Abstract
An understanding of the molecular basis of liver regeneration will open new horizons for the development of novel therapies for chronic liver failure. Such therapies would solve the drawbacks associated with liver transplant, including the shortage of donor organs, long waitlist time, high medical costs, and lifelong use of immunosuppressive agents. Regeneration after partial hepatectomy has been studied in animal models, particularly fumarylacetoacetate hydrolase–deficient (FAH−/−) mice and pigs. The process of regeneration is distinctive, complex, and well coordinated, and it depends on the interplay among several signaling pathways (eg, nuclear factor κβ, Notch, Hippo), cytokines (eg, tumor necrosis factor α, interleukin 6), and growth factors (eg, hepatocyte growth factor, epidermal growth factor, vascular endothelial growth factor), and other components. Furthermore, endocrinal hormones (eg, norepinephrine, growth hormone, insulin, thyroid hormones) also can influence the aforementioned pathways and factors. We believe that these endocrinal hormones are important hepatic mitogens that strongly induce and accelerate hepatocyte proliferation (regeneration) by directly and indirectly triggering the activity of the involved signaling pathways, cytokines, growth factors, and transcription factors. The subsequent induction of cyclins and associated cyclin-dependent kinase complexes allow hepatocytes to enter the cell cycle. In this review article, we comprehensively summarize the current knowledge regarding the roles and mechanisms of these hormones in liver regeneration. Articles used for this review were identified by searching MEDLINE and EMBASE databases from inception through June 1, 2019.
Abbreviations and Acronyms: CDK, cyclin-dependent kinase; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular signal-regulated kinase; FAH, fumarylacetoacetate hydrolase; GH, growth hormone; Ghr-/-, growth hormone receptor gene knockout; HGF, hepatocyte growth factor; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cells; HNF, hepatocyte nuclear factor; HPC, hepatic progenitor cell; IGF, insulinlike growth factor; IL, interleukin; InsP3, inositol 1,4,5-trisphosphate; IR, insulin receptor; JNK, JUN N-terminal kinase; LDLT, living donor liver transplant; LRP, low-density lipoprotein-related protein; MAPK, mitogen-activated protein kinase; mRNA, messenger RNA; mTOR, mammalian target of rapamycin; NF-κβ, nuclear factor κβ; NOS, nitric oxide synthase; NTBC, 2-nitro-4-trifluoro-methyl-benzoyl-1,3-cyclohexanedione; PCR, polymerase chain reaction; PCNA, proliferating cell nuclear antigen; PH, partial hepatectomy; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PKB, protein kinase B; PTU, 6-n-propyl-2-thiouracil; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; T3, triiodothyronine; TGF, transforming growth factor; TNF, tumor necrosis factor; TR, thyroid receptor
Article Highlights.
-
•
Norepinephrine, a hepatic mitogen, increases the production and activity of epidermal growth factor (EGF) and hepatocyte growth factor (HGF). These growth factors excite the Ras-Raf-MEK-ERK and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)–protein kinase B (PKB)–mammalian target of rapamycin (mTOR) cascades, leading to up-regulation of several genes (MYC, FOS, JUN) encoding transcription factors and nuclear factor κβ signaling pathway, which increase protein synthesis and cell division. Furthermore, the canonical Wnt/β-catenin pathway in hepatic stem cells is provoked by this hormone.
-
•
Growth hormone contributes to liver regeneration by activating hepatocyte growth factor, hepatocyte nuclear factor, and EGF, which excite the Ras-Raf-MEK-ERK and PI3K-PKB-mTOR cascades. Resistance to this hormone may block liver regeneration by enhancing the activity of SMAD2/3 and transforming growth factor β, by increasing the susceptibility to hepatocyte apoptosis, and by increasing the possibility of bile acid and cytokine-induced liver injury from an increase in apoptotic factors and reactive oxygen species and a decrease in prosurvival factors (EGF, ONECUT 1).
-
•
Intraportal insulin injection after living donor liver transplant enhances graft regeneration and prevents or even cures small-for-size syndrome. Insulin signaling can partially reverse the harmful effects of portal vein stenosis or ligation. These effects are due to stimulation of Ras-Raf-MEK-ERK and PI3K-PKB-mTOR cascades and to calcium signals from nuclear inositol 1,4,5-trisphosphate.
-
•
Thyroid hormone is a hepatic proliferative factor. It up-regulates E2F transcription factors, which causes overexpression of cell cycle inducers (cyclins and cyclin-dependent kinases). It decreases the activity of p16 and p27, preventing their inhibitory effect on cyclin-dependent kinase. It suppresses tumor suppressor proteins p53 and p73, which abolishes their stimulatory effects on p21 and thus blocks inhibition of hepatic cell cycles. It stimulates the Ras-Raf-MEK-ERK and β-catenin signaling pathways. It minimizes mitochondrial oxidative injuries and the activity of apoptotic caspase genes. It antagonizes the transcriptional activity of transforming growth factor β and SMAD, factors that terminate liver regeneration.
-
•
From a clinical perspective, we believe that for patients with artificial extracorporeal liver support devices, supplemental treatment with norepinephrine, growth hormone, insulin, and thyroid hormone may optimize the hepatocyte regeneration, especially in patients with acute liver failure and small-for-size syndrome.
Tissue engineering and regenerative medicine is a fast-growing area of research that aims to restore the structure and function of damaged organs and cure previously untreatable diseases. This field combines molecular biology, cell biophysics, and engineering techniques to elucidate the molecular intricacies underlying tissue regeneration, and it aims to rebuild new organs by using stem cells.1 The regenerative capacity of the liver has been known since the 19th century. Regeneration may occur after partial hepatectomy (PH) and chemical-induced injury (eg, acetaminophen and galactosamine toxicities).2,3 Liver regeneration is a unique and well-orchestrated phenomenon controlled by growth factors, cytokines, and signaling pathways.2, 3, 4 The process has been studied in rodent and pig models such as the 2/3-PH model and fumarylacetoacetate hydrolase–deficient (FAH−/−) model.2,5, 6, 7 Partial hepatectomy is among the most preferred and acceptable models for studying liver generation; it has been used for many years to elucidate the underlying mechanistic pathways and factors of this process.2,3,8 Evidence suggests that regeneration starts as early as 5 minutes after PH in rodents and proceeds for 5 to 7 days to restore the original liver volume.2,3 Regeneration is initiated by hemodynamic changes that elevate the activity of urokinase plasminogen activator, which represents the initial firing stimulus for growth factors. Additionally, innate immunity induction and increased leukocytic activity trigger 4 important signaling pathways: nuclear factor κβ (NF-κβ), Notch, Wnt/β-catenin, and Hippo.2,3,8 During the priming and proliferation phases of regeneration, the factors associated with these pathways prepare the hepatocytes for DNA synthesis and cellular division, respectively.2,3
Multiple published trials have found that endocrinal hormones (particularly norepinephrine, growth hormone [GH], insulin, and thyroid hormone) strongly influence and trigger the signaling pathways, cytokines, and growth factors involved in liver regeneration. In this review article, we summarize the most important and pertinent randomized controlled trials, basic animal research studies, and observational research studies to shed light on the contribution of these hormones to liver regeneration. Articles were identified by searching MEDLINE and EMBASE databases from inception through June 1, 2019.
Norepinephrine
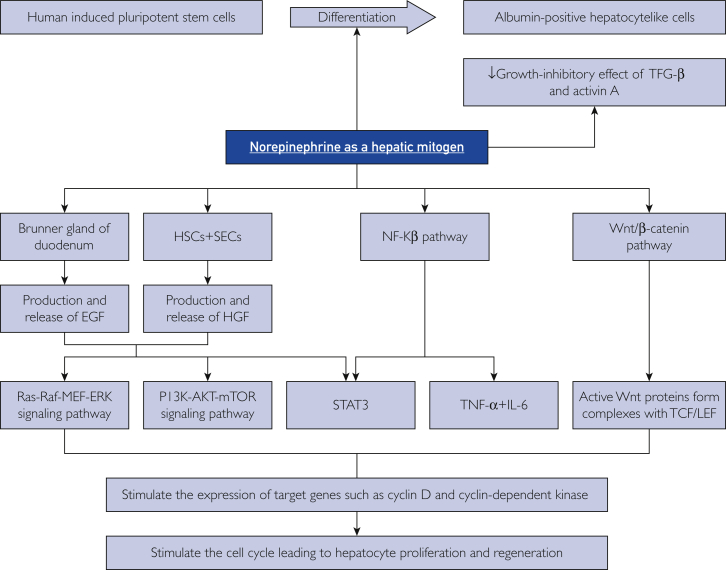
Norepinephrine is an endogenous catecholamine neurotransmitter that is released as a stress hormone from secretory granules of adrenal medullary chromaffin cells, from the locus coeruleus nuclei in the pons, and from postganglionic sympathetic neurons. The process of norepinephrine production starts when phenylalanine hydroxylase catalyzes the transformation of phenylalanine into tyrosine, which is then converted by tyrosine hydroxylase into levodopa. The latter is converted by dihydroxyphenylalanine decarboxylase to dopamine, the direct precursor of norepinephrine.9,10 Norepinephrine is responsible for the fight-or-flight response because it acts as a positive inotropic, chronotropic, and vasoconstrictor agent by decreasing blood flow to the gastrointestinal tract and directing it to the skeletal muscle, triggering glycogenolysis and gluconeogenesis, and raising the level of arousal and alertness.9,10 The following sections review norepinephrine as a hepatic mitogen (Figure 1).
Figure 1.
The mechanisms of norepinephrine in inducing liver regeneration. EGF = epidermal growth factor; ERK = extracellular signal-regulated kinase; HGF = hepatocyte growth factor; HSC = hepatic stellate cell; IL = interleukin; mTOR = mammalian target of rapamycin; NF-κβ = nuclear factor κβ; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; SEC = subendothelial cell; STAT = signal transducer and activator of transcription; TCF/LEF = T-cell and lymphoid enhancer transcription factors; TGF = transforming growth factor; TNF = tumor necrosis factor; ↓ = decreased.
Understanding the Mechanisms of Norepinephrine in Liver Regeneration
Cruise et al11 reported in 1985 that stimulation of α-adrenergic receptors occurs via norepinephrine-induced DNA synthesis. They cultured adult rat hepatocytes in serum-free medium containing epidermal growth factor (EGF) and insulin. Norepinephrine was added, and the effect of norepinephrine concentration on DNA synthesis was measured by the rate that 3H-thymidine was incorporated into trichloroacetic acid−precipitated material. They reported that an increased concentration of norepinephrine (as shown via α1-adrenoreceptor signaling) stimulated DNA synthesis.11 In the same year, Olsen et al12 investigated the influence of norepinephrine on the synthesis and spontaneous secretion of EGF from Brunner glands in the duodenum. Chemical sympathectomy with 6-hydroxydopamine depleted these glands of EGF, but administration of norepinephrine increased the production of EGF in the glands and inhibited its flow rate and output. The impact of norepinephrine on liver regeneration was also observed in a 1988 study by Houck et al.13 They stated that the binding of norepinephrine to the α1-adrenoceptor antagonizes the growth-inhibitory effect of transforming growth factor β (TGF-β) in primary rat hepatocyte cultures. Specifically, their study found that norepinephrine increased the amount of TGF-β required for a 50% inhibition of EGF-induced DNA synthesis (ID50 increased from 2.8 to 14.4 pM, a 5-fold difference). Furthermore, norepinephrine also decreased the sensitivity to inhibition by TGF-β, with a nadir at 48-hour–regenerating cells. The cells subsequently acquired innate resistance to TGF-β, which increased the amount of TGF-β required to establish inhibition of EGF-induced DNA synthesis.
