Abstract
Objectives:
The accuracy of different bioelectrical impedance analysis (BIA) devices for assessing body composition in children with obesity is unclear. We determined the relative accuracy of two BIA devices compared to dual x-ray absorptiometry (DXA) in obese and severely obese children.
Methods:
We measured body composition in a cross-sectional study of 78 obese children by a handheld single frequency tetrapolar BIA device (Omron), a stationary multifrequency octopolar BIA device (InBody 370) and DXA. Inter-method agreement was assessed by intraclass correlations, paired t-tests, and Bland-Altman analyses.
Results:
Participants (37% female, age 14.8±2.7 years) had mean (±SD) BMI of 36.7±7.5 kg/m2, body fat percentage of 46.4±5.2% and appendicular lean mass of 22.5±6.0 kg by DXA. Intraclass correlations with DXA for body fat percentage were 0.39 and 0.87 for single frequency tetrapolar and multifrequency octopolar BIA devices, respectively. The single frequency tetrapolar BIA underestimated body fat percentage by 5.5±2.9% (p<0.0001). Differences between the multifrequency octopolar BIA and DXA for body fat percentage (−1.1±2.8%) and appendicular lean mass (−0.3±1.4 kg) were small, and 95% limits of agreement were approximately ± 5%.
Conclusions:
BIA machines vary in relative accuracy in measuring body composition in children who are obese and severely obese. The multifrequency octopolar BIA device accurately estimated body fat percentage and appendicular lean mass relative to DXA and has the advantage of point of care performance.
Keywords: Body fat percentage, appendicular lean mass, adiposity, fat mass
INTRODUCTION
Obesity is increasingly common among children with gastrointestinal and liver diseases, due to global increases in pediatric obesity (1–3). It is important to accurately assess adiposity to determine to what degree obesity may influence pathophysiology, response to treatment and/or health outcomes across a variety of conditions, including nonalcoholic fatty liver disease, liver transplantation, pancreatic disorders and inflammatory bowel disease (4–6). While body mass index (BMI) is a commonly used, indirect measure of adiposity, it cannot reliably assess body composition. Direct measurement of fat mass, lean body mass and relative adiposity (percent body fat) remains important in clinical care and in research to accurately assess severity of adiposity, its impact on disease pathophysiology, and the efficacy of interventions.
The gold standard for body composition measurements uses multi-compartment models involving estimates of weight, body volume, body density, bone mineral content and total body water, but is not feasible in clinical or community settings (7–9). Dual energy x-ray absorptiometry (DXA) is frequently used to measure body composition, as it has been shown to be relatively accurate compared to 4 compartment models (8, 10, 11). DXA provides measures of body fat mass, lean mass, bone mineral content and body fat percentage of the whole body and of sub-regions, such as the arms and legs (11). However, DXA is not portable, cannot be performed at point of care and involves radiation exposure, limiting broad application in clinical care and in community settings. Bioelectrical impedance analysis (BIA) does not suffer these limitations and is easy to use making it attractive for measurement of body composition in both clinical and non-clinical settings (12).
BIA measures body composition by applying a small alternating current to the body and measuring electrical resistance and reactance. Total body water is calculated from resistance measures and height or limb length and involves assumptions about body shape. In turn, calculation of body composition from total body water involves assumptions of intracellular fluid composition, which may vary according to age (13), disease conditions (12), and obesity (14, 15). BIA devices that utilize electrical currents at multiple frequencies may better distinguish intracellular and extracellular water compared to those using only a single frequency (12). Tetrapolar BIA devices, using 4 electrode contact points (2 per foot or hand), yield estimates of body composition of the whole body, whereas octopolar devices, using 8 electrode contact points (2 per foot and 2 per hand), can estimate both whole body composition as well as body composition of the arms and legs separately. BIA devices from different manufacturers utilize proprietary equations involving height and weight, and sometimes age, sex and assessment of athletic build or health status for estimating percent body fat, fat mass and fat free mass.
Several independent studies have shown BIA to be relatively accurate for estimating body composition in adults, which has resulted in the development of clinical guidelines for use of BIA in several populations, including healthy adults, adults at risk of malnutrition, as well as those who are overweight/obese (12, 16–18). However, the limited data assessing the accuracy of current BIA devices in overweight/obese adults have shown mixed results (19–21). Similarly, in obese and severely obese children, there is no consensus regarding the relative accuracy of various types of BIA devices (22–24). Given the increasingly high global prevalence of pediatric obesity, especially severe obesity (25), it is important to determine the accuracy of different types of BIA in affected children to assess utility in clinical care and research endeavors.
