Abstract
Previous studies have reported that low temperature (LT) constrains plant growth and restricts productivity in temperate regions. However, the underlying mechanisms are complex and not well understood. Over the past ten years, research on the process of adaptation and tolerance of plants during cold stress has been carried out. In molecular terms, researchers prioritize research into the field of the ICE-CBF-COR signaling pathway which is believed to be the important key to the cold acclimation process. Inducer of CBF Expression (ICE) is a pioneer of cold acclimation and plays a central role in C-repeat binding (CBF) cold induction. CBFs activate the expression of COR genes via binding to cis-elements in the promoter of COR genes. An ICE-CBF-COR signaling pathway activates the appropriate expression of downstream genes, which encodes osmoregulation substances. In this review, we summarize the recent progress of cold stress tolerance in plants from molecular and physiological perspectives and other factors, such as hormones, light, and circadian clock. Understanding the process of cold stress tolerance and the genes involved in the signaling network for cold stress is essential for improving plants, especially crops.
Keywords: chilling, cold acclimation, freezing, low temperature, ICE-CBF-COR, tolerance
1. Introduction
Drought, salinity, and low temperature (LT) are the main abiotic stress factors that have strong impacts on plant growth and development [1]. In addition to drought stress, LT is one of the most harmful environmental stresses encountered by higher plants [2,3]. LT stress is divided into chilling stress (<20 °C) and freezing stress (<0 °C) according to the environmental temperature. In addition to affecting the growth and development of the plant, LT stress significantly restrains the geographical distribution of plants [4,5,6,7].
Tropical and subtropical plants are sensitive to chilling stress and lack the capacity of cold acclimation. However, temperate plants have the ability to tolerate freezing temperatures following a period of exposure to non-freezing temperatures, which is termed as cold acclimation [8]. Temperate plants are tolerant to seasonal changes in temperature and can tolerate cold stress during early spring and winter. Many important crops, such as rice, corn, soybean, potato, cotton, and tomato, are chilling sensitive and incapable of cold acclimation. In contrast, some crops, such as oats, are chilling tolerant but freezing sensitive. On the other hand, barley, wheat, and rye are well adapted in freezing temperatures [9]. However, some plants such as Arabidopsis, winter wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) are not able to tolerate nonfreezing temperatures without cold acclimation [10]. Similarly, Santalum album and Betula utilis (Himalayan Birch), as dominant vegetations in cold environments (Eastern Nepal), are also sensitive to cold during the growing season [11,12].
Each plant has different enrichment pathways in different periods of cold stress, such as the amino sugar and nucleotide sugar metabolism pathway, alanine and protein export, and the aspartate and glutamate metabolism are highly enriched during the latter stages of cold stress periods in tea plants. In contrast, cellular components, biological processes, and the molecular function category are highly enriched in the early periods of cold stress in tea plants. The same result was found using transcriptome analysis in which flavonoid biosynthesis, phagosome, plant hormone signal transduction, and fructose were highly enriched at the beginning of cold stress [13]. In tomato, even though the expression of ethylene signaling genes decreased after cold stress, none of the protein genes decreased. This shows the different regulation of gene and protein levels [14]. In addition, abscisic acid (ABA) signaling pathway gene expression improved the cold hardiness in grapevine buds only during the cold acclimation period [15]. Furthermore, the overexpression of GLR1.2 or GLR1.3 enhanced cold tolerance by increasing endogenous jasmonate levels under cold stress. However, they could not interact with each other directly, indicating they interact indirectly to achieve cold tolerance [16]. In banana and Zoysia japonica, MaPIPI2.7 and ZjICE1 improved multilevel stresses, such as cold, salt, and drought stress [17]. These results show that every gene has a different mechanism in different plants to achieve abiotic or biotic stress tolerance. Due to these differences of signal transduction pathways and metabolisms, the mechanisms related to cold stress are complex [18].
The methods plants use to deal with adverse environmental stress, including stress avoidance and stress tolerance, have been examined in the literature [19,20]. Recent studies of LT stress and cold acclimation based on the model plant Arabidopsis contribute substantially to understanding the molecular mechanisms of cold acclimation [6]. In addition, numerous studies have been carried out in other plant species to reveal the molecular mechanism and gene regulatory networks. In this review, we aim to give a comprehensive overview of the current knowledge about plant under LT stress.
2. Genetical Changes during Cold Stress
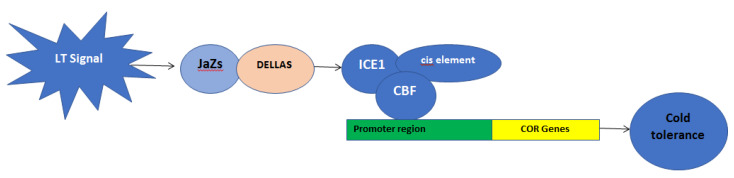
Investigation of transcriptional alterations of plants during cold acclimation is crucial for understanding the underlying molecular mechanism under LT stress. Until now, a significant number of cold responsive genes have been identified and several gene regulated networks have been reported. Among these, ICE-CBF-COR is one of the most wildly reported pathways. In most plant species, the ICE-CBF-COR pathway is induced by LT stress and then activates the appropriate expression of downstream genes, which encode osmoregulation substances [9]. Inducer of CBF Expression (ICE) is a pioneer of cold acclimation, an MYC-type basic helix-loop-helix family transcription factor (TF) [8,21,22]. It is reported that ICE1 plays a central role in C-repeat binding factor 3 (CBF3) cold induction. When plants encounter LT stress, ICE1 could be released from JAZs bound by DELLAs and induce the expression of CBF3. CBF3 activates the expression of GA2ox7 to reduce the bioactive gibberalic acid (GA) level, which promotes the accumulation of DELLAs. Therefore, DELLAs can regulate the cold induction of CBF3 through ICE1 via JAZs.
