Abstract
Sarcocystis is a genus of eucoccidian parasites, which globally infects humans and various animals. In addition to economic losses in livestock industries, the parasite is a zoonosis that infects humans through contaminated beef and pork with the parasite sarcocysts. Therefore, this study was carried out to assess Sarcocystis contamination in beef and industrial raw beef burger samples from butcheries and retail stores in Tehran, Iran. Overall, 180 samples of 90 beefs and 90 raw industrial beef burgers with at least 80% meat were randomly collected in Tehran, Iran. Samples were studied microscopically after peptic digestion. Furthermore, sample genomic DNAs were used in conventional polymerase chain reaction (PCR) to amplify approximately 900-bp fragments from 18S ribosomal DNA. Of 180 samples, 170 samples (94.4%) were microscopically and 161 samples (89.44%) were molecularly positive for Sarcocystis spp. Eucoccidial DNA fragments were detected in 161 samples (89.4%), including 78 (86.6%) beef and 83 (92.2%) beef burger samples. No significant differences were found between the beef and beef burger infestations by Sarcocystis bradyzoites using statistical analysis (P > 0.05). Statistically significant differences were seen between the sample type and the intensity of parasites in samples (P = 0.003). Furthermore, differences between the conventional PCR results (positive/negative) and the intensity of parasites in samples were statistically significant (P < 0.001). The considerable prevalence of Sarcocystis spp. in beef and beef burger samples reflects high transmission of the parasite in meat producing cattle, which is important due to food hygiene. Although the most prevalent bovine species, S. cruzi, is not a zoonosis, it is highly recommended to follow guidelines on the parasite transmission prevention due to the existence of S. hominis as a zoonotic bovine species.
Keywords: Food safety, Food microbiology, Microbiology, Sarcocystis spp, Sarcocystosis, Beef, Beef burger, PCR
Food safety, Food microbiology, Microbiology, Sarcocystis spp, Sarcocystosis, Beef, Beef burger, PCR.
1. Introduction
Sarcocystosis is a parasitic infection caused by the members of Sarcocystis genus, which are obligate intracellular protozoan parasites belonging to Apicomplexa phylum (Dubey et al., 1989). The genus includes more than 189 species with global distribution (Poulsen and Stensvold, 2014). These cyst-forming coccidian parasites infect animals as well as humans. They include a heterogeneous prey-predator life cycle in carnivores and omnivores as definitive hosts and majorly herbivores as intermediate hosts. Definitive hosts are infected through ingestion of the parasite sarcocysts (bradyzoites) in striated-muscle tissues of the intermediate hosts and oocysts are shed in feces of the final hosts. Oocyst contaminated water and vegetables are the major sources of infection for the intermediate hosts (Dubey, 2015; Fayer, 2004). Sarcocystis is reported as the intermediate host-specific parasite. Humans can be infected with intestinal sarcocystosis through consumption of raw or under cooked beef, pork and meat products (hamburgers, sausages and hot dogs), containing bradyzoites of Sarcocystis hominis and S. suihominis (Dubey, 2015).
Sarcocystis prevalence in cattle (Bos taurus) is nearly 100% in most regions of the world (Vangeel et al., 2007; Akhlaghi et al., 2016; Pena et al., 2001; Bottner et al., 1987). Cattle represent as the intermediate hosts for a few species of the parasite, including S. cruzi, S. hirsute and S. hominis with canines, felines and primates as their definitive hosts, respectively (Dubey et al., 1989; Dubey, 2015; Heydorn et al., 1975, 1976). Another species, S. sinensis, has been reported from buffalos and cattle in China and Argentina, but its definitive host is still unknown (Gjerde, 2013; More et al., 2013; Yang et al., 2001a, 2001b). Recently, S. heydorni and S. rommeli have been identified as two newly investigated species in bovines (Dubey, 2015; Dubey et al., 2016). Sarcocystosis, with its severe economic, medical and veterinary consequences, is a major public health issue in many countries (Daryani et al., 2006). Of the bovine species, only S. hominis can survive in humans as its definitive hosts (Dubey, 2015). Infection of S. hominis in humans is usually asymptomatic and self-limiting. However, it may sometimes cause dyspnea, vomiting, bloat, nausea, stomachache, inappetence, and rapid pulse and symptoms continued up to 48 h (Bunyaratvej et al., 2007; Fayer et al., 2015). Animals infected with Sarcocystis spp. suffer from loss of weight, decreased milk production, anemia, abortion and even death in heavy infections (Fayer, 2004).
