Highlights
-
•
CBF impairment is found in T2DM and SCD individuals, which might suggest a preclinical stage of dementia.
-
•
Comparing to HC, lower CBF in T2DM was due to higher rate of multiple cerebrovascular risk factors.
-
•
Unlike T2DM, CBF reduction in AD and VD was due to amyloid deposition and microangiopathy respectively.
-
•
Significant negative correlation between adjusted CBF and HbA1c in all cortical regions in healthy control and T2DM.
Keywords: Type 2 diabetes mellitus, Cerebral blood flow, Arterial spin labeling, Subjective cognitive decline, Dementia, Cerebral autoregulation
Abstract
The link between non-demented type 2 diabetes mellitus (T2DM) and different types of cognitive impairment is controversial. By controlling for co-morbidities such as cerebral macrovascular and microvascular changes, cerebral atrophy, amyloid burden, hypertension or hyperlipidemia, the current study investigated the cerebral blood flow of T2DM individuals as compared to cognitively impaired subjects recruited from a memory clinic.
15 healthy control (71.8 ± 6.1 years), 18 T2DM (62.5 ± 3.7 years), as well as 8 Subjective Cognitive Decline (69.5 ± 7.5 years), 12 Vascular Dementia (79.3 ± 4.2 years) and 17 Alzheimer’s Disease (75.1 ± 8.2 years) underwent multi-parametric MRI brain scanning. Subjects with T2DM and from the memory clinic also had 18-F Flutametamol PET-CT scanning to look for any amyloid burden. Pseudocontinuous Arterial Spin Labeling (PCASL), MR Angiography Head, 3D FLAIR and 3D T1-weighted sequences were used to quantify cerebral blood flow, cerebrovascular changes, white matter hyperintensities and brain atrophy respectively. Vascular risk factors were retrieved from the medical records. The 37 subjects from memory clinic were classified into subjective cognitive decline (SCD), vascular dementia (VD) and Alzheimer’s disease (AD) subgroups by a multi-disciplinary panel consisting of a neuroradiologist, and 2 geriatricians.
Absolute cortical CBF in our cohort of T2DM, SCD, VD and AD was significantly decreased (p < 0.01) as compared to healthy controls (HC) in both whole brain and eight paired brain regions, after age, normalized grey matter volume and gender adjustment and Bonferroni correction.
Subgroup analysis between T2DM, SCD, VD, and AD revealed that CBF of T2DM was not significantly different from AD, VD or SCD. By controlling for co-morbidities, impaired cortical CBF in T2DM was not related to microangiopathy or amyloid deposition, but to the interaction of triple risk factors (such as diabetes mellitus, hypertension, and hyperlipidemia).
There was statistically significant negative correlation (p ≤ 0.05) between adjusted CBF and HbA1c in all brain regions of T2DM and HC (with partial correlation ranging from −0.30 to −0.46).
Taken together, altered cerebral blood flow in T2DM might be related to disruption of cerebrovascular autoregulation related to vascular risk factors, and such oligemia occurred before clinical manifestation due to altered glycemic control.
1. Introduction
Diabetes is a complex metabolic disorder characterized by hyperglycaemia and associated macrovascular and microvascular complications (Sims-Robinson et al., 2010). Type 2 diabetes mellitus (T2DM) is the most common form of this disease, associated with hyperinsulinemia and insulin resistance. It is considered as a risk factor of dementia (Crane et al., 2013, Huang et al., 2014, Peila et al., 2002), but the underlying mechanisms contributing to the pathogenesis remain controversial (Biessels et al., 2006, Daulatzai, 2017, de la Monte, 2014, Exalto et al., 2012, Moran et al., 2019, Sims-Robinson et al., 2010). The roles of comorbid conditions such as hypertension, hypercholesterolemia, atherosclerotic vascular disease, and other risk factors such as demographic variables (age, gender, ethnicity, education), medication and genetic predisposition (such as APOE ε4 allele) (Exalto et al., 2012) have complicated the issue and not been clearly worked out (Roberts et al., 2014).
Prior 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) demonstrated diabetics with elevated haemoglobin A1c (HbA1c) levels (Roberts et al., 2014), and non-diabetics with higher serum glucose levels (Burns et al., 2013), were associated with glucose hypometabolism in Alzheimer’s disease signature regions in the brain. Studies also found insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with pre-diabetes or early type 2 diabetes (Baker et al., 2011), and in late middle-aged adults at risk for Alzheimer disease (Willette et al., 2015). These FDG-PET studies suggested that insulin resistance, and hyperglycemia (including elevated HbA1c and fasting glucose) might mediate an adverse effect on cerebral glucose metabolism.
Since cerebral perfusion has a close coupling to cerebral metabolism, it is often been used as a surrogate marker of metabolism (Paulson et al., 2010). However, a study (van Golen et al., 2013) exploring the contribution of blood flow reduction to glucose metabolic reduction in Type 1 DM found that vascular mechanism explained only partially the relationship between T1DM and glucose metabolism, indicating other factors such as hyperglycemia might play a role.
Diabetes and its associated vascular risk factors constituting metabolic syndrome could also lead to cerebral hypoperfusion (Biessels et al., 2006). Among the studies that utilize non-ionizing Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) to assess cerebral perfusion in diabetes, they reported contradictory results (similar to or reduced) in the regional cerebral hypoperfusion in diabetic as compared to the non-diabetic counterparts (Dai et al., 2017). Different regional patterns were found, such as the resting-state default mode network, visual and cerebellum networks (Dai et al., 2017, Xia et al., 2015); the hippocampus, inferior temporal, inferior parietal, and frontal cortices (Bangen et al., 2018). In these studies (Bangen et al., 2018, Dai et al., 2017, Xia et al., 2015) cerebral hypoperfusion was found to correlate with cognitive decline. In studies that assessed cerebral atrophy in the same setting (Bangen et al., 2018, Xia et al., 2015) showed neither grey matter or brain tissue atrophy exist in T2DM as compared to the non-demented controls, suggesting cortical CBF hypoperfusion precede structural alterations.
Altered cerebral haemodynamics is also found in T2DM, and is attributed to underlying causes such as inflammation (Chung et al., 2015), periventricular white matter hyperintensities (Brundel et al., 2012, Novak et al., 2006, Shen et al., 2017), blood pressure (Kim et al., 2011, Tchistiakova et al., 2014), and reduced sensitivity to carbon dioxide (Rusinek et al., 2015). Impaired cerebral autoregulation is implicated as a common neurovascular pathway in diabetes and diabetes-related AD (Shekhar et al., 2017).
