Summary
Since the first World Health Organization notification on 31 December 2019, coronavirus disease 2019 (COVID‐19), the respiratory disease caused by the coronavirus severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), has been responsible for over four million confirmed infections and almost 300 000 deaths worldwide. The pandemic has led to over half of the world's population living under lockdown conditions. To allow normal life to resume, public health interventions will be needed to prevent further waves of infections as lockdown measures are lifted. As one of the most effective countermeasures against infectious diseases, an efficacious vaccine is considered crucial to containing the COVID‐19 pandemic. Following the publication of the genome sequence of SARS‐CoV‐2, vaccine development has accelerated at an unprecedented pace across the world. Here we review the different platforms employed to develop vaccines, the standard timelines of development and how they can be condensed in a pandemic situation. We focus on vaccine development in the UK and vaccines that have entered clinical trials around the world.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus‐2, vaccine
The SARS‐CoV‐2 pandemic has accelerated vaccine development at an unprecedented pace across the world, whilst utilising novel platform technologies. This review summarises the main platforms employed for vaccine development, the standard timelines of development and how they can be condensed during a pandemic. We focus on vaccine candidate development in the UK, and vaccines which have progressed to phase I clinical trials in the UK and worldwide.
Abbreviations
- ADE
antibody‐dependent enhancement
- APC
antigen‐presenting cell
- CEPI
Coalition for Epidemic Preparedness and Innovations
- ChAd
chimpanzee adenovirus
- COVID‐19
coronavirus disease 2019
- DC
dendritic cell
- DRC
Democratic Republic of the Congo
- EBOV
Ebola virus
- GMP
good manufacturing practice
- hAd
human adenovirus
- IL
interleukin
- LAV
live attenuated vaccine
- MERS
Middle Eastern respiratory syndrome
- S
Spike glycoprotein
- SARS
severe acute respiratory syndrome
- Th2
T helper type 2
- VLP
virus‐like particle
- VSV
vesicular stomatitis virus
Introduction
December 2019 saw the outbreak of a novel beta‐coronavirus from Wuhan, China. This virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and the corresponding disease – coronavirus disease 2019 (COVID‐19) – causes illness ranging from asymptomatic to severe respiratory distress, pneumonia and death. 1 Although its exact origins are unknown, SARS‐CoV‐2 shares genetic homology with other coronaviruses found in bats and with its most closely related human virus, SARS‐CoV‐1. 2 , 3 Beta‐coronaviruses are the causative agents of two previous outbreaks this millennium. The SARS‐CoV‐1 outbreak in 2003 caused similar respiratory pathology and amassed 8098 cases and 774 deaths. 4 , 5 SARS‐CoV‐1 potentially underwent zoonotic transmission between bats and palm civets before human infection. 6 Middle Eastern respiratory syndrome coronavirus (MERS‐CoV) is another related CoV identified in Saudi Arabia in 2012. Consequent outbreaks have determined a mortality rate of approximately 35%, although this does not include likely asymptomatic transmission. 7 , 8 Similarly, genomic evidence suggests that MERS‐CoV circulated between bats and camels before transmission to humans, 9 , 10 suggesting that bats are a natural reservoir species of coronaviruses, and that zoonosis can occur with close human‐to‐animal contact. 8 Two other beta‐coronaviruses, HCoV‐OC43 and HCoV‐HKU1, and two alpha‐coronaviruses, HCoV‐229E and HCoV‐NL63, circulate within the human population and cause mild respiratory pathogenesis. 8
COVID‐19 mortality is currently estimated to be approximately 3·4% globally, 11 with a higher risk of COVID‐19 mortality in older adults and those with pre‐existing co‐morbidities. 12 , 13 Since January 2020, SARS‐CoV‐2 has spread to more than 200 countries and caused over four million infections and over 300 000 deaths. 14 COVID‐19 was declared a global pandemic by the World Health Organization on 11 March 2020. 15
This outbreak of a previously unknown virus has instigated a worldwide effort to develop a vaccine for COVID‐19. The traditional vaccine development pipeline, which takes an average of 10 years from conception to licensing, 16 is unfeasible for a situation of this urgency. Therefore, new strategies for the accelerated development of vaccines, subsequent pre‐clinical and clinical trials, and rapid upscaling of manufacture are necessary to ensure there is no setback in the delivery of potential vaccine candidates. Currently, it is estimated that a vaccine for COVID‐19 will be widely available in 18–24 months if clinical trials demonstrate safety, immunogenicity and protective efficacy. 17
Vaccination against COVID‐19
Vaccination can generate long‐lasting immunity by exposing individuals to antigens, to drive the development of immunological memory before encounter with live pathogen. The resulting immunity can be mediated by humoral antibody induction, and/or cellular T‐cell effector function.
Spike glycoprotein (S) is the sole surface protein of the SARS‐CoV‐2 virion. 18 SARS‐CoV‐2 S mediates viral entry into host cells via angiotensin‐converting enzyme 2, which is expressed at high levels on the surface of pulmonary epithelial cells. 19
Currently, the most clinically advanced COVID‐19 vaccines target the S. In addition, the structure of the SARS‐CoV‐2 S shares homology with SARS‐CoV‐1 S, the primary target of the immune response and vaccine development for SARS. 20 , 21 The correlates of protection against SARS‐CoV‐2 are not yet fully understood, so it may be necessary that vaccination delivers robust humoral and cellular immunogenicity to increase the likelihood of inducing protection.
Humoral protection arises through structural epitope recognition of proteins. In SARS‐CoV‐2 infection, antibodies are most frequently generated against the S protein and the internal nucleoprotein. 22 Potential SARS‐CoV‐2 vaccines would ideally generate a long‐lasting humoral immunity with protective titres of neutralizing antibodies that do not cause antibody‐dependent enhancement (ADE) upon re‐infection. ADE occurs when antibodies to the pathogen, or cross‐reactive antibodies to a closely related pathogen, facilitate viral infection of cells instead of protecting the host and contribute towards an exacerbated pathology. 23 ADE was detrimental in SARS‐CoV‐1 pathogenesis during the 2002/03 outbreak, 23 and pre‐clinical trials demonstrated infection of macrophages via ADE, 24 , 25 and lethal pneumonia in mice. 26 , 27 ADE is therefore of potential concern for a SARS‐CoV‐2 vaccine.
