Abstract
Secreted Protein Acid and Rich in Cysteine (SPARC) is an extracellular glycoprotein secreted by fibroblasts and osteoblasts in normal tissues. SPARC overexpression occurs in multiple tumors including pancreatic ductal adenocarcinoma (PDAC) and may predict favorable response to nab-paclitaxel. The prognostic significance of SPARC expression in PDAC is unclear – some reports indicate SPARC overexpression associates with poor outcomes and others find no correlation. Considering neoadjuvant therapy enhances the stromal fibrosis of PDAC and taking into account that SPARC is a component of PDAC stromal fibrosis, we hypothesized that SPARC expression would be greater in neoadjuvant-treated versus treatment-naive PDAC. Quantitative immunohistochemistry was used to measure SPARC expression in resected PDAC in 74 cases of neoadjuvant treated PDAC and 95 cases of treatment-naïve PDAC. SPARC expression was increased 54% in neoadjuvant treated PDAC compared to treatment-naïve PDAC. These data indicate that increased SPARC expression correlates with neoadjuvant therapy in PDAC.
Keywords: SPARC, Pancreatic cancer, Neoadjuvant therapy, Adjuvant therapy, Immunohistochemistry, Digital image analysis
1. Introduction
Pancreatic adenocarcinoma (PDAC) represents less than 2% of all cancer but is the 4th leading cause of cancer death in the United States with 5-year survival rates less than 5% for all stages combined [1]. Roughly 80% of PDAC presents with metastatic disease or are otherwise unresectable. Patients eligible for surgery have the best prognosis, and in these cases, inclusion of neoadjuvant therapy offers the best overall survival [2,3]. Despite decades of basic science research and clinical trials, PDAC has shown the least improvement in survival among common cancers.
A major area of focus for basic research and novel therapeutics is the PDAC stroma. Evolving concepts include the stroma as a hydrostatic barrier to chemotherapy, the growth signaling of benign stromal cells to malignant PDAC cells, and the role of stromal extracellular matrix (ECM) turnover in PDAC invasion and metastasis [4]. A distinctive, nearly invariant histologic feature of PDAC is an abundant desmoplastic stromal reaction. Formation of the desmoplastic stroma likely represents a host defense mechanism to attenuate neoplastic transformation and metastasis [5]. In this sense, the desmoplastic reaction is akin to wound healing and tissue regeneration following injury. The protein SPARC stands out in as a key stromal component regulating the interaction between malignant cells and the stroma in many cancer types including PDAC [6,7].
SPARC, also known as osteonectin and BM-40, is an extracellular matrix protein mainly secreted by fibroblasts and osteoblasts [8,9]. Expression of SPARC is upregulated in tumors of the breast, lung, melanoma, prostate and pancreas [10]. In PDAC, SPARC is a stromal protein expressed mainly by cancer-associated fibroblasts where it localizes adjacent to malignant ducts, particularly in areas of fibrosis [11]. Despite SPARC’s role in wound healing and its anti-oncogenic functions, SPARC promotes oncogenesis via induction of epithelial-to-mesenchymal transition (EMT) and down-regulation of inflammatory response and immune surveillance [12].
The prognostic significance of SPARC expression in PDAC has largely been determined in adjuvant-treated patients undergoing pancreatectomy with curative intention. In general, nearly all studies evaluating SPARC expression utilize immunohistochemistry and rely on SPARC intensity to determine relative expression levels. In general, high expression is associated with worse survival [[13], [14], [15], [16]]. Furthermore, the negative prognostic value of SPARC has been specifically associated to patients who received adjuvant therapy with gemcitabine suggesting that SPARC is a negative predictive marker for gemcitabine-based adjuvant therapy [17]. SPARC expression shows no significant association with prognosis in advanced, unresected PDAC [18,19] indicating that the prognostic value of SPARC changes in the metastatic setting. Preclinical studies investigating the role of SPARC in a KrasG12D mouse model of PDAC demonstrated no changes in progression from pancreatic intraepithelial neoplasia (PanIN) to PDAC progression, vascularity, proliferation, apoptosis rate, or metastatic frequency in animals deficient for SPARC [20]. Furthermore, gemcatibine accumulation and metabolism was also unaffected by SPARC ablation suggesting increased SPARC and ECM accumulation in PDAC is not a barrier to drug delivery.