Comparable accuracy of ASL-MRI and 18F-FDG PET in distinguishing Alzheimer’s disease (AD) from cognitively normal individuals had been demonstrated (Chen et al., 2011, Musiek et al., 2012, Verclytte et al., 2016). ASL-MRI has gained widespread clinical acceptance in the recent decade (Alsop et al., 2015) and ASL studies on dementia have been increasing. These studies showed strong correlation between cerebral perfusion and cognitive function in AD (Binnewijzend et al., 2013, Mak et al., 2012), reduced cerebral blood flow (CBF) in ischemic vascular dementia (Schuff et al., 2009), in frontotemporal dementia (Du et al., 2006, Hu et al., 2010), as well as different patterns of cerebral hypoperfusion in mild cognitive impairment (MCI) subtypes (Chao et al., 2009).
Taken together, different patterns of cerebral perfusion exist in individuals with different types of dementia and in different phases of cognitive impairment. A direct comparison of CBF in non-demented T2DM with cognitively impaired subjects might shed light on the mechanistic similarities or differences between these diseases. In this study, we hypothesized that different degrees or patterns of impairment of cerebral blood flow exist in T2DM, subjective cognitive decline (SCD), vascular dementia (VD) and AD, as distinct (albeit overlapping) mechanisms might mediate their pathogenesis. Besides cerebral metabolism, cerebral macrovascular and microvascular changes, cerebral atrophy, amyloid burden, hypertension or hyperlipidemia might influence cerebral perfusion in T2DM, SCD or dementia subjects. Hence, we attempted to control for the above factors in order to disentangle the effects of such co-morbidities. Cognitively normal healthy elderly subjects were also recruited as controls in present study. In addition, we explored the relationship of chronic hyperglycemia (glycated hemoglobin HbA1c) and CBF in our cohort of non-demented T2DM and healthy controls, as a possible link of diabetes and dementia (Biessels et al., 2006, Sims-Robinson et al., 2010).
2. Materials and methods
2.1. Participants
Cognitively impaired/dementia subjects were referred by the geriatricians of the memory clinic of a university hospital to participate in a prospective combined PET amyloid/MRI study during the period from June 2017 to June 2019. The clinical diagnoses of the cohort included: subjective cognitive decline (Jessen et al., 2014), Alzheimer’s disease (McKhann et al., 2011), and vascular dementia (Roman et al., 1993). 20 subjects with known T2DM based on the American Diabetes Association (ADA) diagnostic criteria were recruited from the university specialist clinic to participate in the study.
Fifteen healthy elderlies were recruited from community centers. The subjects were excluded for prediabetes and diabetes (based on HbA1c level), previous cerebrovascular events, and claustrophobia. For all subjects, the exclusion criteria also included history of stroke; head injury; seizures; migraine; cancer within 5 years; active infection; end-stage renal or other organ failure; nonambulatory, psychiatric diseases; regular alcohol drinkers; and drug abusers.
Written informed consent was obtained from all subjects. The study logistics complied with the Declaration of Helsinki and ethical approval of the research protocol had been obtained from the Institutional Review Board of the University of Hong Kong and the Hospital Authority Hong Kong West Cluster.
2.2. Clinical and neuropsychological assessment
Each subject, including healthy controls, underwent the local version of Montreal Cognitive Assessment (HK-MoCA) by a trained research assistant (Wong et al., 2009).
Any history of vascular risk factors (such as diabetes status and duration, hypertension and hyperlipidemia) in all subjects (including healthy controls) was obtained either from the medical records of the hospital clinical management system database or interviews conducted by a research nurse.
Glycemic control (HbA1c) from the immediate blood test prior to MRI scan of all T2DM, and healthy controls were obtained.
2.3. MRI acquisition
MR images were acquired by a 3T clinical scanner (Philips Healthcare, Achieva) using a 32-channel head coil at the university imaging center. The following sequences were performed:
Three-dimensional (3D) T1-weighted MPRAGE using repetition time (TR) = 6.8 ms, echo time (TE) = 3.2 ms, thickness = 1.2 mm, flip angle = 8°, field of view (FOV) = 256 × 240 × 204 (mm), matrix = 256 × 240; 3D FLAIR using TR = 6.8 ms, TE = 3.2 ms, thickness = 1.2 mm, field of view (FOV) = 250 × 250 × 184 (mm), matrix = 208 × 207; 2D Pseudo-continuous ASL (PCASL) with background suppression using single shot EPI to cover the whole brain with parameters: TR = 4500 ms, TE = shortest, flip angle 90°, FOV = 240 × 240 × 119 (mm), matrix = 80 × 77, slices thickness = 7 mm, labeling duration = 1650 ms, post-labeling delay (PLD) = 2000 ms. Forty pairs of control and labeled images at seventeen contiguous level of the brain were acquired, leading to 4.5 min acquisition time. In addition, MR angiography (MRA) of head, resting state functional MRI, susceptibility- and diffusion-weighted images were also acquired. Each subject was scanned for 45 min in total.
2.4. PET-CT acquisition
All subjects underwent 18-F Flutametamol PET-CT scan. The dose of 185 MBq 18-F Flutametamol was injected 90 min before image acquisition. Standardized uptake value ratios (SUVR) in 16 cortical regions were calculated with reference to the pons by automated software developed by the manufacturer (General Electric, Cortex ID). Composite Z-score of each subject either above or below the threshold (uptake ratio- 0.62) (Thurfjell et al., 2014) were used as a cut-off to determine the positivity and negativity of the amyloid scans.
2.5. ASL processing
Quality of ASL data of each subject was reviewed by a neuroradiologist (HKFM). Quantification was performed using ASL-MRI Cloud (Li et al., 2019). Assumptions such as tissue (1165 ms) and blood (1650 ms) T1 times, brain/blood partition coffecicient (0.9 ml/g) and labeling efficiency (0.85) were applied in CBF quantification. Images in DICOM format of each patient were converted to Analyze format. ASL images, including M0 and ASL data, as well as MPRAGE images were uploaded for processing. ASL images were transformed, coregistered to the individual T1 space then normalized into an age-matched template in MNI space. For the purpose of this study, absolute CBF of cerebral cortex and eight bilateral brain regions, i.e. precuneus/posterior cingulate, mesial temporal, lateral temporal, parietal, prefrontal, sensorimotor, anterior cingulate, and occipital regions, were used.
2.6. Brain volume calculation
Voxel-based morphometry (VBM) analysis on T1-weighted 3D anatomical images of each individual using Computational Anatomy Toolbox (CAT12.5-rc1) in SPM12 for segmentation into volumes of cerebrospinal fluid (CSF), grey matter (GM) and white matter (WM). Grey matter volume presented in the rest of this article was the normalized GM volume i.e. GM volume divided by intracranial volume (summation of volumes of CSF, GM and WM).
2.7. White matter lesion quantification
Axial FLAIR images of each subject were used to quantify periventricular and subcortical white matter lesions using four-point Fazekas scale (Fazekas et al., 1987). All scoring was performed by a trained scientist (ACMC), which was subsequently confirmed by an experienced neuroradiologist (HKFM).
