Abstract
The chronic ocular toxicity, tolerability, and inflammation following corneal intrastromal injection of saline or escalating doses of an adeno-associated virus (AAV) containing a codon-optimized α-l-iduronidase (AAV-opt-IDUA) expression cassette were evaluated in New Zealand White rabbits. Corneal opacity following corneal intrastromal injection resolved by 24 h. Mild elevation of clinical ocular inflammation was observed 24 h after injection, but it returned to baseline by day 7 and no abnormalities were noted through 6 months of observation after injection. Vector genomes and IDUA cDNA were detected in the injected corneas in a dose-dependent manner. Both the lowest administered AAV-opt-IDUA dose, shown to be effective in mucopolysaccharidosis type I (MPS I) dogs, and a 10-fold higher dose of AAV-opt-IDUA resulted in no detectable immunologic response or adverse effect in rabbits. Vector genomes outside of the eye were rarely detected following corneal intrastromal injection of AAV-opt-IDUA, and neutralizing antibodies to the AAV capsid were not present at the experimental conclusion. This study, combined with our previous studies in MPS I dogs, suggests that AAV-opt-IDUA corneal gene therapy following corneal intrastromal injection of AAV-opt-IDUA has the potential to prevent and reverse blindness in MPS I patients in a safe and effective manner.
Keywords: Adeno-Associated Virus (AAV), Mucopolysaccharidosis (MPS), Gene therapy, Corneal disease, Intrastromal injection
Graphical Abstract
Intrastromal AAV-opt-IDUA resulted in no detectable immunologic response or adverse effect following corneal intrastromal injection in normal rabbits, suggesting that corneal gene therapy with AAV-opt-IDUA has the potential to prevent and reverse blindness in mucopolysaccharidosis type I patients in a safe manner.
Introduction
Mucopolysaccharidosis type I (MPS I) is a rare autosomal recessive metabolic storage disease caused by a deficiency of α-l-iduronidase (IDUA), a ubiquitous intracellular and secreted enzyme that breaks down intracellular and extracellular glycosaminoglycans (GAGs).1, 2, 3 In the absence of IDUA, GAG accumulation results in enlarged cells and thickened tissues that manifest as a progressive multisystem disease resulting in severe mental retardation, ischemic cardiac disease, ocular abnormalities (including retinopathy, optic nerve damage, and corneal opacity), and usually death in early childhood.1 The incidence of MPS I is approximately 1 in 100,000 live births,3,4 and the current standard of care for MPS I patients includes enzyme replacement therapy (ERT) or hematopoietic stem cell transplantation (HSCT), which can significantly extend their lifespan. Although these treatments ameliorate many MPS I symptoms, all MPS I patients regardless of therapeutic intervention eventually develop corneal opacity-associated vision loss.
MPS I corneal disease is characterized as a disease of the corneal stroma. As for all MPS I patient cells, the specialized quiescent cells therein lack the ability to break down heparin and dermatan sulfates, resulting in accumulation of GAGs within the cell and in the extracellular matrix.5 Accumulated GAGs disrupt the precise collagen fibril alignment, which interferes with light transmission and results in corneal opacity.5,6 Additionally, increased and disordered expression of collagen VI and the presence of alpha smooth muscle actin-positive cells, likely myofibroblasts, are also implicated in corneal opacity.7 Unlike MPS I retinopathy and optic nerve edema, which have been reported to be improved by HSCT,8 there remains no effective treatment for MPS I corneal opacity, as cornea transplantations are considered high risk in these patients and therefore have high rates of rejection.9, 10, 11
In previous work, an adeno-associated virus (AAV) vector containing a codon-optimized IDUA expression cassette (opt-IDUA) restored function to primary MPS I patient fibroblasts at both normal and supraphysiological levels.12 Additional ex vivo experiments optimized human corneal gene delivery and demonstrated no acute toxicity of AAV-opt-IDUA in human cadaver corneas.12 A single corneal intrastromal injection of AAV-opt-IDUA in a naturally occurring MPS I canine model resulted in the prevention and complete clearance of the corneal storage disease at all doses and in all animals tested.13 Remarkably, at the higher tested doses resolution of opacity was noted as early as 7 days after injection, whereas at lower doses, clearance was observed by 3 weeks following the vector injection. Corneal clarity in all dogs was maintained until the humane experimental endpoint (>6 months).13 However, an ocular inflammatory response initiating at various times after injection (i.e., 6–17 weeks) and consisting primarily of corneal edema was observed in eyes dosed with either AAV-opt-IDUA or the control vector AAV-green fluorescent protein (GFP). This inflammatory response resolved following the use of corticosteroids and did not interfere with subsequent corneal clarity or transgene expression; however, the cause of this inflammatory response was not determined and is hypothesized to be specific to the MPS I canine cornea, which demonstrates neovascularization unlike the human condition.13
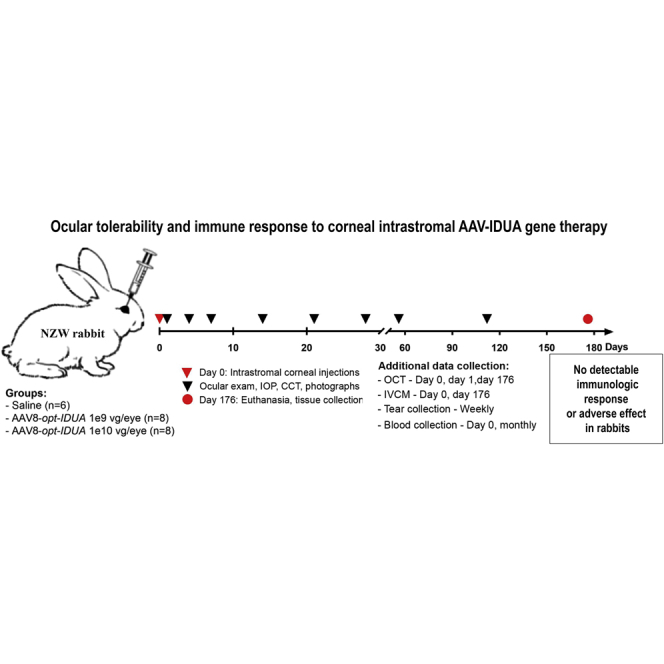
To complement the MPS I canine efficacy data and to help determine the source of the observed inflammatory response in some of the MPS I dogs, chronic ocular toxicity, tolerability, and ocular inflammation associated with the injection procedure or AAV-opt-IDUA in normal New Zealand White (NZW) rabbits were investigated. This model is ideal for corneal drug toxicity studies because NZW rabbits are a common and relevant animal model for ocular toxicology studies.14 Their eyes and corneas are similar to the human eye in both size (e.g., corneal thickness) and structure,14 and they therefore allow for close approximation of viral dosing, injection volume, and injection route, which can be subsequently performed in human clinical trials. To assess the safety of a corneal intrastromal injection, animals were monitored for clinical signs of ocular abnormalities, systemic exposure to viral particles, transgene distribution, and development of capsid serum titers (neutralizing antibodies [nAbs]) during a period of 176 days after corneal intrastromal injection of the viral vector. Results presented herein demonstrate that both the lowest administered dose shown to be effective in MPS I dogs and a 10-fold higher dose of AAV-opt-IDUA resulted in no detectable immunologic response or adverse effects in NZW rabbits. Vector genome distribution outside of the eye was rarely detected in AAV-opt-IDUA-treated animals, and nAbs to the AAV capsid were not present at the experimental conclusion. In combination with the efficacy data obtained from the MPS I dog model, the lack of ocular toxicity in NZW rabbits demonstrated herein suggests that a therapeutic approach using a single corneal intrastromal AAV-opt-IDUA injection appears safe and effective to prevent and reverse MPS I-associated corneal clouding.
