Highlights
-
•
We summarised immunosuppressing anticancer drugs which could worsen COVID 19 infections.
-
•
Other drugs were studied for drug-drug interactions with antiviral medicines.
-
•
A ready-to-use table synthesised these interactions between antiviral and anticancer drugs.
Keywords: COVID-19, Drug interactions, Anticancer drugs, Drug-induced long QT syndrome, Antiviral interactions
To the Editor,
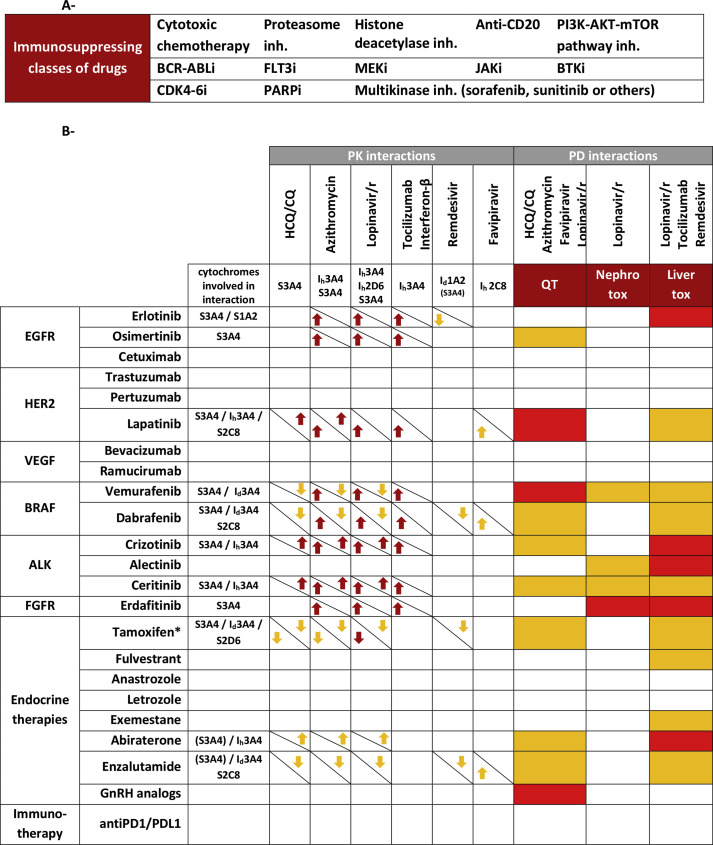
The rapid emergence of the COVID-19 (CoronaVirus Disease) pandemic worldwide is of particular concern for fragile populations who are more at risk of acute respiratory distress syndrome and death. Patients treated for malignant hemopathy and solid cancers have a four times higher risk of hospitalisation due to influenza infection and a ten times higher risk of death. This fragility could be due to their age, multiple associated comorbidities, lymphopenia or the immunosuppressive action of a broad spectrum of anticancer drugs [1]. Therefore, anticancer drugs should be used carefully in this population. Without further safety data, it might be unsafe to treat SARS-CoV-2 (Severe Acute Respiratory Syndrome-associated CoronaVirus)–positive patients who have COVID-19 symptoms with anticancer drugs known to increase infections or harvesting immunosuppressive properties. We summarised in Table (part A) the drug classes that have been reported to increase either neutropenia or infections. Regarding patients tested positive who have recovered from their symptoms, clinical data are missing. It is currently not clear if cancer treatments should be stopped, and if so, the time needed to resume it safely.
Table.
A - Class of anticancer drugs with immunosuppressive properties. Immunosuppressing drugs were defined as drugs associated with significantly more infections or neutropenia compared with the control group or placebo in trials. These drugs were excluded from part B. B - Summary of pharmacokinetic (PK) and pharmacodynamic (PD) interactions of interest concerning non-immunosuppressive anticancer drugs and potential COVID-19 treatments. No interaction driven by other cytochromes was found.
∗Tamoxifen is a prodrug and the reported effect is on the active metabolite endoxifen.