2.8. Intracranial vascular stenosis grading
MRA head of cerebral (anterior, middle and posterior) and vertebrobasilar arteries were graded according to: mild to moderate (≤50%) or moderate to severe (>50%). Significant intracranial atherosclerosis or macrovascular complication was defined as more than 50% stenosis (Adams et al., 1993).
2.9. Dementia/cognitively impaired subtype classification
The final diagnosis of each subject was made by a multi-disciplinary panel, consisting of a neuroradiologist (HKFM) and two geriatricians (YFS, PKCC) based on the following findings i.e. clinical (baseline and follow-up), neuropsychological (HK-MoCA), amyloid PET-CT, structural MRI, MRA and PCASL-MR perfusion.
In summary, the panel made the diagnosis of SCD according to Jessen et al. (2014). For dementia patients, definitive diagnosis of AD was made based on clinical criteria by McKhann et al. (2011) plus a positive amyloid scan, whereas definitive diagnosis of VD was made based on clinical criteria by Román (1993), a negative amyloid scan, as well as any microvascular MRI or macrovascular MRA abnormalities. MR perfusion patterns by PCASL served to provide supplementary information on a case-by-case basis.
2.10. Statistical analysis
Non-parametric Kruskal-Wallis 1-way ANOVA with pairwise comparison was used to compare HC, T2DM, SCD, VD and AD on age, HK-MoCA and Fazeka’s score. Chi-square tests of categorical variables (sex, cardiovascular risk factors such as hypertension and hyperlipidemia, MRA head vascular stenoses) between all groups were also performed. Analysis of covariance (ANCOVA) model plus post hoc t-test with Bonferroni’s correction were used to determine GM volume differences among the groups adjusting for age. Multiple analysis of covariance (MANCOVA) plus post hoc t-test with Bonferroni’s correction were used to compare CBF in whole brain and eight bilateral brain regions adjusting for age, gender and GM volume between the subject groups. Partial correlation evaluated the relationships between HbA1c and CBF (controlled for age, gender and grey matter volume) in all brain regions of all T2DM and healthy controls.
3. Results
20 T2DM were recruited, however, one subject refused MRI scan and another had artefactual ASL scan. 47 subjects were recruited from the memory clinic. 6 of them were contraindicated to MRI (claustrophobia or non-cooperative), and 4 had artefactual ASL. The final cohort consisted of 70 subjects, including 15 cognitively normal healthy controls (HC), 18 T2DM, 8 Subjective Cognitive Decline (SCD), 12 Vascular Dementia (VD), and 17 Alzheimer Disease (AD).
Table 1 showed demographic, neuropsychological and vascular risk status of the cohort. T2DM were significantly (p < 0.01) younger (62.5 ± 3.7 years) than the healthy controls and demented subjects, but not so as compared to SCD (69.5 ± 7.5 years old). There was a significant (p < 0.05) gender difference between the groups. Our HC (26.6 ± 2.1), T2DM (27.6 ± 1.0) and SCD (28.5 ± 1.4) subjects had similar normal cognitive performance on HK-MoCA examination. Their scores were significantly (p < 0.01) higher than VD (17.0 ± 4.7) and AD (14.7 ± 6.7) subjects.
Table 1.
Demographic characteristics and neuropsychological characteristics of the cohort.
| HC (n = 15) |
T2DM (n = 18) |
SCD (n = 8) |
VD (n = 12) |
AD (n = 17) |
|
|---|---|---|---|---|---|
| Age in year | 71.8 ± 6.1a | 62.5 ± 3.7abc | 69.5 ± 7.5 | 79.3 ± 4.2b | 75.1 ± 8.2c |
| Gender (M/F)^ | 3/12 | 15/3 | 2/6 | 8/4 | 5/12 |
| HK-MoCA score# | 26.6 ± 2.1dg | 27.6 ± 1.0eh | 28.5 ± 1.4 fj | 17.0 ± 4.7ghj | 14.7 ± 6.7def |
| No. of vascular risk factor | |||||
|
0 | 18 | 0 | 3 | 0 |
|
7 | 9 | 2 | 9 | 7 |
|
4 | 16 | 2 | 10 | 6 |
| Two vascular risk factors* | 4 | 7 | 0 | 5 | 3 |
| Three vascular risk factors* | 0 | 9 | 0 | 3 | 0 |
Kruskal-Wallis test with pairwise comparison abcdefghjp < 0.01.
Chi-square test with p < 0.05.
Chi-square test with p > 0.05.
Two SCD, one VD and five AD have missing HK-MoCA score.
Two vascular risk factors = DM + HT or DM + HL or HT + HL; three vascular risk factors = DM + HT + HL.
A higher proportion of T2DM and vascular dementia subjects presented with 2 or 3 vascular risk factors than the other groups. 50% of T2DM had diabetes with hypertension and hyperlipidemia (triple risk factors) and 38.9% of them have diabetes and either hypertension or hyperlipidemia (two risk factors) (Table 1). Similarly, 41.7% and 25% of VD had 2 or 3 vascular risk factors respectively. In contrast, only 17.6% AD and 26.7% HC had two vascular risk factors, but none in SCD. Among, the vascular risk factors, only DM and HL showed significant difference among the groups but not hypertension.
MRA head (Table 2) showed the prevalence of significant (moderate to severe) intracranial vascular abnormalities i.e. 0%, 12.5%, 5.6%, 5.9%, and 16.7% in HC, SCD, T2DM, AD and VD respectively. Moreover, intracranial atherosclerosis in T2DM was seen only in those with triple risk factors (Supplementary Table 1).
Table 2.
MRI and PET-CT findings of the cohort.
| HC (n = 15) |
T2DM (n = 18) |
SCD (n = 8) |
VD (n = 12) |
AD (n = 17) |
|
|---|---|---|---|---|---|
| 18-F Flutametamol PET-CT | |||||
| Amyloid positive | NP | 1 | 0 | 0 | 17 |
| MRI | |||||
| Normalized GM volume | 0.40 ± 0.04aj | 0.38 ± 0.03 | 0.40 ± 0.03bc | 0.36 ± 0.02bj | 0.35 ± 0.03ac |
| Fazakes Scale (periventricular/subcortical WM) | 0.67 k/1.33 | 0.44de/1.28g | 0.75f/1.25h | 2.08dfk/2.25gh | 1.41e/1.47 |
| No abnormality in cerebral vessels* | 15 | 9 | 7 | 4 | 6 |
Anterior/middle/posterior cerebral arteries
|
0 | 1 | 1 | 1 | 1 |
Vertebrobasilar arteries
|
0 | 0 | 0 | 1 | 0 |
ANCOVA with age ajustment and Bonferroni correction abcjp < 0.01.
Kruskal-Wallis test with pairwise comparison fhp < 0.05; degkp < 0.01.
