Abstract
The Hippo pathway and its effector protein YAP (a transcriptional coactivator) have been identified as important in the biology of both hepatocellular carcinoma and cholangiocarcinoma. First identified as a tumor suppressor pathway in Drosophila, the understanding of the mammalian YAP signaling and its regulation continues to expand. In its “on” function, the canonical regulatory Hippo pathway, a well-described serine/threonine kinase module, regulates YAP function by restricting its subcellular localization to the cytoplasm. In contrast, when the Hippo pathway is “off,” YAP translocates to the nucleus and drives cotranscriptional activity. Given the role of Hippo/YAP signaling in hepatic malignancies, investigators have sought to target these molecules; however, standard approaches have not been successful based on the pathways’ negative regulatory role. More recently, additional regulatory mechanisms, such as tyrosine phosphorylation, of YAP have been described. These represent positive regulatory events that may be targetable. Additionally, several groups have identified potentiating feed-forward signaling for YAP in multiple contexts, suggesting other experimental therapeutic approaches to interrupt these signaling loops. Herein we explore the current data supporting alternative YAP regulatory pathways, review the described feed-forward signaling cascades that are YAP dependent, and explore targeting strategies that have been employed in preclinical models of hepatic malignancies.
Key words: Hippo pathway, Lck, NUAK2, src family kinase, Tyrosine phosphorylation
INTRODUCTION
Yes-associated protein (YAP) and its ortholog transcriptional coactivator with a PDZ-binding domain (TAZ) function canonically as transcriptional coactivators and are the effector proteins of the Hippo pathway. The Hippo pathway is a serine/threonine kinase module that phosphorylates YAP on serine residues, especially serine 127, the consequence of which is sequestration of YAP in the cytoplasm, limiting its coactivating transcriptional function. In this manner, the Hippo pathway acts as a regulator of YAP function, with the components and function of the pathway well described1–9 (Fig. 1). The recognition that the pathway acts as a tumor suppressor, with mutations/deletions in the pathway associated with tissue overgrowth and oncogenesis [including well-documented association with development of hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and mixed hepatocellular CCA tumors], has led many investigators to seek strategies to target the pathway therapeutically. Given its negative regulatory function, this has proven difficult with standard approaches. More recently, novel, non-Hippo, regulation of YAP has been described. This regulation has focused on tyrosine phosphorylation of YAP, which activates YAP, independent of the Hippo pathway. This regulation has been described in both malignant and benign conditions. Moreover, additional evolving concepts in YAP signaling have recently been reported, namely, the concept of feed-forward oncogenic signaling pathways whereby YAP can drive the transcription of target genes involved in “activating” YAP. Herein we will review briefly the canonical regulatory pathway, focus on evolving concepts of YAP regulation, and review YAP in hepatic malignancies. We also discuss experimental approaches for targeting YAP in cancer.
Figure 1.
The canonical Hippo kinase module. The canonical Hippo pathway kinase module consists of mammalian Sterile 20-like kinase 1/2 (MST1/2), large tumor suppressor 1/2 (LATS1/2), the scaffolding protein Salvador 1 (SAV), and Mps One Binder (MOB), which phosphorylates YAP on serine 127 (S127), restricting YAP to the cytoplasm.
