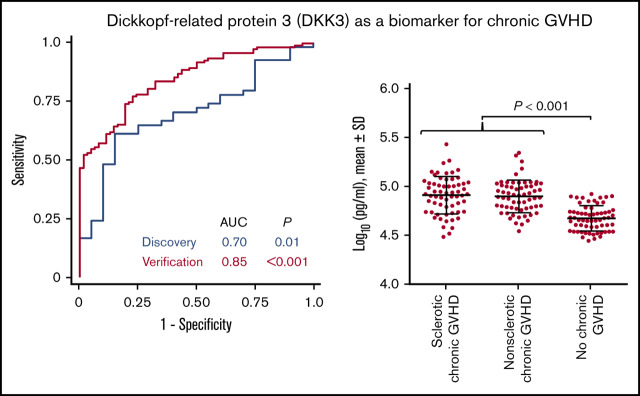
Key Points
Mass spectrometry analysis identified DKK3 as a novel biomarker associated with chronic GVHD.
Plasma DKK3 concentrations were associated with nonrelapse mortality after sample draw in patients with chronic GVHD.
Abstract
To identify plasma biomarkers associated with fibrotic mechanisms of chronic graft-versus-host disease (GVHD), we used multiplex mass spectrometry with pooled samples for biomarker discovery in comparing proteomic profiles between patients with newly diagnosed sclerotic chronic GVHD (n = 21), those with newly diagnosed nonsclerotic chronic GVHD (n = 33), and those without chronic GVHD (n = 20). Immunoassay was used to measure protein concentrations of individual discovery samples and 186 independent verification samples. The discovery mass spectrometry analysis identified 2 candidate proteins with at least 1.5-fold difference in sclerotic GVHD: Dickkopf-related protein 3 (DKK3) and interleukin-1 receptor accessory protein (IL1RAP). Analysis of individual discovery samples by immunoassay showed that DKK3, a modulator of the Wnt signaling pathway, was a biomarker for both sclerotic and nonsclerotic chronic GVHD. Verification analysis of 186 patients confirmed that elevated plasma DKK3 concentrations were associated with chronic GVHD, regardless of the presence or absence of sclerosis, and that the area under the receiver operating characteristic curve was 0.85 for association of DKK3 concentrations with chronic GVHD. Multiple linear regression analysis showed that chronic GVHD with or without steroid treatment and patient age were independently associated with DKK3 concentrations. Patients with high DKK3 concentrations had a higher nonrelapse mortality than those with low concentrations. The lower IL1RAP concentrations in patients with sclerotic GVHD compared with other conditions in the discovery cohort were not confirmed in the verification cohort. DKK3 is a novel biomarker for chronic GVHD. Further studies are needed to determine the biological functions of DKK3 in the pathogenesis of chronic GVHD.
Visual Abstract
Introduction
Chronic graft-versus-host disease (GVHD) is a systemic immunological complication that occurs in approximately one-half of patients who underwent allogeneic hematopoietic cell transplantation (HCT).1 It initially starts from inflammation, eventually leading to extensive tissue fibrosis and significant disability.2 Clinical manifestations of chronic GVHD are highly variable but at least 2 clinically unique phenotypes have been recognized as sclerotic chronic GVHD and bronchiolitis obliterans syndrome (BOS). Sclerosis occurs in more than 20% of patients with chronic GVHD and is characterized by skin thickening or fasciitis resulting from accumulation of collagen and extensive fibrosis.3-5 BOS is characterized by airflow obstruction from progressive circumferential fibrosis and ultimate cicatrization of the small terminal airways.6 Biomarkers that identify pathogenic mechanisms or reflect disease activity in patients with these specific phenotypes of chronic GVHD would help advance the field.
High-throughput mass spectrometry (MS) is a powerful and comprehensive approach to identify proteomic profiles. Prior studies have identified several biomarkers for chronic GVHD such as CXCL9, CXCL10, ST2, MMP-3, osteopontin, BAFF, and CD163.7-13 We hypothesized that comparison of plasma proteomic profiles among patients with different phenotypes of chronic GVHD could identify biomarkers associated with specific phenotypes or specific mechanisms of chronic GVHD that may also serve as treatment targets. Using a novel multiplex MS system, we compared samples among 5 different conditions including several phenotypes of chronic GVHD. This study aimed to identify biomarkers associated with fibrotic mechanisms of chronic GVHD. We hypothesized that such fibrosis-related biomarkers are most likely to be evident in patients with sclerotic chronic GVHD.
Methods
Study design
This study was carried out in 3 phases: (1) an initial discovery phase testing pooled plasma samples using high-throughput MS; (2) confirmation of candidate proteins in individual samples of the discovery cohort using immunoassays; and (3) verification of candidate proteins in an independent cohort using immunoassays.
Patients and sample collection
All samples were collected from patients aged 18 years or older. The discovery samples were identified from 5189 samples in 1237 patients prospectively collected during follow-up visits between March 2003 and August 2013 at the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance from participants in an observational study of patients who had allogeneic HCT. A total of 74 patients in the following 5 mutually exclusive conditions were selected for the discovery cohort (Table 1; supplemental Figure 1A): (1) 21 patients who newly developed sclerotic GVHD5; (2) 16 patients who were taking immunosuppressive treatment and newly developed moderate or severe chronic GVHD other than sclerosis or BOS14; (3) 11 patients who had withdrawn all immunosuppressive treatment and newly developed moderate or severe chronic GVHD other than sclerosis or BOS14; (4) 6 patients with newly diagnosed BOS14; and (5) 20 patients who never developed chronic GVHD and had ended all immunosuppressive treatment without subsequent resumption.15 All samples from patients in groups 1 through 4 were collected before changing systemic treatment. Absence of subsequent development of sclerotic GVHD or BOS after sample draw was confirmed for groups 2 and 3. Blood was collected in EDTA or heparin, and plasma was stored in aliquots at −80°C within 4 hours of phlebotomy.
