Abstract
Multispecific antibodies can be generated in different formats. More than two decades of R&D in the field of bispecific antibody engineering revealed that the design and choice of format can have a profound impact on the antibody functionality. This holds in particular true for entities that elicit (inter-)cellular processes such as receptor activation, receptor internalization, receptor clustering or the formation of immunological synapses between two cells. This review covers design parameters that influence the functionality of multispecific formats, with particular focus on T cell-recruiting bispecific antibodies. We describe formats that display the same size and domain sequences but a varying geometry. The structural composition of (artificial) immune synapses is reviewed and allows conclusions why some formats that share size and domain composition are more effective than others. To support the statement that the geometry matters, we present a recently designed antibody format that is characterized by its compact shape. The TriFab-Contorsbody consists of two tumor cell-targeting entities and one moiety for T cell recruitment. The unique barrel-like shape provides a 35-fold increase in potency compared to an IgG-like molecule with identical domain sequences.
Keywords: T cell bispecific, Antibody format, Protein engineering, Geometry, Immune synapse
1. Background: Different fields, different formats
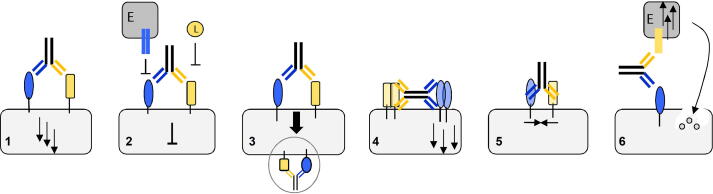
The technical progress in recombinant protein expression and creative antibody engineering brought up many different antibody formats that are reviewed by others in detail [7], [53], [15], [23]. The key message in the literature is that there is no master format that fits all applications. A suited antibody configuration rather depends on the desired biological effect and its underlying structural conditions. Multispecific antibodies are applied for various purposes including (1) receptor–activation [38], (2) –blocking [30], (3) –internalization [42], (4) –clustering [8], (5) the association of membrane-associated proteins [26], or (6) the retargeting of cytotoxic effector cells [35] (Fig. 1). Within these application fields, there are several examples that prove that formats influence the bispecific antibody (bsAb) performance.
Fig. 1.
Application areas of bispecific antibodies: 1) Receptor activation. 2) Receptor blocking and inhibition of soluble ligand (L) or membrane-bound ligand binding, e.g. of effector cells (E). 3) Receptor internalization. 4) Receptor clustering. 5) Receptor association. 6) Bicellular binding and retargeting of effector cells (E).
Receptor activation (1) and induction of downstream signaling to achieve a certain phenotype is the goal of agonistic antibodies. Shi et al. present an IgG-shaped, tetravalent, biparatopic format that contains VH-only binders on each N-terminus. The close distance of paratopes mimics the natural ligand and thereby activates the endocrine fibroblast growth factor (FGF) 21 receptor (FGFR). The distinct antibody geometry was carefully selected and shown to be essential for inducing agonism [52]. The same format was recently published by others who also detected agonism by targeting CD40 [34]. Researchers around Milutinovic have generated a bivalent Y-shaped Surrobody that agonizes the death receptors DR4 and DR5. The molecule showed greater potency than the combination of monospecific DR4 and DR5 antibodies, suggesting its distinct properties to mediate agonism [38]. Just recently, antibody engineers from Genentech showed agonism via the MerTK (Mer Tyrosine Kinase) receptor pathway on macrophages that led to the phagocytosis of CD20 positive B cells. They made use of a simple 1 + 1 IgG format [25].
Receptor blockade (2) takes currently an important part in cancer immunotherapy approaches. Bispecific, antagonistic molecules that target inhibitory immune checkpoints show promising results in overcoming tumor evasion mechanisms. A prominent example is the dual-blockade of immune checkpoints such as LAG-3 and PD-1, which induces antitumor immunity [30], [61]. The number of ongoing clinical trials with diverse bispecific antibody formats is striking [45], [46], [28]. Whereas, the generation of agonistic antibodies is challenging (they depend on both affinity and intrinsic efficacy), designing blocking antibodies mostly relies on finding high affinity binders that compete with the natural ligand and is therefore seen to be less complex [37].
Antibody-induced receptor internalization (3) plays an essential role in antibody-drug-conjugate (ADC) retargeting [10], [56]. It could be shown that the bivalent binding of EGFR and the thereby triggered dimerization leads to a significantly higher internalization of the receptor–antibody complex than seen with the monovalent format [24], [20]. Niewoehner et al. analyzed different formats to achieve blood–brain barrier transcytosis. A monovalent targeting of the transferrin receptor (TfR) lead to a 55-fold higher brain exposure than the bivalent <TfR>-format in vivo. It is hypothesized that the bivalent TfR binding leads to substantial receptor dimerization, and the subsequent routing to the lysosomal pathway. On the other hand, a monovalent binding could allow a simultaneous complexation of the physiological binding partner transferrin and the co-transport of both molecules to the abluminal side without lysosomal degradation of the antibody [42], [60].