Results
AAV-opt-IDUA Construct and Study Design
An AAV-opt-IDUA expression cassette, which includes a codon-optimized human IDUA cDNA (opt-IDUA), was previously described.12 The single-strand AAV cassette relies on the ubiquitous EF1α promoter, which generates the opt-IDUA transcript terminated by the SV40 polyadenylation sequence and the wild-type (WT) inverted terminal repeat serotype 2 sequence. The AAV8 capsid, previously determined to transduce the corneal stroma with high efficiency,12 was used to package the EF1α-opt-IDUA-poly(A) construct evaluated in this study.12,13,15
In prior studies, it was evident that an AAV vector solution in 50 μL results in widespread distribution of rabbit, canine, and human cadaver corneas; therefore, the experiments herein utilized 50 μL of vector solution independent of vector dose.12,13,16 Additionally, unpublished studies using AAV8-EF1α-opt-IDUA in 50 μL in the MPS I canine cornea demonstrated that 1e9 vector genomes (vg) was the lowest effective dose identified to date (data not shown). In this study, the right cornea of NZW rabbits, with a mean baseline central corneal thickness (CCT) of 377.1 ± 17.8 μm, was injected with either 50 μL of saline (n = 6), AAV-opt-IDUA at 1e9 vg (n = 8), or AAV-opt-IDUA at 1e10 vg (n = 8) using a 31G needle as described in Materials and Methods. The left eye of each animal was not dosed and served as a contralateral non-injected control. During the injection, no endothelial perforations or anterior chamber penetrations occurred; however, 15 out of 22 eyes (68%) had mild injection site surface leakage, and 1 eye (5%) had moderate injection site surface leakage (Table S1). A single needle insertion was used to perform the intrastromal injection in 19 out of 22 (86%) eyes; in the remaining three corneas, two or three needle injections were used to ensure stromal injection (Table S1). Rabbits were followed for 176 days post-injection and monitored for ocular inflammation, CCT, intraocular pressure (IOP), endothelial cell counts, complete blood counts (CBCs), serum chemistry, ocular histology, ocular transgene expression, transgene biodistribution, and NAb titers to the AAV8 capsid (serum, aqueous humor, vitreous) in order to provide a comprehensive toxicological safety profile of the AAV8-opt-IDUA therapy delivered via corneal intrastromal injection in a relevant ocular toxicology model, the NZW rabbit.
Clinical Assessment of Ocular Tolerability and Safety
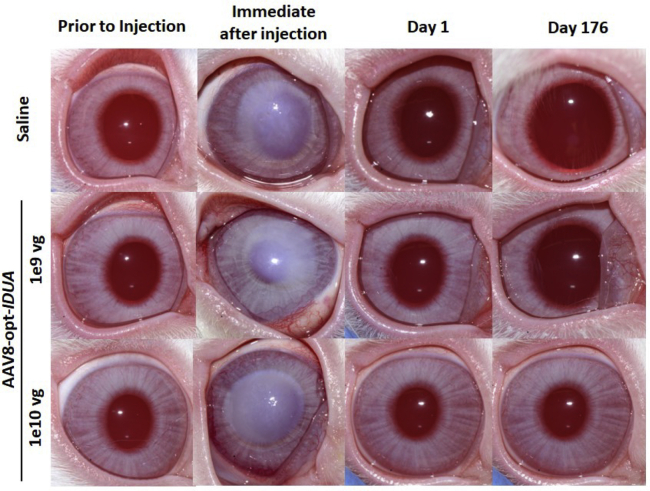
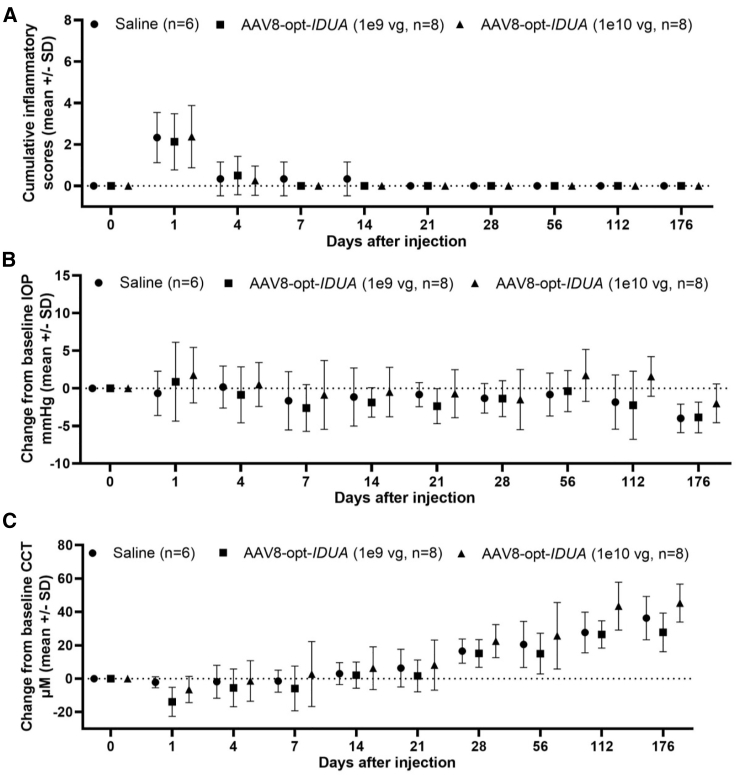
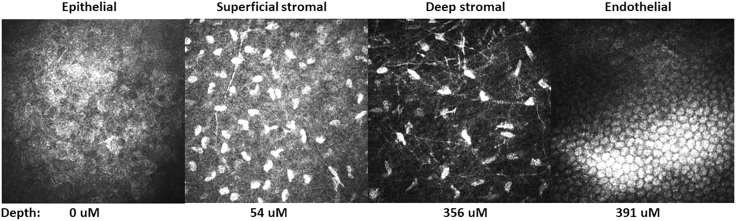
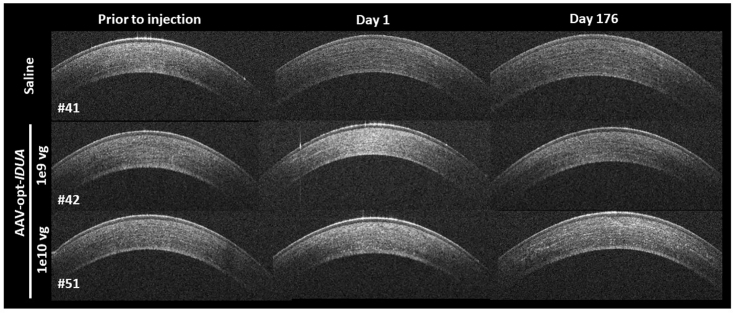
Corneal opacity, noted immediately following injection, resolved within 24 h after injection (Figure 1). Ocular inflammation was assessed by slit lamp biomicroscopy, and scores were recorded according to the Hackett-McDonald (HM) system on days 0 (prior to anesthesia and injection), 1, 4, 7, 10, 14, 18, 21, 24, and 28, and then weekly for 6 months. Mild elevation of HM scores (e.g., mild conjunctival hyperemia and chemosis) was observed within 24 h of injection (mean cumulative scores for all groups was ~2), but scores returned to baseline by day 7 (Figure 2A). No ocular abnormalities were noted throughout the remaining 6 months after injection for all groups. Mean change from baseline of IOP was not significantly different between treatment groups (Figure 2B). Mean CCT was decreased in the AAV8-opt-IDUA groups at 1 day after injection compared to baseline, but the difference was not significant and the values returned to baseline by day 4 post-injection. Due to natural development, as expected,17,18 CCT increased during the 6 months of follow-up as the animals aged and body weight increased (Figure 2C) with no significant differences between saline and AAV8-opt-IDUA groups at any time point. At 6 months following injection, no corneal abnormalities were noted during in vivo confocal microscopy in eyes of any group (Figure 3A). Endothelial counts were normal in each group and comparable to the non-injected contralateral eye, and not significantly different among the groups (Table 1). Finally, corneal optical coherence tomography (OCT) was performed prior to injection (time 0), at 24 h post-injection, and at day 176 post-injection. No corneal abnormalities were observed in any of the groups at each time point, except for artifacts as noted (Figure 4; Figure S1). A CBC and serum chemistry profile were performed prior to injection and then monthly for all rabbits. All CBC and serum chemistry profiles were within the normal rabbit reference range at all time points (Table S2), suggesting that no systemic toxicity was associated with the cornea intrastromal AAV8-opt-IDUA or vehicle injections.
