Abstract
Upper extremity deep vein thrombosis (UEDVT) accounts for ≤10% of DVT and can be associated with morbidity and mortality. Accurate diagnosis and treatment are necessary for safe and effective patient management. We systematically reviewed the accuracy of D-dimer and duplex ultrasonography (US) for the evaluation of suspected first-episode UEDVT. We searched the Cochrane Central Register, OVID MEDLINE, EMBASE, and PubMed for eligible studies, reference lists of relevant reviews, registered trials, and relevant conference proceedings. We included prospective cross-sectional and cohort studies that evaluated test accuracy. Two investigators independently screened and collected data. The risk of bias was assessed using Quality Assessment of Diagnostic Accuracy Studies 2 and certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation framework. We pooled estimates of sensitivity and specificity. The review included 9 studies. The pooled estimates for D-dimer sensitivity and specificity were 0.96 (95% confidence interval [CI], 0.87-0.99) and 0.47 (95% CI, 0.43-0.52), respectively. The pooled estimates for duplex US sensitivity and specificity were 0.87 (95% CI, 0.73-0.94) and 0.85 (95% CI, 0.72-0.93), respectively. Certainty of evidence was moderate. In this review, we summarized the test accuracy (sensitivity and specificity) of D-dimer and duplex US for this indication. The sensitivity and specificity of the tests found in the present review should be considered in the context of whether they are used alone or in combination, which is dependent on the prevalence of disease in the population, the clinical setting in which the patient is being evaluated, cost, potential harms, and patient outcomes. This study was registered at PROSPERO as Systematic Review Registration Number CRD42018098488.
Introduction
Upper extremity deep vein thrombosis (UEDVT) is suspected in patients presenting with acute-onset pain, swelling, erythema, and functional impairment of the upper extremity. They are typically associated with malignancy and central venous access lines.1 These clinical manifestations are highly nonspecific, and objective tests are required to confirm or exclude the diagnosis. UEDVT is clinically important, since it can result in pulmonary embolism (PE) and postthrombotic syndrome, although the exact risk of PE arising from UEDVT is debated.2-4 Postthrombotic syndrome following UEDVT has a quoted incidence between 36% and 50%.4 Early diagnosis and clinical intervention are important for managing DVT and minimizing adverse consequences, as well as to exclude the diagnosis in those who do not have the disease, thereby avoiding the added costs and risks of anticoagulant therapy.
The exact incidence of DVT of the upper extremities is unknown. A prevalence of 2 cases per 1000 hospital admissions has been reported.1 Traditionally regarded as a rare entity, UEDVT is now diagnosed more frequently due to the widespread use of central venous catheters, often in relation to cancer treatment4-6 or for parenteral nutrition. Placement of cardiac implantable devices, such as pacemakers and defibrillators, can also be associated with UEDVT. Other causes include thoracic outlet syndrome, trauma, malignancy, or stasis from extrinsic obstruction. PE related to upper limb thrombosis is a rare but serious complication.7
Diagnostic modalities to identify UEDVT include D-dimer, duplex ultrasonography (US), venography, contrast-enhanced computed tomography (CT), and magnetic resonance imaging (MRI). Highly sensitive D-dimer is frequently elevated in the presence of inflammation, malignancy, and other systemic illness and thus is nonspecific, necessitating additional testing if elevated (positive) or if the clinical probability for DVT is not low. Duplex US, a noninvasive and widely available technique that uses Doppler technology to evaluate flow through vessels, has become the first-line diagnostic tool.1 It has largely replaced contrast venography for this indication, given the potential patient risks and requirement for technical expertise needed for contrast venography.
