Abstract
Objective
To assess whether self-reported physical activity during daily life reduces the mortality risk associated with atherosclerotic burden, as measured by coronary artery calcium (CAC) scanning.
Methods
We assessed 2318 patients aged 65 to 84 years who underwent CAC scanning from August 31, 1998, through November 16, 2016, and had daily life physical activity assessed by a single-item question that was used to divide patients by low, moderate, and high physical activity levels. Patients were followed for a mean ± SD of 10.6±4.9 years for the occurrence of all-cause mortality.
Results
The results indicated a graded relationship between the magnitude of CAC abnormality and mortality and an inverse relationship between physical activity and mortality. Of patients with low CAC scores (0-99), those with low, moderate, and high physical activity levels had similarly low mortality rates. Of patients with high CAC scores (≥400), however, there was a stepwise increase in mortality with decreasing physical activity. Patients with CAC scores of 400 or greater but reporting high physical activity had a mortality rate that was similar vs that observed in patients with CAC scores of only 0 to 99 and low physical activity (19.9 vs 16.3 per 1000 person-years; P=.60).
Conclusion
Combining CAC score with self-reported physical activity level provides a synergistic means for predicting clinical risk in older patients, with high physical activity level substantially attenuating the mortality risk associated with elevated CAC scores. Moreover, the useful prognostic information afforded by just a single-item physical activity questionnaire supports current initiatives to make such assessments into a “fifth vital sign.”
Abbreviations and Acronyms: ACM, all-cause mortality; CAC, coronary artery calcium; CAD, coronary artery disease; CT, computed tomography
Cardiovascular disease increases markedly with age and constitutes a leading cause of death and disabilities in older adults.1,2 The underlying substrate for this increased risk is advancing atherosclerosis, which can be characterized as to its presence and extent by assessing the magnitude of coronary artery calcium (CAC) in the coronary arteries. A growing evidence base indicates that CAC scores predict clinical risk in older patient populations,3, 4, 5, 6 paralleling its utility in younger populations. Accordingly, there is growing interest in using CAC scanning to identify older individuals who are at increased clinical risk and then assessing what interventions might modify the risk associated with high CAC scores in such individuals. A practical lifestyle intervention that may serve this purpose is the promulgation of physical activity in older adults. However, how to best risk stratify older adults for physical activity interventions has not yet been adequately addressed. Therefore, to evaluate a new approach to such risk stratification in older adults on an exploratory basis, in this study we assessed long-term mortality risk in a cohort of older adults aged 65 to 84 years who underwent CAC scanning on a clinical basis and had assessment of physical activity according to a single-item questionnaire.
Methods
For this study, we identified 2653 patients aged 65 to 84 years who were referred for CAC scanning on a clinical basis between August 31, 1998, and November 16, 2016, and who had more than 1 year of follow-up for all-cause mortality (ACM). We excluded 227 patients with known coronary artery disease (CAD) and 108 patients with incomplete clinical information, resulting in a study cohort of 2318 patients. The study was approved by the Cedars-Sinai Medical Center institutional review board.
Each patient completed a questionnaire regarding pertinent baseline clinical and demographic information at the time of CAC scanning. This questionnaire included information regarding chest pain symptoms, cardiac risk factors, physical activity, and medication use. Resting heart rate, blood pressure, height, and weight were recorded at the time of testing. Dyslipidemia was considered present if patients had a preexisting diagnosis of dyslipidemia or were receiving treatment with lipid-lowering agents. Diabetes was considered present if patients had a previous diagnosis of diabetes or were receiving treatment with diabetes medication. Hypertension was considered present if patients had a previous diagnosis of hypertension or were being treated with antihypertensive medications. Current smoking was defined as either currently smoking or having stopped smoking for less than 1 year. A family history of premature CAD was considered present if a primary relative had been diagnosed as having CAD or a cardiac event when younger than 55 years for a male family member or younger than 65 years for a female family member. Patients’ physical activity was assessed according to a single-item question that was incorporated into the standard questionnaire as follows: On a scale of 0-10, how much do you exercise (0 – none, 10 – always)?
