Abstract
In addition to the canonical biological and biochemical framework, blood clots can also be considered as active biomaterials composed of dynamically contracting platelets, nascent polymeric fibrin that functions as a matrix scaffold, and entrapped blood cells. As platelets sense, rearrange, and apply forces to the surrounding microenvironment, they dramatically change the material properties of the nascent clot, increasing its stiffness by an order of magnitude. Hence, the mechanical properties of blood clots are intricately tied to the forces applied by individual platelets. Research has also shown that the pathophysiological changes in clot mechanical properties are associated with bleeding and clotting disorders, cancer, stroke, ischemic heart disease, and more. By approaching the study of hemostasis and thrombosis from a biophysical and mechanical perspective, important insights have been made into how the mechanics of clotting and the forces applied by platelets are linked to various diseases. This review will familiarize the reader with a mechanics framework that is contextualized with relevant biology. The review also includes a discussion of relevant tools used to study platelet forces either directly or indirectly, and finally, concludes with a summary of potential links between clotting forces and disease.
Keywords: Blood clot, platelet, clot contraction, bleeding disorders, thrombotic disease
Introduction
Blood clot signaling and structure
Platelets are an integral part of clot formation and clot contraction, and these two processes both modify and simultaneously are affected by the local clot biochemical and biomechanical environment. The biochemical and biological aspects of clot formation have been well covered by previous reviews1–6, but will be briefly mentioned here. In the absence of vascular injury, clot formation is prevented by the anticoagulant surface of vascular endothelial cells. However, vascular injury damages and denudes the endothelial surface and exposes the circulating blood to subendothelial elements. Hemostasis occurs in two simultaneous steps: primary hemostasis, which mainly involves the anchoring and aggregation of platelets to the wound site, and secondary hemostasis which reinforces the clot via fibrin formation and clot stabilization and contraction.
Upon activation, a cascade of biochemical signaling leads to a concerted process that results in healthy hemostasis. During primary hemostasis, receptors on the surface of platelets interact with adhesive ligands in the subendothelial matrix, including von Willebrand factor (VWF) and collagen, anchoring the platelets to the injured site. Platelet-platelet adhesion is facilitated by the binding of surface integrins, especially integrin αIIbβ3 to fibrin and fibrinogen, VWF, collagen, fibronectin, and vitronectin. This process of platelet aggregation is further modulated by the hydrodynamic environment. At low shear rates, platelet aggregation occurs predominantly via αIIbβ3 and fibrinogen interactions, while at higher shear rates, platelets aggregate in an increasingly VWF-dependent manner7. Further aggregation and platelet plug development occurs in a compounding manner as surrounding platelets are activated by serotonin, thromboxane A2 (TxA2), and adenosine diphosphate (ADP), agonists released by other activated platelets. These processes collectively result in formation of a platelet plug at the site of injury.
During secondary hemostasis, exposed tissue factor, present in the extravascular tissue, interacts with or activates a succession of coagulation factors (VIIa, X, IX, IXa, VIIIa, Xa, Va) thereby activating prothrombin (II), which ultimately generates thrombin (IIa). Thrombin acts to cleave soluble fibrinogen into insoluble fibrin, thus forming a mesh around the platelet plug, and further plays a role in coagulation by converting factors XI, VIII, V and XIII to activated factors XIa, VIIIa, Va, and XIIIa, respectively. Moreover, thrombin also activates platelet G-protein coupled receptors PAR1 and PAR4, which initiate cell-signaling pathways resulting in integrin activation and aggregation, changes in platelet shape, granule secretion, and ultimately results in platelet contraction. The interaction of actin and myosin for retraction is regulated by phosphorylation of myosin light chain (MLC) through calcium-dependent activation of MLC kinase and Rho-kinase dependent inhibition of MLC phosphatase8.
More recent work suggests that upon injury, the platelet plug has a unique hierarchical structure, and specifically consists of an inner, compact core with fully activated platelets, a loose outer shell consisting of less activated platelets, and an intervening transition region. The observed hierarchical difference in packing density results in an agonist concentration gradient expanding out from the inner core and subsequently heterogeneous platelet activation. The high packing density of platelets in the inner core limits transport in this region to diffusion, effectively trapping agonists. Thrombin and fibrin are thus primarily found in the inner core, while smaller agonists such as ADP and TxA2 can diffuse farther into the shell and may play a role in clot stability9,10. Hence, biophysical aspects of clot formation appear to influence biochemical signaling and integrity.
Platelet-driven contracting blood clots as dynamic mechanical materials
In addition, stable blood clots can also be viewed as active materials formed by dynamically contracting platelets, fibrin polymers, and entrapped blood cells. In describing this physical framework, it can be helpful to consider a few common mechanical terms and structures. Stiffness refers to the “springiness” or, more technically, the deformability of a given material or structure. Purely elastic materials will consistently resist deformation and immediately return to their original position when the force is no longer applied. Purely viscous liquids will resist deformation based on how fast that deformation is applied, but do not return to their original shape. In practice, most materials, and especially blood clots, are viscoelastic, and behave as a combination of an elastic solid and viscous liquid.
Each of the primary constituents of blood clots may be considered from a mechanics perspective, especially fibrin and entrapped cells. A substantial number of studies11–16 and excellent reviews16–18 cover fibrin in detail. In broad terms, individual fibrin polymers may be considered as elastic12, with extraordinary extensibility19 and stiffnesses that change with diameter20. Fibrin networks are viscoelastic21 and have some unusual properties which include: stiffening when stretched11,22, alignment with applied forces11, and alignment and thickening with applied fluid forces16. These properties of fibrin clots presumably allow them to withstand the shear forces resulting from blood flow and maintain hemostasis.
Of the cells that become entrapped in a developing clot, red blood cells (RBCs) make up the vast majority of these cells and will dictate the mechanical response. RBCs are softer than other blood components23 and the amount of incorporated erythrocytes will change both the local and bulk clot stiffness. In addition, RBC incorporation is canonically associated with venous clots, and is plasma FXIIIa dependent24,25. These cells can form a core structure within clots and may become significantly compacted due to platelet contractile forces26.
Platelets may be thought of as actuators that dynamically contract and remodel the microenvironment. In conditions with freely moving boundaries, such as in a clot suspended in a liquid, platelets can dramatically shrink a fibrin mesh by over an order of magnitude. In fixed boundary conditions, where the clot is pinned between two plates, studies have demonstrated that platelet-driven contraction dramatically increases the stiffness of these clots27. Similarly, bulk studies have demonstrated that platelets generate substantial forces28 when the overall clot length remains constant. At the microscale, platelets use filipodia to rearrange and densify fibrin29,30. Moreover, in addition to changing their local environment, platelets are capable of sensing the local biochemical and biomechanical environment and can respond by generating substantial forces31–33.
