Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China and rapidly spread worldwide, with a vast majority of confirmed cases presenting with respiratory symptoms. Potential neurological manifestations and their pathophysiological mechanisms have not been thoroughly established. In this narrative review, we sought to present the neurological manifestations associated with coronavirus disease 2019 (COVID-19). Case reports, case series, editorials, reviews, case-control and cohort studies were evaluated, and relevant information was abstracted. Various reports of neurological manifestations of previous coronavirus epidemics provide a roadmap regarding potential neurological complications of COVID-19, due to many shared characteristics between these viruses and SARS-CoV-2. Studies from the current pandemic are accumulating and report COVID-19 patients presenting with dizziness, headache, myalgias, hypogeusia and hyposmia, but also with more serious manifestations including polyneuropathy, myositis, cerebrovascular diseases, encephalitis and encephalopathy. However, discrimination between causal relationship and incidental comorbidity is often difficult. Severe COVID-19 shares common risk factors with cerebrovascular diseases, and it is currently unclear whether the infection per se represents an independent stroke risk factor. Regardless of any direct or indirect neurological manifestations, the COVID-19 pandemic has a huge impact on the management of neurological patients, whether infected or not. In particular, the majority of stroke services worldwide have been negatively influenced in terms of care delivery and fear to access healthcare services. The effect on healthcare quality in the field of other neurological diseases is additionally evaluated.
Keywords: cerebrovascular diseases, COVID-19, healthcare impact, neurological manifestations, SARS-CoV-2
Introduction
In December 2019, a novel coronavirus emerged in Wuhan, China, as the causing factor of pneumonia and severe acute respiratory syndrome.1 As of May 1st, 2020, the currently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread in more than 215 countries, infecting 3,181,642 patients and causing the coronavirus disease 2019 (COVID-19) pandemic, as this outbreak was declared on 11th March 2020 by the World Health Organization.2 During this pandemic, public health is relentlessly facing critical challenges, and the medical community continues to struggle in an uncharted so far territory, especially with regards to reliable therapeutic interventions.3
Neurologists have to navigate through the same dark arena of uncertainty, since novel data are emerging, and the pattern of clinical characteristics of COVID-19 is continuously widening and increasing. However, neurologists are used to uncertainty, but they are also dedicated to thoughtful problem solving, taking one step at a time.4 In fact, the brain has traditionally been the target organ in a variety of infectious diseases and critical illnesses, either as a direct insult or as a secondary result of infection.5 Apart from the central nervous system (CNS), the peripheral nervous system (PNS) is particularly vulnerable during immune-mediated diseases associated with infections, and prolonged immobilization during critical hospitalization can also severely impact nerves and muscles.6,7
In the present narrative review, we sought to present the neurological manifestations associated with SARS-CoV-2 infection and COVID-19. Caution is recommended so that clinicians can differentiate between the cases where neurological disease is directly associated with COVID-19 from those that present as non-etiological comorbidities. We also evaluated the impact of the COVID-19 pandemic on the health care of neurological patients.
Methods
We systematically searched the literature through MEDLINE and EMBASE, based on the combination of keywords: SARS-CoV-2, SARS-CoV, MERS-CoV, COVID-19, coronavirus, neurological manifestations, implications, cerebrovascular diseases, healthcare impact, pandemic. References of retrieved articles were also screened. Case reports, case series, editorials, reviews, case-control and cohort studies were evaluated, and relevant information was abstracted. Duplicate publications were excluded from further evaluation. Reference lists of all articles that met the criteria and references of relevant review articles were examined to identify studies that may have been missed by the database search. Literature search protocol was conducted by three independent authors (GT, LP and AHK). The last literature search was conducted on May 1st, 2020.
Results
What is already known from previous coronavirus epidemics?
SARS-CoV-2 virus is the seventh coronavirus known to infect humans.8 Severe disease can be caused by three of these zoonotic viruses: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), which both have caused epidemics in the new millennium, and SARS-CoV-2. In fact, SARS-CoV and SARS-CoV-2 share common viral characteristics, such as they both target the same receptor, angiotensin-converting enzyme 2 (ACE2), and they cause similar symptoms, predominantly pulmonary.9
Regarding the search for a neurological component of COVID-19 manifestations, one can derive experience and data from the previous epidemics caused by coronaviruses. During the worldwide outbreak of SARS in 2002–2003, limited cases and case series of patients manifesting neurological complications were reported, most of which were associated with prolonged immobilization and septic and cardiogenic shock.10–12 However, one case with possible direct infection of the central nervous system was documented, based on SARS-CoV detection by reverse transcriptase–polymerase chain reaction (RT-PCR) in the cerebrospinal fluid (CSF) of the patient.13 Interestingly, neurotropism of human coronaviruses has been suggested by in vitro and in vivo studies that showed that certain strains of the viruses could persist in the human CNS by targeting oligodendrocytic and neuroglial cell lines.14–16
Cases with neurological complications have also been reported in patients with MERS-CoV.17 Neurological manifestations, such as Bickerstaff’s encephalitis overlapping with Guillain–Barré syndrome, intensive-care-unit-acquired neuropathy, seizures and strokes, both ischemic and haemorrhagic, had all complicated the clinical course of patients with MERS-CoV.18–21 More severe manifestations occurred in two patients with immune-mediated disorders, precisely one with acute disseminated encephalomyelitis and another with encephalitis.21 CSF analysis was performed in the last two cases, but MERS-CoV RT-PCR was negative in both of them.
Coronaviruses can cause serious pulmonary manifestations requiring intensive care unit treatment; thus, infected patients may also suffer from indirect neurological complications of critical illness. The central and peripheral nervous system involvement may be related to hypoxia and endothelial damage, uncontrollable immune reaction and inflammation, electrolyte imbalance, hypercoagulable state and disseminated intravascular coagulation, septic shock and/or multiple organ failure. Neurological manifestations are indeed expected (Table 1), but few robust data exist to suggest direct infection of the nervous system by coronaviruses as yet.
Table 1.
Neurological symptoms potentially associated with COVID-19, according to the localization in the nervous system.
| Localization in the nervous system | Neurological symptoms |
|---|---|
| Central nervous system | Headache |
| Dizziness | |
| Stroke symptoms | |
| Seizures | |
| Confusion | |
| Agitation | |
| Delirium | |
| Stupor | |
| Coma | |
| Peripheral nervous system | Hypogeusia |
| Hyposmia | |
| Generalized Weakness | |
| Muscles | Myalgias |
| Weakness |
What are the preliminary data of the current SARS-CoV-2 outbreak?