Cellular immunogenicity is vital for vaccine‐derived immunity against intracellular pathogens, and for a rapid cytotoxic response to re‐infection. 28 , 29 However, acute SARS‐CoV‐2 infection may be worsened by a skewed immune response towards CD4+ T helper type 2 (Th2) cells involving interleukins 10 and 14 (IL‐10 and IL‐14), which initiates immunosuppression of inflammatory CD8+ T‐cell responses and could contribute towards more severe pathology. 30 , 31 Therefore, vaccines that do not induce a skewed Th2 immune response are thought to be optimal for SARS‐CoV‐2.
An adjuvant is a substance that is often co‐administered with a vaccine to enhance the immunogenicity and duration of protection by stimulating the innate immune system. The choice of adjuvant can also skew the T‐cell response towards a Th1‐ or Th2‐dominant reaction, necessitating careful planning when trying to avoid adverse events such as ADE. 32
Platforms for COVID‐19 vaccine development
Whole virion vaccines (live attenuated and inactivated)
Live attenuated vaccines (LAV) are viruses that are rendered replication‐incompetent through repeated passage in cell culture, and inactivated vaccines use whole pathogen that has typically been killed by exposure to chemicals (e.g. formaldehyde) or heat inactivation. 33 LAV are immunogenic and reproduce the breadth of the humoral and cellular immune protection that would be generated by live viral infection; 32 , 33 however, inactivated vaccines are generally less immunogenic and require more than one dose or an additional adjuvant. 34
Safety issues regarding the generation and subsequent attenuation of the virus, with potential for re‐activation in vaccinated individuals, means LAV are not a tenable vaccine strategy for highly pathogenic viruses. 33 , 35 This also prevents immunization of individuals with weakened immune systems who are at further risk of illness if the pathogen reverts. 36 From the perspective of vaccine distribution, LAV are generally kept refrigerated to preserve immunogenicity, which may be problematic in countries that cannot sustain cold‐chain distribution. 34 , 36
Live attenuated vaccines for SARS‐CoV‐1 were tested in pre‐clinical trials. 37 There is currently one company, Codagenix (Farmingdale, NY), proposing a computationally designed, lab‐made SARS‐CoV‐2 ‘virus’ that is immunogenic but not pathogenic. 38 SinoVac (Beijing, China) demonstrated safety and immunogenicity of an inactivated SARS‐CoV‐1 vaccine in a phase I trial, 39 and have determined efficacy of a formalin‐inactivated SARS‐CoV‐2 vaccine in rhesus macaques 40 (Table 1). Although this vaccine did not demonstrate any ADE‐derived pathogenesis, previous whole virus SARS‐CoV‐1 vaccines trialled in mice induced eosinophil‐derived immunopathology upon viral challenge, 27 and Th2‐driven histopathological changes in the lungs. 31
Table 1.
Categories of vaccine, with licensed and experimental examples
| Vaccine | Licensed vaccines | Example institutions developing COVID‐19 vaccines |
|---|---|---|
| Live attenuated | Smallpox, measles, yellow fever, mumps, influenza, rubella, varicella, polio | Codagenix (Farmingdale, NY) |
| Inactivated | Inactivated polio, rabies, hepatitis A | SinoVac (Beijing, China) |
| Protein subunit | Hepatitis B, Haemophilus influenzae B, pertussis, human papillomavirus, pneumococcal bacteria, meningococcal bacteria |
Generex Biotechnology (Miramar, FL) Vaxart (San Francisco, CA) Medicago (Quebec City, Canada) GSK (London, UK) and Clover Biopharmaceuticals (Chengdu, China) University of Queensland |
| Nucleic acid | None in humans |
Moderna (Cambridge, MA) Inovio Pharmaceuticals (Plymouth Meeting, PA) Pfizer (New York, NY) Imperial College London Takis Biotech (Rome, Italy) BIOCAD (St Petersburg, Russia) |
| Viral‐vectored | Japanese encephalitis (Australia), dengue virus (Mexico and Brazil) |
University of Oxford CanSino Biologics (Tianjin, China) Greffex (Houston, TX) Janssen (Beerse, Belgium) |
Protein subunit vaccines
Protein subunit vaccines include antigenic proteins thought to induce a protective immune response. This vaccine type is produced in vitro and circumvents handling highly pathogenic live viruses. 33 , 41 Subunit vaccines predominantly elicit a humoral antibody response, and most are administered with an adjuvant, which is a prerequisite to stimulate a strong immune response and generate a higher‐quality immune memory in humoral and cellular compartments. However, the inclusion of adjuvants can increase the reactogenicity and production costs, which are important considerations. 41 Virus‐like particles (VLP) are a type of subunit vaccine that present many copies of the relevant antigen in a three‐dimensional virus‐like structure, and may be immunogenic enough to not require the inclusion of adjuvants. 41
Subunit vaccines are an attractive vaccine technology for rapid vaccine development, and multiple institutions worldwide are developing protein subunit‐based vaccines (Table 1). They can be upscaled for mass production at good manufacturing practice (GMP) standards, 42 and distribution has less reliance on cold‐chain systems. 34 However, they can require bespoke manufacturing processes, which can increase cost, and may require specific mammalian cell expression and optimization. 18 , 43
Nucleic acid vaccines
Similar to subunit vaccines, specific proteins from the target pathogen are chosen for their immunogenic epitopes; however, these proteins are delivered as either plasmid DNA or RNA sequences. 44 , 45 Upon vaccination, the host cell manufactures the pathogen protein, which is recognized by the immune system as foreign and generates an immune response. 44 Non‐capsulated RNA vaccines are readily removed by the host cell upon injection, so advances in delivery technology, including encapsulation of RNA in liposomes, have been developed to avoid degradation. 46
RNA vaccines have been shown to induce antigen‐specific antibody and polyfunctional T‐cell responses in phase I clinical trials of cancer vaccines, 46 and functional antibodies against rabies virus glycoprotein; 47 however, there are currently no licensed RNA vaccines for humans. Although DNA vaccines are immunogenic in small animal models, they show less immunogenicity in human clinical trials and require adjuvants or multiple doses. 34 , 48 Four DNA vaccines are available for animal use; 46 however, there are currently none licensed for humans. 49
There are several nucleic acid vaccines in development for COVID‐19 prophylaxis (Table 1). Nucleic acid vaccines are relatively cheap and rapid to manufacture, with the possibility to mass‐produce large‐scale GMP product. 50
Recombinant viral‐vectored vaccines