PET-CT positron emission tomography computed tomography; GM grey matter; WM white matter; NP did not perform.
Chi-square test with p < 0.05.
Table 2 showed PET-CT and MRI findings of the cohort, including amyloid deposition, normalized grey matter volume, and microvascular burden/white matter hyperintensities (WMH). 18-F Flutametamol PET-CT revealed there was only 1 subject in T2DM with amyloid deposition, while the amyloid positivity was found in all AD and none in SCD and VD subjects. There were significant age-adjusted cortical atrophy in AD and VD as compared to SCD and HC, but no significant atrophy comparing to T2DM. There was no significant difference in age-adjusted normalized grey matter volumes between HC, T2DM and SCD.
T2DM scored the lowest in Fazekas scale in periventricular WMH while SCD scored the lowest in subcortical WMH, indicating they had minimal microvascular burden. VD had the highest Fazekas scores in both periventricular and subcortical regions.
3.1. CBF among all groups
Absolute CBF values of eight bilateral brain regions were compared among all groups with age, grey matter volume, and gender adjusted (Supplementary Table 2). MANCOVA test found HC has significantly (p < 0.01) higher absolute global CBF (82.0 ± 30.3 ml/100 g/min) than SCD (41.7 ± 12.1 ml/100 g/min), T2DM (37.2 ± 9.8 ml/100 g/min), VD (46.4 ± 17.3 ml/100 g/min), and AD (39.7 ± 8.9 ml/100 g/min).
However, MANCOVA analysis of absolute CBF did not reveal any significant perfusion difference among all subject (T2DM and cognitively impaired) groups.
3.2. Subgroup analyses of CBF
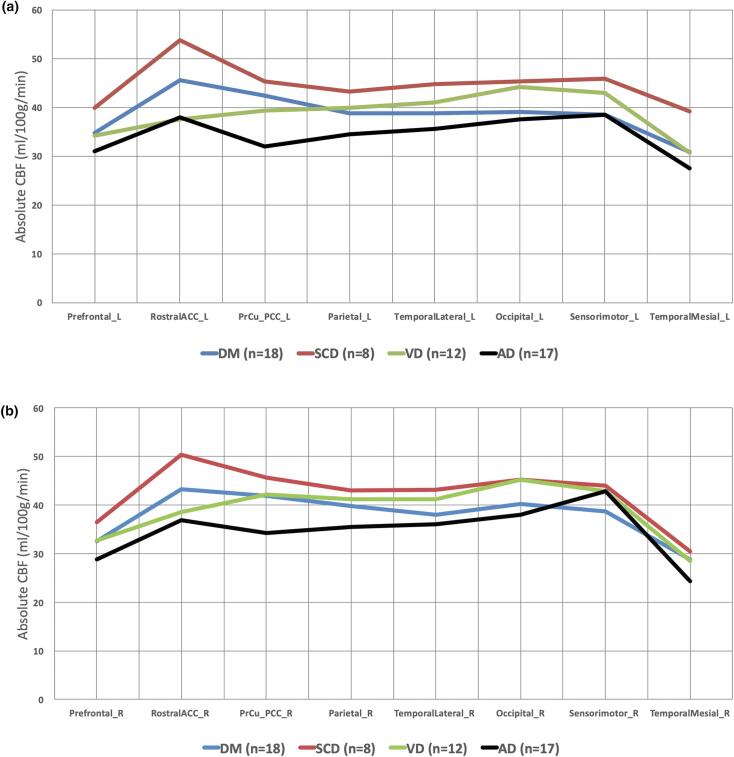
Absolute CBF values of eight bilateral brain regions among four groups (excluding HC) adjusted with age, grey matter volume, and gender (MANCOVA) were seen (Table 3, Fig. 1). SCD demonstrated the highest CBF in all the regions while AD subjects had the lowest. No significant difference was found among all subgroups.
Table 3.
Adjusted mean absolute CBF of eight bilateral brain regions of T2DM, SCD and demented subjects.
| T2DM (n = 18) |
SCD (n = 8) |
VD (n = 12) |
AD (n = 17) |
|
|---|---|---|---|---|
| CBF in ml/100 g/min in the left cortex | ||||
| Cerebral cortex | 39.9 ± 9.8 | 45.3 ± 12.1 | 41.0 ± 17.0 | 36.3 ± 9.3 |
|
34.8 ± 7.4 | 40.0 ± 12.1 | 34.3 ± 15.7 | 31.0 ± 8.4 |
|
45.7 ± 11.8 | 53.9 ± 17.2 | 37.6 ± 15.6 | 38.0 ± 12.1 |
|
42.5 ± 9.5 | 45.4 ± 13.0 | 39.4 ± 21.3 | 32.1 ± 8.7 |
|
38.8 ± 9.1 | 43.3 ± 11.1 | 40.0 ± 16.1 | 34.6 ± 10.1 |
|
38.9 ± 8.7 | 44.8 ± 12.4 | 41.1 ± 16.3 | 35.7 ± 9.6 |
|
39.1 ± 10.2 | 45.4 ± 11.1 | 44.2 ± 20.7 | 37.6 ± 11.5 |
|
38.6 ± 9.3 | 46.0 ± 13.6 | 43.1 ± 17.9 | 38.6 ± 10.1 |
|
30.9 ± 7.7 | 39.2 ± 8.9 | 30.7 ± 11.1 | 27.5 ± 9.1 |
| CBF in ml/100 g/min in the right cortex | ||||
| Cerebral cortex | 39.7 ± 10.1 | 44.0 ± 12.1 | 41.7 ± 17.7 | 36.4 ± 8.7 |
|
32.5 ± 7.6 | 36.4 ± 11.0 | 32.7 ± 14.5 | 28.8 ± 7.6 |
|
43.2 ± 11.8 | 50.3 ± 17.2 | 38.5 ± 14.3 | 36.8 ± 10.7 |
|
41.8 ± 9.7 | 45.6 ± 12.8 | 42.2 ± 23.2 | 34.2 ± 8.7 |
|
39.8 ± 9.3 | 43.0 ± 11.9 | 41.2 ± 17.6 | 35.5 ± 9.1 |
|
38.0 ± 8.7 | 43.1 ± 12.3 | 41.2 ± 18.3 | 36.0 ± 8.5 |
|
40.2 ± 11.6 | 45.2 ± 12.4 | 45.2 ± 21.6 | 38.0 ± 11.2 |
|
38.7 ± 9.6 | 43.9 ± 12.5 | 42.8 ± 19.0 | 42.8 ± 9.2 |
|
28.7 ± 8.9 | 30.4 ± 8.0 | 28.5 ± 10.1 | 24.3 ± 7.6 |
ACC anterior cingulate; PPC precuneus posterior cingulate; Temp temporal.
MANCOVA with age, normalized grey matter volume and gender adjustment and Bonferroni correction.
Fig. 1.