C-repeat binding factors (CBFs), also known as dehydration-responsive element-binding proteins (DREBs), act as a regulating gene that has an important role in cold acclimation [23]. CBF is a member of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) family and regulates the expression of the cold-responsive (COR) gene [24]. The AP2/ERF family is one of the largest TF families in plants and is characterized by having a minimum of one AP2 DNA-binding domain [25]. CBFs activate the expression of COR genes via binding to cis-element in the promoter of COR genes (CRT: TGGCCCGAC) (Figure 1) [23,26]. In addition to LT stress, CBFs also play important roles in other abiotic stresses [7,26,27]. Some of the cold-responsive genes have an ABA responsive element and dehydration responsive element in their promoter regions [28]. EgDREB1 from oil palm might also have a similar regulatory element located in a sequence promoter and are responsive to cold signaling [29]. It was also found in DREB [30] and FtbHLH2 that the transgenic plant promoter was induced by cold and that many cis-elements of the FtbHLH2 promoter collaborate in cold stress conditions [31].
Figure 1.
ICE-CBF-COR pathways in plants tolerance to cold stress. The expression of CBFs is mainly mediated by DELLA signaling and induced by ICE1. DELLAs contribute to the cold induction of CBF genes through interaction with JaZs signaling. CBFs activate the expression of COR genes via binding to cis-elements in the promoter of COR genes and result in the enhancement of cold tolerance in plants.
In temperate plants of wheat and barley, CBFs are found in the Frost Resistance 2 (FR2) locus [4]. Winter barleys planted in early autumn need collaboration between Vernalization 1(VRN1) and CBF genes to enhance cold acclimation. However, for spring barley, vernalization is not required. As a result, spring barley is not freezing tolerant. Similar to spring barley, 214 upregulated genes and 884 downregulated genes were found in grapevine cultivars after LT treatment [25], suggesting that cold stress induced the downregulated genes. This study found that CBF1, CBF2, and CBF3 had no role in cold acclimation in grapevine leaves. This is due to the absence of CBF1 and low levels of CBF2 and CBF3. However, there were some exceptional cases in the mechanisms of the CBF gene family expression. A study found that ICE1 mutation only had a small effect on the CBF transcript [21]. The expression of CBF1 is also regulated by the SVALKA-CBF1 cascade. SVALKA is a long non-coding RNA (lncRNA) that is transcribed by RNA polymerase II (RNAPII). Mutation in SVALKA affects expression of CBF1 and plant freezing tolerance. The SVALKA-CBF1 regulatory network has been found in species other than Arabidopsis [32].
The implementation of CBF-regulated genes is not only for cold stress but also for salt stress, hormone response, and even carbohydrate metabolism [33]. Previous research found that at 3 h or 24 h of cold treatment in Arabidopsis cbfs triple mutant, the number of up-regulated genes was 609 and 1375, respectively, and the number of down-regulated genes was 163 and 1349, respectively [33]. In addition, a total of 1394 cold-induced genes and 1113 cold-repressed genes were recognized in both the wild-type and cbfs triple mutant. This indicates that a large number of COR genes are not influenced in the cbfs triple mutant within cold stress.
In addition, numerous TFs that can regulate cold signaling and cold stress have been identified, such as CBF1, CBF2, CBF3, ICE1, ICE2, CAMTA3, MYB15, ZAT12, COR15a, and COR15b [34]. Some TF families found to be related to LT stress in Pyrus ussuriensis are DREB, WRKY, NAC, MYB, AP2/ERF, and bHLH [35]. Brassinosteroids are plant hormones that have an important role in plant growth and can also protect plants against abiotic stress such as cold stress. Two Brassinosteroids (BRs) TFs, namely, Brassinazole-resistance 1 (BZR1) and CESTA (CES), are direct regulators of CBF [36,37].
Several factors influence the binding affinities of TFs to specific sites, such as chromatin accessibility, DNA methylation, TF cooperativity, and TF interactions with non-binder cofactors and the transcription machinery [38]. A single TF regulates the expression of many downstream genes, so the utilization of TFs proffers many advantages in genetic engineering [39]. Some protein kinases (MEKK1-MKK1/2-MPK4) induce the expression of CBFs, especially CBF2 [6,40]. Another gene that also plays a role in the cold acclimation process is Cold Induced Small Protein 1 (CISP1), which was found in the roots of the Poaceae plant (a case study on barley). Normally, CISP1 is increased after 27 days of low-temperature treatment. Homologous adherents of CISP1 (i.e., CISP2 and CISP3) are also found in several Poaceae, and it also plays a role in the cold acclimation process [41].
Overexpression of FtbHLH2 in the transgenic plant increases the expressional level of CBF1-3 and enhances low temperature tolerance in plants [31]. Other studies suggested that ethylene response factors from Vitis amurensis, VaERF080, and VaERF087 (AP2/ERF Family) regulate the expressional levels of cold-related genes including CBF1, CBF2, ICE1, ZAT12, KIN1, SIZ1, RD29A, COR15A and COR47 [42]. When treated by cold stress, CBF1-8 of Brachypodium distachyon, a herbaceous grass species that can tolerate cold stress, was upregulated at a different time [43].