Recent studies have demonstrated that microenvironmental cues, such as mechanical properties of the underlying matrix substrate31,32, matrix geometry34, biochemical conditions9, and shear stress35,36, all mediate platelet physiology at the single cell level. Particularly, it has been found that platelets sense and directly respond to the stiffness of the surrounding extracellular matrix, as evidenced by changes in adhesion, spreading and activation that occur on substrates of different stiffnesses, with either immobilized fibrinogen or conjugated collagen32,37,38. Further, fibrinogen density has also been found to play a role in platelet response and signaling via the αIIbβ3 integrin, and varying densities were specifically found to result in distinctly different signaling responses via the same αIIbβ3 integrins39.
Part of the challenge associated with studying the mechanics of blood clots lies in the inherent mechanical complexity and heterogeneity of individual platelets and fibrin, as well as the organization of these components. Clots in the hemodynamic environment are innately heterogeneous materials in which shear rate, fibrin architecture, and agonist concentration all vary significantly throughout the same clot. However, despite these challenges, research into various diseases has revealed pathophysiological changes to the forces applied by platelets and by extension to the mechanical properties of whole blood clots. Enhanced and impaired platelet forces have been associated with clotting and bleeding disorders, respectively, although the mechanisms remain wholly unknown and untested at this point. In the following sections, we will discuss both the historic and current tools that are being used to study the mechanics of whole blood clots, the individual components as well as the clinical links that have been elucidated using these tools. As platelets are the primary drivers of clot contraction, our focus will remain on platelet mechanics and how platelets affect clot mechanics.
Bulk clot and platelet contraction assays
Successful hemostasis ultimately occurs due to the formation of resilient clots with bulk mechanical properties that are appropriate to the surrounding environment. It has thus been useful to study these properties at the macroscale, and a variety of tools have been developed and widely used in the past for this purpose. To this end, in the following sections we will describe assays measuring bulk clot contraction, bulk clot forces, and several viscoelastic tests, including rheometry, thromboelastography, and the sonoclot analyzer.
Bulk clot contraction assays
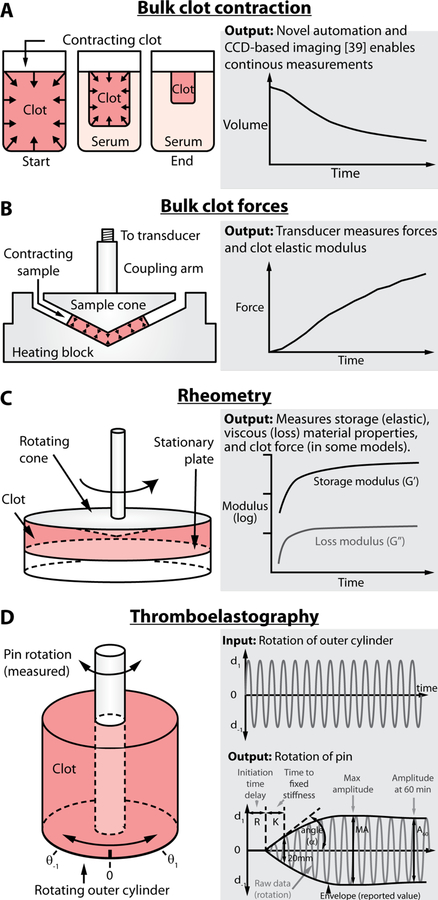
Clot mechanical properties have historically been evaluated at the bulk clot level. In the most basic system, samples of whole blood or platelets and fibrin are placed in an aggregometer tube, clotted with thrombin, and monitored. New automated approaches using digital cameras have enabled continuous measurements40 over time (Figure 1A). Results from these assays depend on multiple parameters such as platelet concentration, fibrin concentration, fibrin structure, RBC stiffness, and more40. This dependence on multiple parameters, which can vary from patient to patient, complicates interpretations. However, a main benefit of these types of assays is the relative speed and ease with which they can be performed. Moreover, they have allowed for the characterization of important signaling pathways vital to clot formation and retraction. For example, bulk clot contraction assays have helped elucidate αIIbβ3 outside in signaling. Suzuki-Inoue et al demonstrated that this signaling is essential for thrombin-stimulated clot retraction and is partially dependent on Src kinases and Phospholipase Cγ2 (PLCγ2)8. This was shown when Src kinase inhibitors were added to bulk clot retraction assays and the rate of clot retraction significantly decreased, though the inhibitor did not prevent the retraction process from reaching completion.
Figure 1.
A number of different tools have been developed to examine bulk clot contraction and material properties. A) Bulk clot contraction represents the simplest measurement in which a clot is formed in a tube and monitored over time. However, new insights have been discovered by using a novel clot imaging approach to automate data collection and provide continuous real-time measurements. B) The well-developed hemostasis analysis system was the first to measure bulk clot contractile forces in a clinical setting and provided key evidence that altered forces are linked to numerous diseases. C) Rheometry represents a powerful method that enables complex material data to be derived from various clots and may include force data similar to B. D) Of the bulk assays, thromboelastography is the most well-known and used in clinical settings. While it can be used to understand clot mechanics, it is mostly used to study coagulation kinetics.
Bulk clot contraction assays have also identified the importance of B Cell Lymphoma 3 (BCL-3) expression in clot retraction and demonstrated modulation of this expression by the mechanistic target of rapamycin (mTOR) signaling pathway. Specifically, Weyrich et al. found that BCL-3 expression was essential for clot retraction and for generation of tight fibrin polymers41. Further, when either BCL-3 or mTOR was inhibited or blocked, clot retraction was found to be impaired. This work identified post-transcription gene expression in platelets as possible targets for molecular therapy of inflammatory and thrombotic disorders.
Assays Assessing Bulk Clot Forces
Additional assays have enabled important assessments of total contraction force and platelet contribution at the bulk clot level, and often make use of force transducers. One example of these assays utilizes a clot strip formed from platelet rich plasma and thrombin and is attached to both a wall on one side and a force transducer on the other. The force transducer then measures the isometric load when platelet contraction occurs28. These assays have helped to reveal the importance and contribution of platelets to clot retraction, and the mechanism by which this occurs. Specifically, these assays helped elucidate the importance of calcium triggered actin-myosin interaction in platelet contraction, and helped demonstrate that cross-linking of fibrin is critical in bulk clot contraction28,42,43.
Later comprehensive studies by Carr44 led to the development and standardization of the Hemostasis Analysis System by Hemodyne. The interested reader is directed to an excellent review on the development, testing, and studies performed with this system45. Briefly, the system relies on placing clots in between rigid surfaces and the platelet contraction forces are measured as depicted in Figure 1B. Unfortunately, although it would appear that this system is no longer commercially available, it provided critical insights into the clinical links between platelet contraction and various diseases, as will be discussed in a further section of this article.
Viscoelastic Tests
Tools have also been developed to measure the viscoelastic properties of blood clots. These properties are commonly measured and understood by applying a stress to the material and looking at the resulting strain. This can be done in a variety of ways, as demonstrated by the tools discussed in the following sections.