Our aim, therefore, was to examine the relative accuracy of two BIA devices, a stationary, 8 electrode, multiple frequencies (MF8 BIA) device and a handheld, single frequency, 4 electrode (SF4 BIA) device, relative to DXA for measuring body composition in obese and severely obese children. In addition, we evaluated the MF8 BIA device relative to DXA for measurement of appendicular lean mass. We chose DXA as our reference method for several reasons: it has been shown to be accurate relative to more detailed reference methods; it is commonly used as a reference method; it is used clinically for assessing body composition; and U.S reference ranges for body composition are based on DXA.(26–28)
METHODS
We conducted a cross-sectional study of children and adolescents recruited from a variety of clinics at Cincinnati Children’s Hospital that see overweight, obese and severely obese children in whom assessment of body composition information may be clinically meaningful. These included the gastroenterology/nonalcoholic fatty liver disease, pediatric weight management, adolescent bariatric surgery, lipid/hypertension and primary care clinics. We also recruited participants via hospital-wide email advertisements. Inclusion criteria were ages 8-19 years with overweight, obese or severely obese status, defined according to current pediatric criteria: overweight BMI ≥ 85th percentile for age and sex; obese BMI ≥ 95th percentile for age and sex; severely obese BMI ≥ 120% of the 95th percentile for age and sex or ≥ 35 kg/m2, whichever is lower.(29) Individuals over 400 pounds, with metal jewelry or devices that could not be removed (except for earrings and braces), and those with a history of chronic illnesses that could affect fluid status were excluded. The study was approved by the Institutional Review Board at Cincinnati Children’s Hospital, and all participants and/or their legal guardians provided informed consent and assent, as appropriate for age.
We obtained information by questionnaire about demographics, medical history, including diabetes type 2, hypertension, dyslipidemia, and current medication use. Weight was measured in duplicate and height was measured in triplicate using a wall-mounted stadiometer following standard techniques by trained study staff. Participants were lightly clothed and without shoes and stockings for measurements. Participants were instructed to fast for 12 hours with the exception of 6-8 ounces of water prior to the measurements.
We obtained BIA measurements using an octopolar (8 electrode) method employing multiple frequencies (5, 50 and 250 kHz) [MF8 BIA] (InBody 370©, Inbody USA, Cerritos, CA). Participants stood on the scale foot pads (2 electrode contact points per foot) and held a handle in each hand (containing 2 electrode contact points per hand) for about 1 minute for the BIA measurement. Height, sex, and age were entered into the device. Results were reported as lean body mass, skeletal muscle mass, body fat mass, body fat percentage, as well as the skeletal muscle mass of each extremity. The latter was summed to obtain an estimate of appendicular lean mass comparable to DXA. We also obtained BIA measurements using a handheld 4 electrode device that employs a single frequency (50 kHz) [SF4 BIA] (Omron Portable Body Fat Analyzer, Omron Healthcare, Inc., Hoffman Estates, IL). The participant held the device with both hands for approximately one minute. Height, weight, sex, and age were entered into the device and the “normal” setting (vs. “athlete”) was selected for calculation of body fat percentage. The device indicated error for body fat percentage values above 50% (device-specific limitation) for 3 participants who were subsequently excluded from the Omron analysis. We could not obtain Omron BIA measurement on 3 children who were less than 10 years of age, as the device’s lower limit for age was 10 years.
Whole body DXA scans were acquired using a Hologic® Horizon (Hologic Inc., Marlborough, MA) densitometer. The high precision mode was used to account for thicker abdomens (> 11 inches) of the overweight and obese participants as recommended by the manufacturer. Scans were analyzed using standard procedures with Hologic software version Apex 5.5. We used the NHANES body composition analysis option, which was based on a study calibrating DXA body composition estimates to those of more detailed reference methods.(26) The NHANES option yields body fat percentage estimates that are about 4.5% higher than when it is turned off. We chose this analysis option because national reference data for body composition were derived using this option, and lean and fat mass results on the DXA report are expressed as age-, sex- and race-specific percentiles compared to national reference data. Appendicular lean soft tissue was derived from the sum of lean mass in the arms and legs excluding bone mineral content. DXA scans were performed within 2 hours of the BIA measurements by technicians who were unaware of the BIA results.
