Abstract
Purpose
To evaluate the short-term outcomes of rotator cuff repair in the presence of a greater tuberosity cyst (GTC) using a transosseous repair technique.
Methods
This study included patients who underwent arthroscopic rotator cuff tear repair with a transosseous technique and were evaluated clinically and by postoperative magnetic resonance imaging (MRI) after 1 year. The inclusion criteria were based on the results of preoperative MRI and were as follows: patients identified as having a repairable full-thickness rotator cuff tear associated with the presence of cystic changes at the tendon insertion site of the greater tuberosity, defined as a GTC involving the footprint area of the torn tendon (supraspinatus and/or infraspinatus tendons).
Results
We evaluated 25 patients. The mean preoperative and postoperative American Shoulder and Elbow Surgeons scores were 39.48 (P = .530) and 84.64 (P = .035), respectively; Constant shoulder scores, 38.96 (P < .005) and 80.28 (P = .425), respectively; and University of California–Los Angeles shoulder rating scale scores, 10.6 (P = .045) and 29.04 (P = .315), respectively. The GTC mapping system was easily adopted in all the MRI examinations independently from the quality of the images. The GTCs were mostly located in the superficial anterolateral section of the humeral head and in both the posterolateral sections (superficial and deep).
Conclusions
Arthroscopic transosseous rotator cuff repair led to significant mid-term improvement and satisfactory subjective outcomes with low complication and failure rates in this study. The GTC mapping system could be useful to evaluate GTCs and to aid surgeons in the choice of the best surgical technique.
Level of Evidence
Level IV, therapeutic case series.
Greater tuberosity cysts (GTCs) are often observed in patients with rotator cuff tears (RCTs), with a reported prevalence of 9%.1 The cysts are occasionally encountered in the anterior (supraspinatus tendon insertion) or posterior (infraspinatus tendon insertion) aspect of the greater tuberosity or in the lesser tuberosity (subscapularis tendon insertion). The anterior location of a cyst is strongly associated with a full-thickness RCT2 regardless of age.1 The etiopathogenesis of these cysts is still not completely understood, and several explanations have been reported. They can arise from congenital abnormalities, age-related degeneration, or rotator cuff (RC) pathology.2, 3, 4 The intact tendon insertion may protect against cyst formation by attaching firmly to bone that creates a barrier for the synovial fluid.5
wIn most cases, the cysts are small and do not interfere with RCT reattachment.6 In this scenario, placing the tendon stump onto the cyst area or medializing the tendon reattachment allows the problem to be easily overcome. However, when a cyst is large enough and in a critical location, its presence poses a serious challenge during arthroscopic RCT repair.6 These GTCs may encompass such a large area of the RC footprint that suture anchor fixation can be compromised.6 Moreover, bone deficiency decreases the biological healing capability of the tendon, particularly in elderly patients.3,7 For these reasons, several surgical techniques have been described in the literature for RCT repair associated with large bone cysts.6, 7, 8, 9 Some authors prefer a 2-stage procedure in which initial bone grafting is followed 3 months later by RCT repair.8 Other authors have described a 1-stage procedure using compaction bone graft with either allogeneic or autogenous bone to fill the greater tuberosity defect during arthroscopic reconstruction, fixing suture anchors to the bone graft itself and enhancing their graft anchor fixation with additional anchors placed into native bone.6
However, many disadvantages have been associated with the use of autograft and allograft, including donor-site morbidity, nonunion, collapse, and resorption of the graft. In addition, the risk of suture anchor pullout from the grafted bone, as well as potential graft necrosis, has been mentioned.6 To address the bone defect, overcoming the problems related to the bony allograft or autograft, a 1-stage arthroscopic technique using a synthetic graft and placing suture anchors distal and lateral to the cuff footprint was described.9
In an attempt to overcome the limitations of anchor repair, arthroscopic transosseous (TO) RC repair techniques have been developed.10, 11, 12 In case of bone deficiency of the greater tuberosity, it is possible to use a lateral cortical augmentation device that is able to protect and reinforce the bone itself,13 without any need to medialize the tendon insertion.14
Several studies have shown that TO tunnels provide an excellent hold. In addition, TO repairs are associated with a higher load to failure and yield less interference motion than suture anchors.15,16 The purpose of this study was to evaluate the short-term outcomes of RC repair in the presence of a GTC using a TO repair technique. The hypothesis was that the TO technique would be a good alternative for fixing an RCT in the presence of an unfavorable condition, such as a GTC.
Methods
Patient Population
The study was designed in a retrospective manner. Prior conservative treatment had failed in all included patients. The inclusion criteria were based on the results of preoperative magnetic resonance imaging (MRI) and were as follows: patients with a repairable full-thickness RCT associated with the presence of cystic changes at the tendon insertion site of the greater tuberosity, defined as a GTC involving the footprint area of the torn tendon (supraspinatus and/or infraspinatus tendons). Patients with previous shoulder surgery, injections, infection, glenohumeral instability, symptomatic acromioclavicular joint pathology, arthritis, and/or stiffness were excluded from the study.
Patient-centered outcome scores were collected preoperatively and postoperatively at 1 year. These included the American Shoulder and Elbow Surgeons (ASES) score,17 Constant shoulder score,18 and University of California–Los Angeles (UCLA) shoulder rating scale.19
Magnetic Resonance Evaluation
Preoperative MRI was analyzed to determine (1) the involved ruptured tendon(s), (2) the amount of fatty infiltration of the torn RC tendon(s) according to the staging system of Fuchs et al.,20 and (3) the location and dimension of the GTC. To elucidate the latter point, a reproducible cyst mapping system was developed: the GTC mapping system.