The phase III MPACT trial demonstrated that gemcitabine together with nab-paclitaxel, the albumin-bound formula of paclitaxel that alters microtubules assembly and impairs mitosis, provides superior survival compared to gemcitabine alone in unresected, metastatic PDAC. SPARC has a high affinity for albumin, and based upon its distribution in PDAC, SPARC overexpression would conceivably enrich the concentration of nab-paclitaxel and enhance its delivery to the tumor environment. Despite a potential role as a positive predictive biomarker for nab-paclitaxel, both preclinical data in mouse models [21] and an exploratory analysis of the MPACT trial [18] demonstrate that SPARC plays no role in either the delivery of nab-paclitaxel to tumor cells or predicting response to nab-paclitaxel in unresected, metastatic PDAC.
To date, little is known regarding either the benefit of adjuvant nab-paclitaxel therapy in resectable PDAC following neoadjuvant chemoradiation therapy or the prognostic value of SPARC in this setting. Since neoadjuvant therapy contributes to stromal desmoplasia in PDAC, we hypothesized that neoadjuvant-treated PDAC would exhibit higher levels of SPARC expression than treatment-naïve PDACs. If neoadjuvant therapy further upregulates SPARC expression in patients with resectable PDAC, it is plausible that these patients become sensitized to nab-paclitaxel-based adjuvant treatment. As an initial exploration of this concept, we utilized quantitative digital analysis to compare the levels of SPARC expression in neoadjuvant and treatment naïve resected PDAC.
2. Methods
2.1. Patients and patient samples
Cases were identified from the pathology department’s clinical database by using appropriate search terms. Pathology reports were reviewed, and H&E slides were examined to identify cases suitable for subsequent analysis. From this, 169 cases of pancreatic adenocarcinoma were selected for analysis. 74 (44%) cases had received neoadjuvant chemotherapy and 95 (56%) were treatment naïve. 60 of 74 (81%) neoadjuvant and 87 of 95 (92%) naïve samples had a paired specimen of histologically normal pancreas available for comparative analysis. When possible, patient demographic and histopathological data was obtained. Table 1 summarizes the clinicohistopathological features of the patients analyzed in this study. Neoadjuvant therapy consisted of chemotherapy and/or radiation therapy, and the specific therapeutic regimen was not available for each patient, although a large number of patients received either 5-fluorouracil or gemcitabine. Paraffin blocks from 153 of these cases were used to create 12 tissue microarrays (TMA). The remaining 16 cases were analyzed as individual slides. 1 mm diameter cores were taken from areas of tumor and, when possible, adjacent normal pancreas. For the majority of cases, 4 to 6 cores from tumor areas and from adjacent normal pancreas were placed onto the TMAs. A protocol to perform this study was approved by the Medical College of Wisconsin Internal Review Board (protocol number PRO15825).
Table 1.
Demographic and Histopathological Features of the Patients Analyzed in this Study.
| Cohort | Treatment Naïve | Neoadjuavnt Treated |
|---|---|---|
| Cases (n) | 95 | 74 |
| Average Age, years (range) | 67.2 (37–90) | 65.3 (40–88) |
| Gendera | ||
| Male | 51 | 30 |
| Female | 34 | 44 |
| Anatomical Site | ||
| Head | 66 | 21 |
| Neck | 0 | 0 |
| Body | 4 | 0 |
| Tail | 7 | 1 |
| Metastatic | 4 | 3 |
| No Data | 14 | 49 |
| Histology | ||
| Adenocarcinoma | 80 | 71 |
| Mucinous variant | 4 | 0 |
| Clear cell variant | 1 | 0 |
| Anaplastic | 0 | 1 |
| No Data | 10 | 2 |
| Grade | ||
| Grade 1 | 15 | 5 |
| Grade 2 | 41 | 43 |
| Grade 3 | 26 | 7 |
| No Data | 13 | 19 |
| AJCC Stage | ||
| IA | 3 | 9 |
| IB | 6 | 22 |
| IIA | 13 | 19 |
| IIB | 54 | 19 |
| III | 0 | 1 |
| IV | 1 | 3 |
| No Data | 18 | 1 |
The treatment cohort of 10 patients (1 female, 9 males) is unknown.
2.2. Immunohistochemistry
TMA slides were stained with mouse anti-human SPARC antibody (Cat MAB3498, Clone 15G12, Abnova) on a DAKO Autostainer Plus instrument. Detection was performed using Envision FLEX High pH kit (Cat K8010, DAKO).