Given the close proximity of EGF to hepatocyte growth factor (HGF) in the liver regeneration process, a clear understanding of the relationship of norepinephrine with HGF in liver regeneration is necessary. Broten et al14 investigated the ability of norepinephrine to stimulate the production of HGF in MRC-5 human embryonic lung fibroblast cultures. They observed that cultures incubated with norepinephrine released HGF into the media at 1.8±0.3 times the amount of control cultures that received no hormone. Norepinephrine appeared to activate protein kinase A, which phosphorylated the Ser299 residue of C/EBPβ and facilitated its translocation into the nucleus, where it triggered expression of the HGF gene.14,15 Additionally, they treated MRC-5 cultures with an α-agonist (phenylephrine), a β-agonist (isoproterenol), or both agonists to confirm which adrenoceptor mediates the hepatic regenerative process. They found that each agonist had similar stimulatory effects on the transcription and release of HGF into the media and that the effect was greater when both agonists were present.
From these studies, we deduce that the interaction of norepinephrine with α1 and β receptors strongly contribute to the mitogenic activity of hepatocytes by stimulating the production and activity of EGF and HGF. These growth factors excite the Ras-Raf-MEK-ERK cascade, which includes the serine-tyrosine-threonine kinase MEK that phosphorylates and activates mitogen-activated protein kinase MAPK 3 and MAPK1, leading to up-regulation of transcription factors MYC, FOS, and JUN.2,4 The growth factors also excite the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)–protein kinase B (PKB)–mammalian target of rapamycin (mTOR) cascade, which activates PI3K and PKB, leading to up-regulation of the mTOR and NF-κβ pathways, thereby increasing protein synthesis and cell division.2,4
To better understand the mechanism of norepinephrine as a hepatic mitogen, Kanamaru et al16 examined the effect of norepinephrine in cultured rat hepatocytes and found that it induced SMAD7 expression. Consequently, EGF-stimulated DNA synthesis was enhanced, and the growth-inhibitory effect of the activin A-induced nuclear accumulation of SMAD2/3 was abolished. They also studied the mechanism of SMAD7 induction by norepinephrine and found that norepinephrine rapidly increased the amount of active NF-κβ complex.16 Additionally, preincubation of cells with NF-κβ inhibitor (N-tosyl-l-phenylalanine chloromethyl ketone) abolished norepinephrine-induced SMAD7 overexpression.16 Nuclear factor κβ is among the most important signaling pathways in liver regeneration because it stimulates expression of tumor necrosis factor (TNF) α and induces transcription of genes encoding cyclin D 1, cyclin-dependent kinase (CDK) 1, and interleukin (IL) 6 which form the basis of hepatocyte cell cycle and proliferation.2,3
In addition, signal transducer and activator of transcription (STAT) 3 mediates norepinephrine-induced liver regeneration. Han et al17 studied the effects of phenylephrine on liver regeneration in primary rat hepatocytes. They noted that phenylephrine induced phosphorylation of STAT3 and increased STAT3 transcriptional activity by enhancing its ability to bind to its DNA response element and provoke expression of target genes such as CCND1 (gene encoding cyclin D1). They also showed that the interaction between norepinephrine and STAT3 was mediated by the EGF receptor (EGFR). Primary rat hepatocytes treated with phenylephrine rapidly phosphorylated EGFR, and this activity coincided with STAT3 phosphorylation. Also, inhibition of EGFR blocked the effects of norepinephrine on STAT3 phosphorylation and activity. These findings support a mechanism in which EGF is necessary for the mitogenic action of norepinephrine on the liver.
Effect of Norepinephrine on Hepatic Stem Cells
Human hepatic pluripotent stem cells could be targeted by up-regulating α1-adrenergic receptors. Kotaka et al18 confirmed that methoxamine hydrochloride, an α1-adrenergic receptor agonist, induced lineage differentiation in human hepatic pluripotent stem cells and mouse embryonic stem cells to form albumin-positive hepatocyte like cells. This differentiation process is mainly mediated by HGF and oncostatin M, which leads to stimulation of the STAT3 pathway. These chemically induced hepatocyte like cells potentially can be explored as a novel and low-cost source of cells for cell therapy, drug discovery efforts, and hepatotoxicity screening of drug compounds.
Hepatic progenitor cells (HPCs) are another important product of hepatic stem cells. In a healthy liver, these bipotential cells reside in the canals of Hering (bile ductules) and are able to proliferate and differentiate into hepatocytes and cholangiocytes when the normal homeostatic regeneration is exhausted.19 Norepinephrine, as a hepatic stem cell modulator, could influence the activity of HPCs. Soeda et al20 reported the rescue of acetaminophen-injured livers in mice by using the β-adrenoceptor agonist isoproterenol to increase the number of HPCs. They found that compared with controls, mice without dopamine β-hydroxylase were genetically deficient in sympathetic nervous system neurotransmitters (norepinephrine and epinephrine) and had a markedly attenuated HPC population, as indicated by immunohistochemical detection of CK19. Surprisingly, HPC numbers in mice without dopamine β-hydroxylase substantially recovered after treatment with isoproterenol. To elucidate the molecular mechanism through which β-adrenoceptor stimulation elicited the amplification of HPCs, they treated immature murine cholangiocytes (603B cells) with isoproterenol. Western blotting revealed overexpression of total β-catenin, dephosphorylated β-catenin (activated β-catenin), and cyclin D1 (a known β-catenin target), and polymerase chain reaction (PCR) quantification of Wnt ligand messenger RNA (mRNA) significantly increased in treated cells. Moreover, they also studied the effect of isoproterenol on liver disease in the acetaminophen-induced acute liver injury mouse model. Sublethal amounts of acetaminophen were administered to induce hepatic necrosis; an hour later, a control group received vehicle solution and an experimental group received isoproterenol. Control mice had massive hepatic necrosis, as evidenced by an elevation in alanine aminotransferase and as histologically observed. Administration of isoproterenol significantly reduced the level of hepatic injury, as evidenced by a reduction in alanine aminotransferase levels, and cells had less hepatic necrosis histologically and an improved survival rate. To determine the relevance of this finding to HPCs, they immunohistochemically analyzed CK19-positive HPCs and noted a considerable increase in the HPC numbers in the experimental group (acetaminophen and isoproterenol) compared with the control group (acetaminophen only). Along these lines, they investigated potential implications of the canonical Wnt pathway as a hepatoprotective mechanism against acetaminophen-induced acute liver injury. Surprisingly, induction of the Wnt/β-catenin pathway appeared to be the main mechanism underlying the expansion of HPCs in the experimental group, as evidenced by the overexpression of total β-catenin, strong β-catenin staining in HPCs and in hepatocytes throughout the liver, and up-regulation of Wnt ligands.
These findings document a possible role for isoproterenol as a β-adrenoceptor agonist in the expansion of HPCs and liver regeneration. The canonical Wnt/β-catenin signaling pathway is an essential driver of the liver regeneration process that commences 1 to 3 hours after partial liver resection, leading to liberation of Wnt proteins from the β-catenin degradation complex; on translocation to the nucleus, these proteins form complexes with T-cell and lymphoid enhancer transcription factors and induce transcription of target genes (eg, cyclin D1) that increase hepatocyte proliferation.2,3
Role of Norepinephrine in Hepatic Tissue Bioengineering
Researchers in hepatic bioengineering have examined the role of norepinephrine as a promoter of the recellularization process in decellularized liver matrix. Recellularized liver may someday be an effective alternative to orthotopic liver transplant for patients with liver cirrhosis. Wen et al21 examined the influence of β-adrenergic receptors on the function of recellularized liver using mouse hepatocytes. Interestingly, up-regulation of the β2-adrenergic receptor with salbutamol increased hepatocyte proliferation, albumin secretion, and urea synthesis in recellularized liver. An analysis of cytokines and transcription factors revealed a significant elevation in the expression of IL-6 and STAT3, which was consistent with findings of previous trials examining the effects of norepinephrine on hepatic regeneration.
Table 1 summarizes the studies that explore norepinephrine-induced liver regeneration.
Table 1.
Norepinephrine-Induced Liver Regeneration
| Reference, year | Species | Experiment | Results |
|---|---|---|---|
| Cruise et al,11 1985 | Rat | Addition of norepinephrine to adult rat hepatocytes cultured in serum-free medium containing EGF and insulin | Norepinephrine treatment leads to: ↑ Concentration of norepinephrine stimulates the incorporation of 3H-thymidine (enhances DNA synthesis) |
| Olsen et al,12 1985 | Rat | Chemical sympathectomy by 6-hydroxydopamine administration | Chemical sympathectomy leads to: Depletion of EGF from Brunner glands Administration of norepinephrine leads to: ↑ Production of EGF in the glands |
| Houck et al,13 1988 | Rat | Treatment of primary rat hepatocyte cultures with norepinephrine | Norepinephrine treatment leads to: ↓ Growth-inhibitory effect of TGF-β |
| Broten et al,14 1999 | Human | Treatment of MRC-5 human embryonic lung fibroblast cultures with norepinephrine | Norepinephrine treatment leads to: ↑ Production and release of HGF |
| Kanamaru et al,16 2001 | Rat | Treatment of primary rat hepatocyte cultures with norepinephrine | Norepinephrine treatment leads to: ↑ Genetic expression of SMAD7 protein leading to ↑ EGF-stimulated DNA synthesis and abolished the growth-inhibitory effects of activin A–induced nuclear accumulation of SMAD2/3 SMAD7 induction depended on increased activity of the NF-κβ pathway by norepinephrine ↑ Activity of the NF-κβ pathway leading to ↑ expression of TNF-α and IL-6 and thus ↑ transcription of genes encoding DK1 and cyclin D1 Preincubation of the cells with NF-κβ inhibitor (N-tosyl-l-phenylalanine chloromethyl ketone) abolished norepinephrine-induced Smad7 overexpression |
| Han et al,17 2008 | Rat | Treatment of primary rat hepatocyte cultures with phenylephrine | Phenylephrine treatment leads to: ↑ Phosphorylation of STAT3 leading to ↑ STAT3 transcriptional activity and ability to bind to its DNA response element to provoke the expression of target genes such as CCND1 |
| Kotaka et al,18 2017 | Human, mouse | Treatment of hiPSCs and mESCs with an α1-adrenergic receptor agonist, methoxamine hydrochloride | Methoxamine hydrochloride treatment: ↑ Hepatic lineage differentiation from hiPSCs and mESCs into albumin-positive hepatocyte like cells |
| Soeda et al,20 2014 | Mouse | Administration of isoproterenol to the acetaminophen-induced acute liver injury mouse model | Massive hepatic necrosis in the control group In the experimental group: ↓ Level of hepatic injury, as evidenced by ↓ in alanine aminotransferase, ↓ in histologic evidence of hepatic necrosis, and improved survival rate Expansion of HPCs by provoking the canonical Wnt/β-catenin pathway |
| Wen et al,21 2016 | Recellularized mouse liver | Treatment of recellularized liver with salbutamol | Salbutamol treatment leads to: ↑ Hepatocyte proliferation, albumin secretion, and urea synthesis in the recellularized liver ↑ Expression of IL-6 and STAT3 |
EGF = epidermal growth factor; HGF = hepatocyte growth factor; hiPSCs = human induced pluripotent stem cells; HPC = hepatic progenitor cell; IL-6 = interleukin 6; NF-κβ = nuclear factor κβ; MESC = mouse embryonic stem cell; STAT = signal transducer and activator of transcription; TGF = transforming growth factor; TNF-α = tumor necrosis factor α; ↑ = increased; ↓ = decreased.
Growth Hormone
Growth hormone, also termed somatotropin, is a 191–amino acid single-chain polypeptide that is synthesized, stored, and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland.22 It is responsible for cellular growth and regeneration, bone mineralization, and protein synthesis, and it increases muscle mass, stimulates the immune system, and promotes gluconeogenesis in the liver.22 The growth-promoting effects of GH occur through an endocrinal mediator, insulin like growth factor (IGF) 1 (somatomedin C) that is produced in the liver after GH stimulates the Janus kinase–STAT5 signaling pathway.22 The following sections review GH as a hepatic mitogen.
