Highlights
-
•
PSMD, a marker of global white matter microstructure disruption, is increased in CAA.
-
•
PSMD in CAA participants is associated with processing speed.
-
•
Changes in PSMD were similar in CAA, NC, MCI, and AD over 1 year.
Keywords: Cerebral amyloid angiopathy, Alzheimer’s disease, Cognition, Diffusion, Magnetic resonance imaging
Abstract
Objectives
To test the hypotheses that peak skeletonized mean diffusivity (PSMD), a measure of cerebral white matter microstructural disruption, is 1) increased in patients with cerebral amyloid angiopathy (CAA) compared to normal control (NC), mild cognitive impairment (MCI), and Alzheimer’s disease (AD); 2) associated with neuropsychological test performance among CAA patients; and 3) increased more quickly over one year in CAA than in AD, MCI, and NC.
Methods
Ninety-two participants provided a medical history, completed a neuropsychological assessment, and had a magnetic resonance (MR) exam including diffusion tensor imaging (DTI) from which PSMD was calculated. A 75-minute neuropsychological test battery was used to derive domain scores for memory, executive function, and processing speed. Multivariable analyses controlling for age and sex (and education, for cognitive outcomes) were used to test the study hypotheses.
Results
PSMD was higher in the CAA group (mean 4.97 × 10−4 mm2/s) compared to NC (3.25 × 10−4 mm2/s), MCI (3.62 × 10−4 mm2/s) and AD (3.89 × 10−4 mm2/s) groups (p < .01). Among CAA patients, higher PSMD was associated with slower processing speed (estimated −0.22 standard deviation (SD) change in processing speed z score per SD increase in PSMD, 95% CI −0.42 to −0.03, p = .03), higher WMH volume [β = 0.74, CI 0.48 to 1.00], and higher CAA SVD score [β = 0.68, CI 0.24 to 1.21] but was not associated with MMSE, executive function, memory, CMB count, or cortical thickness. PSMD increased over 1-year in all groups (p < .01) but without rate differences between groups (p = .66).
Conclusions
PSMD, a simple marker of diffuse global white matter heterogeneity, is increased in CAA. Our findings further support a role for white matter disruption in causing cognitive impairment in CAA.
1. Introduction
Cerebral amyloid angiopathy (CAA) is a small vessel disease in which beta-amyloid aggregates in the media and adventitia of the cerebral small vessels, leading to decreased vascular reactivity and integrity. CAA is diagnosed by the presence of lobar, cortical or cortical-subcortical hemorrhage including intracerebral hemorrhage (ICH), cerebral microbleeds (CMB), or cerebral superficial siderosis (cSS) according to the modified Boston criteria (Greenberg et al., 1995, Greenberg and Charidimou, 2018). CAA is recognized to be a significant risk for future stroke, transient focal neurological episodes, cognitive impairment, psychiatric and motor disabilities (Wermer and Greenberg, 2018).
Disruption of white matter microstructure, as reflected by diffusion-weighted MR imaging measures, has been associated with functional changes in CAA (Charidimou et al., 2017). Recently, a number of brain diffusivity measures have been developed and applied to patients with cerebral small vessel disease (cSVD) including, for example, simple measures such as the peak height of mean diffusivity (MD) (Croall et al., 2017) as well as more complex measures based on alternative decomposition and integration of the tensors (Williams et al., 2017). Another emerging method that is increasingly being applied is the peak width of skeletonized mean diffusivity (PSMD), derived from a histogram of the MD values in the skeletonized white matter tracts (Baykara et al., 2016). PSMD is a quantitative metric of microstructural disruption of white matter over the whole brain.
PSMD was shown to be significantly associated with processing speed in patients with sporadic cSVD and cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and correlated better with cognition than the WMH volume or number of lacunes (Baykara et al., 2016). Within the CADASIL group, longitudinal changes in PSMD could be detected over 18 months. PSMD and whole brain white matter MD have been shown to discriminate between hereditary cerebral hemorrhage with amyloidosis-Dutch type genetic carriers and controls (Schouten et al., 2019). Advantages of PSMD are that it can be calculated automatically, reflects global brain changes, and should be less affected by partial volume effects with cerebrospinal fluid, a particularly acute problem in older populations with brain atrophy, because it is based on skeletonized white matter tract region-of-interest. However, PSMD has yet to be assessed in a CAA population and it has not been compared to a simpler DTI metric, mean diffusivity (MD).
The objectives of this study were to: 1) assess PSMD values in CAA, mild cognitive impairment (MCI), Alzheimer’s disease (AD), and normal control (NC) groups; 2) evaluate the relationship between PSMD and memory, executive function, and processing speed in CAA; 3) compare the discriminative value of PSMD to a simpler DTI measure, MD of the white matter; and 4) determine if increases in PSMD can be detected after one year. We tested the hypotheses that 1) PSMD is higher in CAA; 2) that higher PSMD is associated with lower processing speed, executive function, and memory; 3) PSMD better discriminates between groups and has stronger associations with cognitive functions than MD; and 4) that the rate of increase in PSMD at one year is higher in CAA than in NC, MCI, and AD.
2. Methods
2.1. Study participants
This was a retrospective analysis of the ongoing Functional Assessment of Vascular Reactivity (FAVR) study (Peca et al., 2013), recruiting patients with CAA, MCI, mild AD and neurologically normal controls. All participants were 55 y of age or older, did not have significant neurological or psychiatric disorders, dementia, or contraindications for MR imaging at 3.0 T. Participants provided informed, signed consent prior to participation in accordance with the University of Calgary research ethics board.
NC participants were recruited from the local community through poster advertisements or from spouses of patients seen at the cognitive disorders clinic. CAA participants were recruited from stroke prevention or memory clinics coordinated through the Foothills Medical Centre. CAA participants were assessed >90 days after symptomatic ICH to avoid confounding effects of edema from acute ICH. Eligibility was based on presentation with a CAA-related syndrome (ICH, cognitive symptoms without dementia, CAA-related inflammation, or transient focal neurological symptoms) accompanied by a pattern of lobar-only cerebral microbleeds (CMBs) and superficial siderosis consistent with probable CAA according to modified Boston criteria (Charidimou et al., 2017) or criteria for CAA-related inflammation (Auriel et al., 2016). Patients with CAA-related inflammation were studied during remission, at a time when no vasogenic edema was visible on magnetic resonance (MR) imaging fluid-attenuated inversion recovery (FLAIR). To reduce the confounding effects of concomitant AD pathology, CAA patients with dementia (according to Diagnostic and Statistical Manual-IV criteria) were excluded. CAA participants had to be living in the community and not a long-term care facility, without aphasia or visual field deficits.