Data were analyzed using SAS®, version 9.4 (SAS Institute, Cary, NC). Continuous data were summarized as mean ± standard deviation and categorical data were summarized as frequency counts and percentages. Agreement and limits of agreement between BIA and DXA was assessed by use of Pearson correlations, intraclass correlation coefficients, paired t-tests, and Bland-Altman analyses. Linear regression was used to test whether there was a differential vs. systematic bias between the BIA and DXA estimates of body composition. P <0.05 was considered statistically significant. Specific comparisons were body fat percentage and fat mass by MF8 and SF4 vs. DXA, and appendicular lean mass by MF8 vs. DXA. Sensitivity analyses were also conducted to determine if outcomes differed when the sample was restricted to white, non-Hispanic participants. The smaller sample of black and Hispanic participants precluded separate analyses in these groups.
RESULTS
Participant characteristics
We enrolled 78 participants with a mean age of 14.8 ± 2.7 years and mean BMI of 36.7 ± 7.5 kg/m2 (BMI Z-score: 2.4 ± 0.4). Seventy-eight percent of participants were severely obese (BMI ≥120% above the 95th percentile). The majority of participants were Caucasian (88%), reflecting the regional population. Participants had multiple comorbidities related to obesity including non-alcoholic fatty liver disease, diabetes mellitus type 2, hypertension, hyperlipidemia, and hypertriglyceridemia (Table 1).
Table 1:
Demographic and health characteristics of study participants.
| Participants N = 78 | |
|---|---|
| Age (years) | 14.8 ± 2.7 [8.7–19.2] |
| Sex (male) | 49 (63%) |
| Race | |
| White/Caucasian | 69 (88%) |
| Black/African American | 4 (5%) |
| Other | 5 (6%) |
| Ethnicity (Hispanic or Latino) | 21 (27%) |
| BMI (kg/m2) | 36.7 ± 7.5 |
| BMI Z-score | 2.4 ± 0.4 |
| Obese | 15 (19%) |
| Severely obese | 61 (78%) |
| Diabetes Mellitus | |
| DM type 2 | 4 (5%) |
| Pre-diabetes | 6 (8%) |
| Hypertension | 8 (10%) |
| Dyslipidemia | 29 (37%) |
| Hypertriglyceridemia | 34 (44%) |
| NAFLD | 46 (59%) |
Mean ± SD [range] or n (% of total)
Body composition
Body fat percentage by the SF4 device could not be measured for 3 participants due to body fat above 50% (device-specific limitation) and for 3 other participants who were younger than 10 years of age (device lower age limit). Thus, the analysis cohort for the SF4 device included 72 participants and for the MF8 device included all 78 participants (Table 2). Body fat percentage estimates from the SF4 and MF8 BIA devices were correlated with those from DXA with Pearson correlation coefficients, which considers the linear association, and were 0.82 and 0.90 (both considered good). The intraclass correlation coefficients, which takes into account differences in the means as well as linear association, were 0.39 (considered poor) and 0.87 (good), respectively (Figure 1a and 1b). An intraclass correlation coefficient of < 0.5 is considered to be poor, 0.75-0.90 is considered to be good, and greater than 0.90 is excellent.(30) For fat mass, the Pearson correlation coefficients were 0.97 and 0.99 and the intraclass correlation coefficients were 0.92 and 0.99 (excellent) for the SF4 and MF8 BIA devices compared to DXA, respectively (Figure 1c and 1d). Table 2 shows the mean body fat percentage and fat mass by DXA and the two BIA machines. Although the MF8 BIA fat percentage values were statistically lower than those from DXA (mean difference −1.1 ± 2.8%, p=0.001), the magnitude of this difference was relatively small. The limits of agreement, defined as ± 2 SD, were approximately −5% to +5%. In contrast, body fat percentage by the SF4 BIA device was appreciably lower than by DXA (mean difference −5.5 ± 2.9%, p<0.0001). Similarly, fat mass estimated by the SF4 device was also on average 5.7 ± 2.9 kg lower than by DXA. Restricting the sample to non-Hispanic whites did not appreciably alter the results or conclusions (data not shown). Bland-Altman analyses showed that the SF4 BIA device systematically underestimated body fat percentage and fat mass compared to DXA across the range examined (Figure 2a and 2c). In contrast, there was a small differential bias between the MF8 BIA device and DXA (Figure 2b and 2d). Agreement varied according to percentage body fat: the MF8 BIA device slightly overestimated fat percentage above 46% and slightly underestimated below 46% (p=0.0004).