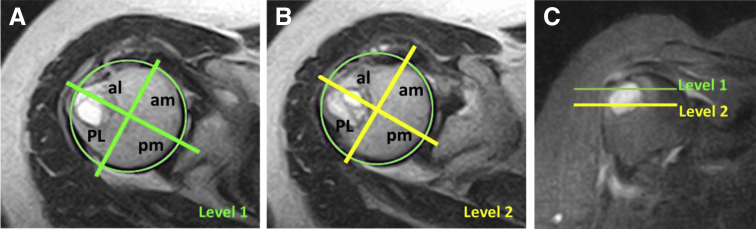
The GTC mapping system identifies cyst depth on the greater tuberosity and has to be performed on 2 axial T2-weighted images with or without fat suppression at the level of footprint and 10 mm below. On both slices, a best circle is placed along the humeral head margins on the axial images, and a horizontal line is placed within the center of the circle and extends onto half of the humeral greater tuberosity (at the 12-o’clock position on the greater tuberosity). A second line, orthogonal to the first one, passing through the center of the circle, is then drawn. The greater tuberosity is divided into 4 sections for each level, in which “A” is anterior, “P” is posterior, “M” is medial, and “L” is lateral: AM, AL, PM, and PL. A number follows these letters, indicating the level: “1” is for the superficial level (at the footprint), or level 1, and “2” is for the deep level (corresponding to 10 mm below level 1), or level 2. Uppercase letters indicate a prevalent width of the cyst in the section, whereas lowercase letters indicates a minor width or absence of the cyst in the section on the axial images (Fig 1). The system also permits the entire axial area of the cyst to be easily measured.
Fig 1.
Magnetic resonance imaging showing greater tuberosity cyst mapping system. (A, B) Axial views of level 1 (green), placed at the footprint, and level 2 (yellow), placed 10 mm below. (C) Coronal view showing levels 1 and 2. The humeral head is divided into 4 sections, named using uppercase letters (major width of the cyst in the section) and lowercase letters (minor width of the cyst in the section). In this case, the cyst mapping was “PL1PL2,” suggesting a prevalent posterior location of the cystic bone reabsorption. The cyst area was 3.2 cm2 at level 1 and 2.4 cm2 at level 2. (al, anterolateral; am, anteromedial; PL, posterolateral; pm, posteromedial.)
Postoperatively, MRI was reviewed to determine (1) the tendon healing process and (2) any modifications in dimension (enlargement vs reduction) of the cyst. The preoperative images were obtained on a 0.3- or 1.5-T magnet in every patient at different radiologic centers (if axial T2-weighted images were not available, patients were not enrolled), whereas all the postoperative examinations were performed 1 year after surgery at a single center with a 1.5-T magnet using routine pulse sequences.
All the images were reviewed by 2 of the authors: a musculoskeletal radiologist with expertise in shoulder pathology (M.O.) and an orthopaedic surgeon dedicated to the shoulder (C.C.). The interobserver agreement was determined, with a κ coefficient21 of 0.85.
Surgical Technique
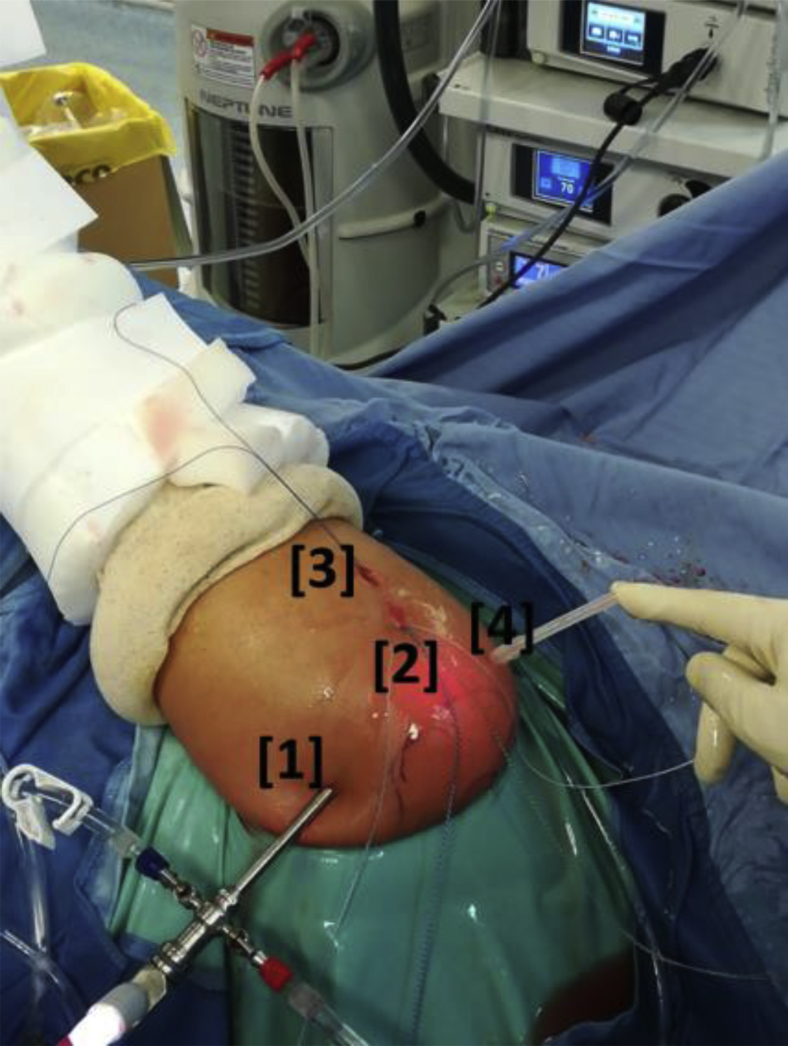
All procedures were performed by the first author with the patient in the lateral decubitus position with an interscalene cervical plexus block according to Alemanno et al.22 A 4-portal surgical technique without cannulas was used, comprising standard posterior (for the scope), superolateral, inferolateral, and anterosuperior (working) portals (Fig 2). The superolateral portal was centered on the lesion and tangential to the greater tuberosity. The inferolateral portal was placed 2 cm below and permitted the entrance of a dedicated instrument called the Taylor Stitcher Evo (NCS Lab–Medical Devices Factory, Carpi, Italy) (Fig 3) developed to create the TO tunnel while avoiding pitfalls and damage to the soft tissues.