Table 1.
Patient characteristics
| Characteristics | Discovery | Verification | ||||||
|---|---|---|---|---|---|---|---|---|
| Sclerotic chronic GVHD* | Chronic GVHD with IST† | Chronic GVHD without IST† | BOS‡ | No chronic GVHD | Sclerotic chronic GVHD | Nonsclerotic chronic GVHD | No chronic GVHD | |
| Total no. | 21 | 16 | 11 | 6 | 20 | 62 | 62 | 62 |
| Median age at transplant (range), y | 51 (26-70) | 50 (18-63) | 42 (24-60) | 42 (35-61) | 44 (20-68) | 53 (16-74) | 52 (29-74) | 51 (22-74) |
| Sex, n (%) | ||||||||
| Male | 10 (48) | 10 (63) | 5 (45) | 3 (50) | 10 (50) | 32 (52) | 32 (52) | 32 (52) |
| Female | 11 (52) | 6 (38) | 6 (55) | 3 (50) | 10 (50) | 30 (48) | 30 (48) | 30 (48) |
| Race, n (%) | ||||||||
| White | 18 (86) | 13 (81) | 10 (91) | 6 (100) | 19 (95) | 58 (94) | 56 (90) | 53 (85) |
| Others | 3 (14) | 3 (19) | 1 (9) | 0 (0) | 1 (5) | 4 (6) | 6 (10) | 9 (15) |
| Primary diagnosis, n (%) | ||||||||
| Acute leukemia | 8 (38) | 6 (38) | 5 (45) | 1 (17) | 11 (55) | 31 (50) | 29 (47) | 31 (50) |
| Chronic leukemia | 0 (0) | 2 (13) | 0 (0) | 2 (33) | 0 (0) | 5 (8) | 8 (13) | 7 (11) |
| MDS/MPN | 6 (29) | 2 (13) | 1 (9) | 0 (0) | 2 (10) | 9 (15) | 12 (19) | 11 (18) |
| Lymphoma | 5 (24) | 4 (25) | 4 (36) | 2 (33) | 5 (25) | 12 (19) | 7 (11) | 8 (13) |
| Others | 2 (10) | 2 (13) | 1 (9) | 1 (17) | 2 (10) | 5 (8) | 6 (10) | 5 (8) |
| Stem cell source, n (%) | ||||||||
| Peripheral blood stem cell | 20 (95) | 13 (81) | 8 (73) | 6 (100) | 13 (65) | 58 (94) | 56 (90) | 48 (77) |
| Bone marrow | 1 (5) | 2 (13) | 1 (9) | 0 (0) | 7 (35) | 3 (5) | 5 (8) | 10 (16) |
| Cord blood | 0 (0) | 1 (6) | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 4 (6) |
| Missing | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Conditioning intensity, n (%) | ||||||||
| Myeloablative | 15 (71) | 8 (50) | 6 (55) | 2 (33) | 11 (55) | 33 (53) | 25 (40) | 38 (61) |
| Reduced intensity | 6 (29) | 8 (50) | 5 (45) | 4 (67) | 9 (45) | 29 (47) | 37 (60) | 24 (39) |
| Donor type, n (%) | ||||||||
| HLA-matched relative | 12 (57) | 5 (31) | 5 (45) | 1 (17) | 13 (65) | 23 (37) | 19 (31) | 26 (42) |
| HLA-mismatched relative | 1 (5) | 1 (6) | 0 (0) | 0 (0) | 3§ (15) | 0 (0) | 3 (5) | 4§ (6) |
| Unrelated donor | 8 (38) | 10 (63) | 6 (55) | 5 (83) | 4 (20) | 39 (63) | 40 (65) | 32 (52) |
| Female donor to a male recipient, n (%) | 4 (19) | 6 (38) | 3 (27) | 2 (33) | 2 (10) | 12 (19) | 14 (23) | 11 (18) |
| Prior grade 2-4 acute GVHD, n (%) | 13 (62) | 15 (94) | 8 (73) | 4 (67) | 8 (40) | 26 (42) | 44 (71) | 38 (61) |
| Median time from HCT to sample draw (range), mo | 12 (2-41) | 2.8 (2.6-7.6) | 11 (7-12) | 13 (11-26) | 12 (11-13) | 29 (8-55) | 29 (8-58) | 13 (9-64) |
| Median time from chronic GVHD to sample draw (range), mo | 0 (0-37) | 0 (0-0) | 0 (0-0.1) | 2.9 (0-15) | NA | 11 (0.1-45) | 17 (0.5-53) | NA |
| Steroid treatment at sample draw, n (%) | 5 (24) | 13 (81) | 0 (0) | 5 (83) | 0 (0) | 54 (87) | 33 (53) | 0 (0) |
BOS, bronchiolitis obliterans syndrome; IST, immunosuppressive treatment; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
Without BOS.
Without sclerosis or BOS.
Without sclerosis.
Among these patients, 3 in the discovery cohort and 2 in the verification cohort had HLA-haploidentical donors.