Figure 1.
Clinical Photographs of Rabbit Corneas before and after Intrastromal Injection
Corneal opacity, noted immediately following the injection, resolved within 24 h. Clinical examination remained normal through 176 days following the corneal intrastromal injections. vg, vector genomes.
Figure 2.
Clinical Examination Data following Intrastromal Injection
(A–C) Inflammatory scores (A), intraocular pressure (IOP) (B), and central corneal thickness (CCT) (C) following corneal instrastromal injection with either 50 μL of saline (n = 6), AAV-IDUA at 1e9 vg (n = 8), or AAV-IDUA at 1e10 vg (n = 8) using a 31G needle. Mild inflammation was observed in all eyes following injection, which resolved by day 7, was not significantly different between groups, and did not recur during the 6-month study period. CCT and IOP also did not differ between groups. Data are presented as the Mean ± SD.
Figure 3.
In Vivo Confocal Cornea Microscopy
Representative images of the ocular surface epithelium, superficial stroma, deep stroma, and endothelium (images from group C, AAV-IDUA at 1e10 vg) at 176 days after injection. The depth of the cornea imaged is listed below each panel. There were no abnormalities noted in any treatment group.
Table 1.
Corneal Endothelial Cell Counts
| Treatment | Endothelial Counts (Mean ± SD) (per mm2) |
|---|---|
| Saline | 3,126 ± 175 |
| AAV-opt-IDUA 1e9 vg | 3,114 ± 125 |
| AAV-opt-IDUA 1e10 vg | 3,522 ± 115 |
| Non-injected eye | 3,360 ± 190 |
Figure 4.
Representative Optical Coherence Tomography (OCT) Images
OCT images of the right corneas prior to intrastromal injection, day 1 after injection, and 176 days after injection of AAV-opt-IDUA. No abnormalities were observed on the OCT scans.
AAV8-opt-IDUA Shedding in Tears
To determine whether AAV8-opt-IDUA could be shedding from the injected eyes, tear samples were collected weekly from all rabbits. Samples collected from days 7 and 14 post-injection were evaluated by probe-specific qPCR, and all groups were found negative for the opt-IDUA transgenic cassette; therefore, no additional tear samples collected at later time points were evaluated (data not shown).
Ocular Transgene Distribution and Expression
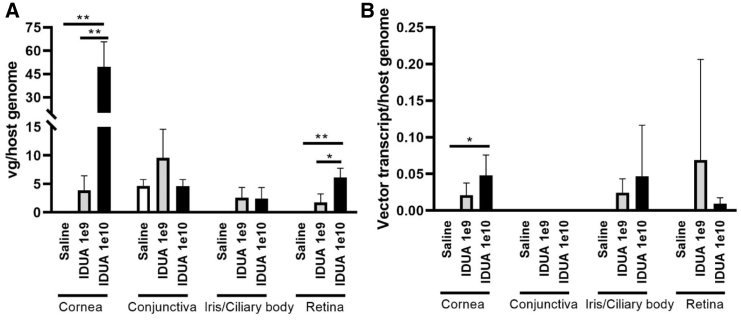
To determine vector genome distribution and transgene expression in ocular tissue, half of the injected and contralateral non-injected eyes (n = 4 in each group) were dissected, and DNA and RNA were recovered from the conjunctival, corneal, iris/ciliary body, and retina/choroidal tissues. The vg were significantly detected by qPCR, using an opt-IDUA-specific probe, in the cornea and in the retina in a dose-dependent manner (Figure 5A). Other tested ocular tissues in the escalating vector-treated groups demonstrated a non-significant trend toward increased vector detection compared to saline control (Figure 5A). No significant vector genomes were detected in the conjunctiva or iris/ciliary body, despite detection of the rabbit GAPDH DNA sequence. All non-injected corneas were negative for vector genome detection (data not shown). Next, opt-IDUA transcripts were analyzed by qRT-PCR using an opt-IDUA-specific probe. Transgenic transcripts were detected with significance only in AAV-opt-IDUA-treated corneas receiving the highest vector dose; however, non-significant trends were noted at both doses in the cornea, iris/ciliary body, and retinal tissues compared to the saline group (Figure 5B), with a trend toward increased detection at the 10-fold higher dose. No opt-IDUA transcripts were detected in the conjunctiva of any animals in the study (however, the GAPDH rabbit control transcript was observed) (Figure 5B).