2.3. Digital analysis of SPARC expression
ACIS III. Stained slides were labeled with barcodes and scanned on the Automated Computerized Imaging System III (ACIS III) (DAKO). Each scanned image from individual TMA slides was reviewed to ensure it was appropriate for subsequent analysis. Slides with out of focus spot(s), dust, or other obstacles were re-scanned or restained as necessary. Each entire core on the TMA was analyzed. ACIS software collects individual, overlapping images at 400X and then tiles these images to create a montage of the entire scanned tissue specimen. The software evaluates each individual 400X image and combines the results into an aggregate quantitative measurement corresponding to the entire tissue specimen. The ACIS system measures the intensity of the staining based on three related color parameters: the color defined by hue, the “darkness” defined as luminosity, and the density of the color defined as saturation. Cores with the strongest intensity of brown staining were identified and used to set the high threshold for the brown color, and regions that demonstrated minimal SPARC immunoreactivity were used to set the low threshold for the brown color. Nuclear hematoxylin blue staining was used to set the high threshold for the blue color. These thresholds were kept constant for all analyses. An experienced user-pathologist (ACM) programmed the ACIS software for the analysis by setting the color-specific thresholds to determine and calculate the ratio of positively stained tissues to the entire area of selection [22,23]. This was used to determine the approximate percentage of positive SPARC staining tissue in each specimen. The percentage of SPARC positive tissue and the intensity of SPARC were measured independently in each core. Obvious artefacts including tissue folding, edge effect, nonspecific chemical precipitation, and dust or debris artefacts were excluded by masking these regions using the ACIS III software. Visiomorph. Two SPARC-stained TMA slides were scanned on a Nanozoomer (Hamamatsu Photonics, Japan) and NDPI image files were created (Nanozoomer image format). Images were analyzed using Visiomorph DP in the Visiomorph Integrator System (Visiopharm, Denmark) to determine percent SPARC expression for each core on the TMAs. For both methods, the percent of SPARC positive tissue and the intensity of SPARC staining were determined by computing the average value of these metrics for all the cores representing tumor or adjacent normal tissue for each patient sample to avoid pseudo-replication of values. The average value was plotted.
2.4. Assessment of tumor response to neoadjuvant therapy
The effects of chemoradiation were determined histologically using two independent systems by two expert gastrointestinal pathologist (D.R., K.O.). Briefly, the system described by Evans et al. [24] consists of a 4-tiered grading scheme that assess the percentage of viable cells: Grade I represents little to no tumor response (<10%), Grade II is subdivided into Grade IIa (10%–50% tumor response) and Grade IIb (50%–90% tumor response), Grade III (>90% tumor response), and Grade IV (no tumor cells identified) represent a spectrum of tumor response to neoadjuvant therapy. Necrosis is not interpreted as evidence of tumor response in this system. Hartman et al. [25] describe a 3-tiered system, which is based in part on the scheme recommended by the College of American Pathologist [26] and integrates some elements of the Evans system: grade 1 (marked response), grade 2 (minimal to moderate response) and grade 3 (poor response).
2.5. Statistics
Two-tail paired or unpaired Student t-Test was used to compare the means among different groups. Two-tailed nonparametric, Mann-Whitney Test or Kruskal-Wallis Test were used for the pooled data analysis. Wilcoxon matched-pair signed rank test for the paired data were used. P < 0.05 is significant.
3. Results
3.1. Digital image analysis for quantitative SPARC expression
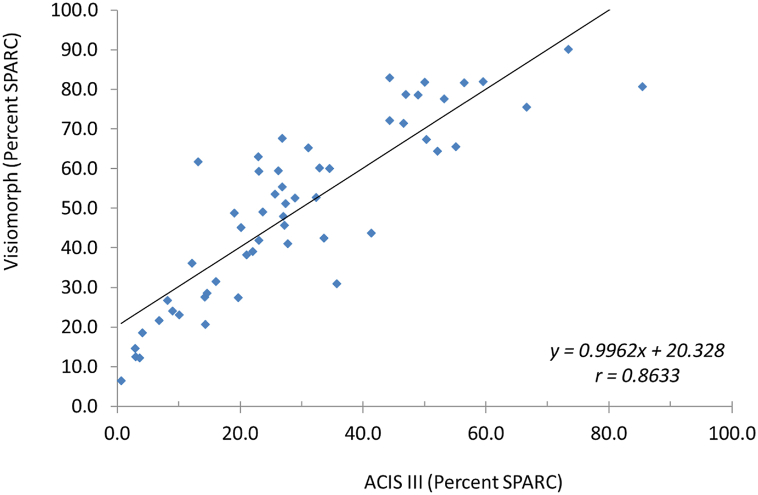
SPARC expression was analyzed in PDAC by IHC using TMAs containing 1 mm cores. Small, patchy SPARC staining is observed by acinar structures and ducts in normal pancreas (Figure 1A and B). In PDAC, uniformly intense SPARC expression is associated with cancer associated fibroblasts in the dense fibrous stroma (Figure 1C and D). SPARC is particularly enriched around glands in a pattern that outlines the malignant growth pattern. SPARC is conspicuously absent from both benign and malignant pancreatic epithelium with only rare and sporadic SPARC expression observed in malignant pancreatic glands. SPARC expression in PDAC was quantified using automated image analysis (ACIS III, DAKO, Carpinteria, CA) that determined both the percentage of SPARC-positive tissue and the intensity of the SPARC staining (Fig. 2). The percent of SPARC-positive tissue ranged from 1.9 to 85.4%, and the intensity of SPARC staining ranged from 68.5 to 100.7 (arbitrary units). To test the validity of these findings, 57 cases were reanalyzed using a different digital imaging system (Visiomorph, Visiopharm, Hoersholm, Denmark). A strong correlation of the percent of SPARC-positive tissue was observed (Pearson r = 0.863; p < 0.001) (Fig. 3). This data indicates that assessing the percent of SPARC-positive PDAC provides a wider dynamic range than assessing the intensity of SPARC staining, and this measurement is reproducible across independent image analysis systems. Therefore, we evaluated SPARC expression by determining the percent of SPARC-positive PDAC using quantitative image analysis.