To estimate the parasite prevalence, Sarcocystis cysts or bradyzoites are detected using various methods, including histopathology, serology (IFA, ELISA), microscopy (peptic digestion, impression or squash squeezing smear) and molecular biology (More et al., 2011; Hamidinejat et al., 2010). Studies have shown that sarcocystosis in slaughtered food animals varies from 3.5 to 100% in different parts of Iran (Daryani et al., 2006; Fard et al., 2009; Nourollahi-Fard et al., 2015). Almost all of these studies have reported a high prevalence of infection in a variety of meat producing livestock, including 100% of goats (Dehaghi et al., 2011), 100% of sheep (Rahdar and Salehi, 2011), 51% of camels (Hamidinejat et al., 2013), 83% of buffalos (Oryan et al., 2010) and 96.8% of cattle (Nourollahi-Fard et al., 2015). Most of these studies have used animal samples from slaughterhouses. However, a few studies have reported Sarcocystis prevalence in beef or other meat products in Iran (Rahdar and Salehi, 2011; Khaniki and Kia, 2006; Nematollahia et al., 2015; Hajimohammadi et al., 2014; Hooshyar et al., 2017). To the best of the authors’ knowledge, no studies have been carried out on Sarcocystis prevalence in beef and beef products from butcheries and retail stores in Tehran. Therefore, the current study was carried out to assess Sarcocystis prevalence, as one of the most prevalent foodborne parasites with zoonotic potency, in beef and raw industrial beef burgers from butcheries and retail stores in Tehran using peptic digestion and conventional PCR for the first time.
2. Materials and methods
2.1. Sample collection
The current study was approved by the Ethical Committee of Tehran University of Medical Sciences. This survey was a cross-sectional study carried out from October 2016 to August 2017. A total number of 180 samples were randomly collected in Tehran, Iran, including 90 beef samples (representing 90 animals) from various butcheries and retail stores and 90 raw industrial beef burger samples (containing >80% meat according to factories) of various brands from retail stores and supermarkets. Samples were purchased from 15 butcheries and major retail stores in five city districts. Weight of samples, date of collections, trade marks, product/expiration dates and proportion of meat contents in hamburgers were recorded. Approximately 50 g of each beef sample were weighted and transferred in zipped plastic bags to the Parasitology Laboratory, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. Beef samples included posterior loin region meats of freshly slaughtered cattle, bought on various days. These samples were stored in standard refrigerators; none of them were processed with preservatives. Moreover, beef burgers were stored at -20 °C; none of them included preservatives according to the manufacturers.
2.2. Macroscopic examination
Beef and beef burger samples were cut into 3–5 mm pieces using sterile blades. These specimens were examined carefully by naked eyes for white rice grain-like Sarcocystis macrocysts.
2.3. Microscopic examination using peptic digestion
Beef samples were minced using meat grinder. Nearly 20 g of each minced sample were processed with 50 mL of the digestion solution, including 1.3 g of pepsin, 2.5 g of NaCl and 3.5 mL of concentrated HCl in 500 mL of sterile distilled water (the whole beef burgers were digested and volumes of the digestion solution were added according to the weight). Suspensions were set at room temperature for 30 min and then filtered using strainer with gauze. Filtrates were collected in sterile 50-mL tubes and centrifuged at 1500 rpm for 15 min. Pellets were washed three times with phosphate buffer saline (PBS). Smears were prepared using one droplet of the washed pellets, fixed with absolute methanol and stained with Geimsa stain. Slides were examined for Sarcocystis bradyzoites using direct light microscopy. Investigating at least 20 fields per slide, the mean number of bradyzoites per slide was calculated for the samples. Then, samples were categorized into three groups of low, medium and high parasitized samples. Samples with less than 10 parasites per slide were reported as low, 10–100 as medium and more than 100 as high parasitized samples.