Figure 5.
Quantitative Analysis of Vector Biodistribution and Transgene Expression
(A) Vector genome copy number in distinct eye compartments (conjunctiva, cornea, retina, and third eyelid) are shown as vector genome copy number/μg of host genome DNA. ∗p < 0.05 (unpaired t test, two-tailed, retina AAV-opt-IDUA 1e10 vg group versus the 1e9 vg group);∗∗p < 0.01 (unpaired t test, two-tailed, cornea AAV-opt-IDUA 1e10 vg group versus the 1e9 vg group; cornea AAV-opt-IDUA 1e10 vg group versus the saline group; cornea AAV-opt-IDUA 1e9 vg groupp versus the saline group; retina AAV-opt-IDUA 1e10 vg group versus the saline group), (B) IDUA transcript abundance examination by qRT-PCR in selected tissues is presented as vector cDNA copy number/host transcript. Data are presented as the mean ± SD. ∗p < 0.05 (unpaired t test, two-tailed, cornea AAV-opt-IDUA 1e10 vg group versus the saline group).
Peripheral Body Biodistribution of opt-IDUA
The biodistribution of persistent AAV vector genomes in non-ocular tissue following cornea intrastromal injections was determined (Table 2). Total DNA was extracted from the liver, kidney, spleen, heart, and contralateral non-injected corneas (saline, n = 6; AAV-opt-IDUA at 1e9 vg, n = 8; AAV-opt-IDUA at 1e10 vg, n = 8). Primers directed toward the transgenic cassette were used to determine vg copy number by qPCR. Three experimental replicates were performed on each tissue isolated from each rabbit; the vast majority of all replicates for all peripheral tissues were negative for vg (Table 2). All rabbits were negative for vg when DNA was isolated from the contralateral non-injected cornea, spleen, and liver. Only two rabbits had detectable vg in the kidney, one in the AAV8-opt-IDUA 1e9 vg group and one in the AAV8-opt-IDUA 1e10 vg group. A single saline-injected rabbit had relatively high levels of vg (>1e9) detected in the heart only and no other tissues, suggesting that the total DNA preparation of heart tissue isolated from this animal was likely contaminated with exogenous AAV-opt-IDUA. Taken together, these data suggest that there is minimal AAV-opt-IDUA present in peripheral organs outside of the injected ocular compartment.
Table 2.
Peripheral Organ Vector Genome Biodistribution
| Rabbit No. | Treatment | vg Detected/Organ | Rabbit No. | Treatment | vg Detected/Organ |
|---|---|---|---|---|---|
| 25 | saline | − | 46 | IDUA (1e9 vg) | − |
| 29 | saline | ++/heart | 49 | IDUA (1e9 vg) | − |
| 33 | saline | − | 53 | IDUA (1e9 vg) | +/kidney |
| 37 | saline | − | 27 | IDUA (1e10 vg) | − |
| 41 | saline | − | 31 | IDUA (1e10 vg) | − |
| 45 | saline | − | 35 | IDUA (1e10 vg) | − |
| 26 | IDUA (1e9 vg) | − | 39 | IDUA (1e10 vg) | − |
| 30 | IDUA (1e9 vg) | − | 43 | IDUA (1e10 vg) | − |
| 34 | IDUA (1e9 vg) | − | 47 | IDUA (1e10 vg) | − |
| 38 | IDUA (1e9 vg) | − | 51 | IDUA (1e10 vg) | − |
| 42 | IDUA (1e9 vg) | − | 54 | IDUA (1e10 vg) | +/kidney |
Organs assayed included non-injected cornea, spleen, liver, heart, and kidney. −, not detected in any organ; +, <2e6 vg/μg host DNA; ++, <1e9 vg/μg host DNA.
AAV8 Capsid NAb Response following Corneal Intrastromal Injection
The NAb titer to the AAV8 capsid was determined 6 months post-injection in the serum or in the aqueous and vitreous humor of the injected eyes using a previously described in vitro transduction assay.13 Only one rabbit had a serum NAb titer (1:2) to the AAV8 capsid at 50% neutralization (Table S3). Furthermore, there were no AAV8 NAbs detected in the aqueous or vitreous humor of eyes injected with saline, AAV8-opt-IDUA at 1e9 vg, or AAV8-opt-IDUA at 1e10 vg.
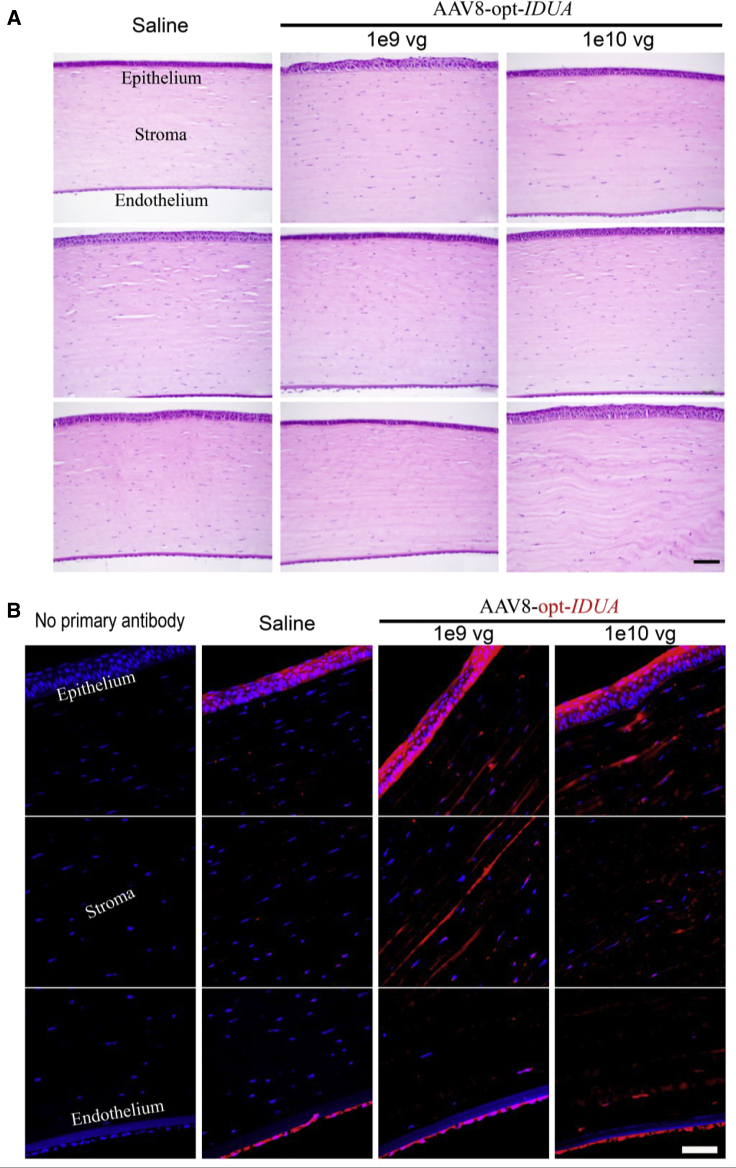
Ocular Histology and IDUA Immunofluorescence
Rabbits were euthanized 176 days after injection and tissues were harvested for further analysis, including the injected eyes, contralateral non-injected eyes, heart, liver, kidney, and spleen. Half of the number of injected eyes in each group were fixed, embedded in paraffin, and sectioned for histopathological and IDUA immunofluorescence analysis. No pathological abnormalities were observed in hematoxylin and eosin (H&E)-stained sections in any of the eyes examined for all groups (Figure 6A). Endothelial cells appeared to be intact and histologically normal. Although slight variations in the thickness of the epithelium and the stroma were observed, this was attributed to a processing/sectioning artifact, because it did not differ among any of the groups and because no abnormalities were observed on ocular examination, CCT measurement, and OCT imaging performed in vivo.