Role of GH in Liver Regeneration After PH
In 1989, Asakawa et al23 used the 2/3-PH rat model to study the role of GH in liver regeneration. After PH, rats received human GH (200 μg) twice a day. Compared with control rats, rats that received human GH had a higher liver mass (3.18±0.13 vs 2.68±0.17 g), increased mitotic activity in hepatocytes, higher levels of serum IGF and albumin, and lower urea nitrogen levels. Başoğlu et al24 studied the impact of GH on hepatic regeneration after PH. They evaluated 30 pathogen-free Sprague-Dawley rats: group 1 received a sham operation and saline solution, group 2 had left lobectomy and saline infusion, and group 3 had left lobectomy and GH injection (0.2 mg/kg per day). On postoperative day 7, animals were euthanized and the total liver was resected. The animals in group 3 had a higher hepatocyte mitotic rate and greater Ki67 staining compared with animals in groups 1 and 2.
To understand the role of GH and the IGF1 axis in liver regeneration, Pennisi et al25 performed 70% PH in 3 groups of mice. The first group consisted of GH-antagonist transgenic mice, in which the effect of GH was genetically blocked. The second group consisted of mice with very low IGF1 activity in the liver but increased GH secretion. The third group (controls) had normal GH and IGF1. The survival rate was significantly lower in the GH-antagonist group (57%) compared with controls (100%) and IGF1-deficient mice (88%). Moreover, liver regeneration was complete in the control group by postoperative day 4, whereas complete regeneration took 7 days in IGF1-deficient mice. In the GH-antagonist group, regeneration plateaued after 4 days and restored only 70% of the original liver mass. These studies highlight the potential mitogenic activity of GH in promoting the replication and regeneration of hepatocytes after PH.
Role of GH in Rescuing the Regenerative Process in Hepatic Steatosis
Hepatic steatosis, a hallmark of nonalcoholic fatty liver disease, is a fatty infiltration condition that precipitates the development of oxidative stress and chronic hepatic inflammation. Left untreated, nonalcoholic steatohepatitis may lead to liver cirrhosis and hepatocellular carcinoma, and it is often associated with insulin resistance, obesity, and type 2 diabetes mellitus.26 In the studies of molecular mechanisms underlying hepatocyte proliferation after PH, a defect in hepatocyte regeneration in nonalcoholic fatty liver disease has been observed.
Collin de l’Hortet et al27 analyzed liver regeneration in 2 mouse models of liver steatosis: (1) leptin-deficient mice (ob/ob mutation) and (2) mice fed a methionine- and choline-deficient diet from age 8 weeks to 13 weeks. They also assessed a third group of normal lean mice (controls). All animals underwent 55% PH, which included removal of the left lateral lobe and the right portion of the median lobe. Bromodeoxyuridine (a synthetic nucleoside) was administered to detect proliferating hepatocytes. Recombinant human GH was given before and after liver resection for some animals in the leptin-deficient ob/ob group until the end of the experiment, and animals were euthanized at 8, 24, 32, 44, 56, or 68 hours after PH.
In that study,27 ob/ob and methionine- and choline-deficient mice had lower levels of circulating GH and underexpression of representative hepatic GH target genes IGF1, IGFALS, and CISH compared with lean mice. The peak in proliferation was observed 56 hours after PH in both steatosis models, whereas it was 44 hours in the control group. Expression of cyclin A, a typical marker of S phase, was substantially reduced in both models of steatosis at 44, 56, and 68 hours compared with controls. The ratio of liver weight to body weight was much lower in both models of steatosis compared with controls. Cell cycle progression from G1 to S phase, which occurs 8 to 32 hours after PH, mainly involves EGFR and the HGF receptor c-Met. Although hepatic levels of c-Met mRNA did not differ between the experimental and control groups, hepatic expression of EGFR was barely detectable in either model of steatosis compared with controls during the first 32 hours after PH. Hepatic EGFR expression and triglyceride content were inversely correlated in both models. They compared the hepatocyte proliferation in ob/ob mice with or without treatment with human GH. The ratio of liver weight to body weight, timing of peak hepatocyte proliferation, EGFR expression, and total EGFR protein level were highly increased in GH-treated mice
These data suggest that the regenerative response to PH and G1 to S phase progression were delayed in both models of hepatic steatosis because of down-regulation of EGFR. Notably, this impairment could be rescued by administering GH. The positive contribution of GH to liver regeneration occurs through activation of EGFR, which induces the Ras-Raf-MEK-ERK and PI3K-PKB-mTOR cascades and leads to induction of transcription factors, protein synthesis, and hepatic cell division and repair.2,4
Effect of GH Resistance on the Regenerative Process in Hepatic Cholestasis
Cholestasis is a pathologic condition that is defined as an arrested or interrupted bile flow. It occurs with bile duct obstruction, impaired hepatocyte uptake of bile from the blood, or failure of hepatocytes to excrete bile into canaliculi, leading to accumulation of bile constituents in the blood. The main symptoms of cholestasis are jaundice, generalized pruritus, dark urine, and clay-colored stools. Laboratory tests indicate hyperbilirubinemia, bile acidemia, choluria, and elevated liver enzymes (eg, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase). Cholestasis can be attributable to intrahepatic and extrahepatic causes. Intrahepatic causes include primary and secondary biliary cirrhosis, primary sclerosing cholangitis, intrahepatic cholangiocarcinoma, primary and secondary liver cancer, some medications (eg, chlorpromazine, azathioprine, oral contraceptives), and intrahepatic cholestasis of pregnancy. Extrahepatic causes include choledocholithiasis, stricture of the common bile duct, hilar and distal cholangiocarcinoma, pancreatic cancer, and chronic pancreatitis.28
Stiedl et al29 elucidated whether GH resistance has a role in establishing liver fibrosis. They examined growth hormone receptor gene (Ghr)−/−/Mdr2−/− double-knockout mice and Mdr2−/− single-knockout mice. Ghr−/− knockouts lack the GH receptor gene and are a model for GH resistance, and Mdr2−/− knockouts have inflammatory cholestasis and liver fibrosis. The double-knockout (Ghr−/−/Mdr2−/−) mice had evidence of GH resistance (higher circulating GH and low serum IGF1 levels). Macroscopic analysis revealed that Ghr−/−/Mdr2−/− mice at age 8 weeks had smaller livers and enlarged gallbladders compared with Mdr2−/− mice. Serum indicators of liver injury (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin) were strongly increased in Ghr−/−/Mdr2−/− mice compared with Mdr2−/− mice. Analysis of hematoxylin-eosin–stained Ghr−/−/Mdr2−/− specimens revealed disruption of liver architecture and a higher degree of collagen deposition. Reverse transcription PCR and immunoblot analysis revealed a considerable increase in expression of α-smooth muscle actin (a marker of activated hepatic stellate cells), p-SMAD2/3 (an element in the TGF-β pathway that blocks liver regeneration), and profibrogenic factors (eg, platelet-derived growth factor β, TGF-β, and TNF-α) in Ghr−/−/Mdr2−/− mice compared with Mdr2−/− mice. Ghr−/−/Mdr2−/− mice had immunohistochemical evidence of greater hepatocyte apoptosis, which was further supported by marked enrichment of genes associated with apoptosis. Furthermore, increased reactive oxygen species (ROS) and diminished hepatocytic expression of hepatoprotective prosurvival genes (EGF, ONECUT1) were observed in Ghr−/−/Mdr2−/− mice relative to Mdr2−/− mice.29
Taken together, these results suggest that GH strongly participates in the process of liver recovery and regeneration. Growth hormone resistance in a mouse model of inflammatory cholestasis (Ghr−/−/Mdr2−/−) dramatically exacerbates liver fibrosis by activating profibrogenic factors (platelet-derived growth factor β, TGF-β, and TNF-α) and blocks liver regeneration by enhancing the activity of SMAD2/3 and TGF-β. Further, GH resistance increases susceptibility of hepatocytes to apoptosis and increases the degree of injury from bile acid and cytokines because apoptotic factors and ROS are elevated and prosurvival factors (EGF, ONECT1) are reduced.29
Role of IGF2 in Hereditary Tyrosinemia Type 1
Hereditary tyrosinemia type 1, also termed hepatorenal tyrosinemia, is an autosomal recessive disease caused by a defect in the fumarylacetoacetate hydrolase enzyme FAH. This protein, which is involved in the metabolism of tyrosine, degrades fumarylacetoacetate into fumarate and acetoacetate. Its absence results in the accumulation of fumarylacetoacetate and succinylacetone in the tissues, leading to liver injury, renal tubular damage, and neurologic manifestations resembling those of porphyrias. These adverse effects can be reversed by administering 2-nitro-4-trifluoro-methyl-benzoyl)-1,3-cyclohexanedione (NTBC), which blocks an earlier step of the catabolic tyrosine pathway and prevents the production of toxic metabolites in hepatocytes.6,30
Wang et al31 used a mouse model of hereditary tyrosinemia type 1 (FAH−/−) to examine pathologic changes in the liver, elucidate the mechanism of therapeutic liver repopulation, and discover mitogens that mediate hepatocyte proliferation. Normal hepatocytes were harvested with collagenase D perfusion, and cells were transplanted into recipient FAH−/− mice with intrasplenic injection. FAH−/− mice survived hepatocyte transplantation and NTBC withdrawal, suggesting that hepatic mitogens released by these cells might be responsible for successful liver repopulation. IGF 2 expression levels in repopulating liver tissues were assessed at 0, 14, and 28 days after NTBC withdrawal. Interestingly, IGF 2 expression had increased by 60-fold at 14 days and was even higher at 28 days after withdrawal of NTBC. Immunohistochemical assays revealed that during repopulation, IGF 2 was localized only to the cytoplasm of FAH−/− hepatocytes, whereas no expression was detected in FAH-positive cells. They also conducted a growth assay using hepatocytes cultured with increasing amounts of IGF 2 supplementation. Hepatocyte proliferation assays (bromodeoxyuridine and Ki67) determined that 100 and 200 ng/mL of IGF 2 markedly induced growth.
IGF 2 is known to bind the IGF1 receptor, which is also activated by IGF1 secreted in response to GH activity. Activation of the IGF1 receptor by both types of IGF leads to induction of the Ras-Raf-MEK-ERK and PI3K-PKB-mTOR signaling pathways. To clarify the potential role of IGF2 in liver repopulation and its impact on these pathways, western blot analysis was used to assess the phosphorylated IGF1 receptor, ERK, and PKB in repopulated livers after hepatocyte transplant. Remarkably, the levels of these factors significantly increased in proliferating hepatocytes and then gradually decreased as the liver repopulation process completed.
These data signify that IGF2 is a paracrine signal released from hepatocytes injured by tyrosinemia. IGF 2 enhances the proliferative capacity of repopulating transplanted hepatocytes by up-regulating the Ras-Raf-MEK-ERK and PI3K-PKB-mTOR signaling pathways.
Further Understanding of the GH Mechanism in Liver Regeneration
Relationship of GH to Growth Factors After PH
Numerous growth factors, especially HGF, EGF, and hepatocyte nuclear factor (HNF), are considered initiators and augmenters of liver regeneration because they prime the parenchymal and nonparenchymal liver cells for replication and facilitate their entry into the cell cycle. Their activity ensures robust cell proliferation and restoration of the original liver size. The association between GH and various growth factors in liver regeneration is well recognized. The association of GH with HGF was clearly shown by Ekberg et al,32 who compared the hepatic HGF mRNA levels in 3 groups of rats after PH. The first group had hypophysectomized rats treated with GH, the second group had rats with intact pituitary glands and no GH treatment, and the third group had hypophysectomized rats and no GH treatment. In the first group, HGF mRNA levels increased 3 hours after PH and reached peak levels after 5 hours, but in the second and third groups, HGF mRNA levels were unchanged or peaked after 10 to 18 hours, respectively. In the first group, DNA synthesis increased from low levels at 10 hours after PH to peak levels after 18 hours, whereas in the second and third groups, DNA synthesis was still low at 18 hours and increased only after 26 hours. Furthermore, treating hypophysectomized rats with IGF1 further increased HGF mRNA levels and DNA synthesis, 3.5 hours and 15 hours after PH, respectively.
