Key Points
Joint outcomes in hemophilia are better in young adults if prophylaxis is started before age 2.5 years compared with after age 6 years.
Standard FVIII prophylaxis is insufficient to fully protect joints from damage through adolescence in severe HA.
Abstract
The Joint Outcome Study (JOS), a randomized controlled trial, demonstrated that children with severe hemophilia A (HA) initiating prophylactic factor VIII (FVIII) prior to age 2.5 years had reduced joint damage at age 6 years compared with those treated with episodic FVIII for bleeding. The Joint Outcome Continuation Study (JOS-C) evaluated early vs delayed prophylaxis effects on long-term joint health, following JOS participants to age 18 years in an observational, partially retrospective study. Index joint magnetic resonance imaging (MRI) scores of osteochondral (OC) damage (primary outcome), joint physical examination scores, and annualized rates of joint/other bleeding episodes (secondary outcomes) were collected. Thirty-seven of 65 JOS participants enrolled in JOS-C, including 15 randomized to prophylaxis at mean age 1.3 years (“early prophylaxis”); 18 initially randomized to episodic treatment, starting “delayed prophylaxis” at mean age 7.5 years; and 4 with high-titer inhibitors. At JOS-C exit, MRI OC damage was found in 77% of those on delayed and 35% of those on early prophylaxis for an odds ratio of OC damage, in the delayed vs early prophylaxis group, of 6.3 (95% confidence interval, 1.3, 29.9; P = .02). Annualized bleeding rates were higher with delayed prophylaxis (mean plus or minus standard deviation, 10.6 ± 6.6 vs 3.5 ± 2.1; P < .001), including when only comparing time periods on prophylaxis (6.2 ± 5.3 vs 3.3 ± 1.9; P < .05). In severe HA, early initiation of prophylaxis provided continued protection against joint damage throughout childhood compared with delayed initiation, but early prophylaxis was not sufficient to fully prevent damage. This trial was registered at www.clinicaltrials.gov as #NCT01000844.
Visual Abstract
Introduction
Joint bleeding in patients with severe hemophilia A (HA; factor VIII [FVIII] activity < 2%) can occur from negligible trauma and result in arthropathy,1 often resulting in acute and chronic pain2 as well as decreased quality of life.3 The Joint Outcome Study (JOS)4 was a randomized controlled trial in young boys with severe HA; the study demonstrated that prophylactic FVIII concentrate administered IV every other day starting before age 2.5 years led to better joint outcomes on magnetic resonance imaging (MRI) at age 6 years than episodic treatment with FVIII for bleeding. The JOS started shortly after safe FVIII products became available following the HIV epidemic of the 1980s. Although prophylaxis had been standard in hemophilia centers such as Malmo, Sweden for many years,5,6 prophylaxis was poorly adopted worldwide because of expense, venous access difficulties, indwelling venous access device complications, and efficacy doubts in the absence of a randomized controlled trial.7 The randomized controlled JOS showed efficacy using MRI and physical examination outcomes at fixed time points, establishing prophylactic FVIII as the standard of care for severe HA. Based on JOS results and because prophylaxis cannot reverse joint osteochondral (bone and cartilage) damage,8 the World Federation of Hemophilia and others recommend starting prophylaxis prior to age 3 years and prior to joint bleeding.5,9-11
Following completion of the JOS, all participants on the episodic arm were encouraged to adopt prophylaxis, allowing an important opportunity to compare outcomes relative to prophylaxis initiation age in the context of a prospective trial. The JOS Continuation (JOS-C) followed the participants of the JOS through adolescence, with a focus on joint outcomes.
Fortunately, hemophilia treatment is in the midst of dramatic improvements. With emicizumab,12,13 other novel nonfactor-based therapies,14 extended half-life FVIII products,15 and an array of gene therapy trials for hemophilia,16 hemophilia prophylaxis is getting easier and possibly more effective. However, long-term outcomes from those novel treatments will not be available for a few decades. This long-term study comparing FVIII prophylaxis initiated before age 2.5 years vs after age 6 years provides a critical baseline against which new therapies can be compared.
Methods
Study design and eligibility
The design and results of JOS are described elsewhere.4 Briefly, 65 boys with severe HA, no evidence of joint damage on screening MRI, and no FVIII inhibitor were randomized prior to age 2.5 years to receive either prophylaxis with 25 IU/kg recombinant FVIII (rFVIII; Kogenate or Kogenate FS; Bayer Healthcare) every other day with an additional 40 IU/kg rFVIII for hemarthroses, or enhanced episodic treatment, consisting of 40 IU/kg rFVIII at the time of joint hemorrhage, followed by 20 IU/kg 24 hours and 72 hours after the first dose, continuing every other day up to 4 weeks until bleed resolution. All JOS participants were eligible to enroll in JOS-C up until age 18 years. The protocol was approved by the ethics board at each participating institution, and each participant or guardian provided informed consent.
Treatment
Hemophilia treatment and FVIII product decisions were made clinically and not dictated by the JOS-C; no medication was provided as part of the continuation study. Factor product and treatment data were collected from chart review, pharmacy records, and log books. Adherence to prophylaxis was calculated as the number of infusions given per year, divided by the number prescribed per year. If prophylaxis frequency (3 times per week vs every other day) was unclear, a 3 times per week frequency was assumed.
