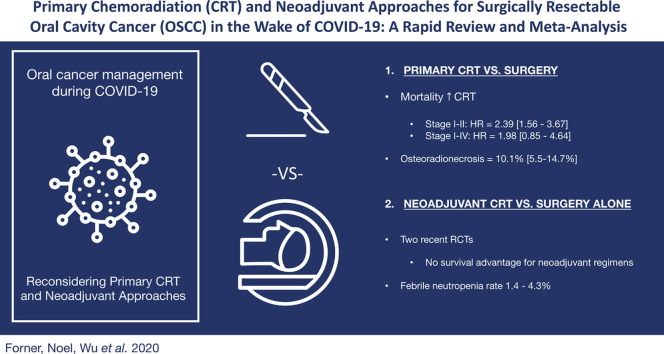
Graphical abstract
Keywords: Oral cavity, Squamous cell carcinoma, Radiotherapy, Chemo-radiotherapy, Immunotherapy, Outcomes, COVID-19, Chemotherapy
Highlights
-
•
COVID-19 resource constraints have resulted in treatment delays for oral cancer.
-
•
Non-surgical treatment may be necessary to provide timely access to care.
-
•
Definitive CCRT is associated with an increased rate of death in early oral cancer.
-
•
Neoadjuvant regimens have not shown survival benefit in resectable oral cancer.
Abstract
Objective
Surgery is the preferred treatment modality for oral squamous cell carcinoma (OSCC). However, due to limited resources, re-assessment of treatment paradigms in the wake of the Coronavirus Disease 2019 (COVID-19) pandemic is urgently required. In this rapid review, we described contemporary oncological outcomes for OSCC using non-surgical modalities.
Methods
A systematic literature search was conducted for articles published between January 1, 2010 and April 1, 2020 on MEDLINE and Cochrane CENTRAL. Studies were included if they contained patients with OSCC treated with either neoadjuvant, induction, or definitive radiotherapy, chemotherapy, immunotherapy, or combination thereof, and an outcome of overall survival.
Results
In total, 36 articles were included. Definitive radiotherapy or chemoradiotherapy were the focus of 18 articles and neoadjuvant chemotherapy or chemoradiotherapy were the focus of the other 18 articles. In early stage OSCC, definitive radiotherapy, with or without concurrent chemotherapy, was associated with a significantly increased hazard of death compared to definitive surgery (HR: 2.39, 95% CI: 1.56–3.67, I2: 63%). The hazard of death was non-significantly increased with definitive chemoradiotherapy in studies excluding early disease (HR: 1.98, 95% CI: 0.85–4.64, I2: 84%). Two recent randomized control trials have been conducted, demonstrating no survival advantage to neoadjuvant chemotherapy.
Conclusion
This review suggests that primary radiotherapy and chemoradiotherapy are inferior to surgical management for OSCC. Strategies for surgical delay warranting consideration are sparse, but may include several neoadjuvant regimens, recognizing these regimens may not offer a survival benefit over definitive surgery alone.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has placed significant strain on healthcare systems across the world. To increase the capacity of these systems, surgical cases, including oncological surgery, have been either delayed or cancelled across various jurisdictions [1], [2]. As COVID-19 pressures mount, head and neck surgeons must adapt to meet healthcare system needs during this unprecedented time. With resource constraints intensifying and surgical waitlists becoming longer, it may become increasingly difficult to meet established targets for treatment initiation. Head and neck oncologists may need to evaluate non-standard management options and weigh the best available evidence.
In oral cavity squamous cell carcinoma (OSCC), surgery has remained first line therapy for decades, with the National Comprehensive Cancer Network (NCCN) recommending primary surgical management in both early and late stage disease [3]. Delays in cancer surgery may risk losing a window of opportunity for resection, potentially worsening both oncologic and functional outcomes [4], [5], [6]. As human resources, including nursing and critical care personnel, become less available during the pandemic, perioperative outcomes may worsen [7]. In the immediate postoperative period, nosocomial spread of COVID-19 is also a significant concern for OSCC patients, who are often elderly and medically complex, both of which correlate with increased COVID-19 related morbidity and mortality [8], [9], [10], [11], [12].
Multiple societies, including the Canadian Association of Head & Neck Surgical Oncology (CAHNSO), have released statements and guidelines for the management of patients with head and neck cancer during COVID-19 [13], [14], [15], [16]. By necessity, these high-level guidelines do not provide specifics on disease management and patient-specific care, given the inherent complexity of such decision making and the variability in resource availability that may exist between institutions. As pandemic related health system changes accumulate, the need for non-surgical treatment of OSCC may become necessary. To better inform such decision making during both this initial surge and possible second wave surges, we have provided a review of contemporary oncological outcomes associated with non-surgical treatment modalities (primary radiation, chemoradiation, and immunotherapy) used for either definitive management or as potential bridging therapies in the management of surgically resectable OSCC.
Methods
Search strategy
This study was informed by the Cochrane COVID-19 Rapid Review templates [17]. A literature search was conducted for articles published between January 1, 2010 and April 1, 2020 on MEDLINE and Cochrane CENTRAL. The search strategy contained head and neck cancer terms and terms related to OSCC, non-surgical treatment modalities, and oncological outcomes. Snowballing and reference review techniques were used, including evaluation of previously published reviews [18], [19], [20], [21], [22]. The full search strategy is outlined in Supplemental Figure S1.
Eligibility criteria
Inclusion and exclusion criteria were defined a priori and applied to identified publications. Studies were included if they contained a population of patients (≥18 years of age) with potentially resectable OSCC, with a treatment intervention of either neoadjuvant, induction, or definitive radiotherapy, chemotherapy, immunotherapy, or combination thereof, and an outcome of overall survival. Studies involving non-OSCC head and neck cancer were considered eligible if OSCC-specific treatment and outcome details could be delineated. Induction therapy was considered to be treatment given before definitive non-surgical treatment, and neoadjuvant therapy was considered to be treatment given before definitive surgical treatment [23]. Publications were excluded if they contained populations of patients with salivary gland cancer or included an exclusive cohort of patients with lip cancer, basaloid squamous cell carcinoma (SCC) or verrucous SCC. Additionally, studies that described a cohort of patients with surgically unresectable disease, or those undergoing palliative therapy were also excluded. Treatments involving intra-arterial chemotherapy, brachytherapy, or photodynamic therapy were also excluded as these treatments would not be practical to administer in a pandemic setting, and would not eliminate the need for perioperative resources. Additionally, studies were excluded if they were non-English, abstract only, protocols, case-reports, case series of less than 10 patients, or studies with no primary data.
Review process and data extraction
Titles and abstracts were independently screened by three reviewers (CN, DF, VW) in duplicate to assess for initial relevance. Full articles were screened by two reviewers (CN, DF) to determine eligibility. All disagreements during the review process were resolved by consensus. Screening was facilitated using the Covidence systematic review software (Veritas Health Innovation, Australia).
Assessment of quality
Risk of study bias was assessed using Version 2 of the Cochrane risk-of-bias tool (RoB 2) for randomized trials and the Newcastle-Ottawa Scale (NOS) for observational studies by a single reviewer (CN). For the RoB 2 tool, studies were categorized as: low risk of bias, some concerns, or high risk of bias across 5 domains and assigned an overall risk of bias judgement [24], [25]. The NOS encompasses 3 subscales, each of which is scored separately and has a different maximum score (selection: 4 stars, comparability: 2 stars, outcome: 3 stars) [25]. Higher scores represent higher-quality studies, with decreased amounts of potential bias. Studies are considered good quality if they score 3–4 on selection, 1–2 on comparability, and 2–3 on outcome; fair quality if 2–3 on selection, 1–2 on comparability, and 2–3 on outcome; and poor quality if 0–1 on selection or 0 on comparability or 1 on outcome.
Statistical analysis
Inter-rater agreement during title and abstract screening was assessed by percent agreement, and inter-rater agreement during full-text review was analyzed by Cohen’s kappa statistic [26]. In studies where overall survival was not directly reported in the body of the text, digitization of the Kaplan-Meier curves using the DigitizeIt software (version 2.3, Germany), allowed for generation of summary survival statistics.