Fig 2.
Arthroscopic portals (external view) in a left shoulder with the patient in the lateral decubitus position. The 4 portals are as follows: (1) a standard posterior portal for the scope; (2) a superolateral portal centered on the lesion and tangential to the greater tuberosity; (3) an inferolateral portal placed 2 cm below the superolateral portal, which permits the entrance of a dedicated instrument to create the transosseous tunnel; and (4) an anterosuperior (working) portal.
Fig 3.
The Taylor Stitcher Evo is a dedicated instrument able to create, with the aid of a nitinol Superelastic Transosseous Needle (1.9 mm in diameter), a curved transosseous tunnel and, at the same time, pass the suture or a shuttle. The instrument consists of 2 arms: The inferior arm is fixed, whereas the superior arm is a sliding targeting frame that permits easy orientation in the shoulder together with proper identification of the lateral entry spot without measuring it in advance. A bone bridge of 18 to 20 mm is provided.
Once the reparability of the RC was assessed, the possible associated pathologies were treated first (subscapularis repair and tenotomy or tenodesis of the long head of the biceps). The tendinous and bony sides of the lesion were accurately prepared, the cuff was adequately mobilized, and the tendon margins were refreshed.23
The greater tuberosity was carefully prepared to maximize RC footprint coverage. A wide surface decortication of the footprint was performed providing maximum spongy bone. In addition, four to six 1.5-mm puncture holes (bone marrow vents or microfractures) were performed to obtain the crimson duvet effect.24 These vascular access channels could contribute to cuff healing25,26 by increasing blood flow in the repaired RC.27
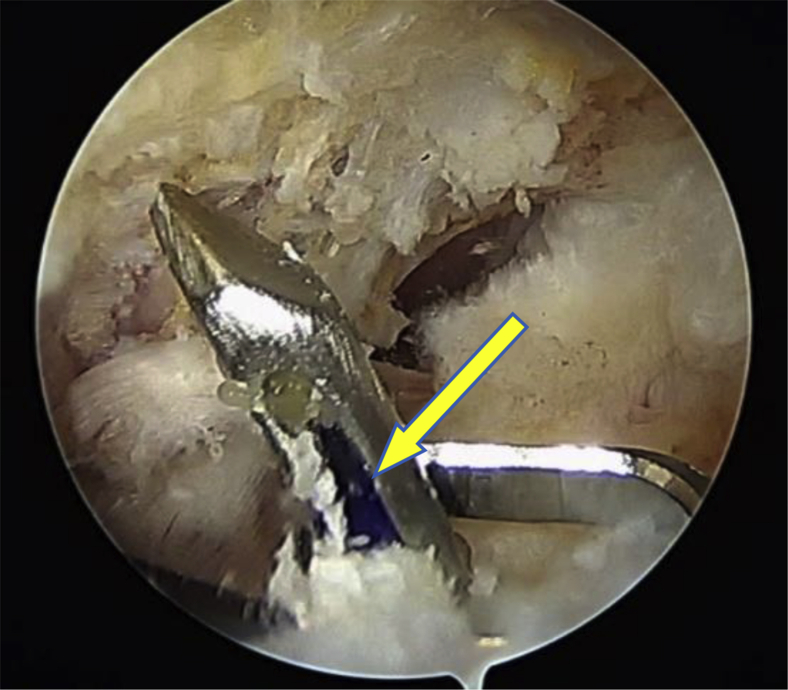
After these steps, the TO tunnel required for this technique was prepared using the Taylor Stitcher Evo. This tunnel is 3 mm in diameter and presents a smooth curved morphology. The shuttle wire was then passed in a single step with the Superelastic Transosseous Needle (STN; NCS Lab - Medical Devices Factory, Carpi [MO], Italy) (having an eyelet close to the tip) through the TO tunnel (Fig 4) so that the suture wires could be pulled through it. This shuttle is capable of pulling up to 6 suture wires connected to the front part of an implant made of PEEK (polyether ether ketone): the Elite-SPK (NCS Lab–Medical Devices Factory) (Fig 5). All 6 stitches were then passed through the cuff to obtain the “2MC” (or “double MC”) configuration with the tape ends passed more anteriorly and posteriorly.13 Schematically, we refer to limb 1 as the most anterior and to limb 6 as the most posterior. We first closed limb 2 with limb 3 (suture 1) and later closed limb 4 with limb 5 (suture 2), leaving limbs 1 and 6 free. After cutting one end of suture 1 and one end of suture 2, we shuttled limb 1 and the remaining end of suture 1 from anterior to posterior in the external eyelet of the Elite-SPK. At this point, to achieve a repair with a closed-loop configuration, knots were tied (laterally) between limbs 1 and 6 (tape), as well as the remaining limbs of sutures 1 and 2. The described technique represents a very tight and stable repair configuration that permits the greater tuberosity to be almost completely covered (Fig 6).