Outcome measures
At study entry, clinical data regarding hemophilia treatments, bleeding, hospitalizations, and surgeries between the time of JOS exit and JOS-C entry were collected retrospectively from medical records and treatment logs. Noncontrast T2* gradient echo MRIs of index joints (ankles, knees, and elbows) were obtained at JOS-C entry and exit. During the JOS-C, bleeding and infusion logs, quality-of-life questionnaires (Haemo-QOL17), and other clinical data were collected annually, in conjunction with annual joint physical examination.
The primary outcome of the JOS-C, as in the JOS, was evidence of osteochondral damage on MRI at study exit. MRIs were scored according to the extended MRI (eMRI) scale (supplemental Table 1); each joint had a 9-point score for soft tissue damage and a 36-point score for osteochondral damage,18 with higher scores being worse. MRIs performed as part of the JOS (up to age 6 years) were originally scored on a different scale,19 so were rescored according to the eMRI scale for comparison. The eMRI scale was chosen because it is better at distinguishing degree of severity of more advanced joint disease, and because of concern for a ceiling effect in the original JOS scoring system.
MRIs were independently scored by 2 board-certified pediatric musculoskeletal radiologists (J.S. and M.F.) blinded to treatment group; scores that differed by >20% were reviewed collaboratively and assigned a consensus score. MRIs that were discrepant regarding the presence of osteochondral damage were adjudicated by a third radiologist (J.D.I.). Two fused ankles and 1 knee requiring synovectomy were counted as hemophilic arthropathy, even though surgery precluded MRI scoring in the joints containing hardware. Joint baseline and exit physical examinations were performed by physical therapists specializing in hemophilia and scored using the Colorado Pediatric Joint Assessment Scale (CPJAS; supplemental Table 2),20 a 31-point per joint scale (max score 27 points for elbows), with higher scores being worse. The CPJAS differed from the original JOS scoring system21 in its inclusion of crepitus and updated age-stratified ankle axial alignment. For longitudinal comparison of CPJAS scores, ankle axial alignment scores at age 6 years (JOS exit) were converted from the original JOS score, and crepitus was excluded. A comparison of physical examination scales is shown with supplemental Table 2.
Numbers of joint and total bleeds were calculated using treatment logs, annual recall, and medical records. For joint, muscle, and soft tissue bleeds, a bleeding episode was defined as pain and swelling treated with additional FVIII.22 Mucosal bleeding was also recorded. Two bleeding treatments for the same body part within 72 hours of each other were counted as a single bleeding episode.22 Average number of bleeds per year, or annualized bleed rate (ABR), for each patient was calculated by dividing the total number of bleeds for the time period using the annualized number of months with adequate bleed records. A time period was considered to have adequate bleed records if log books were available, or if there was evidence in the medical record of monthly contact between the participant and the clinic or study team. The annualized joint bleed rate (J-ABR) excluded nonjoint bleeds from the count.
Physical activity and sports
Sports and physical activity data were collected annually, and each participant’s routine sports activities were categorized according to the National Hemophilia Foundation (NHF) risk category.23 The maximum risk sport that each participant played for >1 season was used for analysis.
Statistical analyses
Odds ratios were calculated using univariate logistic regression. Relative risk was also calculated to make the current results directly comparable to the original JOS findings. Paired comparisons (MRI scores, CPJAS, and ABRs) were made using independent sample Student t tests (continuous normally distributed variables), Mann-Whitney U (continuous skewed variables), χ2, or Fisher's exact tests (counts) as appropriate. Longitudinal analyses for the MRI and CPJAS scores were performed using 2 × 3 mixed-model analyses of variance (ANOVAs), with the prophylaxis group as the between-subjects variable and age group (JOS exit, JOS-C entry, and JOS-C exit) as the within-subjects variable. Repeated measures correlations, which account for the decreased variability between joints within 1 individual,24 measured the relationship between each individual joint’s ABR, CPJAS score, and eMRI score. Participants with high-titer inhibitors were reported as a separate group, too small for statistical analysis. Statistical analyses were performed using SPSS V25.0 and R 3.5.2.
Results
Timing of data collection
Data were collected retrospectively from the time of JOS exit to JOS-C entry, and the retrospective time period ranged from 4.2 to 13.5 years (median, 8.5 years). Participants were followed prospectively after entry into JOS-C for 0 to 7.6 years (median, 3.4 years). Sports and Haemo-QOL surveys were only collected during the prospective data collection periods. Adequate bleed data (monthly logbooks available, or medical records available with at least monthly contact with clinic or research staff) were available at JOS-C exit for a mean of 10.6 years (median, 11.8 years; range, 3.5-12.8 years). When JOS-C data were combined with pre-JOS and JOS bleed data to get “lifetime” bleed data, adequate bleed data were available for a mean of 17.1 years (median, 18.1 years; range, 12.8-18.9 years). The mean percentage of time from birth to JOS-C exit with adequate bleed data was 92.7% (median, 100%; range, 52.4% to 100%).
Participants from the initial JOS study came from 15 sites in the United States. JOS-C participants came from 11 of those sites. Ten participants of the JOS-C were treated at the University of Colorado.