2.4. DNA extraction and PCR amplification
Genomic DNA of the samples was extracted using the following protocol. Briefly, 50 mg of each sample were frozen and thawed for three times (each for 10 min). DNA was extracted using High Pure PCR Template Preparation Kit (Roche, Germany) according to the manufacturer's instructions. Extracted DNA was stored at -20 °C for PCR amplification. The extracted DNA was used as template to detect eucoccidial parasites using conventional polymerase chain reaction (PCR). Fragments of nearly 900-bp from 18S ribosomal DNA genes were amplified using single PCR and forward (SarcoFext 5′-GGTGATTCATAGTAACCCAACG-3′) and reverse (SarcoRext 5′-GATTTCTCATAAGGTGCAGGAG-3′) primers (More et al., 2014). The PCR amplification was carried out with 20-μl reaction volumes, including 10 μl of 2× Master Mix RED (Ampliqon, Denmark) with 1.5 mM of MgCl2, 1 μl of each primer at concentration of 10 pmol, 6.5 μl of sterile distilled water and 1.5 μl of the template DNA. The PCR reactions were amplified using thermal cycler (Peqlab peqSTAR, USA) with the following cycling conditions: initial hot start at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s and extension at 72 °C for 45 s. Final extension was carried out at 72 °C for 5 min. The PCR products were analyzed using electrophoresis on 1% agarose gel and UV visualization.
2.5. Statistical analysis
The SPSS Software v.16.0 (IBM Analytics, USA) and Chi-square and Mann-Whitney tests were used for data analysis. Data were described using calculation of frequencies (%) and 95% confidence intervals. In general, P-values less than 0.05 were statistically considered as significant.
3. Results
3.1. Microscopic examination
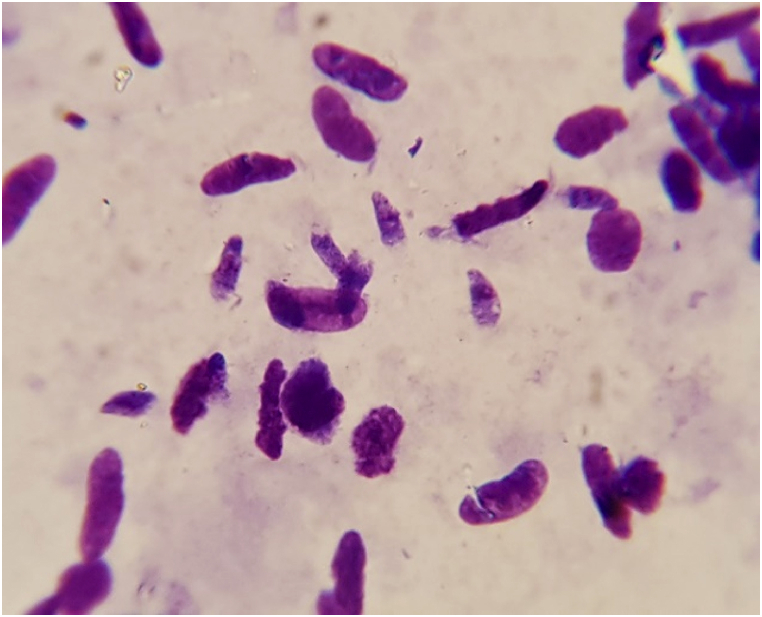
Of 180 beef and beef burger samples, Sarcocystis bradyzoites (Figure 1) were detected in 170 (94.4%) samples. At least one Sarcocystis bradyzoite was seen in 84 (93.3%) beef and 86 (95.5%) beef burger samples (Table 1). No significant differences were found between the beef and beef burger infestations by Sarcocystis bradyzoites (P > 0.05). Furthermore, the mean number of parasites per slide was calculated (Table 2). Statistically significant differences were reported between the sample type and intensity of the parasites in samples (P = 0.003).
Figure 1.
Sarcocystis bradyzoites in digested samples after staining with Geimsa.
Table 1.
Prevalence of Sarcocystis spp. in beef and beef burger samples using direct light microscopy.
| Result | No. of samples Positive (%) |
No. of samples Negative (%) |
Total (%) |
|---|---|---|---|
| Beef | 84 (93.3) | 6 (6.7) | 90 (100) |
| Beef burger | 86 (95.5) | 4 (4.5) | 90 (100) |
| Total | 170 (94.4) | 10 (5.6) | 180 (100) |
Table 2.
The mean number of Sarcocystis bradyzoites per slide.
| Mean No. of parasites/slide | No. of beef samples (%) | No. of beef burger samples (%) |
|---|---|---|
| Low | 15 (17.9) | 22 (25.6) |
| Medium | 48 (57.1) | 60 (69.8) |
| High | 21 (25) | 4 (4.6) |
| Total | 84 (100) | 86 (100) |
Low, samples with <10 parasites per slide; medium, samples with 10–100 parasites per slide; high, samples with >100 parasites per slide.