HIPPO PATHWAY AND YAP/TAZ REGULATION BY SERINE PHOSPHORYLATION (FIG. 1)
Examination of YAP/TAZ regulation typically begins with the canonical regulatory entity the Hippo pathway and its core components, a series of serine/threonine kinases, and other associated proteins. The Hippo pathway acts as a brake, restraining YAP activity, such that “activation” of the pathway culminates in serine phosphorylation of YAP and its cytoplasmic sequestration. This canonical regulatory pathway was first described in Drosophila. Mutagenesis screens, examining potential tumor suppressor genes in mosaic flies, led to recognition of a fly with overgrowth of the imaginal disc. Several components of the Hippo pathway were identified and cloned, including Wts (human ortholog large tumor suppressor 1/2), Hpo (human ortholog mammalian sterile twenty-like 1/2), and Sav (human ortholog Salvador)2,3,8. The fly and human proteins share sufficient homology that expression of the human counterparts of the Hippo pathway compensates for their loss in Drosophila 3,10. Since the discovery of the Hippo pathway, the understanding of its complexity and cross-talk with other pathways has advanced considerably. The Hippo pathway does not have a dedicated cell surface receptor nor ligand; instead, a variety of signaling pathways can interact with and regulate Hippo pathway activation. Hence, YAP integrates multiple signaling pathways. Several of these outside regulatory inputs have been investigated, but many have remained unstudied; however, irrespective of the signal engaging the Hippo pathway, frequently its initiation leads to activation of mammalian STe20-like kinase 1/2 (MST 1/2), which phosphorylates and subsequently activates large tumor suppressor 1/2 (LATS 1/2), followed by phosphorylation of YAP on serine residue 127 by LATS1/211. Phosphorylation at Ser127 on YAP results in its cytosolic retention through recognition and binding by the acidic adapter protein 14-3-3, essentially restricting YAP from entering the nucleus and preventing its transcriptional activity12. Although serine 127 has been identified as the canonical “Hippo” phosphorylation site, additional work has demonstrated that mutant YAP with all five serine residues changed to alanine (5SA-YAP) enhances YAP activity in stimulating cell proliferation and oncogenic transformation as compared to wild-type YAP13. Subsequent work has suggested that serine 127 and 381 are the most important residues for oncogenic transformation13; however, taken together, these results suggest that additional serine residues other than S127 may serve as unique regulatory sites in YAP. For example, the other HXRXXS motif that has been shown to be phosphorylated in vivo by LATS is Ser381 (in isoform 2)/Ser397 (in isoform 1). Phosphorylation of this motif leads to additional phosphorylation of YAP by casein kinase 1 epsilon (CK1δ/ε) and subsequent ubiquitination and degradation9. The specificity of LATS for an individual site, and/or the cellular context regulating this specificity, has yet to be defined. Further complicating the understanding of the regulatory mechanisms for YAP are the observations that other serine kinases such as Akt and Nemo-like kinase (NLK) can also engage these regulatory sites12,14. Since mutations in the Hippo pathway components are rare, much of the recent work performed to date on the Hippo pathway and YAP regulation examines the regulation of Hippo component activity through outside factors influencing Hippo signaling, including YAP target genes.
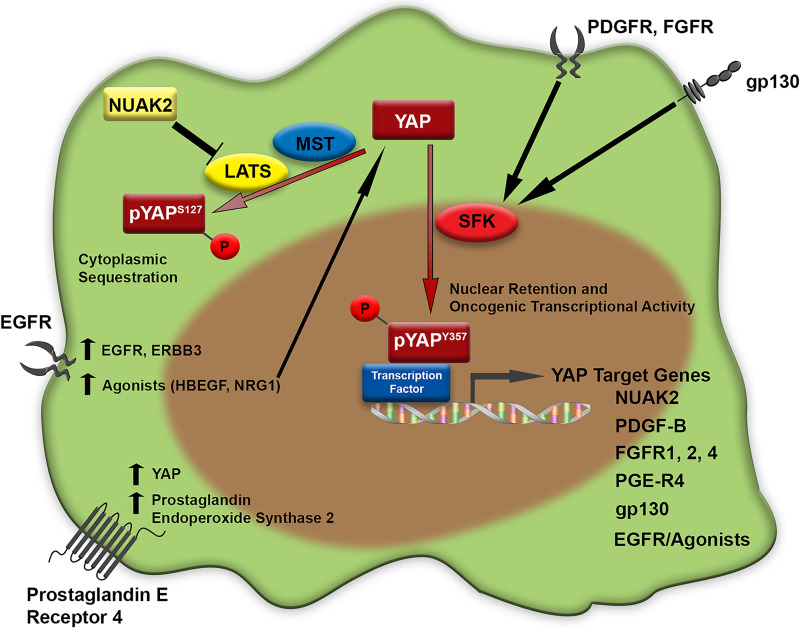
FEED-FORWARD YAP SIGNALING (FIG. 2)
Figure 2.