Baseline characteristics
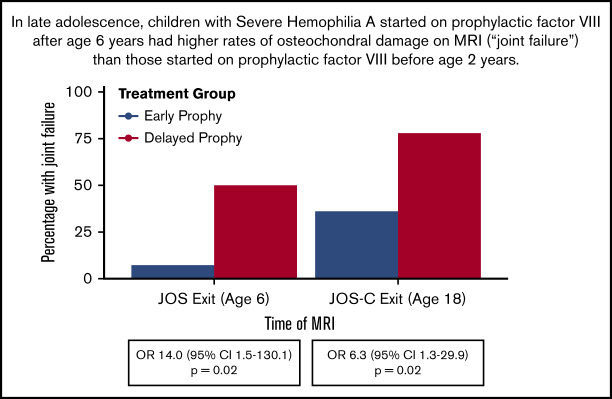
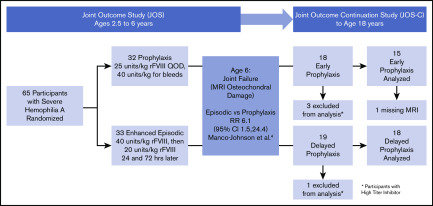
JOS-C participants enrolled between January 2010 and September 2015, and exited between December 2012 and September 2017. Of the initial 65 boys in the JOS, 37 participated in the JOS-C, including 4 with a history of high-titer inhibitor, leaving 15 in the prophylaxis and 18 in the episodic groups (referred to as “early prophylaxis” and “delayed prophylaxis” groups, respectively) (Figure 1). The mean ages of prophylaxis initiation were 1.3 years (standard deviation [SD], 0.4 years; median, 1.1 years; range, 0.9-2.1 years) and 7.6 years (SD, 3.5 years; median, 6.2 years; range, 5.3-17 years) (P < .001). Three participants in the delayed prophylaxis group started prophylaxis after age 10 years. Table 1 shows outcomes at age 6 years in the subset of JOS participants who were enrolled in the JOS-C. Early prophylaxis participants had better outcomes at age 6 years compared with delayed prophylaxis, nearly identical to the original JOS sample. At age 6 years, the odds ratio of osteochondral damage in the delayed vs early prophylaxis group was 14.0 (95% confidence interval [CI], 1.5-130.1; P = .02), with a relative risk of osteochondral damage of 6.09 (95% CI, 0.92-40.21; P = .01). This relative risk was similar to that of the prophylaxis group in the original JOS study at age 6 years (6.1 [95% CI, 1.5-24.4]), indicating that the JOS-C group was representative of the original JOS group. The proportion of total joints with osteochondral changes was also lower in the early prophylaxis group (P = .02).
Figure 1.
Follow-up and analysis of study participants. All participants of the JOS were eligible to enroll in the JOS-C prior to age 18 years. CI, confidence interval; QOD, every other day; RR, relative risk.
Table 1.
Baseline characteristics of JOS-C study participants at the time of JOS study exit (age 6 y)
| Baseline characteristics at age 6 y | Early prophylaxis | Delayed prophylaxis | P |
|---|---|---|---|
| Participants in analysis, n | 15 | 18 | |
| Age of prophylaxis initiation, mean (SD), y | 1.3 (0.4) | 7.6 (3.5) | <.0001 |
| MRI OC damage, n/N (%) | |||
| Boys with OC damage | 1/15 (6.7) | 9/18 (50) | .020 |
| Joints with OC damage | 1/89 (1) | 9/102 (9) | .021 |
| No MRI damage, eMRI score < 1, n/N (%) | |||
| Boys with no damage | 11/15 (73) | 6/18 (33) | .022 |
| Joints with no damage | 85/89 (96) | 81/102 (79) | .001 |
| eMRI, median (IQR) of participant joint averages | |||
| MRI score | 0.08 (0-0.3) | 0.6 (0.02-1.7) | .07 |
| OC MRI score | 0 (0-0) | 1.2 (0.3-3.1) | .05 |
| ABR prior to age 6 y, mean (SD) | |||
| Joint ABR | 0.7 (0.8) | 3.8 (2.8) | .0002 |
| ABR | 3.2 (3.1) | 14.4 (6.6) | .0072 |
| CPJAS, average per joint, mean (SD) | |||
| Physical examination score | 1.0 (0.9) | 1.7 (1.4) | .094 |
Numbers expressed as mean (SD) or median (IQR) for continuous variables depending on normal or skewed distribution, and number (percentage) for count variables. Maximum CPJAS score, 31 points per joint; maximum MRI score, 45 per joint; maximum OC score, 36 per joint.
OC, osteochondral.
When enrolling in the JOS prior to age 2.5 years, all participants had MRI and physical examination scores of zero as part of the enrollment criteria. A summary of JOS entry and exit data for the full JOS population and the JOS-C subset population is shown in Table 2.
Table 2.
Comparison of full JOS population and JOS-C subset
| JOS*† | JOS-C subset‡ | |||
|---|---|---|---|---|
| Prophylaxis | Episodic | Early prophy | Delayed prophy | |
| Bleeds prior to JOS entry, mean (range) | ||||
| Total joint bleeds | 1.0 (0-5) | 0.6 (0-3) | 0.8 (0-3) | 0.4 (0-3) |
| Total bleeds | 6.2 (0-35) | 6.8 (0-32) | 8.1 (0-35) | 8.1 (0-32) |
| Annualized bleeds at age 6 y, mean (SD) | ||||
| Joint bleeds | 0.6 (1.4) | 4.9 (3.57) | 0.7 (0.8) | 3.8 (2.8) |
| Total bleeds | 3.3 (6.2) | 17.7 (9.3) | 3.2 (3.1) | 14.4 (6.6) |
Data from Manco-Johnson et al.4
For JOS, relative risk of MRI OC damage at age 6 years (episodic/prophylaxis) was 6.1 (95% CI, 1.5, 24.4).