CAC Scanning
Patients underwent coronary noncontrast computed tomography (CT) using either an Imatron C-150XL ultrafast CT scanner (GE Imatron) or a Siemens Definition dual-source 64-slice scanner (Siemens Medical Solutions). Tomographic imaging was electrocardiographically gated, and image acquisition occurred at a predetermined time in diastole (50%-80% of the R-to-R cycle depending on heart rate). The coronary arteries were visualized without contrast medium, with 30 to 40 consecutive images obtained at 3-mm intervals from the carina caudally to the diaphragm. A CT threshold of 3 pixels and 130 HU was used for identification of calcified coronary artery lesions. Each focus exceeding the minimum criteria was scored with the algorithm developed by Agatston et al.7
Follow-up Data Collection
Follow-up for ACM was conducted using internal hospital records and the Social Security Death Index, the California Non-comprehensive Death File, and the National Death Index. The last date of access for the Social Security Death Index was April 9, 2012; for the California Non-comprehensive Death File was August 6, 2018; and for the National Death Index was February 12, 2018. Mean ± SD follow-up was 10.6±4.9 years; median follow-up was 11.4 years (interquartile range, 6-15 years).
Statistical Methods
All the data were analyzed using Stata release 14 (StataCorp LP). Participants were categorized into 3 groups based on their self-reported physical activity: 0 to 2 = mild, 3 to 7 = moderate, and 8 to 10 = high physical activity. Continuous variables were compared using the Wilcoxon rank sum test for 2 groups or the Cuzick test for trend across ordered groups. Categorical variables were compared using the Pearson χ2 test or the χ2 test for trend across ordered groups. Mortality rates were visualized using Kaplan-Meier curves and are expressed as annual event rates (dividing number of events by person-years) or per 1000 person-years and were compared using the log-rank test or the log-rank test for trend. Cox proportional hazards models were used to obtain unadjusted and risk-adjusted hazard ratios where the proportional hazard assumption was assessed using Schoenfeld residuals. Multivariable predictors of ACM were assessed using the likelihood ratio χ2 test. Two-tailed P<.05 was considered statistically significant.
Results
Comparison of the clinical characteristics of patients according to self-reported physical activity levels is shown in Table 1. There were only minimal differences in mean age. The high physical activity group had more males and fewer CAD risk factors, including a lower frequency of hypertension, dyslipidemia, smoking, and diabetes. In addition, patients with a high physical activity level had a substantially lower frequency of obesity (7.7%). Conversely, patients reporting a low level of physical activity had the highest frequency of CAD risk factors and the highest frequency of obesity (26.8%). Patients with a low self-reported physical activity level tended to have chest pain symptoms, but the frequencies of typical angina and dyspnea were low and similar among the physical activity groups. The CAC scores had similar distributions among the 3 physical activity groups: 15.7% to 18.3% had CAC scores of 0 and 10.0% to 11.4% had CAC scores greater than 1000, with no significant differences noted either continuously or categorically.
Table 1.
Study Population Characteristics
| Characteristic | All patients (N=2318) | Low physical activity (n=402) | Moderate physical activity (n=1365) | High physical activity (n=551) | Trend P value |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 70.4±4.6 | 70.9±5.0 | 70.6±4.5 | 69.7±4.3 | .001 |
| Male sex (No. [%]) | 1285 (55.4) | 188 (46.8) | 746 (54.7) | 351 (63.7) | <.001 |
| Hypertension (No. [%]) | 1004 (43.3) | 205 (51.0) | 576 (42.2) | 223 (40.5) | <.002 |