Brain and skeletal muscles express ACE2 which may increase their susceptibility as potential targets of SARS-CoV-2.22,23 Proposed neurotropic mechanisms have been published and involve viral access to the CNS through systemic circulation or across the cribriform plate of the ethmoid bone leading to symptoms of hyposmia and hypogeusia.23 Viral neuro-invasion and subsequent central neuronal injury have also been proposed to contribute to the acute respiratory distress of the patients with COVID-19.24 Moreover, the adhesion of SARS-CoV2 to ACE2 receptors gains particular importance in the cases of intracerebral haemorrhage, due to the inactivation of the receptor and subsequent dysfunction in blood pressure regulation.25 In severely infected patients, coagulopathy and prolonged prothrombin time due to disseminated intravascular coagulation may contribute to increased risk of secondary intracranial haemorrhage. In cases of ischemic stroke, potential mechanisms are hypercoagulability associated with inflammation, endothelial and platelet activation, dehydration and cardio-embolism from virus-related cardiac injury.26,27 Hypoxemia can further worsen neuronal damage.
The first cohort study about neurological manifestations of COVID-19 summarized the neurological symptoms among 78 out of the 214 (36.4%) patients hospitalized in three designated COVID-19 hospitals in China.28 Of those patients, 6 suffered from stroke, but milder neurological symptoms were more commonly reported in this cohort, such as dizziness, headache, muscle symptoms, hypogeusia and hyposmia. Interestingly, anosmia, hyposmia and dysgeusia affected such a significant number of patients worldwide that the American Academy of Otolaryngology – Head and Neck Surgery proposed that these symptoms should be added to the list of screening tools for possible COVID-19 infection.29,30 Mild neurological symptoms, such as headache (13.6%) and myalgias (14.9%), were also reported in another large case series, which summarized the clinical characteristics among 1099 COVID-19 patients.31 In addition, the most common (52%) symptoms of adult patients with COVID-19 infection were myalgia and fatigue in a recent Chinese study.32 It is unknown whether these symptoms represent a systemic inflammatory state, neurological disease or both.
Cerebrovascular disease in patients with COVID-19 was studied in a single-centre, cohort study that has been published without peer-review on a pre-print server.33 According to this study, acute stroke may complicate or co-exist with COVID-19; 5%, 0.5% and 0.5% of the patients developed acute ischemic stroke, cerebral venous sinus thrombosis and cerebral haemorrhage, respectively. The main limitation of this report is the lack of comprehensive diagnostic work-up to document the potential aetiopathogenic mechanisms of cerebrovascular diseases.
A more recent study underscored the high incidence of ischemic stroke due to large vessel occlusion in patients younger than 50 years old.34 More importantly, all of those patients (n=5) had confirmed SARS-CoV-2 infection but were not critically ill until the stroke occurrence. Two of them did not have any known risk factors for stroke.
More case reports of neurological manifestations have also followed (Table 2). A patient with COVID-19 developed decreased consciousness during hospitalization in the intensive care unit and tested positive for SARS-CoV-2 by RT-PCR in the CSF.35 The authors diagnosed this patient as a case of SARS-CoV-2 encephalitis. However, caution is highly suggested since positive RT-PCR in the CSF is not necessarily equivalent to CNS infection. A traumatic lumbar puncture with contamination of the CSF sample by the patient’s blood or other biological secretions that contained the genetic material of SARS-CoV-2, cannot be entirely excluded. One more patient with COVID-19 presented with altered mental status and possible encephalopathy and underwent electroencephalogram (EEG), which showed bilateral slowing and focal slowing in the left temporal region with sharply countered waves.36 However, the patient had suffered a stroke in the past and had an underlying left temporal lesion of encephalomalacia, that could have contributed to the abnormal EEG. CSF analysis was also not indicative of CNS infection. More conclusive evidence of SARS-CoV-2 associated meningitis/encephalitis are presented by a case report of a comatose patient with positive RT-PCR for SARS-CoV-2 in the CSF and neuroimaging features indicative of right lateral ventriculitis and encephalitis mainly on right mesial lobe and hippocampus.37 Surprisingly, no genetic material of SARS-CoV-2 was detected during nasopharyngeal swab testing raising the question of primary seeding of the CNS versus residual CNS infection after viral clearance from other sites, a feature observed in other viral infections like Ebola and human immunodeficiency virus. A case of acute haemorrhagic necrotizing encephalopathy in a patient with COVID-19 has also been described as a result of an intracranial cytokine storm, similar to the one occurring in the lungs during severe COVID-19, leading to respiratory failure.38 Large trials have been looking into therapeutic countermeasures that include immune modulation like IL-6 inhibition in an attempt to fight this inflammatory cascade. Another case involved the peripheral nervous system with the development of para-infectious Guillain–Barré syndrome.39 However, the authors acknowledge the fact that SARS-CoV2 infection in their case might as well be a coincidence rather than causality. Five more patients developed Guillain-Barré syndrome 5 to 10 days after the onset of COVID-19 symptoms.40 Severe deficits, axonal involvement and respiratory failure with subsequent need for mechanical ventilation were reported among those patients. PNS involvement has also been documented in two patients who were diagnosed with Miller-Fisher syndrome and polyneuritis cranialis at 3 to 5 days after exhibiting COVID-19-related symptoms.41
Table 2.
Reported cases of COVID-19 patients presenting neurological manifestations, according to the date of publication.