Fig 4.
Transosseous tunnel preparation in a left shoulder with the patient in the lateral decubitus position. The scope is in the posterior portal, and the subacromial space is shown. The shuttle wire (No. 0 polydioxanone, arrow) is transported through the transosseous tunnel by the Superelastic Transosseous Needle.
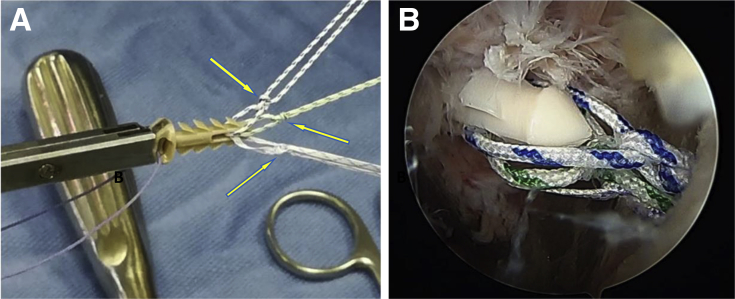
Fig 5.
Lateral cortical augmentation. (A) The Elite-SPK contains 2 separated eyelets: a rear eyelet that remains externally on the lateral cortex of the humerus and a front, smaller eyelet through which sutures are initially loaded. Along the body of the device, 8 stabilizing flaps are attached to the main body; in combination with the wide supporting platform, these flaps have the function to provide optimal primary stability to the implant. Three sutures of different colors (1 white-and-blue suture, 1 white-and-green suture, and 1 tape) were passed. Before this step, to avoid any sliding of the sutures, 2 simple knots (arrows) were performed for each one, in the front part of the implant. (B) Entrance of Elite-SPK in a left shoulder with the patient in the lateral decubitus position. The scope is in the posterior portal, and the subacromial space is shown.
Fig 6.
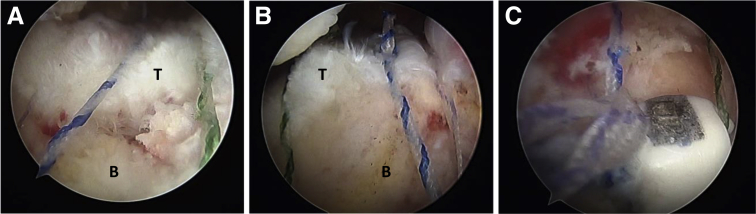
Final arthroscopic view in a left shoulder with the patient in the lateral decubitus position. The scope is in the lateral portal (from superior in A and B and from inferior in C), and the subacromial space is shown. All the sutures are tied and closed in the lateral eyelet of the Elite-SPK. (B, bone; T, tendon.)
Postoperative Rehabilitation Protocol
The postoperative rehabilitation protocol after arthroscopic TO RC repair is designed to protect the repair in the early stages, to prevent postoperative stiffness, and to restore the function of the scapulohumeral joint.28,29 Immediately after surgery, the patient wears a 15° to 20° abduction pillow, which maintains the arm abducted without external rotation (neutral or resting position), for the first 4 postoperative weeks. This pillow is capable of protecting the repair, reducing tension at the suture level, and improving vascularization of the scar during the early weeks.30,31 The postoperative rehabilitation program is conventionally divided into 3 stages that naturally overlap without any break between them (Table 1).
Table 1.
Postoperative Rehabilitation Program
| Period | Aim | Activity |
|---|---|---|
| First stage: from immediately postoperatively until fourth week | Prevent articular stiffness | Passive exercises in abduction, front flexion, and ER (passive ER limited to 0° if subscapular tendon was repaired) |
| Second stage: from fourth week until 12th week (third month) | Achieve progressive recovery of passive ROM without scapular compensation | Assisted or active exercises at a minimum load; removal of sling |
| Third stage: beginning around third month (10th-12th week) and lasting until 12th week or beyond | Recover strength and physiological scapular-humeral rhythm | Toning exercises focusing on recovery of power and strength of rotator cuff tendons |
ER, external rotation; ROM, range of motion.
Statistical Analysis
The sample size was determined a priori by imposing a power value of 90%, α equal to .05, and a minimum difference in the Constant score equal to 4. The data obtained with Minitab 17 (Minitab, State College, PA) were increased by 30% to account for loss of data during the study. Descriptive statistics (mean, standard deviation, minimum, and maximum) were applied for all values. Preoperative and postoperative UCLA, Constant, and ASES scores were evaluated. Comparison of the clinical variables was performed using the dependent-samples t test for normally distributed data and the Wilcoxon signed rank test for non–normally distributed data. Two-sided, paired Student t tests with 95% confidence intervals for the mean differences were computed. P < .05 indicated a statistically significant difference. All analyses were performed using Minitab 17.
The t test was used to compare preoperative versus postoperative data. The normality test was performed on the entire postoperative data set to check for the appropriateness of the method. The null hypothesis for this study was that there would be no difference in the clinical results in the preoperative versus postoperative evaluation and in the applied treatment. Moreover, to assess the clinical relevance of the findings,32,33 the minimal clinically important difference (MCID) for this trial was estimated based on the collected data according to Gum et al.34 using a test-retest reliability coefficient (r) estimated through the Pearson correlation coefficient.