MCI participants were recruited through community advertising or from a memory clinic. Participants were considered to have mild cognitive impairment if they scored 1 or more standard deviations below age-adjusted test scores in 1 or more domains of the neuropsychological tests and instrumental activities of daily living were maintained.
AD participants were recruited from a memory clinic. AD was diagnosed based on National Institute on Aging–Alzheimer’s Association criteria for clinical probable AD (McKhann et al., 2011). They had relatively mild-stage AD-dementia, based on requirements for the Folstein Mini-Mental Status Examination (MMSE) score (Folstein et al., 1975) to be ≥20 and to be living in the community and not a long-term care facility.
2.2. Medical and neuropsychological assessment
Neuropsychological assessments were performed by qualified personnel and included MMSE, digit symbol substitution (processing speed), trail making parts A (processing speed and B (executive function), California Verbal Learning II delayed recall (memory), Rey Osterrieth Complex Figure (memory), and Controlled Oral Word Association (executive function). For further analysis, raw scores were converted to z-scores by pooling the study scores and then calculating means and standards for each test. The individual test scores were combined into domains of memory, executive function, and processing speed as in a previous study (Case et al., 2016).
2.3. MR imaging
MR images were acquired on a 3 T scanner (Signa VH/I or MR750, GE Healthcare, Waukesha, WI) using a 12-channel head and neck coil within 3 months of the cognitive assessment at time of recruitment and one-year follow-up. Three-dimensional, inversion-prepared T1-weighted (3D-T1), T2-weighted fluid attenuated inversion recovery (FLAIR), susceptibility-weighted (SWI), and diffusion image data were collected. The 3D-T1 images were acquired using a 240-mm field of view (FOV), a 256 × 256 × 210 acquisition matrix, with a 1 mm slice thickness and an echo time (TE) of ~3 ms, repetition time (TR) of ~7 ms, inversion time (TI) of 650 ms, a flip angle of 8°, and 1.5 times acceleration factor. FLAIR images were acquired with a 240 mm FOV, 256 × 256 acquisition matrix, 3.5 mm slice thickness, TE = ~145 ms, TR = 9000 ms. TI = 2250 ms, and flip angle = 111°. Susceptibility-weighted images were acquired with a 240 mm FOV, 256 × 256 acquisition matrix, and 2 mm slice thickness, TE = 20 ms, TR = 30 ms, and flip angle = 15°. SWI images were reconstructed with an interpolation factor of 2. Echo planar diffusion images were acquired with a FOV = 220 mm, a 128 × 128 acquisition matrix, 3.5 mm slice thickness, TE = ~88 ms, TR = 11000 ms, flip angle = 15°. Non-collinear diffusion gradients were applied (b-value = 850 s/mm2; 11 directions with 2 excitations averaged or 25 directions with 1 excitation).
Brain and intracranial volumes as well as mean cortical thickness were measured using the T1-w images and FreeSurfer (v6.0, http://surfer.nmr.mgh.harard.edu/) (Dale et al., 1999, Desikan et al., 2006, Fischl and Dale, 2000). WMH volumes were measured by qualified readers using the FLAIR images and a semi-automated, seed-based 3D region growing method in Quantomo (Cybertrial Medical Software, Inc., Calgary, AB). The susceptibility-weighted images were phase-filtered (Haacke et al., 2009) to enhance contrast for cerebral microbleed (CMB) detection (Cheng et al., 2013). A qualified reader (neurologist or radiologist) reviewed these images and counted the number of CMBs and determined the presence or absence of cortical superficial siderosis (cSS). The total CAA SVD score was determined using a 0–4 point scale, where points are added for moderate WMH (Fazekas score (Fazekas et al., 1987) ≥ 2), moderate number of enlarged perivascular spaces (≥21 in the centrum semiovale), presence of CMBs, and presence of lacunes (Charidimou et al., 2016).
Diffusion images were visually review for data quality and processed using the PSMD marker script (http://www.psmd-marker.com) (Baykara et al., 2016) and FSL (http://www.fmrib.ox.ac.uk.fsl) (Smith, 2002). In a subset of 4 individuals, we acquired diffusion images with 11- and 25-directions within a single imaging session. The PSMD values between the diffusion acquisitions were compared using a Wilcoxon signed rank test. PSMD median values (interquartile range) were 2.48 × 10−4 mm2/s (2.45 × 10−4 mm2/s – 3.30 × 10−4 mm2/s) and 2.60 × 10−4 mm2/s (2.59 × 10−4 mm2/s – 3.28 × 10−4 mm2/s) for 11- and 25 directions respectively. No significant difference was detected (p = .25). Since no significant difference was detected, we pooled the 11- and 25-direction diffusion data.
For comparison with PSMD results, we generated the average mean diffusivity (mean MD) from voxels used in the PSMD calculation using FSLstats tools. These voxels were along major white matter tracts excluding voxels with FA < 0.3 after TBSS registration and voxels adjacent to the ventricles to mitigate possible partial volume effects with cerebral spinal fluid (Baykara et al., 2016).
For a secondary analysis of 15 CAA participants with ICH, the major white matter tracks generated as part of the PSMD processing were masked to exclude voxels affected by the ICH and peri-ICH gliosis. The ICH masks were manually generated from the FLAIR images using Quantomo (Cybertrial Medical Software, Inc., Calgary, AB). The FLAIR images were skull stripped and registered to the MNI152 1 mm T1 standard brain and the resulting transformation matrix was applied to the ICH mask. The difference between the 5th and 95th percentile of the MD histogram was recalculated using FSL (http://www.fmrib.ox.ac.uk.fsl) (Smith, 2002).