Receptor hyperclustering (4) is for example required to activate the extrinsic apoptotic pathway that is mediated by receptors belonging to the tumor necrosis factor receptor superfamily (TNFRSF), e.g. death receptors (DR). The generation of agonistic antibodies to induce the signaling cascade is challenging and had limited clinical efficacy in earlier approaches. Yang et al. achieved robust intrinsic agonism with a tetravalent biparatopic antibody. The bivalent biparatopic control molecules did not trigger any activation [63]. Brunker et al. designed a 2 + 2 format targeting both the tumor antigen FAP (Fibroblast Activating Protein) and the DR5. They could show that bivalent binding of both antigens in a cis orientation leads to an avidity-driven hyperclustering of DR5 and subsequently strong induction of apoptosis. In contrast to other clustering approaches that rely on FcγR interactions, they made use of the FAP expression in the tumor stroma to promote hyperclustering of DR5 in a targeted manner, hence reducing the systemic toxicity that has been seen in FcγR-dependent approaches [8].
Another field of application is the antibody-mediated association of two or more proteins on phospholipid membranes (5). Hemophilia A is a genetic bleeding disorder caused by the missing of the clotting protein factor VIII. Under physiological conditions, FVIII acts as a cofactor promoting the association of the enzyme-substrate complex FIXa-FX that results in the activation of FX, which in turn is a key factor in the coagulation cascade [32]. Researchers from Chugai generated an IgG-shape bsAb with a defined geometry that mimics the structure and allosteric properties of FVIII thereby restoring the cascade [26]. This molecule is known as Hemlibra® (emicizumab) and is one of the two approvedbsAb [29].
The redirection of cytotoxic effector cells to malignant tissue represents another mode-of-action of monoclonal antibodies (6). Mimicking antibody-dependent cell-mediated cytotoxicity (ADCC) is probably one of the most established therapeutic strategies to eliminate cancer cells. The concept bases on the decoration of cancer cells with therapeutic antibodies and the subsequent binding of NK cells via their Fc receptor. The activation of NK cells in turn leads to target cell killing [59]. Fc-receptor (FcγRIII, CD16)-mediated recruiting as a function of bsAbs can occur via binding of CD16 on NK cell surfaces to the Fc region of the bsAb, or alternatively by bsAbs that bind tumor specific antigens as well as CD16. In both cases, composition and format of bsAbs can affect the efficacy of ADCC induction. The design of bsAbs for Fc-mediated NK-cell recruiting faces the challenge that chosen formats need to assure accessibility of the Fc domain to CD16 on NK-cells. For example, scFv fusions at either the N-or C-terminus can display different capabilities in inducing ADCC, ranging from full competency to loss of ADCC activity [14]. These effects, most likely caused by sterical hindrance of the FcγRIII interaction with NK cells by added binding regions and/or bound target antigen, depend not only on the format but also on the choice of target antigen (and epitopes), and hence need to be evaluated experimentally on an individual basis. ADCC efficacies of bsAbs that recruit NK cells via binding to tumor cells as well as to CD16 are similarly dependent on choice of binders and formats. For example, Reusch and colleagues have shown that ADCC induction of bsAbs that bind the tumor-associated antigen (TAA) CD30 as well as CD16A are superior in the TandAb (tandem diabody) format with dual CD16A binding, compared to each monovalent CD30/CD16A binding in a Diabody format [47].
Another class of well-characterized and clinically relevant bsAb for effector cell retargeting (6) are T cell-engaging derivatives as they are a central pillar in cancer immunotherapy. The first-in-class bsAb in this field is Blincyto® (blinatumomab). This CD19/CD3 bispecific led to a complete remission in 69% of patients suffering from relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL) in a phase-II study [58].
After the first proof of concept for T cell bispecifics (TCB) in 1985 [54] a flood of new antibody formats arose . On first glance, the crosslinking of two cells seems to be rather trivial and not as format-dependent as examples mentioned earlier. However, in the course of testing various antibodies it became apparent that designing safe and efficacious TCB is not just a plug and play exercise. For instance, a too high affinity of the CD3 binder was shown to be associated with rapid antibody infiltration into secondary lymphatic tissue and a lower tumor penetration [36]. Others report a strong cytokine release after injection with high affinity or bivalent CD3ε binders [57], [40]. The geometry, valency and flexibility are also important parameters that influence functionality, potency and safety of T cell recruiting therapeutic entities [19].
In this mini-review, we focus on TCB formats and their geometries and how an alternative domain architecture can enhance the therapeutic effect without changing domain sequences or valencies. T cell recruiters serve as an example class for format-function relations here, however, the same lessons might also apply to other immune cell modulators that aim to retarget NK cells, macrophages or other phagocytic effector cells.
2. Structural aspects of an artificial immune synapse
The format matters for bispecific T cell engagers. But why does it actually matter? To answer the question why some antibody architectures display a higher potency than others it is essential to understand the underlying biology of where the molecule is supposed to act.