The independent verification cohort included 186 patients (supplemental Figure 1B): (1) 62 patients with sclerotic chronic GVHD who participated in a multicenter randomized phase 2 study of rituximab vs imatinib for treatment of sclerotic chronic GVHD conducted by the Chronic GVHD Consortium.16 Three patients without enrollment samples, 5 with BOS at enrollment and 2 without matched control samples, were excluded from the original cohort of 72 patients. Almost all patients were already on treatment of chronic GVHD, but samples were collected at study enrollment in the clinical trial before starting treatment with rituximab or imatinib; (2) 62 control patients with nonsclerotic chronic GVHD who participated in a prospective, multicenter, longitudinal, observational study of the same Consortium17,18; and (3) 62 control patients with no chronic GVHD who participated in the same observational study. Sclerotic chronic GVHD cases and controls were matched for sex and time after HCT (90-day window for nonsclerotic chronic GVHD and 2-year window for no chronic GVHD). We confirmed that control patients with nonsclerotic chronic GVHD and those with no chronic GVHD did not subsequently develop sclerotic GVHD after samples were collected. Patients gave written consent allowing blood sample collection and the use of medical records for research in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review board of all participating centers.
Acute GVHD was diagnosed and graded according to previously described criteria.19 Chronic GVHD was diagnosed according to the 2014 National Institutes of Health (NIH) consensus criteria.20 Organ involvement was defined as NIH organ score ≥1 except for the lungs. Lung involvement was defined for patients with BOS according to the 2014 NIH consensus criteria.20 Late acute GVHD was not included in this study. The intensity of conditioning was defined as described elsewhere.21
Quantitative MS
Six pooled-plasma samples were individually processed to remove high-abundance proteins (supplemental Figure 2). Remaining low-abundance proteins in each sample were labeled with 6-plex tandem-mass-tag isobaric reagents (Thermo Pierce) individually. The mixture of labeled samples was separated by an orthogonal 2-dimensional high-performance liquid chromatography with anion exchange as the first dimension, and reversed phase as the second dimension. Collected protein fractions were digested with trypsin and analyzed by nano liquid chromatography-HDMSE with ion-mobility (Waters).
Two-hour gradient elution was performed in a capillary column (C18, 3 µm 120Å, 75 µm ID×25 cmL; Column Technology, Inc.) at 500 nL/min (A: 0.1% formic acid in water; B: 0.1% formic acid in acetonitrile). MS was undertaken with a resolving power of at least 20 000 full width at half maximum at m/z 785.843 (+2, Glu1-fibrinopeptide B) nano ESI source with a NanoLockSpray. The lock mass channel was sampled every 60 seconds.
Accurate liquid chromatography-HDMSE data were acquired in an alternating, low-energy (HDMS) and high-energy (HDMSE) mode with mass scan range from m/z 50 to 1800 under a capillary voltage of 2.8 kV, a source temperature of 100°C, and a cone voltage of 30 V. The spectral acquisition in each mode was 1.0 second with a 0.1 second interscan. In HDMS mode, data were collected at collision energy of 2 eV in both the trap and transfer cells. In HDMSE mode, the collision energy was ramped up from 25 to 55 eV in the transfer cell. Acquired data were processed through ProteinLynx Global Server software (Waters) and searched against UniProt at a 4% false discovery rate. The identified proteins were filtered with ≤5 ppm mass accuracy of sequenced peptides. Loess and quantile normalization approaches were used to normalize the peak intensities of reporter ions before protein quantification.
Luminex microbead assay
The Luminex microbead method (Luminex, Austin, TX) was used for measurement of proteins, as previously described.22 Supplemental Table 1 lists the reagents used in these assays along with their respective lower limits of detection. No sample showed values below the lower limit of detection.
Statistical analysis
Protein concentrations were log-transformed for analysis and mean differences between the groups were calculated as fold differences. Concentrations were compared between the groups using the Student t test. Linear regression models were used to examine factors associated with protein concentrations. A backward elimination procedure was used in developing final models, using a P value threshold of .05. Covariates included GVHD conditions (main effect), patient age at sample draw, patient sex, race, stem cell source, conditioning intensity, donor type, a female donor to a male recipient, prior grade 2-4 acute GVHD, duration from HCT to sample draw, NIH global severity at sample draw, and involved sites at sample draw. Patients without chronic GVHD were not treated with steroids. Therefore, 3 categories were analyzed for the main effect: chronic GVHD with steroid treatment at sample draw, chronic GVHD without steroid treatment at sample draw, and no chronic GVHD (no steroid treatment). The cumulative incidence of nonrelapse mortality from sample draw was calculated by the Gray method, treating disease progression as a competing risk. Two-sided P < .05 was considered statistically significant. The receiver operating characteristic area under the curve (AUC) was estimated nonparametrically.
Results
Candidate discovery
Patient characteristics of the discovery cohort are summarized in Table 1. Using the multiplex MS system, we compared pooled plasma of patients with sclerotic chronic GVHD versus those with 4 other conditions without sclerosis. Experiments were performed separately in male and female patients. A total of 617 proteins were identified from a male experiment, and 769 proteins were identified from a female experiment. Of the 377 proteins identified in both male and female experiments, 23 were increased to 1.5-fold or higher among patients with sclerotic chronic GVHD compared with at least 3 other chronic GVHD conditions in both experiments using the Loess normalization, whereas 52 proteins were decreased to 0.67-fold or lower. Among these 75 proteins, the quantile normalization showed similar results for 70 proteins as summarized in supplemental Table 2. Among the 70 proteins, 10 had relevant biology in fibrotic mechanisms of chronic GVHD based on the literature (supplemental Table 3), and 5 (CCL14, DKK3, IL1RAP, IL6ST, LGALS3BP) had commercially available antibodies that were suitable for Luminex assays. Concentrations of these 5 proteins were measured by immunoassays in 74 individual discovery samples and were evaluated in 21 patients with sclerotic chronic GVHD compared with 53 patients with other conditions without sclerosis (Table 2). Two proteins (DKK3 and IL1RAP) met criteria for further evaluation (consistent fold difference between MS and immunoassay as well as between males and females, and AUC ≥0.60).
Table 2.