| Date of publication | Authors | Design | Neurological manifestations | RT-PCR in CSF | Clinical outcome |
|---|---|---|---|---|---|
| February 22nd | Mao et al.28 | Case series | All: 78 (36.4%) CNS: 53 (24.8%) Stroke: 6 (2.8%) PNS: 19 (8.9%) Muscles: 23 (10.7%) |
NA | NA NA Death: 1 NA NA |
| February 28th | Guan et al.31 | Case series | Headache: 150 (13.6%) Myalgia or Arthralgia: 164 (14.9%) |
NA | NA |
| March 5th | Xiang et al.35 | Case report | Encephalitis | (+) | Recovery |
| March 13th | Li et al.33 | Case series (not peer-reviewed) | AIS: 11 (5%) CVT: 1 (0.5%) ICH: 1 (0.5%) |
NA | Death: 5 |
| March 21st | Filatov et al.36 | Case report | Encephalopathy | NA | Critically ill, still hospitalized |
| March 31st | Poyiadji et al.38 | Case report | Acute haemorrhagic necrotizing encephalopathy | NA | NA |
| April 1st | Zhao et al.39 | Case report | Guillain–Barré syndrome | NA | Complete recovery |
| April 3rd | Moriguchi et al.37 | Case report | Meningitis/Encephalitis | (+) | At day 15 still hospitalized with impaired consciousness |
| April 6th | Han et al.32 | Case series | Myalgia and weakness: 13 (52%) | NA | Death: 0% Hospital Discharge: 100% |
| April 15th | Helms et al.42 | Case series | Confusion: 26 (65%) Agitation: 40 (69%) Dysexecutive syndrome: 14 (36%) Abnormal corticospinal tract signs: 39 (67%) Ischemic stroke: 3 (23%) |
(–)* | Hospital Discharge: 45 (78%) Dysexecutive Syndrome: 15 (33%) |
| April 17th | Toscano et al.40 | Case series | Guillain -Barré Syndrome: 5 (0.4-0.5%) | (-) | At 4 weeks Mechanical ventilation: 2 Flaccid paraplegia: 2 Walks independently: 1 |
| April 17th | Gutiérrez-Ortiz et al.41 | Case report | Miller-Fisher Syndrome: 1 Polyneuritis Cranialis: 1 |
(-) | Recovery |
| April 28th | Oxley et al.34 | Case series | Ischemic stroke due to large vessel occlusion (n=5) | NA | Discharged at home:1 Discharged at Rehabilitation Facility: 2 Critically ill, still hospitalized: 2 |
AIS, acute ischemic stroke; CNS, central nervous system; CSF, cerebrospinal fluid; CVT, cerebral venous thrombosis; ICH, intracerebral haemorrhage; NA, not available; PNS, peripheral nervous system; RT-PCR, reverse transcriptase–polymerase chain reaction.
CSF analysis was performed in seven patients (12%).
Another recent study reported the neurological features in a case series of 58 patients hospitalized in intensive care unit because of acute respiratory distress syndrome due to COVID-19.42 Agitation, confusion and abnormal corticospinal tract signs were commonly documented in those critically ill patients and 33% of those discharged had a persistent dysexecutive syndrome. Further diagnostic evaluation was also performed in a subset of patients, consisting of magnetic resonance imaging, electroencephalography and CSF examination. Among neuroimaging features, leptomeningeal enhancement in eight patients, frontotemporal hypoperfusion in 11 patients and cerebral ischemic stroke in three patients were reported. CSF examination was performed in seven patients and was abnormal in three of them, presenting positive oligoclonal bands with an identical electrophoretic pattern in serum or elevated protein and IgG levels. However, RT-PCR testing for SARS-CoV-2 was negative in all examined CSF samples.
The distinction between true causality and non-etiological concomitance may pose a real challenge in some instances, but its importance is unquestionable when presenting case-reports with neurological manifestations attributable to COVID-19. Microbiological assays and temporal association can contribute to one or the other direction. We present the case reports of two stroke patients with clearly identified underlying aetiopathogenic stroke mechanisms, who also suffered from COVID-19 manifestations (Figures 1 and 2). These cases highlight the potential of comorbidity between COVID-19 and stroke. In fact, COVID-19 and stroke share some common risk factors (Table 3) such as age, cardiovascular disease, diabetes mellitus, smoking, cardiac arrhythmia, coronary artery disease and others.43,44 Consequently, COVID-19 and stroke may coexist without any causal association. The CASCADE (Call to Action: SARS-Cov-2 and CerebrovAscular DisordErs) study is a worldwide, multicentre hospital-based study on stroke incidence and outcomes during the COVID-19 pandemic.45 The main aim of this study is to enable the understanding of the changes in models of stroke care, differential hospitalization rate, and stroke severity, as it pertains to the COVID-19 pandemic. Ultimately, this will help guide clinical-based policies surrounding COVID-19 and other similar global pandemics to ensure that treating cerebrovascular comorbidities is appropriately prioritized during the global crisis.
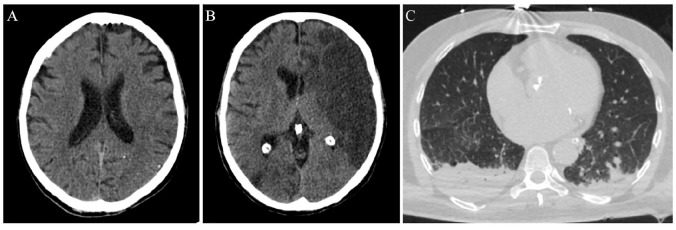
Figure 1.
Imaging evaluation of an ischemic stroke patient with concomitant COVID-19.
This 53-year-old man presented in the emergency department of a comprehensive stroke centre on March 18, 2020. The patient was aphasic with right hemiplegia. The National Institute of Health Stroke Scale (NIHSS) score was 16. He was afebrile and his family members denied any symptoms attributable to COVID-19. Neurological symptoms’ onset-to-presentation time was 1 h. A non-contrast brain computed tomography (CT)-scan was performed, intracranial haemorrhage was excluded and early hyperacute signs of left middle cerebral artery ischemia were disclosed (loss of grey-white matter differentiation (A). No other contraindications existed and intravenous thrombolysis with alteplase was administered. Door-to-needle time was relatively prolonged (70 min), since the CT-scan was occupied at that time with chest examinations of patients with possible SARS-CoV-2 infection. There was no availability for mechanical thrombectomy in this institution (after-hours presentation of the patient and limited personnel in the catheter laboratory). Diagnostic work-up revealed two possible stroke mechanisms: atrial fibrillation of unknown duration with subsequent cardio-embolism, and concomitant atherothrombotic disease, causing haemodynamically significant (>70%) stenosis of the internal carotid arteries bilaterally. During the next 48 h, the patient presented neurological deterioration (NIHSS-score of 22) and follow-up brain CT showed an extensive infarction in the distribution of the left middle cerebral artery (B). At that time, the patient developed a low-grade fever, most probably due to aspiration. Chest CT-scan was indicative of bilateral aspiration pneumonia (C). Oropharyngeal swabs were also examined for SARS-CoV-2 on RT-PCR assay, but the virus was not detected. Three days later, the patient was intubated and transferred to the general intensive care unit. The second oropharyngeal swab test was positive for SARS-CoV-2 RNA. The patient expired on March 24, 2020 due to further neurological deterioration from cerebral oedema.
Figure 2.
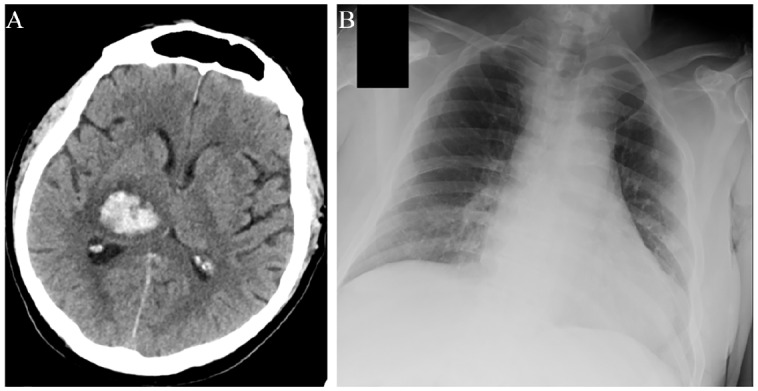
Imaging evaluation of a patient with intracerebral haemorrhage with concomitant COVID-19.