Recombinant viral‐vectored vaccines use the host’s innate immunity to generate self‐adjuvanted immunogenicity, while eliciting a targeted immune response against genetically encoded pathogen antigens. 51 The viral vector ‘backbone’ is constructed from a genetically modified virus, 52 examples being adenoviruses, poxviruses and vesicular stomatitis virus (VSV). 52 , 53 This vector typically has insertion sites for gene(s) of the target pathogen, which are expressed intracellularly in the host upon vaccination. 54
Important considerations for development of virus vectored vaccines is the generation of immunity towards the vector, which could hinder the antigen‐specific response upon a boost vaccination. However, reports from pre‐clinical and clinical studies show sufficient protection can be elicited from a single dose. 55 , 56
Human adenoviruses (hAds) are a frequently used viral vector thaat circulate at high frequency in most populations, 57 contributing towards demographically variable yet significant pre‐existing immunity that can reduce vaccine efficacy. 54 Vectors constructed from chimpanzee adenovirus (ChAd) were developed to elicit immunogenicity that is similar or superior to that of hAd vectors, while having significantly reduced seroprevalence and hence neutralizing antibodies in most populations. 28 , 58 In pre‐clinical studies, ChAd vectors have demonstrated up to 100% efficacy with a single vaccination against several emerging pathogens. 56 , 59 Clinical trials have established that ChAd vectors also have a good safety profile and immunogenicity for influenza A virus, 60 Ebola virus (EBOV), 61 and MERS‐CoV. 62
Adenovirus vectors can be rapidly made to GMP at large scale, and a single vaccination can be sufficient to provide rapid immunity in individuals. 63 This rapid production and distribution pipeline was tested during the 2013–2016 EBOV outbreak in Guinea, Liberia and Sierra Leone, where five viral‐vectored vaccines were rapidly escalated to clinical trials. 63 A recombinant VSV vector expressing the EBOV glycoprotein (rVSV‐ZEBOV) progressed to phase III trials in Guinea and Sierra Leone and provided 100% efficacy across 4359 individuals vaccinated with a single dose. 55 Following the second EBOV outbreak in the Democratic Republic of the Congo (DRC) in 2018, the World Health Organization allowed compassionate use of rVSV‐ZEBOV in the DRC; and it has now been licensed in the DRC, Burundi, Ghana and Zambia. 64 An Ad26‐vectored EBOV vaccine has also been developed by Janssen (Beerse, Belgium) and tested extensively in a prime‐boost regimen in sub‐Saharan Africa for efficacy and immunogenicity. 65
Timelines in vaccine development
Development of vaccines is a long process, taking at least 10 years per vaccine. 66 Most of the duration of vaccine development is determined by clinical trials, which are split into three phases between pre‐clinical exploratory work and licensure of the vaccine: 67
Phase I: First‐in‐human experiments on a small number of healthy volunteers, who have not been exposed to the pathogen. The trial focuses on safety and immunogenicity of the vaccine. 67
Phase II: Vaccines successful in phase I move into phase II trials. Phase II trials have a greater focus on the immunogenicity of the vaccine and expand the cohort across a wider breadth of the population, allowing for immune response to be analysed across age, gender, ethnicity and other variables. 67 Efficacy may also be assessed at this stage, with controlled human microbial infection studies giving a useful early indication of potential efficacy for diseases where robust controlled human microbial infection studies models are available. 68
Phase III: the efficacy of the vaccine is assessed across a larger population. Phase III studies enrol enough participants to ensure statistical power to assess if the immune response stimulated by the vaccine is sufficient to protect against disease. The clinical end point of phase III vaccine studies is often determined by reduction in case numbers or severity of disease in the cohort and requires an active outbreak. 67
Phase overlap
Vaccine trials are extremely expensive, 17 representing a huge financial risk, and as such the vaccine timeline is extensive. 66 It is estimated to cost US$31–68 million to bring a candidate to the end of phase IIa trials. 69 Ethical acceleration of the trial can be achieved by performing phase I/II and phase II/III studies in parallel once sufficient data have been extracted from the preceding phase. 70 This can entail a larger risk from commercial investors and therefore requires philanthropic organizations such as the Coalition for Epidemic Preparedness Innovations (CEPI) and others to fund the parallel phases to accelerate vaccine development through clinical trials at a rapid rate. 70
COVID‐19 vaccine development: the UK perspective
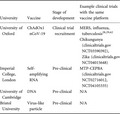
There are a number of COVID‐19 vaccines under clinical development at research institutes in the UK, at least three of which are at the pre‐clinical stage, and one of which has progressed to clinical trial recruitment (Table 2).
Table 2.
COVID‐19 vaccine development in the UK
| University | Vaccine | Stage of development | Example clinical trials with the same vaccine platform |
|---|---|---|---|
| University of Oxford | ChAdOx1 nCoV‐19 | Clinical trial recruitment |
MERS, influenza, tuberculosis 28 , 29 , 62 Chikungunya (clinicaltrials.gov NCT03590392), Zika (clinicaltrials.gov NCT04015648) |
| Imperial College, London | Self‐amplifying RNA | Pre‐clinical | MTP‐CEPBA (clinicaltrials.gov NCT02716012, NCT04105335) |
| University of Cambridge | DNA | Pre‐clinical | N/A |
| Bristol University | Virus‐like particle | Pre‐clinical | N/A |
This table outlines UK universities developing COVID‐19 vaccines, the vaccine platform under development, the stage of development for each vaccine, and examples of previous or ongoing clinical trials for this vaccine platform co‐ordinated by the university.
Pre‐clinical COVID‐19 vaccine research
A self‐amplifying RNA vaccine encoding SARS‐CoV‐2‐S is under development at Imperial College, London. Previous self‐amplifying RNA vaccines against influenza A virus haemagglutinin and Toxoplasma gondii NTPase‐II have induced cellular and humoral immunogenicity in mice, 71 , 72 and a first‐in‐class clinical trial using self‐amplifying RNA against hepatocellular carcinoma encoding the transcription factor C/EBP‐α demonstrated acceptable safety and tolerability. 73 This COVID‐19 vaccine is currently in pre‐clinical testing, with a target clinical trial date set for Summer 2020. 74
Bristol University, with spin‐out company Imophoron (Bristol, UK), is developing a SARS‐CoV‐2‐S VLP vaccine with their adenovirus‐based ADDomer© technology. Imophoron states that the ADDomer© VLP platform has many of the main benefits of VLP‐based vaccines, including rapid development, self‐adjuvant properties and no cold storage requirement. 75 An ADDomer© VLP vaccine expressing Chikungunya virus E2 protein also proved immunogenic in pre‐clinical trials, 76 although this platform has not yet been tested in humans.