Pre-determined pooled analyses were performed. Meta-analysis was performed to examine pooled differences in the hazard of death between definitive radiotherapy, concurrent chemoradiotherapy (CCRT), and primary surgery. Studies which reported adjusted hazard ratios (HRs) underwent analysis. Standard errors were computed from the 95% confidence interval (CI) or p-value and log transformed HRs were used in the analysis [27]. Meta-analysis was performed using a random effects model and the generic inverse variance method; HRs and 95% CIs were reported. Heterogeneity was quantified using the I2 test statistic.[12] Meta-analysis was performed on observational studies with two separate analyses; studies that included only early stage disease were analysed separately from studies that included only advanced disease or that included all stages. Meta-analysis was also used to provide pooled proportions of osteoradionecrosis (ORN) following definitive CCRT through a DerSimonian-Laird binary random effects model. Meta-analysis was completed using Review Manager (version 5.3, Denmark).
Results
Study selection
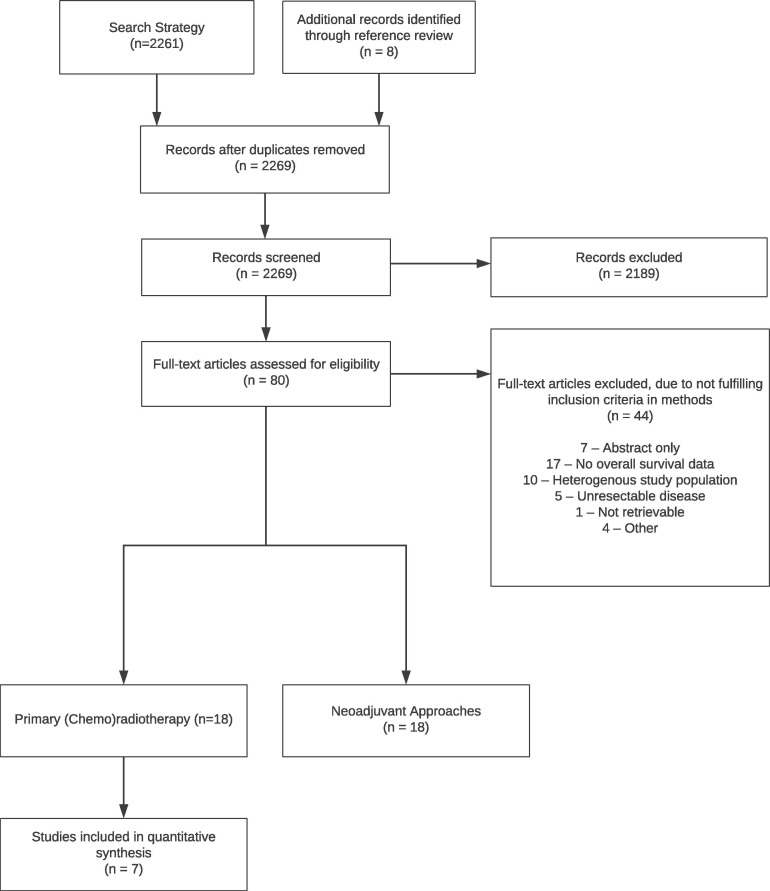
The search strategy yielded 2,261 non-duplicate articles (Figure 1 ). An additional eight studies were identified through snowballing and reference review techniques. After title and abstract screening, 80 studies underwent full text review. Agreement between three independent reviewers during title and abstract screening was 95.9% (2,176 / 2,269). During full text review, 38 articles were excluded (Figure 1). Inter-rater agreement of two independent reviewers during the full text screen was high (Cohen’s kappa = 0.713, percent agreement = 86.8%). Six studies were excluded during the data extraction phase (total excluded, n = 44) due to the inability to differentiate outcomes of unresectable disease (n = 4) or inability to differentiate disease-specific from overall survival (n = 2). Experts in the field ensured there were no missing studies (AP, KC, ZH, AE). Therefore, 36 studies were included in the review [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61].
Fig. 1.
PRISMA flow diagram.
Study characteristics
Study characteristics are summarized in Table 1 . Definitive radiotherapy or CCRT was investigated in 18 studies, of which one was a randomized controlled trial and the remainder were observational studies. Comparatively, induction or neoadjuvant chemotherapy, radiotherapy, or CCRT was also the focus of 18 articles, of which 4 studies were randomized controlled trials reporting on 3 different trials. The majority of studies provided either a comparison against primary surgery, such as in studies where the primary exposure of interest was definitive CCRT, or a comparison against no neoadjuvant treatment, such as in studies where the primary exposure of interest was neoadjuvant treatment (n = 24, 66.7%). Half of the studies presented multivariable adjusted analysis of overall survival (n = 18, 50.0%).
Table 1.
Characteristics of Included Studies (n = 36).
| First Author | Year | Journal | Country | Design | Data Source | Comparator | Matched | N | Adjusted Analysis | Stages |
|---|---|---|---|---|---|---|---|---|---|---|
| Cannon | 2017 | Head & Neck | USA | Retrospective Cohort | SEER | Yes | No | 5856 | Yes | III-IV |
| Crombie | 2012 | Oral Oncology | Australia | Retrospective Cohort | Chart Review | No | No | 54 | No | I-IV |
| Ellis | 2017 | Otolaryngology Head & Neck Surgery | USA | Retrospective Cohort | NCDB | Yes | Yes | 1912 (matched); 20779(unmatched) | Yes | I-II |
| Fujiwara | 2017 | Oral Oncology | USA | Retrospective Cohort | NCDB | Yes | No | 23,459 | Yes | I-IV |
| Gogarty | 2017 | European Archives of Oto-Rhino-Laryngology | Ireland | Retrospective Cohort | National Cancer Registry Ireland (NCRI) | Yes | No | 397 | Yes | I-II |
| Gore | 2014 | Head & Neck | Australia | Retrospective Cohort | Unspecified Cancer Database | Yes | No | 104 | Yes | I-IV |
| Hauswald | 2012 | Acta Oncologica | Germany | Retrospective Cohort | Chart Review | Yes | No | 66 | No | I-IV |
| lyer | 2015 | Cancer | Singapore | Randomized Controlled Trial | N/A | Yes | N/A | 119 | No | III-IV |
| Jenwitheesuk | 2010 | Journal of the Medical Association of Thailand | Thailand | Retrospective Cohort | Chart Review | Yes | No | 107 | No | I-IV |
| Kjems | 2015 | International Journal Radiation Oncology, Biology, Physics | Denmark | Retrospective Cohort | Chart Review | No | No | 942 (100 OSCC) | No | I-IV |
| McDowell | 2014 | Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology | Australia | Retrospective Cohort | Chart Review | Yes | No | 31 | Yes | T4 |
| Pederson | 2011 | American Journal of Clinical Oncology | USA | Prospective Cohort | Chart Review | No | No | 21 | No | II-IV |
| Scher | 2015 | Oral Oncology | USA | Retrospective Cohort | Chart Review | No | No | 73 | No | I-IV |
| Sowder | 2017 | Head & Neck | USA | Retrospective Cohort | SEER | Yes | No | 8274 | Yes | I-II |
| Spiotto | 2017 | JAMA Otolaryngology | USA | Retrospective Cohort | NCDB | Yes | Yes | 2286 (matched); 6900(unmatched) | Yes | III-IV |
| Stenson | 2010 | The Laryngoscope | USA | Retrospective Cohort | Chart Review | No | No | 138 | No | III-IV |
| Studer | 2012 | Radiation Oncology | Switzerland | Retrospective Cohort | Chart Review | Yes | No | 160 | No | I-IV |
| Wang | 2010 | Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology | Taiwan | Retrospective Cohort | Chart Review | Yes | No | 88 | No | I-IV |
| Bossi | 2014 | Annals of Oncology | Italy | Randomized Controlled Trial | N/A | Yes | N/A | 198 | Yes | T2-T4 |
| Chinn | 2014 | JAMA Otolaryngology | USA | Prospective cohort | Chart Review | Yes | Yes | 72 | Yes | III-IV |
| Harada | 2013 | Cancer Chemotherapy and Pharmacology | Japan | Non-Randomized Trial | N/A | No | No | 39 | No | III-IV |
| Hauswald | 2012 | Acta Oncologica | Germany | Retrospective Cohort | Chart Review | Yes | No | 66 | No | I-IV |
| Hirakawa | 2017 | Japanese Journal of Clinical Oncology | Japan | Retrospective Cohort | Chart Review | Yes | No | 164 | Yes | I-IV |
| Inhestern | 2017 | Annals of Oncology | Germany | Non-Randomized Trial | N/A | No | No | 59 | Yes | I-IV |
| Irjala | 2012 | European Archives of Oto-Rhino-Laryngology | Finland | Retrospective Cohort | Chart Review | No | No | 10 | No | I-IV |
| Kies | 2012 | Head & Neck | USA | Non-Randomized Trial | N/A | No | No | 23 | No | T2-3 N0-2 |
| Kina | 2016 | Cancer Chemotherapy and Pharmacology | Japan | Retrospective Cohort | Chart Review | Yes | No | 117 | Yes | I-II |
| Kirita | 2012 | International Journal of Oral & Maxillofacial Surgery | Japan | Prospective cohort | Chart Review | No | No | 154 | No | II-IV |
| Kreppel | 2013 | Journal of Cranio-Maxillo-Facial Surgery | Germany | Retrospective Cohort | Chart Review | No | No | 139 | Yes | II-IV |
| Kreppel | 2012 | Oral Oncology | Germany | Retrospective Cohort | Chart Review | Yes | No | 151 | Yes | IV |
| Lyu | 2014 | Journal of Oral and Maxillofacial Surgery | China | Retrospective Cohort | Chart Review | No | No | 22 | No | III-IV |
| Mucke | 2011 | Annals of Surgical Oncology | Germany | Retrospective Cohort | Chart Review | Yes | No | 926 | Yes | I-IV |
| Myers | 2011 | Otolaryngology Head & Neck Surgery | USA | Retrospective Cohort | Chart Review | Yes | No | 70 | Yes | III-IV |
| Sadighi | 2014 | Acta Medica Iranica | Iran | Randomized Controlled Trial | N/A | Yes | No | 24 | No | III-IVa |
| Zhong | 2015 | Oncotarget | China | Randomized Controlled Trial | N/A | Yes | No | 256 | Yes | I-IV |
| Zhong | 2013 | Journal of Clinical Oncology | China | Randomized Controlled Trial | N/A | Yes | No | 256 | Yes | I-IV |
NCDB: National Cancer Database, SEER: Surveillance, Epidemiology, and End Results
Risk of bias assessment
Observational cohort studies (Supplemental Table S1) and randomized controlled trials (Supplemental Table S2) underwent risk of bias assessment. Observational studies without a comparator arm were considered to be case series and thus no bias can exist. Overall, observational cohort studies were predominately low quality, primarily due to lack of adjusted analysis and insufficient detail on follow-up. Trials were generally of low risk of bias, except one [60], which was considered high risk due to insufficient information regarding the allocation concealment and randomization process, and some concern on how missing outcome data was handled.