A 67-year-old man with a history of hypertension, diabetes, hypercholesterolemia and coronary artery disease presented to the emergency department of a comprehensive stroke centre on April 6, 2020 after waking up with slurred speech, left-sided weakness and numbness. Admission systolic and diastolic blood pressure levels were 178/106 mmHg. The patient was afebrile with no respiratory symptoms. However, history provided by his wife suggested that earlier in the morning, prior to his admission, he had an increased body temperature of 38.5°C. Brain computed tomography scan uncovered right thalamic intracerebral haemorrhage with mass effect and intraventricular extension (A). The patient was admitted in the stroke unit. Due to the history of fever, a nasopharyngeal swab was performed on the day of admission and was negative for SARS-CoV-2. However, on the following day the patient redeveloped fever, so work-up with blood cultures, chest X-ray (B) and urinalysis was performed but did not uncover any obvious source of infection. A second nasopharyngeal swab was ordered and came back positive for SARS-CoV-2 RNA. Despite SARS-CoV-2 infection, the patient never experienced any symptoms of dyspnoea, cough, chest pain or diarrhoea during his hospitalization. He only reported the presence of mild generalized headache and nausea, which could have been attributable to the intracerebral haemorrhage. A certain causal association between COVID-19 and intracerebral haemorrhage cannot be established based on the evidence in this case, especially since the patient had a medical history of three common risk factors of manifesting both the diseases (Table 3). However, it may be postulated that blood pressure dysregulation due to angiotensin converting enzyme 2 (ACE2) inactivation by SARS-CoV-2 could have been at least partially involved in the development of intracerebral haemorrhage.
Table 3.
Risk factors reportedly associated with severe COVID-19 and established risk factors associated with cerebrovascular diseases.
| Risk factor | COVID-19 | Cerebrovascular diseases |
|---|---|---|
| Advanced age | + | + |
| Heart failure | + | + |
| Coronary artery disease | + | + |
| Hypertension | + | + |
| Dyslipidaemia | − | + |
| Diabetes mellitus | + | + |
| Obesity | + | + |
| Chronic obstructive pulmonary disease | + | − |
| Asthma | + | − |
| Chronic kidney failure | + | + |
| Liver disease | + | + |
| Malignancy | + | + |
| Smoking | + | + |
| Immunosuppression | + | − |
In the spectrum of PNS involvement in COVID-19, we present the case report of a patient with confirmed SARS-CoV-2 infection who developed facial nerve palsy during hospitalization (Figure 3). Despite that, RT-PCR in the CSF was negative for SARS-CoV-2 and direct infection of the nervous system could not be established, the temporal association between COVID-19 and facial nerve palsy is highly suspicious for an indirect, possibly immune-mediated mechanism.
Figure 3.
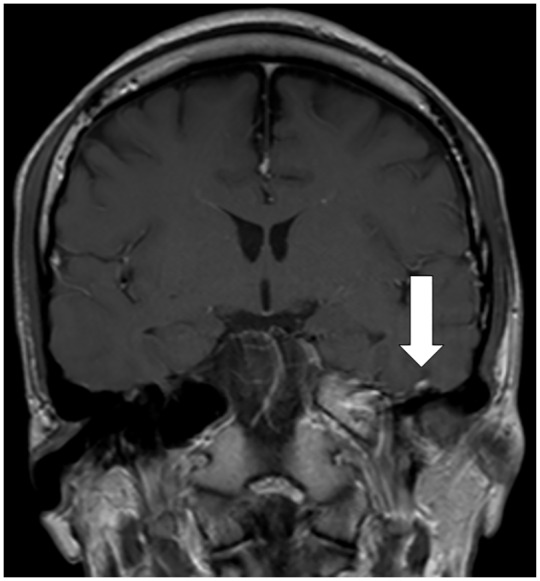
Imaging evaluation of a left facial palsy patient with concomitant COVID-19.
A 27-year-old man was admitted to the isolation ward of a tertiary centre on March 16, 2020, presenting with myalgia, cough, fever and left-sided headache for 4 days. He had just returned from Spain the day before admission. On examination his lungs were clear and neurological examination was unremarkable. Reverse-transcription polymerase-chain-reaction (PCR) performed on the nasopharyngeal swab was positive for SARS-CoV-2. On day 3 of hospitalization, he developed left retro-auricular pain, dysgeusia and left facial weakness. Neurological examination showed a left facial nerve palsy. There was no associated neck stiffness, vesicles in the outer ear, or parotid swelling. Cerebrospinal fluid (CSF) studies showed no cells, and protein and glucose levels were normal. CSF PCR was negative for herpes simplex virus, varicella zoster virus and SARS-CoV-2. His magnetic resonance imaging of the brain showed contrast enhancement of the left facial nerve (Figure). He was treated with lopinavir/ritonavir for reducing SARS-CoV-2 viral replication. He received a 1-week course of prednisolone and valacyclovir for treatment of facial palsy. Upon review 1 week later, his headache had resolved, and improvement was noted in facial weakness.
What is the impact of COVID-19 pandemic on the health care of neurological patients?
Regardless of any direct or indirect neurological manifestations related to SARS-CoV-2, the COVID-19 pandemic has a huge impact on the management of neurological patients, whether infected or not. Normal neurological medical care, as we know it, has been seriously impaired worldwide.46,47 Many neurological wards, among other general medical wards, have been converted into quarantine wards for the treatment of infected patients. Routine neurological outpatient visits are delayed or suspended, and treatment remains available only for emergency conditions and highly selective cases. Even in the emergency setting, neurological departments may be forced to function with fewer staff, either due to staff redeployment to COVID services, prophylactic quarantine or direct illness. The remaining healthy personnel are at high risk of burn-out, due to long shifts, sleep deprivation, psychological distress, shortages of medicine and supplies and discomfort associated with personal protective equipment (PPE) use.48 Characteristically, PPE use has recently been associated with the development of de novo headaches and aggravation of pre-existing headaches among frontline healthcare personnel and these factors can interfere with compliance and workplace safety and productivity.49 Despite those unprecedented conditions, quality of care in neurology should be maintained.