Rheometry
Rheometers are widely applicable tools that enable the measurement of fluidic properties and viscoelastic solid properties, and can be used to apply controlled, consistent shear stresses to in vitro cell cultures. Rheometers rely on the use of a moving and stationary surface, such as between two cylinders or between two round flat plates. Flow can be applied by continuously turning the moving plate, and is useful for measuring fluidic properties and applying constant shear stresses to samples, which arise as a consequence of the cylinder or plate geometries.
Rheometers can also measure the elastic or viscous components of a sample, including blood clots, which are formed between the plates, as shown in Figure 1C. Rather than continuously spinning, the moving plate oscillates the sample back and forth. By measuring either the resulting force on the stationary plate or the resistance to movement on the moving plate, the quantitative elastic and viscous measurements of the clot may be obtained. The elastic component refers to how much the blood clot deforms as it is oscillated, whereas the viscous component refers to how much the blood clot resists the deformation motion. For ease, the elastic and viscous components are combined into a single term, the complex shear modulus, composed of the shear storage modulus (G’) and shear loss modulus (G’’), respectively.
Rheometry thus provides a way to measure clot stiffness and deformation, and has been used to analyze macroscale differences in clot mechanical properties across varying conditions. It has also been used to approximate the gel point or clotting time by determining the time at which the storage modulus (G’) becomes finite17,46–48. Rheometers including the ability to measure forces generated by the sample have found a link between platelet contractile force and the bulk plasma clot elastic modulus27, although the extent and exact mechanism is still unknown. Some research has suggested that clot elastic properties are influenced by platelets synergistically by modifying the fibrin orientation and by applying tension to the fibrin strands21,27.
Thromboelastography
Originally described by Hartert in 194849, thromboelastography is another tool that has enabled measurement of viscoelastic properties similar to rheometry described above. Here, many of the same principles are used to more directly monitor clot formation and elasticity over time. In this case, a cup containing the sample oscillates while a pin suspended from a torsion wire remains stationary. As blood coagulates, the pin begins to oscillate with the cup and the resulting torque is detected. As shown in Figure 1D, this produces a trace with constant period and changing amplitude, representative of the pin movement, where the amplitude correlates to the material stiffness.
Several parameters are commonly identified from a thromboelastograph to characterize coagulation. First, the R time is the amount of time it takes for the pin to begin rotating up to 2 mm, and thus represents the clotting time. The K time is the time needed for the clot to reach a fixed stiffness, defined as a pin rotation of 20 mm, while α (angle) provides a measure of the rate at which this stiffness is reached. The maximal stiffness of the clot is characterized by the maximal amplitude (MA), or the largest amplitude of pin rotation. Eventually, the clot will begin to lyse due to fibrinolysis as indicated by the decrease in amplitude of pin rotation over time. This can be quantified as the time it takes for the amplitude of pin rotation to decrease to 2 mm less than its maximal amplitude, or Time to Lysis (TTL), and can also be quantified as the percentage decrease in amplitude that occurs 30 or 60 minutes after MA is reached (LY30 and LY60, respectively)50.
Rotation thromboelastometry (ROTEM) provides similar information by instead rotating the pin and measuring the resulting motion optically; however, the specific parameters used to characterize test outcomes are slightly different and care should be taken when comparing across tools.
In addition to rheometry, thromboelastography is thus another tool that has enabled characterization of macroscale clot mechanical properties. Its well-defined outcome measures have allowed for comparison across samples, and enabled evaluation of clot mechanical response to different stimulants and conditions46. This in addition to its relative speed and recent availability at the point of care has made it an attractive test for clinical use, though significant variation has been observed between labs51. In general, while thromboelastography detects the influence of changes to clot mechanics and platelet forces, it primarily has been employed to evaluate coagulation and especially the kinetics of clot formation and lysis52.
Sonoclot Analyzer
Sonoclot Analyzer is another viscoelastic test that has also found use in the clinic. Originally developed by von Kaulla in 1975 to enable coagulation analysis at the point of care with a minimal amount of sample53, the test consists of a probe vibrating with an amplitude of less than 1 micron and a frequency of less than 200 hertz suspended in a cuvette containing whole blood or recalcified plasma. The initiation of fibrin and clot formation dampens the amplitude of oscillation of the vibrating probe as the mechanical impedance of the surrounding sample on the probe mobility increases. This change is detected and is represented on a Sonoclot Signature53.
As was the case in thromboelastography, several parameters are commonly identified from a Sonoclot Signature for comparison purposes. This includes the time for clot initiation and fibrin formation, comparable to the R time in thromboelastography, and here called the activated clotting time (ACT). The rate of clot formation (CR) is also identified and is similar to α (angle). Additionally, the peak amplitude and time to peak can be evaluated.
The Sonoclot Analyzer has provided another means to analyze the viscoelastic changes associated with clot formation, and has been used successfully to describe differences in coagulability between healthy individuals and patients. For example, results from the Sonoclot Analyzer were able to confirm the hypercoaguability proposed to occur in pregnant women54–56.
Micro and nanotechnology based platelet contraction
The bulk clot assays described above have thus enabled important insights into the role of platelets in clot contraction and hemostasis. Importantly, they were also used to estimate the average platelet contraction force in the bulk clot by comparing the contraction and elasticity of clots generated from platelet rich plasma and those generated from platelet poor plasma, and then determining an effective force per unit area27. However, as was discovered utilizing micro and single platelet assays, this can widely vary between platelets31.
Platelets specifically have remained difficult to study due to their small size and heterogeneous response to stimuli that vary within a clot. However, new micro and nanoscale approaches that are able to create structures on the same size scale of platelets have enabled new insights into platelet behavior and physiology. Within this framework, springs are an extremely useful component for designing structures that test mechanical properties. A linear spring is governed by Hooke’s law and will displace proportionally to an applied force. In practice, a spring can be designed to have a certain stiffness, and when a biological structure pulls on that spring, the exact force can be deduced from measuring how much the spring moves. This makes springs extremely useful as tools for probing the mechanical properties of blood clots, and have been utilized at different scales and in a variety of ways to measure platelet contraction. To that end, in the following sections, we will cover some of the recent assays developed that enable force measurements at the micro clot, single platelet and sub-platelet levels. Less clinical data is available for these assays given their recent development.
Micro-Assays
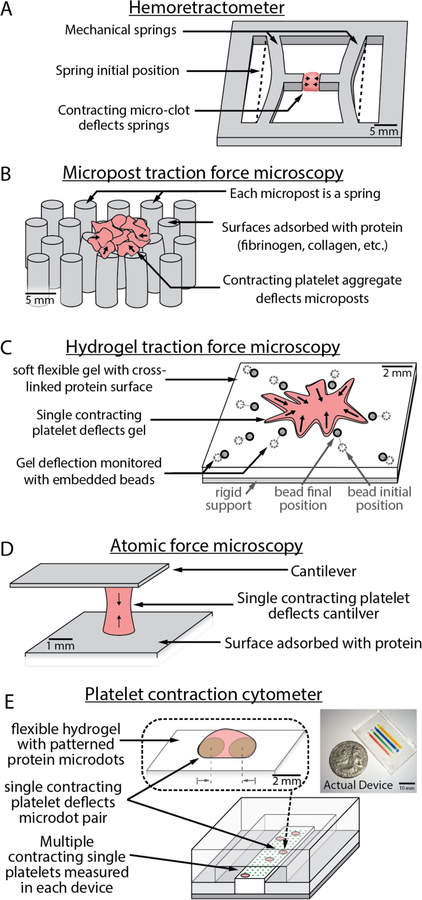
The miniaturized hemoretractometer57 (Figure 2A) uses a clever set of in-plane flexural springs that measure the contractile force of a small drop of whole blood. Specifically, a drop of blood is placed between two protruding flexible posts, and the resulting inward deflection as clotting occurs enables measurement of contractile force. Each test only uses a small amount of blood (12.75 µL), and the device is inexpensive and disposable. As such, this technology could be a promising point-of-care tool for contraction force monitoring that provides much of the same information as the larger bulk clot force tests with far less blood and in a much smaller package.