Adjusted mean absolute CBF distribution in eight left (a) and right (b) cortical regions of the cohort. MANCOVA with age, normalized grey matter volume and gender adjustment and Bonferroni correction. ACC anterior cingulate; PPC precuneus posterior cingulate.
3.3. Comparison of T2DM and VD
The major differences were: only 25% of VD had diabetes, slightly higher prevalence of hypertension (75% versus 50%) and macrovascular complications (16.7% versus 5.6%), and significantly higher age (p < 0.01) and WMH (both periventricular and subcortical) (p < 0.01) in VD.
No significant difference was seen in cerebral atrophy, similar prevalence in amyloid burden (5.6% versus 0%), and hyperlipidemia (88.9% versus 83.3%), in T2DM versus VD. In subgroup analysis, T2DM had higher adjusted absolute CBF than VD in left precuneus posterior cingulate, bilateral rostral anterior cingulate, and left prefrontal regions, but no significant difference (Fig. 1, Table 3).
3.4. Comparison of T2DM and AD
The major differences between the 2 groups were: no diabetes in AD, significantly higher age (p < 0.01), periventricular WMH (p < 0.01), and higher amyloid burden (100% versus 5.6%) in AD; but higher prevalence of hyperlipidemia in DM versus AD (88.9% versus 35.3%). No significant difference in cerebral atrophy, subcortical WMH, and similar prevalence of macrovascular complication or hypertension in T2DM versus AD, being 5.6% versus 5.9%, and 50% versus 41.2% respectively.
T2DM had higher absolute CBF (adjusted) than AD in all regions (except right sensori-motor) but no significant differences (Fig. 1, Table 3).
3.5. Comparison of T2DM and SCD
Higher prevalence of triple risk factors (DM, HT, HL) occurred in T2DM versus SCD i.e. 100%, 50%, and 88.9% versus 0%, 25%, 25%, but lower in macrovascular complication i.e. 5.5% versus 12.5%. There was no significant difference in microvascular WMH, amyloid burden and grey matter volume. In subgroup analysis, T2DM had lower adjusted CBF across all regions, but no significant difference.
3.6. Correlation of CBF and HbA1c
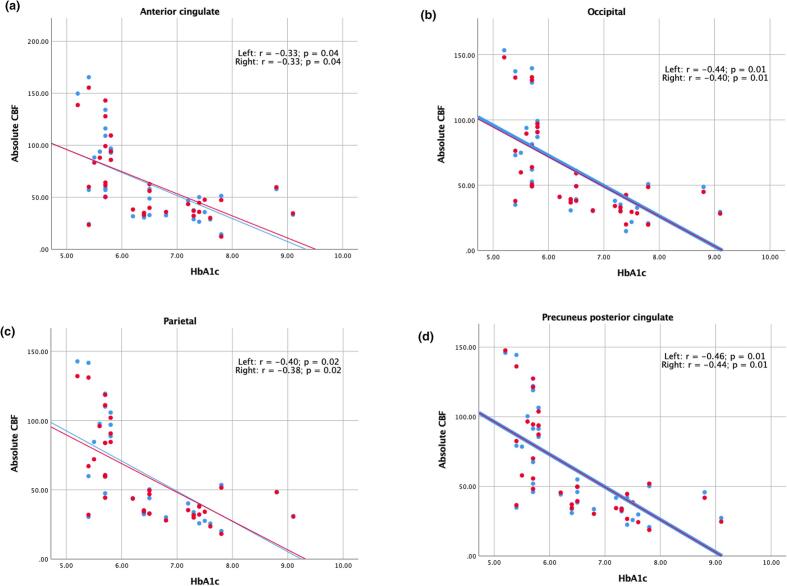
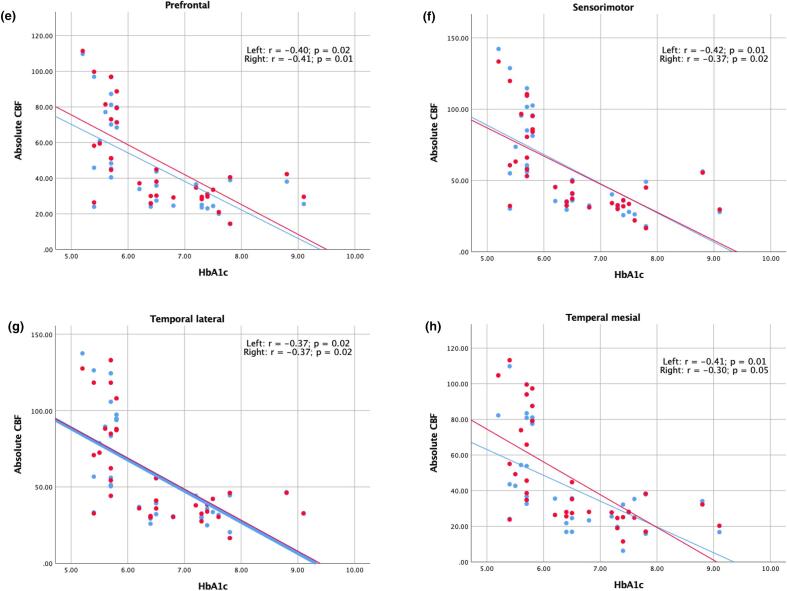
Significant negative correlation ranging from −0.30 to −0.46 (p ≤ 0.05) between absolute CBF (controlled for age, gender and grey matter volume) and HbA1c of the cohort (comprising of 15 HC, and 18 T2DM) was found in all cortical brain regions (Fig. 2).
Fig. 2.
Significant one-way partial correlation (controlling for age, normalized GMV and gender) of HbA1c and absolute CBF of 33 subjects in eight cortical regions (a-h). The left side is presented in red and the right side is presented in blue. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Based on a head-to-head comparison of the clinical features and co-morbidities of T2DM, healthy controls, preclinical cognitive impairment (SCD), vascular dementia and AD, we found that:
-
1.
T2DM versus HC: CBF reduction in T2DM was likely related to higher incidence of cerebrovascular risk factors
In current study, our T2DM subjects showed similar grey matter volume and minimal WMH as in HC. Our results corroborated with previous studies (Bangen et al., 2018, Xia et al., 2015) that CBF alterations in different brain regions precede significant structural changes among adults with T2DM. Although prior studies suggested the role of microangiopathy on cerebral CBF and vaso-reactivity in T2DM (Brundel et al., 2012, Chung et al., 2015, Last et al., 2007, Novak et al., 2006, Shen et al., 2017), it was interesting to note in current study that lower CBF in T2DM was not related to WMH (microangiopathy).