Red arrows are for interactions relying on clinically significant data. Orange arrows are for interactions relying on in vitro data for pharmacokinetic interactions. Cytochromes involved in the drug interaction were specified. Substrates for which induction but not inhibition could lead to significant interaction are between brackets. When the interaction modifies the pharmacokinetics of the anticancer drug, the arrow was on the bottom-left. Antiviral exposition prediction is on the top-right. Red boxes are for anticancer drugs with known torsade de ointes risk and high risk of renal and liver toxicities. Orange boxes are for anticancer drugs prolonging QT without known torsade de pointes risk and moderate risk for renal and liver toxicities. Data from FDA labels [4] were retrieved for drug metabolism, QT prolongation and nephrotoxicity. LiverTox database was used for hepatotoxicity [5].
CQ: chloroquine; GnRH: gonadotrophin-releasing hormone; HCQ: hydroxychloroquine; Id: cytochrome inducer; Ih: cytochrome inhibitor; Lopinavir/r: lopinavir/ritonavir association (KALETRA®); S: substrate.
In any of these settings, clinical trials and incoming standard of care could lead to the prescription of antiviral drugs concomitant to non-immunosuppressive anticancer treatments. Similar to previous works reporting interactions between HIV (human immunodeficiency virus) antiretrovirals and anticancer drugs [2], these two classes of medications have a narrow therapeutic index and can have pharmacological interactions. Some of them are substrates or interact with hepatic cytochrome P450 cytochrome isoenzymes (CYPs), particularly CYP3A4, and pharmacokinetic interactions could lead to supra or infratherapeutic concentrations. For example, enzalutamide, a non-steroidal antiandrogen prescribed for prostate cancer, is both a CYP3A4 substrate and inducer. Ritonavir, on the other hand, is a pharmacokinetic booster of lopinavir contained in Kaletra, which is explored as a COVID-19 treatment. Ritonavir is a substrate and also a potent inhibitor of CYP3A4. Thus, enzalutamide and ritonavir could interfere with each other's metabolism, decrease or increase each other's clearance and be responsible for severe toxicities or decreased efficacy. Favipiravir, an anti-EBOV (Ebola Virus) drug, also a candidate for the COVID-19 treatment, is an inhibitor of CYP2C8 [3] and therefore may increase anticancer drug metabolised through this pathway, such as dabrafenib and enzalutamide. Furthermore, CYP3A4 induction could lead to sustained CYP3A4 increased activity for up to 1 week after discontinuation. Dabrafenib or enzalutamide, two CYP3A4 inducers, could significantly decrease hydroxychloroquine concentration during the first week of washout.
Among pharmacodynamic interactions, QT interval prolongation could be of particular interest. Hydroxychloroquine, which is currently widely prescribed as an anti-coronavirus drug, or azithromycin are two drugs known to prolong QT interval. Concomitant use of QT-prolonging anticancer drugs could lead to torsade de pointes and be fatal. Caution should be observed in this case, and electrocardiographic monitoring should be implemented to monitor QT interval duration during combination therapy.
Similarly, anticancer drugs could potentiate nephrotoxicity and hepatotoxicity of antiviral treatments.
Table (part B) summarises pharmacokinetic and pharmacodynamic interactions between some currently tested drugs against COVID-19 and anticancer drugs.
Funding
This study received no funding.
Conflict of interest statement
J.-P.S. reports serving as a consultant for Roche, MSD and Biogaran and reports attending adboard/symposium in MSD, Roche, AZ, LEO Pharma, Mylan, Pfizer, BMS, Novartis, PFO, Myriads, Gilead and Lilly. All remaining authors have declared no conflicts of interest.
References
- 1.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spano J.-P., Poizot-Martin I., Costagliola D., Boué F., Rosmorduc O., Lavolé A. Non-AIDS-related malignancies: expert consensus review and practical applications from the multidisciplinary CANCERVIH Working Group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27(3):397–408. doi: 10.1093/annonc/mdv606. [DOI] [PubMed] [Google Scholar]
- 3.Liverpool COVID-19 interactions. https://www.covid19-druginteractions.org/
- 4.Drugs@FDA FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/
- 5.LiverTox . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Clinical and research information on drug-induced liver injury. [PubMed] [Google Scholar]