2.4. Statistical analyses
Analysis of variance (ANOVA) with Tukey-Kramer post-hoc testing was used to compare continuous demographic information (e.g. age, years of educations), cognitive performance scores, and MR measures between NC, CAA, MCI, and AD groups. Frequency of categorical sample characteristics (e.g. sex) were compared using Pearson’s chi-squared test. PSMD was compared between groups using a general linear model controlling for age and sex, with least-squares means to estimate mean PSMD in each group and Tukey-Kramer test to adjust for multiple post-hoc comparisons. In a secondary analysis, we compared PSMD separately in CAA subgroups with ICH and without ICH. General linear models were used to determine associations between baseline PSMD and baseline cognitive scores, log-transformed baseline WMH volume, log-transformed baseline number of cerebral microbleeds, total SVD score and baseline cortical thickness adjusting for age, sex, and, for analyses of cognition only, years of education. Covariates were standardized to mean of zero and standard deviation of one for these analyses, such that the beta coefficients represent either the change in standard deviations of PSMD per one standard deviation in the covariate (for models of neuroimaging parameters), or the change in standard deviations of the cognitive domain score per one standard deviation change in PSMD. Separate models were run for each cognitive domain score or neuroimaging covariate. Longitudinal changes in PSMD, WMH volume, and cognitive scores were compared using either Pearson or Spearman correlation, as appropriate. Because history of hypertension differed between groups it was added to the models but was then removed because it was no longer significant (p > .20) after controlling for other variables including group. The threshold for significance was p ≤ 0.05. Analyses were done using SAS version 9.4 (Cary, NC).
2.5. Data availability
Anonymized data will be shared by request from any qualified investigator. Requests should be submitted to either the corresponding author or the senior author.
3. Results
Ninety-nine participants were recruited (23 NC, 38 CAA, 22 MCI and 16 AD). After quality review of the diffusion images for motion or other image artifacts, 7 participants (1 NC, 4 CAA, 1 MCI, 1 AD) were excluded, leaving 92 participants for baseline cross-sectional analyses. Sixty-four participants (12 NC, 24 CAA, 17 MCI and 11 AD) had both baseline and follow-up diffusion scans meeting image quality requirements. Reasons for missing follow-up data were: participants no longer wished to participate or unspecified reasons (n = 11), MR protocol deviations (n = 7), or were no longer eligible due to contraindications for MR (n = 1 pacemaker), progression to severe dementia (n = 1) or death (n = 2).
Group characteristics are summarized in Table 1. CAA participants were older than NC participants (p = .01) but were of similar age to MCI (p = .28) and AD (p = .08) participants. CAA participants more frequently had hypertension, ICH, and superficial siderosis than NC, MCI, or AD groups. Neuropsychological domain scores were lower in CAA, MCI and AD compared with NC; the largest differences were between NC and AD participants (Table 2).
Table 1.
Cohort characteristics at baseline including a summary of MRI findings. Means ± standard deviations are reported for normally distributed continuous measures. Number of individuals with the specified medical history are reported for each cohort. ANOVA group comparison p-values reported.
| Overall (n = 92) | NC (n = 22) | CAA (n = 34) | MCI (n = 21) | AD (n = 15) | p-value | |
|---|---|---|---|---|---|---|
| Age (y) | 71.0 ± 8.0 | 67.8 ± 9.6 | 74.4 ± 7.4 | 70.6 ± 5.9 | 68.7 ± 7.2 | 0.007 |
| Female (%) | 39 (42.4) | 12 (54.6) | 13 (38.2) | 8 (38.1) | 6 (40.0) | 0.62 |
| Education (y) | 14.6 ± 2.9 | 14.8 ± 2.7 | 13.7 ± 2.7 | 15.2 ± 3.1 | 15.3 ± 3.2 | 0.15 |
| Current Smokers (%) | 4 (4.4) | 1 (4.5) | 2 (5.6) | 0 (0) | 1 (6.3) | 0.72 |
| Past Smokers (%) | 43 (46.7) | 9 (40.9) | 18 (52.8) | 7 (33.3) | 9 (62.5) | 0.33 |
| Hypertension (%) | 40 (43.5) | 2 (9.1) | 26 (76.5) | 7 (33.3) | 5 (31.3) | <0.001 |
| Hypercholestero-lemia (%) | 35 (38.0) | 8 (36.4) | 17 (50.0) | 7 (33.3) | 3 (20.0) | 0.18 |
| Diabetes (%) | 15 (16.3) | 1 (4.6) | 8 (23.5) | 4 (19.0) | 2 (13.3) | 0.29 |
| CAD (%) | 10 (10.9) | 1 (4.6) | 4 (11.8) | 4 (19.0) | 1 (6.7) | 0.45 |
| AFib (%) | 8 (8.7) | 1 (4.6) | 4 (11.8) | 1 (4.8) | 2 (13.3) | 0.69 |
| ICH (%) | 15 (16.3) | 0 (0) | 15 (44.1) | 0 (0) | 0 (0) | <0.001 |
Table 2.
Summary of neuropsychological assessment scores and MRI findings. Means ± standard deviations are reported for normally distributed continuous measures. Median and interquartile range are reported for the WMH volume as they were not normally distributed. Number of individuals with 1 or more lacunes or CMBs are reported. ANOVA comparison group p-values reported.