Discovery candidate selection: comparison of sclerotic chronic GVHD (n = 21) with other conditions (n = 53)
| Protein | Mean fold difference in MS | Mean fold difference in Luminex | Consistency | AUC | Candidate | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| Candidates from MS analysis | |||||||
| C-C motif chemokine ligand 14 | 0.5 | 0.6 | 1.1 | 1.0 | No | 0.51 | No |
| DKK3 | 4.5 | 2.5 | 1.3 | 1.2 | Yes | 0.64 | Yes |
| IL1RAP | 0.6 | 0.4 | 0.8 | 1.0 | Yes | 0.62 | Yes |
| Interleukin-6 receptor subunit β | 3.1 | 3.9 | 0.9 | 0.8 | No | 0.67 | No |
| Galectin-3-binding protein | 0.4 | 0.4 | 1.5 | 1.8 | No | 0.69 | No |
Boldface type indicates that the protein passed criteria for further evaluation (consistent fold differences between MS and Luminex as well as between males and females, and AUC ≥0.60).
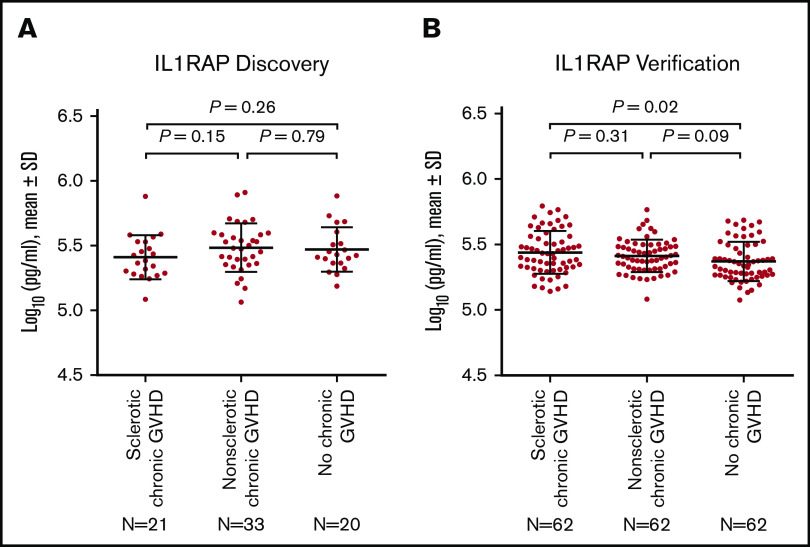
Comparison of IL1RAP concentrations among the groups and verification
To verify the IL1RAP results from the discovery analysis, we tested independent plasma samples from 186 patients: 62 with sclerotic chronic GVHD, 62 with nonsclerotic chronic GVHD, and 62 with no chronic GVHD. Patient characteristics were well balanced among the 3 groups (Table 1). Chronic GVHD samples in the verification cohort were notably collected after longer interval times from HCT and after longer interval times from diagnosis of chronic GVHD when compared with chronic GVHD samples in the discovery cohort.
Although discovery analysis showed that IL1RAP concentrations measured by immunoassays were lower in patients with sclerotic chronic GVHD compared with other conditions (respective AUC, 0.62; Figure 1A), this relationship did not hold true in the verification cohort (Figure 1B).
Figure 1.
Protein concentrations of IL1RAP. (A) Discovery cohort. (B) Verification cohort. The horizontal lines indicate means and "I" bars indicate standard deviation (SD). P values were derived from Student t tests.
Comparison of DKK3 concentrations among the groups and verification
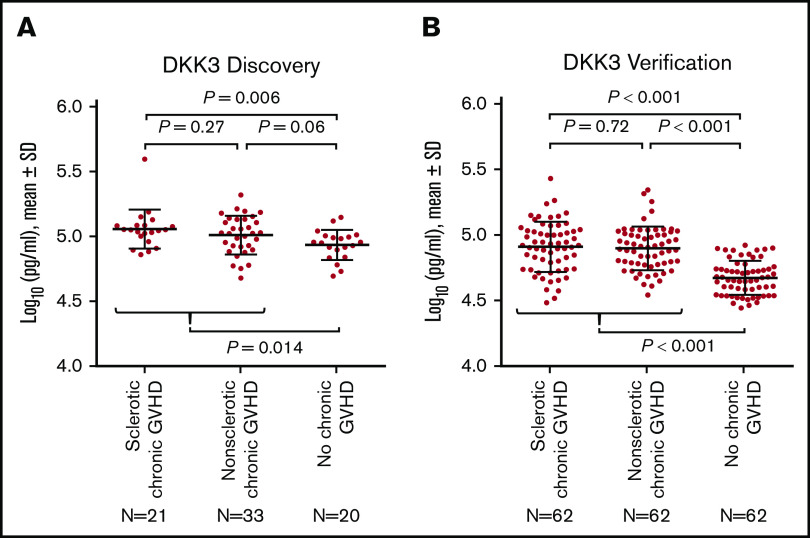
In the individual discovery samples, DKK3 concentrations measured by immunoassays were higher among patients with sclerotic chronic GVHD (P = .006) and those with nonsclerotic chronic GVHD (P = .06) vs those with no chronic GVHD, with no appreciable difference between sclerotic chronic GVHD and nonsclerotic chronic GVHD (Figure 2A), suggesting that DKK3 concentrations measured by immunoassays are a marker for chronic GVHD overall regardless of the presence or absence of sclerotic symptoms. We therefore combined patients with sclerotic chronic GVHD and nonsclerotic chronic GVHD for subsequent analyses.
Figure 2.
Protein concentrations of DKK3. (A) Discovery cohort. (B) Verification cohort. The horizontal lines indicate means and "I" bars indicate SD. P values were derived from Student t test.
