Clinical Implications.
-
•
Infants at risk for development of peanut allergy have very limited access to allergist assessment for the prevention of peanut allergy during the COVID-19 pandemic. Virtually supported infant home introduction represents a practical and safe alternative to avoid delays in peanut introduction.
Recently published North American guidelines for the management of allergic disease during the COVID-19 pandemic recommend service adjustments such as virtual visits or postponing appointments.1 Although it is reasonable to delay treatment for some allergic conditions, the guideline notes that other scenarios such as peanut avoidance in a high-risk infant requires expedited assessment and active management. Therefore, the time-sensitive nature of food introduction presents a dilemma for both parents and clinicians. Although some guidelines recommend that families introduce peanut without physician evaluation,2 National Institute of Allergy and Infectious Disease (NIAID) guidelines have suggested screening and in-office introduction of peanut for at-risk and sensitized populations.3 As many clinics currently offer limited in-person services, the inability to follow these guidelines places these infants at risk of missing the preventative window. Furthermore, parental concerns of reactions requiring emergency department visit with potential COVID-19 exposure compound an already stressful experience.4, 5, 6 With no consistent guidance or concrete plan regarding the reopening of medical services, and variance in COVID-19 risk by region, access to in-office food introduction remains uncertain. We report the first known use of a virtually supported home introduction option for infants at risk of developing peanut allergy.
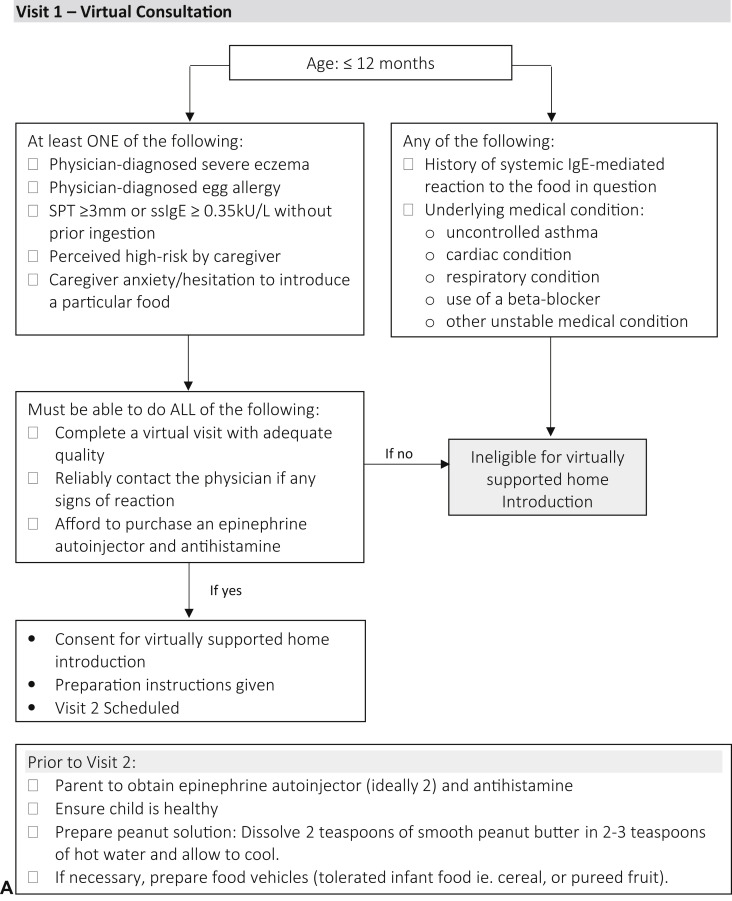
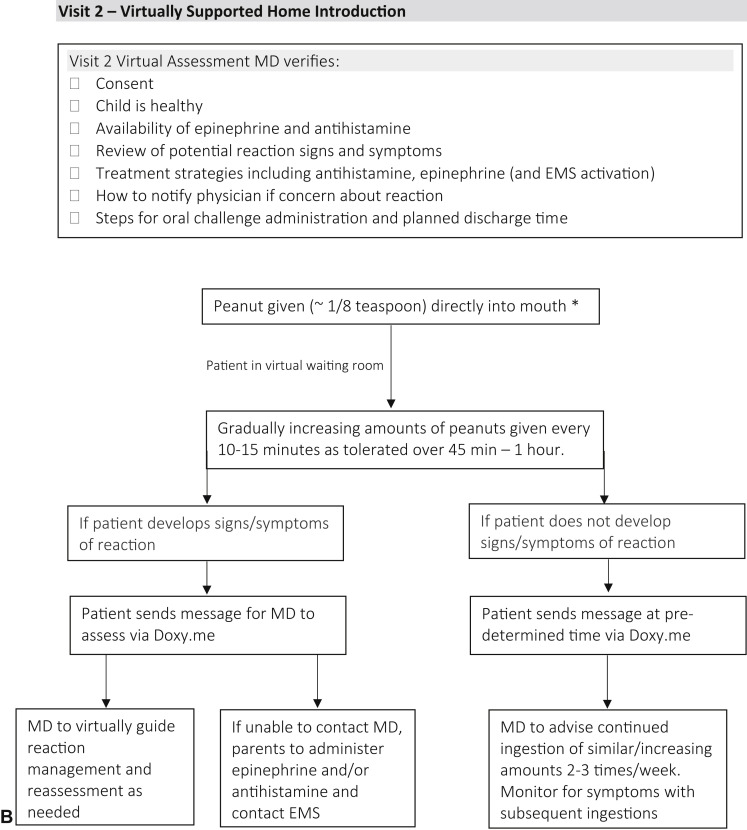
In April 2020, infants were evaluated virtually in a private-practice allergist setting using the telehealth platform Doxy.me. These infants were identified as either being: at risk for the development of peanut allergy (eg, egg allergy or severe eczema)3; tested previously as per guidelines; perceived higher-risk by parents (eg, family history, other food allergy); or there was caregiver hesitation to feed (Figure 1 , A).5 During the initial virtual consultation, shared decision making was used to review the risks and benefits of the following options: peanut avoidance until in-office assessment; or participation in a virtually supported food introduction process. If families chose the latter option, verbal consent was obtained and they were prescribed an epinephrine autoinjector and rupatadine, to be obtained before the virtually supported introduction. Parents were instructed to prepare peanut butter as per Figure 1, A.7 Families subsequently met virtually with the physician on Doxy.me where they were counseled about food introduction, possible symptoms, and treatment (Figure 1, B). Parents then proceeded to gradually introduce 2 g peanut protein over 45 minutes to 1 hour. Families could contact the physician immediately through the virtual platform with questions or concerns, or if the child reacted. Below, 3 cases demonstrate the utility and potential application of this approach.