The physiological immune synapse (IS) between an antigen presenting cell (APC) and a T cell is essential for T cell activation. On the T cell membrane the IS displays a nested ring-shaped structure frequently termed as “bullseye”. The central region cSMAC (central supramolecular activation cluster) contains components such as the T cell receptor (TCR) complexes, the co-stimulatory molecule CD28, (co)-inhibitory immune checkpoints such as PD-1 or CTLA-4, signaling mediators such as Lck (lymphocyte-specific protein tyrosine kinase) and PKC (protein kinase C), and cytotoxic agents such as perforin. The cSMAC is surrounded by the peripheral SMAC (pSMAC) that contains a number of adhesion molecules, which mediate the cell–cell association (e.g. LFA1). The distal ring (dSMAC) includes the inhibitory tyrosine phosphatase CD45 and dynamic actin [17], [44]. The membrane distance between an APC and a T cell is approximately 13–15 nm [18], [12], [21]. An elongated membrane spacing between the membranes decreased the T cell activation in an experimental setup revealing that a closer distance is mandatory for a proper induction of T cell activation [12].
Today, it is common knowledge that TCBs facilitate the formation of a regular cytolytic immune synapse by simultaneously binding to the TCR subunit CD3ε and the TAA on the target cell [39], [48]. Offner et al. used confocal microscopy to prove that the co-localization of synapse-specific markers from all SMAC rings did not vary between a physiological and an antibody-induced artificial synapse [43].
Cartwright and colleagues used quantitative fluorescence microscopy and nanometer-scale dextrans in order to analyze which molecular sizes have access to the IS and whether there is a threshold in size triggering exclusion from the IS. They could indeed prove that dextrans in the range of 4 and 13 nm could penetrate the IS. On the other hand, larger molecules between 32 and 54 nm were excluded. Their hypothesis was further proven with antibodies of different sizes for immune synapses between NK and tumor cells [9]. These findings suggest, that distances in artificial immune synapses matter and should be taken into consideration when designing TCBs that might rather disrupt than trigger the synapse formation.
Li and co-workers generated TCBs that bind to different regions of FcRH5 with similar binding affinities. Chosen epitopes were located close to the membrane (proximal), far from the membrane (distal) or in-between. Co-culturing of myeloma cells with human T cells and antibodies indicated that targeting the proximal epitope led to the strongest induction of the IS and resulting T cell activation and tumor cell lysis. Considering the mechanism, they could prove, that the exclusion of the inhibitory phosphatase CD45 triggers the TCR phosphorylation and induction of the T cell signaling [33]. CD45 is a large transmembrane receptor that has an ectodomain size ranging from approximately 28 to 50 nm [13]. This in turn reveals that smaller intermembrane distances are favorable to facilitate the exclusion of CD45 from the sSMAC.
To the same direction point experiments by Lee et al. who analyzed the structural basis for the interaction of the co-inhibitory receptor CTLA-4 (cytotoxic T-lymphocyte-associated protein-4) on the T cell with its binding partner B7 on the tumor cell within the cSMAC. They quantified the intercellular distance to 14 nm, which is in line with the spacing of an immune synapse that was reported by others. This knowledge is also important for generating antagonistic antibodies. The team for instance hypothesizes that the anti-CTLA-4 antagonist tremelimumab disrupts the inhibitory axis between CTLA-4 and B7 by increasing the intercellular space to 15–19 nm. The two receptors are then not able to interact with each other anymore [31].
Taken together, the artificial immune synapse is a highly organized structure that shares features with the physiological topology. The findings considering the membrane spacing between tumor and T cell indicate that especially the antibody dimension is a critical parameter for synapse formation.
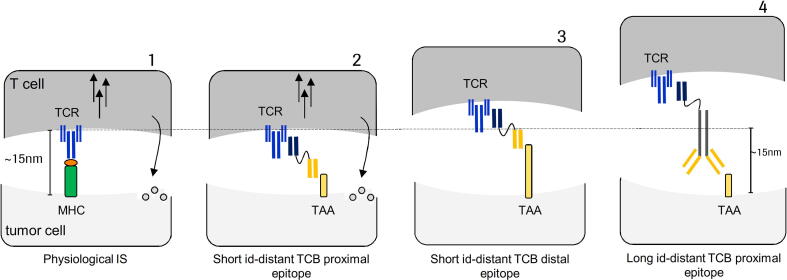
Fig. 2 summarizes different scenarios in which an artificial IS is developed (if the antibody mimics the physiological membrane spanning) or not efficiently formed (in case distal epitope binding or large antibodies are applied).
Fig. 2.
Physiological immune synapse in comparison to TCB-induced artificial synapse formation. 1) Physiological immune synapse between effector T cell and tumor cell. The intermembrane distance is about 15 nm. 2) Short interdomain-distant (id)-antibodies induce the formation of artificial immune synapses that share common features (spanning) with the physiological condition. 3) Using short id-distant antibodies that bind to distal epitopes extends the intermembrane spacing. Molecules are less effective. 4) Long id-distant antibodies extend the intercellular spacing which might lead to decreased T cell activity.
