Abstract
Although several recent studies reported that probiotics might be beneficial for allergic rhinitis (AR), the effect of probiotics on AR is not consistent and have not been reproduced between studies. We aimed to determine the efficacy and safety of probiotic NVP-1703, a mixture of Bifidobacterium longum and Lactobacillus plantarum, in subjects with perennial AR. Adult subjects with perennial AR received either NVP-1703 (n = 47) or placebo (n = 48) for four weeks. Total nasal symptom scores (TNSS), rhinitis control assessment test (RCAT), blood eosinophil count, allergen-specific IgE, and immunological parameters in serum and urine were compared at baseline and after four weeks. TNSS changes from baseline at weeks 1, 3, and 4 were significant between the NVP-1703 and placebo groups (p = 0.033, 0.031, and 0.029, respectively). RCAT score showed significant differences between the NVP-1703 and placebo groups (p = 0.049) at week 4. Dermatophagoides farinae-specific IgE levels and serum IL-10 levels were significantly different between the NVP-1703 and placebo groups (p = 0.033 and p = 0.047, respectively). IL-10/IL-4 and IL-10/IL-13 ratios were different between the NVP-1703 and placebo groups at week 4 (p = 0.046 and 0.018, respectively). NVP-1703 treatment reduced urinary prostaglandin F2α and leukotriene E4 levels (p > 0.05). Therefore, NVP-1703 can be treatment option for perennial AR.
Keywords: allergic rhinitis, probiotics, immunoglobulin E, interleukin-10
1. Introduction
Allergic rhinitis (AR) is one of the most common allergic diseases globally [1]. Typical symptoms include nasal itching, sneezing, rhinorrhea, nasal congestion, and/or red, itchy, and watery eyes, which last throughout the year in perennial AR [1]. Immunoglobin E (IgE)-mediated allergic inflammation is the main pathophysiological mechanism of AR and drives T helper 2 (Th2) cell polarized immune reactions [2]. Th2 cells produce interleukin (IL)-4, IL-5, and IL-13, which activate various effector cells involved in allergic inflammation, such as eosinophils and mast cells [1,2]. Th2-derived allergic inflammation is attributed to a functional defect of T regulatory (Treg) cells, which results in decreased production of IL-10 and transforming growth factor (TGF)-β [3,4]. As IL-10 and TGF- β antagonize the biologic function of Th2-cytokines, decreased IL-10 and TGF-β is considered to subsequently potentiate allergic inflammation [5].
Conventional pharmacological treatments of AR include antihistamines, nasal corticosteroids, and leukotriene receptor antagonists, which are effective in controlling symptoms and allergic inflammation [1]. Allergen-specific immunotherapy is a potential disease-modifying, immune-modulatory treatment option that can activate allergen-specific Treg cells and subsequent production of IL-10 and TGF-β [5]. Although allergen immunotherapy is effective in treating AR, it has some limitations, as some patients experienced moderate to severe adverse events during immunotherapy.
Previous clinical trials have reported the effectiveness of probiotics in controlling symptoms and improving quality of life in patients with AR [6,7,8,9]. However, the study protocols of outcome measurements have been mixed: some probiotics improved rhinitis quality of life questionnaire scores in several studies, while they had no effect on rhinitis total symptom scores or symptom medication scores in others [10]. Furthermore, the mechanism of anti-allergic activity of probiotics has been investigated with peripheral blood mononuclear cells (PBMCs) from patients with AR. Bifidobacterium lactis NCC2818 [6], Lactobacillus gasseri A5 [11], Lactobacillus paracasei ST11 [12], and Lactobacillus casei Shirota [13] reduced IL-5, IL-13, and tumor necrosis factor (TNF)-α production from PBMCs of patients with AR, but did not affect serum total IgE, serum allergen-specific IgE, or IL-10 production. In a randomized, open-label crossover study of Lactobacillus johnsonii EM1 in 7–12-year-old children with perennial AR, treatment with EM1 and levocetirizine combined significantly increased serum TGF-β levels compared with levocetirizine alone at week 12 [14]. We previously reported that the noble probiotic NVP-1703, a mixture of Bifidobacterium longum IM55 and Lactobacillus plantarum IM76, mitigated ovalbumin (OVA)- and house dust mite allergen (HDMA)-induced AR in mice, reducing IL-4, IL-5, IL-13, and IgE levels in nasal mucosa, bronchial alveolar lavage fluid, and blood and increasing the level of IL-10 [15,16], suggesting that probiotics may suppress Th2 response by promoting Treg activity.
We conducted a clinical trial to assess the efficacy and safety of NVP-1703 for treatment of perennial AR by evaluating total nasal symptom scores (TNSS) and rhinitis control assessment test (RCAT). We also investigated the effect of NVP-1703 on blood eosinophil count, allergen-specific IgE, immunological parameters (IL-4, IL-5, IL-10, IL-13, and INF-γ), and leukotriene (LT)E4 and prostaglandin (PG)F2α in serum or urine.
2. Materials and Methods
2.1. Subjects
A total of 95 adult subjects (19–65 years old) with perennial AR were recruited from Hallym University Dongtan Sacred Heart Hospital and Chungbuk National University Hospital. The inclusion criteria were: (1) persistent rhinitis symptoms for at least two consecutive years; (2) presence of two or more rhinitis symptom domains (nasal itching, sneezing, rhinorrhea, and nasal congestion) with ≥2 scores without taking medication; and (3) at least one positive response to skin prick tests for five common perennial allergens (Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df), cat, dog, and cockroach). The subjects did not take any drugs or functional foods to relieve nasal symptoms during the run-in period. Additionally, subjects who could not discontinue drugs that might affect AR symptoms during the study period, who received systemic steroids or nasal cromolyn within two weeks or antihistamines within three days of the screening, or who were taking medicine for gastritis, gastric ulcer, or asthma were excluded. Subjects who were taking rhinitis medication were excluded from the study.
All subjects provided written informed consent. The study protocol was approved by the Institutional Ethics Committee of Hallym University Dongtan Sacred Heart Hospital (IRB file no. HDT 2018–01–007) and Chungbuk National University Hospital (CBNUH 2018–01–004). The study was registered at https://cris.nih.go.kr under the identifier KCT0003760.