NADP-dependent D-sorbitol-6-phosphate dehydrogenase (S6PDH), anthocyanidin synthase (ANS), and phenylalanine ammonia-lyase (PAL) genes might play a vital role in the cold response of the loquat [44]. Studies reveals that the expression levels of S6PDH, ANS, and PAL are upregulated by cold treatment during the first 4 h but suppressed as the stress continues. A previous study shows that SiDHN found in Saussurea involucrata plays a pivotal role in low temperature and drought stress. After being grown under a low-temperature treatment for 24 h, the expression level of SiDHN is increased three-fold. This proved that SiDHN is responsive to cold stress [5]. Recently, Arabidopsis overexpressing RNA-DIRECTED DNA METHYLATION 4 (RDM4) showed the antagonist result with the rdm4 mutant in facing cold stress. Overexpression of RDM4 in plants increases the expression of CBFs and the downstream genes after chilling stress, subsequently improving the survival rate. However, while the rdm4 mutant shows an increase of electrolyte leakage and H2O2 content, the survival rate decreased after chilling treatment [45]. In Arabidopsis, RDM4 promotes the affinity of polymerase II (Pol II) to the promoter of CBF genes and, as a result, increases the cold tolerance of Arabidopsis [45].
In rice (Oryza sativa), during the recovery period from cold stress, transgenic lines overexpressing OsGH3-2 exhibit enhanced cold tolerance compared to wild type plants. Even after 7 days of recovery, more than 80% of the transgenic plants remain vigorous, whereas almost all wild type plants died [46]. Another study shows that OsMADS57 and OsTB1 are directly targets of OsWRKY94 and axillary bud regulated gene D14 during cold adaptation in rice. This provides evidence that OsMADS57 acts as a molecular linker between the developmental response and the tolerance to chilling stress in rice [47]. After the recovery process, OsMADS57 could still preserve cell division during low temperatures [47]. Transgenic tobacco overexpressing GhDREB1 showed improved tolerance to chilling stress compared to wild plants in early seedling and later seedling stages. GhDREB1 was also detected as a transcriptional activator of NtERD10B and NtERD10C after cold stress treatment in transgenic tobacco [48]. A gene that is likely responsible for cold stress was also found in Sorghum bicolor [49]. The results were obtained through significant regions, proxies, or co-localization with single nucleotide polymorphisms (SNPs) and also on homology with photosynthesis and stress-responsive genes. The gene is a GST gene family, namely SB08g007310 (Table 1). The gene function is also related to photosynthesis, and carbon and nitrogen metabolism [49]. Ectopic expression of a CBF pathway independent chilling tolerance gene (AtGRXS17) in tomato enhances chilling tolerance of tomato via collaboration with CBFs [50]. In recent years, transcriptome and bioinformatics has been increasingly used to address complex biological questions [51,52,53,54,55] and more cold stress related genes will be investigated. Moreover, a large number of mutant lines have been developed for the functional study of the genes in the plant genome, including those inserted by Transfer DNA (T-DNA) and RNA Interference (RNAi) [51,52]. In addition, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (Cas9), also known as a genome editing tool, allow scientists to change the DNA of an organism. CRISPR/Cas9 has been widely used, since it can be successfully used to edit multiple genes in a plant [56,57,58,59]. However, the lack of sufficient genomic sequence information in many plants is a limitation of CRISPR/Cas9. In the future, it would be prudent to develop this technology’s ability to elucidate multiple genes, TFs, and protein functions in plants that are yet to be identified and may have a role in cold stress.
Table 1.
Cold stress-related genes in plants and their expression under different type of stress conditions.
| Gene Name | Family | Species | Type of Stress Condition | References |
|---|---|---|---|---|
| FtbHLH2 | bHLH | Fagopyrum tataricum | Cold stress | [31] |
| BpUVR8 | UVR | Betula platyphylla | ABA response and cold stress | [60] |
| FDA2-3 | FDA | Gossypium hirsutum | Cold stress | [30] |
| FDA2-4 | ||||
| FDA8 | Arabidopsis thaliana | Cold stress | [30] | |
| Sb08g007310 | GST | Sorghum bicolor | Cold stress | [49] |
| Sb06g018220 | ZEP | Sorghum bicolor | Epoxidation of zeaxanthin in the xanthophyll cycle | [49] |
| AtGRXS17 | Trx | Solanum lycopersicum | Chilling stress | [50] |
| AtCBF3 | AP2/ERF | Arabidopsis | Cold Stress | [50] |
| VaERF080 | AP2/ERF | Vitis amurensis | Cold stress | [31] |
| VaERF087 | ||||
| SiDHN | DHN | Saussurea involucrata | Freezing stress and drought stress | [5] |
| OsGH3-2 | GH3 | Oryza sativa | Drought and cold stress | [46] |
| MYBS3 | MYB | Oryza sativa | Cold stress | [4] |
| RDM4 | Arabidopsis | Cold stress and freezing stress | [45] | |
| OsMADS57 | Oryza sativa | Chilling stress | [47] | |
| GHDREB1 | DREB |
Gossypium
hirsutum |
Chilling stress | [48] |
| AtHAP5A, AtXTH21 | Arabidopsis thaliana | Freezing stress | [61] | |
| PUB25/26 | Arabidpsis thaliana | Freezing stress | [62] | |
| MaPIP2-7 | AQP | Musa acuminata | Drought, cold and salt stress | |
| MaPIP2-7 | AQP | Musa acuminata | Drought, cold and salt stress | [17] |
| CsCPKs | CPK | Camellia sinensis | Cold tolerance | [63] |
| COR413 | COR | Saussurea involucrata | Cold and drought tolerance | [64] |
| SET, JmJC | Brassica rapa | Heat and cold stress | [65] | |
| TaTPS11 | Triticum aestivum | Cold stress | [66] | |
