Abstract
Coronavirus disease 2019 (COVID‐19) is a pandemic disease currently affecting millions of people worldwide. Its neurological implications are poorly understood, and further study is urgently required. A hypercoagulable state has been reported in patients with severe COVID‐19, but nothing is known about coagulopathy in patients with milder disease. We describe cases of patients in New York City presenting with stroke secondary to large vessel thrombosis without occlusion, incidentally found to have COVID‐19 with only mild respiratory symptoms. This is in contrast to the venous thrombosis and microangiopathy that has been reported in patients with severe COVID‐19. Our cases suggest that even in the absence of severe disease, patients with COVID‐19 may be at increased risk of thrombus formation leading to stroke, perhaps resulting from viral involvement of the endothelium. Further systematic study is needed because this may have implications for primary and secondary stroke prevention in patients with COVID‐19.
Keywords: stroke, thrombosis, coronavirus, infection, carotid artery thrombosis
Essentials
-
•
Thrombosis in the setting of Coronavirus disease 2019 [COVID‐19] is reported but poorly understood.
-
•
We report cases of large‐artery thrombosis in the setting of mild COVID‐19 disease.
-
•
This suggests a previously undescribed large artery coagulopathy in patients with COVID‐19.
-
•
This may be due to viral involvement with the endothelium.
Alt-text: Unlabelled Box
1. INTRODUCTION
Coagulopathy in patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been reported,1 and there is evidence that the extent of coagulopathy correlates with severity of respiratory illness.2 The predominant clinical sequelae of this coagulopathy have been venous thromboembolic events,3 as well as end‐organ failure secondary to a microangiopathy believed to be similar to disseminated intravascular coagulation.4 Stroke is also recognized, and in the largest series to date, was seen in 5.7% of those with critical illness in comparison to 0.8% of those with milder coronavirus disease 2019 (COVID‐19) disease.5 Because severity of COVID‐19 illness correlates with both large artery ischemia and coagulopathy, it would seem plausible to associate the two phenomena. Here, we describe three SARS‐CoV‐2‐infected individuals with large artery stroke as their presenting illness in the absence of advanced COVID‐19 and describe the details of their workup for coagulopathy. One of these patients has been summarized in a recent report of COVID‐related stroke in patients younger than age 50.6