This study was conducted in accordance with the World Medical Association Declaration of Helsinki standards of 1964, as revised in 1983 and 2000. All patients were informed about the study in detail prior to providing written informed consent for enrollment.
Results
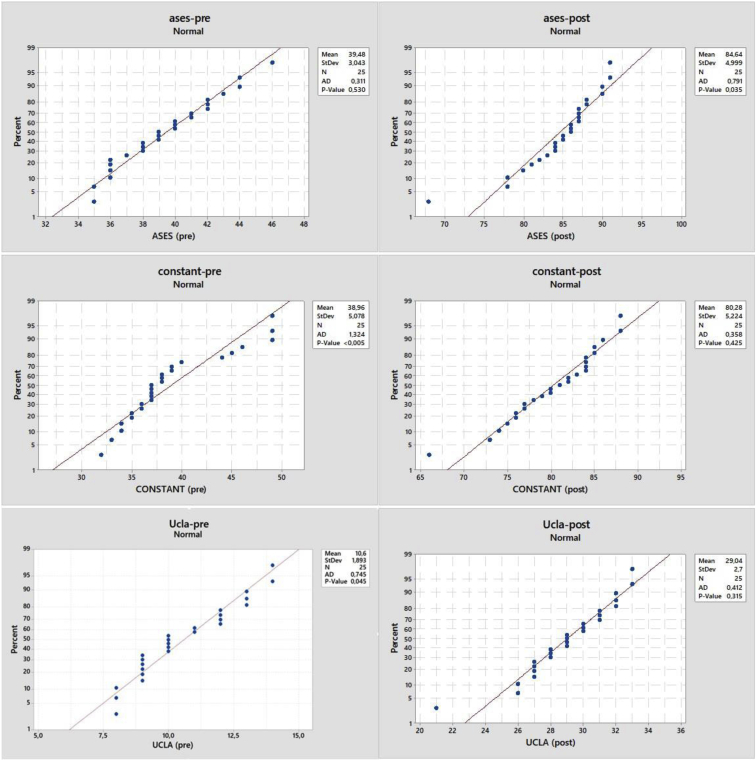
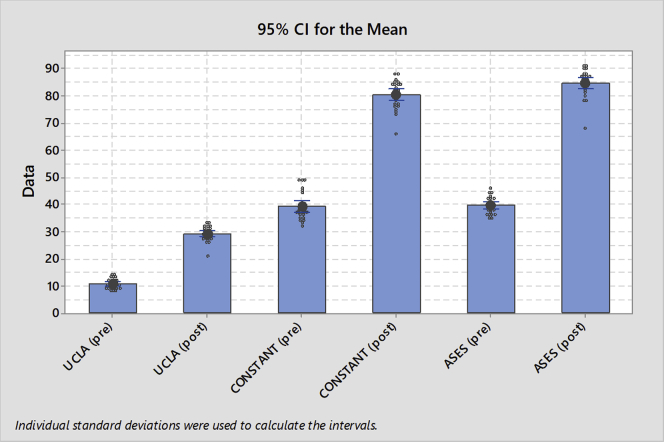
Demographic data and the distribution of the RCT pattern within the study population are reported in Table 2. All 25 patients completed the study. Acromioplasty was not performed in any case. No intraoperative complications were registered. All outcome scores improved at 1 year after surgery (Table 3). MCID threshold values equal to 4.81, 11.49, and 6.13 were found for the UCLA, Constant, and ASES scores, respectively. According to these values, all the patients met the MCID threshold for each clinical score, meaning that the experienced change was always clinically relevant. According to ASES scores, the results of the 25 shoulders available for final follow-up were excellent in 4 (16%), good in 18 (72%), fair in 2 (8%), and poor in 1 (4%), with mean values of 39.48 preoperatively (P = .530) and 84.64 postoperatively (P = .035). The mean Constant shoulder score was 38.96 preoperatively (P < .005) and 80.28 postoperatively (P = .425). The UCLA scores were excellent or good in 22 shoulders (88%) and fair or poor in 3 (12%). The mean UCLA score was 10.6 preoperatively (P = .045) and 29.04 postoperatively (P = .315). The null hypothesis of no difference in the preoperative versus postoperative scores was rejected, and the patients presented with significantly better results postoperatively (Figs 7 and 8). In only 1 case was a failure registered 1 year after surgery.
Table 2.
Patient Demographic Characteristics and RCT Patterns
| Data | |
|---|---|
| Age, mean ± SD (minimum/maximum), yr | 58.4 ± 6.9 (46/75) |
| Male/female sex, n | 13/12 |
| Side affected: right/left (dominant), n | 17/8 (20) |
| Tear pattern, n | |
| Supraspinatus | 1 |
| Supraspinatus and subscapularis | 2 |
| Supraspinatus and infraspinatus | 16 |
| Supraspinatus, infraspinatus, and subscapularis | 6 |
| Status of long head of biceps tendon, n | |
| Healthy | 1 |
| Pathologic (partial rupture or tendinosis) | 19 |
| Absent (spontaneous rupture) | 5 |
| Fuchs grade, n | |
| I | 8 |
| II | 15 |
| III | 2 |
RCT, rotator cuff tear; SD, standard deviation.
Table 3.
Outcome Scores Preoperatively and Postoperatively
| Preoperative |
Postoperative |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients With Available Data, n | Mean | SD | P Value | Patients With Available Data, n | Mean | SD | P Value | |
| ASES score | 25 | 39.48 | 3.04 | .530 | 25 | 84.64 | 5.00 | .035 |
| Constant | 25 | 38.96 | 5.08 | <.005 | 25 | 80.28 | 5.22 | .425 |
| UCLA score | 25 | 10.60 | 1.89 | .045 | 25 | 29.04 | 2.70 | .315 |
ASES, American Shoulder and Elbow Surgeons; SD, standard deviation; UCLA, University of California–Los Angeles.