2.2. Study Products
NVP-1703 is a probiotic mixture of B. longum IM55 and L. plantarum IM76 freeze-dried with maltodextrin. The placebo was made of maltodextrin and manufactured to be indistinguishable in appearance from the NVP-1703 stick pack. According to the instruction of Navipharm Inc. (Suwon, Korea), all study products were produced at a Good Manufacturing Practice-certified manufacturing facility. Both NVP-1703 and placebo were added to 0.5% (w/w) strawberry concentrate powder and 0.5% (w/w) strawberry incense cotton to equalize taste and aroma, and were stored at 2–8 °C.
2.3. Study Design
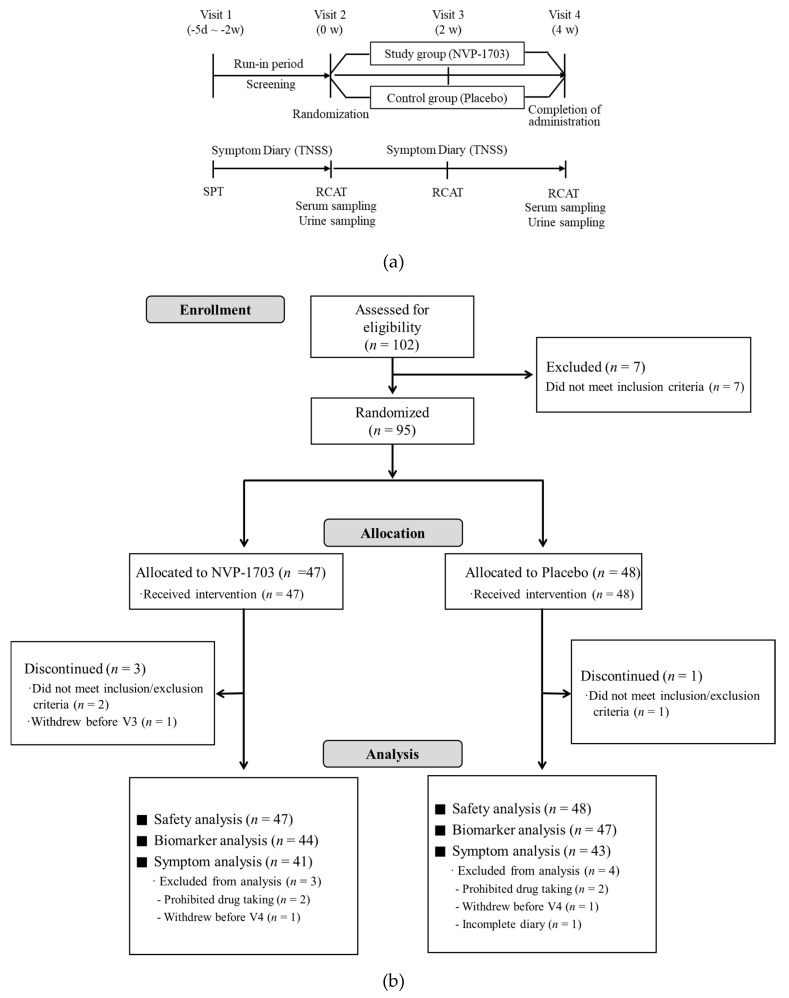
The study was conducted as a multi-center, double-blind, randomized, placebo-controlled clinical trial. The study subjects made a total of four visits during the study (Figure 1). At Visit 1 (screening), demographics, medical history, and medication history were obtained for each participant. Physical examination, laboratory tests, electrocardiogram, and skin prick tests were also performed. After completing a nasal symptom diary during the run-in period of at least 5 days after Visit 1, subjects who met the inclusion criteria were enrolled and assigned to one of the two groups by random numbers generated by the SAS system on Visit 2 (week 0). The randomization ratio for each group was 1:1. NVP-1703 [1.0 × 1010 CFU/day (2 g/stick pack)] or placebo (maltodextrin 2g/stick pack) was taken orally once a day for 4 weeks. After Visit 2, the subjects kept daily nasal symptom diaries while taking the investigational products. Visits 3 (week 2) and 4 (week 4) occurred at 2-week intervals after Visit 2. RCAT scores were assessed at Visit 2 and Visits 3 and 4. Peripheral blood eosinophil counts, serum Dp- or Df-specific IgE, IL-4, IL-5, IL-10, IL-13, interferon (IFN)-γ, and urinary LTE4 and PGF2α were evaluated at Visit 2 and Visit 4. The primary outcome was the difference in change in TNSS between groups before and after the ingestion of NVP-1703, and secondary outcomes were changes in RCAT scores, immunological parameters, blood eosinophil count, and Dp- or Df-specific IgE.
Figure 1.
Description of (a) study protocol and (b) flow diagram of subject disposition. TNSS, total nasal symptom score; SPT, skin prick tests; RCAT, rhinitis control assessment test.
2.4. TNSS
TNSS were expressed as the sum of the scores for the four symptoms (nasal congestion, rhinorrhea, nasal itching, and sneezing). Each symptom was rated on a 4-point scale from 0 (none) through 1 (mild), 2 (moderate), and 3 (severe), and recorded daily in the diary by the subjects during the study. The baseline was the average of the symptom scores recorded during the run-in period, and weekly changes in the symptom scores were analyzed.
2.5. RCAT
RCAT, a tool for assessing the level of rhinitis control, consists of six items: nasal congestion, sneezing, watery eyes, sleep interference, daily activities, and degree of rhinitis control. Each item is assigned 1 to 5 points, and the scores of all items are summed up for evaluation. A higher score indicates better rhinitis control. The subjects were surveyed on Visits 2, 3, and 4, and the RCAT score was evaluated at each visit.
2.6. Skin Prick Test
Allergy skin prick tests were performed with 12 common inhalant allergens, including Dp, Df, mold mixture 1 (Alternaria alternata, Chaetomium globosum, Cladosporium, Helminthosporium sativum), mold mixture 2 (Aspergillus fumigatus, Aspergillus niger), cat, dog, cockroach, tree pollen mixture, birch, grass pollen mixture, ragweed, and mugwort (Allergopharma, Hamburg, Germany or Hollister-Stier, Spokane, WA, USA). Skin prick tests were considered positive if the mean wheal diameters were 3 mm or greater.
2.7. Measurement of Inflammatory Mediators in Serum and Urine
Serum Dp- and Df-specific IgE levels were measured by fluorescent enzyme immunoassay using the ImmunoCAP® system (Thermo Fisher Scientific, Uppsala, Sweden). IL-4, IL-5, IL-10, IL-13, and IFN-γ levels were measured in serum using a Human High Sensitivity T-Cell Magnetic Bead Panel kit (Millipore, Billerica, MA, USA) and Luminex LX200 (Millipore). Urinary LTE4 and PGF2α levels were measured using a commercial ELISA kit (Cayman Chemical, Ann Arbor, MI, USA) and expressed as ng/mmol creatinine. Creatinine level was measured using a CREJ2 kit with Cobas 8000 (Roche Diagnostics, Mannheim, Germany).