Figure 1.
A flowchart and checklist of a virtually supported home peanut introduction in at-risk infants. A, Visit 1: initial virtual consultation and preparation. B, Visit 2: virtually supported home peanut introduction procedure. ∗Barrier cream or petroleum jelly can be applied around mouth if eczema. EMS, Emergency medical services; MD, Medical Doctor; SPT, skin prick test; ssIgE, serum-specific IgE.
Case 1: a 10-month-old infant with severe eczema pre-emptively tested for multiple foods with a 6-mm epicutaneous wheal and 0.95 kU/L serum sIgE for peanut and 5.71 kU/L serum sIgE for whole egg. During virtual peanut introduction, parents had concern about respiratory status, but on virtual reassessment, the child was happy and in no distress, with a normal respiratory rate, no accessory muscle use, and no cutaneous lesions. Introduction proceeded with no reaction, although only 1 g of peanut protein was ingested and parents were instructed to try peanut puffs. At follow-up, parents had successfully given up to 2 g peanut on a regular basis.
Case 2: a 7-month-old with mild eczema, sibling with peanut allergy, and parental reluctance to feed. Barrier cream was applied around mouth pre-emptively and peanut was introduced without difficulty. There was brief virtual communication with the physician during the introduction clarifying the volume to be introduced. After initial introduction, parents continued to give peanut at home regularly without difficulty.
Case 3: a 6-month-old infant had mild eczema with a history of anaphylaxis requiring 2 doses of epinephrine after sesame introduction. Because of the traumatic experience and a fear of emergency department presentation during the COVID-19 pandemic, parents expressed reluctance to introduce peanut. During introduction, the patient had a small exposure to peanut, but expressed distaste, and the full amount could not be introduced. There was no evidence of adverse reaction, and parents continued to offer similar small amounts at home. After this experience, parents reported feeling more comfortable introducing tree nuts and have successfully introduced almond at home.
These 3 cases demonstrate the feasibility and practicality of a virtually supported food introduction program. Case 1 was considered “high-risk” as per NIAID guidelines,3 and although Case 2 was not technically “high-risk,” siblings of patients with a peanut allergy show a higher long-term risk of developing an allergy, especially if families continue to avoid peanut.5 All families expressed significant concern about peanut introduction (as a result of a previous severe reaction in Case 3), and this reluctance is consistent with North American survey data.4 , 5 These patients tolerated peanut during the introduction and on subsequent exposures. Each family virtually interacted with the physician at the beginning of the introduction and 1 to 2 times during the actual procedure. Families could immediately and easily contact the physician during the process through the virtual platform if they had concerns. These interactions were brief and not burdensome to the clinician. All families reported significant appreciation and satisfaction with this virtually supported approach.