Fig 7.
Patient-centered preoperative (pre) and postoperative (post) outcome scores. (AD, Anderson-Darling test; ases, American Shoulder and Elbow Surgeons; StDev, standard deviation; Ucla, University of California–Los Angeles.)
Fig 8.
Ninety-five percent confidence intervals for mean patient-centered preoperative (pre) and postoperative (post) outcome scores. (ASES, American Shoulder and Elbow Surgeons; UCLA, University of California–Los Angeles.)
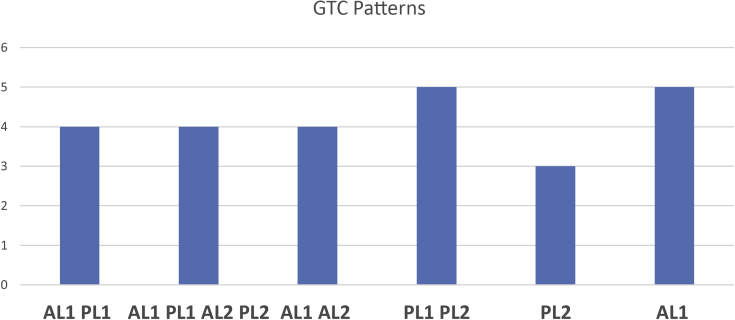
The GTC mapping system was easily adopted in all the MRI examinations independently from the quality (0.3- or 1.5-T magnet) of the images. The GTCs were mostly located in the anterolateral section of the superficial level and in the posterolateral section of both levels (Table 4, Fig 9).
Table 4.
GTC Mapping System Results
| No. of Cysts | Cyst Size, cm2 |
|||
|---|---|---|---|---|
| Mean Area | Minimum Area | Maximum Area | ||
| Level 1 | 22 | 1.96 | 0 (no cyst) | 3.4 |
| Level 2 | 17 | 1.28 | 0 (no cyst) | 2.6 |
GTC, greater tuberosity cyst.
Fig 9.
Distribution pattern of greater tuberosity cysts according to greater tuberosity cyst (GTC) mapping system, in which A indicates anterior; P, posterior, L, lateral; 1, superficial level (i.e., level 1, at the footprint) on axial view; and 2, deep level (i.e., level 2, corresponding to 10 mm below level 1) on axial view. An uppercase letter indicates a prevalent width of the cyst in the section whereas a lowercase letter indicates a minor width or absence of the cyst in the section on the axial images.
Postoperative MRI examinations revealed RC repair integrity after surgery in 24 of 25 cases (Sugaya types I and II) (Fig 10) and the presence of a major discontinuity (i.e., failure) in only 1 case (Sugaya type V). The implant was stable (i.e., no device mobilization) in all patients. The cyst’s size was maintained stable 1 year after surgery (i.e., no enlargement or reabsorption). Tunnel closure occurred only in the case in which the tunnel crossed healthy bone, in which there was no cyst.
Fig 10.
Coronal TSE (turbo spin echo) T2-weighted magnetic resonance image 12 months after transosseous rotator cuff repair showing regular margins of the tendon on the bursal side with lower signal, compatible with fibrous tissue; one should note the position of the Elite-SPK with intact cystic lateral and superior walls.
Discussion
The presence of a bone cyst in the greater tuberosity when associated with an RCT may represent a highly demanding technical challenge. Arthroscopic TO RC repair using a lateral PEEK cortical augmentation leads to significant mid-term improvement and satisfactory subjective outcomes with very low complication and failure rates in patients with RCTs associated with GTCs. On the basis of our investigation, the current literature on this topic includes only technical notes and case reports. Reda et al.35 proposed repairing the cyst with demineralized bone matrix, using metal anchors that are placed in the area near the cyst, and eventually using an augmentation in case of massive tears. Postl et al.7 used an open technique to segment the cyst lesion and repair the cuff with double-row anchor reconstruction. Agrawal and Stinson9 used an OsteoBiologics implant (San Antonio, TX) to fill the cyst; they then repaired the cuff tear.
Our study was a retrospective case-series study, not only showing an all-arthroscopic TO procedure but also reporting clinical and radiologic results at 1 year. In addition, a mapping system for cysts was introduced: the GTC mapping system. This mapping system overcomes some limitations of a previous location system for GTCs,1 in which the proximal humerus was divided into 2 halves by a line drawn at the 12-o’clock position on the greater tuberosity on axial and sagittal oblique images. Cysts located in the anterior quadrant were related to cuff disorder, whereas those in the posterior quadrant were not.36
This kind of classification appears to have some limitations because it is not able to precisely locate and subcategorize cysts.36 The GTC mapping system, which we developed in a simple and reproducible way, is able to define the location (anterior or posterior, medial or lateral) and depth (superficial or deep) of GTCs. The exact positioning easily obtained with the GTC mapping system may help the surgeon in determining the correct choice to manage RCTs.