| Dyslipidemia (No. [%]) | 1536 (66.3) | 265 (65.9) | 932 (68.3) | 339 (61.5) | .089 |
| Smoking (No. [%]) | 119 (5.1) | 31 (7.7) | 72 (5.3) | 16 (2.9) | <.001 |
| Diabetes (No. [%]) | 179 (7.7) | 47 (11.7) | 101 (7.4) | 31 (5.6) | <.001 |
| Family history of CAD (No. [%]) | 650 (28.0) | 112 (27.9) | 414 (30.3) | 124 (22.5) | .031 |
| BMI (mean ± SD) | 25.8±4.2 | 27.2±4.8 | 25.8±4.2 | 24.7±3.6 | <.001 |
| <25 (No. [%]) | 1076 (46.4) | 152 (37.8) | 609 (44.6) | 315 (57.2) | <.001 |
| 25-29 (No. [%]) | 927 (40.0) | 147 (36.6) | 586 (42.9) | 194 (35.2) | .403 |
| ≥30 (No. [%]) | 315 (13.6) | 103 (25.6) | 170 (12.5) | 42 (7.6) | <.001 |
| Chest pain symptoms (No. [%]) | |||||
| Asymptomatic | 1842 (79.5) | 301 (74.9) | 1074 (78.7) | 467 (84.8) | <.001 |
| Nonanginal pain | 208 (9.0) | 40 (10.0) | 132 (9.7) | 36 (6.5) | .048 |
| Atypical angina | 136 (5.9) | 32 (8.0) | 82 (6.0) | 22 (4.0) | .009 |
| Typical angina | 41 (1.8) | 8 (2.0) | 26 (1.9) | 7 (1.3) | .369 |
| Dyspnea only | 91 (3.9) | 21 (5.2) | 51 (3.7) | 19 (3.5) | .188 |
| Medications (No. [%]) | |||||
| β-blockers | 286 (12.3) | 47 (11.7) | 180 (13.2) | 59 (10.7) | .527 |
| Calcium blockers | 212 (9.2) | 46 (11.4) | 117 (8.6) | 49 (8.9) | .230 |
| ACE inhibitors | 324 (14.0) | 66 (16.4) | 190 (13.9) | 68 (12.3) | .077 |
| Lipid-lowering medications | 857 (37.0) | 160 (39.8) | 507 (37.1) | 190 (34.5) | .091 |
| Aspirin | 1135 (49.0) | 178 (44.3) | 689 (50.5) | 268 (48.6) | .267 |
| CAC scores | |||||
| Mean ± SD | 392.6±776.9 | 416.7±789.5 | 391.8±793.6 | 376.9±724.9 | |
| Median (interquartile range) | 106.5 (7.5-430.2) | 128.9 (23.3-501.3) | 101.6 (4.8-416.0) | 98.8 (8.7-404.7) | .196 |
| Category (No. [%]) | |||||
| 0 | 451 (19.5) | 63 (15.7) | 287 (21.0) | 101 (18.3) | .459 |
| 1-99 | 683 (29.5) | 116 (28.9) | 392 (28.7) | 175 (31.8) | .281 |
| 100-399 | 573 (24.7) | 108 (26.9) | 329 (24.1) | 136 (24.7) | .505 |
| 400-999 | 361 (15.6) | 69 (17.2) | 208 (15.2) | 84 (15.3 | .461 |
| ≥1000 | 250 (10.8) | 46 (11.4) | 149 (10.9) | 55 (10.0) | .458 |
| Percentile score (mean ± SD) | 48.0±33.0 | 53.4±32.2 | 46.8±33.5 | 47.1±32.1 | .007 |
| Mortality incidence (No. [%]) | 533 (23.0) | 119 (29.6) | 321 (23.5) | 93 (16.9) | <.001 |
| Annual mortality (% [95% CI]) | 2.3 (2.1-2.5) | 2.9 (2.4-3.4) | 2.3 (2.1-2.6) | 1.7 (1.4-2.1) | <.001 |
ACE = angiotensin-converting enzyme; BMI = body mass index (calculated as weight in kilograms divided by height in meters squared); CAC = coronary artery calcium; CAD = coronary artery disease.
Mortality According to Physical Activity Level and CAC Score
Patients were followed for a mean ± SD of 10.6±4.9 years. During this time, 533 patients (23.0%) died. For the entire cohort, annualized mortality was 2.3% per year, with the highest mortality occurring in patients reporting low physical activity (2.9% per year) and the lowest mortality occurring in patients reporting the highest physical activity (1.7% per year) (P<.001).
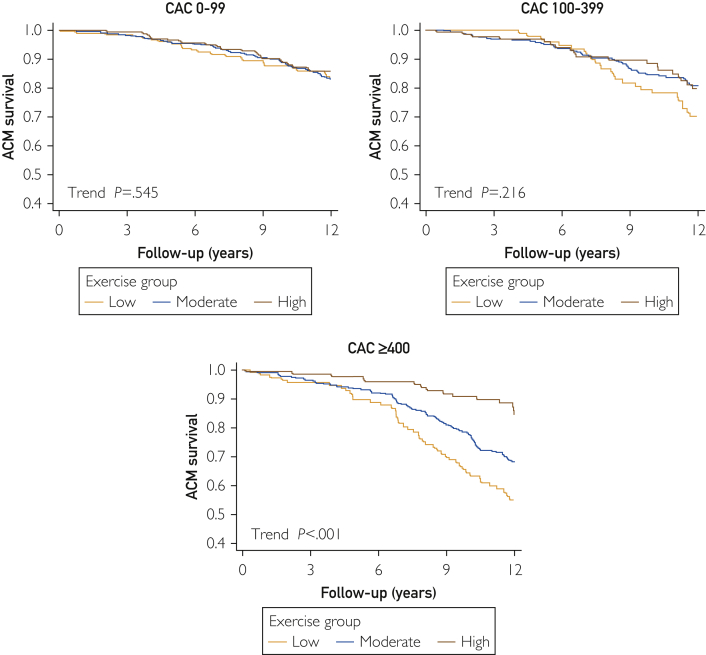
Kaplan-Meier survival curves for patients stratified according to baseline CAC scores and self-reported physical activity levels are shown in Figure 1. In patients with CAC scores of 0 to 99 and 100 to 399, survival was similar in patients with low, moderate, or high physical activity levels. In patients with CAC scores of 400 or greater, a progressive decline in survival was noted with decreasing physical activity. A similar pattern of survival was noted in patients stratified according to CAC percentile scores (Supplemental Figure 1, available online at http://www.mcpiqojournal.org). In patients with percentile CAC scores less than 50%, the survival curves were similar in patients reporting low, moderate, and high physical activity. In contrast, in patients with percentile CAC scores of 75% or greater, there was a progressive inverse relationship between reported physical activity and mortality.