Definitive radiotherapy/Concurrent chemoradiotherapy
Definitive radiotherapy or CCRT for OSCC was examined in 18 studies. Specific chemotherapy regimens varied substantially (Table 2 ). Among included studies there was only one randomized control trial, of which patients with OSCC (stage III-IV) were assigned to either upfront surgery or definitive CCRT [35]. The regimen used included cisplatin at a dose of 20 mg/m2 and 5-fluorouracil at a dose of 1000 mg/m2, as continuous intravenous infusions for 96 h on days 1 and 28 of the radiotherapy course. The primary tumor and upper neck received a radiation dose total of 66 Gy in 33 fractions over 6.5 weeks, whereas involved lymph nodes received at least 60 Gy. For the remaining observational studies, there was a wide variation in specific delivery and dosages of both chemotherapy and radiotherapy (Table 2).
Table 2.
Survival Outcomes for Patients Undergoing Definitive Radiotherapy or Chemoradiotherapy for Oral Cavity Squamous Cell Carcinoma.
| First Author | Stage |
n*** | T (%) |
N (%) |
Treatment Modality | Radiation |
Chemotherapy | Overall Survival (years, %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-II | III-IV | 1–2 | 3–4 | 0 | 1 | 2–3 | Technique | Dose (Gy) |
1 | 2 | 3 | 4 | 5 | ||||||
| Median | Range | Dose per fraction | |||||||||||||||||
| Cannon | x | 5856 | 41 | 58 | 22 | 26 | 39 | RT* | – | – | – | – | – | 11–16 | |||||
| Crombie | x | x | 54 | – | – | 46 | – | – | CCRT | 3DC | 69.7 | 44–73.6 | 2 | Various:
|
29 | ||||
| Ellis | x | 20,779 | 100 | 0 | 100 | 0 | 0 | RT | Various:
|
– | – | – | none | 58.7a | 37.0a,c | ||||
| Fujiwara | x | x | 23,459 | 68 | 32 | 74 | 12 | 15 | RT/CCRT | – | – | – | – | – | 46.0 | 19.2 | |||
| Gogarty | x | 397 | 100 | 0 | 100 | 0 | 0 | RT/CCRT | – | – | – | – | – | 55.4c | |||||
| lyer | x | 119 | 19 | 82 | 30 | 18 | 51 | CCRT | – | 66 | 66 | 2 | Cisplatin 20 mg/m2 IV × 4 days, 5-FU 1000 mg/m2 IV x4 days, day 1 and 28 of RT course | 35 | |||||
| Gore | x | x | 104 | 35 | 36 | – | – | – | CCRT | 3DC | – | – | 2 | Platinum-based, other | 52.1c | 30.9c | |||
| Hauswald | x | x | 66 | 70 | 26 | 26 | 27 | 39 | RT/CCRT | Various:
|
66 | – | – | Platinum-based, immunotherapy | 30.5c | ||||
| Jenwitheesuk | x | x | 117 | – | – | – | – | – | CT/RT | – | – | – | – | – | 0 | ||||
| Kjems | x | x | 942 | 57 | 42 | 22 | 13 | 65 | RT/CCRT | Various:
|
– | 66–68 | 2 | Cisplatin 40 mg/m2 IV q7days | 48c | ||||
| McDowell | x | 31 | 0 | 100 | 32 | 13 | 55 | CCRT, CCRT | – | 70 | 70 | 2 | Various:
|
40 | |||||
| Pederson | x** | 21 | 24 | 76 | 29 | 19 | 52 | CCRT, CT + CCRT | IMRT | – | 72–75 | 1.5–2 | Various:
|
76 | |||||
| Scher | x | x | 73 | 21 | 80 | 33 | 16 | 51 | RT/CCRT | Various:
|
70 | 8.24–73.1 | Various:
|
15c | |||||
| Sowder | x | 8274 | 100 | 0 | 0 | 0 | 0 | RT* | – | – | – | – | – | 34.3c | |||||
| Spiotto | x | 6900 | 29 | 70 | – | – | 44 | CCRT | – | – | – | – | – | 40 | |||||
| Stenson | x | 111 | 22 | 78 | – | – | – | CCRT | Various:
|
– | 60–75 | 1.5–2 | Various: 5-FU NOS and hydroxyurea NOS with paclitaxel, docetaxol, carbo, cisplatin, or other NOS | 65.9 | |||||
| Studer | x | x | 160 | – | – | – | – | – | RT/CCRT/CT + CCRT | IMRT | – | 69.6–70 | 2 | Various:
|
37 | ||||
| Wang | x | x | 88 | 41 | 59 | 78 | 5 | 16 | CCRT | 3DC | – | 68–74 | 1.8–2 | Cisplatin 100 mg/m2 IV q21days then (cisplatin 100 mg/m2 IV × 1 day and 5-FU 1000 mg/m2 IV × 4 days) × 2 cycles | 15 | ||||
a overall survival for matched cohort
b obtained from Licitra et al.
c obtained from digitization of Kaplan-Meier curves
-‘ not reported
2D = Conventional two-dimensional radiation; 3DC = Three-dimensional conformal radiation therapy; 5-FU = 5-fluorouracil; AUC = Area Under the Curve; Carbo = Carboplatin; CT = Chemotherapy; CTX = Cetuximab; CRT = chemoradiotherapy; EBRT = External Beam Radiation Therapy; FHX: 5-Fluorouracil, hydroxyurea, radiation; IMRT = Intensity-modulated radiation therapy; NOS = Not Otherwise Stated, RT = Radiotherapy; TPZ: Tirapazamine
obtained from SEER database which does not capture chemotherapy
stage 2–4
total study cohort, not just those receiving CRT/RT
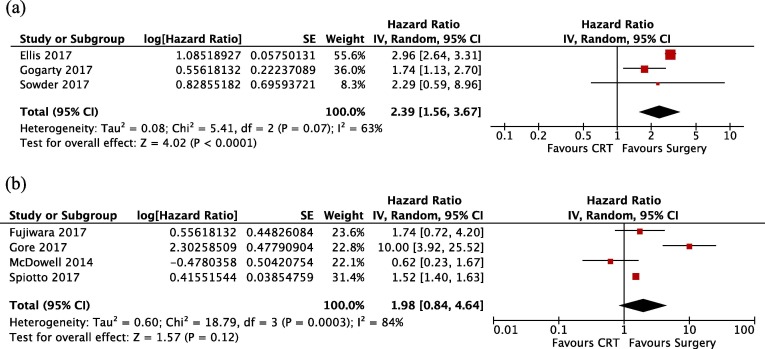
All studies reported survival by year or provided Kaplan-Meier estimated survival curves (Table 2). The 5-year survival ranged from 0% to 76%. A subset of studies directly compared definitive radiotherapy or CCRT against primary surgery. Of these studies, eight performed adjusted analysis of the hazard of death. However, one study did not report the HR and therefore, seven studies were meta-analysed. Three studies restricted recruitment to early stage disease and were analysed separately from studies that included advanced disease or did not restrict participation. In early stage disease, definitive radiotherapy, with or without concurrent chemotherapy, was associated with a significantly increased hazard of death (Figure 2 A, HR: 2.39, 95% CI: 1.56–3.67, I2: 63%).
