Abstract
Life histories can influence the degree of parasite infestations on a host. Pressures exerted on hosts based on age and sex convey varying degrees of parasite prevalence due to differences in host lifestyles, but it is not known how interactions between different host traits affect tick numbers. The objective of this study was to determine if host characteristics (e.g., age, sex, weight, and their interactions) affect the mean number of ticks found on small mammals regardless of host species or habitat. Sherman live traps were placed in forest and grass/forb habitats representative of the southeastern United States. After capture, host characteristics were recorded, and hosts were then searched for ticks. A total of 281 small mammals (148 Peromyscus leucopus, 34 P. maniculatus, 76 Sigmodon hispidus, 16 Microtus pinetorum, and 7 Ochrotomys nuttalli) and 610 ticks (488 Dermacentor variabilis, 114 Ixodes scapularis, 1 Amblyomma americanum, and 7 A. maculatum) were collected in this study. Host's age, sex, and weight affected the number of ticks collected from small mammals and significant interaction effects between host traits occurred (weight by sex, weight by age, and sex by age). For instance, female subadult rodents had significantly more ticks compared to female adults, male subadults had significantly fewer ticks compared to male adults, and the number of ticks on a host increased as host body mass increased. These results support the hypothesis that the number of ticks vary on rodent hosts based on life histories and trait interactions. Therefore, understanding the behavioral mechanisms of a host can aid in the management of parasites in the environment.
Keywords: Rodents, Ticks, Infestation, Age, Sex, Life-history
Graphical abstract
Highlights
-
•
Host life histories predict the number of ticks present on a rodent.
-
•
Male and subadult rodents are infested with an overall greater number of ticks than females and adults.
-
•
Rodents have more ticks as their weight increases.
-
•
Interactions between sex and age predict the number of ticks present on a rodent.
1. Introduction
Ticks are obligate parasites that cause direct damage to their vertebrate hosts by blood feeding, or indirectly when they transmit infectious agents that affect animal health (Gulia-Nuss et al., 2016). Understanding host susceptibility to tick infestation is important to elucidate the links that underlie the ecology of tickborne disease (TBD) in human and animal populations. Variation in parasite burden has been reported for different host classes (Klein, 2004). Parasitic infection may result from influential factors such as host physiology (Moore and Wilson, 2002) or behavioral characteristics that increase or decrease host exposure (Nunn and Dokey, 2006).
Population dynamics of parasites are often formed by host dissimilarities (Morand et al., 1996). In many instances, males have been infested with more parasites than have females, such as male damselflies acquiring more parasites than did females (Córdoba-Aguilar and Munguía-Steyer, 2013) and male mountain ungulates having higher prevalence, richness, and intensity of parasites compared to females (Martínez-Guijosa, 2015). Other studies have found differences in male-biased parasitism where female mice were more parasitized than were male mice (Sciutto et al., 1991) and Daphina magna females were more heavily parasitized compared to males (Duneau et al., 2012). In addition, host age has been shown to be significant in parasite burden (Hämäläinen et al., 2014; Izhar and Ben-Ami, 2015; Lesniak et al., 2017). Similar to host sex, studies have varied in the numbers of parasitic infestations between differing age groups; some have found parasites favoring older hosts, and others found preferences for younger hosts (Cichoń et al., 2003; Tinsley et al., 2012).
In addition, parasites can impose sex-specific consequences on fitness based on differences in selection between each sex; however, the entire impact of parasitism on fitness between the sexes is obscure (Vincent and Sharp, 2014). It was noted that certain life-history traits of hosts are shaped by parasitic infections (Fredensborg and Poulin, 2006). For bats there was no tendency for animals with poor body condition to have more parasites than did healthier bats (Zahn and Rupp, 2004), but being infested with louse flies actually led to poor weight gain (Linhares and Komeno, 2000). Thus, host weight could be an important factor for assessing parasites on a host.
Additional studies investigated differences in parasite abundances that vary between host weights. Sexual size dimorphism was investigated, and an association between male-biased parasitism was identified (Moore and Wilson, 2002). Another study conducted by Krasnov et al. (2005) found that fleas feeding on under-fed rodents produced larger clutches. Tseng and Myers (2014) noted that information is lacking in regard to food limitations on a host and its effect on parasite well-being. Parasites are inherently an important factor in terms of the membership processes in natural communities, but the mechanisms that form distributions of parasite occurrences across host populations are not well understood (Rodríguez and Valdivia, 2017).