2.8. Statistical Analysis
The normality of each group was calculated using the Kolmogorov–Smirnov test. A two-sample t-test was used if the normality was approximated after log transformation of parameters that were not distributed normally. For continuous data among the baseline characteristics, two-sample t-tests were performed to compare between groups, and categorical data were analyzed using chi-square or Fisher’s exact test. A two-sample t-test was used to compare weekly changes in the TNSS and individual symptom scores between groups, and baseline-adjusted ANCOVA was used for the changes in RCAT scores, blood eosinophil count, serum specific IgE, and serum cytokines and urinary LTE4 and PGF2α. Cytokine levels were analyzed after log transformation. Changes in the ratios of IL-10 to IFN-γ, IL-4, IL-5, and IL-13 were analyzed by baseline-adjusted ANCOVA. For each item of data, a paired t-test was employed within groups. All values were calculated as mean ± standard error of the mean (SEM). Statistical analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC, USA), and p values ˂ 0.05 were considered statistically significant.
3. Results
3.1. Study Subject Characteristics
Of the 95 subjects, 44 were included in the NVP-1703 group and 47 in the placebo group. The dropout rates did not differ between the NVP-1703 and placebo groups (6.8% vs. 8.5%, respectively). Finally, 84 subjects who completed the study without protocol violations were evaluated for primary efficacy outcome (Figure 1). Safety analysis included all the enrolled subjects. Analyses of immunological parameters, TNSS, and RCAT scores were conducted for the subjects who completed the study.
Demographics of the study subjects are summarized in Table 1. The mean age was 33.61 ± 1.23 in the NVP-1703 group and 33.49 ± 1.33 in the placebo group. The proportion of males in the NVP-1703 and placebo group were 24.4 and 44.2%, respectively. The duration of AR was 175.41 ± 14.59 months in the NVP-1703 group and 176.09 ± 13.46 months in the placebo group. Most of the study subjects were sensitized to house dust mites (92.86% in the NVP-1703 group; 95.35% in the placebo group). The baseline TNSS was 8.09 ± 0.29 in the NVP-1703 group and 7.55 ± 0.28 in the placebo group, without significant difference.
Table 1.
Baseline characteristics of the study subjects.
| Characteristics | NVP-1703 (n = 41) | Placebo (n = 43) | p-Value |
|---|---|---|---|
| Male [n (%)] | 10 (24.39) | 19 (44.19) | 0.057 |
| Age, years [mean (SEM)] | 33.61 (1.23) | 33.49 (1.33) | 0.947 |
| Duration of allergic rhinitis, months [mean (SEM)] | 175.41 (14.59) | 176.09 (13.46) | 0.923 |
| Family history of allergic diseases [n (%)] | 33 (80.49) | 33 (76.74) | 0.676 |
| Positivity of skin prick test [n (%)] | |||
| D. pteronyssinus | 38 (92.86) | 39 (90.70) | 1.000 |
| D. farinae | 38 (92.68) | 41 (95.35) | 0.672 |
| Mold 1 | 1 (2.44) | 2 (4.65) | 1.000 |
| Mold 2 | 0 | 1 (2.33) | 1.000 |
| Cat | 7 (17.07) | 5 (11.63) | 0.688 |
| Dog | 6 (14.63) | 5 (11.63) | 0.933 |
| Cockroach | 5 (12.20) | 4 (9.30) | 0.735 |
| Tree pollen mixture | 4 (9.76) | 9 (20.93) | 0.265 |
| Birch | 7 (17.07) | 11 (25.58) | 0.494 |
| Grass pollen mixture | 3 (7.32) | 4 (9.30) | 1.000 |
| Ragweed | 4 (9.76) | 3 (6.98) | 0.710 |
| Mugwort | 7 (17.07) | 5 (11.63) | 0.688 |
| Symptom score [mean (SEM)] | |||
| TNSS | 8.09 (0.29) | 7.55 (0.28) | 0.174 |
| Rhinorrhea | 2.29 (0.07) | 2.03 (0.09) | 0.031 |
| Nasal congestion | 2.12 (0.12) | 1.95 (0.12) | 0.292 |
| Sneezing | 1.88 (0.10) | 1.68 (0.12) | 0.203 |
| Itching | 1.81 (0.14) | 1.89 (0.12) | 0.661 |
Data are expressed as mean (SEM). TNSS, total nasal symptom score; Mold 1, Alternaria alternata, Chaetomium globosum, Cladosporium, Helminthosporium sativum; Mold 2, Aspergillus fumigatus, Aspergillus niger.
3.2. Changes from Baseline in TNSS
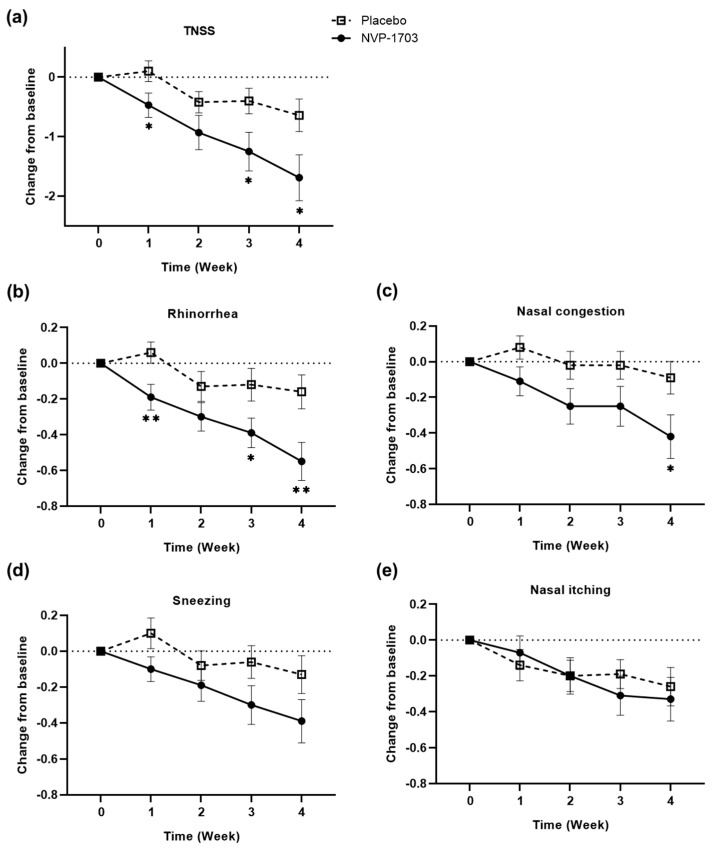
Changes in TNSS and individual nasal symptom scores are summarized in Figure 2 and Table 2. TNSS significantly improved from the baseline to each week (p < 0.05) in the NVP-1703 group. Changes in TNSS from baseline to weeks 1, 3, and 4 were −0.47 ± 0.20, −1.25 ± 0.33, and −1.69 ± 0.39, respectively, in the NVP-1703 group, and 0.10 ± 0.17, −0.40 ± 0.21, and −0.64 ± 0.27, respectively, in the placebo group, resulting in significant difference in change between the groups (p = 0.033, 0.031, and 0.029, respectively).
Figure 2.
Effect of NVP-1703 on (a) TNSS and AR symptoms [(b) rhinorrhea, (c) nasal congestion; (d) sneezing, and (e) nasal itching] scores. Scores are shown as weekly mean change in scores from the baseline for total and individual nasal symptoms during the study period. (*) indicates a statistically significant difference between the two groups (* p < 0.05, ** p < 0.01).
Table 2.
Changes in TNSS and individual symptom scores during the study period.
| Parameter | Group | Symptom Score | ||||
|---|---|---|---|---|---|---|
| 0 w | 1 w | 2 w | 3 w | 4 w | ||
| TNSS | NVP-1703 | 8.09 (0.29) | 7.62 (0.29) * | 7.16 (0.35) *** | 6.84 (0.37) *** | 6.40 (0.41) *** |
| Placebo | 7.55 (0.28) | 7.65 (0.31) | 7.13 (0.33) * | 7.15 (0.34) | 6.90 (0.36) * | |
| p-Value † | 0.033 | 0.135 | 0.031 | 0.029 | ||
| Rhinorrhea | NVP-1703 | 2.29 (0.07) | 2.09 (0.09) * | 1.99 (0.09) *** | 1.90 (0.09) *** | 1.73 (0.11) *** |
| Placebo | 2.03 (0.09) | 2.10 (0.11) | 1.90 (0.11) | 1.91 (0.11) | 1.87 (0.11) | |
| p-Value † | 0.007 | 0.149 | 0.035 | 0.007 | ||
| Nasal congestion | NVP-1703 | 2.12 (0.12) | 2.02 (0.11) | 1.87 (0.12) * | 1.87 (0.12) * | 1.71 (0.12) ** |
| Placebo | 1.95 (0.12) | 2.03 (0.12) | 1.93 (0.11) | 1.93 (0.11) | 1.85 (0.12) | |
| p-Value † | 0.077 | 0.070 | 0.097 | 0.034 | ||
| Sneezing | NVP-1703 | 1.88 (0.10) | 1.77 (0.09) | 1.69 (0.10) * | 1.57 (0.12) ** | 1.49 (0.13) ** |
| Placebo | 1.68 (0.12) | 1.78 (0.10) | 1.60 (0.11) | 1.62 (0.12) | 1.55 (0.12) | |
| p-Value † | 0.069 | 0.374 | 0.087 | 0.103 | ||
| Itching | NVP-1703 | 1.81 (0.14) | 1.74 (0.11) | 1.61 (0.12) | 1.50 (0.13) ** | 1.47 (0.13) ** |
| Placebo | 1.89 (0.12) | 1.75 (0.11) | 1.69 (0.11) * | 1.69 (0.11) * | 1.63 (0.12) * | |
| p-Value † | 0.597 | 0.955 | 0.394 | 0.641 | ||
Data are expressed as mean (SEM) for TNSS and individual symptoms. † p-value for change in symptom score from baseline between NVP-1703 and placebo groups. * p < 0.05, ** p < 0.01, *** p < 0.001 for change in symptom score from baseline within groups. TNSS, total nasal symptom score.
Among individual symptoms, the scores for rhinorrhea were the most markedly reduced from baseline by −0.19 ± 0.07, −0.39 ± 0.08, and −0.55 ± 0.11 at weeks 1, 3, and 4, respectively, in the NVP-1703 group, and 0.06 ± 0.06, −0.12 ± 0.09, and −0.16 ± 0.09, respectively, in the placebo group, showing significant differences between the groups (p = 0.007, 0.035, and 0.007, respectively). Furthermore, NVP-1703 treatment significantly reduced the scores for nasal congestion at weeks 2, 3, and 4 compared with those at baseline (p = 0.017, p = 0.031, and p = 0.001, respectively), and a significant difference between the groups was shown at week 4 (−0.42 ± 0.78 in NVP-1703 vs. −0.09 ± 0.60 in placebo, p = 0.034). For sneezing and nasal itching scores, there were no significant differences between the groups (p > 0.05 each). However, significant improvement from the baseline was observed in the NVP-1703 group at weeks 3 and 4, with changes of −0.30 ± 0.11 and −0.39 ± 0.12, respectively, in sneezing, and −0.31 ± 0.11 and −0.33 ± 0.12, respectively, in itching (p < 0.01 each).
3.3. Changes from Baseline in RCAT
Total RCAT scores increased from baseline by 1.95 ± 0.58 and 2.00 ± 0.54 in the NVP-1703 and placebo groups, respectively, at week 2 (p > 0.05), while at week 4, the scores increased from baseline by 3.83 ± 0.73 and 2.21 ± 0.67, respectively, showing a significant difference in change between the groups (p = 0.049). Among the sub-items, watery eyes showed a significant improvement at week 4 (0.80 ± 0.16 with NVP-1703 vs. 0.16 ± 0.19 with placebo, p = 0.018) and sleep disturbances tended to improve (0.66 ± 0.13 with NVP-1703 vs. 0.33 ± 0.18 with placebo, p = 0.051) (Table 3).
Table 3.