Fig. 2.
Meta-analysis results for A) Early stage disease only and B) Late stage and all-stage disease. CI: confidence interval, SE: Standard error.
In the four studies which excluded early disease, there was no statistically significant increased hazard of death associated with definitive CCRT (Figure 2 B, HR: 1.98, 95% CI: 0.85–4.64, I2: 84%). Only a single study showed a decreased hazard of death with definitive CCRT compared to primary surgery [38].
Four studies, none of which had comparator arms, provided outcomes related to ORN. The proportion of patients experiencing ORN ranged between 6.8 and 18.4%, with a pooled proportion of 10.1% (95% CI: 5.5–14.7%, I2: 14.05%). Only a single study specifically reported the rate of neutropenia with definitive CCRT, in which the rate of febrile neutropenia was 19% and grade was not reported [39].
Neoadjuvant chemotherapy/Concurrent chemoradiotherapy
Seventeen studies investigated neoadjuvant regimens. Nine of these studies explored neoadjuvant radiotherapy or CCRT and 8 evaluated chemotherapy alone (Table 3 ). Among chemotherapy only studies, four reported on three different randomized control trials. Bossi et al. report the long-term results of individuals randomized to three cycles of cisplatin 100 mg/m2 and fluorouracil 1000 mg/m2 (120-h infusion administered every 21 days) compared to upfront surgery in stage T2–T4, N0–N2 [46]. Zhong et al. reported short- and long-term results of a phase III trial of patients receiving TPF (docetaxel, cisplatin, 5-flourouracil) induction in stage III/IVa OSCC [61], [62]. Sadighi et al. also reported trial results of a TPF induction protocol in advanced OSCC [60].
Table 3.
Survival Outcomes for Patients Undergoing Either Neoadjuvant Chemotherapy or Neoadjuvant Chemoradiotherapy for Oral Cavity Squamous Cell Carcinoma.
| First Author | Stage |
n*** | T (%) |
N (%) |
Neoadjuvant Modality | Radiation |
Chemotherapy | Overall Survival (years, %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-II | III-IV | 1–2 | 3–4 | 0 | 1 | 2–3 | Technique | Dose (Gy) |
1 | 2 | 3 | 4 | 5 | ||||||
| Median | Range | Dose per fraction | |||||||||||||||||
| Bossi | x** | 198 | 41 | 59 | 57 | 27 | 16 | CT | none | none | none | None | Cisplatin 100 mg/m2 IV, 5-FU 1000 mg/m2 IV q21days × 3 cycles | 55b | |||||
| Chinn | x** | 72 | 24 | 76 | 26 | 22 | 51 | CT | none | none | none | none | Various: Cisplatin NOS or Carboplatin, 5-FU NOS q21days × 1 cycle then CRT with: Cisplatin 100 mg/m2 IV q21days × 3 cycles; 1. Carbo AUC 6 IV q21days × 3 cycles |
47 | 42 | 32 | |||
| Harada | x | 39 | 26 | 74 | 33 | 28 | 38 | CCRT | – | 40 | 40 | 2 | S-1 50–100 mg PO 5 days/week × 4 weeks | 83.8 | 78.9 | ||||
| Hauswald | x | x | 66 | 70 | 26 | 26 | 27 | 39 | RT/CCRT | IMRT | 40 | 40 | 2 | Platinum agent; immunotherapy (n = 1) | 56.1c | ||||
| Hirakawa | x | x | 164 | 20 | 80 | 34 | 21 | 45 | CT | none | none | none | none | Cisplatin 80 mg/m2 IV day 6, 5-FU 800 mg/m2 IV days 1–5, q21days × 1 cycle | 63.8 | ||||
| Inhestern | x | x | 54 | 43 | 57 | 9 | 4 | 87 | CT | none | none | none | none | Docetaxel 30 mg/m2 IV , Cisplatin 40 mg/m2 IV, 5-FU 2000 mg/m2 IV days 1 and 8, q21days up to 3 cycles | 97.3 responders73.7 non-responders | ||||
| Irjala | x | x | 10 | 20 | 80 | 60 | 10 | 30 | RT/CCRT | – | – | 63–65 | Cisplatin 40 mg/m2 IV q7days × 4–6 cycles | 60 | |||||
| Kies | x** | 23 | 57 | 43 | 43 | – | – | CT | – | – | – | – | Paclitaxel 175 mg/m2 IV, Ifosfamide 1000 mg/m2 IV days 1–3, Carbo AUC 6 IV q21-28 days × 3 cycles | Crude overall survival: 48 | |||||
| Kina | x | 117 | 100 | 0 | 0 | 0 | 0 | CT | none | none | none | none | Met. Bleomycin 15 mg IV twice weekly, S-1 100 mg PO daily or UFT-E 450 mg PO TID × 3 weeks | 90 | |||||
| Kirita | x** | 154 | – | – | – | – | – | CCRT | – | – | – | – | Cisplatin 15 mg/m2 or Carbo 70–100 mg/m2 days 1–3 and peplomycin 5 mg/day or 5-FU 500–750 mg/day days 4–7, q28days × 2 cycles | 83 | 79 | ||||
| Kreppel 2013 | x** | 139 | 41 | 59 | 20 | 17 | 63 | CCRT | – | 39.6 | 39.6 | 1.8 | Carbo 60 mg/m2/day IV × 5 days | 45.5 | |||||
| Kreppel 2012 | x** | 151 | 29 | 71 | – | – | – | CCRT | – | 39.6 | 39.6 | 1.8 | Carbo 70 mg/m2/day IV × 5 days | 46.3 | |||||
| Lyu | x | 22 | 9 | 91 | 18 | 59 | 23 | CT + RT | – | – | – | – | Docetaxel 75 mg/m2 IV, cisplatin 75 mg/m2 IV, 5-FU 750 mg/m2 IV days 1–5, q21days × 2 cycles | 67.2 | |||||
| Mucke | x | x | 926 | 68 | 32 | 60 | 17 | 23 | CCRT | EBRT | 20 | 2 | Cisplatin 12.5 mg/m2 IV × 5 days during first week of RT | 85.3 | 68.6 | ||||
| Myers | x | 70 | – | – | – | – | – | CT + RT/CCRT | – | 68 | 68 | 1.2 | Platinum agent/Taxane +/- 5-FU; CTX | 31.1 | |||||
| Sadighi | x | 24 | – | – | – | – | – | CT | none | none | none | none | Docetaxel 70–80 mg/m2 IV Cisplatin 60 mg/m2 IV, 5-FU 750 mg/m2 IV × 5 days × 2–3 cycles | 46.5c | 23c | ||||
| Zhong 2015 | x | x | 256 | 26 | 74 | 43 | 37 | 20 | CT | none | none | none | none | Docetaxel 75 mg/m2 IV, Cisplatin 75 mg/m2 IV, 5-FU 750 mg/m2 IV on days1-5 q21days × 2 cycles | 61.1c | ||||
‘-‘ not reported. 2D = Conventional two-dimensional radiation; 3DC = Three-dimensional conformational radiation therapy; 5-FU = 5-fluorouracil, Carbo = Carboplatin, CT = Chemotherapy, CTX = Cetuximab, CRT = chemoradiotherapy; EBRT = External Beam Radiation Therapy, IMRT = Intensity-modulated radiation therapy, Met. = Metronomic, RT = Radiotherapy, S-1 = oral 5-FU, UFT-E = oral 5-FU
aoverall survival for matched cohort
obtained from SEER database which does not capture chemotherapy
stage 2–4
total study cohort, not just those receiving CRT/RT
obtained from Lictra et al.
obtained from digitization of Kaplan-Meier curves
One study investigated induction chemotherapy [47]. Chinn and colleagues treated patients with induction cisplatin or carboplatin and fluorouracil. Tumor response was assessed at 3 weeks, and patients with a response of greater than 50% were treated with definitive CCRT. Patients with responses less than 50% were treated with definitive surgical resection. Of the 53% of patients who responded to the initial induction therapy, 30% had complete response after CCRT. Of the 70% who did not have complete response after CCRT, 14% were successfully salvaged with surgery. Overall, patients undergoing induction chemotherapy had a 2.5 times increased hazard of death compared to those undergoing definitive surgery, regardless of initial response status to induction chemotherapy.