Growth hormone also has an impact on HNF, according to a report by Wang et al33 who administered GH to adult wild-type and ONECUT 1−/− knockout mice for 7 days after bile duct ligation. They found that GH in wild-type mice enhanced expression of ONCECUT 1, inhibited caspase-3, caspase-8, and caspase-9 responses, and decreased hepatic injury (apoptosis and fibrosis). In contrast, these effects were abolished in the knockout mice. Twenty-eight days after bile duct ligation, the GH treatment group had substantial improvement in liver function, increased hepatocyte proliferation, decreased fibrosis, and elevated serum albumin levels compared with the knockout group. The interplay between GH and growth factors (HGF, HNF) is important for liver regeneration because these factors are overexpressed with GH and control the expression of genes needed for liver growth and function (Hes1, Hes5, and Hes6), as well as Jagged1, which interacts with Notch receptors and stimulates its downstream effectors. Moreover, we believe that the GH-ONECUT 1-HGF axis may be a possible in vivo mechanism that explains the multiple functions of GH during liver repair after bile duct injury.
Relationship of GH to Transcription Factors and Cell Cycle Regulators
The importance of GH in liver regeneration is not limited to hepatic growth factors; GH also acts on different signaling pathways, cell cycle regulators, and transcription factors involved in hepatic regeneration. Martinez et al34 assessed the signaling mediators, cell cycle regulators, and transcription factors associated with cell growth in transgenic mice overexpressing GH (phosphoenolpyruvate carboxykinase−bGH mice) at age 2, 4, and 9 weeks. Hepatocyte enlargement could be seen as early as age 2 weeks and was more prominent in young adults (liver weight was 0.22±0.01 g in control animals vs 0.27±0.03 g in GH transgenic animals at age 2 weeks; 0.94±0.07 g in control animals vs 1.62±0.06 g in GH transgenic animals at age 4 weeks). To evaluate hepatocellular proliferation, immunohistochemical analysis of the S phase−related proliferating cell nuclear antigen (PCNA) was performed and revealed higher levels of PCNA in transgenic mice compared with control mice, starting from the fourth week of life. Furthermore, expression of cyclin D1, the main activator of the G1 to S phase transition, and MYC and JUN (genes encoding transcription factors implicated in cell cycle progression) were also elevated in transgenic mice at 9 weeks.
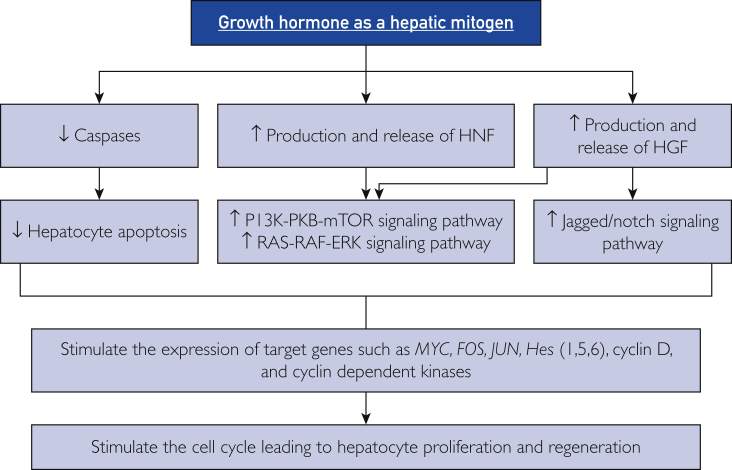
This evidence suggests that GH participates in the initiation and progression of liver regeneration by multiple mechanisms (Figure 2). First, interference with caspases leads to blockage of apoptosis in hepatocytes. Second, induction of cell cycle regulators, particularly cyclin D1, controls the passage of hepatocytes from G1 to S phase in the cell cycle. Third, GH and IGF activate hepatic growth factors such as HGF, EGF, and HNF, which stimulate the Jagged-Notch, Ras-Raf-MEK-ERK, and PI3K-PKB-mTOR signaling pathways and overexpress target genes involved in cell growth such as Hes1, Hes5, and Hes6. The Ras-Raf-MEK-ERK cascade includes phosphorylation and activation of MAPK1 and MAPK3, leading to up-regulation of important genes (such as MYC, FOS, and JUN) encoding several transcription factors.2,4 The PI3K-PKB-mTOR cascade involves activation of PI3K and PKB, leading to up-regulation of mTOR and the NF-κβ pathway, resulting in increased protein synthesis and cell division.2,4 In addition, stimulation of the Notch receptor leads to formation of the Notch intracellular domain, which is translocated into the nucleus and forms a complex with the transcription factor CSL and its coactivator Maml1. This interaction triggers the transcription of Hes1, Hes5, MYC, FOS, JUN, CCND1, and other Notch target genes that boost hepatocyte proliferation and differentiation. The Notch signaling pathway also participates in the regeneration of liver vasculature through the association of NICD with the CSL component RBP-J, thereby mediating the overexpression of specific genes involved in endothelial cell proliferation and revascularization.2,3,8
Figure 2.
The mechanisms of growth hormone in inducing liver regeneration. ERK = extracellular signal-regulated kinase; Hes = hairy and enhancer of split; HGF = hepatocyte growth factor; HNF = hepatocyte nuclear factor; mTOR = mammalian target of rapamycin; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; PKB = protein kinase B; ↑ = increased; ↓ = decreased.
The growth-promoting effect of GH sometimes can stimulate the formation of liver tumors. In the liver of young adult mice overexpressing GH, Miquet et al35 observed precancerous lesions similar to those in populations with high risk of liver cancer, and they evaluated the expression of selected molecules in the signal transduction pathways associated with hepatocellular carcinoma in GH-overexpressing mice. Long-term exposure to GH induced changes in the signaling pathways associated with cell growth, proliferation, and survival in the liver, and these changes were similar to those observed in many human tumors.
Table 2 summarizes the studies that explore GH-induced liver regeneration.
Table 2.
Growth Hormone–Induced Liver Regeneration
| Reference, year | Species | Experiment | Results |
|---|---|---|---|
| Asakawa et al,23 1989 | Rat | 2/3 PH | In hGH treatment group (compared with the control group): ↑ Liver mass, ↑ mitosis of hepatocytes ↑ Levels of serum IGF and albumin ↓ Urea nitrogen levels |
| Ekberg et al,32 1992 | Rat | PH in 3 groups: Group 1: Hypophysectomized rats + GH Group 2: Rats with intact pituitaries without GH Group 3: Hypophysectomized rats without GH |
↑ HGF mRNA levels at 3 h after PH: Group 1: Peak levels observed after 5 h Group 2: Remained unchanged Group 3: Peaked after 10-18 h ↑ DNA synthesis: Group 1: From low levels at 10 h after PH to peak levels after 18 h Groups 2 and 3: Still low at 18 h, increased after 26 h |
| Başoğlu et al,24 2000 | Rat | Three experimental groups: Group 1: Sham operation + saline infusion Group 2: Left lobectomy + saline infusion Group 3: Left lobectomy + GH injection |
On postoperative day 7, group 3 had a higher hepatocyte mitotic rate and greater Ki67 staining than groups 1 and 2 |
| Pennisi et al,25 2004 | Mouse | 70% PH in 3 groups: Group 1: GH-antagonist transgenic mice Group 2: Liver IGF1-deficient mice Group 3: Control mice |
The survival rate in group 1 was significantly lower than that of the other groups Liver regeneration after PH: Group 1: 70% of the original mass after 4 d Group 2: Complete regeneration after 7 d Group 3: Complete regeneration after 4 d |
| Wang et al,33 2008 | Mouse | GH administration for 7 d after bile duct ligation: Adult wild-type ONECUT 1−/− knockout |
Wild-type mice: ↑ Expression of ONECUT 1 ↓ Caspase-3, caspase-8, and caspase-9 responses ↓ Hepatic apoptotic and fibrotic injury. Knockout mice: Effects seen in wild-type mice were abolished |
| Collin de l’Hortet et al,27 2014 | Mouse | PH in 3 groups: Group 1: Lean mice (control) Group 2: Hepatic steatosis model; leptin-deficient ob/ob mice Group 3: Hepatic steatosis model; methionine- and choline-deficient diet |
Compared with controls, the 2 groups with hepatic steatosis showed: Delayed hepatocyte proliferation after PH (peak was 56 h in both models, 44 h in controls) ↓↓ Cyclin A at 44, 56, and 68 h ↓↓ Ratio of LW:BW Barely detectable EGFR during the first 32 h after PH Recombinant hGH was given before and after liver resection for some animals in group 2 Peak hepatocyte proliferation expression of EGFR and LW:BW ratio were highly increased in ob/ob mice injected with GH compared with nontreated animals |
| Stiedl et al,29 2015 | Mouse | Two experimental groups: Ghr−/−/Mdr2−/− double knockout Mdr2−/− single knockout |
GH resistance in Ghr−/−/Mdr2−/− mice results in: ↑ Profibrogenic factors (PDGFβ, TGF-β, and TNF-α) leading to liver fibrosis ↑ SMAD2/3 and TGF-β >> blocks liver regeneration ↑ Apoptotic factors and ROS and ↓ prosurvival factors (EGF, ONECUT 1) >> hepatocyte apoptosis and injury |
| Martinez et al,34 2016 | Mouse | Immunoblotting, qRT-PCR, and immunohistochemistry were used to assess signaling mediators, cell cycle regulators, and transcription factors associated with cell growth at age 2, 4, and 9 wk in normal and GH-overexpressing mice | Compared with normal mice, most mitogenic mediators (cyclin D1, STAT3, MYC,JUN) and the Ras-MEK-ERK and PI3K-PKB-mTOR cascades were gradually up-regulated in the liver of GH-overexpressing mice, resulting in hepatocyte proliferation |
| Wang et al,31 2018 | Mice | Elucidate the mechanism of therapeutic liver repopulation in the FAH−/− mouse model | In spite of NTBC withdrawal, FAH−/− mice: Survived hepatocyte transplant Showed ↑ IGF2 expression after NTBC withdrawal Hepatocytes cultured with increasing amounts of IGF2 had greater proliferation Western blot analysis revealed ↑ levels of phosphorylated IGF1R, ERK, and PKB in repopulated livers after hepatocyte transplant |
EGFR = epidermal growth factor receptor; ERK = extracellular signal-regulated kinase; GH = growth hormone; HGF = hepatocyte growth factor; hGH = human growth hormone; IGF = insulinlike growth factor; LW:BW = liver weight to body weight; mRNA = messenger RNA; mTOR = mammalian target of rapamycin; NTBC = 2-nitro-4-trifluoro-methyl-benzoyl-1,3-cyclohexanedione; PDGF = platelet-derived growth factor; PH = partial hepatectomy; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; PKB = protein kinase B; qRT-PCR = quantitative reverse transcription polymerase chain reaction; ROS = reactive oxygen species; TGF = transforming growth factor; TNF = tumor necrosis factor; STAT= signal transducer and activator of transcription; ↑ = increased; ↓ = decreased; ↓↓ = much decreased; >> = much greater than.
Insulin
Insulin is an anabolic peptide hormone synthesized by β cells in the islets of Langerhans in the pancreas. It acts on a heterotetrameric receptor tyrosine kinase that consists of 2 extracellular α subunits (with ligand-binding activity) and 2 transmembrane β subunits (with tyrosine kinase activity). To decrease the blood glucose concentration back to normal, insulin promotes cellular uptake of glucose and synthesis of glycogen, fat, and protein.36 The following sections review insulin as a hepatic mitogen.
Insulin contributes to liver regeneration and is a metabolic regulator of homeostatic functions of regenerating hepatocytes.2,3,8 As early as 1975, Starzl et al37,38 discovered that insulin in the portal vein is a hepatotrophic factor that is essential for liver regeneration. In 1984, Francavilla et al39 also reported that insulin-binding sites on the hepatocyte membrane increased significantly in rats 24 to 48 hours after 70% PH, suggesting that insulin is closely associated with liver regeneration.