The University of Cambridge and DioSynVax (Cambridge, UK) are using a bioinformatic approach to design an optimal SARS‐CoV‐2 genetic sequence, which will be used to make a vaccine encoding SARS‐CoV‐2‐S. 77
UK COVID‐19 vaccine clinical trials
The University of Oxford COVID‐19 vaccine has recently entered clinical trials in the UK (Table 2). This approach, using viral‐vectored vaccine technology, is being led by the Jenner Institute and the Oxford Vaccine Group. 78 The vaccine, known as ChAdOx1 nCOV‐19, uses a replication‐deficient ChAd viral vector to encode SARS‐CoV‐2‐S. ChAdOx1 is derived from the ChAd isolate Y25 79 and has been tested in many pre‐clinical and clinical trials, demonstrating safety with robust humoral and cellular immunogenicity and durable protection. 80 , 81 , 82 At the beginning of the SARS‐CoV‐2 outbreak, the Jenner Institute was conducting CoV vaccine development against MERS‐CoV with collaborative ChAdOx1‐MERS‐CoV phase I clinical trials in Oxford and the Kingdom of Saudi Arabia. 83 , 84
Screening, recruitment and vaccination for the ChAdOx1 nCoV‐19 phase I/II clinical trial is currently underway and at the time of writing, approximately 900 healthy volunteers aged 18–55 years have been vaccinated with 5 × 1010 viral particles. ChAdOx1 nCoV‐19 or a Meningitis‐ACWY vaccine via intramuscular vaccination, in a randomized 1 : 1 ratio of trial vaccine to control (clinicaltrials.gov: NCT04324606). The primary and co‐primary objectives of this study are to assess the safety and efficacy of the vaccine. The humoral and cellular immune responses to vaccination will be assessed as secondary end points. 85
The current planned phase II clinical trial will recruit volunteers between the ages of 55 and 70, age 70+ and children age 5–12 years to assess the vaccine across broader demographics. A phase III clinical trial is planned to recruit more than 10 000 volunteers over 18 years old to assess the efficacy of the ChAdOx1 nCoV‐19 vaccine. 86
COVID‐19 vaccine development: worldwide clinical trials
In April, CEPI announced the number of COVID‐19 vaccine candidates around the world had exceeded 100. 16 Here we will focus on those candidates that have entered early clinical trials by April 2020. Table 3 shows data on the protocols for each trial.
Table 3.
Selected phase I clinical trials for vaccines against SARS‐CoV‐2
| Vaccine | Vaccine platform | Location | Cohort size (no. groups) | Dose(s) | Vaccination schedule | Immunological objectives |
|---|---|---|---|---|---|---|
|
mRNA‐1273 Moderna Inc. (Cambridge, US) |
mRNA in lipid nanoparticle | USA | 45 (3) |
Group 1: 25 μg RNA Group 2: 100 μg RNA Group 3: 250 μg RNA |
Day 1: Prime Day 29: Boost |
Antibodies up to 28 days post‐boost |
|
BNT162 BioNtech (Mainz, Germany) |
Modified RNA (a1, b1, b2) or self‐amplifying RNA (c2) in a lipid nanoparticle | Germany | 200 (4) | Unknown |
Day 0 (all groups): Prime Day 21 (a1, b1, b2): Boost |
Total and neutralizing antibodies at days 7, 21, 42, 84 and 183 post‐prime |
|
INO‐4800 (Inovio Pharmaceuticals) |
DNA | USA | 40 (2) |
Group 1: 1 mg DNA Group 2: 2 mg DNA |
Day 0: Prime Day 28: Boost |
Antibodies and cellular response from week 0 to week 28 post‐boost |
|
bacTRL‐Spike Symvivo Corporation (Burnaby, Canada) |
DNA with Bifidobacterium longum delivery vector | Canada | 84 (4) |
Group 1a: 1 × 109 Bifidobacterium longum Group 2a: 3 × 109 Bifidobacterium longum Group 3a: 1 × 1010 Bifidobacterium longum Group 1b, 2b, 3b: Placebo |
Day 0: Prime | Antibodies at months 1,3 and 12 post‐prime. Presence of bacTRL‐Spike in stool up to 12 months post‐prime |
|
Ad5‐nCoV ( CanSino Biologics) |
Adenovirus viral vector | China | 108 (3) |
Group 1: 5x1010 vp Group 2: 1 × 1011 vp Group 3: 1·5 × 1011 vp |
Day 0: Prime only | Total and neutralizing antibodies and cellular response at days 14 and 28, and months 3 and 6 post‐prime |
| ChAdOx‐1 nCoV‐19 University of Oxford, AstraZeneca (Cambridge, UK) | Chimpanzee adenovirus viral vector | UK | 510 (5) |
Group 1a, 2a, 3: 5 × 1010 vp Group 1b, 2b: meningitis ACWY standard dose |
Day 0: Prime Week 4 (group 3, 10 participants): boost |
Total and neutralizing antibodies and cellular response up to 6 months post‐vaccination |
|
COVID‐19/APC Shenzhen Geno‐Immune Medical Institute |
Lentivirus‐transduced APC | China | 100 (1) | Group 1: 5 × 106 APCs |
Day 0: Prime Day 14: Boost Day 28: Boost |
Cellular response at days 14 and 28 post‐prime |
| COVID‐19 Synthetic Minigene Vaccine Shenzhen Geno‐Immune Medical Institute | LV‐DCs and antigen‐specific CTLs | China | 100 (1) | Group 1: 5 × 106 LV‐DCs and 1 × 108 CTLs | Day 0: Prime | No immunological outcomes assessed |
| Inactivated SARS‐CoV‐2 Sinovac Research & Development (Beijing, China) | SARS‐CoV‐2 virus inactivated by β‐propiolactone | China | 744 (6) |
Group 1 and 4: 600 SU/0·5 ml Group 2 and 5: 1200SU/0·5 ml Group 3 and 6: Placebo |
Day 0 (all groups): Prime Day 14 (Groups 1,2 and 3): Boost Day 28 (Groups 4, 5 and 6): Boost |
Group 1,2,3: Total and neutralizing antibodies at day 7,14, 21, 28 and 42, cellular response at day 14 and 28 Group 4, 5, 6: Total and neutralizing antibodies at days 28, 35, 42 and 56, cellular response at days 14 and 42 |
Abbreviations: APC, antigen‐presenting cell; COVID‐19, coronavirus disease 2019; CTL, cytotoxic T lymphocyte; LV‐DC, lentivirus‐transduced dendritic cell; MERS, Middle East respiratory syndrome; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
The table lists: vaccine name, vaccine platform, the trial location, the trial size and number of trial groups, dosing size, vaccination regimen and the primary immunological objective of the trial.