Figure 1.
Kaplan-Meier survival curves for all-cause mortality (ACM) among patients divided according to level of reported physical activity in 3 coronary artery calcium (CAC) groups (scores of 0-99, 100-399, and ≥400).
After adjustment for patient, age, and CAD risk factors, the adjusted hazard ratio for ACM increased with both increasing CAC score and decreasing physical activity level (Table 2). When combining CAC scores and reported physical activity level, the multivariable-adjusted hazard ratios for mortality were similar for each physical activity level in patients with CAC scores of 0 to 99. In contrast, in patients with CAC scores of 100 to 399 and 400 or greater, there was a progressive increase in the risk-adjusted hazard ratio for ACM as physical activity diminished (trend P<.001). In patients with moderate CAC scores (100-399), the adjusted hazard ratio for ACM was increased 2.07-fold in patients with low vs high physical activity levels, and in patients with CAC scores of 400 or greater, the adjusted hazard ratio for ACM was increased 2.35-fold in patients with low vs high physical activity levels.
Table 2.
| Group | Patients (No.) | Hazard ratios |
|||
|---|---|---|---|---|---|
| Unadjusted | P value | Risk adjustedc | P value | ||
| CAC score | |||||
| 0-99 | 1134 | 1 (Referent) | 1 (Referent) | ||
| 100-399 | 573 | 1.40 (1.12-1.75) | .003 | 1.21 (0.95-1.55) | .12 |
| ≥400 | 611 | 2.07 (1.70-2.52) | <.001 | 1.59 (1.26-2.01) | <.001 |
| Physical activity level | |||||
| High | 551 | 1 (Referent) | 1 (Referent) | ||
| Moderate | 1365 | 1.39 (1.10-1.74) | .006 | 1.26 (0.98-1.63) | .08 |
| Low | 402 | 1.68 (1.28-2.20) | <.001 | 1.60 (1.18-2.17) | .003 |
| CAC score/physical activity level | |||||
| 0-99/high | 276 | 1 (Referent) | 1 (Referent) | ||
| 0-99/moderate | 679 | 1.19 (0.82-1.73) | .36 | 1.01 (0.67-1.52) | .96 |
| 0-99/low | 179 | 1.14 (0.70-1.85) | .59 | 0.97 (0.57-1.64) | .90 |
| 100-399/high | 136 | 1 (Referent) | 1 (Referent) | ||
| 100-399/moderate | 329 | 1.07 (0.69-1.66) | .77 | 1.15 (0.71-1.87) | .58 |
| 100-399/low | 108 | 1.37 (0.83-2.29) | .22 | 2.07 (1.16-3.69) | .01 |
| ≥400/high | 139 | 1 (Referent) | 1 (Referent) | ||
| ≥400/moderate | 357 | 1.91 (1.28-2.83) | .001 | 1.68 (1.06-2.66) | .03 |
| ≥400/low | 115 | 2.63 (1.69-4.10) | <.001 | 2.35 (1.39-3.97) | .001 |
CAC = coronary artery calcium.
A formal test for interaction showed that the Wald P value interaction between CAC score and physical activity level was significant (P=.013 unadjusted and P=.009 risk adjusted).
Hazard ratios adjusted for age, male sex, hypertension, dyslipidemia, smoking, diabetes, and family history of premature coronary artery disease, lipid-lowering medication use, and blood pressure medication use.