Table 2:
Body Fat Percentage, Fat Mass, and Appendicular Lean Mass Measurements
| SF4 BIA n=72 |
MF8 BIA n=78 |
DXA n=78 |
Difference SF4 – DXA n=72 |
Difference MF8 - DXA n=78 |
|
|---|---|---|---|---|---|
| Body Fat Percentage (%) | 40.6 ± 4.5 [27.2–49.6] |
45.4 ± 6.3 [31.1–56.7] |
46.4 ± 5.2 [32.9–58.4] |
−5.5 ± 2.9* [−15.2–0.6] |
−1.1 ± 2.8* [−8.7–5.1] |
| Fat mass (kg) | 40.5 ± 11.6 [20.2–76.5] |
46.0 ± 16.5 [16.8–97.1] |
47.3 ± 16.2 [19.4–100.9] |
−5.7 ± 2.9* [−12.8–0.1] |
−1.3 ± 2.5* [−7.7–4.8] |
| Upper extremities (kg) | -- | 6.2 ± 1.9 [2.3–11.2] |
5.2 ± 1.5 [2.1–9.3] |
-- | 1.0 ± 0.6* [−0.2–3.1] |
| Lower extremities (kg) | -- | 16.0 ± 4.2 [6.9–29.6] |
17.3 ± 4.6 [6.5–29.6] |
-- | −1.4 ± 1.5* [−5.9–1.7] |
| Appendicular lean (kg) | -- | 22.2 ± 6.0 [9.2–40.4] |
22.5 ± 6.0 [8.6–38.1] |
-- | −0.3 ± 1.4* [−4.7–3.1] |
Mean ± SD [range],
p<0.05
Figure 1:
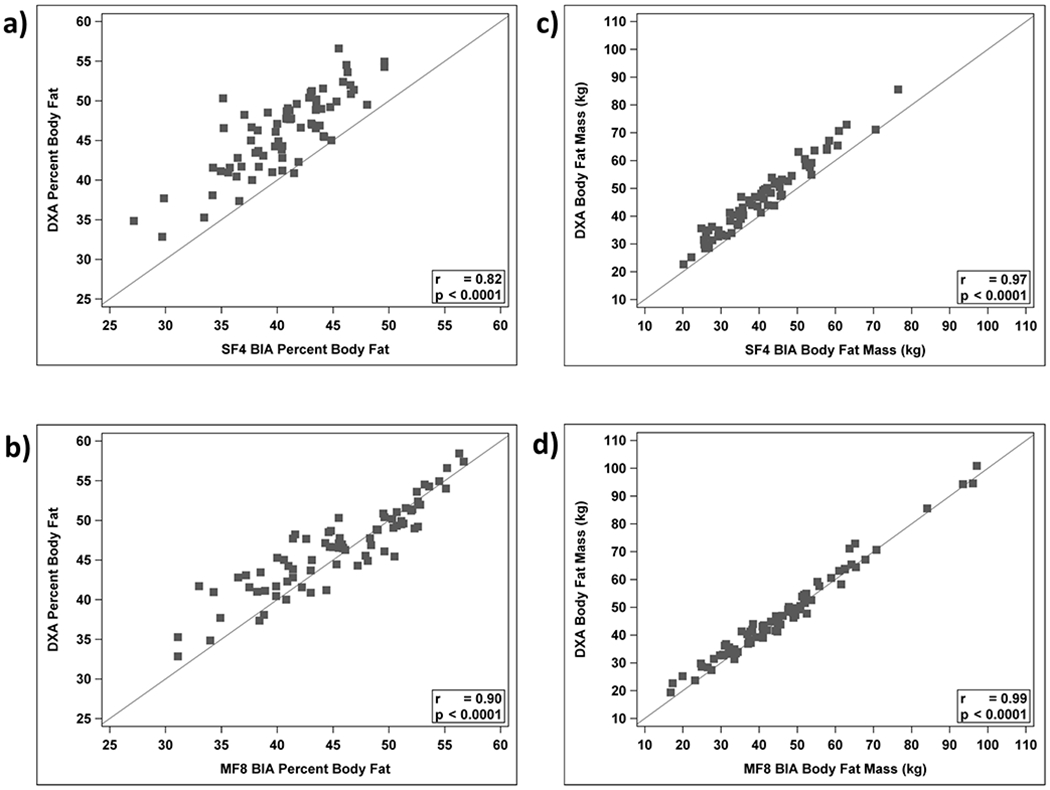
Body fat percentage by a) SF4 BIA (x-axis) b) MF8 BIA (x-axis) versus body fat percentage by DXA (y-axis). Fat mass by c) SF4 BIA (x-axis) d) MF8 BIA (x-axis) versus fat mass by DXA (y-axis). Solid line is the line of identity. Pearson correlations are listed in the graphs. Intraclass correlation coefficients are a) 0.39, b) 0.87, c) 0.92, and d) 0.99, respectively.