Figure 2.
Recent developments in microtechnology have enabled new systems capable of studying micro-clots and single platelet biophysics. A) The hemoretractometer uses mechanical springs and provides data similar to bulk clot contraction but in a quantitative manner with vastly reduced sample size. B) Micropost assays have been used to examine small platelet clumps and have even been miniaturized enough to examine single platelet force. C) Hydrogel traction force microscopy provides similar information to micropost traction force microscopy, but may require significant imaging and computational resources. D) Atomic force microscopy enables single platelet nanomechanical studies but can be time-consuming to perform as only a single platelet can be measured in each experiment. E) Platelet contraction cytometry enables high-throughput single platelet nanomechanical measurements and has begun to show links between impaired single platelet contraction forces and bleeding.
Another microassay, micropost traction force microscopy, relies on the creation of a series of microposts that deflect when force is applied (Figure 2B). Since each micropost may be treated as an independent spring, this clever approach reduces computational needs to calculate applied forces. A key challenge in applying this technology to platelets lies in the mechanical design of the microposts. Creating posts that are sufficiently small enough to interact with platelets yet large enough to visualize, especially with the available material sets, is quite difficult. Remarkably, one group has solved this challenge and created successful micropost systems that measured the forces of platelet clumps58 and even of individual platelets59,60.
Single Platelet Assays
Beyond micropost traction microscopy, other tools have also been adapted for measurement of single platelet contraction force, such as hydrogel traction force microscopy. Hydrogel traction force microscopy reduces initial design complexity at the expense of later computational needs. Rather than rely on a series of independent mechanical springs, platelets interact with a hydrogel of known stiffness (Figure 2C). Beads are embedded throughout the gel in a random fashion to enable tracking of the movement of the gel. By determining the bead position before contraction and the bead final position after contraction, it is possible to calculate the forces that were applied to the gel by the platelet. However, these calculations can be quite complex as the entire gel is connected and meticulous care must be taken during imaging to minimize errors. However, these gels can be an attractive approach as they enable a wider range of stiffness to be tested and can be more accessible to researchers who do not have access to microfabrication resources. Schwarz Henriques et al. successfully translated this method to single platelets61 and were even able to provide sub-cellular data on the evolution of platelet forces on soft 2D surfaces, and provide additional evidence that platelet forces are highly heterogeneous.
Another single-cell platelet contraction force assay developed by our research group31 utilizes Atomic Force Microscopy (AFM) to measure single cell platelet contraction force and their response to various microenvironments (Figure 2D). Here, fluorescently labelled single platelets suspended in buffer are positioned between two opposing fibrinogen coated surfaces (one being the AFM cantilever). When a platelet contacts these opposing surfaces, actin structures form between them and allow for platelet contraction. Using this assay, we were able to demonstrate the wide range of maximum contractile force exhibited by platelets, from 1.5 to 79 nN. We were also able to investigate how individual platelets respond to different microenvironments, which is vital in understanding the role of platelets within the context of mechanically heterogeneous clots. We were able to account for and study this by varying the stiffness of the cantilever tip used representative of different fibrin clot stiffness. It was subsequently found that in stiffer microenvironments as well as in areas with high fibrin densities platelets contracted with higher forces. This work provided the necessary framework to focus on individual platelets to study disease31.
Building on our original work31, our research group recently developed a platform, the platelet contraction cytometer, to perform high-throughput single platelet force measurements (Figure 2E)33. This system utilizes an array of fibrinogen microdot pairs patterned on four parallel microstrips of polyacrylamide (PAA) hydrogels with varying stiffnesses ranging from 25kPa to 75kPa. Thrombin-activated platelets adhere to these microdots, spread to the neighboring microdot, and contract, pulling the microdots closer together. Since the platelet force is directly proportional to the microdot displacement, only a single visual measurement is needed to determine the force applied by each platelet. As such, a plethora of platelets may be measured in a controlled mechanical, biochemical, and shear environment.
Using this system, it was determined that both biochemical and mechanical cues mediate platelet contraction force. This work found that platelets have certain conditions which maximize contractility, specifically, moderately stiff microenvironments and moderate thrombin concentrations (75kPa gels and 1 U/mL thrombin). Using parameters that maximize contractility, we tested the functionality of the cytometer with blood samples from patients with a variety of bleeding disorders, including Wiskott-Aldrich Syndrome and May Hegglin, as well as from patients with bleeding phenotypes yet also with normal laboratory values for complete blood count, coagulation screening, platelet function or von Willebrand disease panels. As expected, the cytometer demonstrated a stark difference between the average contractile force of healthy controls (~30nN) and patients with Wiskott-Aldrich Syndrome (~5, ~10 nN) or MYH9-related disorder (10 nN), who should have lower forces due to mutations related to actin and myosin. Moreover, three out of the five patients with bleeding phenotypes (~15, ~20, and ~20 nN) yet normal laboratory values for hemostatic function had impaired contractile forces, suggesting that bleeding symptoms may be related to low contractile forces. Surprisingly, platelet force was found to be completely independent of the known markers of platelet activation, namely phosphatidylserine exposure, p-selectin exposure, and αIIbβ3 activation. In addition, when comparing the contraction force distributions of healthy controls to patients with bleeding phenotypes/disorders, it was found that approximately 30% of platelets from patients with bleeding phenotypes/disorders had near-zero contractile forces when compared to that of the healthy control (~6%). Taken together, since the individual platelet forces are independent of known markers of platelet activation, and impaired in some bleeding disorders, this exciting research suggests that individual platelet contractile force may be a novel biophysical biomarker.
Sub-Platelet Assays
More recently, several groups have demonstrated the ability to acquire force measures at the sub-platelet level, specifically at the integrin level. One method of measuring sub platelet contraction, developed by Wang et al. and termed the integrative tension sensor (ITS), measured forces exerted by platelets at the subcellular level by converting molecular tensions exerted by integrins to fluorescent signals62. Their ITS system utilizes tensions transmitted by the integrin αIIbβ3 of platelets to create force distributions at the subcellular level allowing for measurements of tensions generated by platelet adhesion and platelet contraction. The investigators found that low-level integrin tensions between 12–54 pN are produced during adhesion and any rise in tension past this occurs during contraction. They also found that the anti-platelet drug tirofiban, an inhibitor of the integrin αIIbβ3, reduces the forces generated by platelets. Even at high concentrations of 5 µg/ml, adhesion still occurred, but the force distribution pattern was found to be more isotropic when compared to lower concentrations of tirofiban and the control. This work demonstrated a new way to study the effects of antiplatelet drugs on platelets by utilizing force mapping and force distributions62.