Stroke, as the cornerstone of neurological emergency and a major cause of mortality and disability, should not be neglected at the expense of extreme community and health-care COVID-19-measures.46 However, published and anecdotal reports of declining stroke admission volumes are accumulating.46,50 Stroke patients with mostly mild symptoms, and for that reason with better chances of recovery if appropriately treated, may be unwilling to seek medical help due to their fear of the virus. Such a practice can significantly narrow down the time-window for available acute treatments and can lead to neurological deterioration, early recurrent stroke and permanent disability.5 In light of those data, several stroke centres have come together to design a study protocol with the aim to investigate the worldwide burden of cerebrovascular disease before, during and after SARS-CoV-2 pandemic (CASCADE study).45
In addition, stroke centres have been guided to implement a ‘protected code stroke’ for the management of acute stroke patients.50,52 ‘Protected code stroke’ actually originates from the acknowledgment that every stroke patient is potentially infected and should be treated accordingly.50,52 However, maintaining the high standards of stroke care is of utmost importance.53 For example, early cerebral imaging should be preserved in order to avoid unnecessary delays, noted in our patient presented in the case report (Figure 1). During stroke patient presentation, doctors should ask patients and their companions whether they have symptoms indicative of COVID-19. If so, they might consider ordering a chest CT-scan, which can be performed at the same time as the brain CT-scan.54 Return visits to the imaging departments run the risk of exposing and being exposed and should be avoided. As early as at the admission process, doctors should establish a discharge plan and perform in-hospital only the most essential diagnostic tests, in order to shorten the duration of hospitalization.53 Finally, early consultation should be asked from an infectious disease specialist in cases of suspected SARS-CoV-2 infection or from an intensive care unit physician, if the patient requires high fractions of inspired oxygen. Stroke patients with confirmed SARS-CoV-2 infection should be transferred to dedicated COVID-19 wards.54 The need for a multidisciplinary team approach and vigorous inter-specialist cooperation is essential.
Notably, the European Stroke Organization (ESO) has recently announced a press release, cautioning for a potential increase in the risk of death or disability from stroke during the COVID-19 pandemic (https://eso-stroke.org/news/). In a survey of 426 stroke care providers from 55 countries, only one in five reported that stroke patients are currently receiving the usual acute and post-acute care at their hospital. This press release concluded that the lack of optimal care is likely to lead to a greater risk of death and a smaller chance of a good recovery (https://eso-stroke.org/news/). The ESO has also emphasized that patients with stroke symptoms should still present to the hospital as soon as possible and that efforts should be made to maintain the usual level of stroke care, including intravenous and endovascular reperfusion strategies, irrespective of the patient’s COVID-19 status, to avoid unnecessary ‘collateral damage’ through the inadequate treatment of this often disabling or life-threatening condition (https://eso-stroke.org/news/).
Other fields of neurology are less obviously, albeit still affected, by the COVID-19 pandemic. Many neurological patients with multiple sclerosis and autoimmune syndromes such as myasthenia gravis, neuromyelitis optica, angiitis and inflammatory polyneuropathies are on a wide variety of immunosuppressive therapies. Whether those therapies can impair the immune response to SARS-CoV-2 infection is a matter of great concern, but few data are currently available.55 However, not only should the disease-modifying therapies not be discontinued, but also neurologists have to ensure that the patients can present safely for their regular treatment appointments. Such patients may need to take extra precautions to prevent exposure to the virus during their hospital visits but also in the community. Any follow-up visits which are unrelated to treatment can be postponed or completed through tele-neurology programs. Once a patient is confirmed with SARS-CoV-2 infection, the physician might consider postponing any second-line immunomodulating (fingolimod, ocrelizumab or natalizumab in particular) or immunosuppressive therapies, especially if the patient is of higher risk for severe COVID-19 disease.56 Treatment should be restarted when symptoms have fully resolved and the repeat testing is negative. When feasible, prompt treatment re-initiation is particularly important in the cases of fingolimod and natalizumab, in order to minimize the risk of the rebound effect that has been associated with the discontinuation of these agents. In the case of a clinical relapse of COVID-19 patients, treatment with intravenous pulse steroids should be carefully considered and potentially avoided, in view of the recent multiple sclerosis scientific organizations’ statements, cautioning against the use of corticosteroids in patients with viral infections and COVID-19.56,57 Fever in the case of a multiple sclerosis patient with COVID-19 can further deteriorate pre-existing symptoms (Uhthoff phenomenon) and this should be differentiated from a ‘true’ clinical relapse. Respectively, in patients with myasthenia gravis, an infection can also induce a myasthenic crisis.58 Those patients are generally at high risk for developing various in-hospital complications and the treating physicians should be extremely vigilant.
Patients with neurodegenerative diseases also belong to a more vulnerable subset of the general population. Patients with dementia, which are generally older and therefore at high mortality risk due to COVID-19, can hardly follow protective measures or use telecommunication when needed.59 Lock-down can also trigger or worsen behavioural symptoms in such patients and their caregivers should be appropriately prepared. In addition, if such patients need prolonged hospitalization or deal with hypoxia, they will be in immediate risk of developing delirium and deteriorate permanently.59 Patients with extrapyramidal disorders are not immunocompromised, however COVID-19 may be particular challenging for those with significant movement restrictions.60 In fact, patients with Parkinson’s disease are more prone to lower respiratory tract infections and, in turn, pneumonia is the leading cause of death in Parkinson patients.60,61 The association of COVID-19 and neurodegenerative diseases is even more complex since a significant proportion of such patients live in nursing homes and are at particular risk of developing the disease or infecting others with SARS-CoV-2. Healthcare- and community-based approaches are urgently needed to minimize the bilateral impact between COVID-19 and neurodegenerative diseases.59
During their clinical practice, neurologists should also be prepared to confront neurological adverse effects and drug interactions related to the use of anti-viral agents and other medications that may be tested and used against SARS-CoV-2. For example, hydroxychloroquine sulphate has been associated with headache, dizziness and extrapyramidal disorders, such as dystonia, dyskinesia and tremor.62 In addition, it interacts with a variety of antiepileptic drugs and can potentially lower the convulsive threshold. Caution is also required when patients with disorders of the neuromuscular junction are prescribed hydroxychloroquine sulphate, especially with co-administration of aminoglycoside antibiotics. Certain drug databases report additional minor interactions of chloroquine with anti-Xa inhibitors, resulting in a decrease of their excretion rate and potentially in higher bleeding risk.63,64 All direct oral anticoagulants also interact with the antiviral agent ritonavir (a protease inhibitor used in conjunction with another PI lopinavir, as the lopinavir/ritonavir combination for COVID-19), which is a strong inhibitor of both cytochrome P450 3A4 and P-glycoprotein transporter and therefore may increase their plasma concentrations to a clinically relevant degree.65–67 Remdesivir, a promising nucleoside analogue currently tested for its efficacy in COVID-19, may be associated with delirium.68 In addition, colchicine that is currently being investigated as a potential therapy for cardiac complications of COVID-19 (ClinicalTrials.gov identifier: NCT04326790), presents with substantial neuromuscular complications that may exacerbate myalgias, weakness and other muscle symptoms of COVID-19.69 Massive, inconsiderate or over-the-counter administration of such drugs may pose a significant morbidity risk for the neurological patient. Since more than 100 trials currently evaluate anti-SARS-CoV-2 agents, we should be aware for potential drug–drug interactions affecting commonly prescribed neurological agents.