Figure 2:
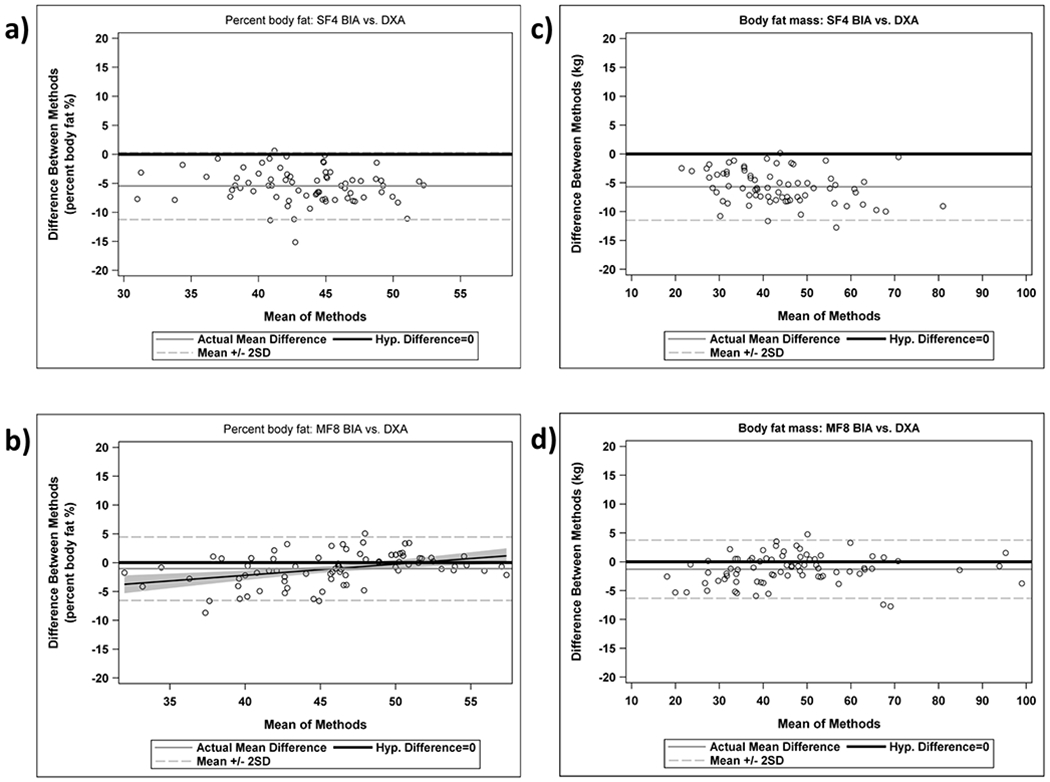
Bland Altman plots for body fat percentage for DXA versus a) SF4 BIA and b) MF8 BIA and fat mass for DXA versus c) SF4 BIA and d) MF8 BIA.
Lean mass of the arms and legs separately and combined (appendicular lean mass) estimated using the MF8 BIA device statistically differed from measurements obtained by DXA (Table 2), however the magnitude of the differences were very small. After restricting the sample to white, non-Hispanic participants, the difference in appendicular lean mass further decreased from −0.3 kg to −0.2 kg and was no longer statistically significant. Supplementary Figure 1 shows the Bland-Altman plot for the differences in the appendicular lean mass measurements, which is often used clinically as an assessment of muscle mass.
DISCUSSION
BIA is an attractive method for measuring body composition of children however the relative accuracy of diverse, yet commonly used, BIA devices in obese and severely obese children has been unclear. We found that the MF8 BIA device (InBody 370) was relatively accurate for estimating body fat percentage (mean difference from reference of −1.1% ± 2.8%) in obese and severely obese children, however the SF4 BIA device (Omron Portable Fat Analyzer) produced estimates of body fat percentage that were systematically 5.5% lower than DXA. Importantly, the handheld SF4 BIA machine, though highly portable and inexpensive, could not be used in patients less than 10 years of age or with a body fat percentage above 50%, limiting applicability in younger children or in those with severe obesity. The MF8 BIA did not have this limitation, but there was a small differential bias so that as body fat percentage increased, the MF8 BIA device slightly underestimated the body fat percentage relative to DXA. However, MF8 BIA was overall a reasonably accurate alternative to DXA for assessing body fat in obese and severely obese children, with 95% limits of agreement within approximately ± 5 for both body fat percentage and body fat mass (Figure 2). Further, the current cost of MF8 BIA devices (several thousands of dollars) are substantially lower than DXA machines that average about 10-fold higher in cost.