Small mammals are known to be reservoir hosts of several Borrelia species and primary hosts for ticks (Oliver et al., 2003; Lynn et al., 2017). Therefore, understanding the quantitative and qualitative associations of tick occurrence or infestation of rodent hosts may be important for modeling maintenance, transmission, and prevention of TBD that influence human and animal health. Knowledge of relationships between hosts and tick species known to harbor TBD could provide crucial information for avoiding pathogen transmission (Sumner et al., 2007; Fritzen et al., 2011; James et al., 2015; Scott et al., 2016).
The purpose of the present study was to assess the magnitude of ticks on small mammals based on host characteristics such as age, sex, and weight, and interactions between traits. Investigations that analyze the effect of parasite load on host life-histories are necessary for understanding pathogen transmission; therefore, this investigation adds new insight towards understanding TBD in relation to a host. We tested the hypothesis that tick infestations are associated with rodent host characteristics from sites in southeastern U.S.
2. Materials and methods
2.1. Host and ectoparasite sampling
Rodents were collected with Sherman live traps baited with rolled oats. In the summer (June and July) of 2013, forest and grass/forb habitats at the Hobart Ames Plantation, a 7446.21-ha facility in Fayette and Hardeman counties near Grand Junction, Tennessee, were assessed. Transects were established and consisted of 20 traps spaced at 10-m intervals. Five transects were set in each of three habitats (pine forest, hardwood forest, grass/forb) resulting in 15 transects, which were trapped for 9 consecutive nights (3 nights per replicate) and resulted in 2700 trap nights.
All small mammals were handled following guidelines of the American Society of Mammalogists (Sikes and Gannon, 2011) and identified to species using keys from (Schwarts and Schwartz, 2016). Methods for mammal capture were approved by The University of Memphis (IACUC #0729). For rodents captured, four standard external measurements (in millimeters), species, sex, age, and total weight (grams) were recorded. Each rodent was examined for ectoparasites and tick species. Ticks were removed from rodents and placed in labeled vials filled with 70% ethanol. Ectoparasite species and life stages (larvae, nymph, and adult) were identified using dichotomous keys (Yunker et al., 1986; Durden, 1996; Keirans and Durden, 1998).
2.2. Statistical analysis
Statistical analysis consisted of a two-tailed analysis (α = 0.05) from Statistical Analysis Software (SAS, ver. 9.4) to test the effect of ectoparasite numbers on host life-histories (age, sex, and weight). Because we analyzed counts of infested and non-infested individuals, a generalized linear-mixed model (PROC GLIMMIX) with a Poisson distribution was used to analyze the number of ticks and rodent age, sex, and weight as main effects, with the addition of weight x sex, weight x age, and age x sex as two-way interactions.
3. Results
A total of 281 rodent hosts (Table 1) were captured, and 610 ticks (Table 2) were collected from the rodent hosts. There were 108 larvae and 6 nymph Ixodes scapularis, 458 larvae and 30 nymph Dermacentor variabilis, 1 larva and 6 nymph Amblyomma maculatum, and 1 larva A. americanum collected. Overall, more adult rodents were captured (N = 244) than subadult rodents (N = 36), there were slightly fewer female (N = 133) rodents than males (N = 145) collected, and most rodents (N = 180) did not have any ectoparasites present. The rodents represented five species (76 Sigmodon hispidus, 148 Peromyscus leucopus, 34 P. maniculatus, 16 Microtus pinetorum, and 7 Ochrotomys nuttalli) of which 100 were infested with ticks (35.58% prevalence; Table 1). Prevalence is the number of hosts infested divided by the number of hosts captured. The 610 ticks represented four tick species (1 Amblyomma americanum, 7 A. maculatum, 488 D. variabilis, and 114 I. scapularis). Dermacentor variabilis was not only the most abundant tick identified, but it was the only tick collected from all five rodent species examined. The least abundant tick species was A. americanum, and it was collected on a single O. nuttalli. The two most abundant rodents captured (S. hispidus and P. leucopus) were infested with different tick species, such that S. hispidus had A. maculatum and D. variabilis while P. leucopus had A. maculatum and I. scapularis. Ixodes scapularis also was collected from P. maniculatus and O. nuttalli.
Table 1.