Similarly, Zhang et al investigated sub-platelet contraction forces to determine how platelets discriminate between soluble and membrane-bound fibrinogen and also provide an understanding of how integrins, primarily αIIbβ3, regulate contraction initiation63. Their system utilizes a supported lipid bilayer (SLB) with anchored ligands through biotin-streptavidin interaction with biotinylated lipids, while controlling mobility of the SLB to attempt activating platelets. They found that platelets only became activated when incubated on non-fluid SLBs with immobilized ligands, demonstrating that platelet integrins activate in response to tangential tension and supporting the notion that platelets utilize membrane-bound fibrinogen as a mechanical checkpoint. This aids the platelet in determining whether activation and adherence is necessary, allowing for the discrimination between soluble and membrane bound fibrinogen and preventing unnecessary activation63.
Brockman et al. mapped the 3D orientation of piconewton integrin traction forces within platelets64. Specifically, fluorescence polarization techniques were integrated with molecular tension probes to enable molecular force microscopy (MFM) and reveal the orientation of the platelet traction forces with piconewton resolution. Using this, the researchers found that during activation forces generated at the platelet edge were radially isotropic, while interior forces within the platelet had axial organization. This showed that two distinct force alignments occur during platelet activation. Applying this same MFM technique to aggregation, to understand if there is a necessary coordination of force orientations between adjacent platelets, they found that force orientations were not aligned in 38% of platelet-platelet boundaries studied and therefore no coordination was found across platelet aggregates64.
Clot contractile force implicated in disease
Using these tools to study clot mechanics, researchers have identified links between clot mechanics and various clinical disorders (Table 1). The majority of reports indicate a decrease in clot contraction, although some notable reports have recorded statistically significant increases in contractile force (grey in table), as in coronary heart disease, where forces are twice that of healthy controls65. Some of these results are expected and serve as important negative controls, as in the case of Glanzmann’s thromboasthenia, in which patients are missing integrin αIIbβ3 and therefore have impaired platelet-platelet or platelet-fibrin binding66. Similarly, patients that have platelets with defective actin and myosin machinery will be unable to generate substantial contractile forces as seen in Wiskott-Aldrich Syndrome or MYH9-related disorders33.
Table 1:
Reported links between various diseases, clot properties, and platelet contraction
| Disease | Device | Findings | Reference |
|---|---|---|---|
| Acute Ischemic Stroke | Automated whole blood clot contraction | impaired whole blood clot contraction | 67 |
| Asthma | Bulk clot contraction | impaired whole blood clot contraction | 87–89 |
| Chest Pain | PCF / Hemostasis Analysis System | increased contractile force | 79 |
| Coagulation Factor Deficiencies | PCF / Hemostasis Analysis System | impaired whole clot contraction, decreased bulk platelet contractile forces | 66 |
| Glanzmann’s Thrombasthenia | PCF / Hemostasis Analysis System | decreased bulk platelet contractile force | 66 |
| MYH9 mutations | Platelet Contraction Cytometer | decreased single platelet forces | 33 |
| Polycythemia vera | Bulk clot contraction, Thromboelastography | increased clot contraction, different fibrin structure | 80,81 |
| Severe Coronary Artery Disease | PCF / Hemostasis Analysis System | increased bulk platelet contractile force, increased clot elastic modulus | 65 |
| Sickle Cell Disease | Automated whole blood clot contraction | impaired whole blood clot contraction | 40 |
| Symptomatic Bleeding | Platelet Contraction Cytometer | decreased single platelet forces | 33 |
| Systemic Lupus Erythematosus | Automated whole blood clot contraction | impaired whole blood clot contraction | 68 |
| Thromboangiitis obliterans | PCF / Hemostasis Analysis System | increased bulk platelet contractile force | 90 |
| Trauma | PCF / Hemostasis Analysis System | decreased bulk platelet contractile force | 91 |
| Wiskott Aldrich Syndome | Platelet Contraction Cytometer | decreased single platelet forces | 33 |
In some cases, explicit links between platelet contractile force and disease pathophysiology have been identified, whereas other instances highlight changes to other clot components or the entire clot in general. For example, excellent work standardizing and simplifying the bulk clot contraction assay has revealed new insights into impaired whole clot contraction in patients with sickle cell disease40, stroke67, or systemic lupus erythematosus68. However, it is unclear whether platelet force is altered in these conditions. Other approaches that measure bulk platelet forces provide more direct evidence of impaired contractile force, although it is unclear whether the platelets themselves have impaired contractile forces or if the platelets are simply responding to a different microenvironment66. Both of these bulk approaches provide critical information that can be useful to better understanding platelet clot contraction and diagnostic implications. However, the most direct evidence for impaired contraction can be gathered using the novel platelet contraction cytometer, which suggests impaired platelet forces are linked to bleeding symptoms. Unlike the previous bulk platelet contractile force measurement, these individual platelet tools suggest that platelets are heterogeneous and sub-populations of platelets with different force properties may exist33. Importantly, these single-platelet tools enable the study of patients with low platelet counts as the results are independent of the total platelet count.
Many of the diseases listed may affect other aspects of clot formation that can in turn affect platelet contraction. For example, patients deficient in clotting factors will have impaired thrombin generation66, which has been shown to decrease platelet forces33. However, once the deficient factor is restored, platelet contractile force increases in a dose-dependent manner69. Similarly, since platelets are mechanosensitive32 and modulate contractile force with the mechanical microenvironment31,33, pathological changes to fibrin or RBC stiffness would be expected to change the applied forces. For example, fibrin formation is altered in multiple myeloma70, diabetes66, nephrotic syndrome71, deep vein thrombosis72, atrial fibrillation, stroke73, and more. The interested reader is directed to a number of excellent reviews on fibrin16,74–76. Canonically, RBCs are thought to be incorporated into venous clots and in vitro studies have demonstrated that these cells segregate into an inner core that is compressed during clot contraction26. As such, increasing the RBC stiffness is expected to modify platelet contraction, although the exact nature of this change is still unknown. Similarly, diseases affecting RBC stiffness, such as the increase seen in sickle cell, may also affect clot contraction40, and the interested reader is directed to other excellent reviews of the role of erythrocytes in clotting77,78.
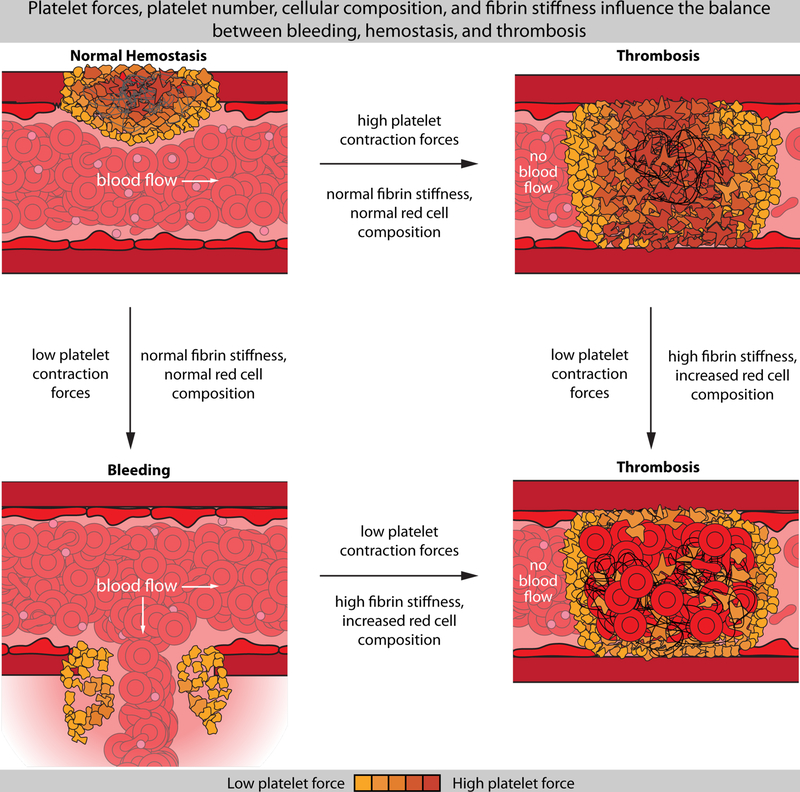
This literature suggests that a complex interplay between platelet forces, platelet number, fibrin content, fibrin stiffness, the RBC composition, and the stiffness of these cells ultimately govern the stiffness of the blood clot. Depending on these factors, both bleeding and thrombosis appear to be related to changes in platelet forces (Figure 3). For example, bleeding has been tied to low platelet forces33 in patients with normal laboratory values. However, low platelet forces are also hypothesized to play a role in stroke, in which impaired bulk clot contraction, reduced platelet count, increased hematocrit, increased fibrinogen, and impaired platelet response to thrombin are observed67. Normal platelet forces can still be tied to thrombosis, as in the case of multiple myeloma70, in which simultaneous measurements of clots stiffness and platelet force demonstrated normal forces with increased stiffness. Here, it was suggested that the clot was simply too stiff for platelets to adequately contract. Finally, increased platelet forces may be thrombotic as well, again, depending on clot composition. In patients with coronary artery disease, chest pain, and electrocardiographic evidence of ischemia, both increased bulk contractile forces and increased clot stiffness were observed79. Similar trends might be expected in polycythemia vera, which is associated with thrombosis, increased hematocrit, and activated platelets. Here, studies on bulk clot contraction have shown increased contractility80,81, although more research is needed to elucidate the exact mechanisms. As such, this work suggests that platelet contractile forces may play an important role in the pathophysiology of diseases, but should be studied in the context of the composition and structure of fibrin, and other entrapped cells.
Figure 3.
Changes in platelet contractile forces are linked to disease, but should be considered in context of the platelet number and other clotting components such as the fibrin stiffness and red cell composition. Here, healthy hemostasis is hypothesized to represent a balance between the platelet force, red cell composition, and fibrin content/stiffness (top left). Low platelet contractile forces have been tied to symptomatic bleeding in patients with normal laboratory values for hemostasis (bottom left), whereas low contraction forces are thought to be involved in ischemic stroke (bottom right), where fibrin and red cell content are increased. Other conditions such as coronary artery disease and ischemia have been linked to increased bulk platelet contractile forces and increased clot stiffness (top right).
Future implications
Although the mechanistic underpinnings related to changes in contractile force with various diseases remain unclear, there does appear to be an optimal platelet contractile force associated with healthy hemostasis33. Noting the recent developments in creating culture derived platelets82–84 and interest in improved ways to examine their function85, measuring bulk clot and single platelet contraction force may be an important avenue for validating function. Similar validations may be useful for platelets that have undergone storage, especially when transfusing into patients that already have platelets with impaired contractile forces and bleeding. Such work would greatly benefit from improved systems that can be manufactured in mass and performed in the clinic with minimal sample pre-processing.
In addition, other exciting work has begun to examine how platelet contractile forces might be leveraged within the body to engineer an outcome. For example, Hansen et al.86 developed a novel system that leverages platelet activation and contraction to break apart capsules loaded with hemostatic agents. Such an approach could have immediate use in providing a local in vivo delivery of factor VIII to wound sites in hemophilia A patients that have developed inhibitory anti-factor VIII antibodies. Early tests in vitro tests indicate that this approach reduces clot formation time and increases fibrin formation.
Conclusions
Taken collectively, we conclude that platelet force may represent a novel, independent, biophysical biomarker that provides important insight into patient health and potential therapeutic treatments. This mechanics-based framework provides a strongly complementary and orthogonal approach to the existing biochemical based assays. However, to enable clinical translation, more research is needed to better identify relevant diseases, uncover mechanisms, and link platelet mechanics to biochemical signaling, as well as to better understand the basic mechanisms that govern whole blood clot and single platelet forces.