3. Bispecific antibodies for T cell recruitment: Same binders, different geometry, different potency
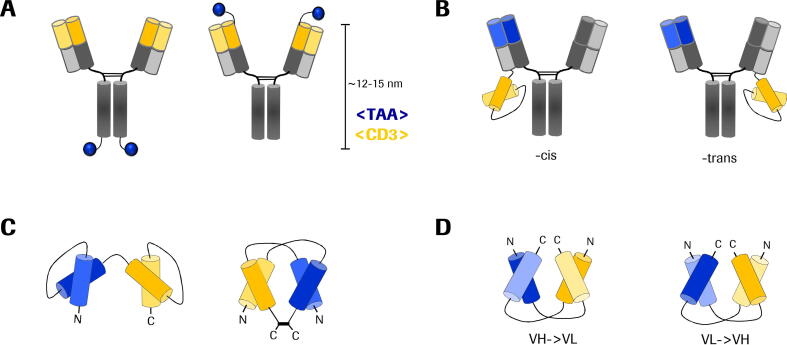
As pointed out earlier we focus here on geometries of different formats that have a major impact on their efficacy. Wuellner et al. compared two antibody formats with identical binders and the same size. The first one, a FynomAb carries the TAA binders (i.e, a fynomer against HER2) N-terminally fused to the <CD3> mAb. In the control molecule, the HER2 binders were C-terminally fused to the Fc (Fig. 3A). Hence, molecules differed by 10–12 nm in the distance between the TAA and CD3ε binder. In co-culturing assays of tumor cells and T cells, the N-terminally fused CD3ε binding entity induced a more than 20-fold (depending on target cell line up to 115-fold) higher potency indicating that a close proximity between TAA and TCR binders is favorable [62]. These observations are in agreement with other studies using tandem scFv targeting different epitopes and thereby varying the spanning of the intermembrane distance [6].
Fig. 3.
T cell-engaging antibodies with same size and domain sequences display different potencies. A) A FynomAb is either C-terminally or N-terminally fused to an IgG. B) A CD3ε-targeting scFv is either fused in cis- or trans- orientation to the light chain of an IgG-based monovalent, tumor-targeting antibody. C) A conventional tandem scFv (left) and a disulfide-stabilized DART molecule with alternating domain composition (right). D) Bispecific diabodies can be designed in four different ways, starting with either VH or VL on each chain. Depicted are VH->VL (both chains start with VH from N terminus to C terminus) and VL->VH. Not shown here are the respective hybrids.
In a similar unpublished study performed by Janssen R&D different bsAb formats were compared in in vitro killing assays. In a 1+1 format, a CD3ε binder was located on one heavy chain, whereas the TAA binder (centyrin) was either fused C-terminally to the Fc, or located in the adjacent hinge region on the other heavy chain. The latter showed a 100-fold higher potency than the format with the larger distance between TAA and CD3ε binder [55].
Recently, Santich and co-workers dissected a symmetric dual bivalent bsAb platform to explore the importance of valency and spatial configuration for bsAb-induced T cell cytotoxicity. They used a monovalent tumor-targeting IgG scaffold and fused a CD3ε-targeting scFv to the C-termini in either cis- (light chain of TAA binder) or trans-orientation (light chain of the non-targeting binder) to result in 1 + 1 bsAb (Fig. 3B). They could show that a cis-orientated version is far more potent in vitro and in vivo compared to the trans-orientated molecule that has a larger interdomain distance. The benefitial cis-configuration and interdomain spacing is also reported by others [4]. Importantly, Santich et al. stress that further shortening of the interdomain distance is not generally advantageous. Experiments with 2 + 2 BiTE-Fc molecules that have a very short interdomain distance revealed that the physical constraint or mechanical coupling between CD3ε and tumor cell is impaired and leads to a decreased T cell response [50].
Moore and colleagues compared DART (dual-affinity re-targeting) antibodies with conventional tandem scFv (e.g. blinatumomab) concerning their potency in tumor cell elimination. Both molecules have the same molecular weight and identical Fv sequences. However, the difference between the two formats is the geometry of the molecule. Whereas in the tandem setup the two scFvs are located one after another on one chain, the DART molecule carries the variable domains of the two specificities in an alternating order on two chains (VLA-VHB, VLB-VHA). Moreover, the construct is disulfide-stabilized in a hinge-like region at the C-terminus (Fig. 3C). The DART format was proven to be up to 60-times more potent in cytotoxicity assays. Furthermore, the DART molecule induced three times more cell–cell associations between T cell and target cell. The authors hypothesize that the increased activity can be explained by the stable architecture and a physiological geometry that supports bicellular associations [41]. Another advantage of the disulfide bridge is the increase in thermal stability [49].
Asano and colleagues published multiple studies that focus on the influence of domain orientation on antibody functionality in the TCB context. They expressed all four possible domain orders of a bispecific diabody targeting EGFR and CD3ε (Fig. 3D). Although all molecules were proven to have similar binding capabilities to the two TAA, tumor growth inhibition varied significantly. They hypothesize that due to their compact and less flexible structure, different diabody orientations face varying sterical hindrances during cellular cross-linking which affects the T cell activity and subsequent effector function [1]. The structural basis for this observation was elucidated in a separate publication [2]. Similar observations were made by rearranging domains in Fc-based diabodies, which besides showing different antitumor effects also had different degradation resistance and in vivo half-life [3].