For the JOS-C subset, relative risk of MRI OC damage at age 6 years (episodic/prophylaxis) was 6.09 (95% CI, 0.92-40.21).
Factor dosing and adherence
Data for adherence calculations were available for an average of 8.9 years (SD, 3.74; range, 0-13 years) per participant in 31 participants without high-titer inhibitors. Table 3 displays adherence rates for the early and delayed prophylaxis groups, which did not differ by treatment group (P = .15). Nine in the early prophylaxis group and 7 in the delayed prophylaxis group had >90% adherence recorded. Six participants had <80% average adherence, with the remaining between 80% and 90% adherent. Due to high and relatively equal adherence rates among participants and the small sample size, it was not possible to relate outcomes to variations in adherence.
Table 3.
Outcome data
| Early prophylaxis | Delayed prophylaxis | P, early vs delayed prophylaxis | High-titer inhibitor | |
|---|---|---|---|---|
| Participants in analysis, n | 15 (14 with MRI) | 18 | N/A | 4 |
| Age of JOS-C exit, mean (SD) | 17.5 (1.7) | 18.4 (0.3) | .1 | 18.7 (0.2) |
| MRI OC damage, n/N (%) | ||||
| Participants with OC damage | 5/14 (35) | 14/18 (77) | .02* | 2/4 (50) |
| Joints with OC damage | 11/84 (13) | 26/108 (24) | .06 | 3/24 (13) |
| Participants with surgery for hemophilic arthropathy | 1 | 2 | N/A | 0 |
| No MRI damage, eMRI < 1, n/N (%) | ||||
| Participants with no damage | 2/14 (14) | 2/18 (11) | .79 | 0/4 (0) |
| Joints with no damage | 40/84 (48) | 48/108 (44) | .66 | 9/24 (38) |
| eMRI, median (IQR) of participant joint averages | ||||
| eMRI score | 1.3 (0.5-2.2) | 2.3 (1.3-5.0) | .17 | 0.8 (0.7-2.0) |
| OC eMRI score | 0.04 (0-0.8) | 1.2 (0.3-3.1) | .08 | 0 (0-1.0) |
| Lifetime, mean (SD) [median (IQR)] | ||||
| J-ABR | 1.5 (1.2) [1.1 (0.7-2.3)] | 4.3 (3.7) [3.9 (2.4-5.2)] | .007* | 2.0 (1.8) |
| ABR | 3.5 (2.1) [2.7 (2.2-4.7)] | 10.6 (6.6) [9.5 (6.5-11.8)] | <.001* | 7.4 (8.8) |
| After prophy start, mean (SD) [median (IQR)] | ||||
| J-ABR | 1.6 (1.4) [1.1 (0.7-2.6)] | 4.0 (4.6) [2.8 (1.7-4.7)] | <.05* | 2.5 (2.5) |
| ABR | 3.3 (1.9) [2.7 (2.1-4.7)] | 6.2 (5.3) [4.6 (2.4-8.6)] | <.05* | 5.0 (4.3) |
| CPJAS, mean (SD) of participant joint averages | ||||
| Physical examination score | 2.4 (1.6) | 3.2 (2.2) | .23 | 2.4 (1.0) |
| Percent adherence, mean (SD) | 91.5 (9.4) | 82.2 (17.4) | .1 |
Numbers expressed as mean (SD) or median (IQR) for continuous variables depending on normal or skewed distribution, and number (percentage) for count variables. ABRs were normally skewed but are shown as both mean (SD) and median (IQR) for comparison with other publications. High-titer inhibitor descriptive statistics are also shown. Maximum CPJAS score 31 points, per joint; maximum MRI score, 45 per joint; maximum osteochondral score, 36 per joint.
P < .05 between groups.
Fourteen participants were maintained on 20 to 30 IU/kg, 6 were increased to 30 to 40 U/kg, 9 to 40 to 50 IU/kg, and 2 to 50 to 60 IU/kg FVIII, all every other day or 3 times per week. On the delayed prophylaxis arm, 8 of 18 participants (44%) were prescribed prophylaxis doses ≥40 to 50 U/kg, compared with 3 of 15 (20%) on the early prophylaxis arm. Six participants (4 delayed prophy, 2 early prophy), used long-acting factor products (Adynovate or Eloctate) for up to 2 years of the JOS-C study period. One participant on the early prophylaxis arm transitioned to on-demand therapy for 2 years, then resumed prophylaxis. The remaining participants on the early prophylaxis arm were on continuous prophylaxis.