The MF8 BIA machine also provided a relatively accurate estimate of segmental (appendicular) lean mass compared to DXA with a mean difference of −0.3 ± 1.4 kg. This finding is advantageous as appendicular lean mass can be used monitor the impact of exercise interventions and to estimate sarcopenia. Readily accessible measurements of appendicular lean mass would facilitate pediatric studies to better understand the role of sarcopenia in mediating the risk of obesity-related conditions, such as type 2 diabetes and nonalcoholic fatty liver disease (NAFLD), a concern that has emerged in adults, but which requires further investigation in children (31, 32).
Prior studies have assessed the agreement of BIA with DXA or the multi-compartment gold standard in various populations and disease states.(18, 33, 34) However, many of these have included participants who were not obese. It is important to establish the accuracy of different types of BIA devices in obese individuals as tissue hydration (which impacts lean mass calculations) increases as adiposity increases (14, 15, 21, 35). Thus, studies assessing the accuracy in obese and severely obese individuals are important to support use of BIA in this population. A recent study of obese adults with a mean BMI of 36.1 ± 5.33 found that body fat percentage measured by two InBody MF8 BIA devices compared to DXA had 95% limits of agreement that were relatively low (< ± 5%), however a small differential bias was also observed (20).
Age also influences free fat mass hydration and needs to be considered in equations used by body composition devices (35). Prior studies have produced inconsistent findings regarding the accuracy of various BIA devices in children and adolescents. A study of obese adolescents, ages 12 to 16 years, found that whole body fat mass percentage measured by a multifrequency octopolar Tanita MC-780 BIA device was 4.8% higher than by DXA and the limits of agreement were wide (−10 to +10%) (23). In contrast, two other studies in obese/severely obese children showed an underestimation of body fat percentage using a SF4 BIA device (Omron Portable Fat Analyzer) or an alternate MF4 BIA (Tanita MC-180MA) device compared to DXA, with similarly wide limits of agreement (−15 to +5%) (22, 24). Using their MF4 BIA machine-generated resistance data with both published pediatric–specific and their own derived equations for estimating body fat and free fat mass, Wan et al. reduced systematic bias to <1% but the limits of agreement remained wide (−9 to +9%) (24). In contrast to these prior studies, we found narrower limits of agreement (~ −5 to 5%) between our MF8 BIA device (Inbody 370) and DXA results, though we also found a slight differential bias with underestimation of body fat as severity of obesity worsened. While our data supports greater relative accuracy of an MF8 BIA device vs. a SF4 device compared to DXA, it also highlights the potential for significant variation in relative accuracy across MF8 devices, which may in part be due to inherent differences in the proprietary equations across manufacturers.
While the device manufactures do not provide details on their proprietary equations and calibration methods, the 8 electrode multifrequency device has technical advantages over a 4 electrode single frequency device. First, the multifrequency device enables the discrimination between intracellular from extracellular water. Furthermore, the MF8 device had electrodes on the hands and feet enabling measurements of 5 circuits, including the whole body. In contrast, the SF4 device had electrodes only on the hands, which resulted in a single hand-to-hand circuit for impedance measures. Results from this hand-to-hand circuit is extrapolated to the whole body. Having multiple circuits enables a better characterization of the whole body.
Limitations of our study include lack of repeat measurements, which prevents us from assessing reproducibility and calculation of the least significant difference, which is important for interpreting follow-up measures within a child. Primary endpoints in interventional clinical trials in children with obesity and overweight have primarily focused on changes in BMI percentiles or z-scores. Therefore, there is no established definition of a clinically meaningful change in body fat percentage in clinical trials in children. However, in a multi-component weight loss intervention among school-aged children, the intervention group had significant mean improvement in BMI z-score of −0.43 (−0.44,−0.42), and a mean decrease in body fat percent of −5.3% (−6.4,−4.2), as assessed by DXA (both p<0.001 vs. control group). This was accompanied by significant increases in insulin sensitivity measures as assessed by oral glucose tolerance testing.(36) In comparison, we found a smaller mean difference of −1.1 ± 2.8% between the MF8-DXA measurements of body fat percentage in our study, suggesting that the measurement error is less than clinically meaningful differences. However longitudinal studies will be important to assess precision as well as accuracy of BIA vs. DXA for assessing changes in body composition in individual participants. Further application of body composition analyses in longitudinal clinical trials or cohort studies in children with obesity will also help establish clinically meaningful changes in body fat percentage and lean body mass changes associated with significant improvements in cardiovascular and metabolic health in children.