Feed-forward autocrine YAP activation. Multiple feed-forward autocrine loops leading to upregulation of YAP levels and/or function are demonstrated.
The model in which YAP transcriptional targets may regulate either the Hippo pathway directly, or YAP directly, has been proposed in several recent studies (Fig. 2). Most recently, an small interfering RNA (siRNA)-based automated kinome screen for targets that may regulate YAP localization was reported15. This work identified NUAK2, a member of the AMP kinase family, as a positive regulator of YAP activity. Further investigation noted that LATS was required for the activity, indicating that NUAK2 was acting on the Hippo pathway as a negative regulator. Interestingly, the investigators also noted that NUAK2 itself was a YAP target gene, as confirmed by chromatin immunoprecipitation assays identifying YAP/TAZ, TEAD, and JUN binding to a known NUAK2 enhancer. This finding was further supported by work, published simultaneously, that identified NUAK2 as a conserved YAP transcriptional target comparing transcriptomes from the human CCA cell line HUCCT-1 and a murine model in which S127A-YAP is overexpressed in the mouse liver16. Thus, NUAK2 was confirmed as a YAP target gene in several tumor cell lines/models and was also shown to negatively regulate the Hippo pathway (at LATS), leading to YAP activation. Additionally, oncogenic cross-talk leading to feed-forward signal propagation has been observed in embryonal rhabdomyosarcoma models between the Notch pathway and YAP17. In these studies, Notch signaling was observed to upregulate YAP at a transcriptional level, with YAP then upregulating Notch ligands and the Notch transcription factor RBPJ. Another recent study demonstrated a novel mechanism of YAP activation in metastatic lymph nodes with an autocrine YAP signaling signature18. Upregulation of YAP via bile acid signaling in lymph nodes harboring metastatic melanoma was noted, in conjunction with upregulation of bile acid production in these nodes, supporting the concept of a potentiating feed-forward signal. These data strengthened this “feed-forward” concept of YAP signaling that has also been previously demonstrated for both G-protein-linked receptors and receptor tyrosine kinases. The first demonstration of this phenomenon was in ovarian cancer cell lines. These investigators demonstrated that the epidermal growth factor (EGF) receptors EGFR and ERBB3 as well as the agonists NRG1 and HBEGF were upregulated by YAP and subsequently demonstrated that the agonists could activate YAP in an autocrine feed-forward loop, promoting oncogenesis19. Similarly, evaluating the role of prostaglandin E2 (PGE2) signaling in colon cancer cell lines and a chemically induced murine colitis model, PGE2 upregulation of YAP mRNA levels and transcriptional activity was identified. Subsequently, these investigators demonstrated that increased YAP transcriptional activity was associated with upregulation of the PGE2-producing enzyme, prostaglandin-endoperoxide synthase 2, and the PGE2 receptor, prostaglandin E receptor 4, via a YAP–TEAD4 mechanism, the end result of which was a feed-forward PGE2 signaling loop mediated through YAP activity20. Additionally, gp130 was identified as a YAP–TEAD4 target gene in colorectal cancer cells. In this model, gp130 augmented activation of YAP via tyrosine phosphorylation by Src family kinases (SFK)21. This feed-forward loop was initiated by APC loss. Signaling through receptor tyrosine kinases leading to feed-forward YAP signaling has been demonstrated in CCA models22,23. Specifically, activation of the fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) pathways have been associated with YAP activation and subsequent ligand or receptor upregulation22,23. Furthermore, this line of investigation was notable for the observation that the regulation of YAP activity by PDGF signaling appeared to be regulated via SFKs, independent of the Hippo pathway itself, unlike what was demonstrated for the target NUAK216. Specifically, tyrosine phosphorylation of YAP on the tyrosine 357 residue appeared to be a nuclear retention signal in CCA.
TYROSINE PHOSPHORYLATION REGULATING YAP (FIG. 3)
Figure 3.