| TaSMT1, TaSMT2 | Triticum aestivum | Cold stress | [67] | |
| 14-3-3ε, 14-3-3ω | Arabidopsis thaliana | Cold and oxidative stress | [68] | |
| CsLEA | LEA | Camellia sinensis | Cold and dehydration stress | [69] |
| MdMYB108L | MYB | Malus domestica | Cold stress | [70] |
| MdHY5 | bZIP | Malus domestica | Cold stress | [70] |
| DlICE1 | bHLH | Dimocarpus longan | Cold stress | [71] |
| ZjICE1 | bHLH | Zoysia japonica | Cold, dehydration and salt stress | [72] |
| VvCBF | DREB | Vitis vinifera | Cold stress | [15] |
| AtGLR1.2 AtGLR1.3 | Arabidopsis thaliana | Cold stress | [16] | |
| STCH4 | Arabidopsis thaliana | Cold stress | [73] |
3. Physiological Changes during Cold Stress
A large number of plant species display physiological or cellular perturbations when encountering LT stress. Under LT stress, plants need to maintain cell behavior and activity, and, in particular, the stability of the cell membrane and structure of the protein with biological activity, for survival in adverse environments [47].The exposure of plants to subzero temperature leads to ice formation in plant tissues [74]. Higher concentrations of active ice nucleators in the apoplastic solution of plants leads to a higher freezing point. As a result, ice crystals first form in the extracellular space of plant cells. Ice formation outside cells reduces the water potential of the apoplastic solution, which leads to water flowing from the cells. Therefore, freezing stress at the cellular level is often followed by dehydration stress. Ice crystals will lead to an increase in electrolyte leakage and membrane lipid phase changes. As the freezing continues, osmotic forces produce cellular dehydration, which facilitates the formation of intracellular ice crystals. At the extreme, ice crystals can puncture plant cells and lead to cytosol outflow, and ultimately cause the plants to die [9,42,75]. Therefore, preventing formation of intracellular ice crystals and avoiding growth of ice crystals are important for plants to tolerate cold stress. The most popular approach used by plants to deal with LT stress is cold acclimation, which allows plants to survive freezing via accumulation of cryoprotective polypeptides (e.g., COR15a) and osmolytes (e.g., soluble sugars and proline). It has been reported that sugar content in Euphorbia resinifera, Echinocactus grusonii, Aloe vera, Crassula lacteal, Bryophyllum pinnatum, Yucca aloifolia, and Sansevieria trifasciata is increased after cold stress [76]. Cold adaptive plants always store more sugar (D-Glucose, D-Glucose 6 Phosphate, amylose, starch, and maltose) in their underground tissues [25,77].
A recent study of grapevine reveals that grapevine leaves have a watery appearance, indicating tissue damage and cell leakage after freezing treatment. After 4 days of recovery, damage was found in the leaves [25]. Necrosis of plants is normally caused by overproduction of Reactive Oxygen Species (ROS). The elevated H2O2 level in plants under LT stress is the result of increased oxygenation reaction in the chloroplasts, which leads to an increased glycolate content. The glycolate is converted to glyoxylate in peroxisomes by glycolate oxidase, which is accompanied by accumulation of H2O2. The physiological process of ROS toxic concentration in plants could be relieved by developing a complicated and efficient ROS scavenging and antioxidant defense system. Plants require the use of low ROS concentrations as mediators for signal transduction. Nitric oxide (NO) is implicated in the response of plants to LT stress. ABA, Ca2+, and H2O2-associated NO are shared by signaling cold stress events [78]. Hemoglobin (Hb) is believed to be a modifier of low-temperature plant response through the transition of NO [79]. Hb over-expressing lines have demonstrated reduced cold-induced gene expression. However, a decrease was only seen in CBF1 and CBF3, not CBF2 [80]. Moreover, an increased malondialdehyde (MDA) content and Ca2+ in the cytosol characterized lipid degradation, while the activity of antioxidant enzymes, such as Catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and peroxidase (POD), was minimized [22,42,81,82]. In addition, ABA and ROS can induce Ca2+ and will affect cold signaling [8].
After methyl jasmonate (MeJa) treatment in bell pepper [34] and ethylene treatment in Arabidopsis [42], bell pepper and Arabidopsis are more tolerant to cold stress and chilling stress. Their physiological function is changed, namely, via decrease of MDA content and increase of antioxidant enzyme activities, such as CAT, SOD, and POD. This proves that these plants have a high tolerance to cold stress [42]. Other research about cold stress revealed that cold could induce the inhibition of CO2 assimilation in Zea mays and was also related to a persistent depression of the photochemical efficiency of PSII [83]. Moreover, NO is known as a signal in an early transduction network. Cold triggers NO a few hours after exposure to low temperature in Arabidopsis [84]. Arabidopsis hemoglobin 1 (AHb1) interferes with NO production before and after exposure to low temperature. However, A. thaliana over-expressing AHb1 resists its capacity to produce NO during cold stress [84].
Nevertheless, lowering the temperature decreased the translocation of xylem N in S. cereale and B. napus by about 60% and 30%, respectively. It is suggested that low temperatures will directly affect the absorption mechanism of nitrates and N accumulation in the roots. This is caused by an obstruction of the N xylem flow greater than the NO absorption [85].