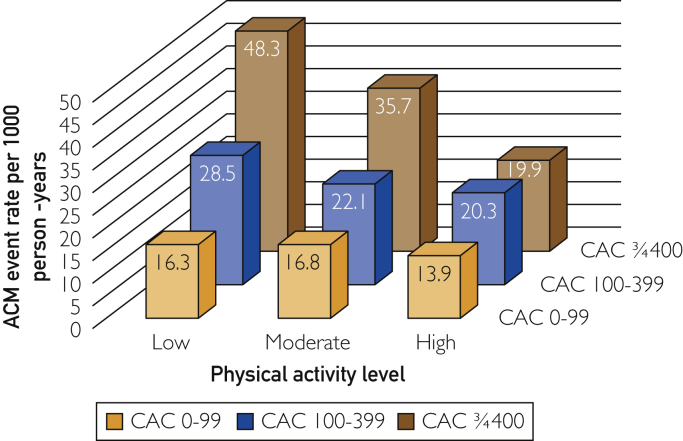
The mortality rate per 1000 person-years varied widely according to the level of CAC and physical activity (Figure 2). Patients with CAC scores of 400 or greater but reporting high physical activity had a mortality rate per 1000 person-years that was similar to the rate observed in patients with CAC scores of only 0 to 99 who reported low physical activity (19.9 vs 16.3 per 1000 person-years; P=.60). The mortality rate per 1000 person-years showed a similar pattern of results for patients who were stratified by CAC percentile score and physical activity level (Supplemental Figure 2, available online at http://www.mcpiqojournal.org). When assessment of mortality rate per 1000 person-years according to patients’ CAC score and physical activity level were confined to patients who were 75 years or older at baseline, similar results were noted (Supplemental Figure 3, available online at http://www.mcpiqojournal.org).
Figure 2.
All-cause mortality (ACM) rates (per 1000 person-years) according to increasing coronary artery calcium (CAC) score and level of reported physical activity.
Comparison of Clinical Predictors of ACM
The multivariable predictors of ACM are shown in Table 3. By χ2 analysis, age was the strongest predictor of mortality, followed by the CAC score. Of the other clinical variables, patient-reported physical activity level was the next most potent predictor of ACM.
Table 3.
Multivariable Predictors of All-cause Mortality
| Variable | χ2 | P value | MVA HR (95% CI) |
|---|---|---|---|
| Age, per 5 years | 120.38 | <.001 | 1.70 (1.55-1.86) |
| CAC score | 18.63 | <.001 | |
| 0 | 1 (Referent) | ||
| 1-99 | 0.95 (0.70-1.30) | ||
| 100-399 | 1.21 (0.89-1.64) | ||
| ≥400 | 1.56 (1.16-2.11) | ||
| Physical activity level | 9.04 | .01 | |
| High | 1 (Referent) | ||
| Moderate | 1.27 (1.00-1.61) | ||
| Low | 1.55 (1.16-2.07) | ||
| Diabetes | 4.50 | .03 | 1.39 (1.04-1.86) |
| Chest pain symptoms | 4.01 | .41 | |
| Asymptomatic | 1 (Referent) | ||
| Nonanginal chest pain | 1.30 (0.96-1.75) | ||
| Atypical angina | 1.13 (0.80-1.60) | ||
| Typical angina | 1.32 (0.76-2.30) | ||
| Dyspnea only | 0.80 (0.25-2.50) | ||
| Smoking | 3.81 | .05 | 1.39 (1.01-1.92) |
| Hypertension | 2.42 | .12 | 1.15 (0.96-1.38) |
| Body mass index, per 5 kg/m2 | 1.97 | .16 | 0.92 (0.82-1.03) |
| Dyslipidemia | 1.30 | .25 | 0.90 (0.75-1.08) |
| Male sex | 0.67 | .41 | 1.09 (0.89-1.33) |
| Family history of premature CAD | 0.06 | .81 | 0.97 (0.79-1.20) |
CAC = coronary artery calcium; CAD = coronary artery disease; HR = hazard ratio; MVA = multivariate analysis.
Discussion
Although the magnitude of CAC, a reflector of the anatomical extent of atherosclerosis, is a strong predictor of clinical risk, recent data reported a synergistic relationship between measures of physical fitness or self-reported physical activity levels and CAC scores in predicting patients’ future risk of adverse clinical events.8, 9, 10, 11 To date, however, such studies have been performed in primarily middle-aged populations, and there are not yet any reported studies regarding how these variables may interact to predict risk in more elderly individuals.
Because of the strong tendency of coronary arteries to calcify with age, we also assessed the relationship between age- and sex-adjusted CAC percentile scores, self-reported physical activity levels, and mortality risk. In patients with CAC percentile scores that were below average (<50% percentile), survival was comparable in those with low, moderate, and high physical activity levels. In contrast, in patients with CAC percentile scores of 75% or greater, there was a stepwise increase in mortality rates with decreasing physical activity, with comparable mortality rates noted in patients with high CAC percentile scores and high physical activity levels vs patients with low CAC percentile scores and low physical activity levels.
Of the clinical parameters, age and the magnitude of CAC abnormality were the most potent predictors of mortality according to χ2 analysis. Of the remaining variables, the patients’ reported physical activity was the next most potent predictor. These results are notable in that physical activity was assessed only according to a single-item question in this study.
Comparison With Previous Studies
Recent studies have examined the interrelationship among physical activity, atherosclerosis, and clinical outcomes in middle-aged populations. Both cardiorespiratory fitness and self-reported physical activity have been examined for their potential effect on clinical risk in patients with differing levels of underlying atherosclerosis as assessed by CAC scanning. In a study of 8425 patients who underwent both treadmill exercise and CAC scanning, a strong association was noted between a continuous measurement of cardiorespiratory fitness and cardiovascular events in all CAC subgroups.8 In patients with CAC scores greater than 400 in that study, the risk of composite cardiac events was increased by approximately 5-fold compared with patients with CAC scores of 0, but this difference narrowed to only a 2-fold increase in the most fit patients with CAC scores greater than 400 compared with those with CAC scores of 0. Similarly, Choi et al9 assessed 25,972 individuals who underwent CAC scanning and treadmill exercise who were followed for a mean of 5.5 years. The risk of ACM in participants with high CAC scores was significantly attenuated in those with high exercise capacity (>10 metabolic equivalents). In further work, Zafrir et al10 noted a synergistic relationship among CAC scores, cardiorespiratory fitness, and clinical outcomes in 600 patients with type 2 diabetes. Finally, in a particularly large study, DeFina et al11 assessed the relationships among self-reported physical activity, CAC scores, and subsequent cardiac mortality and ACM during a 10.4-year mean follow-up of 21,758 generally healthy men with a mean age of 51.7 years. At both low and high levels of CAC abnormality, individuals with a high level of self-reported physical activity had lower mortality and cardiac death rates than did those reporting a low level of physical activity.
In contrast to these studies, assessment of the relationships among physical activity, CAC scores, and outcomes in elderly patients have been sparse. However, in a study of interest, von Bonsdorff et al12 assessed 4074 individuals with a mean age of 76 years who underwent both CAC scanning and measurement of their gait speed over a 6-m distance. During mean follow-up of 5.4 years, the combined presence of low gait speed and high CAC scores increased noncardiac mortality beyond the effects noted with each individual parameter. Although gait speed is a noncardiopulmonary parameter that may have many etiological causes, the results of this study of older adults parallel the results of the present study.
Pathophysiology
There are multiple mechanisms that could help contribute to the observations of the present study. First, it is notable that patients who reported high physical activity levels manifested better health profiles. This included a lower frequency of hypertension, dyslipidemia, smoking, and diabetes and a lower mean body mass index. Of patients reporting high physical activity in this study, only 7.7% had a body mass index consistent with obesity. In contrast, more than one-quarter of patients reporting low physical activity in this study had obesity, as well as manifesting the highest frequency of other CAD risk factors. Thus, patients with high physical activity levels may have had increased survival because of better overall CAD risk profiles in such patients, perhaps mediated by the beneficial effects of physical activity or representing an association between greater physical activity and assumption of other beneficial health behaviors. Still, after risk adjustment for age, sex, and these risk factors, patients reporting a high physical activity level still had a lower hazard ratio for ACM. Thus, other mechanisms are likely contributing to the findings as well.
It is likely that physical activity also benefits health and survival through direct physiologic mechanisms. Older patients become susceptible to clinical risk through both the direct effects of atherosclerosis and the indirect effects of aging per se. The most prominent effects of aging on the cardiovascular system include the development of arterial stiffness and the development of endothelial dysfunction.13,14 The latter can occur on an even nonatherosclerotic basis due to loss of nitric oxide bioavailability with aging.13 Increasing evidence suggests that physical activity can potentially ameliorate these effects by preventing or restoring age-related decline in endothelial function in middle-aged and older individuals15, 16, 17 as well as in old experimental animals.18 In addition, exercise may help improve vascular function by blunting the effects of age-related vasconstrictive factors, such as endothelin-1.19 In addition, it is possible that physical activity may benefit patients by reducing platelet aggregation20 and enhanced fibrinolysis.21 Combined, these effects may help explain recent preliminary evidence linking cardiorespiratory fitness to reduced lipid volume, increased fibrous volume, and thick fibrous cap thickness with coronary plaque lesions.22 In addition, physical activity can benefit metabolic and immune function and lead to the production of myokines during exercise that may potentially benefit skeletal muscle through autocrine and paracrine effects and the liver, bone, gut, brain, and adipose tissue through endocrine effects.23,24 Conversely, low levels of physical activity are associated with important adverse effects, including an increased propensity toward insulin resistance and diabetes, inflammation, and development of visceral obesity, muscle weakness, and sarcopenia.23, 24, 25 Accordingly, an accumulating literature provides multiple potential pathways by which physical activity may decrease the clinical risk associated with a high burden of coronary atherosclerosis, but prospective study is needed to determine which mechanisms are predominant.
Conversely, the finding that patients with relatively low CAC scores (0-99) had similarly low risk regardless of their level of self-reported physical activity suggests a minification of risk posed by sedentariness in elderly patients who do not have significant atherosclerosis.
Limitations
This study contains important limitations. As a single-center study, these findings require prospective validation in other older patient cohorts. The use of a single-item physical activity questionnaire was not validated vs more standard questionnaires or objective measures of physical activity. However, other studies have found a moderate correlation between brief physical activity questionnaires (1-2 items) and more standard subjective or objective measures of physical activity.26, 27, 28 Consistent with these findings, we previously assessed physical activity according to the same 1-item questionnaire used in this study among 10,690 middle-aged patients who underwent CAC scanning and had more than 1 year of follow-up.29 The results of that study directly parallel the present findings in older adults. Because we did not collect information regarding the duration (in years) of patients’ physical activity, it is unknown from this study whether the protective effect of physical activity in this patient population was the result of long-term physical activity or that of recent onset. Another limitation is that we did not collect information regarding important comorbid conditions that may have been present in the patient population, such as the presence of chronic kidney disease or previous stroke, which could have accounted for both a lower level of physical activity and increased mortality risk in some patients; however, it is not common for such patients to be referred for CAC scanning. Finally, because follow-up was limited to ACM only, we could not assess the direct influence of physical activity in modifying cardiovascular events in older patients with atherosclerosis.
Clinical Significance
This study indicates that a simple assessment of self-reported daily life physical activity can substantially improve the effectiveness of CAC scanning for risk stratifying older adults. Most notably, mortality risk was substantially attenuated in older patients with CAC scores of 400 or greater, even in a subanalysis of patients who were 75 years or older at baseline. These results suggest a need to conduct prospective intervention studies to assess the clinical benefits of exercise programs in older individuals with substantial atherosclerosis on CAC scanning.
The present results are also notable in that physical activity in this study was simply assessed by a convenient single-item question. These results are consistent with recent suggestions to make assessment of physical activity a “fifth vital sign” that should be entered into the electronic medical records of patients.30,31 However, prospective studies should be conducted to ascertain which brief clinical questions formulate the best evaluation of physical activity at the time of patient assessment during CAC scanning and other medical encounters.
Conclusion
In this study, combining CAC scores with a single-item self-reported question regarding physical activity significantly improved the assessment of mortality risk in older adults.
Footnotes
Grant Support: This research was supported in part by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Potential Competing Interests: Dr Berman participates in software royalties for QPS Software at Cedars-Sinai Medical Center. The other authors report no competing interests.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
Kaplan-Meier survival curves for all-cause mortality (ACM) in patients divided according to level of reported physical activity in 3 coronary artery calcium (CAC) percentile groups (<50, 50-74, and ≥75th percentiles).
All-cause mortality (ACM) rates (per 1000 person-years) according to increasing coronary artery calcium (CAC) scores and levels of reported physical activity for patients 75 years or older.
All-cause mortality (ACM) rates (per 1000 person-years) according to increasing coronary artery calcium (CAC) percentile scores and levels of reported physical activity.