Tyrosine phosphorylation can regulate YAP localization/activity. Tyrosine phosphorylation of YAP via Src family kinases (SFK) is demonstrated, which inhibits binding to exportin-1 (XPO1). The role of phosphatases in regulating YAP activity/localization has yet to be determined.
While the canonical regulation of YAP activity is via the Hippo pathway, accumulating evidence suggests a central role for tyrosine phosphorylation in regulating YAP subcellular localization, and cotranscriptional activity, at least in certain contexts (Fig. 3). The role of Src family kinases in facilitating YAP phosphorylation has been observed in several studies and is gaining increasing recognition within the field as an important regulator of YAP activity. For example, the Src family kinase YES regulates embryonic cell self-renewal programs via a YAP–TEAD2 pathway24. In these studies, leukemia inhibitory factor (LIF), an interleukin-6 (IL-6) family cytokine, activated YES, which subsequently led to increased tyrosine phosphorylation of YAP. These investigators demonstrated both direct interaction of the SH2 domain of YES with the LIF receptor (gp130 subunit) and also demonstrated direct interaction of YES with YAP via coimmunoprecipitation. Subsequently, others evaluated β-catenin active colon cancer cell lines and suggested a YAP–TBX5 complex was required for proliferation25. Evaluation of YAP regulatory factors demonstrated that YAP and YES interacted in the β-catenin active colon cancer cell line SW480 and that both YES and SRC may phosphorylate YAP on the tyrosine 357 residue. Knockdown studies suggested that while SRC could phosphorylate YAP, only knockdown of YES was associated with inhibition of proliferation. Finally, these investigators rescued YAP knockdown with both wild-type and a tyrosine mutant (Y357F-YAP) and noted that phosphorylation of the Y357 residue was necessary to rescue proliferation. A role for SFK-mediated YAP activation in epithelial regeneration in the setting of inflammation, utilizing multiple models of inflammation and proliferation, has also been reported26. In a transgenic model in which gp130 was activated, intestinal epithelial cells (IECs) demonstrated an increase in serine phosphorylated YAP (Hippo-regulated YAP), even though the cells demonstrated increased YAP transcriptional activity. Further evaluation of alternative YAP regulatory mechanisms suggested that IECs had increased levels of active SFK, and stimulation with IL-6 was associated with increased levels of Y357-YAP phosphorylation. This was also evident when evaluating the tyrosine phosphorylation status of YAP in mouse hepatocytes following partial hepatectomy. These effects could be blocked utilizing a semiselective SFK inhibitor PP2. Similar to previous results, these investigators found that gp130 interacted directly with YES (based on coimmunoprecipitation) and also that SRC coimmunoprecipitated with gp130. Based on these previous observations, our group also explored the role of SFKs in regulating YAP subcellular localization and transcriptional coactivity in several models of CCA23,27. In these models, inhibition of PDGF with a small molecule inhibitor was associated with relocation of YAP from the nucleus to the cytoplasm and a decrease in YAP target gene transcription; however, the levels of S127-YAP phosphorylation were static or even slightly decreased, suggesting the Hippo pathway was not central to the observed effects on YAP. Subsequently, we noted that Y357-YAP phosphorylation was markedly decreased following PDGF inhibition. Focusing on Y357-YAP phosphorylation, and the SFKs as the most likely mediator of YAP tyrosine phosphorylation, we performed siRNA- and CRISPR/Cas9-mediated downregulation of individual SFK and observed a central role for the SFK family member LCK in regulating YAP tyrosine phosphorylation and nuclear retention in CCA27. Evaluation of LATS activation in these models did not identify any change in activity following SFK inhibition, further suggesting tyrosine phosphorylation represented a Hippo-independent mechanism of YAP regulation. These data were supported by recent work utilizing photobleaching combined with mathematical modeling to examine nuclear export rate for YAP; the export rate could be regulated specifically by tyrosine phosphorylation via Src family kinases and was noted to be the major determinant of YAP subcellular distribution28.