3.2. Conventional polymerase chain reaction
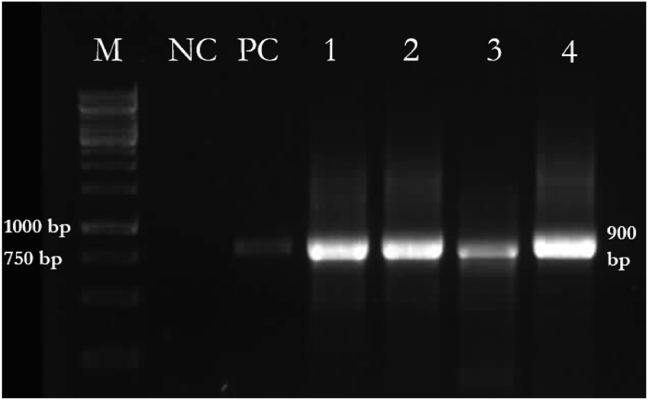
Eucoccidial DNA fragments were detected in 161 (89.4%) of the samples, including 78 (86.6%) beef and 83 (92.2%) beef burger samples (Table 3) (Figure 2). Statistical analysis showed no significant differences between the beef and beef burger infestations (P > 0.05). The mean number of parasites per slide was calculated based on the molecular test results (Table 4). High and moderate intensities of infestation were significantly correlated with positive PCR results. Differences between the conventional PCR results (positive/negative) and intensity of the parasites in samples were statistically significant (P < 0.001).
Table 3.
Prevalence of Sarcocystis spp. in beef and beef burger samples using molecular methods.
| Sample | Positive (%) | Negative (%) | Total (%) |
|---|---|---|---|
| Beef | 78 (86.6) | 12 (13.4) | 90 (100) |
| Beef burger | 83 (92.2) | 7 (7.8) | 90 (100) |
| Total | 161 (89.4) | 19 (10.6) | 180 (100) |
Figure 2.
PCR amplification of eucoccidial DNA fragments in samples. M, DNA ladder (1 kb); NC, negative control; PC, positive control; Lanes 1–4, beef and beef burger samples.
Table 4.
Comparison of the mean number of Sarcocystis bradyzoites per slide from microscopy with that from conventional PCR.
| Mean No. of parasites per slide | No. of PCR positive samples (%) | No. of PCR negative samples (%) |
|---|---|---|
| Not seen | 1 (0.6) | 9 (47.4) |
| Low | 31 (19.3) | 6 (31.6) |
| Medium | 104 (64.6) | 4 (21) |
| High | 25 (15.5) | 0 (0) |
| Total | 161 (100) | 19 (100) |
Low, samples with <10 parasites per slide; medium, samples with 10–100 parasites per slide; high, samples with >100 parasites per slide.
4. Discussion
Meat and meat products are the common sources of food within the world. Sarcocystis is globally distributed as one of the most prevalent foodborne parasites in livestock (Dubey, 2015). Surveys on the prevalence of Sarcocystis spp. in meat producing animals have shown broad infection rates. Therefore, consumption of raw or undercooked meats can transfer parasites to humans (Vangeel et al., 2007; More et al., 2014; Dubey et al., 1989). Humans serve as intermediate or definitive hosts for certain Sarcocystis species (Lindsay and Weiss, 2004). Human muscular sarcocystosis, caused by S. lindemani or S. nesbitti, is often reported from Southeast Asia (Dubey et al., 1989; Tappe et al., 2014). In contrast, humans only serve as definitive hosts for S. hominis and S. suihominis with cattle and swine as intermediate hosts, respectively (Lindsay et al., 1995). Therefore, increased knowledge of Sarcocystis prevalence in beef, pork and relative meat products can be helpful to predict the infection rate in humans and to show the necessity of improving parasite transmission prevention. Of all bovine Sarcocystis species, S. hominis and S. heydorni are zoonoses with microscopically visible cysts. Therefore, S. hominis includes public health risk in countries, where raw or under cooked beefs is traditionally consumed (Dubey, 2015). Based on the Iranian eating habits (common consumption of undercooked beefs), S. hominis infection seems important. To date, a few studies have investigated the prevalence of S. hominis in Iran. However, results of these studies are confusing and controversial. Prevalence of S. hominis in slaughtered cattle diaphragms and burgers has been reported high, including 54.4 and 57.8% respectively (Akhlaghi et al., 2016; Hajimohammadi et al., 2014). Hooshyar et al. has reported that S. hominis prevalence in burgers was 1.7% (Hooshyar et al., 2017). Therefore, further studies with improved methodologies are necessary to accurately assess S. hominis spread in Iran. In the present study, macroscopic cysts were detected neither in beef nor in beef burger samples. However, detection of macroscopic cysts was not expected due to the disposal of infected carcasses in slaughterhouses. Surveys on the prevalence of macroscopic cysts in cattle and hamburgers in Iran have reported 0–0.4 and 0% infection rates, respectively; similar to those from the current study (Faghiri et al., 2019; Hoeve-Bakker et al., 2019; Hooshyar et al., 2017; Nourollahi-Fard et al., 2015; Oryan et al., 2010; Fard et al., 2009; Najafian et al., 2008; Vangeel et al., 2007). Since felids serve as definitive hosts for bovine macroscopic cyst-forming species, a lesser contact of the cattle with fields and hence a lesser oocyst shed can explain the low macroscopic cyst prevalence in cattle. In fact, correct macroscopic cyst detection is questioned because of the cyst long formation time and because usually young cattle are slaughtered before formation of the cysts.