LT also contributed to decreasing chlorophyll content of rice. Putting rice at low temperatures modified the number of chloroplasts, the arrangement of grana (no normal stacked membranous structures), and the lamellar structures in chloroplasts. In addition, temperature-sensitive virescent (TSV) could improve the stability of OsTrxZ at low temperatures, which is critical to the production of chloroplasts during or after cold stress in Oryza sativa [86]. This is due to the abnormal development of chloroplasts during and after low-temperature stress [87].
4. Influence Factors
Plants can sense several parameters of light signals, such as light quality (wavelength), quantity, and duration (daylight), and even direction. The length of the daily photoperiod and moderate subfreezing temperature greatly affects the hardening and dehardening processes in Scots pine [81]. Short day (9 h) and long day (16 h) first frost temperatures are different with 16 h and 9 h daily photoperiod: the first frost is at about −10 °C and frozen at −22 °C. This means that light has a pivotal role in the hardening process. A previous study suggested that light is one of the regulators of FDA2-3 and FDA2-4 gene expression in cotton (Gossypium hirsutum). Light and low temperature induces the expression of these two genes and allows the plant to tolerate cold stress [30]. Meanwhile, LT reduces the utilization of light [82]. Light has a pivotal effect on the relationship between insects and plants, while temperature affects light performance [88]. However, the light also acts as an external signal that influences the growth of the plant [89].
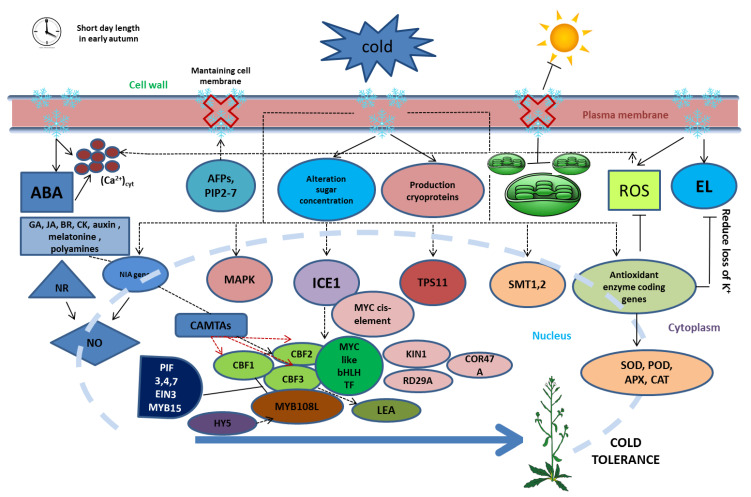
Similar to light, hormones also have a role in the activation process of TFs [35,37]. Although the ICE-CBF-COR pathway plays a key role in the cold tolerance in plants, researchers are currently focusing on the contribution of hormones to cold stress. Major hormones, such as ABA, GA, brassinosteroids (BR), jasmonates (JA), auxin, cytokinin (CK), melatonin, and polyamines, affect CBFs (Figure 2) [37]. One of the most important hormones in cold stress is ABA [90]. ABA increases when stress surge affects plants and, contrary to ABA, the TF is not affected. Furthermore, an overlapping linkage between the ABA-dependent pathway and the ICE-CBF-COR pathway was found in cold tolerance [37]. A number of studies suggest that ABA can induce increases in the transcript levels of CBF genes, perhaps via binding to the CRT/DRE element. It has the potential to encourage CBF activation. In addition, AtHAP5A regulates freezing stress tolerance through binding to the CCAAT motif of AtXTH21 in Arabidopsis. AtHAP5A and AtXTH21 overexpressing plants were more tolerant of freezing stress but less susceptible to ABA than WT plants [61]. Moreover, ABA also plays an important role in the acclimation process and can induce COR gene expression [26]. Some genes in Pyrus ussuriensis also collaborate with ABA, Gibberellin, and Ethylene to up-regulate genes [35]. The ABA signal transduction pathway also has a positive regulator, namely, the BpUVR8 gene, which regulates the expression of a subset of ABA-responsive genes, both in Arabidopsis and Betula platyphylla under ABA treatment [60]. ABA pretreatment was also successful in increasing the mechanism of cold stress tolerance in the root level of Brassica rapa with phase <3 h and decreased the deleterious effect of Paclobutrazol (PBZ), which reduced the root hydraulic conductance of B. rapa [91].
Figure 2.
The mechanism of cold tolerance in plants. A short day in early autumn represents the first initiation of cold stress. AFPs and PIP2-7 slow ice crystal formation to maintain cell membranes and reduce membrane injury. However, low temperature (LT) still causes some changes in membrane structure, sugar concentration, and production in cry proteins. LT initiates the increase of ABA, EL, (Ca2+)cyt, and ROS accumulation but decreases chloroplast number. LT induces the expression of some genes, such as NIA, MAPK, TPS11, SMT1,2, ICE1 and antioxidant enzyme coding genes. Antioxidant enzyme coding genes reduce the EL and increase the activity of the antioxidant enzymes in cold stress plants. Meanwhile, NIA genes and NR initiate NO as a result of LT. ICE1, CAMTAs, NIA, and hormones induce the expression of CBFs, which bind to CRT/DRE cis-elements to enhance cold tolerance. ICE1-CBFs induce expression of cold-responsive genes, such as KIN1, RD29A, COR47A, and LEA, during cold stress.
Other studies have found plant mutants with altered levels of GA. GA will increase rice tolerance in stress. In research of B. napus, the gibberellin 2 oxidase gene was added into a transgenic plant and caused dwarfism. In comparison to the WT plants, the dwarf transgenic plants were 18.3–26.1% shorter in height and had 17.8–33.6% shorter internodes, and smaller and dark green leaves. GA and JA play an efficient role in the ICE-CBF-COR pathway [37]. Jasmonates activate TFs and then TFs will bind with the cis-acting element in the promoter of target genes [92].