YAP IN HEPATIC MALIGNANCIES
Hepatocellular Carcinoma
The role of the Hippo pathway and YAP signaling in HCC is well documented. The seminal paper, clearly defining a kinase cascade in mammals controlling cellular proliferation and organ size, confirmed in vitro findings by overexpressing YAP in murine livers1. Overexpression of YAP in the short term leads to massive overgrowth of the liver, by hyperplasia, and longer-term overexpression of YAP leads to tumor formation consistent with HCC. Similarly MST1/2 and Sav1 conditional knockout mice as well as Mob1a/1b-deficient mice develop tumors, which have demonstrated either HCC, CCA, or mixed histology4,5,7,29. Demonstrating the importance of Hippo signaling to human HCC, evaluation of a gene signature associated with “silencing of the hippo pathway” was developed from MST1/2 and Sav1 mice, and when applied to human HCC patients was a significant negative prognosticator30. Importantly, this signature predicted outcome in both Western and Eastern patient cohorts. Other novel YAP/TAZ-related regulatory mechanisms associated with HCC include noncoding RNAs. While YAP has previously been demonstrated to sequester and limit the activity of p72, a subunit of the microprocessing machinery, there are also multiple specific miRNAs that have been described targeting either YAP/TAZ or components of the Hippo pathway, and associated with HCC31–33.
Furthermore, Hippo pathway signaling (and the effectors YAP/TAZ) has been associated with conditions predisposing to HCC development, such as nonalcoholic steatohepatitis (NASH) and fibrosis. The role of Hippo signaling in the progression from steatosis to NASH and development of HCC was recently explored in the background of hyperactive AKT signaling, a known model of NASH and HCC development. Liver-specific phosphatase and tensin homolog (PTEN) knockout (the negative regulator of AKT) was associated with high levels of p-AKT, steatosis, progression to NASH, and, eventually, HCC (typically at 50 to 60 weeks of age). PTEN and Sav1 double knockout mice were observed to have a markedly accelerated progression of fatty liver disease and tumor formation (by 15 weeks of age). Mechanistic studies on these models suggested that YAP/TAZ activity leads to upregulation of the insulin receptor substrate IRS2, which subsequently further increased the activation of AKT. In these models, downregulation of this signaling circuit via an AKT inhibitor was effective in reducing TAZ expression as well as overall liver size and the size of liver tumors34. TAZ has previously been associated with liver inflammation and progression to fibrosis, with silencing associated with decreases in inflammation and fibrosis, but without any difference in steatosis35. These data suggest a protective role of the Hippo pathway by restraining YAP/TAZ activity, and specific to AKT signaling, this protection likely requires Skp2, suppressing cell ploidy36. The role of the Hippo pathway in limiting progression of NASH to HCC is further supported by the finding that the obesity-associated protein, JCAD, was able to inhibit LATS2 by directly binding the catalytic domain, inhibiting the Hippo kinase module, leading to YAP activation, and the progression of NASH to HCC37. Upregulation of JCAD was observed in the setting of high free fatty acids. The role of YAP/Hippo signaling in the regulation of liver fibrosis has been explored in the setting of hepatic stellate cell (HSC) activation. YAP signaling has been demonstrated to be integral to both HSC activation directly as well as the ability of HSCs to upregulate metabolic pathways necessary for survival and proliferation, specifically glutaminolysis38,39. Finally, the end result of steatohepatitis and liver fibrosis is increasing extracellular matrix (ECM) stiffness, which can lead to changes in YAP/Hippo regulation due to mechanotransduction. The ability of mechanotransduction to regulate YAP has been well covered in previous reviews40–43; however, specific to hepatocarcinogenesis, recent work has linked the ECM proteoglycan Agrin to YAP activation and hepatocarcinogenesis44. Increasing ECM stiffness was associated with a dramatic increase in Agrin levels. Increased Agrin was associated with decreased activity of the Hippo kinase cascade and, subsequently, an increase YAP signaling activity.
Cholangiocarcinoma
The importance of YAP to CCA biology was first suggested in MST1/2 and Sav1 conditional knockout mice4,5,7. All mice in both models developed tumors with the MST1/2 demonstrating more HCC tumors, but intrahepatic CCA tumors were also noted, and the Sav1 knockout mice demonstrating mixed histology tumors. Other Hippo pathway knockout animals have also demonstrated either mixed histology or intrahepatic CCA tumors, while activated YAP models have been developed that also demonstrate either mixed histology or intrahepatic CCA29,45. For example, biliary instillation of a sleeping beauty-based set of transposons expressing myristolated AKT and S127A-YAP (YAP that cannot be negatively regulated by the Hippo pathway) leads to reproducible development of tumors that are histologically and immunophenotypically consistent with intrahepatic CCA45. Development of these tumors is markedly more efficient when administering IL-33, a known biliary mitogen, to the animals, and interestingly, IL-6 can be substituted for IL-33. Multiple investigators have evaluated YAP staining in clinical specimens of human CCA46–50. In these series, the majority of specimens have nuclear localized YAP, a hallmark of YAP activity. Strikingly, although YAP appears to be “activated” in a majority of human CCA specimens, mutations in Hippo pathway components are uncommon in human tumors. For example, in the TCGA patient cohort, only three patients (8%) had a mutation in Hippo pathway-related genes (two patients with a Salvador1 mutation and one patient with an NF2 mutation)51. As such, regulation of YAP via cross-talk from other signaling pathways has been of significant interest with demonstration of receptor tyrosine kinase activation of YAP (via Src family kinases) in CCA, independent of the Hippo pathway. Further delineation of other potential regulatory mechanisms remains an active area of research.
EXPERIMENTAL TARGETING STRATEGIES FOR YAP/HIPPO IN HEPATOBILIARY CANCER MODELS
While no YAP-specific targeting strategies are currently being evaluated in clinical trials, preclinical data are available in various tumor models demonstrating efficacy of novel approaches, including in CCA and HCC. The majority of these strategies have focused on interrupting feed-forward signaling cascades, disrupting the YAP-TEAD interaction, or targeting novel regulatory mechanisms such as noncoding RNAs. We have previously demonstrated efficacy of the pan-SFK inhibitor dasatinib in patient-derived xenograft CCA models27. In these studies, we noted a decrease in tyrosine phosphorylated YAP, decrease in YAP transcriptional activity, an increase in TUNEL-positive cells, and a markedly decreased tumor size as compared to control animals. Interestingly, others have previously demonstrated activity of dasatinib in isocitrate dehydrogenase (IDH)-mutated intrahepatic CCA, including xenograft models52. This study developed from a screen of small-molecule inhibitors against a large panel of cancer cell lines. The most significant effect was noted for dasatinib treatment of IDH-mutant CCA; however, these investigators did not evaluate the role of the Hippo pathway or YAP signaling specifically in their studies. Yuan et al. explored the efficacy of targeting NUAK2 as a means of targeting hepatic YAP activity in a murine knock-in model employing S127A-YAP16. These mice develop hepatomegaly, and employing a semispecific inhibitor in these animals, the investigators demonstrated a significant decrease in hepatomegaly and proliferating hepatocytes. Additionally, the investigators evaluated efficacy of their novel inhibitor in nude mice bearing CCA cell line xenografts. Prolonged treatment (30 days) was well tolerated and demonstrated efficacy manifested as significantly reduced tumor growth rates as compared to vehicle-treated controls. The tolerance of therapy is important to note, as our group has previously observed therapy-limiting toxicity when evaluating verteporfin in murine models of CCA22. Verteporfin, used clinically currently as a photosensitizer for photodynamic therapy, is known to interrupt TEAD–YAP binding; however, it may be limited therapeutically given off-target effects/toxicity. In HCC, targeting of the poly ADP-ribose polymerase (PARP) family members tankyrase 1 and 2 has been explored for therapeutic efficacy. Cell line-based studies have demonstrated therapeutic effect, which was associated with upregulation of the negative YAP regulators angiomotin-like 1 and 2 (AMOTL1/2) as well as decreased YAP protein levels and downregulation of YAP cotranscriptional activity53,54. Additional studies in HCC models have explored inhibition of Aurora kinases as a therapeutic strategy. Efficacy was noted, with evidence supporting downregulation of YAP downstream of the kinases as contributing to effects observed55.