Number of each rodent species captured, number of rodents infested with ticks, mean weight and standard error of each rodent species, and each rodent species captured categorized by sex and age for rodent hosts (Peromyscus leucopus, Sigmodon hispidus, Peromyscus maniculatus, Microtus pinetorum, and Ochrotomys nuttalli) captured at the Hobart Ames Plantation, Fayette and Hardeman counties, Tennessee.
| Rodent species | No. Captured | No. Infested |
Host Weight (grams) | No. Males | No. Females | No. Adults |
No. Subadults |
Infested adults | Infested subadults |
|---|---|---|---|---|---|---|---|---|---|
| S. hispidus | 75 | 23 | 83.77 ± 5.308 | 40 | 34 | 53 | 22 | 17 | 6 |
| P. leucopus | 148 | 58 | 16.35 ± 0.847 | 86 | 61 | 141 | 7 | 53 | 5 |
| P. maniculatus | 34 | 9 | 14.90 ± 0.382 | 11 | 23 | 34 | 0 | 9 | 0 |
| M. pinetorum | 16 | 6 | 20.93 ± 0.835 | 6 | 10 | 15 | 1 | 5 | 1 |
| O. nuttalli | 7 | 4 | 13.78 ± 0.406 | 2 | 5 | 7 | 0 | 4 | 0 |
| Total | 281 | 100 | 35.07 ± 2.354 | 145 | 133 | 250 | 30 | 88 | 12 |
a sex was not recorded on one specimen.
Table 2.
Mean number (±SE) of ticks found on each species of rodent, separated by age and sex and all rodent sex and age interactions.
| Rodent Species | No. Ticks Collected |
Mean Number of Ticks ± SE |
||||
|---|---|---|---|---|---|---|
| All Hosts | Adult | Subadult | Male | Female | ||
| All Rodents | 610 | 2.17 ± 0.519 | 2.04 ± 0.512 | 3.16 ± 2.109 | 3.37 ± 0.992 | 0.81 ± 0.165 |
| Sigmodon hispidus | 395 | 5.19 ± 1.846 | 1.73 ± 1.434 | 13.77 ± 4.987 | 7.65 ± 2.922 | 2.61 ± 2.232 |
| Peromyscus leucopus | 160 | 1.09 ± 0.173 | 0.87 ± 0.145 | 3.38 ± 1.140 | 1.18 ± 0.227 | 0.98 ± 0.277 |
| Peromyscus maniculatus | 17 | 0.5 ± 0.185 | 0.5 ± 0.185 | 0 ± 0.000 | 0.36 ± 0.203 | 0.56 ± 0.257 |
| Microtus pinetorum | 25 | 1.56 ± 0.737 | 1.26 ± 0.635 | 0 ± 0.000 | 1.33 ± 0.988 | 1.7 ± 0.919 |
| Ochrotomys nuttalli | 13 | 1.85 ± 0.961 | 1.85 ± 0.961 | 0 ± 0.000 | 0.5 ± 0.500 | 2.4 ± 1.288 |
| All Rodents | 610 | 2.17 ± 0.519 | 2.04 ± 0.512 | 3.16 ± 2.109 | 3.37 ± 0.992 | 0.81 ± 0.165 |
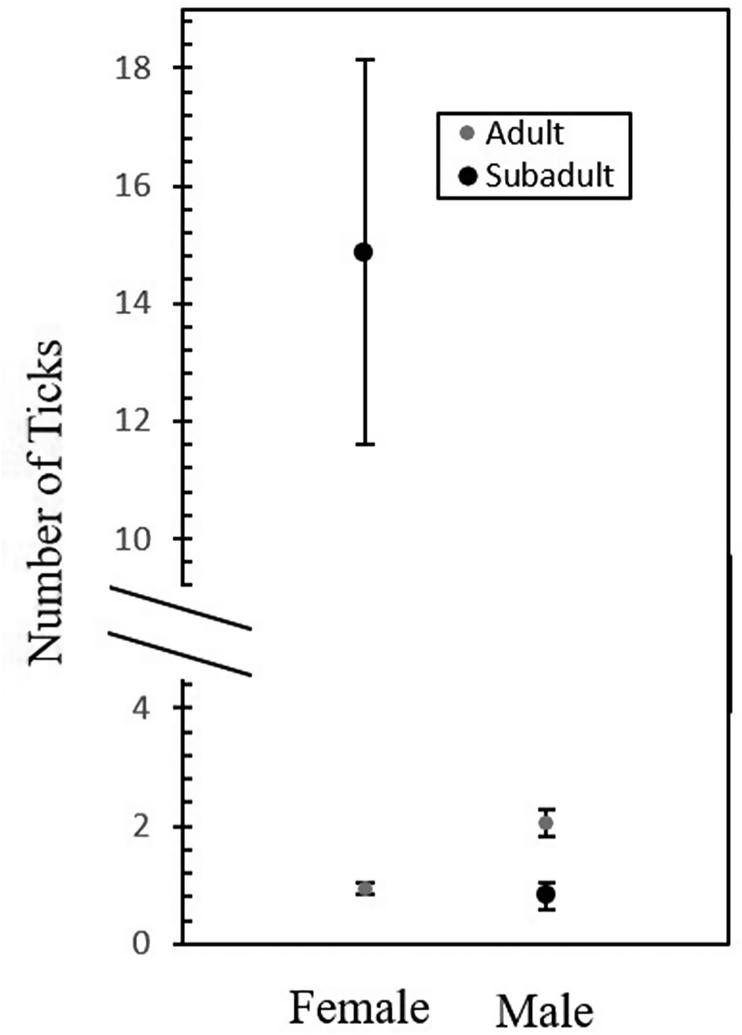
aMean number (±SE) of ticks found on female adults was 0.94 ± 0.106, female subadults 14.86 ± 3.262, male adults 2.05 ± 0.219, and male subadults 0.81 ± 0.226.
The number of ticks per host was analyzed for relationships with life histories of hosts (Table 2). Number of ticks per host was significantly different based on host sex, age, weight, and their interactions. Although more adult rodents were captured than subadult rodents, subadults had an overall higher mean number of ticks per host than adults (F = 13.60; df = 1, 239; P = 0.0003) (Fig. 1). More ticks were collected from male hosts compared to female hosts (F = 94.06; df = 1, 239; P < 0.0001). Males of the two most frequently captured rodent species (S. hispidus and P. leucopus; Table 2) had a greater mean number of ticks than did females, whereas female P. maniculatus, M. pinetorum, and O. nuttalli had more ticks than did males. Heavier rodents also had more ticks compared to lighter rodents (F = 17.72; df = 1, 239; P < 0.0001).
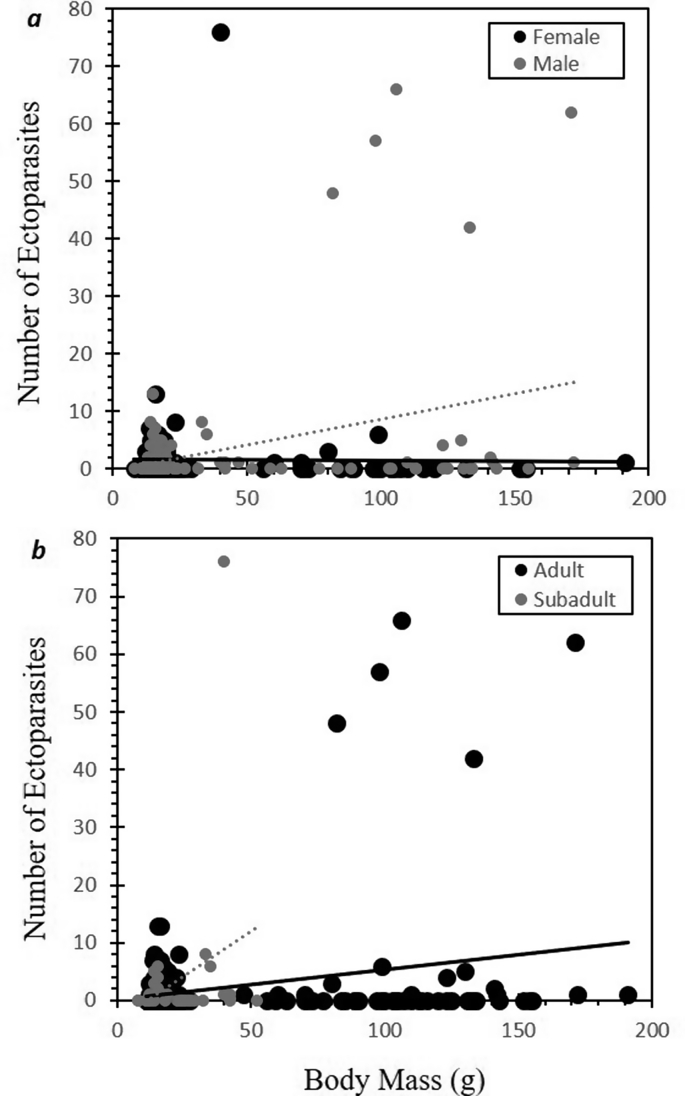
Fig. 1.