The aim of this systematic review is to determine the accuracy of commonly available diagnostic tests for DVT of the upper extremities, which can be used to inform a combined strategy for diagnosis. Pooled estimates of sensitivity and specificity obtained in this systematic review were used to model different diagnostic strategies for patients with suspected UEDVT. The results of modeling were used to inform evidence-based recommendations on diagnostic strategies for DVT in the American Society of Hematology clinical practice guidelines for diagnosis of venous thromboembolism.8
Methods
Search strategy and data sources
We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception until May 2019. We also manually searched the reference lists of relevant articles and existing reviews. Studies published in any language were included in this review. We limited the search to studies reporting data for accuracy of diagnostic tests. The complete search strategy is available in supplemental Material 1. The prespecified protocol for this review is registered with PROSPERO (CRD42018098488). This review is reported in accordance with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.9
Study selection
Studies.
Studies reporting data on diagnostic test accuracy (cohort and cross-sectional studies) for UEDVT were eligible for inclusion in this systematic review.
Participants.
Adult patients (≥18 years of age) presenting to inpatient or outpatient settings with suspected first or recurrent episode of UEDVT were eligible for inclusion.
Index tests for diagnosis.
Duplex US, quantitative high-sensitivity D-dimer assays (Vidas ELISA Assay, STA Liatest D-Di Assay, TinaQuant D-Dimer Assay, Innovance D-Dimer, and HemoSIL D-Dimer Assay) were eligible index tests for diagnosis of UEDVT.
Reference standards.
Venography and/or clinical follow-up were eligible as a reference standard for proximal compression, whole-leg, or serial US strategies. US tests and/or clinical follow-up were considered appropriate reference standards for D-dimer assays. If a reference diagnostic test was not conducted, clinical follow-up for symptoms alone was sufficient as a reference standard.
Exclusion criteria.
Patients who were asymptomatic, had superficial thrombophlebitis with no DVT, or were pregnant (addressed in separate guideline) were excluded. While studies reporting on both adult and pediatric patients were eligible for inclusion, we excluded studies with >80% of the study sample <18 years of age, or if the mean age was <25 years. When possible, we extracted data separately for adult patients from these studies.
We also excluded studies that did not provide sufficient data to determine test accuracy (sensitivity and specificity) and abstracts published prior to 2014, as the complete studies were likely published in peer-reviewed journals. Studies that used an unsuitable reference standard were excluded. D-dimer studies were excluded if they used assays that are no longer in use and/or are not highly sensitive (MDA, Asserachrom, Dimertest I, Enzygnost, Fibrinostika FbDP, Acculot, Wellcotest, or Minutex), if they used a nonquantitative assay (SimpliRed), or if they considered a positive threshold other than the defined clinical cutoffs.
Screening and data extraction
Independent reviewers conducted title and abstract screening and full-text review in duplicate to identify eligible studies. Data extraction was also conducted independently and in duplicate and verified by a third author (R.A.M.). Disagreements were resolved by discussion to reach consensus, in consultation with 2 expert clinician scientists (R.A.M. and W.L.). Data extracted included general study characteristics (authors, publication year, country, and study design), diagnostic index test and reference standard, prevalence of UEDVT, parameters to determine test accuracy (ie, sensitivity and specificity of the index test), and patient outcomes. When the same results were presented in >1 publication, we included the publication with the most complete results. If results were incomplete or unclear, we contacted study authors for additional information.
Risk of bias and certainty of evidence
We conducted the risk of bias assessment for diagnostic test accuracy studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) revised tool.10 QUADAS-2 evaluates study population and characteristics as well as patient selection to help evaluate the quality of evidence presented in the included studies.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to assess overall certainty by evaluating the evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias.11,12
Data synthesis
The accuracy estimates from individual studies were combined quantitatively (pooled) for each test using OpenMetaAnalyst (http://www.cebm.brown.edu/openmeta/). We conducted a bivariate analysis for pooling sensitivity and specificity for each of the test comparisons to account for variation within and between studies. Forest plots were created for each comparison. The Breslow-Day test was used to measure the percentage of total variation across studies due to heterogeneity (I2); however, the results did not influence our judgment of the pooled estimates, as literature has discouraged its use for test accuracy.13
Diagnostic strategies for UEDVT are based on assessment of the pretest probability (PTP) for individual patients, which provides an estimate of the expected prevalence of DVT at a population level. Prevalence estimates for UEDVT were based on 2 studies that classified patients into low/unlikely and high/likely PTP using the Constans clinical decision score.14 Low/unlikely prevalence in a sample of 457 patients was 12%, 9%, and 13% in the derivation, validation, and prospective cohort samples, respectively.15 High/likely prevalence in a sample of 406 patients was 42%.14 We used similar disease prevalence estimates to determine the absolute differences in effects among patients with clinical suspicion of UEDVT (∼10% corresponding to low PTP and 40% to high PTP). We calculated the absolute differences in effects for each comparison as true positives, true negatives, false positives, and false negatives. Here, we present the results for the low and high PTP group, with the GRADE evidence profiles provided in supplemental Material 2.