The CBF of T2DM was lower than HC in all brain regions. A much higher incidence of risk factors (such as DM, and HL) (Table 1) and possibly macrovascular complication (5.6% T2DM versus 0% HC) in T2DM (Table 2) might explain the differences. An interaction of these cerebrovascular factors could lead to an adverse effect on autoregulation of cerebral blood flow (Shekhar et al., 2017). Tchisakova et al. demonstrated that subjects with both T2DM and hypertension had impaired cerebrovascular reactivity and cortical thinning compared to age-matched hypertensive controls (Tchistiakova et al., 2014). Another study (Birdsill et al., 2013) showed the presence of more metabolic syndrome factors being associated with increasingly lower CBF. Kim et al. found that different degrees of impairment of cerebrovascular reactivity in uncomplicated diabetes on tight blood pressure control, and diabetic patients with microvascular complications (Kim et al., 2011). Hence our findings may reflect a relatively uncomplicated T2DM cohort, evidenced by lack of microangiopathic changes in contradistinction to prior studies (Chung et al., 2015, Last et al., 2007).
-
2.
T2DM versus SCD: both conditions reflected CBF impairment in the preclinical stages
In both whole group and subgroup analysis, no significant difference in CBF was found between the 2 cohorts. As SCD cohort size was small, due caution in interpretation was required. Among the 8 SCD, only 4 had no related clinical history, while 2 had depression, 1 with family history of dementia, and 1 with alcoholic abuse. There was significant association between SCD and depression (Zlatar et al., 2018) and higher risk of developing MCI/dementia when depression and SCD co-occurred (Liew, 2019). CBF impairment was found in major depression in a prior study (Wang et al., 2014).
Also, the findings of current studies on CBF in SCD studies were controversial. A recent study demonstrated that CBF was lower in MCI (mild cognitive impairment) and AD compared to SCD subjects (Leijenaar et al., 2017), while another study showed that SCD group showed higher CBF relative to MCI and cognitively normal older adults (West et al., 2016). The presence of premorbid psychiatric conditions in SCD patients recruited in different studies needs to be ascertained prior to any comparison.
-
3.
T2DM versus AD: CBF reduction in T2DM was not due to amyloid deposition, but impaired to a level with no significant difference from AD.
In view of low prevalence of amyloid burden in T2DM, our finding concurred with a previous study that functional decline in T2DM not being related to amyloid accumulation (Roberts et al., 2014).
There was no statistically significant difference between the two entities in whole and subgroup analysis, although T2DM showed less reduction in CBF (corrected for age, gender and cerebral atrophy) compared to AD . In view of T2DM with mean HK-MoCA score of 27.6 and normal cognition, while moderate to severe AD with mean HK-MoCA score of 14.7, such a finding indicated severe preclinical oligemia in T2DM even before cognitive impairment.
Similar prevalence of macrovascular complication or hypertension in T2DM and AD indicated that they were not key factors leading to the differences in CBF between the 2 entities. In T2DM, the cerebral perfusion decline was likely related to the triple risk factors (hyperglycaemia, hypertension and hyperlipidaemia) as previously discussed, while in AD due to amyloid-related pathology. It was interesting to note that significantly higher periventricular WMH was seen in AD as compared to T2DM, which might reflect a co-existing element of cerebrovascular disintegrity in AD (Mak et al., 2012).
-
4.
T2DM versus VD: Impaired CBF in VD was related to microangiopathy, but not in T2DM
T2DM had higher adjusted CBF than VD in left precuneus/ posterior cingulate, bilateral rostral anterior cingulate and left prefrontal regions, though no significant difference. As there were only slightly higher prevalence of hypertension and macrovascular complications in VD versus T2DM (being 75% versus 50% and 16.7% versus 5.6% respectively), these co-morbidities might not contribute to their different CBF patterns.
Nevertheless, there was significantly (p < 0.01) increased WMH (both periventricular and subcortical) in VD as compared to T2DM. Previous study (Schuff et al., 2009) revealed that increased subcortical WMH in subcortical ischemic vascular disease were associated with reduced CBF in the cortex, irrespective of brain atrophy. Hence, unlike VD, impaired CBF in T2DM was unrelated to microangiopathy.
-
5.
Relationship of glycemic control and absolute CBF (adjusted) in T2DM and healthy controls
Among 15 HC, and 18 T2DM, significant negative correlation between adjusted CBF and HbA1c in all cortical regions (Fig. 2) might reflect a risk of preclinical oligemia due to altered glycemic control, leading to potential neuronal injury (Zlokovic, 2011).
Previous studies by 18F-FDG PET demonstrated glucose hypometabolism in Alzheimer’s disease signature regions in diabetic patients with elevated haemoglobin A1c (HbA1c) levels (Roberts et al., 2014), non-diabetics with higher serum glucose levels (Burns et al., 2013); pre-diabetic cognitively normal adults or early type 2 diabetes (Baker et al., 2011), and in late middle-aged adults at risk for Alzheimer disease (Willette et al., 2015) with insulin resistance. Increased plasma glucose and insulin levels could cause the AD-like pattern in both 18F-FDG and 15O-H2O PET images even in normal subjects without insulin resistance (Ishibashi et al., 2015). Roberts et al. (2014) found in their cognitively normal subjects, there was a loss of significance after adjustment for glucose level. The unresolved issue was whether the adverse effect of hyperglycaemia on cerebral hypometabolism being a generalized diabetic effect or a specific AD meta-ROI effect. Our findings showed impaired CBF in both AD-signature and non-AD signature regions in T2DM. Hence, our study lends support to the former explanation.
The mechanism of the susceptibility of AD-signature brain tissues to insulin resistance/hyperglycemia warrants further investigation as this might constitute the link between T2DM and AD, which has been coined as Type 3 DM (de la Monte, 2014). In current study, our cohort of cognitively unimpaired T2DM without white matter microangiopathy or amyloid deposition showed surprisingly low CBF. The level of cerebral perfusion decline was similar to cognitively impaired subgroups of AD and VD, and to SCD subgroup having a high percentage of depressive illness, alcohol abuse and family history of dementia. We hypothesised that an interaction of cerebrovascular risk factors could lead to an adverse effect on autoregulation of cerebral blood flow. Prior studies have shown that the risk of autoregulation was increased with co-existence of hypertension and diabetes (Shekhar et al., 2017). According to the 2-hit hypothesis by Zlokovic: 1. Vascular mediated injuries such as arteriosclerosis due to glycosylation in DM was associated with loss of stretch reflux, resulting in blood–brain-barrier (BBB) leakage and constituted the first hit (non-amyloidogenic pathway); 2. BBB leakage resulted in microinfarction, microbleeds, toxic accumulation and impaired clearance of amyloid protein, resulted in second hit. The increase in β-amyloid amplified neuronal dysfunction, and such self-propagating cascade may acccelerate the development of neurodegeneration and eventually dementia (Zlokovic, 2011). Hence, our relatively uncomplicated T2DM cohort could be in the ‘hit-one’ phase, and a longitudinal study might exemplify the related changes during disease progression.