| Overall (n = 92) | NC (n = 22) | CAA (n = 34) | MCI (n = 21) | AD (n = 15) | p-value | |
|---|---|---|---|---|---|---|
| MMSE | 26.9 ± 3.3 | 29.4 ± 1.2 | 26.6 ± 3.1 | 27.2 ± 2.3 | 23.7 ± 4.1 | <0.001 |
| Memory z-score | −0.02 ± 0.90 | 0.85 ± 0.60 | −0.10 ± 0 0.86 | −0.21 ± 0.65 | −0.89 ± 0.50 | <0.001 |
| Executive Function z-score | 0.01 ± 0.87 | 0.72 ± 0.59 | −0.20 ± 0.86 | 0.07 ± 0.59 | −0.64 ± 0.89 | <0.001 |
| Processing Speed z-score | −0.05 ± 0.92 | 0.72 ± 0.65 | −0.50 ± 0.88 | 0.26 ± 0.49 | −0.59 ± 0.94 | <0.001 |
| WMH Volume (mL) | 8.1 [3.0–22.5] | 2.4 [0.3–4.7] | 26.6 [11.8–52.9] | 6.0 [3.0–11.4] | 7.9 [4.3–10.6] | <0.001 |
| WMH volume (% ICV) | 0.62 [0.2–3.1] | 0.15 [0.11–0.32] | 1.83 [0.86–3.82] | 0.41 [0.26–0.83] | 0.56 [33–0.72] | <0.001 |
| Log WMH volume (% ICV) | 2.10 ± 1.35 | −1.78 ± 1.13 | 0.50 ± 0.98 | −0.75 ± 1.15 | −0.68 ± 0.84 | <0.001 |
| CMB (%) | 42 (46.2) | 3 (13.6) | 33 (97.1) | 4 (19.1) | 2 (14.3) | <0.001 |
| Lacune baseline (%) | 20 (21.7) | 4 (18.2) | 10 (29.4) | 3 (14.3) | 3 (20.0) | 0.56 |
| Lacune follow-up (%) | 17 (25.4) n = 67 | 2 (14.3) n = 14 | 11 (37.5) n = 27 | 2 (13.3) n = 15 | 2 (18.2) n = 11 | 0.23 |
| cSS (%) | 18 (19.8) | 0 | 18 (52.9) | 0 | 0 | <0.001 |
| Mean Cortical Thickness (mm) | 2.36 ± 0.12 | 2.38 ± 0.11 | 2.37 ± 0.10 | 2.39 ± 0.08 | 2.28 ± 0.17 | 0.03 |
| PSMD baseline (×10−4 mm2/s) | 4.07 ± 1.43 | 3.25 ± 0.49 | 4.97 ± 1.69 | 3.62 ± 1.09 | 3.89 ± 1.05 | 0.002 |
| PSMD follow-up (×10−4 mm2/s) | 4.52 ± 1.51 (n = 64) | 3.76 ± 0.82 (n = 15) | 5.50 ± 1.49 (n = 24) | 4.14 ± 1.58 (n = 17) | 4.03 ± 1.13 (n = 11) | <0.001 |
| ΔPSMD (×10−4 mm2/s) | 0.42 ± 0.53 | 0.46 ± 0.63 | 0.52 ± 0.41 | 0.42 ± 0.85 | 0.33 ± 0.15 | 0.71 |
| Annualized ΔPSMD (%) | 10.2 ± 11.2 | 11.7 ± 11.1 | 11.0 ± 9.6 | 10.5 ± 16.7 | 8.8 ± 5.2 | 0.94 |
| MD baseline (×10−4 mm2/s) | 8.57 ± 0.67 | 8.10 ± 0.42 | 9.03 ± 0.68 | 8.42 ± 0.58 | 8.49 ± 0.43 | <0.001 |
| MD follow-up (×10−4 mm2/s) | 8.74 ± 0.70 | 8.36 ± 0.39 | 9.21 ± 0.66 | 8.48 ± 0.70 | 8.42 ± 0.44 | 0.002 |
| ΔMD (×10−4 mm2/s) | 0.11 ± 0.22 | 0.15 ± 0.16 | 0.17 ± 0.21 | 0.04 ± 0.26 | 0.12 ± 0.04 | 0.09 |
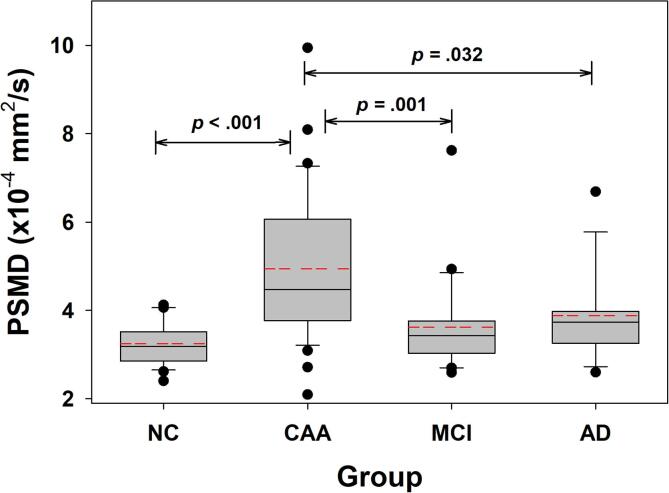
Boxplots of baseline PSMD values are shown in Fig. 1. The baseline PSMD values were higher in CAA than in NC (p = .002), MCI (p = .01), and AD (p = .03). After adjusting for age and sex and controlling for multiple comparisons, PSMD remained higher in CAA (estimated mean 4.7 × 10−4 mm2/s, 95% CI 4.3 to 5.1 × 10−4 mm2/s) than NC (estimated mean 3.5 × 10−4 mm2/s; 95% CI 3.0 to 4.0 × 10−4 mm2/s; p = .005 for comparison with CAA) and MCI (estimated mean 3.6 × 10−4 mm2/s; 95% CI 3.1 to 4.1 × 10−4 mm2/s; p = .005). PSMD was no longer different from AD (estimated mean 4.0 × 10−4 mm2/s; 95% CI 3.4 to 4.6 × 10−4 mm2/s; p = .19) after adjusting for age and sex.
Fig. 1.
Cross-sectional baseline PSMD across study groups. The baseline PSMD data for each group are shown in box plots. Univariate tests of significance between groups are indicated by the brackets with unadjusted p-values. After adjusting for age, sex, and education, PSMD in CAA was higher than NC (adjusted p = .005) and MCI (adjusted p = .005), while AD was no longer significantly different (adjusted p = .19). The median (solid line within each box), mean (dashed red line within each box), 25th and 75th percentiles (extent of each box) as well as the 10th and 90th percentile (error bars) are shown. Outliers are indicated by the symbols for each group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Similar to the PSMD results, after adjusting for age and sex and controlling for multiple comparisons, group mean MD remained higher in CAA (estimated mean 8.91 × 10−4 mm2/s, 95% CI 8.73 – 9.09 × 10−4 mm2/s) than NC (estimated mean 8.24 × 10−4 mm2/s; 95% CI 8.02 – 8.46 × 10−4 mm2/s; p = .006) and MCI (estimated mean 8.41 × 10−4 mm2/s; 95% CI 8.19–8.64 × 10−4 mm2/s; p = . 004). Mean MD for CAA was not different from AD (estimated mean 8.56 × 10−4 mm2/s; 95% CI 8.29–8.81 × 10−4 mm2/s; p = . 44).