Results
Description of studies
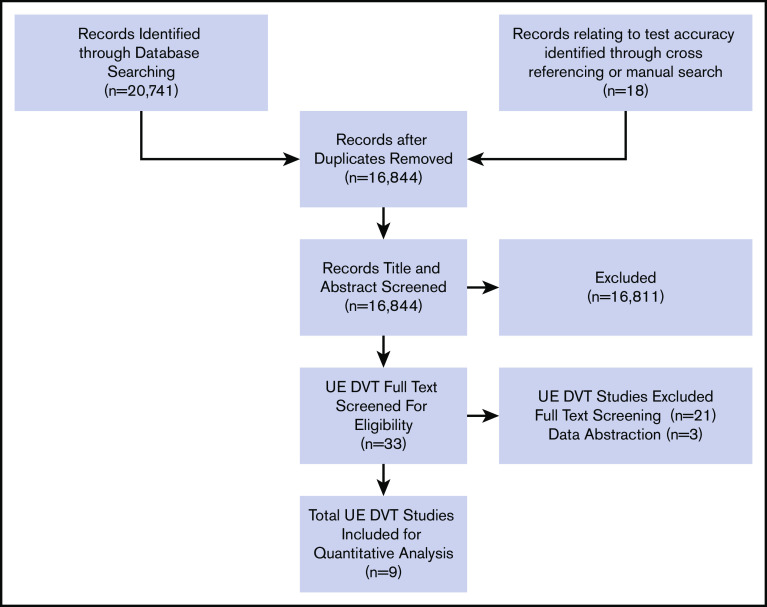
The initial search retrieved 16 844 nonduplicate studies of which 33 were included for full-text review. Following full-text review, 9 were found to be eligible for data abstraction and inclusion in the systematic review. A list of excluded studies and reasons for exclusion is provided in supplemental Material 3. Reasons for exclusion at full-text review were ineligible study design, study population, or diagnostic test, unacceptable reference standards, D-dimer assays that were not highly sensitive or used nonclinical cutoffs, evaluation of superficial thrombophlebitis, and studies that did not provide enough information to determine sensitivity and specificity. Figure 1 shows the study flow diagram for included studies.
Figure 1.
PRISMA flow diagram for study selection.
Of the included studies, 13 reported on mainly patients with first-episode UEDVT.1,2,16-22 First-episode DVT studies reported the test accuracy of 3 studies on D-dimer in comparison with a reference standard16-18 and 7 studies on duplex US for the diagnosis of DVT of the upper extremities.1,2,18-22 Studies assessing the accuracy of US used venography or contrast CT as a reference standard, with some including clinical follow-up, whereas reference standards for D-dimer were primarily US. Table 1 summarizes general characteristics of included studies, as well the index and reference standards. The majority of included studies were judged to be low risk of bias for patient selection, index test, and reference standard interpretation. Although there was unclear reporting regarding flow and timing in some studies, the certainty of evidence was generally not downgraded for risk of bias. The complete risk of bias assessment for individual studies is included in supplemental Material 4.
Table 1.