Outcomes at study exit
By age 18 years, the odds ratio of osteochondral damage in the delayed vs early prophylaxis group was 6.3 (95% CI, 1.3, 29.9; P = .02), with a relative risk of osteochondral damage of 2.63 (95% CI, 1.14, 6.06; P = .02). Lifetime ABR and J-ABR from birth to 18 years in the early prophylaxis group were one-third that of the delayed prophylaxis group (Table 3). If only comparing bleeding rates after initiation of prophylaxis, the early prophylaxis group ABR and J-ABR were also significantly lower than the delayed prophylaxis group ABR and J-ABR (Table 3). The per-patient averages of eMRI and CPJAS (physical examination) scores are shown in Table 3 and used in longitudinal analysis to maximize comparability if single joints were unscorable (eg, due to hardware). The eMRI 6-joint total scores [median (interquartile range [IQR])] were 7.8 (3.3-25.5) in the early prophylaxis and 13.8 (7.9-37.9) in the delayed prophylaxis group. The CPJAS 6-joint totals were normally distributed and had a mean (SD) of 14.3 (9.3) in early prophylaxis and 19.1(13.1) in delayed prophylaxis. The outcomes of participants with high-titer inhibitors (all treated with immune-tolerance induction) are also shown in Table 3 (right column), and were intermediate between the early and delayed prophylaxis arms.
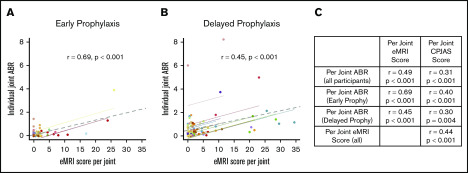
In an effort to relate joint outcomes to bleeding, repeated measures correlations were performed between J-ABR, CPJAS, and eMRI scores at the individual joint level. Figure 2 displays relationships between joint bleeding and physical or imaging examinations, adjusting for decreased joint variation within participants. Figure 2A-B show eMRI scores in relationship to same joint ABR, with the individual joints of a participant depicted in a single color. It is noted that the majority of joints with obligate osteochondral damage (eMRI score > 9) had a corresponding J-ABR of <2. Figure 2C shows significant correlation between variables for all participants, as well as for each prophylaxis group. J-ABR was moderately correlated with CPJAS score (r = 0.31, r = 0.40, and r = 0.30 for all noninhibitor participants, early prophy, and delayed prophy, respectively; all P < .001), and moderately to strongly correlated with eMRI score (r = 0.49, r = 0.69, and r = 0.45 for all noninhibitor participants, early prophy, and delayed prophy, respectively; all P < .001) at the level of individual joints.25,26 The early prophylaxis group showed more significant correlation between MRI and joint physical examination because there was less variability in the joint ABR. The effect was also seen when comparing outcomes at age 6 years (supplemental Figure 1) but was obscured when total body scores were substituted for individual joint scores.
Figure 2.
Repeated measures correlation between individual joint ABR and eMRI scores at age 18 years. (A) Early prophylaxis group. (B) Delayed prophylaxis group. Each color represents a participant, each dot represents an index joint, each colored solid line represents the summary correlation for that participant, and the gray dotted line represents the summary correlation for all data. (C) Additional repeated measures correlations with CPJAS scores.
There were 3 joint surgeries: 1 ankle fusion and 1 knee synovectomy on the delayed prophylaxis arm, and 1 ankle fusion on the early prophylaxis arm.
Longitudinal outcomes
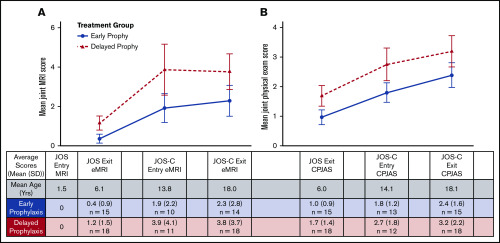
Figure 3 shows changes in joint MRI and physical examination scores over time in the 2 study groups. A 2 × 3 mixed-model ANOVA on MRI scores revealed a main effect of age (F = 10.98; P = .003) but not treatment group (F = 1.91; P = .18), possibly from low numbers (observed power, 0.26). Physical examination scores also increased with age (F = 7.04; P = .002), and differed marginally between treatment groups (F = 3.95; P = .06), but this also suffered from low power (0.48). No interactions between age and treatment group were observed (P = .50, P = .98), suggesting that the rate of joint change over time did not substantially differ between treatment groups.
Figure 3.
Longitudinal comparison. Average joint MRI scores (A) and physical examination scores (B) at JOS exit, JOS-C entry, and JOS-C exit between early prophylaxis (blue) and delayed prophylaxis (red) groups. Table column headers also reflect radiograph time-point labels for graphs. Error bars represent standard error mean.
Physical activity and sports
Sports and physical activity data were available for 24 participants without inhibitors, including 12 early and 12 delayed prophylaxis (Table 4). There was no osteochondral damage in 66% of early prophylaxis boys playing moderate-/high-risk sports (vs 67% of early prophylaxis overall) or in 37.5% of delayed prophylaxis playing moderate-/high-risk sports (vs 23% of delayed prophylaxis overall). All boys were on prophylaxis during sports participation, but factor administration data were not sufficiently detailed to determine whether replacement therapy was immediately prior to sports participation.
Table 4.
Sports participation and OC joint damage
| Maximum NHF risk category of sports | Early prophylaxis participant count | Delayed prophylaxis participant count | ||
|---|---|---|---|---|
| No MRI OC damage | MRI OC damage | No MRI OC damage | MRI OC damage | |
| 1: Low | 1 | 1 | 0 | 3 |
| 1.5: Low to moderate | 1 | 0 | 0 | 1 |
| 2: Moderate | 1 | 0 | 0 | 1 |
| 2.5: Moderate to high | 5 | 3 | 3 | 4 |
| 3: High | 0 | 1 | 0 | 0 |
| Total | 8 | 5 | 3 | 9 |
Sports participation did not seem to contribute to OC joint damage in the 24 participants without inhibitors who provided sports participation data.