Figure 6.
Representative Ocular Histology and IDUA Immunofluorescence
(A) No abnormalities were observed on H&E staining at 6 months after intrastromal injection. Scale bar, 50 μm. (B) Immunofluorescence staining on cross-sections of the cornea given the indicated treatments. IDUA (red) and nuclei counterstaining (DAPI, blue) are shown. Scale bar, 50 μm.
In an effort to visualize the AAV-produced opt-IDUA protein distribution in the corneal tissue, immunofluorescence staining of corneal sections was performed using an anti-IDUA polyclonal antibody. As shown in Figure 6B, the saline-injected rabbit corneal samples exhibited IDUA-positive staining in the endothelial and epithelial layers of the cornea, and due to a lesser degree in the stroma, likely due to cross-reactivity of the polyclonal IDUA primary antibody to both human and rabbit IDUA. Both doses of AAV8-opt-IDUA displayed increased positive IDUA staining in the epithelial and stromal layers of the cornea compared to the saline-injected controls (Figure 6B).
Discussion
An AAV gene therapy approach is ideally suited to treat corneal opacity-associated MPS I blindness in patients and has many advantages, including the following: (1) administration that is accomplished via an outpatient corneal intrastromal injection procedure; (2) long-term transgene expression;13 (3) an immune-privileged status of the ocular compartment that lessens concerns of an adverse immune response to the viral vector; (4) the possibility of an immune response to the IDUA transgene product in MPS I human patients is significantly diminished due to their previous tolerization via HSCT; (5) it has previously been demonstrated that IDUA function can be complemented or restored via AAV gene delivery in human cadaver corneas and MPS I canines;12,13 and (6) AAV-opt-IDUA both prevented and reversed MPS I-associated corneal opacity in a dog model at an extremely low vector dose (1e9 vg, data not shown).13 In addition, as heterozygous IDUA−/+ humans have no ocular abnormalities, 50% of WT IDUA activity is sufficient for normalcy. Despite these advantages, the toxicological safety profile of AAV-opt-IDUA has yet to be rigorously assessed, and it remains a significant barrier to further drug development and clinical trial initiation.
In this study, saline, the predetermined lowest tested effective dose of AAV8-opt-IDUA (1e9 vg), or a 10-fold higher dose of AAV8-opt-IDUA (1e10 vg) was injected into the corneal stroma of normal NZW rabbits and followed for 6 months to assess the potential of AAV8-opt-IDUA to elicit acute or chronic ocular toxicity. Clinical assessments, tolerability, inflammation, endothelial cell density, CBC, serum chemistry, histopathology, IDUA transgene expression, vg biodistribution, and capsid (NAb) titers were evaluated to provide a comprehensive toxicological safety profile. Other than mild corneal surface leakage and corneal cloudiness at the time of injection, as well as mild ocular inflammation immediately following the injection, AAV8-opt-IDUA delivery was well tolerated, and the eyes of all rabbits were clear by 24 h and clinically normal within 4–7 days post-injection (Figures 1 and 2A; Table S1). Furthermore, in vivo confocal microscopy, OCT, and histopathology analyses demonstrated that all AAV8-opt-IDUA-injected corneas were structurally normal and did not differ from the group of rabbits injected with saline or non-injected, contralateral eyes.
The AAV8-opt-IDUA 1e9 vg dose is the lowest efficacious dose identified to date in MPS I canines (DNS) and, more broadly, of clinically administered AAV gene therapy to humans in any context. Other groups have observed immune responses to the AAV capsid and the foreign transgene product and promiscuous biodistribution to unintended off-target organs depending on the dose and route of injection.19 Although these observations occurred at significantly higher doses of AAV, this study aimed to determine whether the therapeutic effective lower doses of AAV8-opt-IDUA injected into the corneal stroma, as used herein, would exhibit any of these undesirable attributes. As shown in Figure 5 and Table 1, the AAV8-opt-IDUA vg and transcripts were largely relegated to the cornea with minimal vg detected outside of the ocular compartment in an inconsistent manner. Furthermore, only a single rabbit exhibited a detectable AAV8 capsid NAb titer, albeit at an extremely low dilution that is below the threshold for candidate elimination in human clinical trials (Table S3).20
The absence of peripheral tissue exposure to AAV vg, as observed in this study in rabbits, may not only be a factor of low vg dose used, but also because no anterior chamber penetrations occurred during the injection procedure. Thus, AAV vectors administered to the cornea were largely restricted to the cornea. However, some AAV vectors were significantly detected in the retina in these rabbits after corneal injection. This may be advantageous therapeutically because some MPS I patients develop retinal degeneration due to GAG accumulation and therefore may benefit from IDUA restoration.21 Furthermore, these results are similar to a separate study by our group evaluating corneal intrastromal injections with 1e10 vg of AAV8-GFP,22 where we demonstrated that anterior chamber perforation resulted in increased detection of AAV vg in nontarget tissues. Taken together, these studies highlight the importance of injection consistency and precision when performing the corneal intrastromal injections. The low effective viral vector dose, along with IDUA tolerance in HSCT-treated patients and ocular immune privilege, decreases immunological concerns to only the AAV8 capsid, which was not detected (other than a very mild capsid antibody response in a single animal).22
In conclusion, these data suggest that AAV8-opt-IDUA corneal stromal injections are well tolerated in the NZW rabbit model, which has comparable corneal anatomy to the human eye. We have demonstrated the therapeutic effect of AAV-IDUA in an MPS I canine model with remarkable MPS I phenotypic correction.13 The present study was designed as a toxicity study to determine treatment adverse effects using NZW rabbits, which do not have MPS disease (thus, no therapeutic efficacy was evaluated), and this was a limitation to the study. The AAV8-opt-IDUA corneal stromal injections appear to be well-tolerated, non-toxic, remain almost entirely in the ocular compartment, and do not appear to be released into the tear film under the tested conditions. Furthermore, the low dose of AAV8-opt-IDUA required for efficacy of 1e9 vg using the exact same construct employed herein (DNS) minimizes concerns of off-target effects and immune responses to the human IDUA protein or the AAV8 capsid itself. Given the collective body of work demonstrating efficacy in the MPS I dog model13 and the comprehensive toxicological safety profile demonstrated herein, AAV8-opt-IDUA is a leading candidate for the likely single-dose prevention and/or reversal of corneal opacity-associated blindness in MPS I patients.
Materials and Methods
All animals in this study were used in accordance with the NIH Guide for the Care and Use of Laboratory Animals with adherence to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. This study was approved and monitored by the North Carolina State University Institutional Animal Use and Care Committee (IACUC). Normal outbred male NZW rabbits (Oryctolagus cuniculus) (N = 22) were used. Rabbits were anesthetized, and 5% betadine solution, sterile saline irrigation, and a topical anesthetic (0.5% proparacaine HCl [Alcaine, Alcon Laboratories, Fort Worth, TX, USA]) were applied to the ocular surface prior to injection. All rabbits were dosed with buprenorphine (0.01–0.05 mg/kg) via subcutaneous injections at the time of corneal injection. A 31G BD ultra-fine II short needle and insulin syringe (1 cc, 5/16 inch; Becton Dickinson, Franklin Lakes, NJ, USA) was used for intrastromal injections, as previously described,22 with use of an operating microscope (Carl Zeiss OPMI VISU 200 operating microscope, Carl Zeiss, Dublin, CA, USA). The left eye was not dosed. The right eye was injected with either 50 μL of saline (n = 6), AAV8-opt-IDUA at 1e9 vg in 50 μL (n = 8), or AAV8-opt-IDUA at 1e10 vg in 50 μL (n = 8). AAV-IDUA viral constructs (see Figure 1) were prepared by the University of North Carolina (UNC) Viral Vector Core, as described previously.13
Assessments of injection leakage (mild, moderate, severe) and the presence of intracameral penetration were recorded at the time of injection for each eye. Prior to injection and on days 1, 4, 7, 10, 14, 18, 21, 24, and 28, and then weekly for 6 months, complete ophthalmic examinations using slit lamp biomicroscopy (Kowa SL-17 slit lamp, Kowa, Torrance, CA, USA), indirect ophthalmoscopy (Vantage Plus, Keeler, Malvern, PA, USA), ultrasonic pachymetry (Pachpen, Keeler, Malvern, PA, USA), and IOP (Tonovet tonometer, Icare, Vantaa, Finland) were performed. Results of examination of ocular surface morphology and anterior and posterior segment inflammation were recorded using the modified HM (i.e., without application of fluorescein) ocular scoring system.23,24 Images of all corneas were collected immediately after injection (time 0) and repeated at each examination time using digital ocular photography (Nikon D200, AF-S DX Micro NIKKOR 85mm f/3.5G lens, Nikon, Tokyo, Japan) with fixed magnification.
Rabbits were also imaged prior to injection, immediately after injection, and then at day 176 after injection using a non-contact spectral domain OCT (SD-OCT) instrument (Envisu R-class SD-OCT, Bioptigen, Morrisville, NC, USA), which contains a superluminescent light-emitting diode (SLED) delivering light at a wavelength of 840 nm. The imaging was performed using the hand-held probe of the SD-OCT device fitted with a non-contact 12-mm telecentric lens for image acquisition. After adjusting the arm reference length on the SD-OCT device by the manufacturer’s recommendations, the SD-OCT was set to 1,000 A scans per B scan, and 100 B scans in total, to generate a radial volume of 8 mm in diameter. B scans and en face reconstructed images were reviewed.
Tears (using a Schirmer tear test strip) were collected weekly after injection for evidence of virus shedding and nAbs generated to the AAV8 capsid or transgene product using a standard protocol.14 Plasma and serum were collected prior to injection and every 30 days after injection for evaluation of standard toxicological parameters, including a CBC (e.g., white count, red blood cell [RBC] count, platelets) and complete chemistry profiles (e.g., liver enzymes, albumin, blood urea nitrogen, creatinine). In addition, at necropsy, the liver, spleen, kidney, heart, and non-injected contralateral eye were harvested for vector biodistribution assays using qPCR.
On day 176, imaging from corneal epithelium to endothelium of the axial cornea was performed in both the injected and opposite-eye non-injected cornea using in vivo confocal microscopy (Heidelberg Retina Tomograph 3 with Rostock corneal module, Heidelberg Engineering, Dossenheim, Germany). Corneal endothelial counts were performed on the endothelial images using the automated feature of the Heidelberg system.
Rabbits were euthanized 176 days after injection and the eyes were collected, fixed, and analyzed histologically or via probe-based qPCR analysis for transgene expression (three eyes in the saline group and four eyes of the AAV-IDUA groups for histology and the remainder from each group for qPCR). Eyes were excised, fixed, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with H&E prior to examination by light microscopy.
Immunofluorescence (Immunohistochemistry)
Eyes were fixed with 10% formaldehyde and then processed and embedded in paraffin. Five-micrometer sections were prepared for immunofluorescence staining following a previously described method.25 Briefly, after deparaffinization, the slides were rehydrated in a series of graded alcohol baths (from 100% to 95% and then to 70%) until water. After antigen retrieval, sections were treated with blocking solution consisting of normal goat serum (10%) and Triton X-100 (0.4%) in PBS for 2 h at room temperature, and antibodies specific for IDUA (1:100 dilution, Biorbyt, orb157615) were diluted in blocking buffer and incubated with samples overnight at 4°C. After washing three times with PBS containing 0.025% Triton X-100, samples were incubated with goat anti-rabbit immunoglobulin G (IgG) (Alexa Fluor 594, 1:1,000 dilution, Abcam, Eugene, OR, USA) for 1 h at room temperature. Slides were mounted with coverslips using mounting medium containing DAPI (p36971, Invitrogen) and observed using a fluorescence microscope.
Whole-Body AAV Biodistribution
To investigate the biodistribution of the AAV8-opt-IDUA vectors delivered via intrastromal injections in the NZW rabbit model, tissue was harvested 176 days after the injections following strict tissue collection and cleaning procedures to minimize the potential for cross-contamination. Upon collection, tissues were frozen on dry ice and then stored at −80°C. DNA from the liver, kidney, spleen, lymph nodes, and brain were isolated using a DNeasy Blood & Tissue kit (QIAGEN, Valencia, CA, USA). Total DNA concentration was quantified via NanoDrop and subsequently diluted with nuclease-free water to a working concentration of 50 ng/μL. Vector genome copy number was analyzed by qPCR based on Universal Probe Library (UPL) technology, as previously described.22,26,27 Briefly, the vector-specific IDUA target was detected using UPL probe #15 and the following primers: forward, 5′-AAAGGGGGCCAGGTCTAGT-3′, reverse, 5′-ATCTGCTGAGCGACCACCT-3′. qPCR reactions were prepared with the LightCycler 480 2× Probes Master Mix (Roche, Mannheim, Germany), 0.2 μM UPL probe #26 (Roche, Mannheim, Germany), 0.5 μM forward primer, 0.5 μM reverse primer, and a total of 200 ng of template genomic DNA diluted to a final volume of 20 μL with nuclease-free water. Serial dilutions of IDUA plasmid DNA ranging from 0.1 to 1000 ng were used to generate a standard curve, and the limit of detection for this assay was 0.1 ng. Reactions were performed at an annealing temperature of 58°C on a LightCycler 480 qPCR instrument (Roche, Mannheim, Germany). All samples were performed in triplicate and the average Cp value was used to calculate the quantity of PCR product (in femtograms) present in each sample from the standard curve. Vector genome copy number was then determined using the following formula:17 Copy number = (fg × 6.022 × 1023)/(650 × size of plasmid [bp] × 1015). Vector genome copy number per nanogram of genomic DNA input was plotted in GraphPad Prism v8.2.1 for each individual tissue sample.
Quantification of Vector Biodistribution and Transgene Expression in the Eye Compartments
DNA/RNA from the cornea, conjunctiva, iris/ciliary body, and retina were extracted with the AllPrep DNA/RNA mini kit according to the kit protocol (QIAGEN, Valencia, CA, USA). Vector genome copy number and transgene expression level were quantitatively analyzed by qPCR and qRT-PCR, respectively, based on UPL technology, as previously described.16,28,29 In short, the vector-specific human leukocyte antigen (HLA)-G target was detected using UPL probe #15 and the following primers: forward, 5′-AAAGGGGGCCAGGTCTAGT-3′, reverse, 5′-ATCTGCTGAGCGACCACCT-3′. The IDUA vg copies and cDNA level were standardized against an amplicon from a single copy rabbit gene, GAPDH, amplified from genomic DNA or cDNA using the following primer set: forward, 5′-GGGTGGTGGACCTCATGGT-3′, reverse, 5′-CGGTGGTTTGAGGGCTCTTA-3′. Vector genome biodistribution data are reported as the number of vector genome copies/μg of gDNA, and the transgene expression level is reported as vector cDNA (HLA-G)/host transcript.
NAb Analysis for Serum, Aqueous Humor, and Vitreous
Serum, vitreous, and aqueous humor were collected from each rabbit prior to corneal injection (time 0) and at euthanasia (day 176 after injection). Serum NAbs to viral capsids were evaluated as previously described.30 Briefly, HEK cells were seeded in a 48-well plate at 50,000 cells/well in duplicate 24 h prior to vector plus serum transduction. Pre-injection and 176-day post-injection sera were used and serially diluted down to 1:2,048 in PBS in a volume of 13 μL and incubated with AAV8-cytomegalovirus (CMV) firefly luciferase in 13 μL of PBS for 2 h at 4°C. A serum/vector mixture was then added to the cells and a luciferase assay was performed 48 h post-transduction using a Promega luciferase assay system (Bright-Glo; Promega, Madison, WI, USA) using a PerkinElmer Victor 3 1420 multi-label counter luminometer. Results were plotted and evaluated to determine the point at which post-injection serum dilution suppressed luciferase expression to less than 50% of pre-injection levels.25,30 Vitreous and aqueous humor were treated the same as serum, with triplicate samples and serial halving dilutions in PBS ranging from 1:2 to 1:4,096.
Statistical and Data Analyses
Wilcoxon tests (nonparametric inflammatory scores) or t tests/ANOVA (parametric data, corneal thickness, IOP, vg copy numbers) were used to determine significance among the treatment groups. Differences were considered significant at p ≤ 0.05, and all probabilities and results were calculated using computerized statistical software (JMP Pro, v13.2; SAS, Cary, NC, USA).
Author Contributions
Conceptualization: M.L.H and B.C.G.; Data Curation: B.C.G., L.S., J.T.B., and T.L.; Methodology: B.C.G., M.L.H., L.S., and J.J.B.; Data Curation: Invention: M.L.H.; Funding Acquisition: B.C.G. and M.L.H.; Resources: J.H.S.; Writing – Original Draft: B.C.G., M.L.H., L.S., and J.J.B.; Writing – Review and Editing: all authors.
Conflicts of Interest
M.L.H. is a co-inventor of technology evaluated herein and has other unrelated technology licensed to Asklepios BioPharmaceuticals for which he has received royalties. B.C.G. and M.L.H. are co-founders of Bedrock Therapeutics, which has licensed an unrelated technology. The remaining authors declare no competing interests.
Acknowledgments
This study was funded by the National MPS Society (to B.C.G. and M.L.H.). Further funding was provided by the NC Biotechnology Center (to B.C.G.), the National Institutes of Health (RO1AR064369-01A1, to M.L.H.), and the NC TraCs Institute (to B.C.G. and M.L.H.). Publication costs were funded by a University Research Council Publication Grant at UNC Chapel Hill (to J.J.B.).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.014.