Summary of included studies for the diagnosis of suspected UEDVT
| Study | Population | Clinical setting | Index test | Reference standard |
|---|---|---|---|---|
| Baarslag et al1 | All patients with suspected UEVTE | Inpatient and Outpatients | Duplex color US | Contrast venography |
| Baxter et al2 | All patients with suspected UEVTE | Outpatients | Duplex color US | Contrast venography |
| Haire et al20 | All patients with suspected UEVTE | Inpatients | Duplex color US and MRI scan | Contrast venography |
| Kleinjan et al18 | All patients with a low and high clinical probability | Inpatients and outpatients | Low probability: D-dimer; high probability: D-dimer, US | Low probability: US or venography; high probability: Doppler US or venography |
| Koskoy et al21 | All patients with suspected UEVTE | Inpatients | Color Doppler US | Contrast venography |
| Merminod et al16 | All patients with suspected UEVTE | Inpatients and outpatients | D-dimer using rapid, highly sensitive, enzyme-linked immunosorbent assay test | Duplex US or CT scan |
| Prandoni et al19 | Patients with suspected UEVTE, and previous history of UEVTE | Inpatients and outpatients | Compression US, color flow Doppler imaging, and Doppler US | Contrast venography |
| Sartori et al17 | All patients with suspected UEVTE | Outpatients | D-dimer testing (cutoff value ≤500 ng/mL) | B-mode and color Doppler US |
| Sottiurai et al22 | All patients with suspected UEVTE | Not reported | Doppler US, phleboreography | Venography |
UEVTE, upper extremity venous thromboembolism.
D-dimer
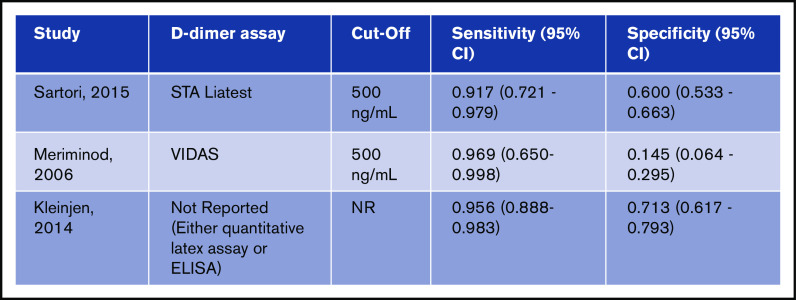
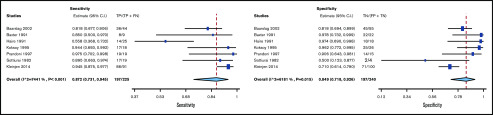
Test accuracy for D-dimer was pooled from 3 studies, with a total of 482 participants.16-18 The pooled estimates for D-dimer sensitivity and specificity were 0.96 (95% confidence interval [CI], 0.87-0.99) and 0.47 (95% CI, 0.43-0.52), respectively. Figure 2 shows the sensitivity and specificity from individual studies and pooled estimates.
Figure 2.
Pooled sensitivity and specificity of D-dimer for diagnosis of UEDVT.
Using the pooled sensitivity and specificity of the included studies, D-dimer results were modeled for 1000 patients from both a low- and high-prevalence population undergoing the test. Table 2 demonstrates this extrapolated data. Overall, the test was shown to be highly sensitive and poorly specific, and the certainty of evidence was moderate. Table 2 shows the summary of findings.
Table 2.
D-dimer test accuracy in a low- and high-prevalence population
| Test result | Number of results per 1000 patients tested (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|
| Prevalence 10%* | Prevalence 40%*† | |||
| True positives | 96 (87-99) | 385 (348-396) | 482 (3) | ⨁⨁⨁◯ Moderate‡§ |
| False negatives | 4 (1-13) | 15 (4-52) | ||
| True negatives | 425 (384-467) | 284 (256-312) | 482 (3) | ⨁⨁⨁◯ Moderate‡§ |
| False positives | 475 (433-516) | 316 (286-344) | ||
| Inconclusive test results | Not reported | |||
| Complications arising from the diagnostic test | Not reported | |||
An interactive summary of findings table is available at: https://gdt.gradepro.org/presentations/#/isof/isof_257cf98c-83bb-488f-b8dd-3c57549fb78e-1590262290785?_k=ad41h5. Patient or population: patients with suspected UEDVT. Setting: inpatient and outpatient. Pooled sensitivity: 0.96 (95% CI, 0.87-0.99). Pooled specificity: 0.47 (95% CI, 0.43-0.52).