For the 9 studies looking at neoadjuvant radiotherapy or CCRT the median radiation dose was 40 Gray. Typically, chemotherapy was administered concurrently, though induction protocols were also described.
Among all studies reporting neoadjuvant or induction regimens, the median length of the neoadjuvant/induction treatment period was 5.0 weeks (IQR 3.75 – 6.75 weeks, range 2 – 12 weeks). The length of time between conclusion of neoadjuvant or induction treatment to initiation of definitive treatment (either CCRT or surgery) was infrequently reported and varied between immediate and longer than 3 weeks. Few studies specifically reported the proportion of patients receiving neoadjuvant treatment who did not undergo surgery. Only one study reported deaths in this interval, of which the death was unrelated to the neoadjuvant treatment or cancer [62]. The majority of observational studies included only those patients who went on to have surgery.
Five studies reported rates of neutropenia, which ranged from 3.7% to 26.1% of varying grades [48], [49], [50], [52], [62]. Febrile neutropenia was specifically reported in three studies, ranging from 1.4% to 4.3% of varying grades [49], [52], [62].
Among all included studies, one study reported 1-year overall survival (47.0%), five studies reported 2-year overall survival (46.5–86.7%), two reported 3-year (42.0%, 83.8%), and 12 reported 5-year overall survival (23.0–90.0%). One study reported crude overall survival (48.0%) and one study reported 10-year survival (46.5%). Meta-analysis of induction CCRT was not performed as insufficient information could be gathered from studies purporting to report adjusted analysis. Additionally, a recent meta-regression has already been published combining the results of the Zhong and Bossi trials [21].
Neoadjuvant immunotherapy
No published studies were identified that reported final results of neoadjuvant immunotherapy trials. Published abstracts reporting preliminary results, as well as ongoing trials, were identified by reference review and snowballing techniques. One manuscript was retrieved as a non-peer reviewed pre-print. Table 4 summarizes ongoing immunotherapy trials that include the oral cavity, the majority of which include other sites within the head and neck. Therefore, this literature is not yet mature for review and the results of these trials are as of yet unknown.
Table 4.
Ongoing Neoadjuvant Immunotherapy Trials for Oral Cavity Squamous Cell Carcinoma.
| Trial | Neoadjuvant/Induction Regimen | Sites | Recruitment Status | Time From 1st Dose to Primary Treatment |
|---|---|---|---|---|
| NCT02296684 | Pembrolizumab | Any | Recruiting | 2–3 weeks |
| NCT02641093 | Pembrolizumab | Any | Recruiting | 1 week |
| NCT02919683 | Nivolumab, Nivolumab + Ipilimumab | OSCC | Active, Not Recruiting | 2 weeks |
| NCT03021993 | Nivolumab | OSCC | Recruiting | 36–50 days |
| NCT02827838 | Durvalumab | OSCC, OPCC | Recruiting | 2 weeks + 3–17 days |
| NCT02488759 | Nivolumab | Any | Active, Not Recruiting | – |
| NCT03174275 | Carboplatin, Paclitaxel, Durvalumab | Any | Recruiting | 8–14 weeks |
| NCT03700905 | Nivolumab, Nivolumab + Ipilimumab | Any | Recruiting | 2 weeks |
| NCT03737968 | Durvalumab, Durvalumab + Tremelimumab | Any | Not Yet Recruiting | 4 weeks |
| NCT02997332 | Durvalumab, Docetaxel, Cisplatin, 5-FU | Any | Recruiting | 7 weeks |
| NCT02882308 | Olarparib Vs. Cisplatin And Olaparib Vs. Olaparib + Durvalumab | Any | Recruitment Complete | 23–29 days |
| NCT03708224 | Atezolizumab, Atezolizumab + Emactuzumab | Any | Recruiting | 3–6 weeks |
| NCT03003637 | Nivolumab, Nivolumab + Ipilimumab | Any | Recruiting | 3 weeks |
| NCT03700905 | Nivolumab | Any | Recruiting | 2 weeks |
| NCT03721757 | Nivolumab | Any | Not Yet Recruiting | 2 weeks |
| NCT03247712 | Nivolumab, Radiation | Any | Recruiting | 2–6 weeks |
| NCT03342911 | Nivolumab, Carboplatin, Paclitaxel | Any | Recruiting | 6 weeks |
| NCT02777385 | Pembrolizumab | Any | Recruiting | – |
‘-‘ not reported
To date, four trials have reported preliminary trial results through published abstracts. One trial was restricted to oral cavity cancer, while the remaining three included all head and neck sites. Preliminary results of the latter trials do not stratify results by site. Checkmate 358 is a larger trial that includes non-head and neck cancer. Patients receive 2 cycles of preoperative nivolumab. Twenty-nine patients with head and neck cancer were reported, with 48% experiencing a reduction in tumor size, with 10% of patients having reductions of 40% or more. Amongst a cohort of 28 head and neck cancer patients enrolled in NCT02641093, a trial in which patients receive neoadjuvant pembrolizumab, followed by surgery and adjuvant pembrolizumab and radiation therapy, 47% of patients experienced a pathologic response greater than 10% of which 68% had a major response (greater than 70%) and one patient had a complete response. Lastly, in nine oral cavity cancer patients enrolled in NCT03021993, where patients receive 3–4 cycles of nivolumab, 44% of patients experienced a reduction in tumor size of more than 30%. Amongst preliminary reports, adverse effects appear infrequent.
In a non-peer reviewed pre-print report, results of NCT02296684 are available [63]. Thirty-six patients were enrolled to receive neoadjuvant pembrolizumab. Tumor response of more than 50% was shown in 22% of patients, and more than 10% in an additional 22% of patients. The one-year relapse rate was 16.7% amongst all-comers, and 0% in patients with low and intermediate risk pathology.
Discussion
The COVID-19 pandemic has placed significant strains on healthcare systems world-wide. Within head and neck oncology, temporary alteration to treatment paradigms may be required given limitations of resources due to the ongoing spread of COVID-19. Our review confirms the accepted notion that surgery remains the preferred treatment modality for OSCC. However, in the context of a pandemic, current standard of care may not be achievable within a preferred time frames, generally accepted as within one month [64]. Depending on regional variation in COVID-19 surge, some centres may be forced to use less than ideal treatments. It is therefore important both in terms of decision-making and patient counselling to have summarized data pertaining to these options.
This review reports on a contemporary repository of observational studies and randomized controlled trials that document survival outcomes for patients with surgically resectable OSCC undergoing non-surgical treatment. In some settings, primary CCRT offers near comparable survival outcomes, albeit with significant toxicity, most commonly and more specifically, ORN.
Definitive radiotherapy/Concurrent chemoradiotherapy
Primary surgical management of OSCC has been a mainstay of therapy for decades. Indeed, one of the included studies using recent National Cancer Data Base data determined that of more than 20,000 early oral cancers, surgery was the modality of choice in 95% of cases [30]. There have been two randomized trials attempting to compare surgery with primary radiotherapy. In 1998, Robertson et al. randomized individuals with a planned sample size of 350 patients, though the study was aborted after the first 30 patients showed inferior survival with definitive radiation [65]. Similarly, in 2015, Iyer et al. terminated their study early due to poor accrual [35]. However, this review does present evidence for primary radiation or CCRT through the assessment of observational studies of low to moderate risk of bias. The pooled hazard of death was assessed in those studies, which reported adjusted analysis, and revealed over a 100% increased instantaneous risk of death amongst early OSCC patients. In advanced disease, the increased hazard of death was non-significant, likely owing to individual studies within the pooled analysis being underpowered, and not a lack of true association.