References
- 1.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Trogdon J.G., Khavjou O.A. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Vliegenthart R., Oudkerk M., Hofman A. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 4.Elias-Smale S.E., Proença R.V., Koller M.T. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56(17):1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Tota-Maharaj R., Blaha M.J., McEvoy J.W. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33(23):2955–2962. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 6.Yano Y., O'Donnell C.J., Kuller L. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2(9):986–994. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 8.Radford N.B., DeFina L.F., Leonard D. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Clinic Longitudinal Study. Circulation. 2018;137(18):1888–1895. doi: 10.1161/CIRCULATIONAHA.117.032708. [DOI] [PubMed] [Google Scholar]
- 9.Choi S.Y., Sung J., Park H.E., Han D., Chang H.J. Combined effects of exercise capacity and coronary atherosclerotic burden on all-cause mortality in asymptomatic Koreans. Atherosclerosis. 2016;251:396–403. doi: 10.1016/j.atherosclerosis.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Zafrir B., Azaiza M., Gaspar T. Low cardiorespiratory fitness and coronary artery calcification: complementary cardiovascular risk predictors in asymptomatic type 2 diabetics. Atherosclerosis. 2015;241(2):634–640. doi: 10.1016/j.atherosclerosis.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 11.DeFina L.F., Radford N.B., Barlow C.E. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4(2):174–181. doi: 10.1001/jamacardio.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Bonsdorff M.B., Groffen D.A., Vidal J.S. Coronary artery calcium and physical performance as determinants of mortality in older age: the AGES-Reykjavik Study. Int J Cardiol. 2013;168(3):2094–2099. doi: 10.1016/j.ijcard.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taddei S., Virdis A., Ghiadoni L. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 14.Paneni F., Diaz Cañestro C., Libby P., Lüscher T.F., Camici G.G. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69(15):1952–1967. doi: 10.1016/j.jacc.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 15.DeSouza C.A., Shapiro L.F., Clevenger C.M. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 16.Smith D.T., Hoetzer G.L., Greiner J.J., Stauffer B.L., DeSouza C.A. Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J Physiol. 2003;546(pt 1):289–298. doi: 10.1113/jphysiol.2002.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoetzer G.L., Van Guilder G.P., Irmiger H.M. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol (1985) 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 18.Durrant J.R., Seals D.R., Connell M.L. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587(pt 13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Guilder G.P., Westby C.M., Greiner J.J., Stauffer B.L., DeSouza C.A. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50(2):403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.S., Li Y.S., Chen J.C., Chen Y.W. Effects of exercise training and deconditioning on platelet aggregation induced by alternating shear stress in men. Arterioscler Thromb Vasc Biol. 2005;25(2):454–460. doi: 10.1161/01.ATV.0000151987.04607.24. [DOI] [PubMed] [Google Scholar]
- 21.Killewich L.A., Macko R.F., Montgomery P.S., Wiley L.A., Gardner A.W. Exercise training enhances endogenous fibrinolysis in peripheral arterial disease. J Vasc Surg. 2004;40(4):741–745. doi: 10.1016/j.jvs.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa D., Ishii H., Kurebayashi N. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardiol. 2013;162(2):123–128. doi: 10.1016/j.ijcard.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 23.Rowe G.C., Safdar A., Aray Z. Running forward: new frontiers in endurance exercise biology. Circulation. 2014;129(7):798–810. doi: 10.1161/CIRCULATIONAHA.113.001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 25.Cartee G.D., Hepple R.T., Bamman M.M., Zierath J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23(6):1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milton K., Bull F.C., Bauman A. Reliability and validity testing of a single-item physical activity measure. Br J Sports Med. 2011;45(3):203–208. doi: 10.1136/bjsm.2009.068395. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton K., White K.M., Cuddihy T. Using a single-item physical activity measure to describe and validate parents' physical activity patterns. Res Q Exerc Sport. 2012;83(2):340–345. doi: 10.1080/02701367.2012.10599865. [DOI] [PubMed] [Google Scholar]
- 28.Schechtman K.B., Barzilai B., Rost K., Fisher E.B., Jr. Measuring physical activity with a single question. Am J Public Health. 1991;81(6):771–773. doi: 10.2105/ajph.81.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnson Y., Rozanski A., Gransar H. Impact of exercise on the relationship between CAC scores and all-cause mortality. JACC Cardiovasc Imaging. 2017;10(12):1461–1468. doi: 10.1016/j.jcmg.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Cowan R.E. Exercise Is Medicine initiative: physical activity as a vital sign and prescription in adult rehabilitation practice. Arch Phys Med Rehabil. 2016;97(9suppl):S232–S237. doi: 10.1016/j.apmr.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Sallis R.E., Baggish A.L., Franklin B.A., Whitehead J.R. The call for a physical activity vital sign in clinical practice. Am J Med. 2016;129(9):903–905. doi: 10.1016/j.amjmed.2016.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier survival curves for all-cause mortality (ACM) in patients divided according to level of reported physical activity in 3 coronary artery calcium (CAC) percentile groups (<50, 50-74, and ≥75th percentiles).
All-cause mortality (ACM) rates (per 1000 person-years) according to increasing coronary artery calcium (CAC) scores and levels of reported physical activity for patients 75 years or older.
All-cause mortality (ACM) rates (per 1000 person-years) according to increasing coronary artery calcium (CAC) percentile scores and levels of reported physical activity.