Ethylene, ABA, and Jasmonates hormones can induce the expression of ethylene-responsive (ERF) genes [93]. These genes can be found in species such as Arabidopsis (AtERF6), Citrus sinensis (CsERF), Lycopersicon esculentum (LeERF3b; SIERF5), Nicotiana tabacum (JERF1; JERF3), Triticum aestivum (TaERF1), and Triticum turgidum (TdSHN1). At low temperature, ERFs will bind with the GCC box and DRE elements and provide some plants with tolerance to cold stress.
In addition, the BpGH3.5 gene also affects Indole-3-acetic acid (IAA) in birch. This is shown by the decrease of auxin, which is indicated by a decrease in IAA. Some research results show that GH3 gene families play an important role in plant growth and development, and the short root phenotype in transgenic birch is caused by changes in IAA levels [94]. In another study, it was also mentioned that BpGH3.5 causes primary short and lateral roots and more root hairs. More root hairs are caused by increased surface area for nutrient and water uptake, and finally lead to short root length (root dwarfism) [95].
Transgenic plants produce more anthocyanins in winter. It is well known that the anthocyanin content in plants will be higher in cold stress conditions. This means that the Gibberellin 2-oxidase gene perhaps has an important role in cold stress [96]. Carbon ion beam irradiation is one of the tools that can upregulate the expression levels of CBFs, ICE1, ICE2, CAMTA3, and COR genes in cold stress [22]. In addition, melatonin is also known as an influencer of the increase of the levels of mRNA, such as COR15a [37]. Expression of those genes is increased by 50 Gy of carbon ion beam irradiation for 6 h and 12 h at 4 °C. Nonetheless, in Arabidopsis, carbon ion beam irradiation of 50 Gy increases the content of AsA and GSH, which play important roles in alleviating ROS generation and oxidative stress under cold stress [22]. The level of soil moisture and stomatal conductivity has been determined by Rihan et al. [26]; these two factors can up-regulate the CBF/DREB1 gene in cauliflower (Brassica oleracea var. botrytis). In fact, the addition of methyl jasmonate to bell pepper can induce POD, CAT, and APX relative gene expression. These genes will reduce the ROS content, so the plant will tolerate chilling stress [34].
In addition to the factors noted above, the circadian clock also plays an important role in cold stress [97]. Circadian clocks are original timekeeping networks that authorize organisms to align their physiology processes with environmental changes at relevant times of the day and year. Recently, researchers have found that there are tissue-specific clocks in the plant’s body. However, further research is still needed to discover how the plant tissues are organized and communicate with each other [97]. Geographical location (latitude) and mutation affect the expression of CBF1 and CBF2 in A. thaliana. When tested under cold treatment, northern areas tend to have higher rates of survival than southern areas [23].
5. Conclusions
Cold tolerance in plants is a complex process. Chilling or freezing temperatures can trigger the formation of ice in plant tissues, which causes cellular dehydration. On the other hand, plants can protect their body by preventing ice formation. As a complex process, the mechanism of cold stress in plants is not only controlled by ICE-CBF-COR genes but also other factors, such as hormones, circadian clocks, and light. ICE is a CBF regulator, and CBFs control the expression of the COR gene when cold stress takes place. The COR gene is a critical gene that is responsible for chilling tolerance and cold acclimation processes in plants. Under normal conditions, CBFs are regulated by the circadian clock and the photoperiod. Under cold stress conditions, however, CBFs induce several cold stress-related genes to regulate the cold tolerance of plants.
Acknowledgments
The authors sincerely thank State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, especially for providing a good environment during the writing process. The authors appreciate the reviewers for comments and suggestions.
Author Contributions
F.N.R. had contributed to writing and original draft preparation, S.C. had contributed to supervision, project administration, funding acquisition, review, and editing manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review is funded by The Fundamental Research Funds for The Central Universities, grant number 2572019CG08 and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mboup M., Fischer I., Lainer H., Stephan W. Trans-species polymorphism and allele-specific expression in the cbf gene family of wild tomatoes. Mol. Biol. Evol. 2012;29:3641–3652. doi: 10.1093/molbev/mss176. [DOI] [PubMed] [Google Scholar]
- 2.Theocharis A., Clément C., Barka E.A. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235:1091–1105. doi: 10.1007/s00425-012-1641-y. [DOI] [PubMed] [Google Scholar]
- 3.Zhou M., Chen H., Wei D., Ma H., Lin J. Arabidopsis I3 and DELLAs positively regulate each other in response to low temperature. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep39819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mickelbart M.V., Hasegawa P.M., Bailey-Serres J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015;16:237–251. doi: 10.1038/nrg3901. [DOI] [PubMed] [Google Scholar]