Although not a primary outcome of this review, definitive CCRT was found to have a high rate of ORN, with pooled analysis suggesting over 10% of patients experience this complication. Previous studies have shown the risk of ORN to be both dose- and target volume-dependent, and as such, the oral cavity has been shown to have a more than four times risk of developing ORN compared to other sites [66], [67]. Clinicians must balance the increased risk of mortality and morbidity associated with definitive CCRT, in light of improving radiotherapy techniques, against pandemic associated threats.
Additionally, while primary CCRT has the advantage of potentially facilitating primarily outpatient management, it poses unique challenges in the COVID-19 setting [68]. Chemotherapy is immunosuppressive and radiotherapy requires visits to a radiation centre. Early reports have noted that patients with a history of cancer were more likely to acquire COVID-19, and that individuals who are immunocompromised or have medical comorbidities are at higher risks for intensive care unit admission, morbidity, and mortality secondary to COVID-19 [12], [69]. Moreover, patients undergoing non-surgical regimens may present with symptoms that mirror COVID-19, including cough and sore throat which may then interrupt treatment and impact survival outcomes [70].
Neoadjuvant chemotherapy/Concurrent chemoradiotherapy
Neoadjuvant and induction chemotherapy have been studied in advanced head and neck cancers since the 1990 s [71], [72]. Over the past decade, two randomized control trials have examined the role of neoadjuvant chemotherapy in OSCC. Bossi, Licitra and colleagues reported on the early and long-term results of a randomized trial of 198 operable oral cancer patients (T2-T4) who were randomized to three cycles of cisplatin and 5-fluorouracil followed by surgery versus surgery alone [46], [73]. The addition of chemotherapy failed to provide a survival benefit, though it resulted in reduced rates of adjuvant radiation (33% vs 46%) as well as a higher incidence of mandibular preservation (52% vs 31%). Zhong et al. conducted a similar trial, albeit with a different triple neoadjuvant chemotherapy regimen (docetaxel, cisplatin and 5-fluorouracil). This trial also failed to demonstrate an overall survival benefit, and was associated with grade 3 hematological toxicity in 6.6% of patients and grade 2 adverse events in over 70% of patients [61]. Marta et al. recently combined these studies in a meta-analysis. The pooled data of 451 patients failed to show any difference in overall survival [21]. A second meta-analysis of 27 randomized trials over a 40 year period also failed to identify any benefit [20].
While neoadjuvant chemotherapy may not have a demonstrated role in routine management of primary OSCC, there is a potential for it to provide symptom relief and even delay the need for surgery for a finite period of time. Indeed, there is even some evidence of benefit for neoadjuvant approach amongst responders [61]. In this systematic review, neoadjuvant regimens prior to surgery included both chemotherapy alone and CCRT. Specific treatment regimens were varied, resulting in an inability to adequately combine studies. Nevertheless, in the event where an institution has a clearly delineated target day to deliver surgical care, therapy delivered in the pre-surgical period could be considered as an option either for neoadjuvant intent, or simply as a bridging option until resource restrictions are loosened – the key difference being in specific dosing regimens delivered.
The avoidance of triple modality therapy through the use of induction chemotherapy has importance in both resource-constrained and resource-abundant settings. In patients who respond to initial induction chemotherapy, definitive CCRT would avoid and preserve the use of surgical treatment. However, only a single contemporary study was included in this review [47], which did not demonstrate survival benefit with the use of induction chemoselection. While over half of patients receiving induction chemoselection had an initial response, this strategy was associated with worse survival than definitive surgery with risk informed adjuvant treatment.
Neoadjuvant immunotherapy
Immunotherapy is governed by the principle that tumors can evade immune detection, and immunotherapies can activate immunologic effector mechanisms to kill cancer cells [74]. Integrating immunotherapeutics into upfront therapies has gained significant traction within the head and neck oncology community. While immunotherapy has shown encouraging results in the salvage setting [75], [76], neoadjuvant and induction immunotherapy remains under investigation. Of the 18 identified studies, only a single trial has produced mature results, which remain to undergo formal peer-review. For the management of patients in extremely resource constrained settings, upfront immunotherapy may afford additional time for definitive treatment, though this is not directly supported by current literature. Despite the potential benefits, limitations must be considered. Immune-mediated toxicity is a concern, and the question of hyperprogression has been raised [77]. In addition, immunomodulation could have implications with regards to post-operative wound healing, potentially necessitating, instead of protecting against, health system utilization [20]. Lastly, neoadjuvant immunotherapy in head and neck oncology is in its early randomized trial years and is far from standard of care, and therefore may not be accessible at many centers. Many centers have closed non-essential randomized trials in the context of COVID-19. It should also be noted that the vast majority of these trials are industry sponsored due to the very expensive cost of these agents and therefore even if these agents prove efficacious, rigorous cost-effectiveness analyses will be required before uptake. Further, some of these trials are window of opportunity studies, and thus the immunotherapy administered is considered experimental and not necessarily considered a component of the planned treatment [78].
Risk of surgical therapy in the wake of COVID-19
Although findings from our study suggest superiority of oncological outcomes for OSCC managed surgically, clinicians must balance the risk to both patients and healthcare providers, as well as limitations of available resources, that surgery would impose during COVID-19. Surgical management may involve increased duration and time spent inside hospitals, during the pre-operative, operative, and post-operative periods, potentially increasing the risk of nosocomial infections. Additionally, intubation, tracheostomy, surgical manipulation of the upper aerodigestive tract, and post-operative care with suctioning of the oral cavity, are all considered aerosol generating medical procedures and known transmission routes for SARS-COV-2 [2], [79], [80], [81]. However, for many OSCCs, particularly early stage cancers, the short-term risks of surgery may be outweighed by the collective risk of daily presentation to a radiation centre over the course of 7 weeks and the increased susceptibility to infection that is associated with CCRT. Furthermore, there has been rapid progression in protocols to allow for safer surgical care of the OSCC patient during COVID-19 including, appropriate use of personal protective equipment, self-isolation prior to surgery, and COVID-19 testing pre-operatively[2].
Limitations
The strengths of this review must be taken in context. Firstly, this was a rapid review of the literature. We did not pursue all elements of a formal systematic review given the evolving nature of the COVID-19 pandemic and need for timely evidence. Despite this, a number of formal systematic review methods were utilized, including the use of multiple reviewers and meta-analytical techniques. Reviews are inherently limited by the evidence quality available in the literature, and as randomized controlled trials were both few in number and high in risk of bias limitations in conclusions drawn from our findings must be noted [82]. However, this study does offer the most comprehensive review of the contemporary literature on the non-surgical management of oral cavity cancer.
Conclusion
How the COVID-19 pandemic affects treatment decisions and resource availability is likely to change as the pandemic unfolds. While head and neck surgeons should strive to provide standard of care therapies throughout the pandemic, reduced access to the operating room and availability of critical care services for post-operative management may necessitate the use of treatment strategies outside of standards of care, particularly in regions with an inability to manage a surge. This review suggests that primary radiation and chemotherapy are inferior to surgical management for oral cavity cancer. Strategies for oncologically safe surgical delay warranting consideration are sparse but may include select neoadjuvant regimens. Our hope is that this systematic review and meta-analysis will shed light on the risks and benefits of contemporary non-surgical options for OSCC within the nuanced COVID-19 pandemic setting. This is particularly important given the expected second wave or surge of COVID-19, which may again put head and neck cancer oncologists in a resource constrained environment. In times of profound uncertainty, the risks and benefits of all strategies must be weighed in the context of patients, healthcare providers, and the healthcare system.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2020.104849.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Emanuel E.J., Persad G., Upshur R. Fair allocation of scarce medical resources in the time of Covid-19. Mass Medical Soc. 2020 doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 2.Wu V., Noel C.W., Forner D., Zhang Z.J., Higgins K.M., Enepekides D.J., Lee J.M., Witterick I.J., Kim J.J., Waldron J.N., Irish J.C. Considerations for head and neck oncology practices during the coronavirus disease 2019 (COVID‐19) pandemic: Wuhan and Toronto experience. Head Neck. 2020 doi: 10.1002/hed.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN. Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology Web site. https://oncolife.com.ua/doc/nccn/Head_and_Neck_Cancers.pdf. Updated February 15, 2018. Accessed March 2, 2020.