Across all participants, higher PSMD was associated with lower memory score (estimated −0.22 SD change in memory per SD increase in PSMD, 95% CI −0.42 to −0.03, p = .03) and lower processing speed score (estimated −0.36 SD change per SD increase in PSMD, 95% CI −0.56 to −0.16, p < .001) but not with lower executive function score (estimated −0.18 SD change per SD increase in PSMD, 95% CI −0.42 to 0.06, p = .15), controlling for age, sex, education, and group. Based on tests of interaction, we failed to find evidence that the association of PSMD with memory, executive function, and processing speed differed by group (p > .05 for all tests). As expected, group was associated with all of the cognitive domains.
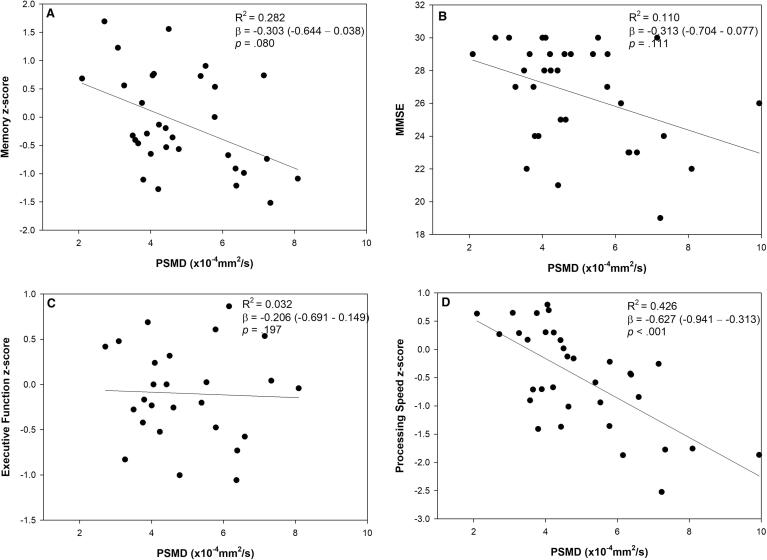
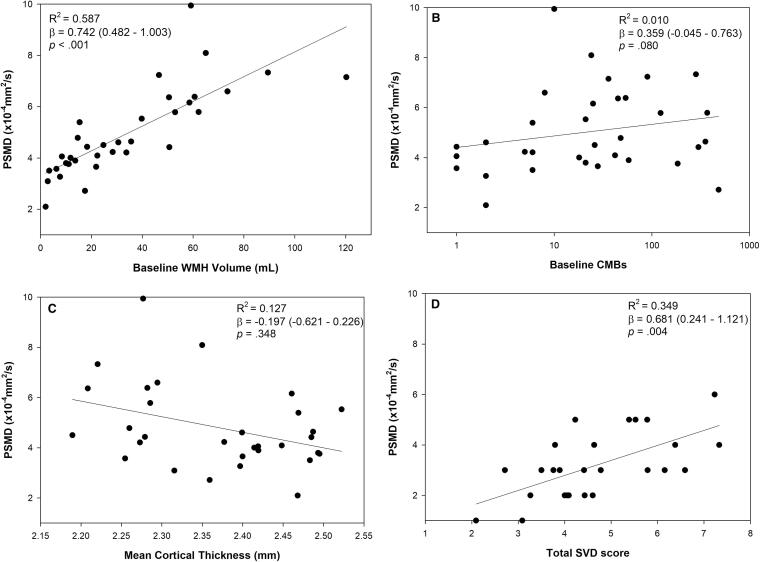
In analyses restricted to CAA participants, higher baseline PSMD was associated with lower processing speed domain score but not with memory or executive function (Fig. 2). Higher PSMD was associated with higher WMH volume and higher total SVD score but not with the number of microbleeds (Fig. 3), adjusting for age. PSMD was not associated with cSS (5.3 ± 1.8 × 10−4 mm2/s in patients with cSS compared to 4.5 ± 1.4 × 10−4 mm2/s in patients without cSS, p = .17).
Fig. 2.
Associations between PSMD and standardized neuropsychological test scores in CAA. The reported coefficients of determination (R2) and beta coefficients (95% confidence interval) are adjusted for age, sex and education. Two memory z-scores (n = 32) and a processing speed z-score (n = 33) were missing.
Fig. 3.
Associations between PSMD and other continuous neuroimaging measures in CAA. The reported coefficients of determination (R2) and beta coefficients (95% confidence interval) are adjusted for age and sex. Eight participants were missing total SVD scores (n = 26).
WMH volume was associated with lower processing speed domain scores but not with MMSE, memory, or executive function. The standardized beta coefficients (95% CI) for associations between WMH volume and cognitive domains were −0.407 (95% CI −0.724 to −0.09; p = .014), −0.140 (95% CI −0.502 to 0.222; p = .43), −0.168 (-0.530 to 0.195; p = .35), and −0.297 (−0.668 – 0.075; p = .11) for processing speed, MMSE, memory, and executive function, respectively.