Data from Constans et al.14
Data from Kleinjan et al.18
Not downgraded for risk of bias, although few studies had unclear information on the standard reference test.
Downgraded for imprecision; small number of patients included in studies.
Duplex US
Test accuracy for duplex US was pooled from 7 studies, with a total of 465 participants.1,2,18-22 The pooled estimates for duplex US sensitivity and specificity were 0.87 (95% CI, 0.73-0.94) and 0.85 (95% CI, 0.72-0.93), respectively. Figure 3 shows the forest plot displaying the sensitivity and specificity from individual studies and pooled estimates.
Figure 3.
Pooled sensitivity and specificity of duplex US for diagnosis of UEDVT. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Using the pooled sensitivity and specificity of the included studies, duplex US results were illustrated for 1000 patients from both a low and high prevalence population undergoing the test. Table 3 demonstrates this extrapolated data. Overall, the test was shown to be highly sensitive and specific, and the certainty of evidence was moderate. Table 3 shows the summary of findings.
Table 3.
Duplex US test accuracy in a low- and high-prevalence population
| Test result | Number of results per 1000 patients tested (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|
| Prevalence 10%* | Prevalence 40%*† | |||
| True positives | 87 (73-95) | 349 (292-378) | 465 (7) | ⨁⨁⨁◯ Moderate‡§ |
| False negatives | 13 (5-27) | 51 (22-108) | ||
| True negatives | 764 (646-833) | 509 (431-556) | 465 (7) | ⨁⨁⨁◯ Moderate‡§ |
| False positives | 136 (67-254) | 91 (44-169) | ||
| Inconclusive test results | Not reported | |||
| Complications arising from the diagnostic test | Not reported | |||
An interactive summary of findings table is available at: https://gdt.gradepro.org/presentations/#/isof/isof_fb2275e1-b029-4dda-a58b-0e5992c809ac-1590262303875?_k=zs2fxi. Patient or population: Patients with suspected UEDVT. New test: Duplex US. Setting: Inpatient and outpatient. Pooled sensitivity: 0.87 (95% CI, 0.73-0.94). Pooled specificity: 0.85 (95% CI, 0.72-0.93)
Data from Constans et al.14
Data from Kleinjan et al.18
Not downgraded for risk of bias, though few studies had unclear information on standard reference test.
One study (Haire et al20) had a wide CI for sensitivity and specificity not overlapping with other studies. In consideration of the inconsistency and imprecision, we downgraded by 1 level.
Discussion
This review presents pooled estimates of test accuracy for commonly available diagnostic methods for DVT of the upper extremities. Moderate quality of evidence was observed with both duplex US and D-dimer. D-dimer had the higher sensitivity at 0.96 (95% CI, 0.87-0.99), with duplex US having a pooled sensitivity of 0.87 (95% CI: 0.73 to 0.94). We did not identify specific data for cost-effectiveness in using D-dimer to diagnose UEDVT. Extrapolating the data from lower extremity DVT, D-dimer can be a cost-effective and accessible approach for excluding DVT in patients with low PTP.23 However, the specificity of D-dimer testing (0.47; 95% CI, 0.43-0.52) is low and, therefore, a positive result must be followed with a more specific diagnostic test (usually US).
This review has several strengths. The comprehensive and systematic approach for identifying studies makes it unlikely that relevant studies were missed. Additionally, we did not limit our review by language and translated articles that were not published in English. All steps, including initial screening, study selection, and data abstraction, were performed independently and in duplicate to minimize any potential biases. Finally, we assessed the certainty of evidence in this area and identified sources of bias.