There are some limitations of the current study. First, the sample sizes of participants in different diagnostic categories were relatively small (especially SCD) and our study should be considered a pilot one. Second, multiple regressions of all the comorbidities on CBF were not feasible because of limited sample sizes and possible collinearity issues. Hence, the effects of vascular risk factors on regional CBF impairment was only a postulation suggested by trend. Third, the healthy controls did not undergo any PET scanning since Flutametamol PET scan involved radiopharmaceutical injection. Fourth, more detailed data on cardiovascular risk factors (e.g. blood pressure and lipid profile) should be obtained for correlative analyses. Our current results were more descriptive in nature and exploratory. Finally, detailed neuropsychological evaluation was not performed in current study, which prevented any correlative study of impaired performance in neuropsychological domains with regional CBF in different subject groups.
5. Conclusions
In summary, cortical CBF in our cohort of T2DM, SCD, VD and AD was significantly decreased compared to HC but not to each other. Although SCD had higher CBF than T2DM, VD and AD, their differences were not significant in whole or subgroup analysis. Based on the occurrence of risk factors, impaired cortical CBF in T2DM was not related to microangiopathy or amyloid deposition, but to the interaction of triple risk factors (DM, HT, HL). Altered glycemic control was found to have significant correlation with impaired CBF in all cortical brain regions in non-demented T2DM and healthy controls. We hypothesised that altered cerebral blood flow in T2DM might be related to disruption of cerebrovascular autoregulation, and such oligemia occurred before clinical manifestation due to altered glycemic control.
CRediT authorship contribution statement
Anson C.M. Chau: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Visualization. Eva Cheung: Formal analysis. K.H. Chan: Investigation, Resources. W.S. Chow: Investigation, Resources. Y.F. Shea: Investigation, Resources. Patrick K.C. Chiu: Investigation, Resources. Henry K.F. Mak: Conceptualization, Validation, Investigation, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgments
Acknowledgement
We would like to thank Ms. Carol HY Fong for providing statistical analysis help.
Funding
This work was supported by State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102302.
Contributor Information
Anson C.M. Chau, Email: ansonc@hku.hk.
K.H. Chan, Email: koonho@hku.hk.
W.S. Chow, Email: wschow01@graduate.hku.hk.
Henry K.F. Mak, Email: makkf@hku.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J., Wang D.J., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.D., Cross D.J., Minoshima S., Belongia D., Watson G.S., Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen K.J., Werhane M.L., Weigand A.J., Edmonds E.C., Delano-Wood L., Thomas K.R., Nation D.A., Evangelista N.D., Clark A.L., Liu T.T., Bondi M.W. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes. Front. Aging Neurosci. 2018;10:270. doi: 10.3389/fnagi.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Binnewijzend M.A., Kuijer J.P., Benedictus M.R., van der Flier W.M., Wink A.M., Wattjes M.P., van Berckel B.N., Scheltens P., Barkhof F. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267:221–230. doi: 10.1148/radiol.12120928. [DOI] [PubMed] [Google Scholar]
- Birdsill A.C., Carlsson C.M., Willette A.A., Okonkwo O.C., Johnson S.C., Xu G., Oh J.M., Gallagher C.L., Koscik R.L., Jonaitis E.M., Hermann B.P., LaRue A., Rowley H.A., Asthana S., Sager M.A., Bendlin B.B. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 2013;21:1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M., van den Berg E., Reijmer Y.D., de Bresser J., Kappelle L.J., Biessels G.J., Utrecht Diabetic Encephalopathy Study, G. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J. Diabetes Complications. 2012;26:205–209. doi: 10.1016/j.jdiacomp.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Burns C.M., Chen K., Kaszniak A.W., Lee W., Alexander G.E., Bandy D., Fleisher A.S., Caselli R.J., Reiman E.M. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013;80:1557–1564. doi: 10.1212/WNL.0b013e31828f17de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L.L., Pa J., Duarte A., Schuff N., Weiner M.W., Kramer J.H., Miller B.L., Freeman K.M., Johnson J.K. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis. Assoc. Disord. 2009;23:245–252. doi: 10.1097/WAD.0b013e318199ff46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wolk D.A., Reddin J.S., Korczykowski M., Martinez P.M., Musiek E.S., Newberg A.B., Julin P., Arnold S.E., Greenberg J.H., Detre J.A. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.C., Pimentel D., Jor'dan A.J., Hao Y., Milberg W., Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–458. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane P.K., Walker R., Larson E.B. Glucose levels and risk of dementia. N. Engl. J. Med. 2013;369:1863–1864. doi: 10.1056/NEJMc1311765. [DOI] [PubMed] [Google Scholar]
- Dai W., Duan W., Alfaro F.J., Gavrieli A., Kourtelidis F., Novak V. The resting perfusion pattern associates with functional decline in type 2 diabetes. Neurobiol. Aging. 2017;60:192–202. doi: 10.1016/j.neurobiolaging.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulatzai M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J. Neurosci. Res. 2017;95:943–972. doi: 10.1002/jnr.23777. [DOI] [PubMed] [Google Scholar]
- de la Monte S.M. Type 3 diabetes is sporadic Alzheimers disease: mini-review. Eur. Neuropsychopharmacol. 2014;24:1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A.T., Jahng G.H., Hayasaka S., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K.P., Miller B.L., Weiner M.W., Schuff N. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–1220. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exalto L.G., Whitmer R.A., Kappele L.J., Biessels G.J. An update on type 2 diabetes, vascular dementia and Alzheimer's disease. Exp. Gerontol. 2012;47:858–864. doi: 10.1016/j.exger.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Hu W.T., Wang Z., Lee V.M., Trojanowski J.Q., Detre J.A., Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75:881–888. doi: 10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.C., Chung C.M., Leu H.B., Lin L.Y., Chiu C.C., Hsu C.Y., Chiang C.H., Huang P.H., Chen T.J., Lin S.J., Chen J.W., Chan W.L. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K., Kawasaki K., Ishiwata K., Ishii K. Reduced uptake of 18F-FDG and 15O–H2O in Alzheimer's disease-related regions after glucose loading. J. Cereb. Blood Flow Metab. 2015;35:1380–1385. doi: 10.1038/jcbfm.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R.E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chetelat, G., Dubois, B., Dufouil, C., Ellis, K.A., van der Flier, W.M., Glodzik, L., van Harten, A.C., de Leon, M.J., McHugh, P., Mielke, M.M., Molinuevo, J.L., Mosconi, L., Osorio, R.S., Perrotin, A., Petersen, R.C., Rabin, L.A., Rami, L., Reisberg, B., Rentz, D.M., Sachdev, P.S., de la Sayette, V., Saykin, A.J., Scheltens, P., Shulman, M.B., Slavin, M.J., Sperling, R.A., Stewart, R., Uspenskaya, O., Vellas, B., Visser, P.J., Wagner, M., Subjective Cognitive Decline Initiative Working, G., 2014. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 10, 844-852. https://doi.org/10.1016/j.jalz.2014.01.001.cz. [DOI] [PMC free article] [PubMed]