- 5.Guo X., Zhang L., Zhu J., Liu H., Wang A. Cloning and characterization of SiDHN, a novel dehydrin gene from Saussurea involucrata Kar. et Kir. that enhances cold and drought tolerance in tobacco. Plant Sci. 2017;256:160–169. doi: 10.1016/j.plantsci.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Zhou J. MAPping Kinase Regulation of ICE1 in Freezing Tolerance. Trends Plant Sci. 2018;23:91–93. doi: 10.1016/j.tplants.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y., Ding Y., Yang S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018;23:623–637. doi: 10.1016/j.tplants.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Chinnusamy V., Zhu J., Zhu J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F., Jiang Y., Bai L.-P., Zhang L., Chen L.-J., Li H.G., Yin Y., Yan W.-W., Yi Y., Guo Z.-F. The ICE-CBF-COR Pathway in Cold Acclimation and AFPs in Plants. Middle-East J. Sci. Res. 2011;8:493–498. [Google Scholar]
- 10.Zhao C., Lang Z., Zhu J.K. Cold responsive gene transcription becomes more complex. Trends Plant Sci. 2015;20:466–468. doi: 10.1016/j.tplants.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Da Silva J.A.T., Niu M., Li M., He C., Zhao J., Zeng S., Duan J., Ma G. Physiological and transcriptomic analyses reveal a response mechanism to cold stress in Santalum album L. Leaves. Sci. Rep. 2017;7:42165. doi: 10.1038/srep42165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey S., Carrer M., Castagneri D., Petit G. Xylem anatomical responses to climate variability in Himalayan birch trees at one of the world’s highest forest limit. Perspect. Plant Ecol. Evol. Syst. 2018;33:34–41. doi: 10.1016/j.ppees.2018.05.004. [DOI] [Google Scholar]
- 13.Hao X., Wang B., Wang L., Zeng J., Yang Y., Wang X. Comprehensive transcriptome analysis reveals common and specific genes and pathways involved in cold acclimation and cold stress in tea plant leaves. Sci. Hortic. 2018;240:354–368. doi: 10.1016/j.scienta.2018.06.008. [DOI] [Google Scholar]
- 14.Mata C.I., Hertog M.L., Van Raemdonck G., Baggerman G., Tran D., Nicolai B.M. Omics analysis of the ethylene signal transduction in tomato as a function of storage temperature. Postharvest Biol. Technol. 2019;155:1–10. doi: 10.1016/j.postharvbio.2019.04.016. [DOI] [Google Scholar]
- 15.Rubio S., Pérez F.J. ABA and its signaling pathway are involved in the cold acclimation and deacclimation of grapevine buds. Sci. Hortic. 2019;256:108565. doi: 10.1016/j.scienta.2019.108565. [DOI] [Google Scholar]
- 16.Zheng Y., Luo L., Wei J., Chen Q., Yang Y., Hu X., Kong X. The glutamate receptors AtGLR1. 2 and AtGLR1. 3 increase cold tolerance by regulating jasmonate signaling in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018;506:895–900. doi: 10.1016/j.bbrc.2018.10.153. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Hu W., Liu J., Song S., Hou X., Jia C., Li J., Miao H., Wang Z., Tie W. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.) Plant Physiol. Biochem. 2020;147:66–76. doi: 10.1016/j.plaphy.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Tolosa L.N., Zhang Z. The Role of Major Transcription Factors in Solanaceous Food Crops under Different Stress Conditions: Current and Future Perspectives. Plants. 2020;9:56. doi: 10.3390/plants9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puijalon S., Bouma T.J., Douady C.J., Groenendael J.V., Anten N.P.R., Martel E., Bornette G. Plant resistance to mechanical stress: Evidence of an avoidance—Tolerance trade-off. New Phytol. 2011;191:1141–1149. doi: 10.1111/j.1469-8137.2011.03763.x. [DOI] [PubMed] [Google Scholar]
- 20.Jutsz A.M., Gnida A. Mechanisms of stress avoidance and tolerance by plants used in phytoremediation of heavy metals. Arch. Environ. Prot. 2015;41:104–114. doi: 10.1515/aep-2015-0045. [DOI] [Google Scholar]
- 21.Zarka D.G., Vogel J.T., Cook D., Thomashow M.F. Cold Induction of Arabidopsis. Plant Physiol. 2003;133:910–918. doi: 10.1104/pp.103.027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Ma R., Yin Y., Jiao Z. Role of carbon ion beams irradiation in mitigating cold stress in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2018;162:341–347. doi: 10.1016/j.ecoenv.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhen Y., Ungerer M.C. Relaxed selection on the CBF/DREB1 regulatory genes and reduced freezing tolerance in the southern range of Arabidopsis thaliana. Mol. Biol. Evol. 2008;25:2547–2555. doi: 10.1093/molbev/msn196. [DOI] [PubMed] [Google Scholar]
- 24.Borba A.R., Serra T.S., Górska A., Gouveia P., Cordeiro A.M., Reyna-Llorens I., Kneřová J., Barros P.M., Abreu I.A., Oliveira M.M., et al. Synergistic binding of bHLH transcription factors to the promoter of the maize NADP-ME gene used in C4photosynthesis is based on an ancient code found in the ancestral C3state. Mol. Biol. Evol. 2018;35:1690–1705. doi: 10.1093/molbev/msy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Londo J.P., Kovaleski A.P., Lillis J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera) Hortic. Res. 2018;5 doi: 10.1038/s41438-018-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rihan H.Z., Al-Issawi M., Fuller M.P. Upregulation of CBF/DREB1 and cold tolerance in artificial seeds of cauliflower (Brassica oleracea var. botrytis) Sci. Hortic. 2017;225:299–309. doi: 10.1016/j.scienta.2017.07.017. [DOI] [Google Scholar]
- 27.Lata C., Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011;62:4731–4748. doi: 10.1093/jxb/err210. [DOI] [PubMed] [Google Scholar]