We note a few limitations in this systematic review. Emerging modalities such as MRI and CT were not included in this review, as limited data are available. Highly sensitive D-dimer is frequently elevated in the presence of inflammation, malignancy, and other systemic illness. Given that patients with upper and lower extremity DVT often have systemic illness and malignancy, specificity of D-dimer is significantly decreased in this population. In addition, it should be noted that not all included patients in the studies had a reference standard of venography. Often, US tests or clinical follow-up was considered acceptable reference standard due to the limited amount of data published on UEDVT. In instances where positive duplex US was deemed diagnostic and no other test was performed, specificity and positive predictive value can be artificially elevated. It is also important to note that while there are multiple techniques sonographers use to identify DVT, the included studies did not evaluate accuracy between techniques. All included studies used Doppler technique, but several incorporated compression into their imaging protocol. Last, the diagnostic test accuracy estimates were determined for a test done in a standalone manner, and we did not consider combinations of tests in a pathway for establishing a diagnosis of UEDVT. This may be required, for example, in patients who have a low pretest probability but have a positive D-dimer and need follow-up duplex US. The pooled sensitivity and specificity estimates of the tests from this review only apply when the test is performed alone. However, they can be used to model various diagnostic strategies to inform clinical decision making as conducted in a separate clinical guideline.8 Ultimately, the diagnostic tests will be used in a strategic approach based on clinical pretest probability and with consideration of availability, cost, potential harms, patient and provider values and preferences, and patient outcomes.
In conclusion, his systematic review synthesizes and evaluates the accuracy of commonly used tests for the diagnosis of DVT of the upper extremities. Estimates of sensitivity and specificity from this review were used to model diagnostic strategies and inform evidence-based recommendations for a clinical practice guideline for the American Society of Hematology.8 For clinical decision making, the prevalence or PTP for DVT in a population will influence how, together with the sensitivity and specificity estimates, patients will be managed. Future research is needed to continue identifying safe and cost-effective diagnostic strategies.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The systematic review team would like to acknowledge Samantha Eiffert and Robin Arnold for their assistance with data management and organization of the manuscript.
This systematic review was conducted to support the development of the American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. The entire guideline development process was funded by the American Society of Hematology. Through the McMaster GRADE Center, some researchers received salary (Payal Patel, C.B., M. Bhatt, W.W., Parth Patel, H.B., and J.V.) or grant support (R.A.M. and H.J.S.); others participated to fulfill requirements of an academic degree or program or volunteered their time.
Footnotes
Send data sharing requests via e-mail to the corresponding author, Reem A. Mustafa (rmustafa@kumc.edu).
Authorship
Contribution: Payal Patel and C.B. contributed to study design, search strategy, study selection, data extraction, statistical analysis, and drafting the report; Parth Patel and M. Bhatt contributed to study design, study selection, data extraction, statistical analysis, and critical revision of the report; H.B., N.M.H., M.A.K., and Y.N.A.J. contributed to the data extraction, statistical analysis, interpretation of results, and critical revision of the report; J.V., D.W., H.J.A., M.T., M. Baig, W.B., R. Khatib, R. Kehar, R.P., A.S., and A.M. contributed to study selection, data extraction, and critical revision of the report; and W.W., R.N., W.L., S.M.B., E.L., G.L.G., M.R., H.J.S., and R.A.M. contributed to the study design, interpretation of the results, and critical revision of the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reem A. Mustafa, Division of Nephrology and Hypertension, Department of Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd, MS3002, Kansas City, KS 66160; e-mail: rmustafa@kumc.edu.