Fig. 1.
SPARC expression in treatment-naïve and neoadjuvant treated resected PDAC. (A) Non-malignant, treatment naïve pancreatic tissue stained with SPARC antibody. (B) Non-malignant pancreatic tissue stained with SPARC antibody from a patient who received neo-adjuvant chemoradiation therapy. (C) PDAC stained with SPARC antibody. The tumor is from the same patient shown in panel A who did not receive any neoadjuvant therapy. (D) Neoadjuvant treated PDAC from same patient that is shown in panel B stained with the SPARC antibody. The insert in each panel is an H&E from the corresponding patient.
Fig. 2.
Quantification of SPARC Expression by Digital Image Analysis. Bar graph of SPARC intensity (left vertical axis) and percent SPARC intensity (right vertical axis) for 21 cases of PDAC stained on a TMA.
Fig. 3.
Correlation of percent SPARC expression determined by two independent digital imaging systems. Percent SPARC expression determined by the Visiomorph and the ACIS III digital imaging systems are plotted on the Y and X axes, respectively. The correlation coefficient (R-squared) is shown.
3.2. SPARC is upregulated in PDAC
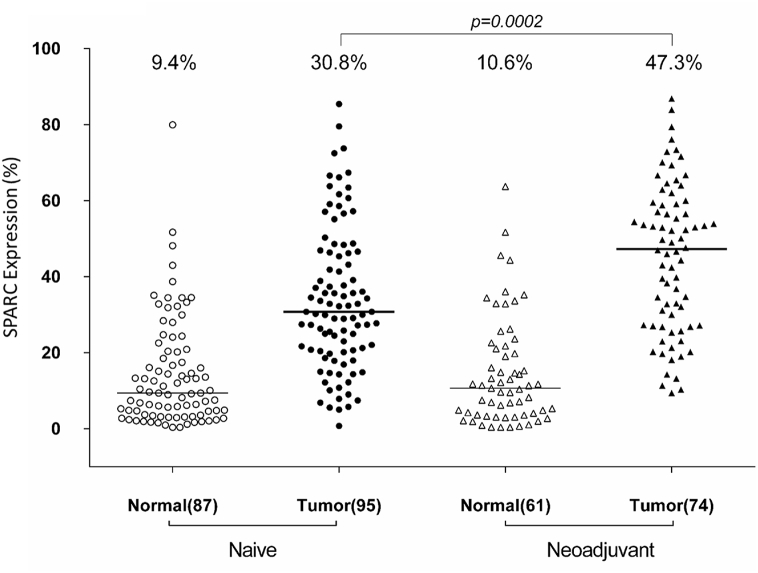
We examined the change in SPARC expression in PDAC by comparing SPARC levels in paired tumor-normal tissue cores. In areas of histologically normal pancreas, the median percent of SPARC-positive tissue was 9.4% in treatment naïve PDAC and 11% in neoadjuvant-treated PDAC. In 60 PDAC cases receiving neoadjuvant chemoradiation therapy, 58 (97%) demonstrated an increase in SPARC expression. In treatment naïve PDAC, SPARC expression increased in a smaller proportion of cases with 72 (83%) demonstrating increased SPARC expression (Fig. 4). Examination of SPARC levels indicate that SPARC expression is significantly higher in neoadjuvant PDAC (47.3% median SPARC expression) compared with treatment-naïve PDAC (30.8% median SPARC expression; p = 0.0002) (Fig. 5). These findings demonstrate SPARC expression increased 4.4-fold and 3.3-fold in neoadjuvant and treatment naïve PDAC, respectively, relative to adjacent, nonmalignant pancreas. Furthermore, SPARC expression is approximately 1.5 fold greater in neoadjuvant-treated PDAC than in treatment naïve PDAC.