- 28.Yadav S.K. Cold stress tolerance mechanisms in plants. Sustain. Agric. 2009;2:605–620. doi: 10.1007/978-94-007-0394-0_27. [DOI] [Google Scholar]
- 29.Azzeme A.M., Abdullah S.N.A., Aziz M.A., Wahab P.E.M. Oil palm drought inducible DREB1 induced expression of DRE/CRT- and non-DRE/CRT-containing genes in lowland transgenic tomato under cold and PEG treatments. Plant Physiol. Biochem. 2017;112:129–151. doi: 10.1016/j.plaphy.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Kargiotidou A., Deli D., Galanopoulou D., Tsaftaris A., Farmaki T. Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum) J. Exp. Bot. 2008;59:2043–2056. doi: 10.1093/jxb/ern065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao P., Sun Z., Li C., Zhao X., Li M., Deng R., Huang Y., Zhao H., Chen H., Wu Q. Overexpression of Fagopyrum tataricum FtbHLH2 enhances tolerance to cold stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2018;125:85–94. doi: 10.1016/j.plaphy.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Kindgren Transcriptional read-through of the long non-coding RNA SVALKA governs plant cold acclimation. bioXriv. 2018;77058:1–31. doi: 10.1038/s41467-018-07010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia Y., Ding Y., Shi Y., Zhang X., Gong Z., Yang S. The cbfs triple mutants reveal the essential functions of CBF s in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- 34.Wang S., Yang C., Zhao X., Chen S., Qu G.-z. Complete chloroplast genome sequence of Betula platyphylla: Gene organization, RNA editing, and comparative and phylogenetic analyses. BMC Genomics. 2018;19:950. doi: 10.1186/s12864-018-5346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang T., Huang X.S. Deep sequencing-based characterization of transcriptome of Pyrus ussuriensis in response to cold stress. Gene. 2018;661:109–118. doi: 10.1016/j.gene.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 36.Barrero-Gil J., Salinas J. CBFs at the Crossroads of Plant Hormone Signaling in Cold Stress Response. Mol. Plant. 2017;10:542–544. doi: 10.1016/j.molp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Wang D.-Z., Jin Y.-N., Ding X.-H., Wang W.-J., Zhai S.-S., Bai L.-P., Guo Z.-F. Gene regulation and signal transduction in the ICE–CBF–COR signaling pathway during cold stress in plants. Biochem. (Mosc.) 2017;82:1103–1117. doi: 10.1134/S0006297917100030. [DOI] [PubMed] [Google Scholar]
- 38.Franco-Zorrilla J.M., Solano R. Identification of plant transcription factor target sequences. Biochim. Biophys. Acta-Gene Regul. Mech. 2017;1860:21–30. doi: 10.1016/j.bbagrm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Yanagisawa S. Dof Domain Proteins: Plant-Specific Transcription Factors Associated with Div. Library. 2004;45:386–391. doi: 10.1093/pcp/pch055. [DOI] [PubMed] [Google Scholar]
- 40.Matsukura S., Mizoi J., Yoshida T., Todaka D., Ito Y., Maruyama K., Shinozaki K., Yamaguchi-Shinozaki K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genom. 2010;283:185–196. doi: 10.1007/s00438-009-0506-y. [DOI] [PubMed] [Google Scholar]
- 41.Ying M., Kidou S.i. Discovery of novel cold-induced CISP genes encoding small RNA-binding proteins related to cold adaptation in barley. Plant Sci. 2017;260:129–138. doi: 10.1016/j.plantsci.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Sun X., Zhu Z., Zhang L., Fang L., Zhang J., Wang Q., Li S., Liang Z., Xin H. Overexpression of ethylene response factors VaERF080 and VaERF087 from Vitis amurensis enhances cold tolerance in Arabidopsis. Sci. Hortic. 2019;243:320–326. doi: 10.1016/j.scienta.2018.08.055. [DOI] [Google Scholar]
- 43.Hao J., Yang J., Dong J., Fei S.z. Characterization of BdCBF genes and genome-wide transcriptome profiling of BdCBF3-dependent and -independent cold stress responses in Brachypodium distachyon. Plant Sci. 2017;262:52–61. doi: 10.1016/j.plantsci.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Lou X., Wang H., Ni X., Gao Z., Iqbal S. Integrating proteomic and transcriptomic analyses of loquat (Eriobotrya japonica Lindl.) in response to cold stress. Gene. 2018;677:57–65. doi: 10.1016/j.gene.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Chan Z., Wang Y., Cao M., Gong Y., Mu Z., Wang H., Hu Y., Deng X., He X.J., Zhu J.K. RDM 4 modulates cold stress resistance in Arabidopsis partially through the CBF-mediated pathway. New Phytol. 2016;209:1527–1539. doi: 10.1111/nph.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du H., Wu N., Fu J., Wang S., Li X., Xiao J., Xiong L. A GH3 family member, OsGH3-2, modulates auxin and methylation and chromatin patterning abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012;63:6467–6480. doi: 10.1093/jxb/ers300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Zhao Y., Xu S., Zhang Z., Xu Y., Zhang J., Chong K. Os MADS 57 together with Os TB 1 coordinates transcription of its target Os WRKY 94 and D14 to switch its organogenesis to defense for cold adaptation in rice. New Phytol. 2018;218:219–231. doi: 10.1111/nph.14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan D.P., Huang J.G., Yang Y.T., Guo Y.H., Wu C.A., Yang G.D., Gao Z., Zheng C.C. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz D., Hu J., Salas Fernandez M.G. Genetic architecture of photosynthesis in Sorghum bicolor under non-stress and cold stress conditions. J. Exp. Bot. 2017;68:4545–4557. doi: 10.1093/jxb/erx276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y., Wu Q., Sprague S.A., Park J., Oh M., