Abstract
Macrophages are mononuclear phagocytes derived from haematopoietic progenitors that are widely distributed throughout the body. These cells participate in both innate and adaptive immune responses and lie central to the processes of inflammation, development, and homeostasis. Macrophage physiology varies depending on the environment in which they reside and they exhibit rapid functional adaption in response to external stimuli. To study macrophages in vitro, cells are typically cultured ex vivo from the peritoneum or alveoli, or differentiated from myeloid bone marrow progenitor cells to form bone marrow-derived macrophages (BMDMs). BMDMs represent an efficient and cost-effective means of studying macrophage biology. However, the inherent sensitivity of macrophages to biochemical stimuli (such as cytokines, metabolic intermediates, and RNS/ROS) makes it imperative to control experimental conditions rigorously. Therefore, the aim of this study was to establish an optimised and standardised method for the isolation and culture of BMDMs. We used classically activated macrophages isolated from WT and nitric oxide (NO)-deficient mice to develop a standardised culture method, whereby the constituents of the culture media are defined. We then methodically compared our standardised protocol to the most commonly used method of BMDM culture to establish an optimal protocol for the study of nitric oxide (NO)-redox biology and immunometabolism in vitro.
Keywords: Macrophage, MCSF, Tetrahydrobiopterin, Nitric oxide
Highlights
-
•
We describe a new and optimised protocol to culture BMDMs.
-
•
We used classically activated macrophages isolated from WT and NO-deficient mice.
-
•
This study emphasises the importance of measuring culture media components and their effects.
-
•
GMCSF priming enhances NO secretion upon inflammatory stimulation.
-
•
Protocol standardises conditions of differentiation, culture, and inflammatory stimulation.
1. Introduction
Macrophages are a heterogeneous population of cells that are derived from haematopoietic progenitors and exhibit functional adaption in response to external biochemical and immunological stimuli [1]. As part of the innate immune response, monocytes are recruited to sites of inflammation, where growth factors such as macrophage colony stimulating factor (MCSF) stimulate their differentiation into macrophages [2]. Inflammatory macrophages infiltrate tissues to eliminate pathogens and secrete cytokines in order to recruit other leukocytes, but are also involved in tissue repair and inflammation resolution. In addition, distinct populations of tissue-resident macrophages carry out specialised homeostatic roles and are maintained by self-renewal throughout adult life [3,4]; these include Kupffer cells in the liver, microglia in the brain, alveolar macrophages in the lungs, and peritoneal macrophages. To varying degrees, depending on the tissue in question, monocyte-derived macrophages replenish populations of tissue-resident macrophages, of foetal origin, throughout adult life [5].
Macrophages display a variety of cell surface receptors that recognise numerous signalling molecules including cytokines, pathogenic fragments, and damaged tissues, which allow for a rapid response to environmental changes. The ability to integrate multiple signals that elicit intracellular changes in transcription, protein expression, and metabolism, lies central to the control of macrophage activation states and the pro-/anti-inflammatory response. The existing paradigm of macrophage polarisation states being either ‘classically’ activated inflammatory, or ‘alternatively’ activated anti-inflammatory, is now changing, with studies revealing a vast range of responses from varying combinations of stimuli leading to a diverse and multi-dimensional array of activation states [6].
Macrophage experimentation typically involves ex vivo culture (e.g. from the peritoneum), or the differentiation from bone marrow progenitor cells to form bone marrow-derived macrophages (BMDMs). As discussed, macrophage provenance, culture conditions, and inflammatory stimuli will naturally affect cell phenotype, function, and inflammatory status. Cell heterogeneity is a major limitation of culturing primary macrophages and it is therefore desirable to robustly control conditions in vitro in order to characterise fully the response of macrophages to specific and quantifiable stimuli [7].
MCSF elicits the differentiation of haematopoietic stem cells into macrophages and has been used to generate in vitro BMDMs from bone marrow progenitor cells for decades [8,9]. However, it is common to differentiate macrophages using whole conditioned media from L929 cells (L-cells), which are known to secrete large amounts of MCSF. L-cell conditioned media (LCM) is easy to self-produce, but suffers from batch variability and is undefined. Thus, using defined concentrations of recombinant MCSF is increasingly preferred [7]. Other common sources of variability in media composition includes the base medium used, FBS source and concentration, glucose content, and antibiotic use—all of which are easily controlled and reported. Given the increasing interest in the emerging concepts of innate immune memory and the understanding that macrophages can be “primed” for stimuli-specific responses, the need for some form of standardisation is apparent.
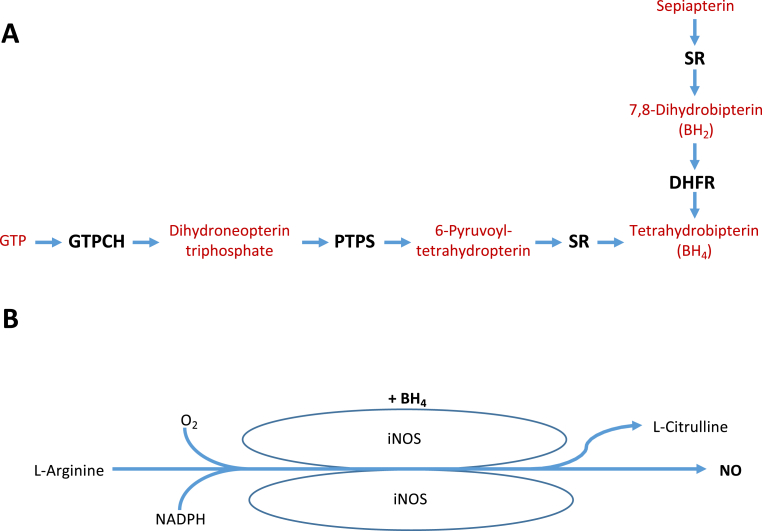
Central to inflammatory macrophage function is the expression of inducible nitric oxide synthase (iNOS), which generates large quantities of nitric oxide (NO) upon activation with lipopolysaccharide (LPS) and interferon gamma (IFNγ), requiring tetrahydrobiopterin (BH4) as a cofactor (Fig. 1) [10]. Our laboratory has recently shown the profound importance of NO production in the regulation of macrophage function, NRF2-dependent redox signalling, and immunometabolism in BH4-deficient and iNOS knockout mice [11,12]. In this study, we propose a standardised protocol for in vitro BMDM generation and show how the production of NO—as a critical modulator inflammatory status—can vary depending on differentiation strategy employed. To this end, we systematically compared different methods of BMDM culture from WT and BH4-deficient Gch1 conditional knockout mice to develop a defined method suitable for the study of NO biology and its impact on redox status and immunometabolism in BMDMs.
Fig. 1.
Schematic showing A) the de Novo BH4 synthetic pathway, and B) BH4-dependent production of NO by iNOS.
2. Methods
2.1. Animal details
All animal procedures were approved and carried out in accordance with the University of Oxford ethical committee and the UK Home Office Animals (Scientific Procedures) Act 1986. All procedures conformed with the Directive 2010/63/EU of the European Parliament.
We generated a Gch1 conditional knockout (floxed) allele using Cre/loxP strategy, as described previously [11,13]. Gch1fl/fl animals were bred with Tie2cre transgenic mice to produce Gch1fl/flTie2cre mice where Gch1 is deleted in endothelial cells and bone marrow-derived cells. The Tie2cre transgene is active in the female germline. Consequently, only male animals are used to establish breeding pairs to maintain conditional expression. Experiments were performed using bone marrow isolated from 10 to 16 weeks old adult male and female Gch1fl/flTie2cre (referred to as Gchfl/flTie2cre) and their Gch1fl/fl (Gchfl/fl) littermates on a pure (>10 generations) C57BL6/J background. Mice were genotyped according to the published protocol [11,13]. Nos2−/− (Nos2tm1Lau) (iNOS KO) and wild type C57BL/6 mice were purchased from The Jackson Laboratory.
2.2. Bone marrow extraction
Male and female mice were sacrificed via cervical dislocation and legs were dissected. Using aseptic technique, bone marrow was extracted from tibia and femur bones following removal of surrounding muscle. To do so, joints were cut using a scalpel and the exposed bone marrow was flushed out the ends of the bones using a 25-gauge needle and a 10 ml syringe filled with PBS. Clumps were gently disaggregated using a needle-less syringe and passed through a 70 μm cell strainer. The cell suspension was centrifuged at 250 g for 5 min at room temperature to pellet cells. The supernatant was discarded and cells were resuspended in ultra-low endotoxin FBS containing 10% DMSO, divided into 4 cryovial aliquots per mouse and stored at −80 °C overnight before transfer to liquid nitrogen storage. This method is illustrated in Fig. S1.
2.3. Established protocol for growing bone marrow derived macrophages
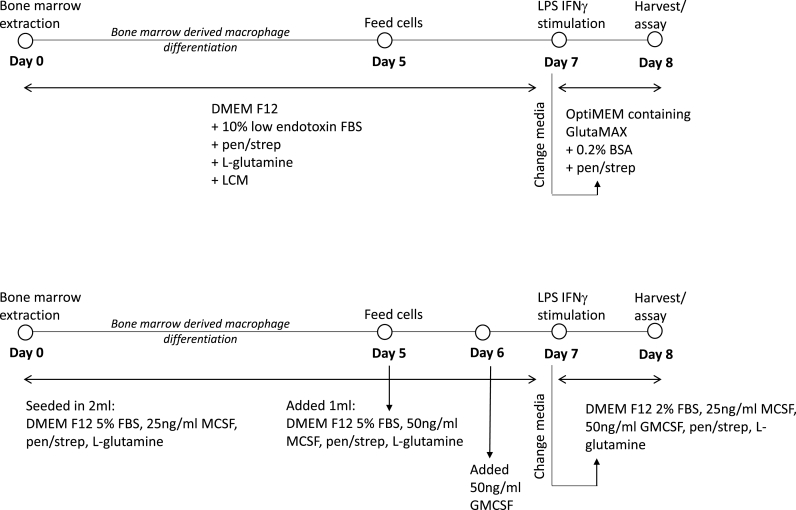
Vials of bone marrow stored in liquid nitrogen were defrosted and resuspended in 5 ml of DMEM F12 media before centrifuging at 250×g for 5 min to pellet cells. The supernatants were discarded and cells were resuspended in 1 ml of DMEM F12 growth media containing l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (0.1 mg/ml), 10% FBS and 10% L-Cell Media (LCM) as a source of MCSF growth factor (DMEM F12 LCM growth media). Conditioned LCM was generated as in the Supplemental File. 500,000 cells/well were seeded into non-tissue culture treated 6 well plates in 2 ml DMEM F12 LCM growth media on Day 0. Cells were cultured for 7 days and fed with addition of 1 ml DMEM F12 LCM growth media on Day 5 of culture. On Day 7 cells were washed with warm PBS to remove unadhered cells and media was replaced with OptiMEM containing GlutaMAX, penicillin (100 units/ml), streptomycin (0.1 mg/ml) and 0.2% BSA. Cells were either left unstimulated or were stimulated with LPS (100 ng/ml) and IFNγ (10 ng/ml) overnight (16 h).
Extensive experiments were carried out to ensure that the FBS used in this study was as high quality as possible. We routinely tested FBS for endotoxin and BH4 levels, and selected FBS from BioWest as this had the lowest levels of BH4, which was not sufficient to support nitrite production in macrophages cultured from GCHfl/flTie2cre KO mice.
2.4. Optimised protocol for growing bone marrow derived macrophages
Vials of bone marrow stored in liquid nitrogen were defrosted and resuspended in 5 ml of DMEM F12 media before centrifuging at 250×g for 5 min to pellet cells. The supernatants were discarded and cells were resuspended in 1 ml of DMEM F12 growth media containing l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (0.1 mg/ml), 5% FBS and recombinant MCSF (25 ng/ml) growth factor (DMEM F12 MCSF growth media). 500,000 cells/well were seeded into non-tissue culture treated 6 well plates in 2 ml DMEM F12 MCSF growth media on Day 0 (Fig. 2, and summarised in S2). Cells were cultured for 7 days and fed with addition of 1 ml DMEM F12 MCSF growth media containing MCSF (50 ng/ml) on Day 5 of culture, and addition of GMCSF (50 ng/ml) on Day 6. On Day 7 cells were washed with warm PBS to remove unadhered cells and media was replaced with DMEM F12 containing l-glutamine (2 mM), penicillin (100 units/ml), streptomycin (0.1 mg/ml), 2% FBS, MCSF (25 ng/ml) and GMCSF (50 ng/ml). Cells were either left unstimulated or were stimulated with LPS (100 ng/ml) and IFNγ (10 ng/ml) overnight (16 h).
Fig. 2.
Optimisation of BMDM culture in vitro. The schematic describes A) the well-established LCM dependent culture of BMDMs and B) the newly optimised BMDM culture method using a combination of MCSF and GMCSF.
2.5. Biopterin quantification by HPLC with electrochemical detection
BH4, BH2, and biopterin levels in cell and mitochondrial lysates were determined by HPLC followed by electrochemical and fluorescent detection, as described previously [14,15]. Macrophage pellets were resuspended in PBS (50 mmol/L), pH 7.4, containing dithioerythritol (1 mmol/L) and EDTA (100 μmol/L) and subjected to three freeze-thaw cycles. Following centrifugation (15 min at 17,000 g, 4 °C), the samples were transferred to new, cooled microtubes and precipitated with ice-cold extraction buffer containing phosphoric acid (1 mol/L), trichloroacetic acid (2 mol/L), and dithioerythritol (1 mmol/L). The samples were vigorously mixed and then centrifuged for 15 min at 17,000 g, 4 °C. The samples were injected onto an isocratic HPLC system and quantified using sequential electrochemical (Coulochem III, ESA Inc.) and fluorescence (Jasco) detection. HPLC separation was performed using a 250 mm, ACE C-18 column (Hichrom) and mobile phase comprising of sodium acetate (50 mmol/L), citric acid (5 mmol/L), EDTA (48 μmol/L), and dithioerythritol (160 μmol/L) (pH 5.2) (all ultrapure electrochemical HPLC grade), at a flow rate of 1.3 ml/min. Background currents of +500 μA and −50 μA were used for the detection of BH4 on electrochemical cells E1 and E2, respectively. 7,8-BH2 and biopterin were measured using a Jasco FP2020 fluorescence detector. Quantification of BH4, BH2, and biopterin was made by comparison with authentic external standards and normalised to sample protein content.
2.6. Western blotting
Cell lysates were prepared by homogenisation in ice-cold CelLytic™ M buffer (Sigma) containing protease inhibitor cocktail (Roche Applied Science). Lysates were centrifuged at 17,000 g for 10 min at 4 °C, and samples were prepared using LDS sample buffer (Invitrogen). Western blotting was carried out using standard techniques with antibodies as previously described.
2.7. NOx measurements
The levels of nitrite/nitrate (NOx) produced by bone marrow derived macrophages from Gchfl/flTie2cre and Gchfl/fl control mice were determined using the CLD88 NO analyser (Ecophysics), as previously described [13]. Quantification of NOx accumulation was obtained by comparison with external standards and normalised to protein concentration, determined by the bicinchoninic acid (BCA) protein assay.
2.8. Cytokine measurements by ELISA
DuoSet enzyme-linked immunosorbent assays (ELISAs) (R&D Systems) were used to measure TNF-α, IL-6, IL-10 and IL-1β in condition media supernatants collected from macrophages re-plated at 1 × 106 cells per well of a 6 well plate on day 7 of culture and stimulated with LPS/IFNγ for 16 h, as per manufacturer's instructions. To measure cytokines neat supernatants were used for IL-10 and IL-1β, and supernatants were diluted 1:100 and 1:400 for TNFα and IL-6 respectively to allow comparison with standard curves.
2.9. FACS
On Day 7 of culture, BMDMs were washed once in PBS before harvesting into PBS containing 5 mM EDTA by flushing with a pipette. 500,000 cells were transferred into FACS tubes, centrifuged at 250×g for 5 min at 4 °C and the supernatants discarded. Cells were resuspended in 100 μl of FACS buffer (PBS containing BSA (0.2%) and EDTA (2 mM)) containing anti-mouse FcγRII/III CD16/32 (5 μg/ml; BD Bioscience) for 15 min on ice to block Fc receptors and non-specific antibody binding. Cells were then stained with fluorochrome conjugated antibodies specific for cell surface markers by incubation for 30 min on ice covered in foil. The antibody mixture of macrophage markers consisted of anti-CD45-PE-Cy7 (1 μg/ml), anti-CD11b-PerCP (1 μg/ml), anti-MHCII-PE (1 μg/ml) and anti-F4/80-APC (1 μg/ml). In control samples, parallel unstained and isotype control antibodies were included at the same concentration as the antigen-specific conjugates. Cells were washed in FACS buffer and centrifuged at 250×g for 5 min at 4 °C and then fixed in 250 μl formaldehyde (2%). FACS was carried out using a CyAn Analyser flow cytometer (Dako) and data were analysed using FlowJo (Tree Star Inc.).
2.10. Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). A Student's t-test was used to compare two groups affected by one single variable. Two-way ANOVA was used to compare multiple data groups affected by two independent variables, with Tukey's post-test to compare groups with each other. Differences were considered statistically significant at P values of × P < 0.05.
3. Results
Characterising Gchfl/flTie2cre bone marrow derived macrophages grown using the established protocol.
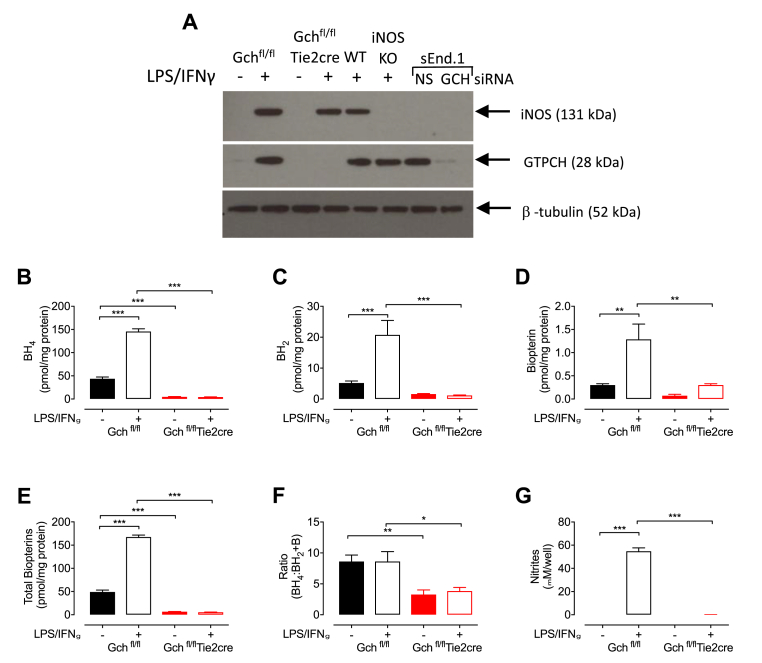
In order to optimise a new protocol for the culture of BMDMs, we directly compared two methods, as shown in Fig. 2. Initially, bone marrow from WT and Gchfl/flTie2cre mice was cultured using the established L-cell protocol previously used by our laboratory to generate macrophages [11] and GTPCH and BH4 levels were assessed. As expected, there was a small amount of GTPCH in control Gchfl/fl macrophages when unstimulated and stimulation with LPS/IFNγ led to a greater abundance in control cells, whereas GTPCH was absent in Gchfl/flTie2cre macrophages (Fig. 3A). iNOS protein was measured in all cells stimulated with LPS/IFNγ, regardless of genotype, apart from iNOS knockout (iNOS KO) negative controls (Fig. 3A). Biopterin measurements revealed lower amounts of BH4, BH2 and biopterin in Gchfl/flTie2cre macrophages compared with Gchfl/fl cells (Fig. 3B–D) and this led to significantly lower total biopterin content (Fig. 3E). The ratio of BH4:BH2+biopterin was also decreased, due to the higher proportion of oxidised biopterins in Gchfl/flTie2cre macrophages (Fig. 3F). Analysis of media supernatants revealed that although production of iNOS was induced by LPS/IFNγ in both Gchfl/fl and Gchfl/flTie2cre macrophages, only Gchfl/fl cells produced NO as indicated by nitrite secretion (Fig. 3G).
Fig. 3.
Characterisation of Gchfl/flTie2cre BMDMs grown in media containing L-cell conditioned media. (A) Western blot analysis of GTPCH and iNOS in Gchfl/fl and Gchfl/flTie2cre macrophages following overnight stimulation with LPS and IFNγ. Stimulated wild type (WT) and iNOS knock out (iNOS KO) macrophages were used as positive and negative controls for iNOS, and NS and Gch siRNA sEnd.1 cells were used as positive and negative controls for GTPCH. (B-F) HPLC was used to quantify (B) BH4, (C) BH2 and (D) biopterin and the results were used to calculate (E) total biopterins (BH4+BH2+biopterin) and (F) the ratio BH4:BH2+biopterin (n = 7). (G) Nitrite was measured in media supernatants using the Griess assay. All data are presented as means ± SEM. Statistics were calculated using 2-way ANOVA and Tukey's post-test (n = 7, *P < 0.05, **P < 0.01, ***P < 0.001).
3.1. Culturing macrophages in low-serum media results in rounding and detachment
During the culture of BMDMs using the previously established protocol (Fig. S2), detachment of unstimulated cells on day 8 of culture was observed and was particularly apparent when using them for assays that took place over a number of hours. Overnight stimulation was considered to be ~16 h; however, by 19 h (representative of the required length of time in many assays), a large number of cells became visibly rounded and detached in unstimulated—but not stimulated—cells (Figs. S2B and C). It was therefore hypothesised that these cells were beginning to die due to serum starvation, which was lowered to 0.2% BSA in OptiMEM on day 7 (Fig. S2A) and is known to trigger apoptosis in cells. Healthy unstimulated cells are a vital control for all experiments and, although MLPS/IFNγ cells appeared healthy, it was is likely they were similarly affected by low serum, as it is known that apoptotic changes and commitment to cell death occur hours before morphological changes become apparent. The cell culture protocol was modified and the media changed to fresh DMEM F12 containing 10% FBS rather than low serum OptiMEM on day 7 (Fig. S2D), which resulted in cells that were well attached and observably healthy on day 8 in both unstimulated and stimulated conditions (Figs. S2E and F).
3.2. FBS and L-cell conditioned media contain BH4 and BH2
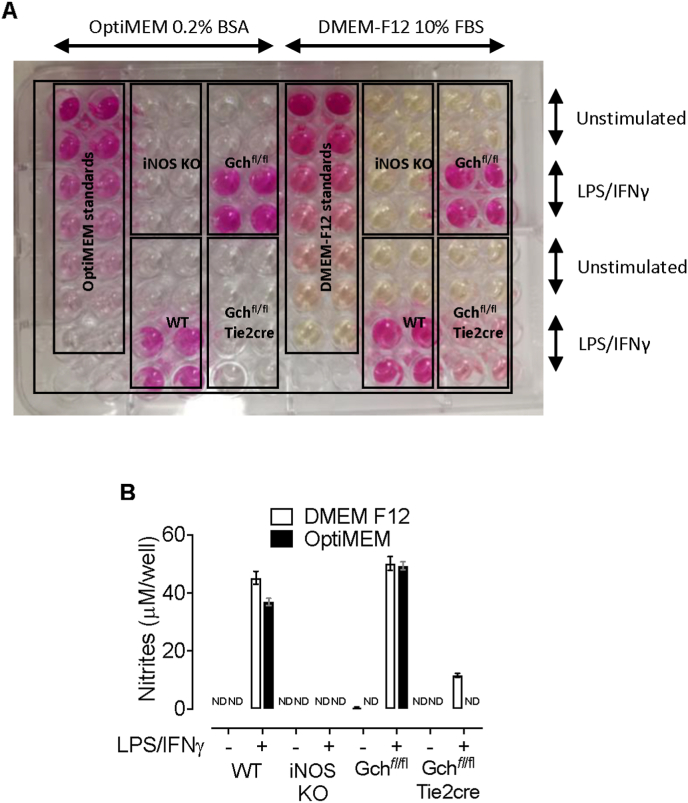
Nitrite levels were measured in media supernatants collected from macrophages following overnight stimulation with LPS/IFNγ and revealed that large amounts of nitrite was secreted from MLPS/IFNγ WT and Gchfl/fl cells when incubated in OptiMEM containing 0.2% BSA and DMEM F12 containing 10% FBS media (Fig. 4A and B). However, whereas no nitrite was detected in media from stimulated Gchfl/flTie2cre macrophages incubated in OptiMEM containing 0.2% BSA, significant levels (11.62 ± 0.99 μM) were found in media from Gchfl/flTie2cre macrophages incubated in DMEM F12 containing 10% FBS (Fig. 4B). This was probably due to functional iNOS in Gchfl/flTie2cre macrophages incubated in DMEM F12 containing 10% FBS despite Gch1 knockout because these data were corrected for any measurable nitrites in fresh media used as blank samples and nitrites were undetectable in media from stimulated iNOS KO cells in both media types (Fig. 4A and B).
Fig. 4.
Gchfl/flTie2cre macrophages stimulated with LPS/IFNγ in DMEM F12 media containing 10% FBS produce NO. (A) Image showing the results of a Griess assay carried out on media supernatants from wild type (WT), iNOS knockout (iNOS KO), Gchfl/fl and Gchfl/flTie2cre macrophages following overnight incubation in OptiMEM containing 0.2% BSA or DMEM F12 media containing 10% FBS and stimulation with LPS/IFNγ. The intensity of pink colouring correlates with nitrite levels. (B) A plate reader (absorbance 550 nm) was used to quantify the concentration of nitrites in media on the plate shown above. All data are presented as means ± range. ND – not detectable.
Hypothesising that BH4 was supplemented by a component of DMEM F12 media containing 10% FBS at a sufficient level to allow iNOS to produce NO, experiments were carried out to identify the source. HPLC was used to quantify levels of biopterin species in constituents of macrophage cell culture media. This showed that DMEM F12, OptiMEM, and DMEM base media alone contained detectable, but very low levels of BH4 and BH2; although biopterin measurements were much higher, the inability of cells to convert this into BH4 means that this is unlikely to supplement cells (Fig. S3A). Much higher levels of BH4 and BH2 were found in 2 batches of low endotoxin FBS diluted to 10% working concentrations (Fig. S3B), and the majority comprised of BH2, which can be converted into BH4 intracellularly by the DHFR salvage pathway. This suggests that FBS was predominantly supplementing Gchfl/flTie2cre macrophages incubated in DMEM F12 containing 10% FBS with BH2, and this was converted into BH4 allowing iNOS to produce NO. Interestingly, similarly substantial levels of BH4 and BH2 were present in 10% LCM but not preparations of recombinant MCSF growth factor (Fig. S3C). Thus, the presence of both FBS and LCM in media during the first 7 days of BMDM culture were providing cells with BH2 and BH4 and it was fortuitous that eliminating LCM and serum starvation removed both sources during the overnight stimulation in the established laboratory protocol.
3.3. Optimising BMDM cell culture in low biopterin conditions
Having demonstrated that the standard media contained substantial levels of BH4, we next sought to minimise the biopterin content of the media throughout the BMDM protocol. As one prominent source of biopterins, FBS concentrations were optimised to the lowest level possible in cell culture media while maintaining healthy cells. Bone marrow grown for 7 days in DMEM F12 containing 10% LCM and 10, 5 or 2% FBS revealed adherent cells indicative of differentiated macrophages in all instances on Day 7 (Fig. S4). Cells grown in 5 or 10% FBS conditions were equally confluent; however, when grown in 2% FBS, cells grew much more slowly. This led to a protocol culturing cells for 7 Days in 5% FBS and switching to 2% FBS during overnight stimulation.
Next, conditions were optimised to culture BMDMs using recombinant MCSF (which had proven to be biopterin free (Fig. S5C)), rather than biopterin rich LCM as the source of growth factor. Culturing macrophages using a range of seeding densities and feeding protocols (data not shown) revealed that bone marrow seeded at 500,000 cells/well of a 6 well plate in DMEM F12 media containing 5% FBS and 25 ng/ml MCSF for 7 days, with addition of 50 ng/ml MCSF media on Day 5, was optimal to produce confluent macrophages on day 7 (Figs. S5A and B). Removing MCSF from media overnight between day 7 and 8 resulted in detachment of cells (Fig. S5C), but with MCSF in the media cells were adherent in both 5 and 2% FBS media (Fig. S5C), so DMEM F12 media containing 2% FBS and 25 ng/ml MCSF was chosen for overnight stimulation (Fig. S5A).
3.4. Treating macrophages with GMCSF leads to increased LPS/IFNγ induced production of NO
To investigate the effect of GMCSF treatment on NO generation, cells grown in LCM, MCSF alone, and MCSF cells treated with GMCSF (Fig. S6A) were compared. After demonstrating that cells in all conditions were confluent and adherent on day 8 (Fig. S6B) nitrite levels were measured in media supernatants from Gchfl/fl and Gchfl/flTie2cre macrophages using the Griess assay. Nitrite measurements showed that LPS/IFNγ induced NO synthesis in Gchfl/fl cells from the three cell culture protocols with indications of greater amounts in GMCSF treated MCSF cells, and there were no detectable accumulation of nitrite in media from Gchfl/flTie2cre macrophages (Fig. S6C). This was an important confirmation that lowering FBS levels to 2% during stimulation had sufficiently decreased biopterin content of the media to maintain BH4 deficiency in Gchfl/flTie2cre macrophages. However, the effect of GMCSF treatment on NO generation in Gchfl/fl cells was less clear and a more thorough investigation carried out.
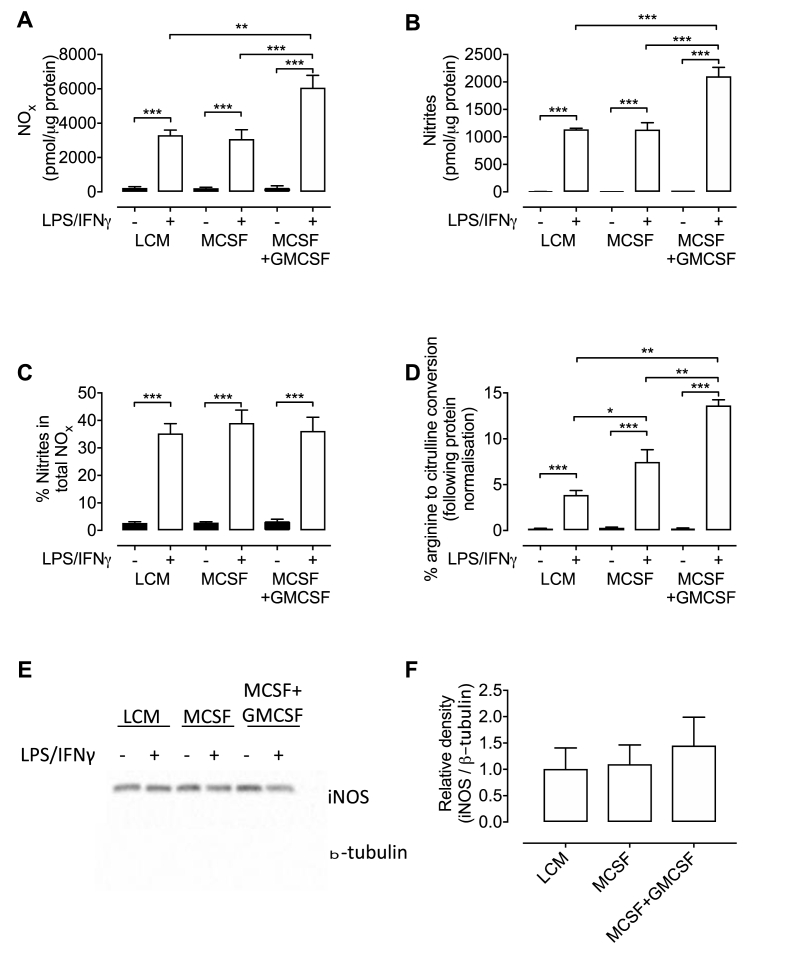
NO generated by NOS may be oxidised to nitrite but also further to form nitrate and so to measure the abundance of both (termed NOx) a NO analyser was used. NOx levels were almost undetectable in unstimulated cells and elevated in all MLPS/IFNγ Gchfl/fl macrophages and were around 2-fold higher in GMCSF treated MLPS/IFNγ cells compared with those cultured in MCSF or LCM only (Fig. 5A). The same pattern was observed when the NO analyser was modified to measure nitrites (Fig. 5B), but the proportion of nitrite remained consistent at 35–39% in all stimulated macrophages regardless of media composition (Fig. 5C).
Fig. 5.
Macrophages cultured in MCSF and treated with GMCSF produce more nitric oxide upon stimulation with LPS/IFNγ. (A) NOx (nitrite + nitrate) and (B) nitrites were measured in media supernatants from LPS/IFNγ Gchfl/fl macrophages grown in different growth media. (C) The proportion of nitrite as a percentage of NOx in media. (D) Nitric oxide synthase activity was determined as a percentage of radiolabelled arginine converted into radiolabelled citrulline. (E) Western blot analysis of iNOS protein using β-tubulin as a loading control. (F) Quantification of the abundance of iNOS protein relative to β-tubulin. All data are presented as means ± SEM. Statistics were calculated using 2-way ANOVA and Tukey's post-test (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001).
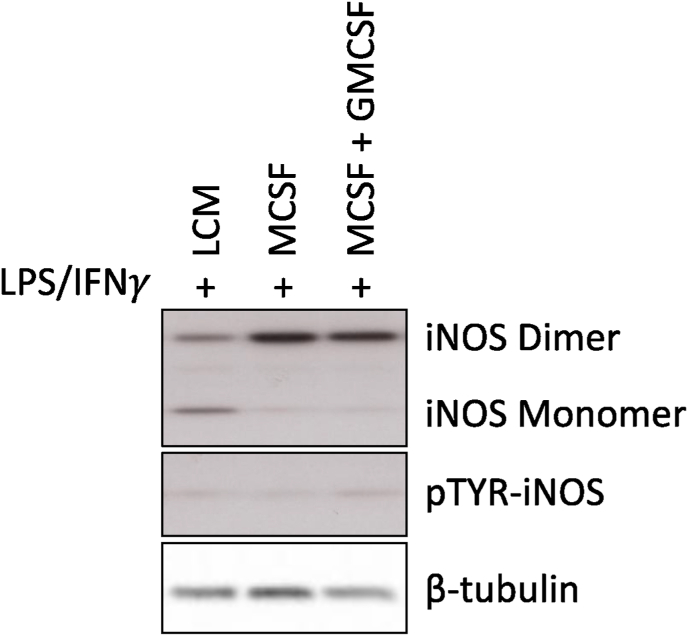
Conversion of arginine into citrulline was used to determine iNOS activity in stimulated Gchfl/fl macrophages, revealing that iNOS was more active in stimulated macrophages grown in MCSF than LCM, but was highest in GMCSF treated cells (Fig. 5D). Western blot analysis showed the abundance of iNOS protein was not significantly different between stimulated macrophages grown in different media types (Fig. 5E and F). Together, these results indicated that increased NO production in GMCSF treated MLPS/IFNγ macrophages did not result from increased iNOS protein abundance, but from increased enzyme activity. This was supported by the increased amount of iNOS dimer, versus monomer that was observed in MCSF and GMCSF containing media, compared to LCM alone. This difference in monomer/dimer ratio is likely to be the mechanism of increased iNOS activity as the phosphorylation status of iNOS remained unchanged (Fig. 6). BH4 levels were not a limiting factor in cells that were not treated with GMCSF, as measurements showed similar levels between stimulated GMCSF treated and untreated MCSF cells, and were significantly elevated in cells grown in LCM (Fig. 7A). GMCSF affected BH4 production in unstimulated MCSF cells, as levels were significantly higher than those measured in MCSF cells not treated with GMCSF (Fig. 7A). Total biopterin levels were similarly affected; however, BH2, biopterin, and the ratio of BH4 to oxidised biopterins were unchanged (Fig. 7B–E).
Fig. 6.
iNOS regulation is dependent on the macrophage culture conditions. Western blots were carried out to determine the monomer/dimer ratio and phosphorylation status of the iNOS enzyme. BMDMs were cultured in either LCM, MCSF alone, or MCSF + GMCSF and harvested for Western blot analysis and determination of Monomer/Dimer ratio as an indicator of iNOS activity. Western Blots are representative images of three separate experiments.
Fig. 7.
Biopterin measurements in macrophages cultured in LCM, MCSF and MCSF + GMCSF media. (A) BH4, (B) BH2 and (C) biopterin were measured in Gchfl/fl macrophages grown in different growth media. (D) Total biopterins and (E) the ratio BH4:BH2+B were calculated. All data are presented as means ± SEM. Statistics were calculated using 2-way ANOVA and Tukey's post-test (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
These results confirmed that BMDM culture using recombinant MCSF and reduced FBS concentrations produced macrophages that induced NO production by Gchfl/fl, but not Gchfl/flTie2cre cells upon stimulation with LPS/IFNγ. GMCSF treatment was also shown to increase NO generation.
3.5. Characterising macrophages grown in MCSF media and treated with GMCSF
Macrophages grown in LCM, MCSF and MCSF + GMCSF were at least 95% positive for the leukocyte cell surface receptor CD45, as well as macrophage markers F4/80, CD11b and MHC II on day 7 of culture (Fig. S7), confirming their purity and maturation into macrophages. Gchfl/flTie2cre macrophages showed the same profile as Gchfl/fl cells grown in MCSF media and treated with GMCSF, showing that knocking out Gch1 did not affect macrophage differentiation according to these markers (Fig. S7).
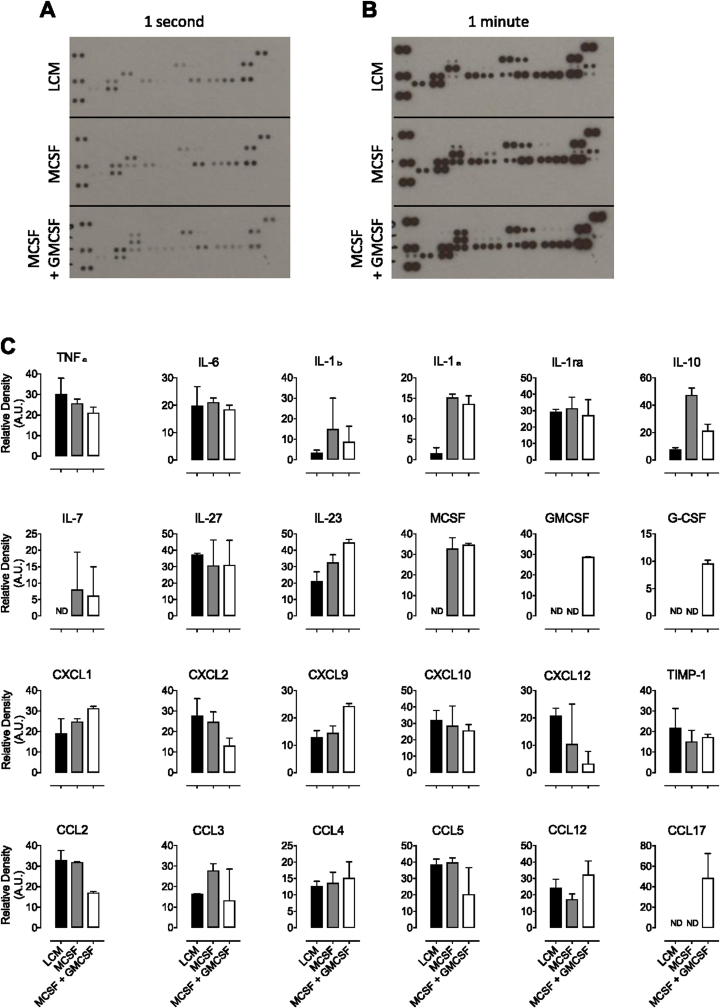
A major part of the macrophage inflammatory response is the production of pro-inflammatory cytokines and a cytokine array was used to confirm production of inflammatory mediators by MLPS/IFNγ Gchfl/fl macrophages grown using the three cell culture protocols (Fig. 8A and B). Unstimulated cells resulted in an absence of signal (only MCSF and GMCSF were visible as these were supplemented into the culture media, as demonstrated in Fig. 9), presumably as these cells either did not produce cytokines, or their levels were below the detection limit of the assay. Quantification of these blots showed secretion of many pro-inflammatory cytokines from MLPS/IFNγ stimulated cells grown using all three types of media (Fig. 8C). In particular, TNFα and IL-6 (commonly used to confirm the phenotype of inflammatory macrophages) were secreted by all cells and the fact that MCSF was only detected in media from MCSF and MCSF + GMCSF cells—and GMCSF only in media from MCSF + GMCSF cells—confirms the robustness and controlled nature of using recombinant CFSs. There were variations such as increased IL-1β and IL-1α in MCSF and MCSF + GMCSF cells compared with LCM and decreased CCL2 in MCSF + GMCSF cells. IL-10—a key anti-inflammatory cytokine—appeared to be elevated in MCSF cells compared with GMCF treated cells or those grown in LCM, which would indicate a more anti-inflammatory phenotype in these cells than the other macrophages.
Fig. 8.
Measurement of cytokines secreted by LPS/IFNγ stimulated macrophages grown in LCM, MCSF and MCSF + GMCSF media. Cytokines were measured in media supernatants from LPS/IFNγ treated Gchfl/fl macrophages grown in different culture media using a cytokine profiler spotted with specific capture antibodies. Following incubation with biotinylated detection antibodies, blots were visualised for varying times using chemiluminescence and representative blots exposed for (A) 1 s and (B) 1 min are shown. Unstimulated cells resulted in an absence of signal, presumably as these cells either did not produce cytokines, or their levels were below the detection limit of the assay. (C) Relative densities of duplicate spots were quantified. Data are presented as means ± range. (n = 3).
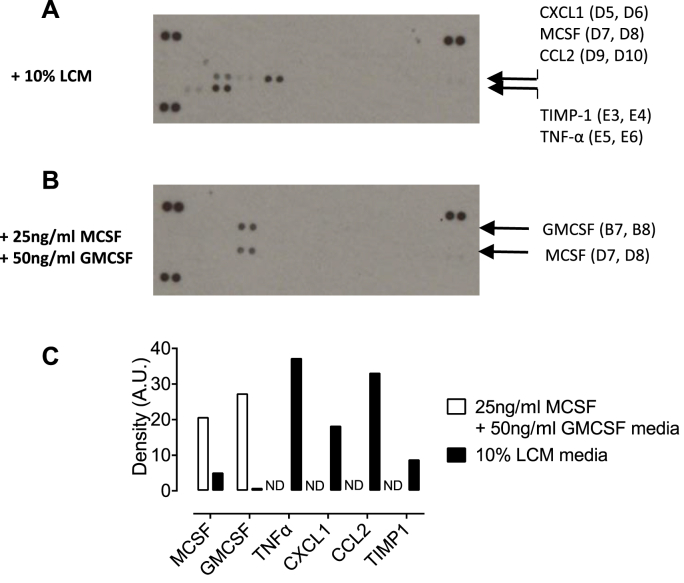
Fig. 9.
Measurement of cytokines in L-cell conditioned media. Cytokines were measured in DMEM F12 media containing 5% FBS and (A) 10% LCM or (B) MCSF and GMCSF using a cytokine profiler spotted with specific capture antibodies. Following incubation with biotinylated detection antibodies, blots were visualised using chemiluminescence. A1, A2, A23, A24, F1 and F2 were Streptavidin-HRP positive control reference spots and F23 and F24 were PBS negative control spots. (C) Relative densities of duplicate spots were quantified. ND – not detectable. (n = 3).
3.6. Defining the composition of cytokines in L-cell conditioned media
LCM contains MCSF and an undefined mixture of constituents present in media from L929 cells. To further define the composition of this media, DMEM F12 media containing 10% LCM was analysed using a cytokine profiler array which revealed that in addition to MCSF, this media contained TNFα, CXCL1, CCL2 and tissue inhibitor of metalloproteinases-1 (TIMP1), whereas MCSF + GMCSF media only contained MCSF and GMCSF (Fig. 9A- C).
3.7. Characterising Gchfl/flTie2cre BMDMs grown in MCSF media and treated with GMCSF
BMDMs grown from Gchfl/flTie2cre mice using the newly optimised protocol using recombinant MCSF, GMCSF treatment, and low FBS concentration were characterised. As expected, there was a small amount of GTPCH in control Gchfl/fl macrophages when unstimulated and stimulation with LPS/IFNγ led to a greater abundance in control cells, whereas GTPCH was absent in Gchfl/flTie2cre macrophages (Fig. 10A). iNOS protein was found in all cells stimulated with LPS/IFNγ regardless of genotype apart from iNOS KO negative controls (Fig. 10A). Biopterin measurements revealed dramatically lower amounts of BH4, BH2 and biopterin in Gchfl/flTie2cre macrophages compared with Gchfl/fl cells (Fig. 10B–D) and this led to significantly lower total biopterin content (Fig. 10E). The ratio of BH4:BH2+biopterin was unchanged between Gchfl/fl and Gchfl/flTie2cre macrophages (Fig. 10F). Analysis of media supernatants revealed that although production of iNOS was induced by LPS/IFNγ in both Gchfl/fl and Gchfl/flTie2cre macrophages, only Gchfl/fl cells produced NO, as indicated by NOx secretion (Fig. 10G). As these results confirmed the expected decreased GTPCH, BH4 and NO generation by Gchfl/flTie2cre macrophages, this model was used for subsequent experiments.
Fig. 10.
Characterisation of Gchfl/flTie2cre and iNOS KO bone marrow derived macrophages grown in media containing recombinant MCSF and treated with GMCSF. (A) Western blot analysis of GTPCH and iNOS in Gchfl/fl and Gchfl/flTie2cre macrophages following overnight stimulation with LPS and IFNγ. Anti-β-tubulin was used as the loading control. (B-F) HPLC was used to quantify (B) BH4, (C) BH2 and (D) biopterin and the results were used to calculate (E) total biopterins (BH4+BH2+biopterin) and (F) the ratio BH4:BH2+biopterin. (G) NOx was measured in media supernatants using an NO analyser. (H-M) All analyses were repeated in iNOS WT and KO mice accordingly. (All data are presented as means ± SEM. Statistics were calculated using 2-way ANOVA and Tukey's post-test (n = 3–6, *P < 0.05, **P < 0.01, ***P < 0.001).
3.8. Characterising iNOS−/- BMDMs grown in MCSF media and treated with GMCSF
Having discovered a NOS-independent role for BH4 in endothelial redox signalling and previous work from our laboratory indicating elevated ROS in Gchfl/flTie2cre macrophages from a NOS-independent source [11], we used Nos2−/− (iNOS KO) BMDMs to distinguish the potential NO-independent effects of BH4 deficiency in activated macrophages. GTPCH was detected in unstimulated WT and iNOS KO macrophages and was increased upon stimulation. Although stimulated WT cells contained iNOS, it was absent in iNOS KO cells (Fig. 10A). WT and iNOS KO macrophages contained comparable levels of BH4, BH2, and biopterin (Fig. 10H–J). BH4, total biopterins, and the ratio of BH4:BH2+biopterin were significantly increased upon stimulation (Fig. 10B, E and F). Analysis of media supernatants showed that, unlike WT cells, iNOS KO macrophages were unable to produce NO, as indicated by NOx secretion (Fig. 10G). Thus, the effects observed in iNOS KO cells are due to loss of NO-producing capacity and not due to any other NO-independent, biopterin-dependent, effects.
4. Discussion
The aim of this study was to address the issue of standardisation of macrophage differentiation, culture, and activation conditions, by developing an optimised and defined protocol that maximised cell health and viability. The primary observation that serum starving during the final stages of the LCM protocol caused cells to detach was easily remedied by maintaining serum in the culture throughout. We then determined that both FBS and LCM contained significant levels of biopterins to supplement cells, leading to changes in NO-redox metabolism. Thus, in order to control the NO-redox environment, we aimed to minimise biopterin supplementation of macrophages, whilst maintaining optimal cell health. Thus, a new protocol using decreased FBS concentrations in combination with recombinant MCSF was optimised. In addition, treating macrophages with GMCSF prior to, and during, stimulation was found to enhance NO generation and was included as described herein. This important point should be noted, as we have shown the significance of NO production in controlling the immunometabolic response to pro-inflammatory stimuli [12]. This new protocol was used to culture BH4-deficient Gchfl/flTie2cre and iNOS KO BMDMs and showed that both were unable to generate NO, proving the suitability of this modified protocol for the investigation of NO-redox metabolism in BMDMs.
Serum starving (or lowering) is commonly used in cell culture systems prior to carrying out certain assays. As an abundant source of growth factors, removal of serum has been viewed as an important step in cell cycle synchronisation and the removal of a variety of extracellular signalling molecules in serum has become a routine way of reducing interfering signals in a range of assays [16,17]. However, serum starving is also known to induce certain signalling pathways, including apoptosis, at time points that vary between cell types and potentially interfere with experimental procedures and interpretation of results [[18], [19], [20]]. In this study, unstimulated macrophages detached after overnight incubation in very low serum media (0.2% BSA), indicative of decreased cell viability, thus proving an inadequate protocol. However, MLPS/IFNγ macrophages remained attached, suggesting a possible resistance to the effects of reduced serum. The inclusion of 10% FBS in media during overnight stimulation maintained adherence of both unstimulated and stimulated cells; however, the unexpected production of NO by stimulated Gchfl/flTie2cre cells unable to produce BH4 led to the discovery of notable BH4 and BH2 levels in the FBS. It is appreciated that FBS contains a vast mixture of undefined molecules that vary between batches and our observation of substantial biopterin levels highlights this important fact. The biopterins in media containing 10% FBS were sufficient to supplement BH4-deficient cells and allowed NO generation. FBS levels were therefore lowered to 5% throughout the 7-day differentiation of bone marrow into macrophages and to 2% during overnight stimulation, as these concentrations minimised biopterins whilst maintaining cell growth and adherence. Bone marrow incubated in 2% FBS media for 7 days were shown to grow slower but with normal morphology. These results demonstrate the need to use a defined cell culture media with optimised serum levels to minimise interference with the experimental model.
MCSF has been used to generate in vitro BMDMs from bone marrow progenitor cells for decades, as it is a cytokine that causes differentiation of haematopoietic stem cells into macrophages and is involved in their proliferation and survival [8,9]. GMCSF in another growth factor commonly used in cell culture systems to generate bone marrow derived dendritic cells; however, evidence suggests that these cells display macrophage markers and are transcriptionally more similar to macrophages than dendritic cells [[21], [22], [23]]. Thus, GMCSF derived macrophages have become another commonly used, but distinct, BMDM model [24,25]. In this study, GMCSF was used to treat macrophages differentiated using the better understood MCSF cell culture system, as GMCSF has been shown to have a ‘priming’ effect leading to enhanced inflammatory cytokine and NO production when added prior to stimulation [21,26,27]. Differences in cytokine levels and increased NO generation were observed in GMCSF treated macrophages compared with untreated cells, and are discussed further below.
The observation that LCM was found to contain significant amounts of BH4 and BH2 has consequences for any studies of NO-redox biology in macrophages. LCM is used as a convenient source of MCSF, which is required for differentiation and maintenance of macrophages, but is undefined and suffers from batch variability [8]. Cytokine analysis revealed that, in addition to MCSF, our current batch of LCM contained TNFα, CXCL1, CCL2, and TIMP1—all of which affect macrophage differentiation and activation. Although it was not measurable on this array, L929 cells have also been shown to produce IFNβ [28]. These molecules are likely to affect differentiation and the ‘unstimulated’ state of BMDMs, as TNFα is a potent inflammatory mediator, whilst CCL2 (also known as monocyte chemoattractant protein-1) plays a key role in macrophage/monocyte migration and infiltration, CXCL1 is a neutrophil chemoattractant, and TIMP-1 is a metalloproteinase inhibitor. To control for these factors, using MCSF is preferable. The concentration of MCSF in culture media was optimised to 25 ng/ml and, although MCSF levels appeared relatively much lower in media containing 10% LCM, the cells in LCM grew at the same rate (not shown). This is possibly a consequence of the additional cytokine cocktail in LCM.
Cytokines were measured to show that LPS/IFNγ stimulation activated an inflammatory response in the new BMDM model. A cytokine blot array showed secretion of the inflammatory cytokines TNFα and members of the interleukin, CCL, and CXCL families by cells grown in all media types. It was of interest to note higher anti-inflammatory IL-10 in cells grown in MCSF alone than either those grown in LCM or treated with GMCSF. Notable IL-10 secretion by MCSF derived BMDMs compared with GMCSF treated cells has been described previously [27]. These results show that all three culture models produce BMDMs secrete pro-inflammatory cytokines upon stimulation, but that un-primed MCSF cells also produce increased amounts of anti-inflammatory IL-10.
In accordance with previous reports [27], GMCSF treatment of MCSF-differentiated macrophages led to higher NO production by stimulated macrophages compared with un-primed cells (LCM- or recombinant MCSF-derived). The mechanisms involved are unexplored, but similar levels of iNOS protein in stimulated macrophages grown in different media, accompanied by increased iNOS activity in GMCSF-treated cells in this study, strongly suggests that up-regulation of enzyme activity is the cause. This is supported by evidence in this study showing an increased amount of iNOS dimer (vs. monomer) in the macrophages cultured in MCSF and GMCSF, compared to LCM. It is also known that iNOS is limited by availability of arginine, NADPH, and BH4, but it is unlikely that either arginine or NADPH were limiting either under LCM conditions, or in MCSF-derived cells not treated with GMCSF.
5. Conclusions
Our defined murine macrophage culturing protocol standardises conditions of cell differentiation, culture, and inflammatory stimulation. It also maximises potential culture timeframes for experiments post stimulation. This method aids reproducibility across the field and allows for better interpretation of results. Moreover, this study emphasises the vital importance of measuring culture media components and their effects.
The principal disadvantage of this method is the greater expense of commercially available recombinant MCSF and GMCSF. However, costs can be mitigated by opting to grow cells in MCSF alone without GMCSF treatment, although the fact that GMCSF priming enhances NO secretion upon inflammatory stimulation should be noted. This ability to boost NO production was considered advantageous for investigating the effects of NO signalling on redox biology and immunometabolism. In this study, we describe a new and optimised protocol to culture BMDMs in more defined media using recombinant CSF proteins to ensure optimal macrophage health and viability, whilst allowing for standardisation of differentiation strategy across the field.
Author Contributions
Concept - JDB, AS, KMC, MJC; experimental work - JDB, AS, EM, MD, SC, JP, AH; wrote manuscript – JB, AS, MJC.
Acknowledgements
This work was supported by the British Heart Foundation Intermediate Fellowship awarded to M.J.C. (FS/14/56/31049), British Heart Foundation Programme grants (RG/17/10/32859 and RG/12/5/29576), Wellcome Trust (090532/Z/09/Z), and the NIH Research (NIHR) Oxford Biomedical Research Centre. The authors would also like to acknowledge support from the BHF Centre of Research Excellence, Oxford (RE/13/1/30181 and RE/18/3/34214).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.niox.2020.04.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Koo S.J., Garg N.J. Metabolic programming of macrophage functions and pathogens control. Redox Biol. 2019;24:101198. doi: 10.1016/j.redox.2019.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton T.A. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front. Immunol. 2014;5:554. doi: 10.3389/fimmu.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto D. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yona S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honold L., Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ. Res. 2018;122(1):113–127. doi: 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue J. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray P.J. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley E.R., Heard P.M. Factors regulating macrophage production and growth - purification and some properties of colony stimulating factor from medium conditioned by mouse L cells. J. Biol. Chem. 1977;252(12):4305–4312. [PubMed] [Google Scholar]
- 9.Wiktorjedrzejczak W. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op op) mouse. Proc. Nat. Acad. Sci. U. S. A. 1990;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tayeh M.A., Marletta M.A. Macrophage oxidation of L-arginine to nitric-oxide, nitrite, and nitrate - tetrahydrobiopterin is required as a cofactor. J. Biol. Chem. 1989;264(33):19654–19658. [PubMed] [Google Scholar]
- 11.McNeill E. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015;79:206–216. doi: 10.1016/j.freeradbiomed.2014.10.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey J.D. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28(1):218–+. doi: 10.1016/j.celrep.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuaiphichai S. Cell-autonomous role of endothelial GTP cyclohydrolase 1 and tetrahydrobiopterin in blood pressure regulation. Hypertension. 2014;64(3):530–+. doi: 10.1161/HYPERTENSIONAHA.114.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabtree M.J. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status INSIGHTS FROM CELLS WITH TET-REGULATED GTP CYCLOHYDROLASE I EXPRESSION. J. Biol. Chem. 2009;284(2):1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree M.J. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling relative importance OF the DENOVO biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009;284(41):28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardee A.B. Restriction point for control of normal animal-cell proliferation. Proc. Nat. Acad. Sci. U. S. A. 1974;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J.C. Role of PI3 kinase/Akt and downstream Bad(ser112) phosphorylation in serum-dependent survival of erythroid cells. Blood. 2003;102(11):834a–835a. [Google Scholar]
- 18.Gonzalez-Polo R.A. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J. Cell Sci. 2005;118(14):3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 19.Messam C.A., Pittman R.N. Asynchrony and commitment to die during apoptosis. Exp. Cell Res. 1998;238(2):389–398. doi: 10.1006/excr.1997.3845. [DOI] [PubMed] [Google Scholar]
- 20.Pirkmajer S., Chibalin A.V. Serum starvation: caveat emptor. Am. J. Physiol. Cell Physiol. 2011;301(2):C272–C279. doi: 10.1152/ajpcell.00091.2011. [DOI] [PubMed] [Google Scholar]
- 21.Lacey D.C. Defining GM-CSF- and macrophage-CSF dependent macrophage responses by in vitro models. J. Immunol. 2012;188(11):5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 22.Mabbott N.A. Meta-analysis of lineage-specific gene expression signatures in mouse leukocyte populations. Immunobiology. 2010;215(9–10):724–736. doi: 10.1016/j.imbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Na Y.R. GM-CSF grown bone marrow derived cells are composed of phenotypically different dendritic cells and macrophages. Mol. Cell. 2016;39(10):734–741. doi: 10.14348/molcells.2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilliams M. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton J.A. GM-CSF-Dependent inflammatory pathways. Front. Immunol. 2019;10:2055. doi: 10.3389/fimmu.2019.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Na Y.R. GM-CSF induces inflammatory macrophages by regulating glycolysis and lipid metabolism. J. Immunol. 2016;197(10):4101–4109. doi: 10.4049/jimmunol.1600745. [DOI] [PubMed] [Google Scholar]
- 27.Sorgi C.A. GM-CSF priming drives bone marrow-derived macrophages to a pro-inflammatory pattern and downmodulates PGE(2) in response to TLR2 ligands. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren M.K., Vogel S.N. Bone marrow-derived macrophages - development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 1985;134(2):982–989. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.