Number of ticks present on rodent hosts (Peromyscus leucopus, Sigmodon hispidus, Peromyscus maniculatus, Microtus pinetorum, and Ochrotomys nuttalli) captured at the Hobart Ames Plantation, Fayette and Hardeman counties, Tennessee. (a) Number of ticks by host sex x weight interaction (males y = 0.009x + 2.135; females y = 0.022x - 0.09). (b) Number of ticks by host age x weight interaction (adult y = 0.051x + 0.203; subadult = 0.310x - 3.499).
Interaction effects between host sex and age, host weight and sex, and weight and age for the number of ticks per host also were statistically significant. Female subadult rodents had more ticks per host compared to male subadults and male adult rodents had more ticks per host than female adult rodents (F = 113.38; df = 1, 239; P < 0.0001). Relationships between weight x age and between weight x sex for the number of ticks per host (Fig. 2) showed tick numbers increased for adults and subadults as host weight increased (F = 45.23; df = 1, 239; P < 0.0001). Additionally, the number of ticks per host increased with male rodent weight, while the slope for the female hosts remained relatively stable (F = 61.15; df = 1, 239; P < 0.0001).
Fig. 2.
Mean number of ticks per host by sex and age of rodent hosts (Peromyscus leucopus, Sigmodon hispidus, Peromyscus maniculatus, Microtus pinetorum, and Ochrotomys nuttalli) captured at Hobart Ames Plantation, Fayette and Hardeman counties, Tennessee.
4. Discussion
Parasites represent principal constituents of natural communities; however, it remains unknown how parasite occurrence and influence form skewed distributions over host populations (Rodríguez and Valdivia, 2017). In the present study, male adult rodents had more ticks than female adult rodents. This difference could be due to contrasts in life histories between the two sexes. The male rodents in this study have larger home ranges for acquiring mates (Frafjord, 2016), which could lead to greater contact with ticks in the environment. Sex-specific life histories and affiliations between trade-offs stem from sex differences in immune responses between immune function and reproductive contributions (Hämäläinen et al., 2015). The grooming of offspring done by maternal rodents could promote decreases in parasite populations within the female rodent nest (Champagne et al., 2003).
There is contrasting literature concerning parasite occurrence on hosts based on host sex. In our study, males were infested with an overall greater number of ticks than were females and males had more ticks as weight increased (Fig. 1a). Klein (2004) noted that males are more resistant to some parasites contrary to females, but male parasitism occurs more frequently than female parasitism. Males may have more ticks than do females due to growth and reproduction (Moore and Wilson, 2002) or due to larger home ranges (Krasnov et al., 2005). Halliday et al. (2014) reported that yarrow's spiny lizard males with higher testosterone levels have more ectoparasites due to immunosuppression. Alternatively, female bats have more ectoparasites due to females gathering in nursery colonies and while their immune system is suppressed during reproduction; whereas, males tend to occupy less dense or isolated areas (Christe et al., 2007). Kuris et al. (1980) noted host size could be a function of increased resource availability for parasites to avoid competition. The contrasting literature between host gender and parasite burden and among different taxa further exemplifies the need to understand how species life histories corresponds to ectoparasite prevalence and burden.
Analogous to sex, numbers of parasites based on host age indicated that subadult rodents supported larger parasite communities than did adults. Similar to our findings that subadults had more parasites than did adults (Fig. 1b), Hämäläinen et al. (2015) found parasite burdens in older gray mouse lemur tended not to be as prevalent as in younger hosts and noted that the substantially higher ectoparasite burden on younger hosts could potentially stem from differences in host behavior, nutrition, or age-related immunity that differentiate them from older rodents. Alternatively, Ujvari and Madsen (2006) found decreases in immune response of tropical pythons with increasing age and an increased parasite burden.
Host size also showed differing effects on the number of ectoparasites in the present study, which is suggested to be indicative of overall health (Summerbell et al., 1993). Ectoparasite numbers increased as small-mammal weight increased for males, females, adults, and subadults. Similarly, Chu et al. (2019) found a trend in which louse prevalence was positively correlated with body mass of birds, and Rodríguez and Valdivia (2017) found increases in prevalence of the parasite Profilicollis altmani in molecrabs. Also, Arneberg (2002) found increasing helminth parasite burdens as mammalian host weight increased and noted that the trend was notably due to larger hosts having longer lifespans and increased parasite survival. It was noted by Tseng and Myers (2014), while using the cabbage looper as a host to viral parasites, that weight is often considered a major determinant of host condition, and deficient food supplies often lead to diminishing host quality. In a study analyzing fleas on rodent hosts, Hawlena et al. (2007) noted parasite populations depend on host prosperity such as fecundity, offspring condition, and rate of survival. Tseng and Myers (2014) found virus fitness increased in response to sufficient food availability of their host; the virus benefitted from host resource availability. A similar situation could have occurred in our study where ectoparasites benefitted by the favorable environment on their larger host.