Additionally, our measurements were obtained in a fasting state as recommended by the device manufacturers, but patients are frequently not fasting in clinical settings. Obtaining measurements under non-fasting conditions may better simulate use in clinics. Participants in our study were largely Caucasian and predominantly 10 to 19 years old. Further investigation is needed in younger ages as well as other ethnicities and races, given the earlier onset of obesity in children and increasing prevalence among diverse ethnic and racial groups (25). Lastly, we did not include children with medical conditions that significantly alter body hydration, fluid distribution or the ratio of extracellular to intracellular water (e.g., congestive heart failure, cirrhosis, nephrotic syndrome, hypoalbuminemia or lymphedema) as BIA is generally not recommended for body composition assessment in these conditions.(37, 38) In children with chronic liver disease, BIA can still be considered for body composition assessment if fluid retention is not present.(39, 40)
Severe obesity now affects 1 in 10 adolescents in the United States (41). These children carry higher risk for developing comorbidities, including gastrointestinal and liver disease, and may require intensive multidisciplinary, pharmacological or surgical weight management interventions (29). Assessing changes in body composition after intervention and the relationship to health outcomes can be helpful in both clinical and research settings. In particular, in a pediatric clinical setting, favorable changes in body composition (reduced body fat, increased muscle mass) may demonstrate benefits from changes in lifestyle that may not always be reflected by changes in weight or BMI status due to ongoing growth.(42) While our findings underscore the relative accuracy of MF8 BIA in obese and severely obese children to assess adiposity and lean body mass compared to DXA, further validation in younger children less than 10 years of age, among diverse ethnicities and races, and in longitudinal analyses, are important future directions.
Supplementary Material
Supplementary Figure 1: Bland Altman plot for differences in appendicular lean mass.
WHAT IS KNOWN/WHAT IS NEW.
What is Known:
Severe obesity is increasing among children and affects health outcomes.
Measuring fat mass, lean mass, and relative adiposity is important to assess the efficacy of interventions to treat obesity-related conditions.
Bioelectrical impedance devices (BIA) utilize proprietary equations for estimating body composition but the relative accuracy in children with severe obesity is unclear.
What is New:
The relative accuracy of BIA for assessing body composition in severely obese children varies across devices.
Relative to dual energy x-ray absorptiometry, multifrequency octopolar BIA is more accurate than single frequency tetrapolar BIA for assessing body composition in severely obese children.
ACKNOWLEDGEMENTS
We would like to acknowledge our clinical research program coordinators, April Carr, Meghan McNeill, and Ann Popelar, for their help with recruitment and study visits. We also thank the Schubert Research Clinic and Bionutrition Core for their assistance in research visits. We gratefully acknowledge funding from the Clinical Center for Translational Science and Training and the National Institutes of Diabetes and Digestive and Kidney Diseases T32 NIH Training Grant.
Funding: Dr. Khan was funded by the National Institutes of Health (NIH) T32 Training Grant DK007727. This work was supported by the Clinical Center for Translational Science and Training, Clinical and Translational Science Award (CTSA) program grant 5UL1TR001425-04 from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interests: None. No support was received from the manufacturers of the devices utilized in this study.
REFERENCES
- 1.Tambucci R, Quitadamo P, Ambrosi M, et al. Association Between Obesity/Overweight and Functional Gastrointestinal Disorders in Children. J Pediatr Gastroenterol Nutr 2019;68:517–20. [DOI] [PubMed] [Google Scholar]
- 2.Koebnick C, Smith N, Black MH, et al. Pediatric obesity and gallstone disease. J Pediatr Gastroenterol Nutr 2012;55:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swenson SM, Perito ER. Weight Gain Trajectory Predicts Long-term Overweight and Obesity After Pediatric Liver Transplant. J Pediatr Gastroenterol Nutr 2019;68:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain A, Nguyen NH, Proudfoot JA, et al. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am J Gastroenterol 2019;114:630–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uc A, Zimmerman MB, Wilschanski M, et al. Impact of Obesity on Pediatric Acute Recurrent and Chronic Pancreatitis. Pancreas 2018;47:967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymsfield SB, Ebbeling CB, Zheng J, et al. Multi-component molecular-level body composition reference methods: evolving concepts and future directions. Obes Rev 2015;16:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells JC, Williams JE, Chomtho S, et al. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr 2012;96:1316–26. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZM, Pierson RN Jr., Heymsfield SB. The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr 1992;56:19–28. [DOI] [PubMed] [Google Scholar]