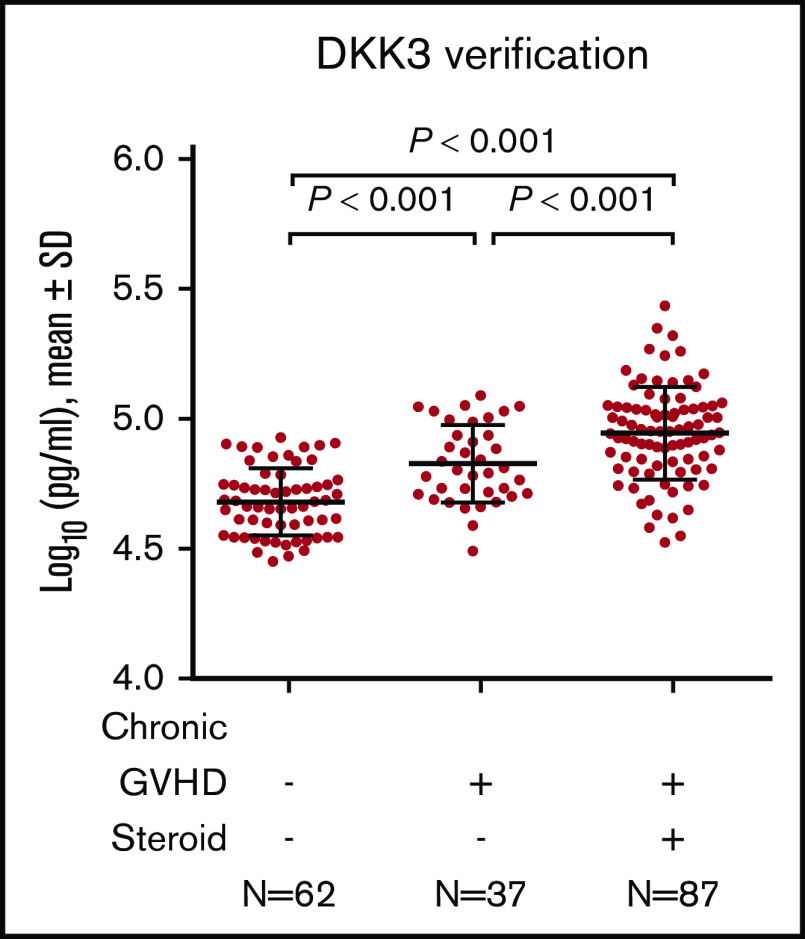
In the verification cohort, DKK3 concentrations were higher among 124 patients with chronic GVHD compared with 62 patients with no chronic GVHD (P < .001), with no statistically significant difference between sclerotic chronic GVHD and nonsclerotic chronic GVHD (Figure 2B). DKK3 concentrations were slightly higher in the discovery cohort compared with the verification cohort. Although longer time intervals from HCT to sample draw and from the onset of chronic GVHD to sample draw were associated with slightly lower concentrations of DKK3 in simple regression analysis among patients with chronic GVHD (supplemental Figure 3), these associations were attenuated and were no longer statistically significant when these factors were entered in the multiple regression model together with cohort type (supplemental Table 4). Compared with the discovery cohort, the verification cohort included larger proportions of patients with mild or severe NIH global severity and those with joint/fascia involvement, and smaller proportions of patients with oral or hepatic involvement (supplemental Table 5). To adjust for those differences between the cohorts, another multiple regression model was constructed (supplemental Table 6). The adjusted and unadjusted fold differences for the verification cohort compared with the discovery cohort were identical (0.77). Taken together, the lower DKK3 concentrations in the verification cohort were independent of differences in time intervals to sample draw, NIH global severity, or organ involvement between the cohorts.
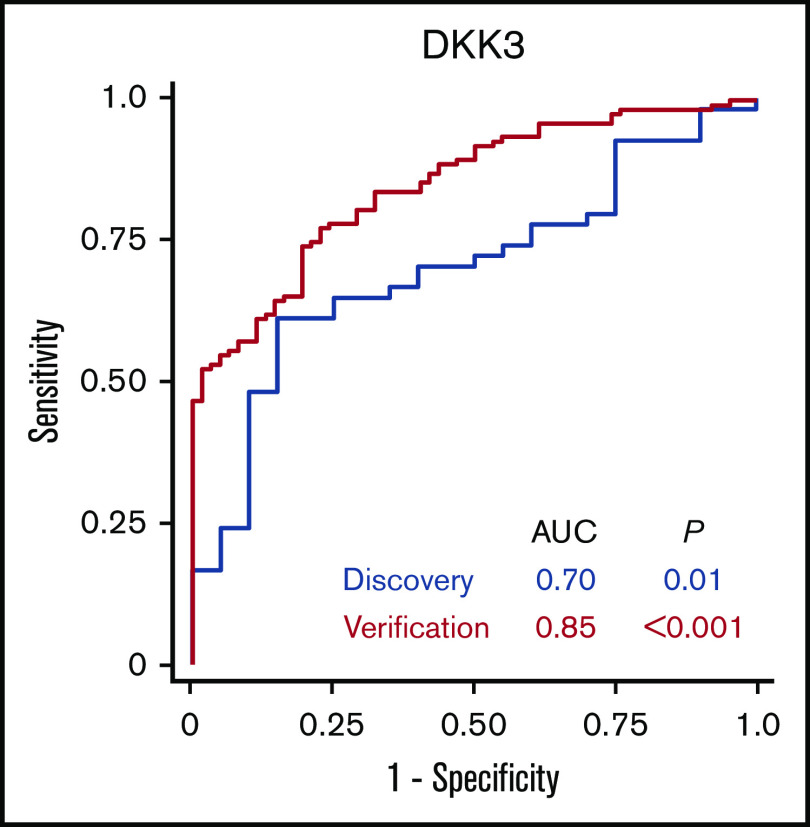
In the discovery cohort, the AUC of the association of DKK3 concentrations with chronic GVHD was 0.70 (Figure 3). In the verification cohort, the AUC of the association of DKK3 concentrations with chronic GVHD was 0.85. Sensitivities, specificities, positive predictive values, and negative predictive values according to different thresholds are shown in supplemental Table 7.
