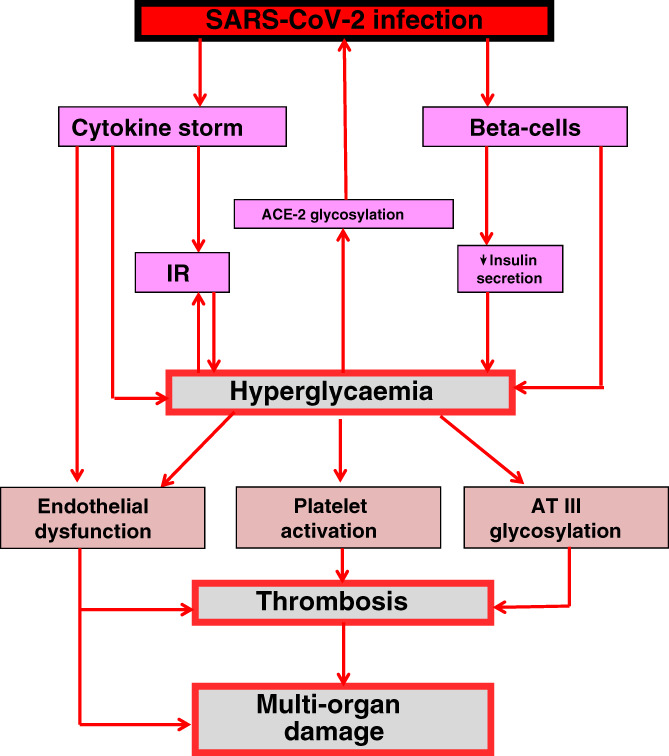
How hyperglycaemia may worse COVID‐19 and its prognosis. SARS‐CoV‐2 may affect beta‐cells, producing a reduction in insulin secretion. SARS‐CoV‐2 infection is also accompanied by a huge production of cytokines, which can induce insulin resistance (IR). Both reduced insulin secretion and IR may hesitate in hyperglycaemia, which, in turn, may further decrease insulin secretion and increase IR. Hyperglycaemia also generates non‐enzymatic glycosylation. Glycosylation of the angiotensin‐converting enzyme‐2 (ACE‐2) receptor can facilitate the entry of SARS‐CoV‐2 into the host cells. On the other hand, glycosylation of antithrombin III (AT III) may favour thrombus formation. Acute hyperglycaemia, through cytokine production or directly, may provoke endothelial dysfunction and thrombus formation, which in turn can lead to organ damage and fatal outcome of the disease
When generated, hyperglycaemia may also play a direct role in worsening the SARS‐CoV‐2 infection. Non‐enzymatic glycosylation is a reaction exacerbated by hyperglycaemia; the glycosylation of the ACE‐2 is needed for the linkage of the virus to this cellular receptor. 7 Therefore, high and aberrantly glycosylated ACE‐2 in the tissues in uncontrolled hyperglycaemia might favour the cellular intrusion of SARS‐CoV2, leading to a higher propensity to COVID‐19 infection and a higher severity of disease. 7 However, hyperglycaemia may not only worsen the COVID‐19 infection, but may also be directly involved in favouring a bad outcome of the disease. An increase in glycaemia is accompanied by overproduction of inflammatory mediators, 8 which are very dangerous for the cardiovascular system. Acute hyperglycaemia, through the glycosylation process, may also alter the function of some key proteins (e.g. antithrombin III) involved in protection from thrombus generation. 9 At the same time, through oxidative stress generation, an acute increase in glycaemia may induce endothelial dysfunction and thrombosis, 8 , 9 , 10 which, in turn, may produce generalized organ damage, as described in COVID‐19. 1
According to this view, it is evident that fast, tight control of hyperglycaemia during the early phase of COVID‐19 might be decisive. Normalizing blood glucose levels may stop the vicious circle resulting from hyperglycaemia, which leads to a worsening of the disease, as illustrated in Figure 1. This should probably improve the situation, thus instilling more optimism with regard to recovering from the disease.
CONFLICT OF INTEREST
AC, VDN and FP does not have any conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
AC wrote, revised and approved the final version of the manuscript. VDN and FP revised and approved the final version of the manuscript.
REFERENCES
- 1. Zhang Y, Li H, Zhang J, et al. The clinical characteristics and outcomes of diabetes mellitus and secondary hyperglycaemia patients with coronavirus disease 2019: A single‐centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020. 10.1111/dom.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID‐19. Diabetes Obes Metab. 2020;22(10):1897‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321. 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prete M, Favoino E, Catacchio G, Racanelli V, Perosa F. SARS‐CoV‐2 inflammatory syndrome. Clinical features and rationale for immunological treatment. Int J Mol Sci. 2020;21:E3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816‐823. [DOI] [PubMed] [Google Scholar]
- 7. Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID‐19 epidemic. J Med Virol. 2020;92(7):770‐775. 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceriello A, Standl E, Catrinoiu D, et al. Diabetes and cardiovascular disease (D&CVD) EASD study Group.Issues of cardiovascular risk management in people with diabetes in the COVID‐19 era. Diabetes Care. 2020;dc200941. 10.2337/dc20-0941. [DOI] [Google Scholar]
- 9. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119‐1125. [DOI] [PubMed] [Google Scholar]
- 10. Ceriello A, Zarich SW, Testa R. Lowering glucose to prevent adverse cardiovascular outcomes in a critical care setting. J Am Coll Cardiol. 2009;53(5 Suppl):S9‐S13. [DOI] [PubMed] [Google Scholar]