- 10.Sopher AB, Thornton JC, Wang J, et al. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics 2004;113:1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendler DL, Borges JL, Fielding RA, et al. The Official Positions of the International Society for Clinical Densitometry: Indications of Use and Reporting of DXA for Body Composition. J Clin Densitom 2013;16:496–507. [DOI] [PubMed] [Google Scholar]
- 12.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr 2004;23:1226–43. [DOI] [PubMed] [Google Scholar]
- 13.Wells JC, Fuller NJ, Dewit O, et al. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr 1999;69:904–12. [DOI] [PubMed] [Google Scholar]
- 14.Wells JC, Fewtrell MS, Williams JE, et al. Body composition in normal weight, overweight and obese children: matched case-control analyses of total and regional tissue masses, and body composition trends in relation to relative weight. Int J Obes (Lond) 2006;30:1506–13. [DOI] [PubMed] [Google Scholar]
- 15.Bray GA, DeLany JP, Harsha DW, et al. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children’s Study. Am J Clin Nutr 2001;73:687–702. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr 2004;23:1430–53. [DOI] [PubMed] [Google Scholar]
- 17.Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr 2019;73:194–99. [DOI] [PubMed] [Google Scholar]
- 18.McLester CN, Nickerson BS, Kliszczewicz BM, et al. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J Clin Densitom 2018. [DOI] [PubMed] [Google Scholar]
- 19.Jensky-Squires NE, Dieli-Conwright CM, Rossuello A, et al. Validity and reliability of body composition analysers in children and adults. Br J Nutr 2008;100:859–65. [DOI] [PubMed] [Google Scholar]
- 20.Nickerson BS, McLester CN, McLester JR, et al. Agreement Between 2 Segmental Bioimpedance Devices, BOD POD, and DXA in Obese Adults. J Clin Densitom 2019. [DOI] [PubMed] [Google Scholar]
- 21.Guida B, Trio R, Pecoraro P, et al. Impedance vector distribution by body mass index and conventional bioelectrical impedance analysis in obese women. Nutr Metab Cardiovasc Dis 2003;13:72–9. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh MG, Crabtree NJ, Shaw NJ, et al. Body fat estimation using bioelectrical impedance. Horm Res 2007;68:8–10. [DOI] [PubMed] [Google Scholar]
- 23.Verney J, Metz L, Chaplais E, et al. Bioelectrical impedance is an accurate method to assess body composition in obese but not severely obese adolescents. Nutr Res 2016;36:663–70. [DOI] [PubMed] [Google Scholar]
- 24.Wan CS, Ward LC, Halim J, et al. Bioelectrical impedance analysis to estimate body composition, and change in adiposity, in overweight and obese adolescents: comparison with dual-energy x-ray absorptiometry. BMC Pediatr 2014;14:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr 2005;81:1018–25. [DOI] [PubMed] [Google Scholar]
- 27.Petak S, Barbu CG, Yu EW, et al. The Official Positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom 2013;16:508–19. [DOI] [PubMed] [Google Scholar]
- 28.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
- 30.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khadra D, Itani L, Tannir H, et al. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J Diabetes 2019;10:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016;63:776–86. [DOI] [PubMed] [Google Scholar]
- 33.de Castro JAC, de Lima LRA, Silva DAS. Accuracy of octa-polar bioelectrical impedance analysis for the assessment of total and appendicular body composition in children and adolescents with HIV: comparison with dual energy X-ray absorptiometry and air displacement plethysmography. J Hum Nutr Diet 2018;31:276–85. [DOI] [PubMed] [Google Scholar]
- 34.Lee LW, Liao YS, Lu HK, et al. Validation of two portable bioelectrical impedance analyses for the assessment of body composition in school age children. PLoS One 2017;12:e0171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Deurenberg P, Wang W, et al. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr 1999;69:833–41. [DOI] [PubMed] [Google Scholar]
- 36.Harder-Lauridsen NM, Birk NM, Ried-Larsen M, et al. A randomized controlled trial on a multicomponent intervention for overweight school-aged children - Copenhagen, Denmark. BMC Pediatr 2014;14:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J 2008;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan MY, Madden AM, Jennings G, et al. Two-component models are of limited value for the assessment of body composition in patients with cirrhosis. Am J Clin Nutr 2006;84:1151–62. [DOI] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouzaki M, Bronsky J, Gupte G, et al. Nutrition Support of Children With Chronic Liver Diseases: A Joint Position Paper of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2019;69:498–511. [DOI] [PubMed] [Google Scholar]
- 41.Skinner AC, Ravanbakht SN, Skelton JA, et al. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mager DR, Iniguez IR, Gilmour S, et al. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). JPEN J Parenter Enteral Nutr 2015;39:73–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Bland Altman plot for differences in appendicular lean mass.