Figure 3.
Receiver operating characteristic curves for association of DKK3 with chronic GVHD.
Factors associated with DKK3 concentrations
We used linear regression models to identify factors associated with DKK3 concentrations in the verification cohort (Table 3). In multiple regression models, independent factors associated with DKK3 concentrations were chronic GVHD with steroid treatment, chronic GVHD without steroid treatment, and older patient age. The results also show that DKK3 concentrations were 1.31-fold higher among chronic GVHD patients treated with steroids than among those not treated with steroids (95% confidence interval, 1.14-1.50; P < .001) (Figure 4). Separate simple linear regression analyses in patients without chronic GVHD and those with chronic GVHD showed similar fold differences associated with patient age in both subgroups (supplemental Table 8). Stem cell source, conditioning intensity, duration from HCT to sample draw, NIH global severity, and involved sites did not remain in multiple regression models. Prior acute GVHD was not statistically associated with DKK3 concentrations. In addition, DKK3 concentrations did not differ according to early (<9 months) vs late onset (≥9 months) of chronic GVHD, onset type, subcategory of chronic GVHD, skin features, or involved sites among patients with chronic GVHD (supplemental Figure 4).
Table 3.
Linear regression analysis for DKK3 concentrations in the verification cohort
| Factor | n | Simple regression | Multiple regression | ||
|---|---|---|---|---|---|
| Fold difference* (95% CI) | P | Fold difference* (95% CI) | P | ||
| Main comparison | <.001† | <.001† | |||
| No GVHD | 62 | Reference | Reference | ||
| Chronic GVHD without steroid | 37 | 1.40 (1.21-1.63) | <.001 | 1.40 (1.21-1.61) | <.001 |
| Chronic GVHD with steroid | 87 | 1.84 (1.63-2.07) | <.001 | 1.83 (1.63-2.05) | <.001 |
| Patient age at sample, continuous (per decade) | 186 | 1.08 (1.03-1.13) | .003 | 1.07 (1.03-1.12) | .001 |
| Patient sex | |||||
| Male | 96 | Reference | |||
| Female | 90 | 0.99 (0.87-1.13) | .92 | ||
| Race | |||||
| White | 167 | Reference | |||
| Others | 19 | 0.92 (0.74-1.14) | .45 | ||
| Stem cell source | .06† | ||||
| Peripheral blood stem cell | 162 | Reference | |||
| Bone marrow | 18 | 0.82 (0.66-1.03) | .083 | ||
| Cord blood | 5 | 0.65 (0.44-0.97) | .035 | ||
| Missing | 1 | 0.74 (0.31-1.80) | .51 | ||
| Conditioning intensity | |||||
| Myeloablative | 96 | Reference | |||
| Reduced intensity | 90 | 1.17 (1.03-1.33) | .02 | ||
| Donor type | .29† | ||||
| HLA-matched relative | 68 | Reference | |||
| HLA-mismatched relative | 7 | 0.94 (0.66-1.34) | .75 | ||
| Unrelated donor | 111 | 1.10 (0.96-1.27) | .16 | ||
| Female donor to a male recipient | 37 | 1.05 (0.89-1.24) | .57 | ||
| Prior grade 2-4 acute GVHD | 108 | 0.99 (0.86-1.13) | .84 | ||
| Duration from HCT to sample draw per y | 186 | 1.08 (1.02-1.15) | .007 | ||
| NIH global severity | <.001† | ||||
| None or mild | 101 | Reference | |||
| Moderate | 35 | 1.41 (1.20-1.66) | <.001 | ||
| Severe | 50 | 1.51 (1.31-1.75) | <.001 | ||
| Involved sites | |||||
| Skin | 74 | 1.40 (1.24-1.58) | <.001 | ||
| Mouth | 58 | 1.34 (1.17-1.54) | <.001 | ||
| Eye | 72 | 1.51 (1.34-1.71) | <.001 | ||
| Gastrointestinal tract | 24 | 1.32 (1.09-1.60) | .005 | ||
| Liver | 5 | 1.24 (0.83-1.86) | .29 | ||
| Lung (bronchiolitis obliterans) | 4 | 1.38 (0.88-2.16) | .16 | ||
| Joint/fascia | 57 | 1.38 (1.21-1.58) | <.001 | ||
| Genital | 15 | 1.15 (0.91-1.47) | .24 | ||
CI, confidence interval.
Fold difference values represent the anti-log10 of the regression coefficient.
Overall P value.
Figure 4.
DKK3 concentrations according to chronic GVHD condition and steroid treatment in the verification cohort. The horizontal lines indicate means and "I" bars indicate SD. P values were derived from Student t test.
Nonrelapse mortality according to DKK3 concentrations
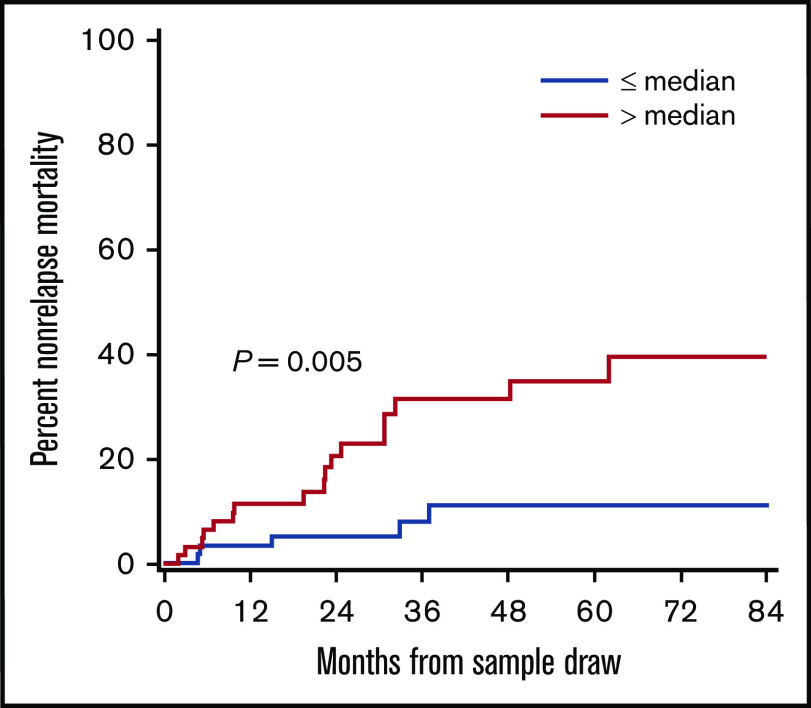
We compared the cumulative incidence of nonrelapse mortality from sample draw according to DKK3 concentrations among patients with chronic GVHD in the verification cohort (Figure 5). The cumulative incidence of nonrelapse mortality was higher in patients with DKK3 concentrations above the median (4.91 Log10 pg/mL) than in those with low concentrations (32% vs 11% at 48 months, P = .005). Infection was the most common cause of mortality, and the distributions of mortality causes were similar between the 2 groups. Associations of DKK3 concentrations with mortality were independent from patient age (data not shown).
Figure 5.
The cumulative incidence of nonrelapse mortality from sample draw according to DKK3 concentrations among patients with chronic GVHD in the verification cohort.
Discussion
Using high-throughput MS, we identified a novel protein DKK3 as a diagnostic biomarker for chronic GVHD. Notably, DKK3 has relevant biology in fibrotic mechanisms in conditions other than chronic GVHD.23-26 The association of DKK3 concentrations with chronic GVHD was independent of steroid treatment at sample draw, suggesting its broad utility during steroid treatment. The associations of DKK3 concentrations with subsequent nonrelapse mortality after sample draw suggest the prognostic utility of this biomarker.
The 4-member family of Dickkopf-related proteins are evolutionarily conserved and contain 2 cysteine rich regions. They modulate signaling through Wnt pathways involved in development and in many pathologic conditions including fibrosis and autoimmunity.27,28 DKK1 and DKK3, the most studied members of this family, inhibit the Wnt signaling and have been considered as potential targets in diseases with aberrant Wnt signaling activity.29 DKK3 attenuates cardiac hypertrophy and fibrosis in murine models,24 and DKK3 overexpression suppressed collagen synthesis through transforming growth factor–β1 (TGF-β1)/Smad signaling in TGF-β1–induced human keloid fibroblasts.25 On the other hand, evidence from murine models indicates that DKK3 promotes kidney fibrosis, and urinary DKK3 concentrations correlate with the extent of renal fibrosis in patients with progressive chronic kidney disease.23 DKK3 also modulates B-cell fate and function and suppresses autoimmunity in a murine model of systemic lupus erythematosus.26 Thus, the elevated DKK3 plasma concentrations in patients with chronic GVHD in the current study suggests that Wnt signaling, TGF-β1/Smad signaling, and B-cell dysfunction may be involved in chronic GVHD. The elevated DKK3 concentrations in patients with nonsclerotic chronic GVHD may indicate the involvement of fibrotic process in those patients even in the absence of sclerotic symptoms.
Concentrations of plasma proteins may be affected by factors other than chronic GVHD. In the current study, multiple regression analysis showed that chronic GVHD, patient age, and steroid treatment at the time of sample procurement were independently associated with DKK3 concentrations. Corticosteroids are frequently used for treatment of chronic GVHD and can affect the concentrations of some plasma proteins.30 Our results showed that steroid treatment was associated with higher DKK3 concentrations among patients with chronic GVHD, whereas the association of DKK3 concentrations with chronic GVHD was independent of steroid treatment.
Our study has several limitations. First, our original goal was to identify plasma proteins associated with fibrotic mechanisms of chronic GVHD by comparing patients with sclerotic chronic GVHD vs those without sclerotic GVHD. Because the immunoassay results unexpectedly showed that DKK3 was associated with both sclerotic chronic GVHD and nonsclerotic chronic GVHD, we combined these groups for subsequent analyses. Results of MS and immunoassays can be inconsistent because immunoassays are based on antibodies directed at specific epitopes, whereas MS has no epitope limitations. Therefore, the discrepancies could reflect different forms of proteins being quantified by the 2 methods. We used immunoassays for verification because immunoassays are widely available in clinical practice. Second, our proteomic analysis did not identify biomarker proteins published in prior studies, probably because of differences in study design. Third, we did not account for the effects of immunosuppressive medications other than steroids. Last, DKK3 concentrations were generally lower in the verification cohort than in the discovery cohort, especially in patients without chronic GVHD. We examined influences of different time intervals to sample draw, different degrees of GVHD severity, and different involved sites between the cohorts, but these factors did not fully account for the concentration differences. Therefore, the concentration differences could be related to batch effects.