The healthcare of neurosurgical patients is also affected by the COVID-19 pandemic.70 Intensive care units are being overcrowded by COVID-19 patients, and post-operative neurosurgical patients cannot be admitted for monitoring. The less emergent surgeries are being postponed. Patients are being triaged according to case severity and are transported to a limited number of designated neurosurgical centres, since the majority have been transformed to COVID-19 wards. Fortunately, but not surprisingly, cases with traumatic brain lesions, which require urgent surgery, are being reduced in number due to the public lockdown and social distancing measures.70
Drastic times call for drastic measures and the safety of neuroscience specialists should be preserved. Neurologists are first responders to stroke and other neurological emergencies and have to be protected.71 One management approach could be that only neurology subspecialists who do procedures or surgeries should be going to the emergency room, stroke units and neuro-intensive care units (i.e. neuro-intensivists and neuro-endovascular specialists, vascular neurologists).71 Other non-urgent neurological evaluations may be performed remotely.72 Tele-neurology has emerged as a fundamental component of solutions to sustain neurological healthcare quality.73 Its expanding use ranges from the simplest drug prescriptions to the more complicated tele-triage of patients before they get admitted to an emergency department. It has already been implemented with success in stroke care over the past decade and in pilot projects for epilepsy care but can also be applied to other neurological fields.74 We expect that information technology experts would scientifically support this endeavour and that authorities would reimburse it appropriately. More optimistically, after the management of the SARS-CoV-2 outbreak and the recovery of global health, the COVID-19 pandemic may act as a stimulus for a new era of tele-neurology to be established. Finally, medical education for neurological residents and the younger generation of neurologists needs to adapt to e-learning activities in these times of extraordinary needs.
Discussion
Our narrative review summarized the so far documented neurological complications of COVID-19 that involve the central and the peripheral nervous system. The neurological manifestations include dizziness, headache, myalgias, hypogeusia and hyposmia, but also highlights less common but more serious disorders including polyneuropathy, myositis, cerebrovascular diseases and rarely encephalitis. These neurological manifestations have also been reported in previous coronavirus epidemics with the SARS-CoV and the MERS-CoV viruses. More data are needed to establish the incidence, outcomes and causal mechanisms between COVID-19 and stroke, encephalitis or polyneuropathy. However, direct infection of the neurological system appears to be extremely rare.
Regardless of any direct or indirect neurological manifestations, the COVID-19 pandemic has had a huge impact on the management of neurological patients, whether infected or not. In particular, the majority of stroke services worldwide have been negatively influenced by the pandemic and the lack of optimal care is likely to lead to a greater risk of death and higher odds of disability in acute stroke patients. This includes treatments for vessel recanalization (intravenous thrombolysis and mechanical thrombectomy), securing brain vascular malformations (aneurysm coiling and clipping), specialized stroke unit care, secondary stroke prevention strategies and rehabilitation. Moreover, patients with neuroimmunological disorders receiving immunosuppressive therapies may have a higher risk of COVID-19 infection than the one recognized so far. Similarly, patients with severe neurodegenerative disorders are prone to pulmonary infections and may reside in nursing homes that have so far proven to be hotspots for SARS-CoV-2 infections associated with high case-fatality rates. In addition, neurologists should also be prepared to treat neurological adverse effects related to the use of anti-viral agents and other medications that may be tested and used against SARS-CoV-2.
Similar to the general population, infectious disease outbreaks are known to have a psychological impact on various healthcare workers. A recent study on healthcare workers from two tertiary care institutions showed that 10.8% of medically trained (doctors and nurses) and 20.7% of allied healthcare workers suffer from moderate anxiety, mostly related to the uncertainty of contracting the disease as well as transmitting it to their colleagues, patients and family members.75 Healthcare workers in neurology departments are particularly vulnerable, since most neurological disorders often need close contact and repeated evaluations of the patients by neurologists, nurses, physiotherapists, technicians, as well as personnel from allied departments. The prevalence of psychological impacts may increase further as the pandemic continues. Important clinical and policy strategies are needed to support healthcare workers on the frontline, especially for those who are susceptible to psychological distress. Studies have shown that the availability and accuracy of health information can alleviate stress levels. Regular communication and stringent educational and psychological interventions should target all healthcare personnel involved in the care of neurology patients to ensure their understanding and proper use of infectious disease control measures, and to boost a sense of security among them. Finally, psychological distress, ‘burn out’ symptoms and headaches associated with the long use of PPE may negatively affect the performance of neurologists that are involved in the management of COVID-19 patients hospitalized in quarantine wards.
In conclusion, COVID-19 presents with predominantly mild neurological manifestations in the majority of infected patients, while there are multiple comorbidities with more severe neurological disorders. New data will be continuously emerging in the upcoming period, so the neurologists have to be prepared in this largely unknown territory. At the same time, they must stand by their neurological patients, whose needs do not disappear during the COVID-19 pandemic but become even more demanding. We should stand united against this challenge and view it as a unique opportunity for preservation of our management planning, for inter-specialist collaboration, for innovative thinking and telemedicine adoption that can all contribute to quality healthcare services during and after the COVID-19 pandemic.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
Andrei V. Alexandrov  https://orcid.org/0000-0001-8871-1023
https://orcid.org/0000-0001-8871-1023
Contributor Information
Georgios Tsivgoulis, Second Department of Neurology, National & Kapodistrian University of Athens, School of Medicine, Rimini 1, Chaidari, Athens 12462, Greece; Department of Neurology, The University of Tennessee Health Science Center, Memphis, TN, USA.
Lina Palaiodimou, Second Department of Neurology, National and Kapodistrian University of Athens, School of Medicine, “Attikon” University Hospital, Athens, Greece.