Data sourced from clinicaltrials.gov identifiers: Moderna: NCT04283461, BioNTech: EudraCT 2020‐001038‐36, Inovio: NCT04336410, Symvivo: NCT04334980, CanSino: NCT04313127, University of Oxford: NCT04324606, Shenzhen Geno‐Immune Medical Institute: NCT04299724 & NCT04276896, Sinovac Biotech: NCT04352608.
mRNA platforms
mRNA1273 (Moderna) and BNT162 (BioNTech)
The Moderna and BioNTech platforms are messenger RNA (mRNA) molecules expressing SARS‐CoV‐2‐S, contained within lipid nanoparticles to facilitate entry of mRNA into the host cells. 87 , 88 Once inside the host cell, the S protein will be expressed and induce antibody responses. The Moderna vaccine was the first to progress to phase I clinical trials in humans. 89
The Moderna platform has been used in phase I clinical trials for several pandemic potential diseases, including MERS, Zika (clinicaltrials.gov NCT04064905) and pandemic influenza. 87 Phase I data for a pandemic influenza vaccine focused on a cohort vaccinated with 25, 50 or 100 µg of vaccine. The vaccine had a good safety profile and showed seroconversion in volunteers who received the highest dose. Crucially, 87% of volunteers who seroconverted developed neutralizing antibodies. The platform did not however generate any measurable cell‐mediated cytokine responses. 87
The BioNTech platform has been used in Zika virus vaccine development. Vaccination with a 30‐µg or 50‐µg dose in C57BL/6 mice and rhesus macaques respectively induced neutralizing antibody titres against Zika virus, and protected challenged animals from detectable viraemia. 88 This mRNA platform is also being used in phase I/II clinical trials for several cancer vaccines. 90
DNA vaccines
INO‐4800 (Inovio Pharmaceuticals) and bacTRL‐Spike (Symvivo Corporation)
INO‐4800, and the previous Inovio vaccine INO‐4700, express either SARS‐CoV‐2‐S or MERS‐CoV‐S respectively within an identical DNA vaccine vector. 91 The vaccine is administered through intramuscular injection, followed by electroporation of the injection site. The need for electroporation may limit the ability of INO‐4800 to be extended to the scales needed for a global pandemic and may be difficult to administer worldwide.
INO‐4700 showed promising immunogenicity in a phase I clinical trial after multiple immunizations. 92 The trial administered three doses of vaccine, and was split across high, medium and low groups. Initial seroconversion peaked at 86% of the total cohort before falling slightly by the trial end point. Titres of neutralizing antibodies showed similar patterns, peaking at 43% of the cohort 2 weeks after final vaccination, before being detected in only 3% of the cohort at the trial end point. Seventy‐six per cent of the total cohort also showed an interferon‐γ T‐cell response against MERS‐specific peptides. 92
Pre‐clinical animal models of INO‐4800 have been published in pre‐print. These demonstrate seroconversion in all animals and robust T‐cell interferon‐γ responses 10 days post‐vaccination against peptides spanning SARS‐CoV‐2‐S (pre‐print ahead of publication, https://doi.org/10.21203/rs.3.rs‐16261/v1).
The bacTRL platform from Symvivo Corporation uses engineered probiotic Bifidobacterium longum to deliver a DNA vaccine expressing SARS‐CoV‐2‐S into intestinal cells. The phase I trial of a COVID‐19 vaccine will also be the first‐in‐man study of the bacTRL platform, so no previous immunological data are available (clinicaltrials.gov NCT04334980).
Viral vectored vaccines
Ad5‐nCoV (CanSino Biologics), APCs and LV‐DC/CTLs (Shenzhen Geno‐Immune Medical Institute)
The Ad5 platform from CanSino Biologics demonstrated safety in phase I/II trials for Ad5‐EBOV, 54 a vaccine against the Zaire strain of EBOV. In addition, Ad5‐EBOV induced humoral immune responses, with 100% seroconversion of vaccines in a phase I trial. Volunteers also exhibited an interferon‐γ T‐cell response, significantly different from the placebo group, suggesting the induction of limited cell‐mediated immunity. 54 This vaccine has subsequently been licenced for emergency use in China against EBOV.
In the phase I Ad5‐EBOV trial, the study found that participants with pre‐existing Ad5 neutralizing antibodies showed significantly lower humoral and cellular responses to the EBOV glycoprotein than participants that were seronegative against Ad5. 54
The Shenzhen Geno‐Immune institute are using lentiviruses to transduce dendritic cells (DCs) and antigen‐presenting cells (APCs) to induce cytotoxic T‐cell responses in individuals who have developed COVID‐19. Dendritic cells are a subset of APCs that can be modified to express and present antigens, and this property has been used in phase I trials with tumour‐associated and neo‐antigen cancer vaccines. 93 The trials will test both the efficacy of APCs as a vaccine alone (clinicaltrials.gov NCT04299724), and administration of modified DCs with donor cytotoxic T cells as a combination therapeutic and vaccination (clinicaltrials.gov NCT04276896).