Role of ROS-Mediated Insulin-IGF1 Resistance in Liver Regeneration After PH
The liver usually has oxidative stress after PH, exposure to chemical toxins (eg, acetaminophen), and viral infection. These stressors alter liver function, induce hepatocyte apoptosis, and impair regeneration. However, quiescent injured hepatocytes potentially can still reenter the cell cycle and proliferate until the original liver mass is regained. A crucial transcription factor that protects hepatocytes from oxidative stress is Nrf2. This protein regulates the expression of antioxidant enzymes (eg, glutathione S-transferase, NQO1) that are the first step of the detoxification process.40
Beyer et al41 studied mice lacking the antioxidant transcription factor Nrf2 and assessed the effect of oxidative stress on liver regeneration after 2/3 PH. The mice had significantly reduced expression of cytoprotective antioxidant proteins, resulting in elevated ROS. Compared with control animals, Nrf2 knockout mice had enhanced hepatocyte apoptosis and a marked delay in liver cell proliferation at 48 hours after PH. Furthermore, in vitro and in vivo studies revealed that impairment of liver regeneration was mediated by oxidative stress−induced insulin-IGF1 resistance. To elucidate the mechanisms underlying this impairment, total protein (60 μg) from liver lysates of Nrf2 knockout and wild-type mice were analyzed by immunoblotting with antibodies directed against the phosphorylated forms and total levels of p38, PI3K, Akt (or PKB), EGFR, HGFR, STAT3, ERK, and JNK. Interestingly, the levels of phosphorylated (active) p38 mitogen-activated kinase, Akt (or PKB) kinase, and downstream targets were significantly lower in Nrf2 knockout mice. However, no differences were observed in the levels of phosphorylated (active) EGFR, HGFR, STAT3, ERK, p38, and JNK between wild-type and Nrf2-deficient mice after PH. These experiments suggest a novel role for Nrf2 as an essential redox regulator in liver regeneration; it prevents ROS accumulation by up-regulating expression of ROS-detoxifying enzymes. Therefore, its deficiency precipitates oxidative stress, resulting in insulin resistance by reducing the tyrosine phosphorylation of insulin receptor (IR) substrate proteins. Consequently, activation and phosphorylation of the PI3K-PKB-mTOR cascade and its downstream targets is abolished. Thus, Nrf2 deficiency appears to be strongly linked with oxidative stress, insulin-IGF1 resistance, and impairment of hepatic regeneration. Furthermore, impedance of the PI3K-PKB-mTOR cascade after establishing insulin-IGF1 resistance by ROS may explain how insulin-IGF1 affects liver regeneration. Activation of IRs appears to induce tyrosine phosphorylation of IR substrate proteins, which leads to activation of the PI3K-PKB-mTOR cascade and increased survival and proliferation of hepatocytes. Pharmacological activation of insulin-IGF1 and Nrf2 could be a promising new strategy to ameliorate liver regeneration in patients with acute or chronic liver injury (especially if patients also have type 2 diabetes mellitus and insulin resistance).
Clinical Effects of Intraportal Insulin Injection on Liver Graft Regeneration
Living donor liver transplant (LDLT) involves transplant of a portion of the liver from a living donor into a recipient with end-stage liver disease. This approach is associated with several benefits, including bypassing the donor organ waiting list, and the surgery can be scheduled at a time that is convenient for the recipient and donor. Living donor liver transplant is increasingly accepted, especially for patients who are too sick to wait for a deceased donor transplant. However, one problem of LDLT is small-for-size syndrome, in which the liver graft parenchymal mass is too small to adequately meet the functional needs of the recipient.42 The syndrome is characterized by persistently elevated bilirubin levels and large ascites during the early period after LDLT, in the absence of other potential causes.43
Because insulin is considered a hepatic mitogen in several animal models, Xu et al44 assessed the clinical effects of intraportal administration of insulin on liver graft regeneration in 15 patients after LDLT and compared them with 15 control recipients who did not receive insulin. They intraoperatively placed a tube in the right gastroepiploic vein of recipients, and insulin was perfused to the portal vein (2 U/h) on postoperative days 1 through 7. Liver function, serum insulin levels, and computed tomographic volumetric analysis of the grafts were evaluated on postoperative days 7 and 30. The growth rate (the ratio of graft volumes from the day of harvest and postoperative day 7) in the insulin perfusion group was significantly higher than that of the control group (186.07%±35.40% vs 160.61%±22.11%; P<.05). The liver function index was also significantly better than that of the control group. The investigators concluded that continuous intraportal insulin perfusion after LDLT may promote early (1 week) liver regeneration and functional recovery after transplant. Clinically, it might be a safe method of enhancing liver graft regeneration; further, it may prevent the occurrence of or even cure small-for-size syndrome. However, only 15 patients were enrolled in that study, and thus further investigation with a larger patient group should be conducted to investigate the underlying mechanisms and confirm the clinical significance of intraportal insulin administration in eliminating small-for-size syndrome.
Role of Insulin in Liver Regeneration After Inhibiting Portal Blood Flow
The dual blood supply of the liver is a unique feature of this organ. About 75% of the blood supply is delivered by the portal vein, which carries deoxygenated blood, rich in nutrients, from the intestine; the remaining 25% of blood is delivered via hepatic arteries that nourish the liver with oxygenated blood. Therefore, portal blood flow is among the most crucial factors needed to launch the liver regeneration process.45 After PH, hemodynamic changes in the portal vein (not affecting hepatic arteries) elevate the portal contribution per unit of liver tissue. The increased portal contribution increases the availability of growth factors and cytokines from the intestine and pancreas. Furthermore, these changes augment the portal blood pressure, causing a turbulent blood flow that applies mechanical shear stress on the endothelium and thereby increases the activity of urokinase plasminogen activator by endothelial cells. As a result, plasmin is produced and matrix metalloproteinases are activated, leading to matrix remodeling. Remodeling activates signaling pathways, cytokines, and growth factors necessary for liver regeneration. Therefore, any interference of portal blood flow could markedly preclude liver regeneration.2,3,8
Two studies have assessed the effect of insulin on reversing the harmful effects of portal vein ligation and stenosis on liver regeneration. Tseng et al46 performed portal vein ligation on normal rats and rats with streptozotocin-induced insulin deficiency. They also studied the role of insulin signaling in liver regeneration by determining volumetric shifting, performing cell cycle analyses, and detecting cell cycle markers and apoptosis-related genes. The lobes became atrophic with portal vein ligation, whereas nonligated lobes became hypertrophic. Rats with insulin deficiency had significantly lower restituted liver mass and redistributed volume ratios compared with normal rats. Flow cytometry revealed a remarkably increased fraction of S-phase hepatocytes, indicating the failure of G1 to S-phase hepatocytes to enter into the G2 to M phase, causing cell cycle progression to stagnate in S phase. Quantitative PCR of insulin-deficient rats revealed underexpression of cell cycle−related genes (CCND1, CCNA2, CCNB1, PCNA) and overexpression of apoptosis-related genes (BAX, DAXX, MAPK8). Consequently, rats with insulin deficiency had increased hepatocyte apoptosis compared with normal rats. This study found that the insulin-signaling pathway had an important role in liver regeneration caused by portal vein ligation. The net effect of reduced hepatocyte proliferation was due to inhibited cell cycle−related genes, telomerase activity, and S-phase stagnation, and increased expression of hepatocyte apoptosis-related genes.
The other study, by Backes et al,47 used a new model to study liver regeneration. They divided experimental animals into 5 groups: (1) sham operation, (2) 70% hepatectomy, (3) 70% hepatectomy plus portal vein stenosis, (4) 70% hepatectomy plus portal vein stenosis plus insulin application, and (5) 70% hepatectomy plus portal vein stenosis plus tacrolimus application. The residual liver after surgery showed that the reduction of portal vein blood flow caused by stenosis adversely affected liver regeneration. Compared with the simple portal vein stenosis group, the residual liver mass, mitotic activity, and proliferation-related indicators IL- 6 and Ki67 were significantly increased in the insulin group and the tacrolimus group, indicating that insulin and tacrolimus can partially reverse the harmful effects of portal vein stenosis in liver regeneration.
Further Understanding of the Mechanisms of Insulin in Liver Regeneration
The mechanisms underlying the ability of insulin to enhance hepatocyte regeneration also must be elucidated. Amaya et al48 analyzed the SkHep-1 human hepatoma cell line and used immunofluorescence microscopy and immunoblotting to monitor localization of the IR before and after insulin stimulation. Before insulin stimulation, the IR was localized to the plasma membrane and nearly absent in the nucleus. After 5 minutes of insulin exposure, the IR was diffusely distributed inside the nucleus and cytoplasm. Further analysis revealed that nuclear and cytosolic inositol 1,4,5-trisphosphate (InsP3) and Ca2+ signals were overexpressed with insulin exposure. To verify the contribution of nuclear vs cytosolic InsP3 to insulin-induced Ca2+ signals, the same cells were loaded with a Ca2+-sensitive dye and examined microscopically under nuclear and cytosolic InsP3-buffering conditions after insulin stimulation. Nuclear and cytosolic Ca2+ signals were nearly eliminated by buffering InsP3 in the nucleus, but buffering InsP3 in the cytosol had no effect on nuclear Ca2+ signals and only minimally decreased cytosolic Ca2+ signals. To verify whether nuclear InsP3 regulated insulin-induced liver regeneration, the same cells were synchronized in G0, transfected with a gene that buffered nuclear InsP3, stimulated with insulin, and assayed for bromodeoxyuridine incorporation. Nuclear InsP3-buffered cells had significantly less bromodeoxyuridine uptake than control cells, indicating that insulin-induced cell proliferation depends on nuclear formation of InsP3 in vivo.
Hepatocyte proliferation experiments were conducted to determine the physiologic relevance of the findings from SkHep-1 cells. Bromodeoxyuridine uptake was measured in rats after 70% PH and under nuclear or cytosolic InsP3-buffering conditions. After PH, animals with nuclear InsP3 buffering had impaired bromodeoxyuridine uptake and a reduced liver: body weight ratio compared with control animals. Indeed, when comparing animals that underwent PH vs a sham treatment, immunohistochemical analysis and immunoblotting revealed greater IR levels in the nucleus 24 hours after PH.
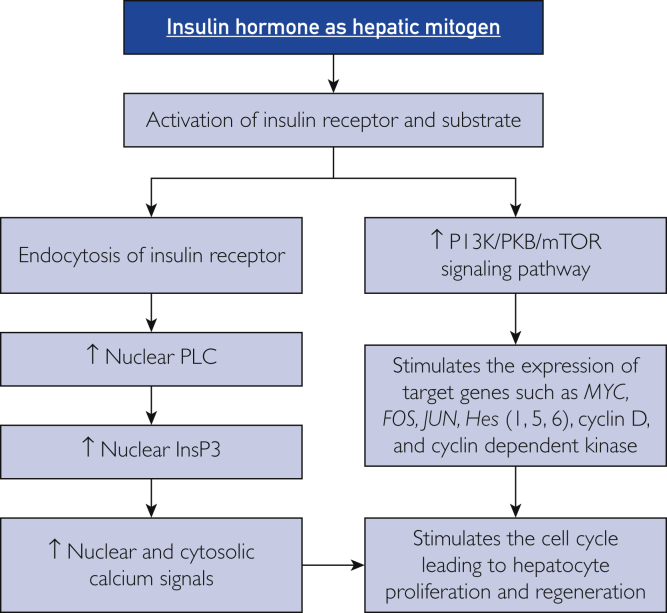
Collectively, these observations provide a mechanistic understanding of insulin-induced liver regeneration (Figure 3). Interaction of insulin with the IR leads to activation and internalization of IRs via endosomes, followed by their translocation into the nucleus, where phospholipase C is activated. This enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate to form diacylglycerol, which activates protein kinase C and InsP3. The latter initiates nuclear and cytosolic Ca2+ signals after binding to the InsP3 receptor on a ligand-gated Ca2+ channel. The process of liver regeneration depends on Ca2+ signals that are triggered only by nuclear (not cytosolic) InsP3. In addition, activation and phosphorylation of the PI3K-PKB-mTOR cascade represents another mechanism that mediates the mitogenic effect of insulin in the liver. Altogether, these factors have broad clinical implications and could be targeted for controlling hepatocellular growth and enhancing the regenerative capacity of hepatocytes after PH or LDLT to prevent the occurrence of small-for-size syndrome.