Aristeidis H. Katsanos, Second Department of Neurology, National and Kapodistrian University of Athens, School of Medicine, “Attikon” University Hospital, Athens, Greece Division of Neurology, McMaster University/Population Health Research Institute, Hamilton, ON, Canada.
Valeria Caso, Stroke Unit, University of Perugia - Santa Maria della Misericordia Hospital, Perugia, Italy.
Martin Köhrmann, Department of Neurology, University of Essen, Essen, Germany.
Carlos Molina, Department of Neurology, Stroke Unit, Hospital Universitari Vall d’Hebrón, Barcelona, Spain.
Charlotte Cordonnier, Inserm, CHU Lille, U1172 - LilNCog - Lille Neuroscience & Cognition, Univ. Lille, Lille, France.
Urs Fischer, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Peter Kelly, HRB Stroke Clinical Trials Network Ireland and Stroke Service/Department of Neurology, Mater University Hospital/University College, Dublin, Ireland.
Vijay K. Sharma, Department of Medicine, Division of Neurology, National University Hospital, Singapore
Amanda C. Chan, Department of Medicine, Division of Neurology, National University Hospital, Singapore
Ramin Zand, Department of Neurology, Neuroscience Institute, Geisinger Health System, Danville, PA, USA.
Amrou Sarraj, Department of Neurology, The University of Texas at Houston, Houston, TX, USA.
Peter D. Schellinger, Department of Neurology and Neurogeriatry, Johannes Wesling Medical Center Minden, University Clinic RUB, Minden, Germany
Konstantinos I. Voumvourakis, Second Department of Neurology, National and Kapodistrian University of Athens, School of Medicine, “Attikon” University Hospital, Athens, Greece
Nikolaos Grigoriadis, Second Department of Neurology, “AHEPA” University Hospital, Aristotelion University of Thessaloniki, Thessaloniki, Macedonia, Greece.
Andrei V. Alexandrov, Department of Neurology, The University of Tennessee Health Science Center, Memphis, TN, USA
Sotirios Tsiodras, 4th Department of Internal Medicine, Attikon University Hospital, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece.
References
- 1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Corona-virus disease (COVID-19) outbreak, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/ (accessed May 1st, 2020).
- 3. Fauci AS, Lane HC, Redfield RR. Covid-19 - Navigating the Uncharted. N Engl J Med 2020; 382: 1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shellhaas RA. Neurologists and Covid-19: a note on courage in a time of uncertainty. Neurology. Epub ahead of print 1 April 2020. DOI: 10.1212/WNL.0000000000009496. [DOI] [PubMed] [Google Scholar]
- 5. Stevens RD, Nyquist PA. Types of brain dysfunction in critical illness. Neurol Clin 2008; 26: 469–486. [DOI] [PubMed] [Google Scholar]
- 6. Kieseier BC, Lehmann HC, Meyer Zu, Hörste G. Autoimmune diseases of the peripheral nervous system. Autoimmun Rev 2012; 11: 191–195. [DOI] [PubMed] [Google Scholar]
- 7. Rubinos C, Ruland S. Neurologic complications in the intensive care unit. Curr Neurol Neurosci Rep 2016; 16: 57. [DOI] [PubMed] [Google Scholar]
- 8. Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med 2020; 26: 450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv 2020. DOI: 10.1101/2020.01.26.919985. [DOI] [Google Scholar]
- 10. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan 2005; 14: 113–119. [PubMed] [Google Scholar]
- 11. Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol 2004; 61: 1669–1673. [DOI] [PubMed] [Google Scholar]
- 12. Chao CC, Tsai LK, Chiou YH, et al. Peripheral nerve disease in SARS: report of a case. Neurology 2003; 61: 1820–1821. [DOI] [PubMed] [Google Scholar]
- 13. Lau KK, Yu WC, Chu CM, et al. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 2004; 10: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arbour N, Day R, Newcombe J, et al. Neuroinvasion by human respiratory coronaviruses. J Virol 2000; 74: 8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arbour N, Talbot PJ. Persistent infection of neural cell lines by human coronaviruses. Adv Exp Med Biol 1998; 440: 575–581. [DOI] [PubMed] [Google Scholar]
- 16. Desforges M, Le Coupanec A, Brison E, et al. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol 2014; 807: 75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chafekar A, Fielding BC. MERS-CoV: understanding the latest human coronavirus threat. Viruses 2018; 10: pii: E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with middle east respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 2014; 29: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol 2017; 13: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med 2016; 2016: 3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with middle east respiratory syndrome corona virus (MERS-CoV). Infection 2015; 43: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11: 995–998. [DOI] [PubMed] [Google Scholar]
- 24. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. Epub ahead of print 27 February 2020. DOI: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang HY, Li XL, Yan ZR, et al. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord 2020; 13: 1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. Epub ahead of print 27 March 2020. DOI: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-Segment Elevation in Patients with Covid-19 — A Case Series. [Published online ahead of print, 2020 Apr 17]. N Engl J Med. 2020. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. Epub ahead of print 20 April 2020. DOI: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Academy of Otolaryngology - Head and Neck Surgery. AAO-HNS: anosmia, hyposmia, and dysgeusia symptoms of coronavirus disease, https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease. (2020, accessed 4 April 2020).