In this study, prevalence of the microscopic Sarcocystis spp. in beef and beef burger samples was estimated as 95.5 and 93.3% using peptic digestion and 86.6 and 92.2% using molecular methods, respectively. Although differences between the Sarcocystis prevalence rates were not statistically significant using these two methods, the peptic digestion method detected Sarcocystis spp. in both meat samples with a higher rate. Although molecular methods are regularly very accurate, PCR did not detect all parasites in the current study. This conflict could occur due to the insufficient quantities of samples (50 mg) in DNA extraction or use of conventional PCR instead of further sensitive methods such as nested PCR. However, the current study achieved much better results, compared to those similar studies with conventional PCR did. More et al. detected Sarcocystis spp. in 35.5% of cattle loin samples in Argentina using conventional PCR, while fresh examinations detected the parasite in 73.1% of the samples (More et al., 2011). The major difference between the protocol used by More et al. and that used in the present study included use of freeze and thaw steps before DNA extraction in the present study. In contrast, conventional PCR in 257 German loin samples showed a 67.7% contamination rate of Sarcocystis. The infection rate reported as 69.6% using 5 g of the loin samples and multiplex real-time PCR (More et al., 2014). Comparing the mean numbers of parasites in certain samples detected by molecular method, PCR was unable to detect parasites belonged to the low-number parasite groups. Therefore, increased sample volume in DNA extraction can greatly improve the molecular detection rate. Moreover, differences of sarcocyst infestation were not significant between the beef and beef burgers. To the best of the authors’ knowledge, no studies have been carried out on differences between the sarcocyst infestations in beef and beef burgers. Beef sarcocyst prevalence was calculated as 95.5% in the present study; similar to that in other studies from various regions of Iran and most regions of the world. Studies have reported 100% (Rahdar and Salehi, 2011; Fard et al., 2009), 96.8% (Nourollahi-Fard et al., 2015), 92.2% (Faghiri et al., 2019) and 99.9% (Najafian et al., 2008) of the parasite infections in cattle meats in Iran. In other regions of the world, 97.4% of minced beef in Belgium (Vangeel et al., 2007), 69.9% of beef in Germany and 82.7% of fresh cattle diaphragm samples in Netherlands (Hoeve-Bakker et al., 2019) have been reported as infested by sarcocysts (More et al., 2014). In the present study, 20% of beef samples were highly contaminated and results were similar to a recent study in Lithuania that estimated the beef contamination as 19.1% (Januskevicius et al., 2019). Furthermore, 93.3% of the beef burger samples were infested by sarcocysts. These were not similar to those from previous studies on hamburgers in Iran that reported less prevalence rates. Khaniki and Kia reported that 6.25% of hamburgers in Garmsar, Iran, were infested by sarcocysts using histopathology (Khaniki and Kia, 2006). Comparison of these results demonstrates that histopathological techniques are not appropriate enough to detect sarcocysts since the chance of parasite detection in a certain sections is possibly low, compared to that in 20 g of beef samples. In other studies in Iran, use of microscopic methods with peptide digestions or impression smears could detect bradyzoites in 56 (Rahdar and Salehi, 2011), 56.25 (Nematollahia et al., 2015) and 68% (Hajimohammadi et al., 2014) of the beef burger samples in Ahwaz, Tabriz and Yazd, respectively. Hooshyar et al. detected Sarcocystis spp. in 29% of hamburger samples using PCR in Kashan, Iran (Hooshyar et al., 2017). In methodology of the current study, a whole beef burger was digested to investigate bradyzoites rather than 20 g of the beef burger; therefore, this increased sample volume might be the major reason for better results. From the economic point of view, bovine sarcocystosis includes significant effects on livestock industries, including animal weight loss, milk production decrease, anemia, abortion and stillbirth (Dubey et al., 1989; Rassouli et al., 2014; Wee and Shin, 2001). Previous studies have shown toxin production by Sarcocystis bradyzoites in tissue cysts (Gracey, 1992). Although toxin production by the bovine parasite species has not been verified yet, S. fayeri tissue cysts have been shown to toxicate horsemeat by production of a 15-kDa toxin that results in food poisoning (Kamata et al., 2014). Therefore, possibility of food poisoning through these toxic meats despite their freezing and cooking (especially in heavily infected beefs) is still a big concern. In general, development of efficient hygienic techniques is necessary to minimize contamination of animal water, feed and bed with the feces of canids at lawns and farms raising cattle.
5. Conclusion
Overall, results from the current study highlight the high prevalence rate of Sarcocystis spp. in beef and raw beef burgers from butcheries and retail stores in Tehran, Iran. Although the most prevalent bovine species (S. cruzi) is not zoonotic, it is highly recommended to cook beef and beef products completely or to store them at -20 °C at least for 3–5 days before use, especially for people in high-risk groups such as immunocompromised patients, due to the existence of S. hominis as a zoonotic bovine species.
Declarations
Author contribution statement
Sara A. Mavi, Aref Teimouri: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Saeedeh Shojaee, Mostafa Rezaian, Mahboobeh Salimi: Contributed reagents, materials, analysis tools or data.
Mehdi Mohebali, Mohammad K. S. Yazdi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Hossein Keshavarz: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Tehran University of Medical Sciences and Health Services (Project No. 27780).
Competing interest statement
The authors declare no conflict of interest.
Additional information
The data that supports the findings of this study are available on reasonable request to the corresponding author.
References
- Akhlaghi M., Razavi M., Hosseini A. Molecular differentiation of bovine sarcocysts. Parasitol. Res. 2016;115:2721–2728. doi: 10.1007/s00436-016-5020-7. [DOI] [PubMed] [Google Scholar]
- Bottner A., Charleston W.A., Pomroy W.E., Rommel M. The prevalence and identity of Sarcocystis in beef cattle in New Zealand. Vet. Parasitol. 1987;24:157–168. doi: 10.1016/0304-4017(87)90036-7. [DOI] [PubMed] [Google Scholar]
- Bunyaratvej S., Unpunyo P., Pongtippan A. The Sarcocystis-cyst containing beef and pork as the sources of natural intestinal sarcocystosis in Thai people. J. Med. Assoc. Thai. 2007;90:2128–2135. [PubMed] [Google Scholar]
- Daryani A., Alaei R., Dehghan M.H., Arab R., Sharif M., Ziaei H. Survey of Sarcocystis infection in slaughtered sheep and buffaloes in Ardabil, Iran. J. Anim. Vet. Adv. 2006;1:60–62. [Google Scholar]
- Dehaghi M.M., Fathi S., Asl E.N. Survey of sarcocystis infection in slaughtered goats in Kerman abattoir, Southeast of Iran. J. Anim. Vet. Adv. 2011;10:1205–1208. [Google Scholar]
- Dubey J.P., Speer C.A., Fayer R. CRC Press; Boca Raton, FL: 1989. Sarcocystosis of Animals and Man; p. 215. [Google Scholar]
- Dubey J.P. Foodborne and waterborne zoonotic sarcocystosis. Food and Waterborne Parasitology. 2015;1:2–11. [Google Scholar]
- Dubey J.P., More G., van Wilpe E., Calero-Bernal R., Verma S.K., Schares G. Sarcocystis rommeli, n. sp. (Apicomplexa: Sarcocystidae) from cattle (Bos taurus) and its differentiation from Sarcocystis hominis. J. Eukaryot. Microbiol. 2016;63:62–68. doi: 10.1111/jeu.12248. [DOI] [PubMed] [Google Scholar]
- Faghiri E., Davari A., Nabavi R. Histopathological survey on sarcocystis species infection in slaughtered cattle of Zabol-Iran. Turk. Parazitoloji Derg. 2019;43(4):182–186. doi: 10.4274/tpd.galenos.2019.6480. [DOI] [PubMed] [Google Scholar]
- Fard S.R.N., Asghari M., Nouri F. Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Trop. Anim. Health Prod. 2009;41:1633–1636. doi: 10.1007/s11250-009-9358-z. [DOI] [PubMed] [Google Scholar]
- Fayer R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004;17:894–902. doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R., Esposito D.H., Dubey J.P. Human infections with sarcocystis species. Clin. Microbiol. Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome C oxidase subunit I gene. Int. J. Parasitol. 2013;43:579–591. doi: 10.1016/j.ijpara.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Gracey J.F. Meat Hygiene. ninth ed. Bailliere Tindall; London: 1992. p. 635. [Google Scholar]
- Hajimohammadi B., Dehghani A., Ahmadi M.M., Eslami G., Oryan A., Khamesipour A. Prevalence and species identification of Sarcocystis in raw hamburgers distributed in Yazd, Iran using PCR-RFLP. J. Food Qualt. Hazards Control. 2014;1:15–20. [Google Scholar]
- Hamidinejat H., Jalali M.H.R., Nabavi L. Survey on sarcocystis infection in slaughtered cattle in South-West of Iran, emphasized on evaluation of muscle squash in comparison with digestion method. J. Anim. Vet. Adv. 2010;9:1724–1726. [Google Scholar]
- Hamidinejat H., Hekmatimoghaddam S., Jafari H., Sazmand A., Molayan P.H., Derakhshan L., Mirabdollahi S. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd province, Iran. J. Parasit. Dis. 2013;37:163–165. doi: 10.1007/s12639-012-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A.O., Gestrich R., Mehlhorn H., Rommel M. Proposal for a new nomenclature of the Sarcosporidia. Z. Parasitenkd. 1975;48:73–82. doi: 10.1007/BF00389639. [DOI] [PubMed] [Google Scholar]
- Heydorn A.O., Gestrich R., Janitschke K. The life cycle of the Sarcosporidia. VIII. Sporocysts of Sarcocystis bovihominis in the feces of rhesus monkeys (Macaca rhesus) and baboons (Papio cynocephalus)] Berl. Münchener Tierärztliche Wochenschr. 1976;89:116–120. [PubMed] [Google Scholar]
- Hoeve-Bakker B.J.A., van der Giessen J.W.B., Franssen F.F.J. Molecular identification targeting cox1 and 18S genes confirms the high prevalence of Sarcocystis spp. in cattle in The Netherlands. Int. J. Parasitol. 2019;49:859–866. doi: 10.1016/j.ijpara.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Hooshyar H., Abbaszadeh Z., Sharafati-Chaleshtori R., Arbabi M. Molecular identification of Sarcocystis species in raw hamburgers using PCR–RFLP method in Kashan, central Iran. J. Parasit. Dis. 2017;41:1001–1005. doi: 10.1007/s12639-017-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januskevicius V., Januskeviciene G., Prakas P., Butkauskas D., Petkevicius S. Prevalence and intensity of Sarcocystis spp. Infection in animals slaughtered for food in Lithuania. Vet. Med. 2019;64(4):149–157. [Google Scholar]
- Kamata Y., Saito M., Irikura D., Yahata Y., Ohnishi T., Bessho T., Inui T., Watanabe M., Sugita-Konishi Y. A toxin isolated from Sarcocystis fayeri in raw horsemeat may be responsible for food poisoning. J. Food Protect. 2014;77:814–819. doi: 10.4315/0362-028X.JFP-13-351. [DOI] [PubMed] [Google Scholar]
- Khaniki G.J., Kia E.B. Detection of Sarcocystis cysts from meat supplied for hamburger in Iran by histological method. J. Med. Sci. 2006;6:18–21. [Google Scholar]
- Lindsay D.S., Blagburn B.L., Braund K.G. Sarcocystis spp. and sarcocystosis. Br. Med. J. 1995;5:249–254. [Google Scholar]
- Lindsay D.S., Weiss L.M. Kluwer; Boston: 2004. Opportunistic Infections: Toxoplasma, Sarcocystis, and Microsporidia; pp. 111–118. [Google Scholar]
- More G., Abrahamovich P., Jurado S., Bacigalupe D., Marin J., Rambeaud M., Venturini L., Venturini M. Prevalence of sarcocystis spp. in Argentinean cattle. Vet. Parasitol. 2011;177:162–165. doi: 10.1016/j.vetpar.2010.11.036. [DOI] [PubMed] [Google Scholar]
- More G., Schares S., Maksimov A., Conraths F.J., Venturini M.C., Schares G. Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet. Parasitol. 2013;197:85–94. doi: 10.1016/j.vetpar.2013.04.024. [DOI] [PubMed] [Google Scholar]
- More G., Pantchev A., Skuballa J., Langenmayer M., Maksimov P., Conraths F., Venturini M., Schares G. Sarcocystis sinensis is the most prevalent thick-walled Sarcocystis species in beef on sale for consumers in Germany. Parasitol. Res. 2014;113:2223–2230. doi: 10.1007/s00436-014-3877-x. [DOI] [PubMed] [Google Scholar]