Changes in RCAT and individual symptom scores during the study period.
| Parameter | Group | Symptom Score | ||
|---|---|---|---|---|
| 0 w | 2 w | 4 w | ||
| Total | NVP-1703 | 15.44 (0.50) | 17.39 (0.62) ** | 19.27 (0.70) *** |
| Placebo | 15.26 (0.44) | 17.26 (0.51) *** | 17.47 (0.56) ** | |
| p-Value † | 0.957 | 0.049 | ||
| Nasal congestion | NVP-1703 | 1.95 (0.14) | 2.20 (0.15) | 2.63 (0.16) *** |
| Placebo | 2.00 (0.11) | 2.33 (0.12) ** | 2.35 (0.13) ** | |
| p-Value † | 0.523 | 0.086 | ||
| Sneezing | NVP-1703 | 2.27 (0.12) | 2.56 (0.12) ** | 2.83 (0.15) ** |
| Placebo | 2.19 (0.13) | 2.63 (0.13) ** | 2.51 (0.14) | |
| p-Value † | 0.483 | 0.143 | ||
| Watery eyes | NVP-1703 | 2.68 (0.15) | 3.05 (0.14) * | 3.49 (0.16) *** |
| Placebo | 2.88 (0.15) | 3.12 (0.16) | 3.05 (0.17) | |
| p-Value † | 0.933 | 0.018 | ||
| Interference of Sleep |
NVP-1703 | 3.07 (0.12) | 3.29 (0.16) | 3.73 (0.14) *** |
| Placebo | 3.02 (0.14) | 3.26 (0.12) | 3.35 (0.14) | |
| p-Value † | 0.960 | 0.051 | ||
| Daily activities | NVP-1703 | 3.15 (0.15) | 3.39 (0.15) | 3.59 (0.18) * |
| Placebo | 2.91 (0.12) | 3.28 (0.13) * | 3.42 (0.12) ** | |
| p-Value † | 0.955 | 0.679 | ||
| Degree of rhinitis control |
NVP-1703 | 2.32 (0.10) | 2.90 (0.12) *** | 3.00 (0.13) *** |
| Placebo | 2.26 (0.09) | 2.65 (0.12) * | 2.79 (0.12) ** | |
| p-Value † | 0.161 | 0.237 | ||
Data are expressed as mean (SEM) of symptom score. † p-value for change in symptom score from baseline between NVP-1703 and placebo groups. * p < 0.05, ** p < 0.01, *** p < 0.001 for change in symptom score from baseline within groups. RCAT, rhinitis control assessment test.
3.4. Effect of NVP-1703 on Blood Eosinophil Count and Serum Specific IgE
There was no significant difference in baseline blood eosinophil counts between the two groups. Although both groups showed significant decreases in blood eosinophil counts at four weeks (p < 0.001), there was no significant difference in the changes in counts between the two groups (p > 0.05).
The baseline levels of Dp-specific IgE were 7.61 ± 1.14 and 8.92 ± 1.18 KU/L in the NVP-1703 and placebo groups, respectively (p > 0.05), and those of Df-specific IgE were 14.75 ± 2.17 and 16.86 ± 2.20 KU/L, respectively (p > 0.05). The serum levels of Df-specific IgE decreased to 12.83 ± 1.78 KU/L at week 4 in the NVP-1703 group (p = 0.008), which was significant compared with the change in the placebo group (p = 0.033) (Table 4). The serum level of Dp-specific IgE decreased to 7.15 ± 1.04 KU/L at week 4 in the NVP-1703 group (p > 0.05 compared with baseline), but there was no significant difference compared with the placebo group.
Table 4.
Changes in immunological parameters.
| Parameter | NVP-1703 | Placebo | |||
|---|---|---|---|---|---|
| 0 w | 4 w | 0 w | 4 w | p-Value † | |
| Blood eosinophil count (/μL) | 175.42 (16.21) | 96.82 (25.24) *** | 178.24 (17.14) | 56.61 (14.35) *** | 0.116 |
| D. pteronyssinus-specific IgE (KU/L) | 7.61 (1.14) | 7.15 (1.04) | 8.92 (1.18) | 8.93 (1.22) | 0.331 |
| D. farinae-specific IgE (KU/L) | 14.75 (2.17) | 12.83 (1.78) ** | 16.86 (2.20) | 17.19 (2.35) | 0.033 |
| IL-4 (log pg/mL) | 2.29 (0.12) | 2.31 (0.12) | 2.28 (0.12) | 2.28 (0.12) | 0.940 |
| IL-5 (log pg/mL) | 1.65 (0.12) | 1.59 (0.14) | 1.36 (0.12) | 1.34 (0.11) | 0.605 |
| IL-10 (log pg/mL) | 2.22 (0.11) | 2.16 (0.11) | 2.23 (0.09) | 1.97 (0.11)** | 0.047 |
| IL-13 (log pg/mL) | 1.49 (0.10) | 1.47 (0.12) | 1.55 (0.10) | 1.51 (0.10) | 0.936 |
| IFN-γ (log pg/mL) | 2.99 (0.08) | 2.98 (0.09) | 2.85 (0.08) | 2.84 (0.08) | 0.488 |
| IL-10/IL-4 | 1.21 (0.15) | 1.17 (0.16) | 1.42 (0.28) | 0.90 (0.09) | 0.046 |
| IL-10/IL-5 | 2.15 (0.24) | 2.27 (0.27) | 3.30 (0.47) | 2.27 (0.22) * | 0.088 |
| IL-10/IL-13 | 2.73 (0.42) | 2.77 (0.44) | 2.71 (0.41) | 1.95 (0.17) | 0.018 |
| IL-10/IFN-γ | 0.53 (0.05) | 0.52 (0.05) | 0.64 (0.08) | 0.49 (0.04) | 0.177 |
| Urinary LTE4 (ng/mmol Cr) | 601.06 (32.53) | 585.78 (31.24) | 546.95 (32.18) | 541.23 (32.31) | 0.701 |
| Urinary PGF2α (ng/mmol Cr) | 77.23 (7.66) | 69.90 (7.90) | 74.19 (6.82) | 72.13 (6.27) | 0.809 |
Data are expressed as mean (SEM). Cytokine levels were analyzed after logarithmic transformation. † p-value for change from baseline at 4 weeks between NVP-1703 and placebo groups. * p < 0.05, ** p < 0.01, *** p < 0.001 for change from baseline at 4 weeks within groups.
3.5. Effect of NVP-1703 on Serum Cytokines
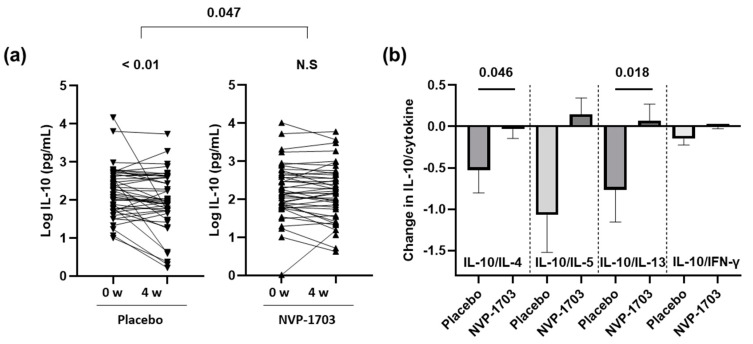
The serum levels of IL-4, IL-5, IL-13 and IFN-γ were unchanged after four weeks of treatment with NVP-1703, and there were no statistically significant differences compared with the placebo group. However, the serum level of IL-10 in the NVP-1703 group was maintained, while it was significantly decreased in the placebo group at week 4 (p < 0.01), showing significant differences in changes between the two groups (p = 0.047). Furthermore, in the NVP-1703 group, the ratio of IL-10 to IL-4 was maintained, with a change of −0.03 ± 0.11 from baseline at week 4, while it decreased by 0.52 ± 0.28 in the placebo group with statistical significance (p = 0.046). The ratio of IL-10 to IL-13 was also significantly decreased in the placebo group (0.76 ± 0.39) compared with the NVP-1703 group (0.07 ± 0.20, p = 0.018) (Figure 3).
Figure 3.
Effects of NVP-1703 on blood levels of IL-10 expression as well as ratios of IL-10 to IL4, IL-5, IL-13, and IFN-γ. (a) IL-10 expression at each time point (baseline, 4 weeks) after log transformation; (b) change in the ratios of IL-10 to IL-4, IL-5, IL-13, and IFN- γ from baseline at week 4.
The ratio of IL-10 to IL-5 increased by 0.15 ± 0.20 in the NVP-1703 group (p > 0.05), while it decreased by 1.05 ± 0.44 in the placebo group (p < 0.05). However, there was no significant difference in change between the groups (p = 0.088) (Table 4).
3.6. Effect of NVP-1703 on Urinary LTE4 and PGF2α
Baseline urinary levels of LTE4 were 601.06 ± 32.54 and 546.95 ± 32.18 ng/mmol creatinine in the NVP-1703 and placebo groups, respectively, and those of PGE2α were 77.23 ± 7.66 and 74.19 ± 6.82 ng/mmol creatinine, respectively, without statistical significance between the groups (p > 0.05). Although urinary LTE4 and PGE2α tended to decrease four weeks after NVP-1703 treatment initiation, compared with the changes in LTE4 and PGF2α before and after NVP-1703 treatment, no significant difference in change in urinary levels of LTE4 and PGF2α (−22.42 ± 33.47 and −5.38 ± 8.50, respectively) was observed between the groups (p > 0.05) (Table 4).
3.7. Safety and Compliance
Safety and tolerability (subject-reported symptoms, vital signs, and laboratory tests) were monitored at every visit during the study. No significant difference in any of the vital signs, laboratory tests, or electrocardiograms was observed between the groups. Six adverse events were documented during the study: abdominal pain (one case, 2.1%), muscle strain (one case, 2.1%), nasopharyngitis (one case, 2.1%), and elevation of alanine aminotransferase and aspartate aminotransferase (one case, 2.1%) in the NVP-1703 group, and abdominal pain (one case, 2.1%) and dyspepsia (one case, 2.1%) in the placebo group. No adverse event was regarded to be associated with the study product or placebo. No serious adverse events or dropouts due to adverse events occurred in either the NVP-1703 or placebo group. Compliance with ingestion was 99.87% ± 1.34% and 96.73% ± 1.73% in the NVP-1703 and placebo groups, respectively, without significant difference.
4. Discussion
In the present study, NVP-1703 significantly improved TNSS and RCAT scores in subjects with perennial AR. NVP-1703 was especially effective in reducing rhinorrhea, nasal congestion, watery eyes, and sleep disturbances. NVP-1703 also significantly improved allergic immunologic profiles such as Df-specific IgE, IL-10, IL-10/IL-4 ratio, and IL-10/IL-13 ratio. These results suggest that NVP-1703 regulates IL-10, one of the key immunological markers associated with AR, thereby improving symptoms of perennial AR.
Several studies have evaluated the efficacy of probiotics in AR and reported significant improvement in AR symptoms. However, these evaluations of the efficacies of probiotics were limited, because the study subjects continued other medications for AR [7,11,17,18,19,20,21,22,23]. Furthermore, most of the previous studies only used the Quality of Life assessment rather than TNSS to assess efficacy [9,22,23,24,25]. The patient-rated, instantaneous, and reflective TNSS is generally preferred as a primary measure of efficacy at least four weeks in AR trials for drug development [26], because the quality of life assessment may insufficiently or inaccurately evaluate the efficacies of anti-AR drugs and probiotics [10]. In the present study, we independently evaluated the efficacy of NPV-1703 without anti-AR medications and performed both TNSS and quality of life assessments. NVP-1703 showed significant improvement in TNSS as well as RCAT scores. To our knowledge, NVP-1703 is the first probiotic mixture that has been proven to be effective in patients with symptomatic AR without any additional medication or the need for rescue medication. Our results contribute reliable and powerful evidence for the efficacy of probiotics in AR treatment.
Previous clinical trials for AR have evaluated the efficacies of single probiotic strains reported to be effective in preliminary animal studies, including B. lactis, B. longum, L. plantatrum, and L. paracasei. However, the effects of a single probiotic strain on AR have not yet been sufficiently evaluated and not consistent between studies. Recently, there have been attempts to mix probiotic strains that have been shown to have anti-allergic effects in animal studies [9,21]. These studies combined different species from within the same genus [9,21]. On this point, our study has some distinct advantages over previous studies. NVP-1703 is a probiotic mixture of B. longum IM55 (from the human gut bacteria collection) as a T-cell regulator and L. plantarum IM76 (from the fermented food bacteria collection) as a macrophage NF-κB inhibitor. We previously reported that NPV-1703 regulates innate and adaptive immunity in animal and in vitro studies [15,16]. In the present study, NVP-1703 treatment significantly improved TNSS and RCAT scores by increasing levels of IL-10, which corresponds to the anti-allergic effect of B. longum IM55 and L. plantarum IM76.
The improvement in clinical outcomes in the NVP-1703 group seems to be related to the improvement in anti-allergic immunologic profiles. NVP-1703 treatment significantly increased serum IL-10 levels. The blood IL-4, IL-5, and IL-13 levels were not significantly changed in the NVP-1703 group; however, the ratios of IL-10/IL-4, IL-10/IL-5, and IL-10/IL-13 were significantly increased in the NVP-1703 group. IL-10 is an important counter-regulatory cytokine required to control allergic inflammation. IL-10-mediated regulation of T-cell responses directly regulates Th2 cells and subsequently decreases IL-4, IL-5, and IL-13 levels in the blood [4]. The administration of NVP-1703 significantly suppressed the expression of IL-4 and IL-5 and differentiation of T-cells into Th2 cells in mice with OVA- and HDMA-induced AR, while the expression of IL-10 and differentiation of T-cells into Treg cells increased [15,16]. These results suggest that NVP-1703 suppresses the expression of Th2 cytokines IL-4, IL-5, and IL-13 by inducing IL-10 expression and Treg cell differentiation.
NVP-1703 treatment also reduced serum Df-specific IgE levels in individuals with AR. IL-10 indirectly facilitates IL-4-induced IgG4 production in human B cells, suppresses IgE, and inhibits IgE-induced activation of human mast cells directly in vitro [27,28]. The induction of IL-10 expression suppresses the expression of IgE receptors in mast cells [29]. Therefore, in this study, IL-10 expression induced by NVP-1703 may have suppressed the production of allergen-specific IgE.
The changes in cytokine profiles and allergen-specific IgE levels with NVP-1703 treatment are similar to those with allergen immunotherapy, which is a disease-modifying, immune-modulatory treatment [5]. Thus, regular ingestion of NVP-1703 may also have long term effects for allergic rhinitis, which requires further studies.
The levels of LTE4 and PGF2α in the urine tended to decrease after NVP-1703 treatment compared with the placebo at week 4. Lipid mediators such as cysteinyl LTs and PGD2, which are released from eosinophils and mast cells in the early phase of allergic inflammation, play important roles in the pathogenesis of AR [30,31]. These mediators increase venous engorgement and tissue swelling/edema, leading to concomitant rhinorrhea and nasal congestion [30,31]. Their metabolites, urinary LTE4 and PGF2α, are useful biomarkers of eosinophil or mast cell activation [32]. Leukotriene receptor antagonists, a major type of anti-allergic medicine, exert their action by blocking receptors for cysteinyl LTs [33]. Although our study did not show statistically significant differences, these results suggest that NPV-1703 may be a safe potential anti-allergic medication to treat AR, contributing to an improvement in symptoms, including nasal discharge and nasal congestion.
A major limitation exists in our study. At first, the duration of probiotic intake was four weeks, which was relatively shorter than the usual eight weeks in previous studies. However, we observed significant symptom improvement at week 4, along with improvement in cytokine profiles, which may be due to the strong effects of the probiotic mixture. In addition, the beneficial effects of the probiotics were observed at least four weeks after continuous administration [34]. To elucidate the mechanisms of their anti-allergic effects, further studies with long-term administration in larger populations will be required. Secondly, we only enrolled the participants with mild to moderate persistent rhinitis who could endure the symptoms without medications. Even though NVP-1703 alone was significantly ameliorate the rhinitis symptoms in our study, we cannot say that NVP-1703 is a better controller than other conventional medications for AR. Further study will be needed to evaluate the comparative or add-on effect of NVP-1703 to other conventional medications.
5. Conclusions
In conclusion, NVP-1703 improves AR symptoms, which results from IL-10 expression in Treg cells. NVP-1703 may reduce the secretion of IL-4, IL-5, IL-13, allergen-specific IgE, PGD2, and cysteinyl LTs by suppressing the activation of mast cells, eosinophils, Th2 cells, and B cells through the induction of Treg cells. However, further studies are required to elucidate this mechanism.
Author Contributions
J.-H.C., H.-R.K., and S.-W.H. contributed study conceptualization; J.-H.C., and D.-H.K. contributed methodology; J.-H.C., and S.-W.H. performed validation; M.-G.K. and S.-W.H. conducted data analysis; M.-G.K. and S.-W.H. wrote and prepared the original draft; J.-H.C., contributed writing, reviewing, and editing; J.-H.C., and D.-H.K. provided supervision; S.-W.H. oversaw funding acquisition; M.-G.K., J.-H.C., and S.-J.H. enrolled subjects. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Medical Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2017M3A9G2049280) and Hallym University Research Fund 2017 (HURF-2017-76).
Conflicts of Interest
Han SW is employee of Navipharm Inc. Other authors declare that there are no potential conflicts of interest.
References
- 1.Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., Zuberbier T., Baena-Cagnani C.E., Canonica G.W., van Weel C., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Baraniuk J.N. Pathogenesis of allergic rhinitis. J. Allergy Clin. Immunol. 1997;99:S763–S772. doi: 10.1016/S0091-6749(97)70125-8. [DOI] [PubMed] [Google Scholar]
- 3.Akdis M., Verhagen J., Taylor A., Karamloo F., Karagiannidis C., Crameri R., Thunberg S., Deniz G., Valenta R., Fiebig H., et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coomes S.M., Kannan Y., Pelly V.S., Entwistle L.J., Guidi R., Perez-Lloret J., Nikolov N., Muller W., Wilson M.S. CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10:150–161. doi: 10.1038/mi.2016.47. [DOI] [PubMed] [Google Scholar]
- 5.Głobińska A., Boonpiyathad T., Satitsuksanoa P., Kleuskens M., van de Veen W., Sokolowska M., Akdis M. Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Ann. Allergy Asthma Immunol. 2018;121:306–312. doi: 10.1016/j.anai.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Singh A., Hacini-Rachinel F., Gosoniu M.L., Bourdeau T., Holvoet S., Doucet-Ladeveze R., Beaumont M., Mercenier A., Nutten S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: An exploratory, randomized, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2013;67:161–167. doi: 10.1038/ejcn.2012.197. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y., Nakamura F., Kanzato H., Sawada D., Hirata H., Nishimura A., Kajimoto O., Fujiwara S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy Sci. 2005;88:527–533. doi: 10.3168/jds.S0022-0302(05)72714-4. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y., Li A., Peng Y., Li A., Yu L., Qin G. The role of probiotics in prevention and treatment for patients with allergic rhinitis: A systematic review. Am. J. Rhinol. Allergy. 2015;29:292–298. doi: 10.2500/ajra.2015.29.4192. [DOI] [PubMed] [Google Scholar]