Hammerschmidt and Kurtz (2005) noted that parasites performed better when they were able to avoid detection by their host's immune systems. It could be that parasites would more readily parasitize larger healthier hosts rather than unhealthy hosts with over-active immune systems. Here, subadult females had the most ticks, while subadult males had the fewest. Also, Hawlena et al. (2007) found large ectoparasite densities on juveniles and proposed that it was most likely a consequence of greater survival and reproductive output of the parasites on these rodents, noting that juveniles spent less time grooming than adults. Grooming is the most important animal behavior used to reduce the number of parasites present on a host (Mooring et al., 2004). The occurrence of subadult female rodents with the greatest number of ticks is not well understood. Polygynous parents devote more energy into male offspring than female offspring under favorable conditions (McGuire et al., 2014). Investments for male and female offspring in polygynous rodents shifted among litters, based on food availability and the mother's body weight (Shibata and Kawamichi, 2009). Because the majority of rodent species examined have promiscuous-mating systems, subadult female rodents in this study might have higher parasite burdens due to decreased rearing effort by parents (Ribble and Millar, 1996; Becker et al., 2012).
Differences in the number of ticks on a host are likely a product of the host's behavior and life-history characteristics. Further investigations, such as the addition of ecological factors and the rate at which hosts become infected following treatment, are needed to better understand this model. The present study provides insights into the occurrence of ticks on natural populations of rodent hosts.
Declaration of competing interest
The author declared that there is no conflict of interest.
Acknowledgments
We are thankful to the Hobart Ames Foundation for its support, and to James Morrow, Larry Teague, and Jimmy Simons at Ames Plantation for their field assistance. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Arneberg P. Host population density and body mass as determinants of species richness in parasite communities: comparative analyses of directly transmitted nematodes of mammals. Ecography. 2002;25:88–94. [Google Scholar]
- Becker E.A., Petruno S., Marler C.A. A comparison of scent marking between a monogamous and promiscuous species of Peromyscus: pair bonded males do not advertise to novel females. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Francis D.D., Mar A., Meaney M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Christe P., Glaizot O., Evanno G., Bruyndonckx N., Devevey G., Yannic G., Patthey P., Maeder A., Vogel P., Arlettaz R. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 2007;76:703–710. doi: 10.1111/j.1365-2656.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- Chu X., Dik B., Gustafsson D.R., Che X., Zhang Q., Zou F. The influence of host body size and food guild on prevalence and mean intensity of chewing Lice (Phthiraptera) on birds in southern China. J. Parasitol. 2019;105:334. [PubMed] [Google Scholar]
- Cichoń M., Sendecka J., Gustafsson L. Age-related decline in humoral immune function in Collared Flycatchers. J. Evol. Biol. 2003;16:1205–1210. doi: 10.1046/j.1420-9101.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Duneau D., Luijckx P., Ruder L.F., Ebert D. Sex-specific effects of a parasite evolving in a female-biased host population. BMC Biol. 2012;10:104. doi: 10.1186/1741-7007-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden L.A. Entomological Society of America; 1996. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/veterinary Importance (Thomas Say Publications in Entomology) [Google Scholar]