CONCLUSIONS
The understanding of the roles and regulation of YAP, either directly or via the Hippo pathway, continues to expand. Multiple examples of feed-forward signaling in a variety of cancer models have been identified. Further delving into the regulation of YAP in these models has led to accumulating data supporting a role for SFK in regulating YAP directly via tyrosine phosphorylation, an interesting finding with potential therapeutic implications, although strategies targeting specific SFKs are presently limited by the significant similarities between family members and the lack of specific inhibitors. Finally, the importance of the YAP/Hippo pathway in the biology of hepatobiliary malignancies has been well described and underscores the continued interest in novel therapeutic targeting strategies, especially for these tumor types, strategies that are currently in preclinical testing phases.
REFERENCES
- 1. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007;130:1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003;114:457–67. [DOI] [PubMed] [Google Scholar]
- 3. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 1995;9:534–46. [DOI] [PubMed] [Google Scholar]
- 4. Lee KP, Lee JH, Kim TS, et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA 2010;107:8248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA 2010;107:1437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–97. [DOI] [PubMed] [Google Scholar]
- 7. Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA 2010;107:1431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tapon N, Harvey KF, Bell DW, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002;110:467–78. [DOI] [PubMed] [Google Scholar]
- 9. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP. Genes Dev. 2010;24:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao W, Zhang S, Turenchalk GS, et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21:177–81. [DOI] [PubMed] [Google Scholar]
- 11. Thompson BJ, Sahai E. MST kinases in development and disease. J Cell Biol. 2015;210:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 2003;11:11–23. [DOI] [PubMed] [Google Scholar]
- 13. Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–98. [DOI] [PubMed] [Google Scholar]
- 14. Moon S, Kim W, Kim S, et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill MK, Christova T, Zhang YY, et al. A feed forward loop enforces YAP/TAZ signaling during tumorigenesis. Nat Commun. 2018;9:3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan WC, Pepe-Mooney B, Galli GG, et al. NUAK2 is a critical YAP target in liver cancer. Nat Commun. 2018;9:4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slemmons KK, Crose LES, Riedel S, Sushnitha M, Belyea B, Linardic CM. A novel notch-YAP circuit drives stemness and tumorigenesis in embryonal rhabdomyosarcoma. Mol Cancer Res. 2017;15:1777–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CK, Jeong SH, Jang C, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019;363:644–9. [DOI] [PubMed] [Google Scholar]
- 19. He C, Lv X, Hua G, et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 2015;34:6040–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim HB, Kim M, Park YS, et al. Prostaglandin E2 Activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology 2017;152:616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taniguchi K, Moroishi T, de Jong PR, et al. YAP–IL–6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc Natl Acad Sci USA 2017;114:1643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizvi S, Yamada D, Hirsova P, et al. A Hippo and fibroblast growth factor receptor autocrine pathway in cholangiocarcinoma. J Biol Chem. 2016;291:8031–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smoot RL, Werneburg NW, Sugihara T, et al. Platelet-derived growth factor regulates YAP transcriptional activity via Src family kinase dependent tyrosine phosphorylation. J Cell Biochem. 2018;119:824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamm C, Bower N, Anneren C. Regulation of mouse embryonic stem cell self-renewal by a Yes–YAP–TEAD2 signaling pathway downstream of LIF. J Cell Sci. 2011;124:1136–44. [DOI] [PubMed] [Google Scholar]