- 9.Miraglia Del Giudice M., Indolfi C., Capasso M., Maiello N., Decimo F., Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017;43:25. doi: 10.1186/s13052-017-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajac A.E., Adams A.S., Turner J.H. In a systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int. Forum Allergy Rhinol. 2015;5:524–532. doi: 10.1002/alr.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.S., Jan R.L., Lin Y.L., Chen H.H., Wang J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010;45:1111–1120. doi: 10.1002/ppul.21296. [DOI] [PubMed] [Google Scholar]
- 12.Wassenberg J., Nutten S., Audran R., Barbier N., Aubert V., Moulin J., Mercenier A., Spertini F. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin. Exp. Allergy. 2011;41:565–573. doi: 10.1111/j.1365-2222.2011.03695.x. [DOI] [PubMed] [Google Scholar]
- 13.Ivory K., Chambers S.J., Pin C., Prieto E., Arques J.L., Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin. Exp. Allergy. 2008;38:1282–1289. doi: 10.1111/j.1365-2222.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 14.Lue K.H., Sun H.L., Lu K.H., Ku M.S., Sheu J.N., Chan C.H., Wang Y.H. A trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7–12 years. Int. J. Pediatr. Otorhinolaryngol. 2012;76:994–1001. doi: 10.1016/j.ijporl.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim W.G., Kang G.D., Kim H.I., Han M.J., Kim D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes. 2019;10:55–67. doi: 10.3920/BM2017.0146. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.-K., Kim J.-Y., Kim H.I., Han M.J., Kim D.H. Bifidobacterium longum and Lactobacillus plantarum alleviate house dust mite allergen-induced allergic rhinitis by regulating IL-4, IL-5, and IL-10 expression. Food Agric. Immunol. 2019;30:581–593. doi: 10.1080/09540105.2019.1608161. [DOI] [Google Scholar]
- 17.Lin T.Y., Chen C.J., Chen L.K., Wen S.H., Jan R.H. Effect of probiotics on allergic rhinitis in Df, Dp or dust-sensitive children: A randomized double blind controlled trial. Indian Pediatr. 2013;50:209–213. doi: 10.1007/s13312-013-0068-2. [DOI] [PubMed] [Google Scholar]
- 18.Yonekura S., Okamoto Y., Okawa T., Hisamitsu M., Chazono H., Kobayashi K., Sakurai D., Horiguchi S., Hanazawa T. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30:397–405. doi: 10.2500/aap.2009.30.3256. [DOI] [PubMed] [Google Scholar]
- 19.Nagata Y., Yoshida M., Kitazawa H., Araki E., Gomyo T. Improvements in seasonal allergic disease with Lactobacillus plantarum No. 14. Biosci. Biotechnol. Biochem. 2010;74:1869–1877. doi: 10.1271/bbb.100270. [DOI] [PubMed] [Google Scholar]
- 20.Jan R.-H., Chen C.-J., Chen L.-K., Wen S.-H., Lin T.-Y. Is the effect of probiotics on allergic rhinitis confined to Dermatophagoides farinae, Dermatophagoides pteronyssinus, or dust-sensitive children? A randomized prospective double-blind controlled trial. Tzu Chi Med. J. 2011;23:51–54. doi: 10.1016/j.tcmj.2011.05.003. [DOI] [Google Scholar]
- 21.Kawase M., He F., Kubota A., Hiramatsu M., Saito H., Ishii T., Yasueda H., Akiyama K. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int. J. Food Microbiol. 2009;128:429–434. doi: 10.1016/j.ijfoodmicro.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Costa D.J., Marteau P., Amouyal M., Poulsen L.K., Hamelmann E., Cazaubiel M., Housez B., Leuillet S., Stavnsbjerg M., Molimard P., et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: A double-blind, randomized, placebo-controlled trial (GA2LEN Study) Eur. J. Clin. Nutr. 2014;68:602–607. doi: 10.1038/ejcn.2014.13. [DOI] [PubMed] [Google Scholar]
- 23.Lin W.Y., Fu L.S., Lin H.K., Shen C.Y., Chen Y.J. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6–13 years old) with perennial allergic rhinitis: A 12-week, double-blind, randomized, placebo-controlled study. Pediatr. Neonatol. 2014;55:181–188. doi: 10.1016/j.pedneo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang M.F., Lin H.C., Wang Y.Y., Hsu C.H. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr. Allergy Immunol. 2004;15:152–158. doi: 10.1111/j.1399-3038.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 25.Peng G.C., Hsu C.H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr. Allergy Immunol. 2005;16:433–438. doi: 10.1111/j.1399-3038.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Allergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry. Draft Guidance. [(accessed on 4 April 2020)];2018 Available online: https://www.fda.gov/downloads/drugs/guidances/ucm071293.pdf.
- 27.Lin A.A., Freeman A.F., Nutman T.B. IL-10 Indirectly Downregulates IL-4-Induced IgE Production by Human B Cells. Immunohorizons. 2018;2:398–406. doi: 10.4049/immunohorizons.1800076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royer B., Varadaradjalou S., Saas P., Guillosson J.J., Kantelip J.P., Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin. Exp. Allergy. 2001;31:694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy Norton S., Barnstein B., Brenzovich J., Bailey D.P., Kashyap M., Speiran K., Ford J., Conrad D., Watowich S., Moralle M.R., et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J. Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 30.Peters-Golden M., Gleason M.M., Togias A. Cysteinyl leukotrienes: Multi-functional mediators in allergic rhinitis. Clin. Exp. Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano M., Fujiwara T., Sugata Y., Gotoh D., Masaoka Y., Sogo M., Tanimoto W., Yamamoto M., Matsumoto R., Eguchi N., et al. Presence and characterization of prostaglandin D2-related molecules in nasal mucosa of patients with allergic rhinitis. Am. J. Rhinol. 2006;20:342–348. doi: 10.2500/ajr.2006.20.2865. [DOI] [PubMed] [Google Scholar]
- 32.Kumlin M. Measurement of leukotrienes in humans. Am. J. Respir. Crit. Care Med. 2000;161:S102–S106. doi: 10.1164/ajrccm.161.supplement_1.ltta-20. [DOI] [PubMed] [Google Scholar]
- 33.Wilson A.M., O’Byrne P.M., Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: A systematic review and meta-analysis. Am. J. Med. 2004;116:338–344. doi: 10.1016/j.amjmed.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Güvenç I.A., Muluk N.B., Mutlu F.Ş., Eşki E., Altıntoprak N., Oktemer T., Cingi C. Do Probiotics have a role in the Treatment of Allergic Rhinitis? A Comprehensive Systematic Review and Meta-analysis. Am. J. Rhinol. Allergy. 2016;30:e157–e175. doi: 10.2500/ajra.2016.30.4354. [DOI] [PubMed] [Google Scholar]