References
- 1.Davì G, Patrono C. Platelet Activation and Atherothrombosis. New England Journal of Medicine 2007;357(24):2482–2494 [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. The growing complexity of platelet aggregation. Blood 2007;109(12):5087. [DOI] [PubMed] [Google Scholar]
- 3.M Hoffman DMr. A cell-based model of hemostasis. Thrombosis and haemostasis 2001;85(6):8. [PubMed] [Google Scholar]
- 4.Gale AJ. Current Understanding of Hemostasis. Toxicologic pathology 2011;39(1):273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furie B, Furie BC. Mechanisms of thrombus formation. The New England journal of medicine 2008;359(9):938–949 [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Favaloro EJ. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin Thromb Hemost 2018;44(3):10. [DOI] [PubMed] [Google Scholar]
- 7.Jackson SP, Nesbitt WS, Westein E. Dynamics of platelet thrombus formation. Journal of Thrombosis and Haemostasis 2009;7(s1):17–20 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki-Inoue K, Hughes CE, Inoue O, et al. Involvement of Src kinases and PLCγ2 in clot retraction. Thrombosis Research 2007;120(2):251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 2013;121(10):1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brass LF, Diamond SL, Stalker TJ. Platelets and hemostasis: a new perspective on an old subject. Blood Advances 2016;1(1):5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science (New York, NY) 2009;325(5941):741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collet J-P, Shuman H, Ledger RE, Lee S, Weisel JW. The elasticity of an individual fibrin fiber in a clot. Proceedings of the National Academy of Sciences of the United States of America 2005;102(26):9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thrombosis and haemostasis 2009;102(6):1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hategan A, Gersh KC, Safer D, Weisel JW. Visualization of the dynamics of fibrin clot growth 1 molecule at a time by total internal reflection fluorescence microscopy. Blood 2013;121(8):1455–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophysical journal 1999;77(5):2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolberg AS. Thrombin generation and fibrin clot structure. Blood reviews 2007;21(3):131–142 [DOI] [PubMed] [Google Scholar]
- 17.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophysical Chemistry 2004;112(2):267–276 [DOI] [PubMed] [Google Scholar]
- 18.Weisel JW. Enigmas of Blood Clot Elasticity. Science 2008;320(5875):456–457 [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Jawerth LM, Sparks EA, et al. Fibrin Fibers Have Extraordinary Extensibility and Elasticity. Science (New York, NY) 2006;313(5787):634–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Sigley J, Pieters M, et al. Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation. Biophysical journal 2016;110(6):1400–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah JV, Janmey PA. Strain hardening of fibrin gels and plasma clots. Rheologica Acta 1997;36(3):262–268 [Google Scholar]
- 22.Piechocka IK, Bacabac RG, Potters M, MacKintosh FC, Koenderink GH. Structural Hierarchy Governs Fibrin Gel Mechanics. Biophysical Journal 2010;98(10):2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fay ME, Myers DR, Kumar A, et al. Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proceedings of the National Academy of Sciences 2016;113(8):1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin α-chain crosslinking. Blood 2015;126(16):1940–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kattula S, Byrnes JR, Martin SM, et al. Factor XIII in plasma, but not in platelets, mediates red blood cell retention in clots and venous thrombus size in mice. Blood advances 2018;2(1):25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cines DB, Lebedeva T, Nagaswami C, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 2014;123(10):1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jen Chauying J, McIntire Larry V. The structural properties and contractile force of a clot. Cell Motility 1982;2(5):445–455 [DOI] [PubMed] [Google Scholar]
- 28.Cohen ADVI Platelet contractile regulation in an isometric system. Nature 1973;246(5427):2. [DOI] [PubMed] [Google Scholar]
- 29.Kim OV, Litvinov RI, Alber MS, Weisel JW. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nature Communications 2017;8(1):1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wufsus AR, Macera NE, Neeves KB. The hydraulic permeability of blood clots as a function of fibrin and platelet density. Biophysical journal 2013;104(8):1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam WA, Chaudhuri O, Crow A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nature materials 2011;10(1):61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Y, Brown AC, Myers DR, et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proceedings of the National Academy of Sciences 2014;111(40):14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers DR, Qiu Y, Fay ME, et al. Single-platelet nanomechanics measured by high-throughput cytometry. Nature materials 2017;16(2):230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita A, Sakurai Y, Myers DR, et al. Microenvironmental geometry guides platelet adhesion and spreading: a quantitative analysis at the single cell level. PloS one 2011;6(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature medicine 2009;15(6):665–673 [DOI] [PubMed] [Google Scholar]
- 36.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood 1996;88(5):1525–1541 [PubMed] [Google Scholar]
- 37.Qiu Y, Ciciliano J, Myers DR, Tran R, Lam WA. Platelets and physics: How platelets “feel” and respond to their mechanical microenvironment. Blood Reviews 29(6):377–386 [DOI] [PubMed] [Google Scholar]
- 38.Kee MF, Myers DR, Sakurai Y, Lam WA, Qiu Y. Platelet Mechanosensing of Collagen Matrices. PLoS ONE 2015;10(4):e0126624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiroušková M, Jaiswal JK, Coller BS. Ligand density dramatically affects integrin αIIbβ3-mediated platelet signaling and spreading. Blood 2007;109(12):5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tutwiler V, Litvinov RI, Lozhkin AP, et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood 2016;127(1):149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weyrich AS, Denis MM, Schwertz H, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood 2007;109(5):1975–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen I The contractile system of blood platelets and its function. Methods Achiev Exp Pathol 1979;9:47. [PubMed] [Google Scholar]
- 43.Isaac Cohen JMG, White James G. Ultrastructure of clots during isometric contraction. The Journal of Cell Biology 1982;93(3):775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr ME. Measurement of platelet force: the Hemodyne hemostasis analyzer. Clin Lab Manage Rev 1995;9:9. [PubMed] [Google Scholar]
- 45.Carr ME. In vitro assessment of platelet function. Transfusion Medicine Reviews 1997;11(2):106–115 [DOI] [PubMed] [Google Scholar]
- 46.Tran R, Myers DR, Ciciliano J, et al. Biomechanics of haemostasis and thrombosis in health and disease: from the macro- to molecular scale. Journal of Cellular and Molecular Medicine 2013;17(5):579–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers DR, Fletcher DA, Lam WA. Towards High-Throughput Cell Mechanics Assays for Research and Clinical Applications. In: Bao HDEaG, ed. Nano and Cell Mechanics: Fundamentals and Frontiers First ed.: John Wiley & Sons; 2013 [Google Scholar]
- 48.Bussolari Steven R and Forbes Dewey C. J. Apparatus for subjecting livig cells to fluid shear stress. Rev Sci Instrum 1982;53(12):4. [DOI] [PubMed] [Google Scholar]
- 49.Hartert H Blutgerinnungsstudien mit der thrombelastographie, einem neuen untersuchungsverfahren. Klinische Wochenschrift 1948;26(37–38):7. [DOI] [PubMed] [Google Scholar]
- 50.Milind Thakur ABA. A Review of Thromboelastography. International Journal of Perioperative Ultrasound and Applied Technologies 2012;1(1):5 [Google Scholar]
- 51.Michelson AD. Platelets San Diego, UNITED STATES: Elsevier Science & Technology; 2012 [Google Scholar]
- 52.Bolliger D, Seeberger MD, Tanaka KA. Principles and Practice of Thromboelastography in Clinical Coagulation Management and Transfusion Practice. Transfusion Medicine Reviews 2012;26(1):1–13 [DOI] [PubMed] [Google Scholar]
- 53.Kaulla KNv. The impedance machine: a new bedside coagulation recording device. Journal of medicine: clinical, experimental and theoretical 1975;6(1):16. [PubMed] [Google Scholar]
- 54.Phyllis L Steer HBK. Thromboelastography and Sonoclot Analysis in the Healthy Parturient. J Clin Anesth 1993;5(6):6. [DOI] [PubMed] [Google Scholar]
- 55.Saleem A Viscoelastic measurement of clot formation: a new test of platelet function. Annals of clinical and laboratory science 1983;13(2):10. [PubMed] [Google Scholar]
- 56.Furuhashi M, Ura N, Hasegawa K, et al. Sonoclot coagulation analysis: new bedside monitoring for determination of the appropriate heparin dose during haemodialysis. Nephrology Dialysis Transplantation 2002;17(8):1457–1462 [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Li X, McCracken B, Shao Y, Ward K, Fu J. A Miniaturized Hemoretractometer for Blood Clot Retraction Testing. Small 2016;12(29):3926–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang XM, Han SJ, Reems J-A, Gao DY, Sniadecki NJ. Platelet Retraction Force Measurements using Flexible Post Force Sensors. Lab on a chip 2010;10(8):991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feghhi S, Munday Adam D, Tooley Wes W, et al. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophysical Journal 2016;111(3):601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feghhi S, Tooley WW, Sniadecki NJ. Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase. Journal of Biomechanical Engineering 2016;138(10):104506–104506-104504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz Henriques S, Sandmann R, Strate A, Köster S. Force field evolution during human blood platelet activation. Journal of Cell Science 2012;125(16):3914. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, LeVine DN, Gannon M, et al. Force-activatable biosensor enables single platelet force mapping directly by fluorescence imaging. Biosensors and Bioelectronics 2018;100:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Qiu Y, Blanchard AT, et al. Platelet integrins exhibit anisotropic mechanosensing and harness piconewton forces to mediate platelet aggregation. Proceedings of the National Academy of Sciences 2018;115(2):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brockman JM, Blanchard AT, Pui-Yan Ma V, et al. Mapping the 3D orientation of piconewton integrin traction forces. Nature Methods 2017;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greilich PE, Carr ME, Zekert SL, Dent RM. Quantitative assessment of platelet function and clot structure in patients with severe coronary artery disease. The American journal of the medical sciences 1994;307(1):15–20 [DOI] [PubMed] [Google Scholar]
- 66.Carr ME. Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochemistry and Biophysics 2003;38(1):55–78 [DOI] [PubMed] [Google Scholar]
- 67.Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of Blood Clots is Impaired in Acute Ischemic Stroke. Arteriosclerosis, thrombosis, and vascular biology 2017;37(2):271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Minh G, Peshkova Alina D, Andrianova Izabella A, et al. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clinical Science 2018;132(2):243. [DOI] [PubMed] [Google Scholar]
- 69.Brophy DF, Martin EJ, Christian Barrett J, et al. Monitoring rFVIIa 90 μg kg⁻¹ dosing in haemophiliacs: comparing laboratory response using various whole blood assays over 6 h. Haemophilia : the official journal of the World Federation of Hemophilia 2011;17(5):57. [DOI] [PubMed] [Google Scholar]
- 70.Carr ME, Zekert SL. Abnormal clot retraction, altered fibrin structure, and normal platelet function in multiple myeloma. American Journal of Physiology-Heart and Circulatory Physiology 1994;266(3):H1195–H1201 [DOI] [PubMed] [Google Scholar]
- 71.Collet J-P, Mishal Z, Lesty C, et al. Abnormal Fibrin Clot Architecture in Nephrotic Patients Is Related to Hypofibrinolysis: Influence of Plasma Biochemical Modifications. Thrombosis and haemostasis 1999;82(11):1482–1489 [PubMed] [Google Scholar]
- 72.Cieslik J, Mrozinska S, Broniatowska E, Undas A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: a cohort study. Blood 2018;131(7):797–807 [DOI] [PubMed] [Google Scholar]
- 73.Drabik L, Wołkow P, Undas A. Fibrin Clot Permeability as a Predictor of Stroke and Bleeding in Anticoagulated Patients With Atrial Fibrillation. Stroke 2017;48(10):2716–2722 [DOI] [PubMed] [Google Scholar]
- 74.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophysical chemistry 2004;112(2–3):267–276 [DOI] [PubMed] [Google Scholar]
- 75.Undas A Fibrin clot properties and their modulation in thrombotic disorders. Thrombosis and haemostasis 2014;112(1):32–42 [DOI] [PubMed] [Google Scholar]
- 76.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood 2013;121(10):1712–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT science series 2017;12(1):176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood 2017;130(16):1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishnaswami A, Carr ME Jr., Jesse RL, et al. Patients with coronary artery disease who present with chest pain have significantly elevated platelet contractile force and clot elastic modulus. Thrombosis and haemostasis 2002;88(5):739–744 [PubMed] [Google Scholar]
- 80.Rusak T, Ciborowski M, Uchimiak-Owieczko A, Piszcz J, Radziwon P, Tomasiak M. Evaluation of hemostatic balance in blood from patients with polycythemia vera by means of thromboelastography: The effect of isovolemic erythrocytapheresis. Platelets 2012;23(6):455–462 [DOI] [PubMed] [Google Scholar]
- 81.Rusak T, Piszcz J, Misztal T, Branska-Januszewska J, Tomasiak M. Platelet-related fibrinolysis resistance in patients suffering from PV. Impact of clot retraction and isovolemic erythrocytapheresis. Thromb Res 2014;134(1):192–198 [DOI] [PubMed] [Google Scholar]
- 82.Lu S-J, Li F, Yin H, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Research 2011;21:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng Q, Shabrani N, Thon Jonathan N, et al. Scalable Generation of Universal Platelets from Human Induced Pluripotent Stem Cells. Stem Cell Reports 2014;3(5):817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panuganti S, Schlinker AC, Lindholm PF, Papoutsakis ET, Miller WM. Three-Stage Ex Vivo Expansion of High-Ploidy Megakaryocytic Cells: Toward Large-Scale Platelet Production. Tissue Engineering Part A 2012;19(7–8):998–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamat V, Muthard RW, Li R, Diamond SL. Microfluidic assessment of functional culture-derived platelets in human thrombi under flow. Experimental hematology 2015;43(10):891–900.e894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansen CE, Myers DR, Baldwin WH, et al. Platelet–Microcapsule Hybrids Leverage Contractile Force for Targeted Delivery of Hemostatic Agents. ACS Nano 2017;11(6):5579–5589 [DOI] [PubMed] [Google Scholar]
- 87.Tomasiak‐Lozowska MM, Misztal T, Rusak T, Branska‐Januszewska J, Bodzenta‐Lukaszyk A, Tomasiak M. Asthma is associated with reduced fibrinolytic activity, abnormal clot architecture, and decreased clot retraction rate. Allergy 2016;72(2):314–319 [DOI] [PubMed] [Google Scholar]
- 88.Tomasiak-Lozowska MM, Rusak T, Misztal T, Bodzenta-Lukaszyk A, Tomasiak M. Reduced clot retraction rate and altered platelet energy production in patients with asthma. Journal of Asthma 2016;53(6):589–598 [DOI] [PubMed] [Google Scholar]
- 89.Misztal T, Rusak T, Tomasiak M. Peroxynitrite may affect clot retraction in human blood through the inhibition of platelet mitochondrial energy production. Thrombosis research 2014;133(3):402–411 [DOI] [PubMed] [Google Scholar]
- 90.Carr ME, Hackney MH, Hines SJ, Heddinger SP, Carr SL, Martin EJ. Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans--two case reports. Vascular and endovascular surgery 2002;36(6):473–480 [DOI] [PubMed] [Google Scholar]
- 91.White NJ, Newton JC, Martin EJ, et al. Clot Formation Is Associated With Fibrinogen and Platelet Forces in a Cohort of Severely Injured Emergency Department Trauma Patients. Shock 2015;44 Suppl 1:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]