In each case, parents were counseled beforehand about the possibility of severe reaction. No reactions were reported during these introductions, which is consistent with our prior clinic experience. Our findings are consistent with current evidence that severe anaphylaxis to peanut in infants is rare, with no life-threatening reactions reported on first ingestion in infancy, and mild reactions can generally be managed with antihistamine and/or observation.8 Recent clinical guidelines suggest a framework for at-home management of anaphylaxis.9 Should a severe reaction have occurred during this procedure, the physician and family would have used the guidance of those recommendations.
Even before COVID-19, lack of allergist resources presented significant barriers to the introduction of peanut to at-risk patients where there was hesitancy. During COVID-19, the need to provide alternative forms of care is heightened. Virtually supported introduction may represent a future option after COVID-19 to improve access for patients who live in remote areas, or otherwise have limited access to allergists, or for clinicians with overburdened clinics. However, the successful implementation of such a strategy requires formal evaluation of safety, cost-effectiveness, caregiver/physician acceptability, sustainability, and patient satisfaction. This pandemic will persist for the foreseeable future and has highlighted the need to move toward a virtual platform. There is simply too much at stake for prolonged peanut avoidance due to lack of in-office access.
Footnotes
No funding was received for this work.
Conflicts of interest: D. P. Mack has provided consultation and speaker services for Pfizer, Aimmune, Kaleo, Merck, Covis, and Pediapharm, and has been part of an advisory board for Pfizer and Bausch Health. He sits on the editorial board for the Journal of Food Allergy. M. A. Hanna has provided speaker services for Pfizer and Pediapharm. E. M. Abrams is a member of the health care advisory board for Food Allergy Canada; and is on the National Advisory Committee for Food Allergy Canada. T. Wong has provided speaking engagements for Pfizer and Stallergenes-Greer, and has been part of an advisory board for Leo Pharma. L. Soller participates in research with DBV Technologies. S. C. Erdle declares no relevant conflicts of interest. S. Jeimy has been on speaker's bureaus for Aralez, Astra Zeneca, Sanofi, and Novartis. J. L. P. Protudjer sits on the steering committee of the National Food Allergy Action Plan. E. S. Chan has received research support from DBV Technologies; has been a member of advisory boards for Pfizer, Pediapharm, Leo Pharma, Kaleo, and DBV; is a member of the health care advisory board for Food Allergy Canada; was co-lead of the CSACI oral immunotherapy guidelines; and was an expert panel and coordinating committee member of the National Institute of Allergy and Infectious Diseases (NIAID)-sponsored Guidelines for Peanut Allergy Prevention.
References
- 1.Shaker M.S., Oppenheimer J., Grayson M., Stukus D., Hartog N., Hsieh E.W.Y. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8:1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netting M.J., Campbell D.E., Koplin J.J., Beck K.M., McWilliam V., Dharmage S.C. An Australian consensus on infant feeding guidelines to prevent food allergy: outcomes from the Australian infant feeding summit. J Allergy Clin Immunol Pract. 2017;5:1617–1624. doi: 10.1016/j.jaip.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Togias A., Cooper S.F., Acebal M.L., Assa’ad A., Baker J.R.J., Beck L.A. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhawt M., Chan E.S., Fleischer D.M., Hicks A., Wilson R., Shaker M. Caregiver and expecting caregiver support for early peanut introduction guidelines. Ann Allergy Asthma Immunol. 2018;120:620–625. doi: 10.1016/j.anai.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Bégin P., Graham F., Killer K., Paradis J., Paradis L., Des Roches A. Introduction of peanuts in younger siblings of children with peanut allergy: a prospective, double-blinded assessment of risk, of diagnostic tests, and an analysis of patient preferences. Allergy. 2016;71:1762–1771. doi: 10.1111/all.12956. [DOI] [PubMed] [Google Scholar]
- 6.Abrams E.M., Greenhawt M. Special article: risk communication during COVID-19. J Allergy Clin Immunol Pract. 2020;8:1791–1794. doi: 10.1016/j.jaip.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food Allergy Canada and Canadian Society of Allergy and Clinical Immunology, Early Introduction of Allergens: FAQs for Families. https://foodallergycanada.ca/wp-content/uploads/EarlyIntro_Web.pdf Available from:
- 8.Chan J.C.K., Peters R.L., Koplin J.J., Dharmage S.C., Gurrin L.C., Wake M., HealthNuts Study Food challenge and community-reported reaction profiles in food-allergic children aged 1 and 4 years: a population-based study. J Allergy Clin Immunol Pract. 2017;5:398–409. doi: 10.1016/j.jaip.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Casale T.B., Wang J., Nowak-Wegrzyn A. Acute at home management of anaphylaxis during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8:1795–1797. doi: 10.1016/j.jaip.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]