Figure 3.
The mechanisms of insulin in inducing liver regeneration. Hes = hairy and enhancer of split; mTOR = mammalian target of rapamycin; HGF = hepatocyte growth factor; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; PKB = protein kinase B; PLC = phospholipase C; InsP3 = inositol 1,4,5-trisphosphate; ↑ = increased.
Table 3 summarizes the studies that explore insulin-induced liver regeneration.
Table 3.
Insulin-Induced Liver Regeneration
| Reference, year | Species | Experiment | Results |
|---|---|---|---|
| Francavilla et al,39 1984 | Rat | 70% PH | ↑ Insulin-binding sites on hepatocyte membranes, 24-48 h after PH |
| Beyer et al,41 2008 | Mouse | 2/3 PH in mice lacking the antioxidant transcription factor Nrf2 | Marked delay in liver regeneration because oxidative stress enhanced insulin resistance |
| Xu et al,44 2009 | Human | Intraoperative, intraportal insulin infusion through the right gastroepiploic vein of living donor liver transplant recipients | Growth rate of the transplanted liver volume in the insulin-perfusion group was significantly higher than that of the control group The liver function index was significantly better than that of the control group |
| Tseng et al,46 2011 | Rat | Portal vein ligation for rats with streptozotocin-induced insulin deficiency | Compared with normal rats, those with insulin deficiency had significantly lower indices of liver regeneration, including restituted liver mass and redistributed volume ratio |
| Backes et al,47 2014 | Rat | Five groups of experimental animals: Group 1: Sham operation Group 2: 70% PH Group 3: 70% PH + portal vein stenosis Group 4: 70% PH + portal vein stenosis + insulin Group 5: 70% PH + portal vein stenosis + tacrolimus |
Reduction of portal vein blood flow from stenosis adversely affected the liver regeneration Compared with the simple portal vein stenosis group, the insulin and tacrolimus groups had greater residual liver mass, mitotic activity, and proliferation-related indicators (IL-6, Ki67) Insulin and tacrolimus can partially reverse the harmful effects of portal vein stenosis in liver regeneration |
| Amaya et al,48 2014 | Rat | SkHep-1 human hepatoma cell line 70% PH |
The mitogenic effect of insulin on liver regeneration was achieved by transferring the insulin receptor into the nucleus Formation of nuclear InsP3 initiates nuclear and cytosolic Ca2+ signals |
IL = interleukin; InsP3 = inositol 1,4,5-trisphosphate; PH = partial hepatectomy.
Thyroid Hormone
Triiodothyronine (T3) and thyroxine are tyrosine-based hormones produced by follicular cells in the thyroid gland. They are responsible for regulating carbohydrate, protein, and lipid metabolism, elevating the basal metabolic rate and oxygen consumption, thermal regulation, increasing gut motility, stimulating heart and muscle function, and regulating tissue growth, brain development, and bone maintenance.49 The secretion of thyroid hormones is also regulated by thyroid-stimulating hormone, which is produced by thyrotropes of the anterior pituitary gland.49 The following sections review thyroid hormone as a hepatic mitogen.
Understanding the Mechanism of Thyroid Hormone in Liver Regeneration
Effect of Thyroid Hormone on Cell Cycle Regulators and Apoptosis
Several important studies have elucidated the mechanisms by which thyroid hormones have mitogenic effects on hepatocytes. Pibiri et al50 compared mitotic activity of hepatocytes from 2 groups of mice. One group was treated with T3 and the other underwent 2/3 PH. They assessed the activation of transcription factors AP-1, NF-κβ, and STAT3 with electrophoretic mobility shift assays, changes in the mRNA levels of the immediate early genes (FOS, JUN, MYC, and E2F) with Northern blot analysis, levels of proteins involved in the progression from G1 to S phase of the cell cycle with Western blot analysis, and mitotic activity with immunohistochemistry and injection of bromodeoxyuridine. They noted that mitotic activity peaked at 18 hours and 24 hours after T3 treatment and PH, respectively, indicating the accelerated entry of hepatocytes into DNA synthesis after T3 treatment compared with PH. Their findings about the molecular mechanisms of T3 mitotic effects revealed that the regeneration of hepatocytes after T3 treatment occurred without changes in the activity of transcription factors (AP-1, NF-κβ, STAT3) or mRNA levels of immediate early genes (FOS, JUN, MYC), even though all these factors had vital roles in liver regeneration after PH. Triiodothyronine treatment also increased the expression of transcription factors of the E2F family, which transactivate the expression of S-phase genes (eg, cyclin D), leading to the transition of hepatocytes from G1 to S phase; this increase explains the rapid entry of hepatocytes into the DNA synthesis phase after T3 treatment. No signficant modification in the activity of E2F factors was observed in PH rats for 24 hours. Cyclin-dependent kinases (CDK2, CDK4, and CDK6) and cyclin E also were similarly activated in both groups.
Additional experiments have further clarified the mechanisms implicated in the proregenerative effects of thyroid hormones on hepatocytes. Alisi et al51 carried out 70% PH on 2 groups of mice. Group 1 had hypothyroidism that was chemically induced by a 6-week exposure to 0.95% 6-n-propyl-2-thiouracil (PTU) in the drinking water. Group 2 had hyperthyroidism that was induced by daily intraperitoneal injection of T3. Their study found that hyperthyroidism in partially hepatectomized animals increased the levels of cyclins A, D1, and E, increased the activity of cyclin A-CDK2 and cyclin D1-CDK4 complexes, and reduced the levels of CDK inhibitors p16 and p27. In contrast, hypothyroidism down-regulated the activity of associated cyclin-dependent kinase (cyclin-CDK) complexes and decreased the levels of cyclins. Furthermore, thyroid hormone also decreased the levels of p53 and p73 (tumor suppressor proteins involved in growth arrest and apoptosis), which caused the failure to activate p21 and blocked the inhibition of CDK enzymes. These changes led to induction of hepatic cell cycles and stimulation of the Ras-Raf-MEK-ERK signaling pathway, which strongly contribute to hepatic regeneration. López-Fontal et al52 studied the role of thyroid hormones in liver regeneration by performing PH in thyroid receptor (TR) α1/TRβ double-knockout mice. The absence of TR resulted in a delayed commitment to the initial round of hepatocyte proliferation caused by delayed expression of cyclins D1 and E. They also reported transient but intense apoptosis 48 hours after PH that affected approximately 30% of remaining hepatocytes. In murine hepatocytes lacking TR, higher activity of DDAH-1 caused a transient decrease in the concentration of asymmetric dimethylarginine, a potent nitric oxide synthase (NOS) inhibitor. The increased activity of NOS2 and NOS3 resulted in apoptosis.
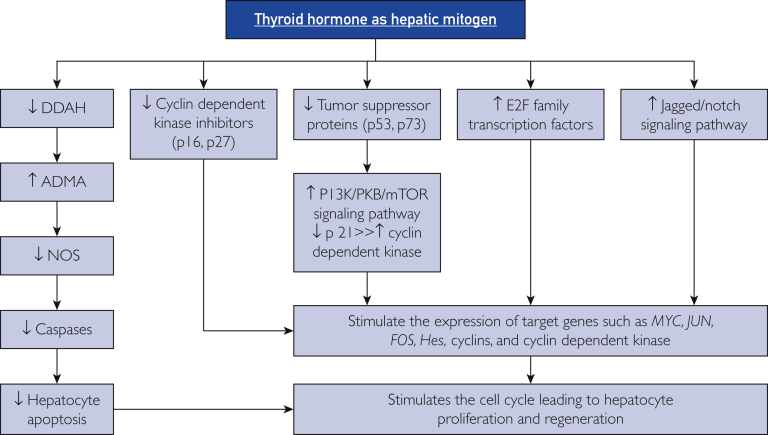
Taken together, thyroid hormone appears to be a hepatic proliferative factor that induces the mitogenic activity of hepatocytes through multiple points (Figure 4). First, up-regulation of E2F transcription factors causes overexpression of cell cycle inducers (cyclins and CDKs). Furthermore, these inducers are directly activated by thyroid hormone. Second, decreased activity of p16 and p27 diminish their inhibitory effect on CDK. Third, suppression of tumor suppressor proteins p53 and p73 abolishes their stimulatory effects on p21, blocks inhibition of CDK enzymes, and causes induction of hepatic cell cycles and stimulation of the Ras-Raf-MEK-ERK signaling pathway, which strongly regulates hepatic regeneration. Fourth, reduced DDAH-1 activity inhibits asymmetric dimethylarginine, which induces NOS activity and triggers hepatocyte apoptosis through up-regulation of caspase genes.
Figure 4.
The mechanisms of thyroid hormone in inducing liver regeneration. DDAH = dimethylarginineaminohydrolase; ADMA = asymmetric dimethylarginine; mTOR = mammalian target of rapamycin; NOS = nitric oxide synthase; PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase; PKB = protein kinase B; ↑ = increased; ↓ = decreased.
Effect of Thyroid Hormone on the Termination Phase of Liver Regeneration
Liver regeneration is a well-orchestrated sequence of events that requires balancing proliferation-inducing factors against proliferation-inhibiting factors to achieve optimal results. Replicating hepatocytes must enter a termination phase at the completion of regeneration; this process is governed by antiproliferative factors TGF-β, SMAD, and activin, which stop cell division, correct “overshooting” in regeneration, and prevent carcinogenic formation. Multiple mechanisms explain how TGF-β terminates liver regeneration. First, stimulation of the TGF receptor phosphorylates SMAD proteins, which are then translocated into the nucleus. SMAD proteins up-regulate the expression of CDK inhibitors and halt the activity of cell cycle inducers such as CDK2, CDK4, and cyclins D and E, causing the cell cycle to arrest at the G1 to S transition. Second, TGF-β induces cellular apoptosis by releasing ROS via a JUN−independent mechanism.2,3,8 Alonso-Merino et al53 reported that the binding of thyroid hormone to TRs in rats antagonizes the transcriptional activity of TGF-β and SMAD and thus prevents termination of the liver regeneration process.
Effect of Thyroid Hormone on Liver Regeneration Through β-Catenin Signaling Pathway
The Wnt/β-catenin pathway is among the essential regulators of the hepatic regeneration process, and it becomes active 1 to 3 hours after PH. The pathway involves the release of Wnt ligands from Kupffer cells, sinusoidal endothelial cells, and hepatic stellate cells. The released, active Wnt proteins activate low-density lipoprotein-related protein (LRP) 5 and LRP6 on hepatocytes, inhibit the β-catenin degradation complex, and prevent the breakdown of β-catenin. After translocation of β-catenin into the nucleus, it combines with transcription factors TCF and LEF and induces cyclin D expression and cell proliferation.
Alvarado et al54 described activation of the β-catenin signaling pathway as an important molecular mechanism underlying T3’s mitogenic effect in liver regeneration. They assessed the regenerative advantage associated with treatment with GC-1 (a TRβ agonist, also termed sobetirome) in normal mice, a mouse model of PH, and mice with a liver-specific β-catenin knockout and liver-specific double-knockouts of Wnt coreceptors LRP5 and LRP6. As evidenced by PCR, immunohistochemistry, and Western blots, mice receiving GC-1 had increased expression or levels (or both) of pSer675–β-catenin, cyclin D1, bromodeoxyuridine incorporation, and PCNA compared with control animals. In contrast, GC-1 injection in mice with β-catenin and LRP5-LRP6 knockouts showed decreased hepatocyte proliferation compared with wild-type littermates. Furthermore, mice treated with GC-1 for 7 days before PH had significantly greater hepatocyte proliferation at time 0 and 24 hours after the procedure. These data clearly indicate that stimulation of TRβ induces the regenerative capacity of the liver in normal and PH mice by activating the Wnt/β-catenin signaling pathway.