- Najafian H.R., Mohebali M., Keshavarz H. Study on frequency of Sarcocystis spp. by macroscopic and microscopic methods in slaughtered cattle in Shahriar district and their public health importance. Paj Saz. 2008;77:15–19. [Google Scholar]
- Nematollahia A., Khoshkerdar A., Helan J.A., Shahbazi P., Hassanzadeh P. A study on rate of infestation to Sarcocystis cysts in supplied raw hamburgers. J. Parasit. Dis. 2015;39:276–279. doi: 10.1007/s12639-013-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourollahi-Fard S.R., Kheirandish R., Sattari S. Prevalence and histopathological finding of thin-walled and thick-walled Sarcocysts in slaughtered cattle of Karaj abattoir, Iran. J. Parasit. Dis. 2015;39:272–275. doi: 10.1007/s12639-013-0341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oryan A., Ahmadi N., Mousavi S.M.M. Prevalence, biology, and distribution pattern of Sarcocystis infection in water buffalo (Bubalus bubalis) in Iran. Trop. Anim. Health Prod. 2010;42:1513–1518. doi: 10.1007/s11250-010-9601-7. [DOI] [PubMed] [Google Scholar]
- Pena H.F., Ogassawara S., Sinhorini I.L. Occurrence of cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of Sao Paulo, Brazil, and experimental transmission to humans. J. Parasitol. 2001;87:1459–1465. doi: 10.1645/0022-3395(2001)087[1459:OOCSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Poulsen C.S., Stensvold C.R. Current status of epidemiology and diagnosis of human sarcocystosis. J. Clin. Microbiol. 2014;52:3524–3530. doi: 10.1128/JCM.00955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar M., Salehi M. The prevalence of Sarcocystis infection in meat-production by using digestion method in Ahvaz, Iran. Jundishapur J. Microbiol. 2011;4:295–300. [Google Scholar]
- Rassouli M., Ahmadpanahi J., Alvandi A. Prevalence of Sarcocystis spp. and Hammondia spp. microcysts in esophagus tissue of sheep and cattle, emphasized on their morphological differences. Parasitol. Res. 2014;113:3801–3805. doi: 10.1007/s00436-014-4047-x. [DOI] [PubMed] [Google Scholar]
- Tappe D., Stich A., Langeheinecke A., von Sonnenburg F., Muntau B., Schäfer J., Slesak G. Suspected new wave of muscular sarcocystosis in travellers returning from Tioman Island, Malaysia, May 2014. Euro Surveill. 2014;19:20816–20818. doi: 10.2807/1560-7917.es2014.19.21.20816. [DOI] [PubMed] [Google Scholar]
- Vangeel L., Houf K., Chiers K., Vercruysse J., D'Herde K., Ducatelle R. Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J. Food Protect. 2007;70:1523–1526. doi: 10.4315/0362-028x-70.6.1523. [DOI] [PubMed] [Google Scholar]
- Wee S.-H., Shin S.-S. Experimental induction of the two-host life cycle of Sarcocystis cruzi between dogs and Korean native calves. Kor. J. Parasitol. 2001;39:227–232. doi: 10.3347/kjp.2001.39.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Q., Zuo Y.X., Ding B., Chen X.W., Luo J., Zhang Y.P. Identification of Sarcocystis hominis-like (Protozoa: Sarcocystidae) cyst in water buffalo (Bubalus bubalis) based on 18S rRNA gene sequences. J. Parasitol. 2001;87:934–937. doi: 10.1645/0022-3395(2001)087[0934:IOSHLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yang Z.Q., Zuo Y.X., Yao Y.G., Chen X.W., Yang G.C., Zhang Y.P. Analysis of the 18S rRNA genes of Sarcocystis species suggests that the morphologically similar organisms from cattle and water buffalo should be considered the same species. Mol. Biochem. Parasitol. 2001;115:283–288. doi: 10.1016/s0166-6851(01)00283-3. [DOI] [PubMed] [Google Scholar]