Fig. 4.
SPARC is upregulated in PDAC relative to paired normal pancreatic ducts. SPARC expression in paired normal and tumor samples from treatment-naïve (left) and neoadjuvant (right) PDAC. Hatched lines connect the normal and malignant SPARC expression values for each case. Values on horizontal axis represent median percent observed for each group. The p-value refers to the mean comparison for each cohort.
Fig. 5.
SPARC expression is enhanced in neoadjuvant-treated PDAC. Quantitative expression of SPARC is plotted in scatter plots for 169 cases of PDAC. 95 cases were treatment naïve and 74 cases received neoadjuvant chemoradiation prior to resection. Many of the cases of PDAC had paired histologically normal appearing pancreatic tissue that could also be analyzed. Bars indicate median of each group with values shown on the top, as well as p value for comparison of treatment-naïve with adjuvant treated PDAC.
3.3. SPARC expression does not correlate with PDAC stage or response to neoadjuvant therapy
Increase in SPARC expression did not correlate with tumor stage in either the neoadjuvant or treatment naive cohorts (p = 0.7752 and p = 0.1396, respectively). Neoadjuvant therapy is frequently associated with a robust tumor response characterized by a loss of neoplastic cells and reduction in tumor volume. Therefore in neoadjuvant treated PDAC, it is possible that SPARC expression remains constant, and the tumor simply shrinks. This in turn would give the appearance of increased SPARC expression when instead SPARC expression per unit of starting area is actually constant. To address this, we determined tumor response to neoadjuvant therapy using two independent methods of assessment and correlated this change to SPARC expression (Fig. 6). Using either assessment system, SPARC expression is independent of tumor response to neoadjuvant therapy indicating that the observed increase in SPARC expression does not reflect a decrease in tumor volume but rather reflects an increase in SPARC-positive stromal cells. Another consideration is that lower stage tumors have less area to retract due to their smaller size resulting in less tumor fibrosis. In turn, lower stage PDAC may appear to have less SPARC expression and/or less response to therapy relative to a high stage PDAC, and both of these could potentially contribute to an overlap of these cases in either the Evans or CAP groups. To examine this possibility, we correlated the tumor response score for both the Evans and CAP scoring systems with tumor stage and did not find a significant correlation (p = 0.1802 and p = 0.376, respectively).
Fig. 6.
SPARC expression is independent of PDAC response to neoadjuvant therapy. Grade refers to the extent of tumor response to neoadjuvant therapy based on the Evans grading system (panel A) or CAP tumor response system (panel B). SPARC expression and tumor response grades show no correlation.
3.4. Prognostic value of SPARC expression in PDAC
In our cohort we observed improved overall survival in the neoadjuvant treated cohort (34.1 months) compared to the treatment naïve group (21 months) [3], however, using various cutoffs for SPARC expression, increased SPARC expression was not associated with overall survival in either the neoadjuvant or treatment naïve cohorts.
4. Conclusion
4.1. Measuring the percent of SPARC expression in tumor stroma provides greater dynamic range
The use of different scoring systems for immunohistochemistry for SPARC in PDAC stroma, based on intensity and/or percentage of expression has complicated the investigation of this biomarker. We have shown that the SPARC clone 15G12 is highly specific to the stroma, and measuring the percent SPARC-positive tissue provides greater dynamic range than measuring intensity. Our findings reveal a potential drawback with the use of intensity in a variety of other reports, where in some studies, it was the only metric to determine the level of SPARC expression in tumor stroma [17]. Our method, like that of Sinn et al., reveals a majority of PDAC cases demonstrate moderate to strong intensity of stromal staining, forcing a binary cut off at the strong end of the staining spectrum and thereby obscuring the more dynamic range available in the extent of tumor staining. Further, a majority of studies choose an arbitrary binary cutoff for SPARC expression rather than exploiting the opportunity to analyze expression as a continuous variable, as permitted with automated systems. Although digital image analysis systems provide advantages for quantifying biomarker expression, they are not without drawbacks and limitations for routine utilization. Some of these include: requirement for specialized equipment, delayed turnaround times, and the need to establish and validate thresholds for meaningful, clinically relevant interpretation of SPARC expression.