Supplemental Information
References
- 1.Giugliani R., Federhen A., Rojas M.V., Vieira T., Artigalás O., Pinto L.L., Azevedo A.C., Acosta A., Bonfim C., Lourenço C.M. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment. Genet. Mol. Biol. 2010;33:589–604. doi: 10.1590/S1415-47572010005000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S.A., Peracha H., Ballhausen D., Wiesbauer A., Rohrbach M., Gautschi M., Mason R.W., Giugliani R., Suzuki Y., Orii K.E. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab. 2017;121:227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum. Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 4.Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 5.Fenzl C.R., Teramoto K., Moshirfar M. Ocular manifestations and management recommendations of lysosomal storage disorders I: mucopolysaccharidoses. Clin. Ophthalmol. 2015;9:1633–1644. doi: 10.2147/OPTH.S78368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotlier E. Letter: corneal cloudiness and retinitis pigmentosa in the mucopolysaccharidoses. N. Engl. J. Med. 1975;292:812. doi: 10.1056/NEJM197504102921521. [DOI] [PubMed] [Google Scholar]
- 7.Yuan C., Bothun E.D., Hardten D.R., Tolar J., McLoon L.K. A novel explanation of corneal clouding in a bone marrow transplant-treated patient with Hurler syndrome. Exp. Eye Res. 2016;148:83–89. doi: 10.1016/j.exer.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers C.G., Purple R.L., Krivit W., Pineda R., 2nd, Copland G.T., Ramsay N.K., Kersey J.H., Whitley C.B. Ocular changes in the mucopolysaccharidoses after bone marrow transplantation. A preliminary report. Ophthalmology. 1989;96:977–984. doi: 10.1016/s0161-6420(89)32795-3. [DOI] [PubMed] [Google Scholar]
- 9.Bothun E.D., Decanini A., Summers C.G., Orchard P.J., Tolar J. Outcome of penetrating keratoplasty for mucopolysaccharidoses. Arch. Ophthalmol. 2011;129:138–144. doi: 10.1001/archophthalmol.2010.341. [DOI] [PubMed] [Google Scholar]
- 10.Di Zazzo A., Kheirkhah A., Abud T.B., Goyal S., Dana R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017;62:816–827. doi: 10.1016/j.survophthal.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohden K.L., Pitz S., Ashworth J., Magalhães A., Marinho D.R., Lindahl P., Teär Fahnehjelm K., Summers C.G. Outcomes of keratoplasty in the mucopolysaccharidoses: an international perspective. Br. J. Ophthalmol. 2017;101:909–912. doi: 10.1136/bjophthalmol-2016-308807. [DOI] [PubMed] [Google Scholar]
- 12.Vance M., Llanga T., Bennett W., Woodard K., Murlidharan G., Chungfat N., Asokan A., Gilger B., Kurtzberg J., Samulski R.J., Hirsch M.L. AAV gene therapy for MPS1-associated corneal blindness. Sci. Rep. 2016;6:22131. doi: 10.1038/srep22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyadera K., Conatser L., Llanga T.A., Carlin K., O’Donnell P., Bagel J., Song L., Kurtzberg J., Samulski R.J., Gilger B., Hirsch M.L. Intrastromal gene therapy prevents and reverses advanced corneal clouding in a canine model of mucopolysaccharidosis I. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2020.04.004. S1525-0016(20)30185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilger B., Abarca E., Salmon J. Selection of appropriate animal models in ocular research: ocular anatomy and physiology of common animal models. In: Gilger B.C., editor. Ocular Pharmacology and Toxicology. Methods in Pharmacology and Toxicology. Humana Press; 2013. pp. 7–32. [Google Scholar]
- 15.Hermonat P.L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch M.L., Conatser L.M., Smith S.M., Salmon J.H., Wu J., Buglak N.E., Davis R., Gilger B.C. AAV vector-meditated expression of HLA-G reduces injury-induced corneal vascularization, immune cell infiltration, and fibrosis. Sci. Rep. 2017;7:17840. doi: 10.1038/s41598-017-18002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Qin X., Cao X., Zhang D., Li L. Age-related variations of rabbit corneal geometrical and clinical biomechanical parameters. BioMed Res. Int. 2017;2017:3684971. doi: 10.1155/2017/3684971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riau A.K., Tan N.Y., Angunawela R.I., Htoon H.M., Chaurasia S.S., Mehta J.S. Reproducibility and age-related changes of ocular parametric measurements in rabbits. BMC Vet. Res. 2012;8:138. doi: 10.1186/1746-6148-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman B.E., Ertl H.C., Terhorst C., High K.A., Herzog R.W. Gene therapy research at the frontiers of viral immunology. Front. Microbiol. 2012;3:182. doi: 10.3389/fmicb.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Longo A., Piozzi E., Schweizer F. Ocular features in mucopolysaccharidosis: diagnosis and treatment. Ital. J. Pediatr. 2018;44(Suppl 2):125. doi: 10.1186/s13052-018-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilger B.C., Crabtree E., Song L., Llanga T., Cullen M., Blanchard A., Salmon J., Patel S., Zarnitsyn V., Hirsch M. A fixed-depth microneedle enhances reproducibility and safety for corneal gene therapy. Cornea. 2020;39:362–369. doi: 10.1097/ICO.0000000000002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackett R., McDonald T. Ophthalmic toxicology and assessing ocular irritation. In: Marzulli F., Maibach H., editors. Dermatoxicology. Fifth Edition. Hemisphere Publishing; 1996. pp. 299–305. 557–566. [Google Scholar]
- 25.Song L., Llanga T., Conatser L.M., Zaric V., Gilger B.C., Hirsch M.L. Serotype survey of AAV gene delivery via subconjunctival injection in mice. Gene Ther. 2018;25:402–414. doi: 10.1038/s41434-018-0035-6. [DOI] [PubMed] [Google Scholar]
- 26.Song L., Li X., Jayandharan G.R., Wang Y., Aslanidi G.V., Ling C., Zhong L., Gao G., Yoder M.C., Ling C. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS ONE. 2013;8:e58757. doi: 10.1371/journal.pone.0058757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L., Bower J.J., Hirsch M.L. Preparation and administration of adeno-associated virus vectors for corneal gene delivery. In: Ahearne M., editor. Corneal Regeneration: Methods and Protocols. 2020. Springer. [DOI] [PubMed] [Google Scholar]
- 28.Song L., Song Z., Fry N.J., Conatser L., Llanga T., Mei H., Kafri T., Hirsch M.L. Gene delivery to human limbal stem cells using viral vectors. Hum. Gene Ther. 2019;30:1336–1348. doi: 10.1089/hum.2019.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crabtree E., Song L., Llanga T., Bower J.J., Cullen M., Salmon J.H., Hirsch M.L., Gilger B.C. AAV-mediated expression of HLA-G1/5 reduces severity of experimental autoimmune uveitis. Sci. Rep. 2019;9:19864. doi: 10.1038/s41598-019-56462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Wu S., Albright B., Hirsch M., Li W., Tseng Y.S., Agbandje-McKenna M., McPhee S., Asokan A., Samulski R.J. Development of patient-specific aav vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol. Ther. 2016;24:53–65. doi: 10.1038/mt.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.