The three patients presented to the emergency department with stroke‐like symptoms and were found to have sub‐occlusive severe stenosis of the common carotid artery and stroke within that distribution. All three presented within 2 weeks in New York City during the SARS‐CoV‐2 outbreak in April 2020. None had severe respiratory symptoms, but all three tested positive for SARS‐CoV‐2 by reverse transcriptase–polymerase chain reaction assay.
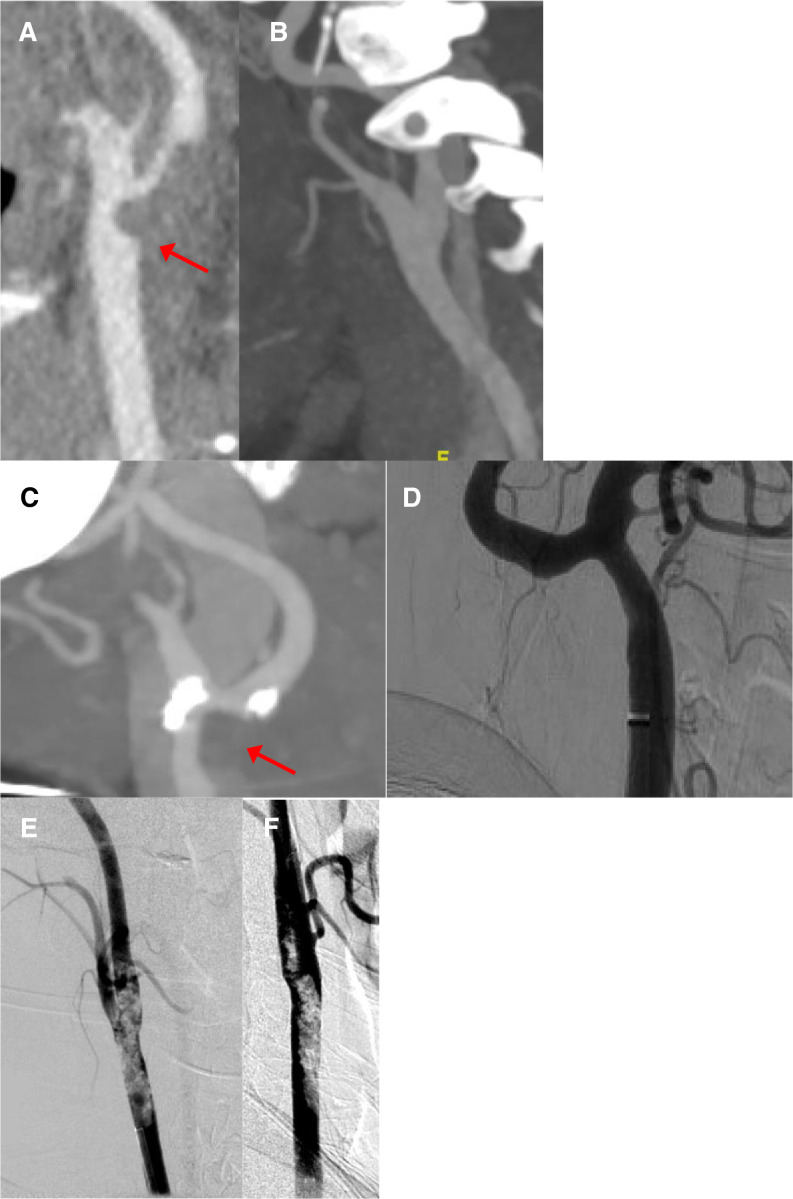
One patient is a previously healthy 33‐year‐old woman who developed numbness of her left hand that quickly progressed to hemiplegia and hemisensory loss of the left face, arm, and leg. Computed tomography (CT) angiography revealed nonocclusive thrombus in the right common carotid artery, extending into the internal carotid artery, as well as lung findings suggestive of viral pneumonia (Figure 1A ). Subsequent magnetic resonance imaging showed acute stroke within the territory of the right middle cerebral artery, with magnetic resonance angiography showing no evidence of underlying arterial dissection. She complained of a recent cough, but did not develop fever or require supplemental oxygenation. She was treated initially with aspirin and clopidogrel, but later switched to apixaban 5 mg twice daily in accordance with our institution's emerging guidelines about venous thromboembolism prevention in the setting of SARS‐CoV‐2 infection. Repeat CT angiography 10 days after presentation showed near‐complete resolution of the thrombosis and no sign of an underlying vascular abnormality (Figure 1B).
Figure 1.
For the first patient, CT angiography images show (A) thrombus within the common carotid artery extending into the internal carotid artery, (B) with subsequent near‐total resolution. For the second patient, CT angiography images (C) show thrombus within the distal right common carotid artery; (D) conventional angiography 1 week later shows resolution of the thrombus and no evidence of arterial dissection. For the third patient, digital subtraction angiography shows (E) severe nonocclusive thrombus within the distal common carotid artery, with (F) subsequent mild improvement in thrombus burden after local infusion of eptifibatide
The second patient is a 77‐year‐old woman with hypertension, hyperlipidemia, and bilateral thromboses of the deep leg veins discovered 2 months prior, prescribed aspirin, clopidogrel, and warfarin (although subtherapeutic on presentation), who developed the sudden onset of aphasia and left hemiparesis, and found to have nonocclusive thrombosis of the distal right common carotid artery (Figure 1C). She was coughing on presentation and was found to be infected with SARS‐CoV‐2, but she did not develop a fever or require supplemental oxygenation. She was treated with enoxaparin 1 mg/kg every 12 hours, and on conventional angiography 1 week later, the thrombosis had completely resolved and the vessel appeared normal (Figure 1D).
