Atypical lymphocytes are reactive lymphocytes which are common to a large variety of etiologies. While infections like Epstein-Barr virus (EBV) and cytomegalovirus are well-known causes of atypical lymphocytes in the peripheral blood, sporadic reports indicate that lymphocytes with atypical morphology are seen in patients infected with the newly emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[1], [2], [3], [4]]. To explore the extent and possible significance of lymphocytic atypia related to SARS-CoV-2, we assessed the spectrum of atypical lymphocytic features in peripheral smears collected from 33 SARS-CoV-2 patients from Mount Sinai Hospital in New York City, the epicenter of the pandemic in the United States.
The study was conducted in compliance with institutional IRB protocols. We reviewed lymphocytes morphology in 33 peripheral blood smears which were flagged for pathologist review by medical technologists in the clinical hematology laboratory and compared to another group of 20 smears flagged from non- SARS-CoV-2 patients as a control. The SARS-CoV-2 cohort patients were confirmed positive for the infection by SARS-CoV-2 nucleic acid testing of throat swab specimens using RT-PCR. Atypical lymphocytic features were identified, tabulated, and compared with their presence in peripheral smears from the negative-control cohort. Absolute lymphocyte counts and morphological features of the two groups were compared and analyzed for statistical significance at 0.05 alpha by Student’s t test using IBM SPSS Statistics Subscription (Armonk, NY, USA).
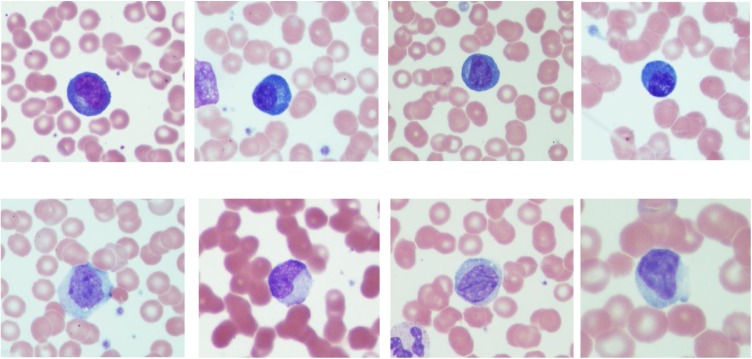
As expected, 79 % (n = 26) of SARS-CoV-2 patients were lymphopenic (<1.0 × 103 μL). Atypical lymphocytic morphology was identified in 75 % (n = 25) as follows: sixteen exhibited plasmacytoid features only (Fig. 1 upper row); five showed EBV-like features (Downey II-like cells) (Fig. 1, lower row), and four showed both features. Collectively, plasmacytoid features were present in smears from 20 patients while the Downey II like-cells were present in only nine. Among non- SARS-CoV-2 patients, lymphocytes with plasmacytoid morphology were absent while six showed lymphocytes with Downey II-like cells features. The frequency of plasmacytoid lymphocytes in SARS-CoV-2 patients was significantly different when compared to non- SARS-CoV-2 (p < .05; Student’s t test). Of particular interest among SARS-CoV-2 patients was an association between Downey II-like cells features and normal absolute lymphocyte counts (p < .05; Student’s t test) in SARS-CoV-2 patients.
Fig. 1.
Upper row: Atypical lymphocytes showing plasmacytoid features including small size, eccentric nucleus and dark blue cytoplasm. Lower row: Atypical lymphocytes with Downey II-like cells features showing large size, ample cytoplasm, indented nucleus, and occasional cytoplasmic granules.
In this brief report of lymphocyte morphology in peripheral smears of SARS-CoV-2 patients, we confirm atypical lymphocytes as a common finding in blood smears from SARS-CoV-2 patients even in the setting of lymphopenia. Plasmacytoid lymphocytes are most commonly found and are highly associated with SARS-CoV-2 infection, at least in high SARS-Cov-2 prevalence environments, in this case New York City.