4.2. SPARC expression is greater in neoadjuvant treated compared to treatment-naïve PDAC
PDAC cohorts studied for stromal SPARC expression have included both neoadjuvant treated and treatment-naïve patients, but none have compared the treatment groups for differences in SPARC expression, despite the common observation that stromal fibrosis is increased in neoadjuvant-treated PDACs. Pursuing this anecdotal difference in stromal fibrosis, we found support for our hypothesis that SPARC expression is increased in neoadjuvant-treated PDAC. To our knowledge this is a novel finding.
A limitation of this study is that it was not designed to investigate the mechanism for the relative increase in SPARC expression observed in the neoadjuvant cohort. A common tumor response to neoadjuvant therapy is the reduction in tumor volume due to loss of tumor cells along with stromal remodeling leading to the frequent observation of rare malignant glands entrapped in densely fibrotic stroma at the time of resection. The apparent relative increase in SPARC expression that we observe in the neoadjuvant-treated PDAC cohort may reflect this effect as a passive accumulation of SPARC due to an increased shift in SPARC-positive stroma to SPARC-negative epithelium in neoadjuvant PDAC. SPARC expression was measured similarly as a percentage of precisely defined area of PDAC (0.785 mm2) in both PDAC cohorts allowing a relative comparison between neoadjuvant and treatment naïve PDAC. If increased SPARC expression reflects decreased tumor volume, then we would expect to see SPARC expression increase proportionally with tumor response to neoadjuvant therapy, and this was not observed.
We support the model that SPARC expression is upregulated in response to neoadjuvant therapy-associated tissue damage and subsequent remodeling and repair [[27], [28], [29]]. PDAC is responsible for the initial increase in SPARC, and enhanced SPARC seen in the neoadjuvant cohort is likely secondary to additional tissue injury caused by the chemoradiation received prior to resection. In essence, the neoadjuvant cohort shows “super enhanced” SPARC due to neoadjuvant-associated tissue injury superimposed on generalized PDAC-associated increased SPARC. We would therefore expect to see increased SPARC in settings of other causes of chronic inflammation, such as chronic pancreatitis [30,31]. In support of this, high expression of SPARC is seen in acinar structures in the Winstar-Bonn/Kobori rat model of chronic pancreatitis [32]. Analysis of human chronic pancreatitis demonstrates increased SPARC in acinar cells during early phases of inflammation [32]. In PDAC, we observe SPARC expression restricted to the tumor stroma and little to no acinar localization. Taken together, these findings along with our results support the concept that SPARC expression correlates with tissue injury, however the pattern and distribution of SPARC expression depends upon the disease context.
4.3. SPARC is expressed nearly exclusively in PDAC stroma
We find that SPARC is nearly exclusively expressed in tumor stroma rather than in the normal or tumor epithelium, which is inconsistent with previous reports that document cytoplasmic staining of malignant epithelial cells [17,18]. Hidalgo, et al., used a different antibody (ON1-1 monoclonal mouse antibody, Invitrogen, Camarillo, California) than us. The faint cytoplasmic SPARC staining observed by Sinn, et al., in tumor epithelium occurring in a minority of cases may be non-specific background staining as the 15G12 monoclonal mouse was obtained from a different vendor (Novocastra, Wetzlar, Germany). As well, the lack of tumor cytoplasmic staining in our cases could be attributed to a variety of methodological differences such as variation in antigen retrieval methods [32]. Notwithstanding this difference, we confirm the prior finding that SPARC is upregulated in tumor stroma compared to paired normal tissue [33].
4.4. Neoadjuvant therapy might sensitize PDAC for adjuvant nab-paclitaxel therapy
Based on increased SPARC expression in the resected PDAC following neoadjuvant therapy, as demonstrated in our study, it is tempting to speculate whether neoadjuvant-treated PDAC might be sensitized to benefit from adjuvant nab-paclitaxel therapy following resection in the non-metastatic setting. Analysis of the largest multicenter trial of nab-paclitaxel and Gemcitabine versus Gemcitabine alone failed to demonstrate any association of SPARC expression in PDAC and efficacy of either therapy [18]. Based on this, Hidalgo et al. recommend that treatment decisions regarding nab-paclitaxel should be not be based on SPARC IHC. However, these patients had previously untreated metastatic pancreatic cancer. Perhaps patients with non-metastatic, resectable PDAC receiving neoadjuvant therapy could derived additional benefit from nab-paclitaxel adjuvant therapy. In the neoadjuvant treated cohort of this study, we were unable to assess this prediction because very few patients received nab-paclitaxel in the adjuvant setting [3]. As such, prospective studies of neoadjuvant-treated PDAC are needed to address whether SPARC IHC might predict nab-paclitaxel response in the adjuvant setting.