Different factors play an important role during the tendon healing process.37 Among these, mechanical factors,38 suturing techniques,39 and biological factors40,41 are considered the crucial points.42
In the management of RC repair, the main goals are pain relief, improvement in function, and successful tendon healing. It has been stated that the ideal repair should have high initial strength, allow minimal gap formation, and maintain stability until solid healing occurs.38 All these features are represented in the TO approach. In a previous study, the usefulness of this approach has been demonstrated for the treatment of RC failure14 in which the bone is weakened by the presence of anchors.43 Similarly, in our study, the TO approach appeared valid when an RCT was associated with GTCs. In this particular condition, anchor placement in a defect-free zone with good bone stock may affect the quality and position of the RC footprint reconstruction. In addition, an open technique to augment the cystic lesion with bone void filler in combination with RCT reconstruction has been suggested, but the long-term results regarding biological integration with this material are still missing.7 Most important, the evidence supporting the use of the aforementioned surgical techniques is lacking because no reports exist describing the clinical results after RCT repair in the presence of large GTCs.
Limitations
We report short-term outcomes, complications, and technical pearls encountered in a series of 25 patients with 1 year of follow-up after arthroscopic TO RC repair associated with large GTCs performed by the same surgeon at a single institution. Complication (0%) and failure (4%) rates were extremely low, and the 1 case of failure was not associated with the surgical technique. A weakness of this study is that the consecutive group of patients presented with a heterogeneous distribution of RCTs; thus, the associated arthroscopic procedures (subscapularis repair and biceps tenotomy or tenodesis) could have served as a confounding variable.
Conclusions
Arthroscopic TO RC repair led to significant mid-term improvement and satisfactory subjective outcomes with low complication and failure rates in this study. The GTC mapping system could be useful to evaluate GTCs and to aid surgeons in the choice of the best surgical technique.
Acknowledgment
The authors thank Mr. John Winslow (Denver, Colorado) for reviewing the English in the manuscript.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.M. has patents issued for Taylor Stitcher EVO, Superelastic Transosseous Needle, and Elite-SPK. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Suluova F., Kanatli U., Ozturk B.Y., Esen E., Bolukbasi S. Humeral head cysts: Association with rotator cuff tears and age. Eur J Orthop Surg Traumatol. 2014;24:733–739. doi: 10.1007/s00590-013-1247-5. [DOI] [PubMed] [Google Scholar]
- 2.Gwark J.-Y., Park T.-S., Park H.B. Association between the location of tuberosity cysts and rotator cuff tears: A comparative study using radiograph and MRI. J Orthop Surg. 2019;27 doi: 10.1177/2309499019825762. 2309499019825762. [DOI] [PubMed] [Google Scholar]
- 3.Levy D.M., Moen T.C., Ahmad C.S. Bone grafting of humeral head cystic defects during rotator cuff repair. Am J Orthop (Belle Mead NJ) 2012;41:92–94. [PubMed] [Google Scholar]
- 4.Sano A., Konnoxy N., Toi E., Kido T., Urayama M., Sato K. Cystic changes of the humeral head on MR imaging: Relation to age and cuff-tears. Acta Orthop Scand. 1998;69:397–400. doi: 10.3109/17453679808999054. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin M., Toumi H., Ralphs J., Bydder G., Best T., Milz S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhart S.S., Klein J.R. Arthroscopic repair of rotator cuff tears associated with large bone cysts of the proximal humerus: Compaction bone grafting technique. Arthroscopy. 2005;21:1149.e1–1149.e5. doi: 10.1016/j.arthro.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Postl L., Braunstein V., von Eisenhart-Rothe R., Kirchhoff C. Footprint reconstruction in a rotator cuff tear associated cyst of the greater tuberosity: Augmented anchorage. Arch Orthop Trauma Surg. 2013;133:81–85. doi: 10.1007/s00402-012-1620-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.C., Rhee K.J., Shin H.D., Kim Y.M. Arthroscopic footprint reconstruction of a bone cyst-associated rotator cuff tear. Knee Surg Sports Traumatol Arthrosc. 2007;15:1486–1488. doi: 10.1007/s00167-007-0326-8. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal V., Stinson M. Arthroscopic grafting of greater tuberosity cyst and rotator cuff repair. Arthroscopy. 2007;23:904.e1–904.e3. doi: 10.1016/j.arthro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Garofalo R., Castagna A., Borroni M., Krishnan S.G. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20:1031–1035. doi: 10.1007/s00167-011-1725-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda S., Ishige N., Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471:3514–3522. doi: 10.1007/s11999-013-3148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aramberri-Gutiérrez M., Martínez-Menduiña A., Valencia-Mora M., Boyle S. All-suture transosseous repair for rotator cuff tear fixation using medial calcar fixation. Arthrosc Tech. 2015;4:e169–e173. doi: 10.1016/j.eats.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chillemi C., Mantovani M. Arthroscopic trans-osseous rotator cuff repair. Muscles Ligaments Tendons J. 2017;7:19–25. doi: 10.11138/mltj/2017.7.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chillemi C., Dei Giudici L., Mantovani M., Osimani M., Gumina S. Rotator cuff failure after surgery: An all-arthroscopic transosseous approach. Musculoskelet Surg. 2018;102:3–12. doi: 10.1007/s12306-018-0560-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee T.Q. Current biomechanical concepts for rotator cuff repair. Clin Orthop Surg. 2013;5:89–97. doi: 10.4055/cios.2013.5.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad C.S., Stewart A.M., Izquierdo R., Bigliani L.U. Tendon-bone interface motion in transosseous suture and suture anchor rotator cuff repair techniques. Am J Sports Med. 2005;33:1667–1671. doi: 10.1177/0363546505278252. [DOI] [PubMed] [Google Scholar]