Inactivated vaccines
Inactivated SARS‐CoV‐2 (Sinovac Research and Development)
Sinovac has published pre‐clinical data on the efficacy of an inactivated SARS‐CoV‐2 vaccine in a macaque challenge model. 40 Rhesus macaques received three vaccine doses of either 3 or 6 µg at 1‐week intervals. Titres of IgG and neutralizing antibody after the third vaccination were similar to those induced by natural infection in recovered patients. T‐cell responses were not reported. Macaques were challenged 8 days after the third vaccination and displayed a reduction in viral load compared with unvaccinated animals; encouragingly, there was no evidence of ADE or Th2‐skewed immune responses in vaccinated animals. 40
COVID‐19 vaccine directions
The COVID‐19 pandemic has driven vaccine research and development into unprecedented territory. Non‐clinical suppression strategies involving contact tracing and social distancing have been employed globally to varying degrees. 17 There is growing evidence that these interventions have had considerable impact on ‘flattening the curve’ of the epidemic, thus reducing the burden on overstretched health‐care systems, and allowing time for vaccine and antiviral development. However, relaxing social distancing measures too soon may result in a second peak in infections. 94 The social, economic and health effects of these measures on society will be fully realized over the coming months, although it is likely that the cost of these strategies will be steep. Furthermore, healthcare facilities have been subjected to intense pressure and high demand, with supplies of personal protective equipment in short supply across the UK and internationally. 95 Together, these limitations put the lives of health‐care workers and patients at higher risk. 96 The length of natural protection post‐exposure is currently unknown, and could result in regular circulation of SARS‐CoV‐2 if immunity is not long‐lived. 97 The development of a safe and effective vaccine, therefore, is vital for mass protection of those most at risk from COVID‐19‐induced disease. This will reduce the number of hospitalized cases, subsequently relieving the burden on health‐care systems, and will allow for relaxation of physical distancing interventions.
What is needed from a vaccine for COVID‐19?
The ideal candidate COVID‐19 vaccine would have good safety and immunogenicity profiles in all age groups and demographics including pregnant women and immunocompromised individuals, and would generate robust cellular and humoral immunity with a single vaccination, which could potentially be boosted for long‐lasting memory durability. 52 , 63 Single‐shot efficacy was demonstrated in clinical trials during the latter half of the 2013–2016 EBOV outbreak, where one vaccination of rVSV‐ZEBOV conferred up to 100% protection for at least 84 days. 55 A ChAd vectored vaccine (ChAd3 EBOZ GP) induced similar immunogenicity, also with a single dose, suggesting that multiple viral vector vaccines are effective at inducing high levels of immunity after a single dose. 98 Single‐dose protective efficacy would offer fast protection to frontline health‐care workers and those in close contact with infected individuals, with additional booster vaccines to extend duration of immunogenicity if needed. 97 Public concerns surrounding vaccine safety may be heightened during the outbreak of an unknown pathogen and unclear scientific reporting. Vaccine hesitancy because of perceived risk is a globally observed phenomenon, 99 and care must be taken to ensure that the public is aware that full safety and regulatory requirements of a new and rapidly developed vaccine against SARS CoV‐2 have been completed with due care.
The ability to generate large quantities of GMP vaccine in a short duration of time is also essential. The cost of producing COVID‐19 vaccines for public use will be steep and will exceed CEPI’s $2 billion fundraising goal, which will establish GMP manufacturing sites, but not cover eventual vaccine manufacturing. 17 Global input will be necessary to fund and produce vaccines at multiple sites across the world, while ensuring fair and equitable distribution. It is hoped that more than one vaccine candidate undergoing current development and clinical testing will be suitable for mass vaccination in the coming year. In this manner, global need will hopefully be adequately addressed.
Unparalleled vaccine research and development is ongoing for the SARS‐CoV‐2 epidemic. An efficacious and publicly available vaccine will significantly reduce the impact of the current and possible future epidemic peaks, so reducing the burden on national health‐care systems. It is vital, however, that we continue to develop tenable vaccines that are both safe and immunogenic, that can be manufactured at scale and that can be distributed to both economically stable countries and to low‐ and middle‐income countries, ensuring equal access.
Disclosures
Teresa Lambe is named as an inventor on a patent application covering a SARS‐CoV‐2 (nCoV‐19) vaccine. The remaining authors declare no competing interests.
Acknowledgements
We would like to thank Professor Sarah Gilbert for help with reviewing, structure and content.
References
- 1. Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID‐19): a clinical update. Front Med 2020; 14:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang JT, Chang SC. Severe acute respiratory syndrome. Curr Opin Infect Dis 2004; 17:143–8. [DOI] [PubMed] [Google Scholar]
- 5. Kapp C. WHO lowers figures on SARS infections. Lancet 2003; 362:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH et al Severe acute respiratory syndrome coronavirus‐like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA 2005; 102:14040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han HJ, Wen HL, Zhou CM, Chen FF, Luo LM, Liu JW et al Bats as reservoirs of severe emerging infectious diseases. Virus Res 2015; 205:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fung TS, Liu DX. Human coronavirus: host–pathogen interaction. Annu Rev Microbiol 2019; 73:529–57. [DOI] [PubMed] [Google Scholar]
- 9. Azhar EI, El‐Kafrawy SA, Farraj SA, Hassan AM, Al‐Saeed MS, Hashem AM et al Evidence for camel‐to‐human transmission of MERS coronavirus. N Engl J Med 2014; 370:2499–505. [DOI] [PubMed] [Google Scholar]
- 10. van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM et al Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012; 3 6:e00473‐12 10.1128/mBio.00473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. worldometers.info . Coronavirus death rate, 2020. URL https://www.worldometers.info/coronavirus/ [accessed on 14 May 2020].
- 12. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID‐19 in elderly patients: A comparison with young and middle‐aged patients. J Infect 2020; 80:e14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. worldometers.info . Countries where Coronavirus has spread 2020. URL https://www.worldometers.info.coronavirus/countries‐where‐coronavirus‐has‐spread/ [accessed on 15 May 2020].
- 15. WHO.int . WHO Director‐General's opening remarks at the media briefing on COVID‐19 11 March 2020, 2020. URL https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020 [accessed on 21 April 2020].
- 16. Le Thanh T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M et al The COVID‐19 vaccine development landscape. Nat Rev Drug Discov 2020; 19:305–6. [DOI] [PubMed] [Google Scholar]
- 17. Yamey G, Schäferhoff M, Hatchett R, Pate M, Zhao F, McDade KK. Ensuring global access to COVID‐19 vaccines. Lancet 2020; 395:1405–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O et al Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 2020; 367:1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K et al A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004; 428:561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang YD, Sin WY, Xu GB, Yang HH, Wong TY, Pang XW et al T‐cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T‐cell immune response in patients who recover from SARS. J Virol 2004; 78:5612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC et al Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis 202020 5:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tetro JA. Is COVID‐19 receiving ADE from other coronaviruses? Microbes Infect 2020; 22:72–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yip MS, Leung NH, Cheung CY, Li PH, Lee HH, Daëron M et al Antibody‐dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J 2014; 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang ZY, Werner HC, Kong WP, Leung K, Traggiai E, Lanzavecchia A et al Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc Natl Acad Sci USA 2005; 102:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK et al Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe 2016; 19:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M et al A double‐inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 2011; 85:12201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol 2016; 11:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ewer K, Sebastian S, Spencer AJ, Gilbert S, Hill AVS, Lambe T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum Vaccin Immunother 2017; 13:3020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseng CT, Sbrana E, Iwata‐Yoshikawa N, Newman PC, Garron T, Atmar RL et al Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 2012; 7:e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011; 12:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol 2010; 28:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J, Arun Kumar S, Jhan YY, Bishop CJ. Engineering DNA vaccines against infectious diseases. Acta Biomater 2018; 80:31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amanat F, Krammer F. SARS‐CoV‐2 vaccines: Status report. Immunity 202052 4:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. PublicHeath.org . How Vaccines Work, 2020. URL https://www.publichealth.org/public‐awareness/understanding‐vaccines/vaccines‐work/ [accessed on 16 April 2020].