CRediT authorship contribution statement
Christopher Hartley: Writing - original draft. Daniel Rowan: Formal analysis. Xiuxu Chen: Data curation, Formal analysis, Methodology. Luisa Gomez-Arellano: Data curation. Anna Marie West: Data curation. Kiyoko Oshima: Formal analysis. Alexander Craig Mackinnon: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
On behalf of my authors, none of us have any conflict of interest to report related to the submission of this manuscript.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Neuzillet C., Tijeras-Raballand A., Bourget P., Cros J., Couvelard A., Sauvanet A. State of the art and future directions of pancreatic ductal adenocarcinoma therapy. Pharmacol. Therapeut. 2015;155:80–104. doi: 10.1016/j.pharmthera.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Tsai S., Christians K.K., George B., Ritch P.S., Dua K., Khan A. A phase II clinical trial of molecular profiled Neoadjuvant therapy for localized pancreatic ductal adenocarcinoma. Ann. Surg. 2018;268(4):610–619. doi: 10.1097/SLA.0000000000002957. [DOI] [PubMed] [Google Scholar]
- 4.McDonald O.G., Maitra A., Hruban R.H. Human correlates of provocative questions in pancreatic pathology. Adv. Anat. Pathol. 2012;19(6):351–362. doi: 10.1097/PAP.0b013e318273f998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang C., Shi S., Meng Q., Liang D., Ji S., Zhang B. Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer? Cellular and molecular life sciences. CMLS. 2018;75(6):1001–1012. doi: 10.1007/s00018-017-2678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Infante J.R., Matsubayashi H., Sato N., Tonascia J., Klein A.P., Riall T.A. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2007;25(3):319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 7.Mantoni T.S., Schendel R.R., Rodel F., Niedobitek G., Al-Assar O., Masamune A. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Canc. Biol. Ther. 2008;7(11):1806–1815. doi: 10.4161/cbt.7.11.6846. [DOI] [PubMed] [Google Scholar]
- 8.Termine J.D., Robey P.G., Fisher L.W., Shimokawa H., Drum M.A., Conn K.M. Osteonectin, bone proteoglycan, and phosphophoryn defects in a form of bovine osteogenesis imperfecta. Proc. Natl. Acad. Sci. U.S.A. 1984;81(7):2213–2217. doi: 10.1073/pnas.81.7.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremble P.M., Lane T.F., Sage E.H., Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J. Cell Biol. 1993;121(6):1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B., Chen K., Xu W., Chen D., Tang W., Xia T.S. Integrative genomic analyses of secreted protein acidic and rich in cysteine and its role in cancer prediction. Mol. Med. Rep. 2014;10(3):1461–1468. doi: 10.3892/mmr.2014.2339. [DOI] [PubMed] [Google Scholar]
- 11.Bloomston M., Ellison E.C., Muscarella P., Al-Saif O., Martin E.W., Melvin W.S. Stromal osteonectin overexpression is associated with poor outcome in patients with ampullary cancer. Ann. Surg Oncol. 2007;14(1):211–217. doi: 10.1245/s10434-006-9128-3. [DOI] [PubMed] [Google Scholar]
- 12.Neuzillet C., Tijeras-Raballand A., Cros J., Faivre S., Hammel P., Raymond E. Stromal expression of SPARC in pancreatic adenocarcinoma. Canc. Metastasis Rev. 2013;32(3–4):585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- 13.Gundewar C., Sasor A., Hilmersson K.S., Andersson R., Ansari D. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand. J. Gastroenterol. 2015;50(9):1170–1174. doi: 10.3109/00365521.2015.1024281. [DOI] [PubMed] [Google Scholar]
- 14.Murakawa M., Aoyama T., Miyagi Y., Kobayashi S., Ueno M., Morimoto M. The impact of SPARC expression on the survival of pancreatic ductal adenocarcinoma patients after curative resection. J. Canc. 2019;10(3):627–633. doi: 10.7150/jca.28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H., Lee Y., Lee H., Kim J.W., Hwang J.H., Kim J. The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumour Biol. : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39(10) doi: 10.1177/1010428317718403. 1010428317718403. [DOI] [PubMed] [Google Scholar]