Quality of life
There was no difference in the total score or any score domain, including physical, of the Haemo-QOL when comparing scores at JOS-C exit for participants in early vs delayed prophylaxis (mean [SD] total score, 18.7 [9.4] vs 14.3 [8.1] of 100, higher scores worse, P = .16), or scores for participants with vs without osteochondral damage (mean [SD] total score, 15.2 [7.4] vs 17.2 [10.8]; P = .56). In the free response section, most participant comments centered on the inconvenience and pain of infusion, or on limitations related to entering certain professions (ie, the military).
Participants with inhibitors
By the end of the JOS, all patients except 2 on the episodic arm had exposure to >50 doses of factor, and those 2 participants did not enroll in the JOS-C. Three participants of the JOS-C had a history of high-titer inhibitor, with peak titers 70 and 30 Bethesda units (BU) in the early prophylaxis arm and 21 BU in the delayed prophylaxis arm. An additional 3 children in the early prophylaxis arm developed inhibitors after JOS exit, including a high-titer inhibitor peaking at 16.4 Bethesda units (BU) that developed after 1751 factor exposures and 2 transient low-titer inhibitors (1.4 and 0.8 BU) that developed after 979 and 1001 factor exposures. Those with a history of high-titer inhibitors (>5 BU) were excluded from the outcome analysis of the JOS-C, as their outcomes were not felt to be a true reflection of early vs delayed prophylaxis, and their data are presented separately (Table 3). Two of 5 boys on early prophylaxis with osteochondral damage had a low-titer inhibitor.
Discussion
In this study, children who had initiation of FVIII prophylaxis delayed beyond 6 years of age continued to have significantly increased risk of MRI osteochondral damage and higher ABR and J-ABR than those who started prophylaxis prior to age 2.5 years, and prior to any joint damage on MRI.4 This supports the recommendation of the World Federation of Hemophilia that early initiation of prophylaxis, prior to the first joint bleed, is critical to maintaining joint health in patients with severe hemophilia.11 This age is critical as most 6-year olds can receive peripheral infusions every other day without a port, whereas many 2-year olds cannot; central venous access devices make infusion easier but require surgery and can lead to infection, thrombosis, and mechanical malfunction. These results confirm previous retrospective studies showing that joint outcomes are better with earlier initiation of prophylaxis.9,27-29 In addition, the ESPRIT trial, in which Italian children ages 1 to 7 years with severe HA were randomized to prophylactic or episodic treatment of bleeding, showed that the 8 children who started prophylaxis prior to age 3 years had no radiograph evidence of joint damage by Pettersson score at age 12 years, whereas many who started prophylaxis later did have joint damage on radiograph.30 Nijdam et al31 showed decreased radiograph evidence of hemophilic arthropathy in patients starting prophylaxis before vs after age 6 years, although they did not see a difference in radiograph outcomes comparing prophylaxis initiation before and after age 3 years. The JOS-C used MRI, which is more sensitive than a radiograph to earlier but clinically significant joint damage.32 This study is the first to our knowledge with long-term follow-up of children with severe HA whose treatment regimen was initially randomized.
These results are striking in part because only a very small proportion of participants and joints survived to adolescence without damage, despite full early prophylaxis (Table 3). Equally striking in both early and delayed prophylaxis groups was the relatively low individual joint ABR that resulted in joint damage on MRI and examination (Figure 2), emphasizing that it is important to prevent every joint bleed.
Joint MRI scores progressed more rapidly prior to age 14 years in children in delayed prophylaxis. This demonstrates the importance of bleeding on bone and cartilage development during the growing years and argues for the critical need of early institution of prophylaxis. Joint physical examination scores increased progressively through childhood and adolescence but were consistently higher in participants on delayed prophylaxis. Figure 3 shows that MRI is more sensitive than physical examination in detecting early damage. This suggests that early prophylaxis is more efficacious than delayed prophylaxis in limiting joint damage, but that joint damage may occur despite early initiation of prophylaxis using every other day prophylaxis with conventional recombinant FVIII. Standard prophylaxis is inadequate to completely protect joints in severe HA, and improved treatment regimens are required to achieve an optimal end point. No interactions between age and treatment group were observed in ANOVA analysis, suggesting that the rate of joint change in the treatment groups did not substantially differ from each other.
Individual joint eMRI scores for a participant were significantly related to that same joint’s ABR. This relationship between joint-specific bleeding rate and joint damage was stronger for the early prophylaxis group than for the delayed prophylaxis group (Figure 2C). This is due in part to the reduced variability in individual joint ABRs for the early prophylaxis group, with the majority of joint ABRs being very low. Individual joint physical examination and eMRI scores also correlated well, which indicates that joint physical examination is useful for joint monitoring in hemophilia, albeit delayed beyond evidence of damage on MRI. A specific threshold for physical examination score indicative of MRI joint damage could not be determined in this relatively small cohort.
Sports participation was common in children and adolescents with severe HA on prophylaxis and did not lead to an overall increase in hemophilic arthropathy (Table 4). This evidence supports the data of Broderick et al that physical activity is related to a transiently increased bleeding risk and mitigated by the factor level at the time of participation.33 This study confirms that sports participation on prophylaxis in the absence of joint bleeding does not worsen joint outcomes in severe hemophilia.