- Kim Y.S., Davis S.C., Truijen J., Stok W.J., Secher N.H., van Lieshout J.J. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension. 2011;57:738–745. doi: 10.1161/HYPERTENSIONAHA.110.160523. [DOI] [PubMed] [Google Scholar]
- Last D., Alsop D.C., Abduljalil A.M., Marquis R.P., de Bazelaire C., Hu K., Cavallerano J., Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijenaar J.F., van Maurik I.S., Kuijer J.P.A., van der Flier W.M., Scheltens P., Barkhof F., Prins N.D. Lower cerebral blood flow in subjects with Alzheimer's dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimers Dement (Amst) 2017;9:76–83. doi: 10.1016/j.dadm.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew T.M. Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimers Res Ther. 2019;11:70. doi: 10.1186/s13195-019-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak H.K., Chan Q., Zhang Z., Petersen E.T., Qiu D., Zhang L., Yau K.K., Chu L.W., Golay X. Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer's disease and cognitively normal Elderly adults at 3-tesla. J. Alzheimers Dis. 2012;31:33–44. doi: 10.3233/JAD-2012-111877. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, C., Beare, R., Wang, W., Callisaya, M., Srikanth, V., Alzheimer's Disease Neuroimaging, I., 2019. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology 92, e823-e830. https://doi.org/10.1212/WNL.0000000000006955. [DOI] [PMC free article] [PubMed]
- Musiek E.S., Chen Y., Korczykowski M., Saboury B., Martinez P.M., Reddin J.S., Alavi A., Kimberg D.Y., Wolk D.A., Julin P., Newberg A.B., Arnold S.E., Detre J.A. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer's disease. Alzheimers Dement. 2012;8:51–59. doi: 10.1016/j.jalz.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V., Last D., Alsop D.C., Abduljalil A.M., Hu K., Lepicovsky L., Cavallerano J., Lipsitz L.A. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson O.B., Hasselbalch S.G., Rostrup E., Knudsen G.M., Pelligrino D. Cerebral blood flow response to functional activation. J. Cereb. Blood Flow Metab. 2010;30:2–14. doi: 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peila R., Rodriguez B.L., Launer L.J., Honolulu-Asia Aging S. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Roberts R.O., Knopman D.S., Cha R.H., Mielke M.M., Pankratz V.S., Boeve B.F., Kantarci K., Geda Y.E., Jack C.R., Jr., Petersen R.C., Lowe V.J. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 2014;55:759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., Amaducci L., Orgogozo J.M., Brun A., Hofman A. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Rusinek H., Ha J., Yau P.L., Storey P., Tirsi A., Tsui W.H., Frosch O., Azova S., Convit A. Cerebral perfusion in insulin resistance and type 2 diabetes. J. Cereb. Blood Flow Metab. 2015;35:95–102. doi: 10.1038/jcbfm.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N., Matsumoto S., Kmiecik J., Studholme C., Du A., Ezekiel F., Miller B.L., Kramer J.H., Jagust W.J., Chui H.C., Weiner M.W. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S., Wang S., Mims P.N., Gonzalez-Fernandez E., Zhang C., He X., Liu C.Y., Lv W., Wang Y., Huang J., Fan F. Impaired Cerebral Autoregulation-A Common Neurovascular Pathway in Diabetes may Play a Critical Role in Diabetes-Related Alzheimer's Disease. Curr Res Diabetes Obes J. 2017;2 [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhao B., Yan L., Jann K., Wang G., Wang J., Wang B., Pfeuffer J., Qian T., Wang D.J.J. Cerebral hemodynamic and white matter changes of type 2 diabetes revealed by multi-TI arterial spin labeling and double inversion recovery sequence. Front. Neurol. 2017;8:717. doi: 10.3389/fneur.2017.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C., Kim B., Rosko A., Feldman E.L. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6:551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchistiakova E., Anderson N.D., Greenwood C.E., MacIntosh B.J. Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. Neuroimage Clin. 2014;5:36–41. doi: 10.1016/j.nicl.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurfjell L., Lilja J., Lundqvist R., Buckley C., Smith A., Vandenberghe R., Sherwin P. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J. Nucl. Med. 2014;55:1623–1628. doi: 10.2967/jnumed.114.142109. [DOI] [PubMed] [Google Scholar]
- van Golen L.W., Huisman M.C., Ijzerman R.G., Hoetjes N.J., Schwarte L.A., Lammertsma A.A., Diamant M. Cerebral blood flow and glucose metabolism measured with positron emission tomography are decreased in human type 1 diabetes. Diabetes. 2013;62:2898–2904. doi: 10.2337/db12-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verclytte S., Lopes R., Lenfant P., Rollin A., Semah F., Leclerc X., Pasquier F., Delmaire C. Cerebral hypoperfusion and hypometabolism detected by arterial spin labeling MRI and FDG-PET in early-onset Alzheimer's disease. J. Neuroimaging. 2016;26:207–212. doi: 10.1111/jon.12264. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Tang S., Liu X., O'Neil A., Turner A., Chai F., Chen F., Berk M. Assessing regional cerebral blood flow in depression using 320-slice computed tomography. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0107735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.D., Risacher S.L., Gandhi P.K., Wu Y.-C., Tallman E.F., McDonald B.C., Glazier B.S., Apostolova L.G., Farlow M.R., Brosch J.R., Gao S., Brown S.A., Unverzagt F.W., Wang D., Saykin A.J. Elevated cerebral blood flow in participants with subjective cognitive decline. Alzheimer's Dementia. 2016;12:P70–P71. doi: 10.1016/j.jalz.2016.06.121. [DOI] [Google Scholar]
- Willette A.A., Bendlin B.B., Starks E.J., Birdsill A.C., Johnson S.C., Christian B.T., Okonkwo O.C., La Rue A., Hermann B.P., Koscik R.L., Jonaitis E.M., Sager M.A., Asthana S. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for alzheimer disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A., Xiong Y.Y., Kwan P.W., Chan A.Y., Lam W.W., Wang K., Chu W.C., Nyenhuis D.L., Nasreddine Z., Wong L.K., Mok V.C. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement. Geriatr. Cogn. Disord. 2009;28:81–87. doi: 10.1159/000232589. [DOI] [PubMed] [Google Scholar]
- Xia W., Rao H., Spaeth A.M., Huang R., Tian S., Cai R., Sun J., Wang S. Blood pressure is associated with cerebral blood flow alterations in patients with T2DM as revealed by perfusion functional MRI. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar Z.Z., Muniz M., Galasko D., Salmon D.P. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2018;73:1198–1202. doi: 10.1093/geronb/gbw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.