- 37. Luo F, Liao FL, Wang H, Tang HB, Yang ZQ, Hou W. Evaluation of antibody‐dependent enhancement of SARS‐CoV infection in rhesus macaques immunized with an inactivated SARS‐CoV vaccine. Virol Sin 2018; 33:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Codagenix.com . Platform Overview, 2020. URL https://codagenix.com/technology/platform‐overview/ [accessed on 16 April 2020].
- 39. Lin JT, Zhang JS, Su N, Xu JG, Wang N, Chen JT et al Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther 2007; 12:1107–13. [PubMed] [Google Scholar]
- 40. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M et al Rapid development of an inactivated vaccine candidate for SARS‐CoV‐2. Science 2020; 10.1126/science.abc1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karch CP, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol 2016; 120:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClean S. Prospects for subunit vaccines: technology advances resulting in efficacious antigens requires matching advances in early clinical trial investment. Hum Vaccin Immunother 2016; 12:3103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plotkin S, Robinson JM, Cunningham G, Iqbal R, Larsen S. The complexity and cost of vaccine manufacturing – an overview. Vaccine 2017; 35:4064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geall AJ, Mandl CW, Ulmer JB. RNA: the new revolution in nucleic acid vaccines. Semin Immunol 2013; 25:152–9. [DOI] [PubMed] [Google Scholar]
- 45. Blakney AK, McKay PF, Christensen D, Yus BI, Aldon Y, Follmann F et al Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J Control Release 2019; 304:65–74. [DOI] [PubMed] [Google Scholar]
- 46. Ulmer JB, Mason PW, Geall A, Mandl CW. RNA‐based vaccines. Vaccine 2012; 30:4414–8. [DOI] [PubMed] [Google Scholar]
- 47. Alberer M, Gnad‐Vogt U, Hong HS, Mehr KT, Backert L, Finak G et al Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open‐label, non‐randomised, prospective, first‐in‐human phase 1 clinical trial. Lancet 2017; 390:1511–20. [DOI] [PubMed] [Google Scholar]
- 48. Martin JE, Louder MK, Holman LA, Gordon IJ, Enama ME, Larkin BD et al A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine 2008; 26:6338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kramps T, Elbers K. Introduction to RNA vaccines. Methods Mol Biol 2017; 1499:1–11. [DOI] [PubMed] [Google Scholar]
- 50. Lee LYY, Izzard L, Hurt AC. A review of DNA vaccines against influenza. Front Immunol 2018; 9:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vitelli A, Folgori A, Scarselli E, Colloca S, Capone S, Nicosia A. Chimpanzee adenoviral vectors as vaccines – challenges to move the technology into the fast lane. Expert Rev Vaccines 2017; 16:1241–52. [DOI] [PubMed] [Google Scholar]
- 52. Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol 2016; 41:47–54. [DOI] [PubMed] [Google Scholar]
- 53. Capone S, D'Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A et al Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines 2013; 12:379–93. [DOI] [PubMed] [Google Scholar]
- 54. Wu L, Zhang Z, Gao H, Li Y, Hou L, Yao H et al Open‐label phase I clinical trial of Ad5‐EBOV in Africans in China. Hum Vaccin Immunother 2017; 13:2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henao‐Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M et al Efficacy and effectiveness of an rVSV‐vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open‐label, cluster‐randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Doremalen N, Haddock E, Feldmann F, Meade‐White K, Bushmaker T, Fischer RJ et al A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv 2020. Published ahead of print. 10.1126/sciadv.aba8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jartti T, Jartti L, Ruuskanen O, Söderlund‐Venermo M. New respiratory viral infections. Curr Opin Pulm Med 2012; 18:271–8. [DOI] [PubMed] [Google Scholar]
- 58. Dudareva M, Andrews L, Gilbert SC, Bejon P, Marsh K, Mwacharo J et al Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine 2009; 27:3501–4. [DOI] [PubMed] [Google Scholar]
- 59. Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau‐Kilby AW, Johnson JC et al Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–9. [DOI] [PubMed] [Google Scholar]
- 60. Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T et al Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther 2014; 22:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Venkatraman N, Ndiaye BP, Bowyer G, Wade D, Sridhar S, Wright D et al Safety and immunogenicity of a heterologous prime‐boost Ebola virus vaccine regimen – ChAd3‐EBO‐Z followed by MVA‐EBO‐Z in healthy adults in the UK and Senegal. J Infect Dis 2018219 8:1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Folegatti PM, Bittaye M, Flaxman A, Lopez FR, Bellamy D, Kupke A et al Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral‐vectored vaccine: a dose‐escalation, open‐label, non‐randomised, uncontrolled, phase 1 trial. Lancet Infect Dis 2020. 10.1016/S1473-3099(20)30160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lambe T, Bowyer G, Ewer KJ. A review of phase I trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Philos Trans R Soc Lond B Biol Sci 2017; 372(1721): 10.1098/rstb.2016.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. WHO.int . Four countries in the African region license vaccine in milestone for ebola prevention. https://www.who.int/news‐room/detail/14‐02‐2020‐four‐countries‐in‐the‐african‐region‐license‐vaccine‐in‐milestone‐for‐ebola‐prevention [accessed on 10 May 2020].