- 4.Caudell J.J., Locher J.L., Bonner J.A. Diagnosis-to-treatment interval and control of locoregionally advanced head and neck cancer. Archives of Otolaryngology-Head & Neck Surgery. 2011;137(3):282–285. doi: 10.1001/archoto.2011.20. [DOI] [PubMed] [Google Scholar]
- 5.Schutte H.W., Heutink F., Wellenstein D.J. Impact of Time to Diagnosis and Treatment in Head and Neck Cancer: A Systematic Review. Otolaryngology-Head and Neck Surgery. 2020;162(4):446–457. doi: 10.1177/0194599820906387. [DOI] [PubMed] [Google Scholar]
- 6.Murphy C.T., Galloway T.J., Handorf E.A. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169. doi: 10.1200/JCO.2015.61.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher N., Engelman D. Postoperative care: who should look after patients following surgery? Anaesthesia. 2020;75:e5–e9. doi: 10.1111/anae.14887. [DOI] [PubMed] [Google Scholar]
- 8.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. 2020:;EClinicalMedicine doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du R.-H., Liang L.-R., Yang C.-Q. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur Respir J. 2020 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccirillo J.F. Importance of comorbidity in head and neck cancer. The Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Association of Head & Neck Surgical Oncology (CAHNSO) guidelines for management of Head & Neck Cancer during the COVID-19 Pandemic. https://cahnso.com/covid-19-resources/. Published 2020. Accessed April 8, 2020.
- 14.Guidance for ENT surgeons during the COVID-19 pandemic. The Australian Society of Otolaryngology Head and Neck Surgery. http://www.asohns.org.au/about-us/news-and-announcements/latest-news?article=78. Published 2020. Accessed April 8, 2020.
- 15.Topf MC. A Framework for Prioritizing Head and Neck Surgery during the COVID-19 Pandemic. Head & Neck. 2020(In press). [DOI] [PMC free article] [PubMed]
- 16.Chaves Aline Lauda Freitas, Castro Ana Ferreira, Marta Gustavo Nader, Junior Gilberto Castro, Ferris Robert L., Giglio Raúl Eduardo, Golusinski Wojciech, Gorphe Philippe, Hosal Sefik, Leemans C. René, Magné Nicolas, Mehanna Hisham, Mesía Ricard, Netto Eduardo, Psyrri Amanda, Sacco Assuntina G., Shah Jatin, Simon Christian, Vermorken Jan B., Kowalski Luiz Paulo. Emergency changes in international guidelines on treatment for head and neck cancer patients during the COVID-19 pandemic. Oral Oncol. 2020;107:104734. doi: 10.1016/j.oraloncology.2020.104734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garritty C, Co-Convenor CR. Cochrane Rapid Review (RR) Methods: A Look at Interim Recommendations (Part 2). 2020.
- 18.Furness S., Glenny A.M., Worthington H.V. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database of Systematic Reviews. 2011(4). doi: 10.1002/14651858.CD006386.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Glenny A.M., Furness S., Worthington H.V. Interventions for the treatment of oral cavity and oropharyngeal cancer: radiotherapy. Cochrane Database of Systematic Reviews. 2010(12). doi: 10.1002/14651858.CD006387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau A., Li K.-Y. Yang W-f, Su Y-X. Induction chemotherapy for squamous cell carcinomas of the oral cavity: a cumulative meta-analysis. Oral Oncol. 2016;61:104–114. doi: 10.1016/j.oraloncology.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Marta G.N., Silva V., de Andrade Carvalho H. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol. 2014;110(1):9–15. doi: 10.1016/j.radonc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Sayers A. Tips and tricks in performing a systematic review. Br J Gen Pract. 2007;57(538):425. [PMC free article] [PubMed] [Google Scholar]
- 23.Devisetty K., Wong S.J. Neoadjuvant versus induction chemotherapy: more than semantics. J Clin Oncol. 2013;31(23):2971–2972. doi: 10.1200/JCO.2013.50.2674. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366. [DOI] [PubMed]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 27.Higgins J., Wells G. Cochrane handbook for systematic reviews. of interventions. 2011 [Google Scholar]
- 28.Cannon R.B., Sowder J.C., Buchmann L.O. Increasing use of nonsurgical therapy in advanced-stage oral cavity cancer: A population-based study. Head Neck. 2017;39(1):82–91. doi: 10.1002/hed.24542. [DOI] [PubMed] [Google Scholar]
- 29.Crombie A.K., Farah C., Tripcony L., Dickie G., Batstone M.D. Primary chemoradiotherapy for oral cavity squamous cell carcinoma. Oral Oncol. 2012;48(10):1014–1018. doi: 10.1016/j.oraloncology.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Ellis M.A., Graboyes E.M., Wahlquist A.E. Primary surgery vs radiotherapy for early stage oral cavity cancer. Otolaryngology-Head and Neck Surgery. 2018;158(4):649–659. doi: 10.1177/0194599817746909. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara R.J., Burtness B., Husain Z.A. Treatment guidelines and patterns of care in oral cavity squamous cell carcinoma: primary surgical resection vs. nonsurgical treatment. Oral Oncol. 2017;71:129–137. doi: 10.1016/j.oraloncology.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Gogarty D.S., Lennon P., Deady S. Variation in treatment and outcome in the early stage oral cavity squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2017;274(2):953–960. doi: 10.1007/s00405-016-4267-z. [DOI] [PubMed] [Google Scholar]
- 33.Gore S.M., Crombie A.K., Batstone M.D., Clark J.R. Concurrent chemoradiotherapy compared with surgery and adjuvant radiotherapy for oral cavity squamous cell carcinoma. Head Neck. 2015;37(4):518–523. doi: 10.1002/hed.23626. [DOI] [PubMed] [Google Scholar]
- 34.Hauswald H., Zwicker F., Rochet N., Jensen A.D., Debus J., Lindel K. Treatment of squamous cell carcinoma of the mobile tongue or tongue margins: An interdisciplinary challenge. Acta Oncol. 2013;52(5):1017–1021. doi: 10.3109/0284186X.2012.722678. [DOI] [PubMed] [Google Scholar]
- 35.Iyer N.G., Tan D.S., Tan V.K. Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer. 2015;121(10):1599–1607. doi: 10.1002/cncr.29251. [DOI] [PubMed] [Google Scholar]
- 36.Jenwitheesuk K., Surakunprapha P., Chowchuen B. Results of multidisciplinary therapy of squamous cell carcinoma of the buccal mucosa at Srinagarind Hospital, Thailand. Medical journal of the Medical Association of Thailand. 2010;93(11):1262. [PubMed] [Google Scholar]
- 37.Kjems J., Gothelf A.B., Håkansson K., Specht L., Kristensen C.A., Friborg J. Elective nodal irradiation and patterns of failure in head and neck cancer after primary radiation therapy. International Journal of Radiation Oncology* Biology*. Physics. 2016;94(4):775–782. doi: 10.1016/j.ijrobp.2015.12.380. [DOI] [PubMed] [Google Scholar]
- 38.McDowell L., Collins M., Kleid S., Rischin D., Corry J. T4 squamous cell carcinoma of the oral tongue without mandibular involvement: surgery or chemoradiotherapy? Oral surgery, oral medicine, oral pathology and oral radiology. 2014;117(2):163–169. doi: 10.1016/j.oooo.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Pederson A.W., Salama J.K., Witt M.E. Concurrent chemotherapy and intensity-modulated radiotherapy for organ preservation of locoregionally advanced oral cavity cancer. Am J Clin Oncol. 2011;34(4):356–361. doi: 10.1097/COC.0b013e3181e8420b. [DOI] [PubMed] [Google Scholar]
- 40.Scher E.D., Romesser P.B., Chen C. Definitive chemoradiation for primary oral cavity carcinoma: a single institution experience. Oral Oncol. 2015;51(7):709–715. doi: 10.1016/j.oraloncology.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowder J.C., Cannon R.B., Buchmann L.O. Treatment-related determinants of survival in early-stage (T1–2N0M0) oral cavity cancer: A population-based study. Head Neck. 2017;39(5):876–880. doi: 10.1002/hed.24679. [DOI] [PubMed] [Google Scholar]
- 42.Spiotto M.T., Jefferson G., Wenig B., Markiewicz M., Weichselbaum R.R., Koshy M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer: a national cancer database analysis. JAMA Otolaryngology-Head & Neck Surgery. 2017;143(7):691–699. doi: 10.1001/jamaoto.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenson K.M., Kunnavakkam R., Cohen E.E. Chemoradiation for patients with advanced oral cavity cancer. The Laryngoscope. 2010;120(1):93–99. doi: 10.1002/lary.20716. [DOI] [PubMed] [Google Scholar]