To analyze the extent to which effects of ICH contributed to PSMD signal in CAA, we compared PSMD in CAA patients to controls after masking out voxels affected by post-ICH changes in the 15 CAA patients with ICH. In the 15 patients with ICH we found that PSMD was lower when ICH-affected voxels were excluded than when they were included (difference −0.38 ± 0.52 × 10−4 mm2/s, p = .01). After removing the ICH-affected voxels, PSMD in CAA patients with ICH still differed from controls (adjusted mean 5.0 × 10−4 mm2/s; 95% CI 4.45 to 5.6 × 10−4 mm2/s; vs 3.54 × 10−4 mm2/s; 95% CI 3.1 to 4.0 × 10−4 mm2/s; p = .008), but was no longer different than CAA patients without ICH (adjusted mean 5.01 × 10−4 mm2/s; 95% CI 4. 5 to 5.6 × 10−4 mm2/s; vs 4.3 × 10−4 mm2/s; 95% CI 3.8 to 4.8 × 10−4 mm2/s; p = .14). Repeating the analyses in Fig. 1 after masking out the effects of ICH, PSMD in 34 patients with CAA (adjusted mean 4.6 × 10−4 mm2/s; 95% CI 4.2 to 4.9 × 10−4 mm2/s) remained higher than controls (adjusted mean 3.5 × 10−4 mm2/s; 95% CI 3.1 to 4.0 × 10−4 mm2/s; p = .004) and MCI (adjusted mean 3.6 × 10−4 mm2/s; 95% CI 3.2 to 4.1 × 10−4 mm2/s; p = .009) but not AD (adjusted mean 4.0 × 10−4 mm2/s; 95% CI 3.5 to 4.6 × 10−4 mm2/s; p = .36).
For comparison between PSMD and mean MD, Table 3 summarizes the standardized beta-coefficients and age- and gender-adjusted R2 values for associations with cognitive scores and other neuroimaging markers of CAA. Processing speed, WMH volume and CAA SVD score were significantly associated with both PSMD and mean MD. The beta-coefficients were equivalent to or higher with PSMD than MD for processing speed, WMH, and SVD score. The adjusted R2 values were higher between mean MD and WMH volume and CAA score than between PSMD and WMH volume and CAA score. The adjusted R2 value was higher between PSMD and processing speed z-score than between mean MD and processing speed z-score.
Table 3.
PSMD and mean MD and their associations with cognitive scores. Standardized beta-coefficients and p-values are shown.
| β-coefficients PSMD (95% CI) | PSMD p-value | PSMD Adj R2 | β coefficients mean MD (95% CI) | Mean MD p-value | Mean MD Adj R2 | |
|---|---|---|---|---|---|---|
| Memory z-score | −0.303 (−0.644 to 0.038) | 0.080 | 0.282 | −0.128 (−0.476 to 0.219) | 0.455 | 0.210 |
| MMSE | −0.313 (−0.704 to 0.077) | 0.111 | 0.110 | −0.202 (−0.574 to 0.170) | 0.276 | 0.067 |
| Executive Function z-score | −0.206 (−0.692 to 0.149) | 0.197 | −0.032 | −0.205 (−0.601 to 0.190) | 0.296 | −0.053 |
| Processing Speed z-score | −0.627 (−0.941–0.314) | <0.001 | 0.426 | −0.480 (−0.780 to −0.162) | 0.004 | 0.318 |
| log(WMH) volume | 0.743 (0.482 to 1.004) | <0.001 | 0.587 | 0.722 (0.484 to 0.960) | <0.001 | 0.614 |
| Number of CMBs | 0.086 (−0.332 to 0.503) | 0.677 | −0.056 | 0.227 (−0.159 to 0.613) | 0.239 | −0.014 |
| Cortical thickness | −0.197 (−0.621 to 0.226) | 0.348 | 0.127 | −0.047 (−0.446 to 0.351) | 0.810 | 0.099 |
| CAA SVD score | 0.681 (0.241 to 1.121) | 0.004 | 0.349 | 0.684 (0.338 to 1.030) | <0.001 | 0.459 |
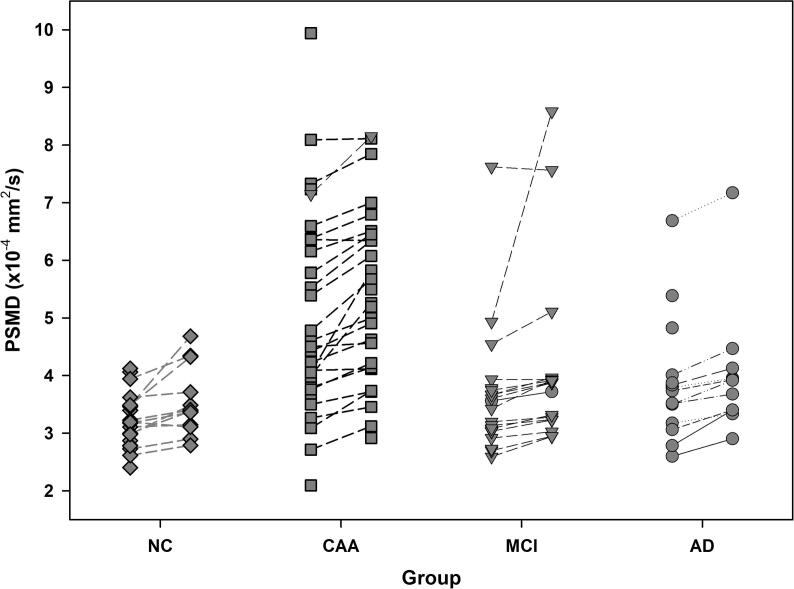
Change in PSMD over time is graphically displayed in Fig. 4. Over the mean (±SD) time to follow-up of 1.1 ± 0.2 years, the mean PSMD increase was 0.42 ± 0.53 × 10−4 mm2/s (p < .001). The mean MD increase was 0.11 ± 0.21 × 10−4 mm2/s (p = .001). However, neither the change in PSMD nor change in mean MD differed by group (p = .71 and p = .09 for ΔPSMD and ΔMD, respectively). PSMD change was not associated with age (p = .28) or sex (p = .49). MD change was not associated with age (p = .54) or sex (p = .85).
Fig. 4.
Changes in PSMD across study groups after 1 year. A significant increase in PSMD between baseline and follow-up time points was detected and was similar across all groups. Between group differences at follow-up were the same as baseline differences.