Cheng et al. generated a TCB that targets the disialoganglioside GD2 on melanoma cells. They observed that the variable domain orientation (VH->VL or VL->VH) in tandem scFv formats significantly affects the binding affinity to the antigen and the cytotoxic efficacy in presence of tumor and T cells. They provide molecular modelling and antigen docking data that confirms that key residues of CDR loops which significantly contribute to antigen binding have different conformations depending on VH and VL orientation [11].
4. TriFab Contorsbody: A compact domain architecture leads to increased potency
To highlight the importance of the antibody architecture for the efficacy of a therapeutic antibody, we directly compared two formats. They share identical binding domains and have the same molecular weight. The only difference is the domain architecture.
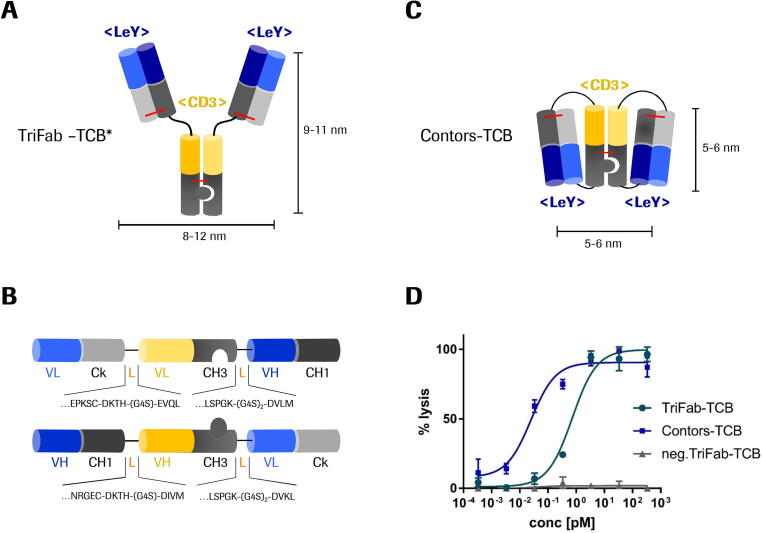
We recently published an antibody format, named TriFab, in which the CH2 domains in the Fc part were substituted by T cell-engaging domains. As depicted in Fig. 4A the variable fragments of a CD3ε binder are incorporated into the stem region of the antibody, rather than N- or C-terminally fused as found in the majority of TCB formats [16].
Fig. 4.
A) TriFab format with two Fab moieties recognizing the Lewis Y antigen (blue) and one Fv directed against the CD3ε antigen (yellow) according to Dickopf et al. (*)[16]. Interdomain disulfide bridges are indicated in red. B) The equivalent binders described in the TriFab format were used to design a two-chained format that is related to the Contorsbody technology by Georges et al. 2020 [64]. Molecules were produced in HEK suspension culture expression systems by transient transfection. C) After kappa-select affinity capture, 85% of the protein presented as folded TriFab-Contorsbody. As side product a tandem-like Fab molecule (9%) and high-molecular weight species (aggregates) were also observed which could be removed by SEC. Preferential assembly of the Contors-TriFab format from these input molecules occurs because during translation and folding, intrachain assembly/and disulfides appear to form earlier than the interchain assembly of knob-into-hole CH3 and VH/VL. The final yields of purified TriFab-Contorsbodies was 8 mg per liter culture. D) Lewis Y targeting TriFab-TCB and Contorsbody-TCB were applied to co-culturing assays of LeY-positive MCF-7 cells and human PBMC (E:T ratio = 10:1). Results are expressed as mean and SD from triplicate wells and plotted as 3-parameter non-linear regression fitting using Graphpad Prism software. Representative plot of three independent experiments is shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Another and novel format which is related to both the TriFab and the Contorsbody architecture [64] is the “TriFab-Contorsbody”. This molecule is composed of two chains (Fig. 4B) that assemble into a trivalent molecule (Fig. 4C). The CD3ε binding motif is placed in a trans orientation, i.e. the <CD3> pseudo-Fab is in a head-to-tail fashion with the targeting moieties. When we compared the efficacy of the two TCB formats in a co-culturing assay with human PBMC, we found that the Contors-TCB was 35-fold more active compared to the TriFab format (Fig. 4D). All sequences, materials and methods were used as described elsewhere [16].
In the Contorsbody the two targeting moieties (blue) are in closer proximity, which could lead to a higher local accumulation on the target cell membrane. Hence, the simultaneous trans-binding of the TCR would lead to more dense TCR clustering compared to the condition with regular Y-shape, IgG-like molecules. This increased activity can be explained by the permissive geometry model which describes that TCR clustering leads to enhanced activation due to induced structural changes in the cytoplasmic CD3ε domains [22], [39].
The Contorsbody format is more compact compared to the IgG-shaped molecule. The gyration radius of a Y-shape format is about 6 nm whereas the one of a Contorsbody is about 3 nm; dividing a radius by two is reducing the volume by factor 8. Because of that, Contorsbody is likely more suited to penetrate the IS. This leads to higher local concentrations as described by Cartwright et al. and thereby elicits increased cytotoxic potencies [9].
The TriFab-Contorsbody format is not only bringing two binding motifs closer together but does also provide the third binding motif in an ideal trans-configuration. The CD3ε binding domain is better accessible which allows an efficient simultaneous bicellular binding of both T cell and target cell.