- 17.Michener L.A., McClure P.W., Sennett B.J. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11:587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 18.Constant C., Murley A. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160–164. [PubMed] [Google Scholar]
- 19.Amstutz H.C., Sew A.H., Clarke I. UCLA anatomic total shoulder arthroplasty. Clin Orthop Relat Res. 1981;155:7–20. [PubMed] [Google Scholar]
- 20.Fuchs B., Weishaupt D., Zanetti M., Hodler J., Gerber C. Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 21.Viera A.J., Garrett J.M. Understanding interobserver agreement: The kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 22.Alemanno F., Ghisi D., Fanelli A., et al. Tramadol and 0.5% levobupivacaine for single-shot interscalene block: Effects on postoperative analgesia in patients undergoing shoulder arthroplasty. Minerva Anestesiol. 2012;78:291–296. [PubMed] [Google Scholar]
- 23.Gigante A., Marinelli M., Chillemi C., Greco F. Fibrous cartilage in the rotator cuff: A pathogenetic mechanism of tendon tear? J Shoulder Elbow Surg. 2004;13:328–332. doi: 10.1016/j.jse.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Snyder S.J., Burns J. Rotator cuff healing and the bone marrow “crimson duvet” from clinical observations to science. Tech Shoulder Elbow Surg. 2009;10:130–137. [Google Scholar]
- 25.Galatz L.M., Ball C.M., Teefey S.A., Middleton W.D., Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Jost B., Zumstein M., Pfirrmann C.W., Gerber C. Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2006;88:472–479. doi: 10.2106/JBJS.E.00003. [DOI] [PubMed] [Google Scholar]
- 27.Urita A., Funakoshi T., Horie T., Nishida M., Iwasaki N. Difference in vascular patterns between transosseous-equivalent and transosseous rotator cuff repair. J Shoulder Elbow Surg. 2017;26:149–156. doi: 10.1016/j.jse.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Bruzga B., Speer K. Challenges of rehabilitation after shoulder surgery. Clin Sports Med. 1999;18:769–793. doi: 10.1016/s0278-5919(05)70184-8. [DOI] [PubMed] [Google Scholar]
- 29.Jackins S. Postoperative shoulder rehabilitation. Phys Med Rehabil Clin N Am. 2004;15:643–682. doi: 10.1016/j.pmr.2004.01.002. vi. [DOI] [PubMed] [Google Scholar]
- 30.Hersche O., Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: A preliminary report. J Shoulder Elbow Surg. 1998;7:393–396. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 31.Millett P.J., Wilcox R.B., III, O'Holleran J.D., Warner J.J. Rehabilitation of the rotator cuff: An evaluation-based approach. J Am Acad Orthop Surg. 2006;14:599–609. doi: 10.5435/00124635-200610000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Harris J.D., Brand J.C., Cote M.P., Faucett S.C., Dhawan A. Research pearls: The significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy. 2017;33:1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 33.Katz N.P., Paillard F.C., Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. doi: 10.1186/s13018-014-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gum J.L., Glassman S.D., Carreon L.Y. Clinically important deterioration in patients undergoing lumbar spine surgery: A choice of evaluation methods using the Oswestry Disability Index, 36-Item Short Form Health Survey, and pain scales. J Neurosurg Spine. 2013;19:564–568. doi: 10.3171/2013.8.SPINE12804. [DOI] [PubMed] [Google Scholar]
- 35.Reda B., Coady C., Wong I. Revision of failed rotator cuff reconstruction with a large humeral head cyst. Arthrosc Tech. 2017;6:e2023–e2030. doi: 10.1016/j.eats.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritz L.B., Ouellette H.A., O'Hanley T.A., Kassarjian A., Palmer W.E. Cystic changes at supraspinatus and infraspinatus tendon insertion sites: Association with age and rotator cuff disorders in 238 patients. Radiology. 2007;244:239–248. doi: 10.1148/radiol.2441050029. [DOI] [PubMed] [Google Scholar]
- 37.Chillemi C., Petrozza V., Garro L., et al. Rotator cuff re-tear or non-healing: Histopathological aspects and predictive factors. Knee Surg Sports Traumatol Arthrosc. 2011;19:1588–1596. doi: 10.1007/s00167-011-1521-1. [DOI] [PubMed] [Google Scholar]
- 38.Gerber C., Schneeberger A.G., Beck M., Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76:371–380. [PubMed] [Google Scholar]
- 39.Apreleva M., Özbaydar M., Fitzgibbons P.G., Warner J.J. Rotator cuff tears: The effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18:519–526. doi: 10.1053/jars.2002.32930. [DOI] [PubMed] [Google Scholar]
- 40.Meyer D.C., Pirkl C., Pfirrmann C.W., Zanetti M., Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res. 2005;23 doi: 10.1016/j.orthres.2004.06.010. 254-248. [DOI] [PubMed] [Google Scholar]
- 41.Rebuzzi E., Coletti N., Schiavetti S., Giusto F. Arthroscopic rotator cuff repair in patients older than 60 years. Arthroscopy. 2005;21:48–54. doi: 10.1016/j.arthro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Castagna A., Conti M., Markopoulos N., et al. Arthroscopic repair of rotator cuff tear with a modified Mason-Allen stitch: Mid-term clinical and ultrasound outcomes. Knee Surg Sports Traumatol Arthrosc. 2008;16:497–503. doi: 10.1007/s00167-007-0461-2. [DOI] [PubMed] [Google Scholar]
- 43.Athwal G.S., Shridharani S.M., O'Driscoll S.W. Osteolysis and arthropathy of the shoulder after use of bioabsorbable knotless suture anchors: A report of four cases. J Bone Joint Surg Am. 2006;88:1840–1845. doi: 10.2106/JBJS.E.00721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.