Among CAA participants, there were no detectable one-year changes in memory, executive function, or mean cortical thickness; therefore, these were not analyzed further. Although the mean psychomotor speed score decreased by 0.24 ± 0.47 (p = .02), we failed to find that this decrease was associated with either baseline PSMD (p = .49) or PSMD change (p = .22). Median WMH as a percentage of intracranial volume increased by 0.22% (interquartile range −0.1 to 0.61%, p < 0.001) but we failed to find associations with baseline PSMD (r = 0.38, p = .07) or change in PSMD (r = 0.01, p = .95). The 14 patients who had new microbleeds had higher baseline PSMD than the 12 patients without new microbleeds, controlling for age (1.5 × 10−4 mm2/s higher, 95% CI 0.9 to 2.1 × 10−4 mm2/s, p < .001). However, the patients with new CMBs did not have higher PSMD change (p = .53).
4. Discussion
The main findings of this study are that PSMD was higher at baseline in the CAA group, where it was associated with processing speed, WMH volume, and total CAA score, and though the change in PSMD over time was detectable, it did not differ between groups. Within the CAA group, PSMD and WMH volume were significantly associated with processing speed domain z-score, however, the standardized beta coefficient was higher for PSMD than for WMH indicating a stronger association between PSMD and processing speed. Analyses of the MD histogram showed that both the width (as reflected by PSMD) and mean were shifted toward higher values in CAA compared with other groups. While, PSMD explained more variance in psychomotor processing speed than mean MD, its association with CAA markers was not uniformly stronger. Mean MD was more strongly associated with CAA SVD score and similarly associated with WMH volume. The finding that both the mean and the width of the MD histogram are altered shows that there is a general tendency toward higher white matter diffusivity as well as greater heterogeneity of diffusivity values in CAA. This heterogeneity may reflect the local and remote effects of hemorrhages, microbleeds, and microinfarcts in CAA that interfere with processing speed.
Higher PSMD reflects greater variation in white matter mean diffusivity, which is typically seen when pathological processes disrupt the white matter microarchitecture. Because mean diffusivity is a physical property of tissue it can be directly compared between studies in a similar manner as other physical properties (e.g. blood pressure), although it could still be affected by how the measurements are made. In the current study, PSMD in sporadic CAA (4.97 × 10−4 mm2/s) was similar or slightly higher than PSMD reported for other types of cerebral small vessel disease (SVD) including patients with sporadic SVD (hypertensive arteriopathy) (3.28 × 10−4 mm2/s) (Baykara et al., 2016), patients with SVD from a memory clinic (4.24 × 10−4 mm2/s) (Baykara et al., 2016), and CADASIL (3.9–5.47 × 10−4 mm2/s) (Baykara et al., 2016, Vinciguerra et al., 2019). However, we did not include a sporadic SVD cohort for direct comparison to CAA, a limitation of this study. The PSMD values measured in NC were also similar to previously reported values, (Baykara et al., 2016, Vinciguerra et al., 2019, Wei et al., 2019) supporting that PSMD measurements are relatively robust to differences in diffusion acquisition parameters, MR system manufacturer, and field strength.
These findings are consistent with previous studies showing that white matter damage is a common feature of CAA. A previous case-control study showed that patients with CAA have reduced fractional anisotropy in the temporal white matter and splenium of the corpus callosum (Viswanathan et al., 2008). Another study showed that a globally increased apparent diffusion coefficient was associated with pre-hemorrhage cognitive impairment in patients with lobar hemorrhage due to CAA (Viswanathan et al., 2008). White matter damage in CAA likely causes cognitive impairment by interrupting white matter connectivity (Reijmer et al., 2015). Multiple pathophysiological processes (including WMH, post-ICH changes, inflammation, increased blood-brain barrier permeability, and others) likely contribute to changes in PSMD and should be the subject of further study. CMBs are not likely to directly alter PSMD in CAA because they occur in the cerebral cortex or just under it, and the number of CMBs was not associated with PSMD in our study. We found that PSMD was lower, but still higher than controls, after excluding ICH-affected voxels. This suggests that both ICH-related changes (such as tissue destruction, gliosis, and hemosiderin deposition) and chronic processes in the white matter contribute to the abnormal PSMD in CAA.
There are few studies on change in PSMD over time. In this study, we found an increase of approximately 10% over one year. A prior study of CADASIL patients found an increase in PSMD at 18 months, but the amount of increase was not reported (Baykara et al., 2016). The similar rate of change in PSMD across the study cohorts was unexpected, given that PSMD was higher at baseline in CAA and that WMH increase was detectable. Two potential reasons may be offered. First, the differing rates of change in PSMD may be small and not significant over one year, but when aggregated over a long period of time could lead to large differences. In genetic forms of AD, alterations in PSMD in white matter tracts can be detected in presymptomatic mutation carriers years before the onset of symptoms (Araque Caballero et al., 2018), suggesting that white matter changes occur early and could accumulate over a long period of time. The latent period between accumulation of vascular beta-amyloid and onset of symptoms in CAA is essentially unknown, but if it is similar to genetic forms of CAA then the latent period is probably years or even decades. Second, the similar PSMD increase in CAA and controls contrasts with the detectable increase in WMH seen in CAA (but not other groups) but could be because white matter changes are not uniform in CAA, and if they are largely confined to the WMH “penumbra” then they may be averaged out by a global white matter metric like PSMD.
Similar to other studies of SVD (Baykara et al., 2016), we found a strong association between higher PSMD and lower cognitive processing speed. This association between PSMD and processing speed was stronger than the association between higher MD and lower cognitive processing speed as indicated by higher standardized beta coefficients and adjusted R2 values. In a study of patients with white matter lesions with and without vascular cognitive impairment, PSMD correlated with executive function and with global cognition in the participants with vascular cognitive impairment (Wei et al., 2019). In a community-based study of 73-year old persons PSMD was significantly associated with all cognitive domains examined except crystallized ability (Deary et al., 2019). In our study it is possible that we were unable to detect significant relationships with MMSE, memory, or executive function due to the limited sample size in each group and the limited range in cognitive z-scores within our study.