Although the TriFab-Contorsbody is quite compact, it can bind bivalently to tumor targets, which enables avidity effects. Noteworthy, the bispecific format can be easily transformed into a trispecific format by just exchanging the variable domains on one side without affecting the compact character of the novel format.
5. Summary and outlook
To date two bispecific antibodies are marketed, however, the high number of ongoing clinical trials probably indicates that there are more to come. Aside from that, the simultaneous binding of two distinct antigens is of relevance in many different therapeutic fields (examples above) and not only limited to co-blocking strategies that evolve from combinational therapies of two existing monospecific antibodies [51], [27].
The design and selection of suitable binder-format combinations is essential for generation of bsAbs with desired functionalities. There is no ‘standard procedure’ to achieve that goal, it rather has to be tackled on a case-by-case basis depending on the underlying biology and structural conditions.
When the first-in-class molecule blinatumomab was proven to be functioning in human [5], a hype in generating T cell bispecifics started. Many T cell engaging antibodies in all kinds of formats and geometries were tested and it quickly became apparent, that they vary in efficacy and safety. By using microscopy and crystallography, the structural basis of immune synapses formation has been unraveled in the meanwhile. Combining this knowledge with the outcome of many (pre-) clinical trials, it is now explainable why some formats are more potent than others are. These lessons will ease the design of safe and efficient biologicals for T cell activation in future approaches. An example that underlines the importance of format design is the presented TriFab-Contorsbody with its unique geometry and architecture designed to fit into the tight space of an immune synapse. The barrel-like composition of that molecule was shown to be 35-fold more potent than the IgG-shaped TriFab format that shares the same domain sequence and size. The TriFab-Contorsbody is an excellent example of how the domain composition, molecule shape and geometry matters and hence extends the format space for antibody-based therapeutics – not only for T cell redirecting strategies.
6. Authorship contributions
SD, GG and UB contributed to the conception of the TriFab-Contorsbody and wrote, reviewed the manuscript.
7. Competing interests
SD, UB and GG are employees of Roche. Roche has an interest in targeted therapies.
Acknowledgment
SD is supported by the Roche Postdoc Fund (RPF) and is member of the international doctoral program ‘i-Target: Immunotargeting of Cancer’ funded by the Elite Network of Bavaria.
References
- 1.Asano R., Kumagai T., Nagai K., Taki S., Shimomura I., Arai K. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: the case of the hEx3 diabody. Protein Eng Des Sel. 2013;26:359–367. doi: 10.1093/protein/gzt009. [DOI] [PubMed] [Google Scholar]
- 2.Asano Ryutaro, Nagai Keisuke, Makabe Koki, Takahashi Kento, Kumagai Takashi, Kawaguchi Hiroko. Structural considerations for functional anti-EGFR × anti-CD3 bispecific diabodies in light of domain order and binding affinity. Oncotarget. 2018;9:13884–13893. doi: 10.18632/oncotarget.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano Ryutaro, Shimomura Ippei, Konno Shota, Ito Akiko, Masakari Yosuke, Orimo Ryota. Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics. mAbs. 2014;6(5):1243–1254. doi: 10.4161/mabs.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacac Marina, Colombetti Sara, Herter Sylvia, Sam Johannes, Perro Mario, Chen Stanford. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 5.Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 6.Bluemel C., Hausmann S., Fluhr P., Sriskandarajah M., Stallcup W.B., Baeuerle P.A. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann Ulrich, Kontermann Roland E. The making of bispecific antibodies. mAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunker P., Wartha K., Friess T., Grau-Richards S., Waldhauer I., Koller C.F. RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers FAP-dependent, avidity-driven DR5 hyperclustering and tumor cell apoptosis. Mol Cancer Ther. 2016;15:946–957. doi: 10.1158/1535-7163.MCT-15-0647. [DOI] [PubMed] [Google Scholar]
- 9.Cartwright Adam N.R., Griggs Jeremy, Davis Daniel M. The immune synapse clears and excludes molecules above a size threshold. Nat Commun. 2014;5:5479. doi: 10.1038/ncomms6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chari Ravi V.J., Martell Bridget A., Gross Jonathan L., Cook Sherrilyn B., Shah Sudhir A., Blättler Walter A. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52:127–131. [PubMed] [Google Scholar]