- 44.Studer G., Brown M., Bredell M. Follow up after IMRT in oral cavity cancer: update. Radiation oncology. 2012;7(1):84. doi: 10.1186/1748-717X-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T.C., Hua C.H., Lin C.C., Tsou Y.-A., Tseng H.C., Tsai M.H. Risk factors affect the survival outcome of hard palatal and maxillary alveolus squamous cell carcinoma: 10-year review in a tertiary referral center. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2010;110(1):11–17. doi: 10.1016/j.tripleo.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 46.Bossi P., Lo Vullo S., Guzzo M. Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Ann Oncol. 2014;25(2):462–466. doi: 10.1093/annonc/mdt555. [DOI] [PubMed] [Google Scholar]
- 47.Chinn S.B., Spector M.E., Bellile E.L. Efficacy of induction selection chemotherapy vs primary surgery for patients with advanced oral cavity carcinoma. JAMA Otolaryngology-Head & Neck Surgery. 2014;140(2):134–142. doi: 10.1001/jamaoto.2013.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harada H., Omura K., Tomioka H. Multicenter phase II trial of preoperative chemoradiotherapy with S-1 for locally advanced oral squamous cell carcinoma. Cancer Chemother Pharmacol. 2013;71(4):1059–1064. doi: 10.1007/s00280-013-2101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirakawa H., Hanai N., Suzuki H. Prognostic importance of pathological response to neoadjuvant chemotherapy followed by definitive surgery in advanced oral squamous cell carcinoma. Jpn J Clin Oncol. 2017;47(11):1038–1046. doi: 10.1093/jjco/hyx097. [DOI] [PubMed] [Google Scholar]
- 50.Inhestern J., Schmalenberg H., Dietz A. A two-arm multicenter phase II trial of one cycle chemoselection split-dose docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1) Ann Oncol. 2017;28(8):1917–1922. doi: 10.1093/annonc/mdx202. [DOI] [PubMed] [Google Scholar]
- 51.Irjala H., Kinnunen I., Aitasalo K. Mandibular reconstruction using free bone flap after preoperative chemoradiation. Eur Arch Otorhinolaryngol. 2012;269(5):1513–1518. doi: 10.1007/s00405-011-1795-4. [DOI] [PubMed] [Google Scholar]
- 52.Kies M.S., Boatright D.H., Li G. Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults. Head Neck. 2012;34(9):1255–1262. doi: 10.1002/hed.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kina S., Nakasone T., Kinjo T., Maruyama T., Kawano T., Arasaki A. Impact of metronomic neoadjuvant chemotherapy on early tongue cancer. Cancer Chemother Pharmacol. 2016;78(4):833–840. doi: 10.1007/s00280-016-3141-4. [DOI] [PubMed] [Google Scholar]
- 54.Kirita T., Yamanaka Y., Imai Y. Preoperative concurrent chemoradiotherapy for stages II–IV oral squamous cell carcinoma: A retrospective analysis and the future possibility of this treatment strategy. Int J Oral Maxillofac Surg. 2012;41(4):421–428. doi: 10.1016/j.ijom.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Kreppel M., Eich H.-T., Brüggenolte C. Preoperative vs. postoperative radiochemotherapy in patients with N2 squamous cell carcinoma of the oral cavity. Oral Oncol. 2012;48(10):1019–1024. doi: 10.1016/j.oraloncology.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Kreppel M., Dreiseidler T., Rothamel D. The role of clinical versus histopathological staging in patients with advanced oral squamous cell carcinoma treated with neoadjuvant radiochemotherapy followed by radical surgery. Journal of Cranio-Maxillofacial Surgery. 2013;41(1):22–27. doi: 10.1016/j.jcms.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Lyu J., Li C., Wu Y., Wang R., Ren G., Guo W. Sequential Therapy of Advanced Buccal Mucosa Squamous Cell Carcinoma: Three-Year Outcome. J Oral Maxillofac Surg. 2014;72(3):606–610. doi: 10.1016/j.joms.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Mücke T., Konen M., Wagenpfeil S., Kesting M.R., Wolff K.-D., Hölzle F. Low-dose preoperative chemoradiation therapy compared with surgery alone with or without postoperative radiotherapy in patients with head and neck carcinoma. Ann Surg Oncol. 2011;18(10):2739–2747. doi: 10.1245/s10434-011-1643-1. [DOI] [PubMed] [Google Scholar]
- 59.Myers L.L., Sumer B.D., Truelson J.M. Impact of treatment sequence of multimodal therapy for advanced oral cavity cancer with mandible invasion. Otolaryngology-Head and Neck Surgery. 2011;145(6):961–966. doi: 10.1177/0194599811417550. [DOI] [PubMed] [Google Scholar]
- 60.Sadighi S., Keyhani A., Harirchi I. Neoadjuvant Chemotherapy for Locally Advanced Squamous Carcinoma of Oral Cavity: a Pilot Study. Acta Medica Iranica. 2015;380–386 [PubMed] [Google Scholar]
- 61.Zhong L-p, Zhang C-p, Ren G-x. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31(6):744. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong L-p, Zhang C-p, Ren G-x. Long-term results of a randomized phase III trial of TPF induction chemotherapy followed by surgery and radiation in locally advanced oral squamous cell carcinoma. Oncotarget. 2015;6(21):18707. doi: 10.18632/oncotarget.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uppaluri. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase 2 Trial In. medRxiv2020. [DOI] [PMC free article] [PubMed]
- 64.Trama A., Botta L., Foschi R. Indicators for measuring integrated quality of care for head and neck cancers: the experience of the European project RARECAREnet. Front Oncol. 2019;9:837. doi: 10.3389/fonc.2019.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson A., Soutar D., Paul J. Early closure of a randomized trial: surgery and postoperative radiotherapy versus radiotherapy in the management of intra-oral tumours. Clinical Oncology. 1998;10(3):155–160. doi: 10.1016/s0936-6555(98)80055-1. [DOI] [PubMed] [Google Scholar]
- 66.Huang S.H. Oral cancer: Current role of radiotherapy and chemotherapy. Medicina oral, patologia oral y cirugia bucal. 2013;18(2) doi: 10.4317/medoral.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caparrotti F., Huang S.H., Lu L. Osteoradionecrosis of the mandible in patients with oropharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer. 2017;123(19):3691–3700. doi: 10.1002/cncr.30803. [DOI] [PubMed] [Google Scholar]
- 68.Day A.T., Sher D.J., Lee R.C. Head and neck oncology during the COVID-19 pandemic: Reconsidering traditional treatment paradigms in light of new surgical and other multilevel risks. Oral Oncol. 2020;104684 doi: 10.1016/j.oraloncology.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for cancer patients. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fesinmeyer M.D., Mehta V., Blough D., Tock L., Ramsey S.D. Effect of radiotherapy interruptions on survival in medicare enrollees with local and regional head-and-neck cancer. International Journal of Radiation Oncology* Biology*. Physics. 2010;78(3):675–681. doi: 10.1016/j.ijrobp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Domenge C., Hill C., Lefebvre J. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. Br J Cancer. 2000;83(12):1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paccagnella A., Mastromauro C., D'Amanzo P., Ghi M.G. Induction chemotherapy before chemoradiotherapy in locally advanced head and neck cancer: the future? Oncologist. 2010;15 doi: 10.1634/theoncologist.2010-S3-08. [DOI] [PubMed] [Google Scholar]
- 73.Licitra L., Grandi C., Guzzo M. Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial. J Clin Oncol. 2003;21(2):327–333. doi: 10.1200/JCO.2003.06.146. [DOI] [PubMed] [Google Scholar]
- 74.Couzin-Frankel J. Cancer Immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science:342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 75.Cohen E.E., Soulières D., Le Tourneau C. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 76.Ferris R.L., Blumenschein G., Jr, Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saâda-Bouzid E., Defaucheux C., Karabajakian A. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 78.Bernal M.O., Chepeha D., Prawira A. Abstract CT124: Sitravatinib and nivolumab in oral cavity cancer window of opportunity study (SNOW) AACR. 2019 doi: 10.1136/jitc-2021-003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Judson S.D., Munster V.J. Nosocomial Transmission of Emerging Viruses via Aerosol-Generating Medical Procedures. Viruses. 2019;11(10):940. doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): A Chinese perspective. J Med Virol. 2020 doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Misra Anoop. Doctors and healthcare workers at frontline of COVID 19 epidemic: Admiration, a pat on the back, and need for extreme caution. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(3):255–256. doi: 10.1016/j.dsx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noel C.W., McMullen C., Yao C. The fragility of statistically significant findings from randomized trials in head and neck surgery. The Laryngoscope. 2018;128(9):2094–2100. doi: 10.1002/lary.27183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.