- 16.Shintakuya R., Kondo N., Murakami Y., Uemura K., Nakagawa N., Okano K. The high stromal SPARC expression is independently associated with poor survival of patients with resected pancreatic ductal adenocarcinoma treated with adjuvant gemcitabine in combination with S-1 or adjuvant gemcitabine alone. Pancreatology. 2018;18(2):191–197. doi: 10.1016/j.pan.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Sinn M., Sinn B.V., Striefler J.K., Lindner J.L., Stieler J.M., Lohneis P. SPARC expression in resected pancreatic cancer patients treated with gemcitabine: results from the CONKO-001 study. Ann. Oncol. : official journal of the European Society for Medical Oncology / ESMO. 2014;25(5):1025–1032. doi: 10.1093/annonc/mdu084. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo M., Plaza C., Musteanu M., Illei P., Brachmann C.B., Heise C. SPARC expression did Not predict efficacy of nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT trial. Clin. Canc. Res. 2015;21(21):4811–4818. doi: 10.1158/1078-0432.CCR-14-3222. [DOI] [PubMed] [Google Scholar]
- 19.Ormanns S., Haas M., Baechmann S., Altendorf-Hofmann A., Remold A., Quietzsch D. Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials. Br. J. Canc. 2016;115(12):1520–1529. doi: 10.1038/bjc.2016.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramu I., Buchholz S.M., Patzak M.S., Goetze R.G., Singh S.K., Richards F.M. SPARC dependent collagen deposition and gemcitabine delivery in a genetically engineered mouse model of pancreas cancer. EBioMedicine. 2019;48:161–168. doi: 10.1016/j.ebiom.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neesse A., Frese K.K., Chan D.S., Bapiro T.E., Howat W.J., Richards F.M. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–983. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao N., Mackinnon A.C., Routes J.M. Granulomatous and lymphocytic interstitial lung disease: a spectrum of pulmonary histopathologic lesions in common variable immunodeficiency--histologic and immunohistochemical analyses of 16 cases. Hum. Pathol. 2015;46(9):1306–1314. doi: 10.1016/j.humpath.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma P.C., Tretiakova M.S., MacKinnon A.C., Ramnath N., Johnson C., Dietrich S. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008;47(12):1025–1037. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans D.B., Rich T.A., Byrd D.R., Cleary K.R., Connelly J.H., Levin B. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. 1992;127(11):1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 25.Hartman D.J., Krasinskas A.M. Assessing treatment effect in pancreatic cancer. Arch. Pathol. Lab Med. 2012;136(1):100–109. doi: 10.5858/arpa.2011-0144-RA. [DOI] [PubMed] [Google Scholar]
- 26.Washington K., Berlin J., Branton P., Burgart L., Carter D., Compton C. Endocrine Tumors and Tumors of the Ampulla of Vater Are Not Included. College of American Pathologist [Internet]; 2013. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas: protocol applies to all endocrine tumors of the pancreas. [Google Scholar]
- 27.Bradshaw A.D., Sage E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Invest. 2001;107(9):1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan E., Ahluwalia A., Tarnawski A.S. Role of SPARC--matricellular protein in pathophysiology and tissue injury healing. Implications for gastritis and gastric ulcers. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : international medical journal of experimental and clinical research. 2007;13(2):RA25–30. [PubMed] [Google Scholar]
- 29.Strandjord T.P., Madtes D.K., Weiss D.J., Sage E.H. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am. J. Physiol. 1999;277(3 Pt 1):L628–L635. doi: 10.1152/ajplung.1999.277.3.L628. [DOI] [PubMed] [Google Scholar]
- 30.Binkley C.E., Zhang L., Greenson J.K., Giordano T.J., Kuick R., Misek D. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29(4):254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Friess H., Ding J., Kleeff J., Liao Q., Berberat P.O., Hammer J. Identification of disease-specific genes in chronic pancreatitis using DNA array technology. Ann. Surg. 2001;234(6):769–778. doi: 10.1097/00000658-200112000-00008. discussion 78-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reding T., Wagner U., Silva A.B., Sun L.K., Bain M., Kim S.Y. Inflammation-dependent expression of SPARC during development of chronic pancreatitis in WBN/Kob rats and a microarray gene expression analysis. Physiol. Genom. 2009;38(2):196–204. doi: 10.1152/physiolgenomics.00028.2009. [DOI] [PubMed] [Google Scholar]
- 33.Guweidhi A., Kleeff J., Adwan H., Giese N.A., Wente M.N., Giese T. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann. Surg. 2005;242(2):224–234. doi: 10.1097/01.sla.0000171866.45848.68. [DOI] [PMC free article] [PubMed] [Google Scholar]