- 11.Cheng Ming, Ahmed Mahiuddin, Hong Xu., Cheung Nai-Kong V. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int J Cancer. 2015;136:476–486. doi: 10.1002/ijc.29007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhuri K., Wiseman D., Brown M.H., Gould K., van der Merwe P.A. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 13.Cordoba Shaun-Paul, Choudhuri Kaushik, Zhang Hao, Bridge Marcus, Basat Alp Bugra, Dustin Michael L. The large ectodomains of CD45 and CD148 regulate their segregation from and inhibition of ligated T-cell receptor. Blood. 2013;121:4295–4302. doi: 10.1182/blood-2012-07-442251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croasdale R., Wartha K., Schanzer J.M., Kuenkele K.P., Ries C., Mayer K. Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Arch Biochem Biophys. 2012;526:206–218. doi: 10.1016/j.abb.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Cuesta A.M., Sainz-Pastor N., Bonet J., Oliva B., Alvarez-Vallina L. Multivalent antibodies: when design surpasses evolution. Trends Biotechnol. 2010;28:355–362. doi: 10.1016/j.tibtech.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Dickopf S., Lauer M.E., Ringler P., Spick C., Kern P., Brinkmann U. Highly flexible, IgG-shaped, trivalent antibodies effectively target tumor cells and induce T cell-mediated killing. Biol Chem. 2019;400:343–350. doi: 10.1515/hsz-2018-0338. [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann Nele M.G., Frazer Gordon L., Asano Yukako, Stinchcombe Jane C., Griffiths Gillian M. The cytotoxic T lymphocyte immune synapse at a glance. J Cell Sci. 2016;129:2881–2886. doi: 10.1242/jcs.186205. [DOI] [PubMed] [Google Scholar]
- 18.Dustin Michael L., Chakraborty Arup K., Shaw Andrey S. Understanding the structure and function of the immunological synapse. Cold Spring Harbor Perspect Biol. 2010;2:a002311–a2411. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellerman D. Bispecific T-cell engagers: Towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods. 2019;154:102–117. doi: 10.1016/j.ymeth.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Fan Z., Lu Y., Wu X., Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269:27595–27602. [PubMed] [Google Scholar]
- 21.Garboczi D.N., Ghosh P., Utz U., Fan Q.R., Biddison W.E., Wiley D.C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 22.Huehls A.M., Coupet T.A., Sentman C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–296. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husain B., Ellerman D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs. 2018;32:441–464. doi: 10.1007/s40259-018-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J., Park G., Park J.B., Kim S., Kim H., Chung J. An anti-EGFR x cotinine bispecific antibody complexed with cotinine-conjugated duocarmycin inhibits growth of EGFR-positive cancer cells with KRAS mutations. Exp Mol Med. 2018;50:67. doi: 10.1038/s12276-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedage V., Ellerman D., Chen Y., Liang W.C., Borneo J., Wu Y. Harnessing MerTK agonism for targeted therapeutics. mAbs. 2020;12:1685832. doi: 10.1080/19420862.2019.1685832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitazawa T., Igawa T., Sampei Z., Muto A., Kojima T., Soeda T. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570–1574. doi: 10.1038/nm.2942. [DOI] [PubMed] [Google Scholar]
- 27.Kontermann R.E. Dual targeting strategies with bispecific antibodies. mAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraman Matthew, Fosh Natalie, Kmiecik Katarzyna, Everett Katy, Zimarino Carlo, Faroudi Mustapha. Abstract 2719: Dual blockade of PD-L1 and LAG-3 with FS118, a unique bispecific antibody, induces CD8+ T-cell activation and modulates the tumor microenvironment to promote antitumor immune responses. Cancer Res. 2018;78:2719. [Google Scholar]
- 29.Labrijn Aran F., Janmaat Maarten L., Reichert Janice M., Parren Paul W.H.I. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Disc. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 30.LaMotte-Mohs Ross, Shah Kalpana, Smith Doug, Gorlatov Sergey, Ciccarone Valentina, Tamura James. Abstract 3217: MGD013, a bispecific PD-1 x LAG-3 Dual-Affinity Re-Targeting (DART®) protein with T-cell immunomodulatory activity for cancer treatment. Cancer Res. 2016;76:3217–3317. [Google Scholar]
- 31.Lee J.Y., Lee H.T., Shin W., Chae J., Choi J., Kim S.H. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat Commun. 2016;7:13354. doi: 10.1038/ncomms13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenting Peter J., Denis Cécile V., Christophe Olivier D. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130:2463–2468. doi: 10.1182/blood-2017-08-801662. [DOI] [PubMed] [Google Scholar]
- 33.Li Ji, Stagg Nicola J., Johnston Jennifer, Harris Michael J., Menzies Sam A., DiCara Danielle. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell. 2017;31:383–395. doi: 10.1016/j.ccell.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljungars A., Schiott T., Mattson U., Steppa J., Hambe B., Semmrich M. A bispecific IgG format containing four independent antigen binding sites. Sci Rep. 2020;10:1546. doi: 10.1038/s41598-020-58150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anja Löffler, Kufer Peter, Lutterbüse Ralf, Zettl Florian, Daniel Peter T., Schwenkenbecher Jan M. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 36.Mandikian D., Takahashi N., Lo A.A., Li J., Eastham-Anderson J., Slaga D. Relative Target Affinities of T-Cell-Dependent Bispecific Antibodies Determine Biodistribution in a Solid Tumor Mouse Model. Mol Cancer Ther. 2018;17:776–785. doi: 10.1158/1535-7163.MCT-17-0657. [DOI] [PubMed] [Google Scholar]
- 37.Mayes P.A., Hance K.W., Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov. 2018;17:509–527. doi: 10.1038/nrd.2018.75. [DOI] [PubMed] [Google Scholar]