References
- 1.Baarslag HJ, van Beek EJ, Koopman MM, Reekers JA. Prospective study of color duplex US compared with contrast venography in patients suspected of having deep venous thrombosis of the upper extremities [published correction appears in Ann Intern Med. 2003;138(5):438]. Ann Intern Med. 2002;136(12):865-872. [DOI] [PubMed] [Google Scholar]
- 2.Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781. [DOI] [PubMed] [Google Scholar]
- 3.Kröger K, et al. . Colour Doppler sonographic diagnosis of upper limb venous thromboses. Clin Sci. 1998;94:657-661. [DOI] [PubMed] [Google Scholar]
- 4.Kommareddy A, Zaroukian MH, Hassouna HI. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2002;28(1):89-99. [DOI] [PubMed] [Google Scholar]
- 5.Rooden CJ, Tesselaar MET, Osanto S, Rosendaal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters: a review. J Thromb Haemost. 2005;3(11):2409-2419. [DOI] [PubMed] [Google Scholar]
- 6.Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21(19):3665-3675. [DOI] [PubMed] [Google Scholar]
- 7.Chao A, Yung W, Robinson J, Chong P, Crozier J. Duplex ultrasonography in the detection of superficial and deep venous thrombosis in the upper limb: an analysis of 34 patients. J Vasc Tech. 2001;25(1):27-30. [Google Scholar]
- 8.Lim W, Le Gal G, Bates SM, et al. . American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. [DOI] [PubMed] [Google Scholar]
- 10.Whiting PF, Rutjes AWS, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. [DOI] [PubMed] [Google Scholar]
- 11.Schünemann HJ, Oxman AD, Brozek J, et al. ; GRADE Working Group . Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schünemann HJ, Oxman AD, Brozek J, et al. . GRADE: assessing the quality of evidence for diagnostic recommendations. ACP J Club. 2008;149(6):2. [PubMed] [Google Scholar]
- 13.Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 09.0. London, United Kingdom: The Cochrane Collaboration; 2010. [Google Scholar]
- 14.Constans J, Salmi LR, Sevestre-Pietri MA, et al. . A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost. 2008;99(1):202-207. [DOI] [PubMed] [Google Scholar]
- 15.Geersing GJ, Zuithoff NPA, Kearon C, et al. . Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ. 2014;348(mar10 3):g1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merminod T, Pellicciotta S, Bounameaux H. Limited usefulness of D-dimer in suspected deep vein thrombosis of the upper extremities. Blood Coagul Fibrinolysis. 2006;17(3):225-226. [DOI] [PubMed] [Google Scholar]
- 17.Sartori M, Migliaccio L, Favaretto E, et al. . D-dimer for the diagnosis of upper extremity deep and superficial venous thrombosis. Thromb Res. 2015;135(4):673-678. [DOI] [PubMed] [Google Scholar]
- 18.Kleinjan A, Di Nisio M, Beyer-Westendorf J, et al. . Safety and feasibility of a diagnostic algorithm combining clinical probability, D-dimer testing, and ultrasonography for suspected upper extremity deep venous thrombosis: a prospective management study. Ann Intern Med. 2014;160(7):451-457. [DOI] [PubMed] [Google Scholar]
- 19.Prandoni P, Polistena P, Bernardi E, et al. . Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157(1):57-62. [PubMed] [Google Scholar]
- 20.Haire WD, Lynch TG, Lund GB, Lieberman RP, Edney JA. Limitations of magnetic resonance imaging and ultrasound-directed (duplex) scanning in the diagnosis of subclavian vein thrombosis. J Vasc Surg. 1991;13(3):391-397. [DOI] [PubMed] [Google Scholar]
- 21.Köksoy C, Kuzu A, Kutlay J, Erden I, Ozcan H, Ergîn K. The diagnostic value of colour Doppler ultrasound in central venous catheter related thrombosis. Clin Radiol. 1995;50(10):687-689. [DOI] [PubMed] [Google Scholar]
- 22.Sottiurai VS, Towner K, McDonnell AE, Zarins CK. Diagnosis of upper extremity deep venous thrombosis using noninvasive technique. Surgery. 1982;91(5):582-585. [PubMed] [Google Scholar]
- 23.Norlin JM, Elf JL, Svensson PJ, Carlsson KS. A cost-effectiveness analysis of diagnostic algorithms of deep vein thrombosis at the emergency department. Thromb Res. 2010;126(3):195-199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.