Atypical lymphocytes are a heterogeneous group of lymphocytes with variable morphological characteristics associated with a polyclonal immune response to antigenic stimulation. The pioneering work of McKinley and Downey in 1923 categorizing the atypical lymphocytes seen in infectious mononucleosis was one of the first to study such changes in lymphocytes [9]. Based on our findings, atypical lymphocyte morphology in SARS-CoV-2 patients could be grouped into two main categories: 1. Plasmacytoid features (Fig. 1, upper row): which are small mature lymphocytes with an eccentric nucleus and deep blue cytoplasm; included in this category are cells with plasmablastic features in which the cell is slightly larger with open chromatin and prominent nucleolus; and 2. Downey II-like cells (Fig. 1, lower low): large lymphocytes abutting the surrounding red blood cells with abundant cytoplasm, and occasional cytoplasmic granules similar in their morphology to the original Downey II cells [5]. A peripheral smear may show either feature or a spectrum of both. Although atypical lymphocyte morphology is not uncommon in viral infections in general, pneumonia-causing viruses including influenza A, SARS-1, and swine flu are not commonly associated with atypical lymphocytic morphology [3,6,7]. A few viral infections are asscoaited with plasmacytoid lymphocytes including Dengue fever and to a lesser extent rubella [8]. Therefore, the results from this study point to the unique characteristics of SARS-CoV-2 virus which may be attributed to mechanisms of immune reaction and dysregulation not frequently seen with other viruses.
Our results are in line with reports from different countries affected by the SARS-CoV-2 pandemic, in which atypical lymphocytes were described [[1], [2], [3], [4]]. However, by performing a systematic review with detailed morphological assessment on the lymphocyte morphology, we provide evidence of the changes that may be seen in SARS-CoV-2. We have shown that atypical lymphocytic morphology is common in SARS-CoV-2 patients’ peripheral blood. Specifically, atypical plasmacytoid lymphocytes are highly associated with SARS-CoV-2, which is unusual among viral infections. The classic Downey II-like cells, which are generally common in viral infections, are less frequently found in SARS-CoV-2 infection.
Financial support
No funds were received for this study.
CRediT authorship contribution statement
Siraj M. El Jamal: Methodology, Investigation, Data curation, Formal analysis, Writing - original draft. Christian Salib: Methodology, Data curation. Aryeh Stock: Methodology, Data curation. Norlita I. Uriarte-Haparnas: Methodology, Investigation. Benjamin S. Glicksberg: Methodology. Julie Teruya-Feldstein: Methodology. Francine R. Dembitzer: Methodology, Writing - review & editing. Girish N. Nadkarni: Methodology. Adolfo Firpo-Betancourt: Methodology, Investigation, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interests.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prp.2020.153063.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Foldes D., Hinton R., Arami S., Bain B.J. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19) Am. J. Hematol. 2020 doi: 10.1002/ajh.25834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gérard D., Henry S., Thomas B. SARS-CoV-2: A new aetiology for atypical lymphocytes. Br. J. Haematol. 2020 doi: 10.1111/bjh.16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg S.E., Behdad A., Ji P. Atypical lymphocytes in peripheral blood of patients with COVID-19. Br. J. Haematol. 2020 doi: 10.1111/bjh.16848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feder H.M., Rezuke W.N. Infectious mononucleosis diagnosed by Downey cells: sometimes the old ways are better. Lancet. 2020;395:225. doi: 10.1016/S0140-6736(19)32962-9. [DOI] [PubMed] [Google Scholar]
- 6.Chng W.J., Lai H.C., Earnest A., Kuperan P. Haematological parameters in severe acute respiratory syndrome. Clin. Lab. Haematol. 2005;27:15–20. doi: 10.1111/j.1365-2257.2004.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 8.Gawoski J.M., Ooi W.W. Dengue fever mimicking plasma cell leukemia. Arch. Pathol. Lab. Med. 2003;127:1026–1027. doi: 10.5858/2003-127-1026-DFMPCL. [DOI] [PubMed] [Google Scholar]
- 9.Downey H., McKinlay C.A. Acute lymphadenosis compared with acute lymphatic leukemia. Arch. Intern. Med. (Chic) 1923;32(1):82–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.