- 38.Milutinovic S., Kashyap A.K., Yanagi T., Wimer C., Zhou S., O'Neil R. Dual Agonist surrobody simultaneously activates death receptors DR4 and DR5 to induce cancer cell death. Mol Cancer Ther. 2016;15:114–124. doi: 10.1158/1535-7163.MCT-15-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minguet S., Swamy M., Alarcon B., Luescher I.F., Schamel W.W. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Moore Gregory L., Lee Sung-Hyung, Schubbert Suzanne, Miranda Yvonne, Rashid Rumana, Pong Erik. Tuning T cell affinity improves efficacy and safety of anti-CD38 × anti-CD3 bispecific antibodies in monkeys – a potential therapy for multiple myeloma. Blood. 2015;126:1798–1898. [Google Scholar]
- 41.Moore P.A., Zhang W., Rainey G.J., Burke S., Li H., Huang L. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 42.Niewoehner J., Bohrmann B., Collin L., Urich E., Sade H., Maier P. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 43.Offner S., Hofmeister R., Romaniuk A., Kufer P., Baeuerle P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Onnis Anna, Baldari Cosima T. Orchestration of immunological synapse assembly by vesicular trafficking. Front Cell Dev Biol. 2019:7. doi: 10.3389/fcell.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puhr H.C., Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO Open. 2019;4 doi: 10.1136/esmoopen-2018-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin Shuang, Linping Xu., Yi Ming, Shengnan Yu., Kongming Wu., Luo Suxia. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reusch U., Burkhardt C., Fucek I., Le Gall F., Le Gall M., Hoffmann K. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. mAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roda-Navarro Pedro, Álvarez-Vallina Luis. Understanding the spatial topology of artificial immunological synapses assembled in T cell-redirecting strategies: a major issue in cancer immunotherapy. Front Cell Dev Biol. 2020:7. doi: 10.3389/fcell.2019.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Root A.R., Cao W., Li B., LaPan P., Meade C., Sanford J. Development of PF-06671008, a highly potent anti-P-cadherin/anti-CD3 bispecific DART molecule with extended half-life for the treatment of cancer. Antibodies (Basel) 2016:5. doi: 10.3390/antib5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santich Brian H., Park Jeong A., Tran Hoa, Guo Hong-Fen, Huse Morgan, Cheung Nai-Kong V. Interdomain spacing and spatial configuration drive the potency of IgG-[L]-scFv T cell bispecific antibodies. Sci Transl Med. 2020;12:eaax1315. doi: 10.1126/scitranslmed.aax1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedykh S.E., Prinz V.V., Buneva V.N., Nevinsky G.A. Bispecific antibodies: design, therapy, perspectives. Drug Des Dev Ther. 2018;12:195–208. doi: 10.2147/DDDT.S151282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi S.Y., Lu Y.W., Liu Z., Stevens J., Murawsky C.M., Wilson V. A biparatopic agonistic antibody that mimics fibroblast growth factor 21 ligand activity. J Biol Chem. 2018;293:5909–5919. doi: 10.1074/jbc.RA118.001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiess C., Zhai Q., Carter P.J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Staerz U.D., Kanagawa O., Bevan M.J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- 55.Strohl William R., Naso Michael. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies. 2019;8:41. doi: 10.3390/antib8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang Haichao, Liu Yan, Yu YuZhaojin, Lin Lu, Sun Mingli, Liu Wensi. The analysis of key factors related to ADCs structural design. Front Pharmacol. 2019:10. doi: 10.3389/fphar.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibben J.G., Boerman O.C., Massuger L.F., Schijf C.P., Claessens R.A., Corstens F.H. Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab’)2 in ovarian carcinoma patients. Int J Cancer. 1996;66:477–483. doi: 10.1002/(SICI)1097-0215(19960516)66:4<477::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 58.Topp M.S., Gokbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S., Viardot A., Marks R., Diedrich H., Faul C., Reichle A., Horst H.A., Bruggemann M., Wessiepe D., Holland C., Alekar S., Mergen N., Einsele H., Hoelzer D., Bargou R.C. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 59.Wang Wei, Erbe Amy K., Hank Jacquelyn A., Morris Zachary S., Sondel Paul M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368–468. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber F., Bohrmann B., Niewoehner J., Fischer J.A.A., Rueger P., Tiefenthaler G. Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 2018;22:149–162. doi: 10.1016/j.celrep.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wuellner Ulrich, Klupsch Kristina, Buller Fabian, Attinger-Toller Isabella, Santimaria Roger, Zbinden Irene. Bispecific CD3/HER2 targeting FynomAb induces redirected T cell-mediated cytolysis with high potency and enhanced tumor selectivity. Antibodies. 2015;4:426–440. [Google Scholar]
- 63.Yang Y., Yeh S.H., Madireddi S., Matochko W.L., Gu C., Pacheco Sanchez P. Tetravalent biepitopic targeting enables intrinsic antibody agonism of tumor necrosis factor receptor superfamily members. mAbs. 2019;11:996–1011. doi: 10.1080/19420862.2019.1625662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guy Georges J., Dengl Stefan, Bujotzek Alexander, Hesse Friederike, Jens Fischer A.A., Gärtner Achim. The Contorsbody, an antibody format for agonism: Design, structure, and function. Computational and Structural Biotechnology. 2020 doi: 10.1016/j.csbj.2020.05.007. doi: 10.1016/j.csbj.2020.05.007. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]