Overall Haemo-QOL scores reflected a good quality of life. The lack of difference in mean scores between treatment groups and osteochondral damage categories may reflect Haemo-QOL’s low sensitivity to detect differences in quality of life in generally healthy adolescents, or the small contribution of early joint damage to lessened quality of life.
There are weaknesses of the study to be noted. Only 57% of those in the JOS continued in the JOS-C. The interim data between JOS exit and JOS-C entrance were collected retrospectively, which was less accurate than continuous prospective data collection would have been. Because of the technology available when the study began, infusion data were collected using paper logs, which may have been less accurate and more easily lost than electronic logs. Although bleeding episode data were corroborated with medical records, it is possible that not all bleeding episodes were captured, and that bleeding rates may be underestimated. In addition, for adherence calculations, the frequency of prophylaxis (3 times per week or every other day) was not always clear retrospectively; assuming prophylaxis 3 times per week could have overestimated adherence.
Another potential weakness is the choice of physical examination scale. Complete psychometric validity has not yet been established for the pediatric Colorado Joint Assessment Scale; however, the related Colorado Adult Joint Assessment Score (CAJAS) has been fully validated.34 The CPJAS has been shown to have good interrater reliability, and normal values have been established in age-matched healthy controls.20 The CPJAS was chosen because it preserved all of the elements of the original JOS score.21 The complete psychometric validation in pediatrics of the Hemophilia Joint Health Score (HJHS), more commonly used today, was published after the JOS-C began35 and has more scoring element differences from the original JOS scoring system than the CPJAS, which would have compromised longitudinal analysis. Additional information on these scores is provided in the supplemental Data.
Initiation of prophylaxis prior to age 2.5 years is critical to protect the joints of patients with severe hemophilia. Those who have delayed prophylaxis initiation continued with a greater bleeding frequency and subsequent arthropathy development after prophylaxis initiation. Equally critical is the confirmation that every joint bleed count and low J-ABR can be associated with joint damage reflected in eMRI and physical examination.
The short-term bleeding outcomes of new hemophilia treatments such as emicizumab12,13 are quite promising for reducing bleeding; thus far, MRI or physical examination scores have not been reported, and the longest published follow-up period has been <1 year.36 The outcomes reported by the JOS-C, which followed joint development to the end of childhood, reveal the benefits and limitations of standard recombinant prophylaxis for severe hemophilia, and provide a reference standard against which outcomes of novel therapies could be compared.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Rick Shearer for maintenance of the study databases since 2005. The authors thank the study participants and their families most of all.
The JOS-C was supported by an investigator-initiated grant from Bayer Healthcare, which had no role in study design, data accrual, data analysis, or manuscript preparation. Clinical research personnel and study database management were funded in part by the Health Resources and Services Administration of the US Department of Health and Human Services Maternal and Child Health Bureau 340B Program (2H30MC24049; Mountain States Hemophilia Network).
Footnotes
The deidentified data set can be made available to other investigators by e-mailing the corresponding author, Beth Boulden Warren, at beth.warren@cuanschutz.edu.
Authorship
Contribution: B.B.W. processed, reviewed, and interpreted the data and wrote the first draft of the manuscript; D.T. performed all statistical analyses; J.S., M.F., and J.D.I. evaluated and scored all MRI images; M.J.M.-J., A.S., and S.F. developed the research plan and performed the research; H.D.L., C.M.B., A.D., and M.R. performed the research; K.L.N. coordinated the data collection, processing, and review; and all authors participated in manuscript revision.
Conflict-of-interest disclosure: B.B.W. has received research funding from the HTRS/Novo Nordisk Clinical Fellowship Award, the Bayer Hemophilia Awards Program Fellowship Project Award, and the CSL Behring Professor Heimburger Award, and has served as a consultant for Bayer Healthcare. M.R. receives research support from Bioverativ, Genentech, Novo Nordisk, and Shire, and serves on consulting/advisory boards for Bioverativ, CSL Behring, Genentech, Kedrion, Novo Nordisk, Pfizer, Shire, and uniQure. S.F. serves on a speakers’ bureau for Sanofi Genzyme. M.J.M.-J. has received research support from Bayer and serves on advisory boards for CSL Behring, HEMA Biologics, Genentech, Novo Nordisk, and Shire. The remaining authors declare no competing financial interests.
Correspondence: Beth Boulden Warren, University of Colorado Anschutz Medical Center, 13199 E Montview Blvd, Suite 100, Aurora, CO 80045; e-mail: beth.warren@cuanschutz.edu.