- 30. Lechien J, Chiesa-Estomba C, De Siati D, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild to moderate forms of the coronavirus disease (COVID-19): a multicenter European study, https://www.entnet.org/sites/default/files/uploads/lechien_et_al._-_covid19_-_eur_arch_otorhinolaryngol_.pdf (2020, accessed 4 April 2020). [DOI] [PMC free article] [PubMed]
- 31. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. [Published online ahead of print, 2020 Feb 28]. N Engl J Med. 2020. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han YN, Feng ZW, Sun LN, et al. A comparative-descriptive analysis of clinical characteristics in 2019-Coronavirus-infected children and adults. J Med Virol. Epub ahead of print 6 April 2020. DOI: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550025 (2020, accessed 4 April 2020). [DOI] [PMC free article] [PubMed]
- 34. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. [Published online ahead of print, 2020 Apr 28]. N Engl J Med. 2020. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed]
- 35. Xiang P, Xu XM, Gao LL, et al. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv 2020: T20200300015. [Google Scholar]
- 36. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020; 12: e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. Epub ahead of print 3 April 2020. DOI: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. Epub ahead of print 31 March 2020. DOI: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao H, Shen D, Zhou H, et al. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. Epub ahead of print 1April 2020. DOI: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. [published online ahead of print, 2020 Apr 17]. N Engl J Med. 2020. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. [published online ahead of print, 2020 Apr 17]. Neurology. 2020. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed]
- 42. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. Epub ahead of print 15 April 2020. DOI: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) - people who are at higher risk, https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions (2020, accessed 4 April 2020). [PubMed]
- 44. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. [Published online ahead of print, 2020 May 1]. N Engl J Med. 2020. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Abootalebi S, Aertker BM, Andalibi MS, Asdaghi N, Aykac O, Azarpazhooh MR, et al. Call to Action: SARS-CoV-2 and Cerebrovascular Disorders (CASCADE). J Stroke Cerebrovasc Dis.2020. Accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao J, Rudd A, Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. Epub ahead of print 31 March 2020. DOI: 10.1161/STROKEAHA.120.029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Federico A. Brain awareness week, CoVID-19 infection and neurological sciences. Neurol Sci 2020; 41: 747–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Low ZX, Yeo KA, Sharma VK, et al. Prevalence of burnout in medical and surgical residents: a meta-analysis. Int J Environ Res Public Health 2019; 16: 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jy Ong J, Bharatendu C, Goh Y, et al. Headaches associated with personal protective equipment - a cross-sectional study amongst frontline healthcare workers during COVID-19 (HAPPE Study). Headache 2020; 60: 864–877. [DOI] [PubMed] [Google Scholar]
- 50. Lyden P. AHA/ASA Stroke Council Leadership. Temporary emergency guidance to US stroke centers during the COVID-19 pandemic on behalf of the AHA/ASA stroke council leadership. Stroke. Epub ahead of print 31 March 2020. DOI: 10.1161/STROKEAHA.120.030023. [DOI] [Google Scholar]
- 51. Bersano A, Pantoni L. On being a neurologist in Italy at the time of the COVID-19 outbreak. [published online ahead of print, 2020 Apr 3]. Neurology. 2020. doi: 10.1212/WNL.0000000000009508. [DOI] [PubMed] [Google Scholar]
- 52. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke. Stroke. Epub ahead of print 1 April 2020. DOI: 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. NHS England and NHS Improvement. Clinical guide for the management of stroke patients during the coronavirus pandemic, https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C033-Specialty-guide_-Stroke-and-coronavirus-v1-24March_.pdf (2020, accessed 4 April 2020).
- 54. Jin H, Hong C, Chen S, et al. Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc Neurol 2020: svn-2020-000382. DOI: 10.1136/svn-2020-000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nath A. Neurologic complications of coronavirus infections. Neurology. Epub ahead of print 30 March 2020. DOI: 10.1212/WNL.0000000000009455 [DOI] [PubMed] [Google Scholar]
- 56. Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. [published online ahead of print, 2020 Apr 2]. Neurology. 2020. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- 57. Multiple Sclerosis Trust. Coronavirus, Covid-19 and multiple sclerosis, https://www.mstrust.org.uk/a-z/coronavirus-covid-19-and-multiple-sclerosis#covid-19-and-steroids-for-ms-relapses. (2020, accessed 9 April 2020).
- 58. Zhang P. Invited commentary: be cautious of comorbidities of COVID-19 and neurologic diseases, https://blogs.neurology.org/global/invited-commentary-be-cautious-of-comorbidities-of-covid-19-and-neurologic-diseases/ (2020, accessed 4 April 2020).
- 59. Wang H, Li T, Barbarino P, et al. Dementia care during COVID-19. Lancet 2020; 395: 1190–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parkinson’s Foundation. Top questions and answers on COVID-19 and Parkinson’s disease, https://www.parkinson.org/blog/COVID-19-questions. (2020, accessed 4 April 2020).
- 61. Chang YP, Yang CJ, Hu KF, et al. Risk factors for pneumonia among patients with Parkinson’s disease: a Taiwan nationwide population-based study. Neuropsychiatr Dis Treat 2016; 12: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Medicines.org.uk. Plaquenil-Hydroxychloroquine sulfate 200mg film-coated tablets-summary of product characteristics (SPC). EMC, https://www.medicines.org.uk/emc/product/1764/smpc (2015, accessed 4 April 2020).
- 63. Drugbank.ca. Apixaban. Drugbank, https://www.drugbank.ca/drugs/DB06605 (2020, accessed 4 April 2020).
- 64. Drugbank.ca. Rivaroxaban. Drugbank, https://www.drugbank.ca/drugs/DB06228 (2020, accessed 4 April 2020).
- 65. Medicines.org.uk. Eliquis 5 mg film-coated tablets-summary of product characteristics (SPC). EMC, https://www.medicines.org.uk/emc/product/2878/smpc (2015, accessed 4 April 2020).
- 66. Medicines.org.uk. Pradaxa 150 mg hard capsules-summary of product characteristics (SPC). EMC, https://www.medicines.org.uk/emc/product/4703/smpc (2015, accessed 4 April 2020).
- 67. Medicines.org.uk. Xarelto 15 mg film-coated tablets-summary of product characteristics (SPC). EMC, https://www.medicines.org.uk/emc/product/6402/smpc. (2015, Accessed 4 April 2020).
- 68. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. Epub ahead of print ahead of print 10 April 2020. DOI: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cocco G, Chu DC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med 2010; 21: 503–508. [DOI] [PubMed] [Google Scholar]
- 70. Zoia C, Bongetta D, Veiceschi P, et al. Neurosurgery during the COVID-19 pandemic: update from Lombardy, Northern Italy. Acta Neurochirurgica. Epub ahead of print 28 March 2020. DOI: 10.1007/s00701-020-04305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hurley D. COVID-19 neurology heroes: a neurology resident in memphis— ‘they didn’t have anything to tell me’. NeurologyToday, https://journals.lww.com/neurotodayonline/blog/breakingnews/pages/post.aspx?PostID=930. (2020, accessed 12 April 2020).
- 72. Waldman G, Mayeux R, Claasen J, Agarwal S, Willey J, Anderson E, et al. Preparing a neurology department for SARS-CoV-2 (COVID-19): Early experiences at Columbia University Irving Medical Center and the New York Presbyterian Hospital in New York City. [published online ahead of print, 2020 Apr 6]. Neurology. 2020. doi: 10.1212/WNL.0000000000009519. [DOI] [PMC free article] [PubMed]
- 73. Klein BC, Busis NA. COVID-19 is catalyzing the adoption of teleneurology. Neurology. Epub ahead of print 1 April 2020. DOI: 10.1212/WNL.0000000000009494. [DOI] [PubMed] [Google Scholar]
- 74. Guzik AK, Switzer JA. Teleneurology is neurology. Neurology 2020; 94: 16–17. [DOI] [PubMed] [Google Scholar]
- 75. Tan BYQ, Chew NWS, Lee GKH, et al. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med. Epub ahead of print 6 April 2020. DOI: 10.7326/M20-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]