Further preclinical and clinical studies are needed to determine biological functions of DKK3 in chronic GVHD. We speculate that Wnt or TGF-β1/Smad signaling could serve as a novel target in the treatment of chronic GVHD. Because both pro-fibrotic and anti-fibrotic effects of DKK3 have been reported in other clinical contexts, the role of DKK3 in chronic GVHD needs to be determined in preclinical models and in patients. Many inhibitors of the Wnt signaling pathway are under development for cancer treatment and therapeutic potential of these drugs could be tested in patients with chronic GVHD.31
Studies are also needed to elucidate longitudinal changes of biomarkers across time from HCT to the development and evolution of chronic GVHD to resolution. Previous studies have shown that chronic GVHD requiring resumption of systemic treatment occurs in approximately one-half of patients after the initial attempt to withdraw immunosuppressive treatment.32 Therefore, studies are needed to determine whether high DKK3 concentrations persisting after clinical resolution of the disease are associated with an increased risk of recurrent chronic GVHD after systemic treatment has ended.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Theodore A. Gooley for statistical advice and all members in the Chronic GVHD Consortium (U54 CA163438), which was funded as part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Disease Research (ORDR), NCATS, funded through collaboration between NCATS and the National Cancer Institute.
This study was supported by grants from the National Institutes of Health, National Cancer Institute (CA118953 and CA163438), and from the Japan Society for the Promotion of Science (18K08345).
Footnotes
Data sharing requests can be e-mailed to the corresponding author, Yoshihiro Inamoto (yinamoto@ncc.go.jp).
Authorship
Contribution: Y.I., P.J.M., S.H., and J.A.H. designed the study, analyzed data, and wrote the paper; Y.I. and L.E.O. performed statistical analyses; A.A.M., R.L.L., H.K., and S.H. performed experiments and wrote the paper; S.J.L., L.T., J.P., and M.E.D.F. collected data and wrote the paper; and all authors critically revised the manuscript for important intellectual content and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshihiro Inamoto, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; e-mail: yinamoto@ncc.go.jp.
References
- 1.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215-233. [DOI] [PubMed] [Google Scholar]
- 2.Cooke KR, Luznik L, Sarantopoulos S, et al. . The biology of chronic graft-versus-host disease: a Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(2):211-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skert C, Patriarca F, Sperotto A, et al. . Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006;91(2):258-261. [PubMed] [Google Scholar]
- 4.Martires KJ, Baird K, Steinberg SM, et al. . Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118(15):4250-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamoto Y, Storer BE, Petersdorf EW, et al. . Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013;121(25):5098-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302(3):306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarantopoulos S, Stevenson KE, Kim HT, et al. . High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitko CL, Levine JE, Storer BE, et al. . Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123(5):786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Storer BE, Kushekhar K, et al. . Biomarker panel for chronic graft-versus-host disease. J Clin Oncol. 2016;34(22):2583-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariminia A, Holtan SG, Ivison S, et al. . Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood. 2016;127(24):3082-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, Flynn R, Paz K, et al. . Murine chronic graft-versus-host disease proteome profiling discovers CCL15 as a novel biomarker in patients. Blood. 2018;131(15):1743-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozmus J, Kariminia A, Abdossamadi S, et al. . Comprehensive B cell phenotyping profile for chronic graft-versus-host disease diagnosis. Biol Blood Marrow Transplant. 2019;25(3):451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamoto Y, Martin PJ, Paczesny S, et al. . Association of plasma CD163 concentration with de novo-onset chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(8):1250-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inamoto Y, Flowers ME, Sandmaier BM, et al. . Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inamoto Y, White J, Ito R, et al. . Comparison of characteristics and outcomes of late acute and NIH chronic GVHD between Japanese and white patients. Blood Adv. 2019;3(18):2764-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai S, Pidala J, Pusic I, et al. . A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin Cancer Res. 2016;22(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chronic GC; Chronic GVHD Consortium . Rationale and design of the chronic GVHD cohort study: improving outcomes assessment in chronic GVHD. Biol Blood Marrow Transplant. 2011;17(8):1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chronic GC; Chronic GVHD Consortium . Design and patient characteristics of the chronic graft-versus-host disease response measures validation study. Biol Blood Marrow Transplant. 2018;24(8):1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 20.Jagasia MH, Greinix HT, Arora M, et al. . National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giralt S, Ballen K, Rizzo D, et al. . Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JA, Hanash SM, Tabellini L, et al. . A novel soluble form of Tim-3 associated with severe graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(9):1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Federico G, Meister M, Mathow D, et al. . Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight. 2016;1(1):e84916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai CG, Xu YY, Tie YY, et al. . DKK3 overexpression attenuates cardiac hypertrophy and fibrosis in an angiotensin-perfused animal model by regulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways. J Mol Cell Cardiol. 2018;114:243-252. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Liu H, Liang Y, Peng P, Ma X, Zhang X. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-β1/Smad signaling pathway. Biomed Pharmacother. 2017;91:174-180. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig J, Federico G, Prokosch S, et al. . Dickkopf-3 acts as a modulator of B cell fate and function. J Immunol. 2015;194(6):2624-2634. [DOI] [PubMed] [Google Scholar]
- 27.Krupnik VE, Sharp JD, Jiang C, et al. . Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238(2):301-313. [DOI] [PubMed] [Google Scholar]
- 28.Maruotti N, Corrado A, Neve A, Cantatore FP. Systemic effects of Wnt signaling. J Cell Physiol. 2013;228(7):1428-1432. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J Immunol Res. 2016;2016:9392132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattori Y, Kida D, Kaneko A. Steroid therapy and renal dysfunction are independently associated with serum levels of matrix metalloproteinase-3 in patients with rheumatoid arthritis. Mod Rheumatol. 2018;28(2):242-248. [DOI] [PubMed] [Google Scholar]
- 31.Haseeb M, Pirzada RH, Ain QU, Choi S. Wnt Signaling in the regulation of immune cell and cancer therapeutics. Cells. 2019;8(11):1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Nguyen TD, Onstad L, et al. . Success of immunosuppressive treatments in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2018;24(3):555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.