The third patient is a 55‐year‐old man with diabetes but no other medical history who presented with isolated left hand weakness that progressed to weakness of the left face, arm, and leg. CT angiography showed thrombosis within the right common carotid artery extending into the internal carotid artery, which appeared occlusive or near‐occlusive, in addition to lung parenchymal changes suggestive of viral pneumonia (Figure 1E). He was taken for emergent conventional angiography, where the thrombosis was found to be nonocclusive. Eptifibatide was given intra‐arterially, and the degree of stenosis was noted to lessen (Figure 1F). No mechanical endovascular treatment was given, and he was subsequently treated with a heparin infusion. He had low‐grade fever at presentation and required supplemental oxygenation but did not develop significant respiratory distress.
Standard serological tests, as well as coagulation markers sent by our institution on all patients testing positive for SARS‐CoV‐2, were sent on all three patients. Notably, only one (patient 2) had significantly elevated D‐dimer levels, whereas all three had elevated C‐reactive protein levels. Two of the three patients are young and without any evident source of thromboembolism; echocardiography was unremarkable and without evidence of patent foramen ovale. One patient had mildly elevated anti‐cardiolipin antibodies, but otherwise none had serological evidence of the antiphospholipid syndrome or of hyperhomocysteinemia, conditions known to be associated with arterial thrombosis.7 The third patient, although older and with vascular risk factors, also did not have radiographic or echocardiographic signs of a proximal source of thromboembolism.
Taken together, these cases raise the possibility that, in addition to the previously characterized venous thrombosis and microangiopathy potentially associated with SARS‐CoV‐2 infection, there may also be a tendency toward large‐vessel arterial thrombosis, which has been reported in patients with SARS from a previous strain of the coronavirus.8 There is an emerging hypothesis that virally mediated disruption of the endothelium may be playing a key role in thrombus formation in patients infected by SARS‐CoV‐2; recently, viral particles have been identified in systemic endothelia along with accumulations of inflammatory cells and apoptosis (endotheliitis).9., 10. Notably, all three of our patients had only mild symptoms of infection, suggesting a possible difference in pattern of thrombophilia in patients with mild as opposed to severe disease. Whether there is a connection between SARS‐CoV‐2 and arterial thrombosis leading to stroke, and, if so, what the pathogenetic mechanism of this is, will require further study.
CONFLICT OF INTEREST
None of the authors has any real or potential conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Michael G. Fara is responsible for study design, literature review, drafting the initial manuscript, and critical revising for intellectual content. Laura K. Stein is responsible for study design, literature review, and critical revising for intellectual content. Maryna Skliut is responsible for patient selection and collection of clinical data. Susan Morgello is responsible for study design, literature review, and critical revising for intellectual content. Johanna T. Fifi is responsible for study design, patient selection, and collection of clinical data. Mandip S. Dhamoon is responsible for critical revising for intellectual content.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap and 23 May 2020
REFERENCES
- 1.Han H., Yiang L., Liu R., et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y., Wang X., Yang P., Zhang S. COVID‐19 complicated by acute pulmonary embolism. Radiol Cardiothor Imaging. 2020;2 doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell C., Kahwash R. Will complement inhibition be the new target in treating COVID‐19 related systemic thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 5.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxley T.J., Mocco J., Majidi S., et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiatt B., Lentz S. Prothrombotic states that predispose to stroke. Curr Treat Options Neurol. 2002;4:417–425. doi: 10.1007/s11940-002-0009-1. [DOI] [PubMed] [Google Scholar]
- 8.Umapathi T., Kor A., Venketasubramanian N., et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;241:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sardu C, Gambardella J, Morelli M, Wang X, Marfella R, Santulli G. Is COVID‐19 an endothelial disease? Clinical and basic evidence. Preprints. 2020, 2020040204. [DOI] [PMC free article] [PubMed]
- 10.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]