- Frafjord K. Influence of reproductive status: home range size in water voles (Arvicola amphibius) PLoS One. 2016;11:1–13. doi: 10.1371/journal.pone.0154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredensborg B.L., Poulin R. Parasitism shaping host life-history evolution: adaptive responses in a marine gastropod to infection by trematodes. J. Anim. Ecol. 2006;75:44–53. doi: 10.1111/j.1365-2656.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- Fritzen C.M., Huang J., Westby K., Freye J.D., Dunlap B., Yabsley M.J., Schardein M., Dunn J.R., Jones T.F., Moncayo A.C. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 2011;85:718–723. doi: 10.4269/ajtmh.2011.10-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M., Nuss A.B., Meyer J.M., Sonenshine D.E., Roe R.M., Waterhouse R.M., Sattelle D.B., De La Fuente J., Ribeiro J.M., Megy K., Thimmapuram J., Miller J.R., Walenz B.P., Koren S., Hostetler J.B., Thiagarajan M., Joardar V.S., Hannick L.I., Bidwell S., Hammond M.P., Young S., Zeng Q., Abrudan J.L., Almeida F.C., Ayllón N., Bhide K., Bissinger B.W., Bonzon-Kulichenko E., Buckingham S.D., Caffrey D.R., Caimano M.J., Croset V., Driscoll T., Gilbert D., Gillespie J.J., Giraldo-Calderón G.I., Grabowski J.M., Jiang D., Khalil S.M.S., Kim D., Kocan K.M., Koči J., Kuhn R.J., Kurtti T.J., Lees K., Lang E.G., Kennedy R.C., Kwon H., Perera R., Qi Y., Radolf J.D., Sakamoto J.M., Sánchez-Gracia A., Severo M.S., Silverman N., Šimo L., Tojo M., Tornador C., Van Zee J.P., Vázquez J., Vieira F.G., Villar M., Wespiser A.R., Yang Y., Zhu J., Arensburger P., Pietrantonio P.V., Barker S.C., Shao R., Zdobnov E.M., Hauser F., Grimmelikhuijzen C.J.P., Park Y., Rozas J., Benton R., Pedra J.H.F., Nelson D.R., Unger M.F., Tubio J.M.C., Tu Z., Robertson H.M., Shumway M., Sutton G., Wortman J.R., Lawson D., Wikel S.K., Nene V.M., Fraser C.M., Collins F.H., Birren B., Nelson K.E., Caler E., Hill C.A. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016;7:10507. doi: 10.1038/ncomms10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday W.D., Paterson J.E., Patterson L.D., Cooke S.J., Blouin-Demers G. Testosterone, body size, and sexual signals predict parasite load in Yarrow's Spiny Lizards (Sceloporus jarrovii) Can. J. Zool. 2014;92:1075–1082. [Google Scholar]
- Hämäläinen A., Dammhahn M., Aujard F., Eberle M., Hardy I., Kappeler P.M., Perret M., Schliehe-Diecks S., Kraus C. Senescence or selective disappearance? Age trajectories of body mass in wild and captive populations of a small-bodied primate. Proc. R. Soc. B Biol. Sci. 2014;281:20140830. doi: 10.1098/rspb.2014.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A., Raharivololona B., Ravoniarimbinina P., Kraus C. Host sex and age influence endoparasite burdens in the gray mouse lemur. Front. Zool. 2015;12:1–14. doi: 10.1186/s12983-015-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K., Kurtz J. Evolutionary implications of the adaptation to different immune systems in a parasite with a complex life cycle. Proc. R. Soc. B Biol. Sci. 2005;272:2511–2518. doi: 10.1098/rspb.2005.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawlena H., Abramsky Z., Krasnov B.R. Ultimate mechanisms of age-biased flea parasitism. Oecologia. 2007;154:601–609. doi: 10.1007/s00442-007-0851-7. [DOI] [PubMed] [Google Scholar]
- Izhar R., Ben-Ami F. Host age modulates parasite infectivity, virulence and reproduction. J. Anim. Ecol. 2015;84:1018–1028. doi: 10.1111/1365-2656.12352. [DOI] [PubMed] [Google Scholar]
- James A.M., Burdett C., Mccool M.J., Fox A., Riggs P. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med. Vet. Entomol. 2015;29:178–188. doi: 10.1111/mve.12099. [DOI] [PubMed] [Google Scholar]
- Keirans J.E., Durden L. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998;35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Krasnov B.R., Morand S., Hawlena H., Khokhlova I.S., Shenbrot G.I. Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia. 2005;146:209–217. doi: 10.1007/s00442-005-0189-y. [DOI] [PubMed] [Google Scholar]
- Kuris A.M., Blaustein A.R., Alio J.J. Hosts as islands. Am. Nat. 1980;116:570–586. [Google Scholar]
- Lesniak I., Heckmann I., Heitlinger E., Szentiks C.A., Nowak C., Harms V., Jarausch A., Reinhardt I., Kluth G., Hofer H., Krone O. Population expansion and individual age affect endoparasite richness and diversity in a recolonising large carnivore population. Sci. Rep. 2017;7:41730. doi: 10.1038/srep41730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares A.X., Komeno C.A. Trichobius joblingi, Aspidoptera falcata, and Megistopoda proxima (Diptera : Streblidae) parasitic on Carollia perspicillata and Sturnira lillium (Chiroptera : Phyllostomidae) in southeastern Brazil: sex ratios, seasonality, host site preference. J. Parasitol. 2000;86:167–170. doi: 10.1645/0022-3395(2000)086[0167:TJAFAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lynn G.E., Oliver J.D., Cornax I., O'Sullivan M.G., Munderloh U.G. Experimental evaluation of Peromyscus leucopus as a reservoir host of the Ehrlichia muris-like agent. Parasites Vectors. 2017;10:1–9. doi: 10.1186/s13071-017-1980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B., Bemis W.E., Vermeylen F. Parental behaviour of prairie voles (Microtus ochrogaster) and meadow voles (M. pennsylvanicus) in relation to sex of offspring. Behaviour. 2014;151:535–553. [Google Scholar]