References
- 1.Su Y, Wong WY, Lail A, Donfield SM, Konzal S, Gomperts E; Hemophilia Growth And Development Study . Long-term major joint outcomes in young adults with haemophilia: interim data from the HGDS. Haemophilia. 2007;13(4):387-390. [DOI] [PubMed] [Google Scholar]
- 2.Buckner TW, Wang M, Cooper DL, Iyer NN, Kempton CL. Known-group validity of patient-reported outcome instruments and hemophilia joint health score v2.1 in US adults with hemophilia: results from the Pain, Functional Impairment, and Quality of life (P-FiQ) study. Patient Prefer Adherence. 2017;11:1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsyth AL, Witkop M, Lambing A, et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. 2015;9:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-544. [DOI] [PubMed] [Google Scholar]
- 5.Löfqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients–a long-term follow-up. J Intern Med. 1997;241(5):395-400. [DOI] [PubMed] [Google Scholar]
- 6.Astermark J, Petrini P, Tengborn L, Schulman S, Ljung R, Berntorp E. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105(4):1109-1113. [DOI] [PubMed] [Google Scholar]
- 7.Gruppo RA. Prophylaxis for hemophilia: state of the art or state of confusion? J Pediatr. 1998;132(6):915-917. [DOI] [PubMed] [Google Scholar]
- 8.Manco-Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115-2124. [DOI] [PubMed] [Google Scholar]
- 9.Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99(7):2337-2341. [DOI] [PubMed] [Google Scholar]
- 10.Bladen M, Main E, Hubert N, Koutoumanou E, Liesner R, Khair K. Factors affecting the Haemophilia Joint Health Score in children with severe haemophilia. Haemophilia. 2013;19(4):626-631. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia . Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. [DOI] [PubMed] [Google Scholar]
- 12.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809-818. [DOI] [PubMed] [Google Scholar]
- 13.Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811-822. [DOI] [PubMed] [Google Scholar]
- 14.Callaghan MU, Sidonio R, Pipe SW. Novel therapeutics for hemophilia and other bleeding disorders. Blood. 2018;132(1):23-30. [DOI] [PubMed] [Google Scholar]
- 15.Konkle BA, Shapiro A, Quon D, et al. BIVV001: the first investigational factor VIII therapy to break through the VWF ceiling in hemophilia A, with potential for extended protection for one week or longer [abstract]. Blood. 2018;132(suppl 1). Abstract 636. [Google Scholar]
- 16.Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65(2): [DOI] [PubMed] [Google Scholar]
- 17.Pollak E, Mühlan H, VON Mackensen S, Bullinger M; HAEMO-QOL Group . The Haemo-QoL Index: developing a short measure for health-related quality of life assessment in children and adolescents with haemophilia. Haemophilia. 2006;12(4):384-392. [DOI] [PubMed] [Google Scholar]
- 18.Hong W, Raunig D, Lundin B. SPINART study: validation of the extended magnetic resonance imaging scale for evaluation of joint status in adult patients with severe haemophilia A using baseline data. Haemophilia. 2016;22(6):e519-e526. [DOI] [PubMed] [Google Scholar]
- 19.Nuss R, Kilcoyne RF, Geraghty S, et al. MRI findings in haemophilic joints treated with radiosynoviorthesis with development of an MRI scale of joint damage. Haemophilia. 2000;6(3):162-169. [DOI] [PubMed] [Google Scholar]
- 20.Hacker MR, Funk SM, Manco-Johnson MJ. The Colorado Haemophilia Paediatric Joint Physical Examination Scale: normal values and interrater reliability. Haemophilia. 2007;13(1):71-78. [DOI] [PubMed] [Google Scholar]
- 21.Manco-Johnson MJ, Funk SM. Joint evaluation instruments in haemophilia In: Rodriguez-Merchan EC, ed.. The Haemophilic Joints: New Perspectives. Malden, MA: Blackwell Publishing; 2003. [Google Scholar]
- 22.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A; Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis . Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935-1939. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AM, Forsyth A. Playing it Safe; Bleeding Disorders, Sports and Exercise. New York, NY: National Hemophilia Foundation; 2005. [Google Scholar]
- 24.Bakdash JZ, Marusich LR. Repeated measures correlation [published correction appears in Front Psychol. 2019;10:1201]. Front Psychol. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis PD. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. Cambridge, United Kingdom: Cambridge University Press; 2010. [Google Scholar]
- 26.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. [DOI] [PubMed] [Google Scholar]
- 27.Coppola A, Tagliaferri A, Di Capua M, Franchini M. Prophylaxis in children with hemophilia: evidence-based achievements, old and new challenges. Semin Thromb Hemost. 2012;38(1):79-94. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(1):25-32. [DOI] [PubMed] [Google Scholar]
- 29.Fischer K, Ljung R. Primary prophylaxis in haemophilia care: guideline update 2016. Blood Cells Mol Dis. 2017;67:81-85. [DOI] [PubMed] [Google Scholar]
- 30.Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM; ESPRIT Study Group . A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700-710. [DOI] [PubMed] [Google Scholar]
- 31.Nijdam A, Foppen W, van der Schouw YT, Mauser-Bunschoten EP, Schutgens RE, Fischer K. Long-term effects of joint bleeding before starting prophylaxis in severe haemophilia. Haemophilia. 2016;22(6):852-858. [DOI] [PubMed] [Google Scholar]
- 32.Manco-Johnson MJ, Pettersson H, Petrini P, et al. Physical therapy and imaging outcome measures in a haemophilia population treated with factor prophylaxis: current status and future directions. Haemophilia. 2004;10(suppl 4):88-93. [DOI] [PubMed] [Google Scholar]
- 33.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308(14):1452-1459. [DOI] [PubMed] [Google Scholar]
- 34.Funk SM, Engelen S, Benjamin K, et al. Validity and reliability of the Colorado Adult Joint Assessment Scale in adults with moderate-severe hemophilia A. J Thromb Haemost. 2020;18(2):285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken). 2011;63(2):223-230. [DOI] [PubMed] [Google Scholar]
- 36.Shima M, Hanabusa H, Taki M, et al. Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors. Blood Adv. 2017;1(22):1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.