- 25. Rosenbluh J, Nijhawan D, Cox AG, et al. Beta-catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 2012;151:1457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugihara T, Werneburg NW, Hernandez MC, et al. YAP tyrosine phosphorylation and nuclear localization in cholangiocarcinoma cells are regulated by LCK and independent of LATS activity. Mol Cancer Res. 2018;16:1556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ege N, Dowbaj AM, Jiang M, et al. Quantitative analysis reveals that actin and Src-family kinases regulate nuclear YAP1 and its export. Cell Syst. 2018;6:692–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishio M, Sugimachi K, Goto H, et al. Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci USA 2016;113:E71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sohn BH, Shim JJ, Kim SB, et al. Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mori M, Triboulet R, Mohseni M, et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell 2014;156:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi X, Zhu HR, Liu TT, Shen XZ, Zhu JM. The Hippo pathway in hepatocellular carcinoma: Non-coding RNAs in action. Cancer Lett. 2017;400:175–82. [DOI] [PubMed] [Google Scholar]
- 33. Hu Y, Yang C, Yang S, Cheng F, Rao J, Wang X. miR-665 promotes hepatocellular carcinoma cell migration, invasion, and proliferation by decreasing Hippo signaling through targeting PTPRB. Cell Death Dis. 2018;9:954. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Jeong SH, Kim HB, Kim MC, et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J Clin Invest. 2018;128:1010–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Zheng Z, Caviglia JM, et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang S, Chen Q, Liu Q, et al. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell 2017;31:669–84 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye J, Li TS, Xu G, et al. JCAD promotes progression of nonalcoholic steatohepatitis to liver cancer by inhibiting LATS2 kinase activity. Cancer Res. 2017;77:5287–300. [DOI] [PubMed] [Google Scholar]
- 38. Du K, Hyun J, Premont RT, et al. Hedgehog–YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology 2018;154:1465–79.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu HX, Yao Y, Bu FT, et al. Blockade of YAP alleviates hepatic fibrosis through accelerating apoptosis and reversion of activated hepatic stellate cells. Mol Immunol. 2019;107:29–40. [DOI] [PubMed] [Google Scholar]
- 40. Dobrokhotov O, Samsonov M, Sokabe M, Hirata H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin Transl Med. 2018;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343:42–53. [DOI] [PubMed] [Google Scholar]
- 42. Yu FX, Guan KL. The Hippo pathway: Regulators and regulations. Genes Dev. 2013;27:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell 2016;29:783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chakraborty S, Njah K, Pobbati AV, et al. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017;18:2464–79. [DOI] [PubMed] [Google Scholar]
- 45. Yamada D, Rizvi S, Razumilava N, et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 2015;61:1627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H, Wolfe A, Septer S, et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pei T, Li Y, Wang J, et al. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget 2015;6:17206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sugimachi K, Nishio M, Aishima S, et al. Altered expression of Hippo signaling pathway molecules in intrahepatic cholangiocarcinoma. Oncology 2017;93:67–74. [DOI] [PubMed] [Google Scholar]
- 49. Wu H, Liu Y, Jiang XW, et al. Clinicopathological and prognostic significance of Yes-associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumour Biol. 2016;37:13499–508. [DOI] [PubMed] [Google Scholar]
- 50. Marti P, Stein C, Blumer T, et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology 2015;62:1497–510. [DOI] [PubMed] [Google Scholar]
- 51. Farshidfar F, Zheng S, Gingras MC, et al. integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saha SK, Gordan JD, Kleinstiver BP, et al. Isocitrate dehydrogenase mutations confer dasatinib hypersensitivity and SRC dependence in intrahepatic cholangiocarcinoma. Cancer Discov. 2016;6:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jia J, Qiao Y, Pilo MG, et al. Tankyrase inhibitors suppress hepatocellular carcinoma cell growth via modulating the Hippo cascade. PLoS One 2017;12:e0184068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma L, Wang X, Jia T, Wei W, Chua MS, So S. Tankyrase inhibitors attenuate WNT/beta-catenin signaling and inhibit growth of hepatocellular carcinoma cells. Oncotarget 2015;6:25390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu F, Wang G, Wang X, et al. Targeting high Aurora kinases expression as an innovative therapy for hepatocellular carcinoma. Oncotarget 2017;8:27953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]