A limitation of this study is that we pooled 11- and 25-direction diffusion data. The number of diffusion gradient directions changed for most individuals between the baseline and follow-up scans. The number of participants in each group with 11 diffusion gradient directions at baseline and 25 diffusion gradient directions at follow-up was approximately equally distributed across groups (100% NC, 91% CAA, 90% MCI, and 80% in AD). This protocol change potentially reduced the ability to detect differences in the rate of change in PSMD between groups or may contribute to the difference in PSMD detected between baseline and follow-up. The change in PSMD detected between baseline and follow-up measurements is unlikely to be solely due to this protocol change because: 1) there was no significant difference within the four individuals that had PSMD calculated using both 11- and 25-diffusion gradient directions (see Methods: MR Imaging), 2) of the five individuals who had PSMD calculated using 25-diffusion gradient directions at both baseline and follow-up the mean percent increase (10.3 ± 6.3%) was similar to that reported for all participants (Table 1), and 3) prior studies show that increasing the number of diffusion directions from 10 to 20 does not affect regional estimates of mean diffusivity (Jones, 2004, Ni et al., 2006). Acquiring more directions may nominally reduce the variance in MD estimates and thus result in lower PSMD, as PSMD is based on the histogram of MD. However, in our study we found that PSMD increased over time despite acquiring more directions at follow-up. Ultimately, new prospectively designed studies will be needed to better define PSMD change over time and the effect of variation in acquisition parameters. Additionally, future studies should compare PSMD to other DTI metrics to identify which are most sensitive to effects of stroke, cognitive change, and change over time. Another limitation is that although we controlled post-hoc for multiple comparisons between participants groups, we did not perform other adjustments for comparisons with multiple imaging markers and cognitive domains. Therefore, the results should be considered exploratory and warrant confirmation in other studies.
These findings show that CAA is associated with changes in the shape of the MD histogram in white matter. Given the demonstrated robustness of PSMD as a marker of diffuse global white matter heterogeneity and lower processing speed, it has potential to detect early changes which warrants further longitudinal research in populations at possible risk of CAA and SVD.
Credit authorship contribution statement
Cheryl R. McCreary: Conceptualization, Investigation, Data curation, Formal analysis, Writing - original draft. Andrew E. Beaudin: Formal analysis, Writing - review & editing. Arsenije Subotic: Formal analysis, Writing - review & editing. Angela M. Zwiers: Investigation, Writing - review & editing. Anna Charlton: Data curation, Writing - review & editing. Bradley G. Goodyear: Methodology, Writing - review & editing. Richard Frayne: Methodology, Writing - review & editing. Eric E. Smith: Conceptualization, Funding acquisition, Investigation, Formal analysis, Supervision, Writing - review & editing.
Acknowledgements
Sources of funding for the FAVR study were the Canadian Institutes of Health Research (Grant # MOP-142175 and FDN-154317), Brain Canada, Canadian Stroke Network (Grant #MIRI2015-3994), Heart and Stroke Foundation of Alberta, and the Alzheimer Society of Canada. Dr. Beaudin is supported by postdoctoral fellowships from the Alzheimer Society of Canada and Campus Alberta Neurosciences. Dr. Frayne is supported by the Hopewell Professorship in Brain Imaging. Dr. Smith is supported by the University of Calgary Katthy Taylor Chair in Vascular Dementia (Grant #END611975).
References
- Araque Caballero M.A. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer's disease. Brain. 2018;141(10):3065–3080. doi: 10.1093/brain/awy229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriel E. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016;73(2):197–202. doi: 10.1001/jamaneurol.2015.4078. [DOI] [PubMed] [Google Scholar]
- Baykara E. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann. Neurol. 2016;80(4):581–592. doi: 10.1002/ana.24758. [DOI] [PubMed] [Google Scholar]
- Case N.F. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke. 2016;47(8):2010–2016. doi: 10.1161/STROKEAHA.116.012999. [DOI] [PubMed] [Google Scholar]
- Charidimou A. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging-pathologic study of concept validation. JAMA Neurol. 2016;73(8):994–1001. doi: 10.1001/jamaneurol.2016.0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829–1850. doi: 10.1093/brain/awx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A.L. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013;44(10):2782–2786. doi: 10.1161/STROKEAHA.113.002267. [DOI] [PubMed] [Google Scholar]
- Croall I.D. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin. Sci. (Lond.) 2017;131(12):1361–1373. doi: 10.1042/CS20170146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deary I.J. Brain Peak Width of Skeletonized Mean Diffusivity (PSMD) and cognitive function in later life. Front. Psychiatry. 2019;10:524. doi: 10.3389/fpsyt.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fazekas F. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am. J. Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenberg S.M. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol. 1995;38(2):254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- Greenberg S.M., Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the boston criteria. Stroke. 2018;49(2):491–497. doi: 10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E.M. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am. J. Neuroradiol. 2009;30(1):19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn. Reson. Med. 2004;51(4):807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- McKhann G.M. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am. J. Neuroradiol. 2006;27(8):1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Peca S. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81(19):1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer Y.D. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138(Pt 1):179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten T.M. Multiple approaches to diffusion magnetic resonance imaging in hereditary cerebral amyloid angiopathy mutation carriers. J. Am. Heart Assoc. 2019;8(3) doi: 10.1161/JAHA.118.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra C. Peak width of skeletonized mean diffusivity (PSMD) as marker of widespread white matter tissue damage in multiple sclerosis. Mult. Scler. Relat. Disord. 2019;27:294–297. doi: 10.1016/j.msard.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Viswanathan A. Tissue microstructural changes are independently associated with cognitive impairment in cerebral amyloid angiopathy. Stroke. 2008;39(7):1988–1992. doi: 10.1161/STROKEAHA.107.509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N. A neuroimaging marker based on diffusion tensor imaging and cognitive impairment due to cerebral white matter lesions. Front. Neurol. 2019;10:81. doi: 10.3389/fneur.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermer M.J.H., Greenberg S.M. The growing clinical spectrum of cerebral amyloid angiopathy. Curr. Opin. Neurol. 2018;31(1):28–35. doi: 10.1097/WCO.0000000000000510. [DOI] [PubMed] [Google Scholar]
- Williams O.A. Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. Neuroimage Clin. 2017;16:330–342. doi: 10.1016/j.nicl.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator. Requests should be submitted to either the corresponding author or the senior author.