- 65. Anywaine Z, Whitworth H, Kaleebu P, Praygod G, Shukarev G, Manno D et al Safety and immunogenicity of a 2‐dose heterologous vaccination regimen with Ad26.ZEBOV and MVA‐BN‐filo ebola vaccines: 12‐month data from a phase 1 randomized clinical trial in Uganda and Tanzania. J Infect Dis 2019; 220:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bregu M, Draper SJ, Hill AV, Greenwood BM. Accelerating vaccine development and deployment: report of a Royal Society satellite meeting. Philos Trans R Soc Lond B Biol Sci 2011; 366:2841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh K, Mehta S. The clinical development process for a novel preventive vaccine: An overview. J Postgrad Med 2016; 62:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roestenberg M, Kamerling IMC, de Visser SJ. Controlled human infections as a tool to reduce uncertainty in clinical vaccine development. Front Med (Lausanne) 2018; 5:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gouglas D, Le Thanh T, Henderson K, Kaloudis A, Danielsen T, Hammersland NC et al Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob Health. 2018; 6:e1386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid‐19 vaccines at pandemic speed. N Engl J Med 2020; 382:1969–73. [DOI] [PubMed] [Google Scholar]
- 71. Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC et al Self‐amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther 2018; 26:446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luo F, Zheng L, Hu Y, Liu S, Wang Y, Xiong Z et al Induction of protective immunity against. Front Microbiol 2017; 8:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sarker D, Plummer R, Meyer T, Sodergren M, Basu B, Chee CE et al MTL‐CEBPA, a small activating RNA therapeutic up‐regulating C/EBP‐α, in patients with advanced liver cancer: a first‐in‐human, multi‐centre, open‐label, phase I trial. Clin Cancer Res 2020; DOI: 10.1158/1078-0432.CCR-20-0414. [DOI] [PubMed] [Google Scholar]
- 74. Wilson J. In pictures: the Imperial lab developing a COVID‐19 vaccine, 2020. URL https://www.imperial.ac.uk/news/196313/in‐pictures‐imperial‐developing‐covid19‐vaccine/ [accessed on 16 April 2020].
- 75. bristol.ac.uk . New vaccine platform used to develop COVID‐19 candidates, 2020. URL http://www.bristol.ac.uk.news/2020/april/covid‐19‐vaccine‐platform.html [accessed on 20 May 2020].
- 76. Vragniau C, Bufton JC, Garzoni F, Stermann E, Rabi F, Terrat C et al Synthetic self‐assembling ADDomer platform for highly efficient vaccination by genetically encoded multiepitope display. Sci Adv 2019; 5:eaaw2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. DioSynVax . DioSynVax COVID Response, 2020. URL https://www.worldometers.info/coronavirus/ [accessed on 15 May 2020].
- 78. covid19vaccinetrial.co.uk . COVID‐19 vaccine trial 2020. URL https://covid19vaccinetrial.co.uk
- 79. Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC et al A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One 2012; 7:e40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Doremalen N, Lambe T, Sebastian S, Bushmaker T, Fischer R, Feldmann F et al A single‐dose ChAdOx1‐vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Negl Trop Dis 2019; 13:e0007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wilkie M, Satti I, Minhinnick A, Harris S, Riste M, Ramon RL et al A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime – MVA85A boost in healthy UK adults. Vaccine 2020; 38:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coughlan L, Sridhar S, Payne R, Edmans M, Milicic A, Venkatraman N et al Heterologous two‐dose vaccination with simian adenovirus and poxvirus vectors elicits long‐lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine 2018; 29:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. clinicaltrials.gov . A clinical trial to determine the safety and immunogenicity of healthy candidate MERS‐CoV vaccine (MERS002) 2019. URL https://clinicaltrials.gov.ct2/show/NCT04170829 [accessed on 5 June 2020].
- 84. clinicaltrials.gov . Safety and immunogenicity of a candidate MERS‐CoV vaccine (MERS001) 2019. https://clinicaltrials.gov.ct2/show/NCT03399578 [accessed on 16 May 2020].
- 85. clinicaltrials.gov . Study of a Candidate COVID‐19 vaccine (COV001), 2020. https://clinicaltrials.gov/ct2/show/NCT04324606?cond=covid‐19+vaccine&draw=2&rank=1 [accessed on 16 May 2020].
- 86. covid19vaccinetrial.co.uk . How long will it take to get the Oxford vaccine to deployment? 2020. URL https://covid19vaccinetrial.co.uk/blog‐how‐long‐will‐it‐take‐get‐oxford‐vaccine‐deployment [accessed on 5 June 2020].
- 87. Feldman RA, Fuhr R, Smolenov I, Mick Ribeiro A, Panther L, Watson M et al mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019; 37:3326–34. [DOI] [PubMed] [Google Scholar]
- 88. Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR et al Zika virus protection by a single low‐dose nucleoside‐modified mRNA vaccination. Nature 2017; 543:248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. WHO . DRAFT landscape of COVID‐19 candidate vaccines, 2020. URL https://www.who.int/blueprint/priority‐diseases/key‐action/novel‐coronavirus‐landscape‐ncov.pdf?ua=1 [accessed on 5 May 2020].
- 90. BioNTech . BioNTech Pipeline, 2019. URL https://biontech.de/science/pipeline [accessed on 5 June 2020].
- 91. Inovio . Inovio Pharmaceuticals selected by CEPI to develop vaccine against new coronavirus, 2020. http://ir.inovio.com/news‐and‐media/news/press‐release‐details/2020/Inovio‐Selected‐by‐CEPI‐to‐Develop‐Vaccine‐Against‐New‐Coronavirus/default.aspx [accessed on 15 May 2020].
- 92. Yoon IK, Kim JH. First clinical trial of a MERS coronavirus DNA vaccine. Lancet Infect Dis 2019; 19:924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sarivalasis A, Boudousquié C, Balint K, Stevenson BJ, Gannon PO, Iancu EM et al A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP‐DC) or with tumor lysate (OC‐DC) in patients with advanced high‐grade ovarian serous carcinoma. J Transl Med 2019; 17:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferguson N, Laydon D, Nedjati Gilani G, Imai N, Ainslie K, Baguelin M et al Report 9: Impact of non‐pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand, 2020.
- 95. Rimmer A. Covid‐19: Third of surgeons do not have adequate PPE, Royal College warns. BMJ 2020; 369:m1492. [DOI] [PubMed] [Google Scholar]
- 96. Haines A, de Barros EF, Berlin A, Heymann DL, Harris MJ. National UK programme of community health workers for COVID‐19 response. Lancet 2020; 395:1173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science 2020368(6493):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T et al A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med 2016; 374:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Geoghegan S, O'Callaghan KP, Offit PA. Vaccine safety: myths and misinformation. Front Microbiol 2020; 11:372. [DOI] [PMC free article] [PubMed] [Google Scholar]