- Moore S.L., Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- Mooring M.S., Blumstein D.T., Stoner C.J. The evolution of parasite-defence grooming in ungulates. Biol. J. Linn. Soc. 2004;81:17–37. [Google Scholar]
- Nunn C.L., Dokey A.T.W. Ranging patterns and parasitism in primates. Biol. Lett. 2006;2:351–354. doi: 10.1098/rsbl.2006.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J.H., Lin T., Gao L., Clark K.L., Banks C.W., Durden L.A., James A.M., Chandler F.W. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11642–11645. doi: 10.1073/pnas.1434553100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble D.O., Millar J.S. The mating system of northern populations of Peromyscus maniculatus as revealed by radiotelemetry and DNA fingerprinting. Ecoscience. 1996;3:423–428. [Google Scholar]
- Rodríguez S.M., Valdivia N. Mesoscale spatiotemporal variability in a complex host-parasite system influenced by intermediate host body size. PeerJ. 2017:1–17. doi: 10.7717/peerj.3675. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarts C.W., Schwartz E.R. third. ed. University of Missouri press; Columbia, Missouri: 2016. The Wild Mammals of Missouri. [Google Scholar]
- Sciutto E., Fragoso G., Diaz M.L., Valdez F., Montoya R.M., Govezensky T., Lomeli C., Larralde C. Murine Taenia crassiceps cysticercosis: H-2 complex and sex influence on susceptibility. Parasitol. Res. 1991;77:243–246. doi: 10.1007/BF00930866. [DOI] [PubMed] [Google Scholar]
- Scott J.D., Anderson J.F., Durden L.A., Smith M.L., Manord J.M., Clark K.L. Prevalence of the Lyme disease spirochete, Borrelia burgdorferi, in blacklegged ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario. Int. J. Med. Sci. 2016;13:316–324. doi: 10.7150/ijms.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata F., Kawamichi T. Female-biased sex allocation of offspring by an Apodemus mouse in an unstable environment. Behav. Ecol. Sociobiol. 2009;63:1307–1317. doi: 10.1007/s00265-009-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes R.S., Gannon W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011;92:235–253. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell C.D., Perrett J.P., Gazzard B.G. Causes of weight loss in human immunodeficiency virus infection. Int. J. STD AIDS. 1993;4:234–236. doi: 10.1177/095646249300400412. [DOI] [PubMed] [Google Scholar]
- Sumner J.W., Durden L.A., Goddard J., Stromdahl E.Y., Clark K.L., Reeves W.K., Paddock C.D. Gulf Coast Ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007;13:30–32. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley R., Stott L., York J., Everard A., Chapple S., Jackson J., Viney M., Tinsley M.C. Acquired immunity protects against helminth infection in a natural host population: long-term field and laboratory evidence. Int. J. Parasitol. 2012;42:931–938. doi: 10.1016/j.ijpara.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Tseng M., Myers J.H. The relationship between parasite fitness and host condition in an insect -virus system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujvari B., Madsen T. Age, parasites, and condition affect humoral immune response in tropical pythons. Behav. Ecol. 2006;17:20–24. [Google Scholar]
- Vincent C.M., Sharp N.P. Sexual antagonism for resistance and tolerance to infection in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2014;281:20140987. doi: 10.1098/rspb.2014.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker C.E., Keirans J.E., Clifford C.M., Easton E.R. Dermacentor ticks (Acari: Ixodoidae: Ixodidae) of the new world: a scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 1986;88:609–627. [Google Scholar]
- Zahn A., Rupp D. Ectoparasite load in European vespertilionid bats. J. Zool. 2004;262:383–391. [Google Scholar]