Further Evidence of the Hepatoproliferative Role of Thyroid Hormone After PH
Bockhorn et al55 conducted research with rats to determine whether a single preoperative injection of T3 improved liver regeneration after 70% PH. They noted that T3-treated rats had an increased ratio of liver weight to body weight, higher expression of vascular endothelial growth factor, and a higher Ki67 index after PH compared with placebo-treated rats (P<.05). Biondo-Simões et al56 investigated the influence of hypothyroidism on liver regeneration in male Wistar rats. One group of 20 euthyroid rats (termed group C) and a second group of 20 hypothyroid rats (group H) who had the thyroid gland completely resected underwent PH. The regeneration rate after 24 hours was 58.49% for group C and 50.42% for group H (P=.02). After 7 days, regeneration rates were 93.04% for group C and 93.74% for group H (P=.22). The average number of mitotic figures after 24 hours was 14±1.5 for group C and 9.8±2.2 for group H (P<.001). After 7 days, the number of mitotic figures was 5.4±1.1 for group C and 5.1±1.2 for group H (P=.63). On the basis of these findings, we believe that hypothyroidism in rats delays hepatic regeneration during the first 24 hours after PH.
Effect of Thyroid Hormone on Hepatic Stem Cells
Given the importance of thyroid hormone in liver regeneration, we recognize that this hormone also influences hepatic stem cell self-renewal and differentiation. Cvoro et al57 reported that thyroid receptor activation induces KLF9 to control proliferation and differentiation of hepatocytes and early stages of organogenesis. They treated mice for 3 days with thyroid hormone and then collected liver tissue for RNA purification and analysis. In mice treated with thyroid hormone, KLF9 was overexpressed in primary hepatocytes in a dose-dependent fashion. Furthermore, they found that activation of thyroid receptors leads to KLF9 induction in HepG2 cells (a liver carcinoma cell line), human induced pluripotent stem cells (hiPSCs), and human embryonic stem cells (hESCs); these effects persisted during hiPSC and hESC differentiation to definitive mature hepatocytes.
To define the roles of KLF9 and TR, Cvoro et al57 used small interfering RNA to silence KLF9 expression in HepG2 cells and then treated small interfering control and small interfering KLF9 HepG2 cells with thyroid hormone. Microarray analysis revealed that T3 regulates hundreds of hESC and hiPSC target genes that cluster into many of the same signaling pathways implicated in T3 and KLF9 regulation, including Wnt and Notch signaling events. Further analysis of T3-responsive hESC or hiPSC genes indicated that T3 controls multiple early steps in hESC differentiation. These signaling pathways represent the primary drivers of liver regeneration. Stimulation of Notch receptor by its ligand Jagged leads to formation of the Notch intracellular domain, which forms a complex with the transcription factor CSL and its coactivator Maml1. This interaction triggers the transcription of Hes 1, Hes 5, MYC, and CCND 1 genes, and thus augmenting the replication of hepatocytes. Furthermore, this pathway is involved in endothelial cell proliferation and revascularization by activating RBP-J. We suggest that the interplay between thyroid hormone, KLF9, and Notch/Wnt signaling pathways have essential roles in several stages of hepatocyte development, in regulating differentiation of hepatic stem cells into mature hepatocytes, and during liver regeneration.
Effect of Thyroid Hormone on Hepatic Mitochondrial Integrity and Activity
Mukherjee et al58 delineated the impact of PTU-induced hypothyroidism and its reversal by T3. In their study, rats were randomly assigned to 3 groups, each consisting of 5 animals. Rats in group 1 (euthyroid) received a standard diet and drinking water. Rats in group 2 (hypothyroid) and group 3 (hypothyroid plus T3) had PTU in their drinking water. After 6 weeks, PTU was withdrawn; group 2 rats then received daily injections of NaOH (vehicle for T3), and group 3 rats received T3. Injections were administered for 3 consecutive days. Compared with the euthyroid group, rats with PTU-induced hypothyroidism had higher mitochondrial H2O2 levels due to reduced matrix glutathione peroxidase activity, increased membrane lipid peroxidation (indicated by the formation of thiobarbituric acid−reactive substances), increased oxidative damage to mitochondrial proteins (indicated by reduced protein carbonyl content in submitochondrial particles), altered antioxidant defenses in the mitochondrial matrix fraction, and eventually, more hepatocyte apoptosis. Surprisingly, T3 (administered after withdrawing PTU) exerted antioxidative, antiapoptotic, and proproliferative effects.
Thyroid hormone levels critically regulate the functional integrity of hepatic mitochondria by up-regulating electron transport chain complexes, removing accumulated H2O2 (by activating the matrix glutathione peroxidase), and reversing oxidative injury to mitochondrial membrane lipids (by decreasing the formation of thiobarbituric acid−reactive substances and proteins through increased protein carbonylation of submitochondrial particles). All these effects substantially help the hepatocytes to recover from hypothyroidism-induced acute injury and apoptosis. These findings support the idea that thyroid hormone acts as a hepatic mitogen because it helps in decreasing mitochondrial oxidative injury and cellular apoptosis and thus enables hepatocytes to proliferate and regenerate in an environment with minimal ROS.
Table 4 summarizes the studies that explore thyroid hormone−induced liver regeneration.
Table 4.
Thyroid Hormone−Induced Liver Regeneration
| Reference, year | Species | Experiment | Results |
|---|---|---|---|
| Pibiri et al,50 2001 | Mouse | Compared the hepatocyte mitotic activity in 2 groups: Group 1: Treated with T3 hormones Group 2: 2/3 PH |
Mitotic activity peaked at 18 h after T3 treatment and 24 h after PH Activity of transcription factors (NF-κβ, STAT3) and mRNA of immediate early genes FOS, JUN, and MYC had a vital role only with PH (not T3) In T3 treatment (relative to PH): ↑ Expression of cyclin D1 leading to rapid entry of hepatocytes into DNA-synthesis phase ↑ Expression of E2F family of transcription factors leading to overexpression of S-phase genes and ↑ G1-S transition |
| Alisi et al,51 2005 | Mouse | 2/3 PH and then divided into 2 groups: Group 1: Hypothyroidism that was chemically induced (6-wk of 0.95% PTU in the drinking water) Group 2: Hyperthyroidism that was induced by daily intraperitoneal injection of T3 |
Hypothyroidism: Down-regulated the activity of cyclin-CDK complexes and levels of cyclins Hyperthyroidism: ↑ Levels of cyclin A, D1, and E and activity of cyclin A-CDK2 and cyclin D1-CDK4 complexes ↓ Levels of CDK inhibitors p16 and p27 |
| López-Fontal et al,52 2010 | Mouse | PH in TR-α1/TR-β double knockouts | Absence of TR: ↓ Expression of cyclins D1 and E ↑ DDAH-1 leading to ↓ ADMA, which stimulates NOS resulting in ↑ hepatocyte apoptosis |
| Alonso-Merino et al,53 2016 | Rat | Effect of thyroid hormone on the termination phase of liver regeneration | Binding of thyroid hormone to TRs antagonizes transcriptional activity of TGF-β/SMAD and prevents termination of the liver regeneration process |
| Bockhorn et al,55 2007 | Rat | Effect of a single preoperative injection of T3 on liver regeneration after 70% PH | T3 treatment increased liver weight to body weight ratio and increased VEGF and Ki67 index after PH compared with placebo treatment |
| Biondo-Simões et al,57 2007 | Rat | PH in 2 groups: Group 1: 20 euthyroid rats Group 2: 20 hypothyroid rats (complete resection of the thyroid) |
Group 1 had a higher regeneration rate and more mitotic figures at 24 h after PH |
| Alvarado et al,54 2016 | Mouse | GC-1 (TR-β agonist; also termed sobetirome) in 4 groups: Group 1: Normal mice Group 2: Model of PH Group 3: Liver-specific β-catenin knockout Group 4: Liver-specific double knockouts of Wnt coreceptors LRP5 and LRP6 |
Groups 1 and 2: ↑ β-catenin, cyclin D1 BrdU, and PCNA Groups 3 and 4: ↓ Cyclin D1, BrdU, and PCNA |
| Mukherjee et al,58 2014 | Rat | Three experimental groups: Group 1: Euthyroid rats received a standard diet and drinking water Group 2: Hypothyroid Group 3: Hypothyroid + T3 rats were treated with PTU in the drinking water for 6 wk After PTU treatment (group 3 only)—daily injections for 3 days: Group 2: NaOH (vehicle for T3) Group 3: T3 |
PTU-induced hypothyroidism (compared with euthyroid group): ↑ Mitochondrial H2O2 levels due to ↓ matrix GPx activity ↑ Oxidative damage to mitochondrial proteins and lipids ↑ Hepatocyte apoptosis. All effects were reversed on administration of T3, which exerted antioxidative, antiapoptotic, and proproliferative effects |
ADMA = asymmetric dimethylarginine; BrdU = bromodeoxyuridine; CDK = cyclin-dependent kinase; DDAH = dimethylarginineaminohydrolase; GPx = glutathione peroxidase; LRP = low-density lipoprotein-related protein; mRNA = messenger RNA; NF-κβ = nuclear factor κβ; NOS = nitric oxide synthase; PCNA = proliferating cell nuclear antigen; PH = partial hepatectomy; PTU = 6-n-propyl-2-thiouracil; STAT = signal transducer and activator of transcription; TGF = transforming growth factor; TR = thyroid receptor; VEGF = vascular endothelial growth factor; ↑ = increased; ↓ = decreased.
Conclusion
Strong and substantial advances in the molecular and biologic understanding of liver regeneration and its clinical applications have occurred in the past several decades. The endocrine system is one of multiple areas that have been investigated in various animal models to further understand the interplay between different systems, pathways, and molecules that assure optimal hepatic regeneration. On the basis of the aforementioned studies, we conclude that endocrinal hormones, particularly norepinephrine, GH, insulin, and thyroid hormone, are important hepatic mitogens with vital roles in inducing the process of liver regeneration. They directly and indirectly trigger the activity of signaling pathways, cytokines, growth factors, and transcription factors, leading to induction of cyclins and cyclin-CDK complexes that activate the entry of hepatocytes into the cell cycle for regeneration. Only limited evidence has been published about the role of other endocrinal hormones such as cortisol, parathormone, vasopressin, sex hormones, and leptin in liver regeneration. Therefore, further investigations are necessary to delineate the effects of other endocrinal hormones on liver regeneration. From a clinical perspective, we believe that incorporating the hormones discussed previously as a supplemental treatment to artificial extracorporeal liver support devices may optimize hepatocyte regeneration, especially in patients with acute liver failure and small-for-size syndrome.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Drs Abu Rmilah and Zhou contributed equally to this work.
Footnotes
Grant Support: This project was supported in part by the Mayo Foundation for Medical Education and Research, grant R01 DK10667 from the National Institutes of Health, and Regenerative Medicine Minnesota.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Zhou W., Nelson E.D., Abu Rmilah A.A., Amiot B.P., Nyberg S.L. Stem cell-related studies and stem cell-based therapies in liver diseases. Cell Transplant. 2019;28(9-10):1116–1122. doi: 10.1177/0963689719859262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Rmilah A., Zhou W., Nelson E., Lin L., Amiot B., Nyberg S.L. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8(3):e340. doi: 10.1002/wdev.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384–1392. doi: 10.1002/hep.28988. [DOI] [PubMed] [Google Scholar]
- 4.Gilgenkrantz H., Collin de l'Hortet A. Understanding liver regeneration: from mechanisms to regenerative medicine. Am J Pathol. 2018;188(6):1316–1327. doi: 10.1016/j.ajpath.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Chen H.S., Joo D.J., Shaheen M., et al. Randomized trial of spheroid reservoir bioartificial liver in porcine model of posthepatectomy liver failure. Hepatology. 2019;69(1):329–342. doi: 10.1002/hep.30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey R.D., Mao S.A., Glorioso J., et al. Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci Transl Med. 2016;8(349):349ra99. doi: 10.1126/scitranslmed.aaf3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azuma H., Paulk N., Ranade A., et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25(8):903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao S.A., Glorioso J.M., Nyberg S.L. Liver regeneration. Transl Res. 2014;163(4):352–362. doi: 10.1016/j.trsl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rang H.P., Ritter J.M., Flower R.J., Henderson G. 8th ed. Elsevier Health Sciences; New York, NY: 2016. Rang & Dale's Pharmacology; pp. 177–196. [Google Scholar]
- 10.Tank A.W., Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5(1):1–15. doi: 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- 11.Cruise J.L., Houck K.A., Michalopoulos G.K. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science. 1985;227(4688):749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- 12.Olsen P.S., Poulsen S.S., Kirkegaard P. Adrenergic effects on secretion of epidermal growth factor from Brunner's glands. Gut. 1985;26(9):920–927. doi: 10.1136/gut.26.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houck K.A., Cruise J.L., Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988;135(3):551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- 14.Broten J., Michalopoulos G., Petersen B., Cruise J. Adrenergic stimulation of hepatocyte growth factor expression. Biochem Biophys Res Commun. 1999;262(1):76–79. doi: 10.1006/bbrc.1999.1183. [DOI] [PubMed] [Google Scholar]
- 15.Chinery R., Brockman J.A., Dransfield D.T., Coffey R.J. Antioxidant-induced nuclear translocation of CCAAT/enhancer-binding protein β: a critical role for protein kinase A-mediated phosphorylation of Ser299. J Biol Chem. 1997;272(48):30356–30361. doi: 10.1074/jbc.272.48.30356. [published correction appears in J Biol Chem. 1998;273(24):15308] [DOI] [PubMed] [Google Scholar]
- 16.Kanamaru C., Yasuda H., Takeda M., et al. Smad7 is induced by norepinephrine and protects rat hepatocytes from activin A-induced growth inhibition. J Biol Chem. 2001;276(49):45636–45641. doi: 10.1074/jbc.M105302200. [DOI] [PubMed] [Google Scholar]
- 17.Han C., Bowen W.C., Michalopoulos G.K., Wu T. Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol. 2008;216(2):486–497. doi: 10.1002/jcp.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotaka M., Toyoda T., Yasuda K., et al. Adrenergic receptor agonists induce the differentiation of pluripotent stem cell-derived hepatoblasts into hepatocyte-like cells. Sci Rep. 2017;7(1):16734. doi: 10.1038/s41598-017-16858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Haele M., Roskams T. Hepatic progenitor cells: an update. Gastroenterol Clin North Am. 2017;46(2):409–420. doi: 10.1016/j.gtc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Soeda J., Mouralidarane A., Ray S., et al. The β-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology. 2014;60(3):1023–1034. doi: 10.1002/hep.27266. [DOI] [PubMed] [Google Scholar]
- 21.Wen X., Huan H., Wang X., et al. Sympathetic neurotransmitters promote the process of recellularization in decellularized liver matrix via activating the IL-6/Stat3 pathway. Biomed Mater. 2016;11(6):065007. doi: 10.1088/1748-6041/11/6/065007. [DOI] [PubMed] [Google Scholar]
- 22.Lu M., Flanagan J.U., Langley R.J., Hay M.P., Perry J.K. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther. 2019;4:3. doi: 10.1038/s41392-019-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asakawa K., Hizuka N., Takano K., et al. Human growth hormone stimulates liver regeneration in rats. J Endocrinol Invest. 1989;12(5):343–347. doi: 10.1007/BF03350004. [DOI] [PubMed] [Google Scholar]
- 24.Başoğlu M., Balik A.A., Kavak I., et al. Effects of growth hormone on hepatic regeneration. Turk J Med Sci. 2000;30:529–534. [Google Scholar]
- 25.Pennisi P.A., Kopchick J.J., Thorgeirsson S., LeRoith D., Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology. 2004;145(10):4748–4755. doi: 10.1210/en.2004-0655. [DOI] [PubMed] [Google Scholar]
- 26.Ayoub F., Trillo-Alvarez C., Morelli G., Lascano J. Risk factors for hepatic steatosis in adults with cystic fibrosis: similarities to non-alcoholic fatty liver disease. World J Hepatol. 2018;10(1):34–40. doi: 10.4254/wjh.v10.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collin de l’Hortet A., Zerrad-Saadi A., Prip-Buus C., et al. GH administration rescues fatty liver regeneration impairment by restoring GH/EGFR pathway deficiency. Endocrinology. 2014;155(7):2545–2554. doi: 10.1210/en.2014-1010. [DOI] [PubMed] [Google Scholar]
- 28.Li T., Chiang J.Y.L. In: Cellular Injury in Liver Diseases. Ding W.-X., Yin X.-M., editors. Springer International Publishing; Cham, Switzerland: 2017. Bile acid-induced liver injury in cholestasis; pp. 143–172. [Google Scholar]
- 29.Stiedl P., McMahon R., Blaas L., et al. Growth hormone resistance exacerbates cholestasis-induced murine liver fibrosis. Hepatology. 2015;61(2):613–626. doi: 10.1002/hep.27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaikh S., Qureshi A., Faiq S.M. Revisiting hereditary tyrosinemia Type 1—spectrum of radiological findings. BJR|Case Rep. 2018;5(2):20180001. doi: 10.1259/bjrcr.20180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M.-J., Chen F., Liu Q.-G., et al. Insulin-like growth factor 2 is a key mitogen driving liver repopulation in mice. Cell Death Dis. 2018;9(2):26. doi: 10.1038/s41419-017-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekberg S., Luther M., Nakamura T., Jansson J.O. Growth hormone promotes early initiation of hepatocyte growth factor gene expression in the liver of hypophysectomized rats after partial hepatectomy. J Endocrinol. 1992;135(1):59–67. doi: 10.1677/joe.0.1350059. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Chen M., Zheng G., et al. Transcriptional activation by growth hormone of HNF-6-regulated hepatic genes, a potential mechanism for improved liver repair during biliary injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G357–G366. doi: 10.1152/ajpgi.00581.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez C.S., Piazza V.G., González L., et al. Mitogenic signaling pathways in the liver of growth hormone (GH)-overexpressing mice during the growth period. Cell Cycle. 2016;15(5):748–759. doi: 10.1080/15384101.2016.1148844. [published correction appears in Cell Cycle. 2016;15(10):1386] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miquet J.G., Freund T., Martinez C.S., et al. Hepatocellular alterations and dysregulation of oncogenic pathways in the liver of transgenic mice overexpressing growth hormone. Cell Cycle. 2013;12(7):1042–1057. doi: 10.4161/cc.24026. [published correction appears in Cell Cycle. 2015;14(15):2537] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokarz V.L., MacDonald P.E., Klip A. The cell biology of systemic insulin function. J Cell Biol. 2018;217(7):2273–2289. doi: 10.1083/jcb.201802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl T.E., Porter K.A., Putnam C.W. Intraportal insulin protects from the liver injury of portacaval shunt in dogs. Lancet. 1975;2(7947):1241–1242. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 38.Starzl T.E., Watanabe K., Porter K.A., Putnam C.W. Effects of insulin, glucagon, and insuling/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1(7964):821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 39.Francavilla A., Di Leo A., Wu S.Q., et al. Discordance between glucokinase activity and insulin and glucagon receptor changes occurring during liver regeneration in the rat. Horm Metab Res. 1984;16(suppl 1):47–50. doi: 10.1055/s-2007-1014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sireesh D., Dhamodharan U., Ezhilarasi K., Vijay V., Ramkumar K.M. Association of NF-E2 related factor 2 (Nrf2) and inflammatory cytokines in recent onset type 2 diabetes mellitus. Sci Rep. 2018;8(1):5126. doi: 10.1038/s41598-018-22913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer T.A., Xu W., Teupser D., et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27(1):212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim P.T.W., Testa G. Living donor liver transplantation in the USA. Hepatobiliary Surg Nutr. 2016;5(2):133–140. doi: 10.3978/j.issn.2304-3881.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoreem H., Gad E.H., Soliman H., et al. Small for size syndrome difficult dilemma: lessons from 10 years single centre experience in living donor liver transplantation. World J Hepatol. 2017;9(21):930–944. doi: 10.4254/wjh.v9.i21.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu M.Q., Yan L.N., Li B., et al. Augmented hepatic regeneration of living donor liver graft by intraportal insulin administration [in Chinese] Zhonghua Wai Ke Za Zhi. 2009;47(11):821–824. [PubMed] [Google Scholar]
- 45.Hou C.-T., Chen Y.-L., Lin C.-C., et al. Portal venous velocity affects liver regeneration after right lobe living donor hepatectomy. PloS One. 2018;13(9):e0204163. doi: 10.1371/journal.pone.0204163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng J.H., Ouyang C.H., Lin K.J., Yeh T.S. Significance of insulin signaling in liver regeneration triggered by portal vein ligation. J Surg Res. 2011;166(1):77–86. doi: 10.1016/j.jss.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 47.Backes A.N., Tannuri A.C., Backes F.N., et al. Effects of tacrolimus and insulin in a liver regeneration model in growing animals with portal vein stenosis: immunohistochemical and molecular studies. Pediatr Surg Int. 2014;30(4):423–429. doi: 10.1007/s00383-014-3464-3. [DOI] [PubMed] [Google Scholar]
- 48.Amaya M.J., Oliveira A.G., Guimarães E.S., et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014;59(1):274–283. doi: 10.1002/hep.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visser T.J. In: Thyroid Diseases: Pathogenesis, Diagnosis, and Treatment. Vitti P., Hegedüs L., editors. Springer International Publishing; Cham, Switzerland: 2018. Regulation of thyroid function, synthesis and function of thyroid hormones; pp. 1–30. [Google Scholar]
- 50.Pibiri M., Ledda-Columbano G.M., Cossu C., et al. Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3) FASEB J. 2001;15(6):1006–1013. doi: 10.1096/fj.00-0416com. [DOI] [PubMed] [Google Scholar]
- 51.Alisi A., Demori I., Spagnuolo S., Pierantozzi E., Fugassa E., Leoni S. Thyroid status affects rat liver regeneration after partial hepatectomy by regulating cell cycle and apoptosis. Cell Physiol Biochem. 2005;15(1-4):69–76. doi: 10.1159/000083639. [DOI] [PubMed] [Google Scholar]
- 52.López-Fontal R., Zeini M., Través P.G., et al. Mice lacking thyroid hormone receptor β show enhanced apoptosis and delayed liver commitment for proliferation after partial hepatectomy. PloS One. 2010;5(1):e8710. doi: 10.1371/journal.pone.0008710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alonso-Merino E., Martín Orozco R., Ruíz-Llorente L., et al. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc Natl Acad Sci U S A. 2016;113(24):E3451–E3460. doi: 10.1073/pnas.1506113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarado T.F., Puliga E., Preziosi M., et al. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: implications in hepatic regeneration. Gene Expr. 2016;17(1):19–34. doi: 10.3727/105221616X691631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bockhorn M., Frilling A., Benko T., et al. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39(1):58–63. doi: 10.1159/000098443. [DOI] [PubMed] [Google Scholar]
- 56.Biondo-Simões M.L.P., Castro G.R., Montibeller G.R., Sadowski J.A., Biondo-Simões R. The influence of hypothyroidism on liver regeneration: an experimental study in rats. Acta Cir Bras. 2007;22(suppl 1):52–56. doi: 10.1590/s0102-86502007000700011. [DOI] [PubMed] [Google Scholar]
- 57.Cvoro A., Devito L., Milton F.A., et al. A thyroid hormone receptor/KLF9 axis in human hepatocytes and pluripotent stem cells. Stem Cells. 2015;33(2):416–428. doi: 10.1002/stem.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee S., Samanta L., Roy A., Bhanja S., Chainy G.B. Supplementation of T3 recovers hypothyroid rat liver cells from oxidatively damaged inner mitochondrial membrane leading to apoptosis. Biomed Res Int. 2014;2014:590897. doi: 10.1155/2014/590897. [DOI] [PMC free article] [PubMed] [Google Scholar]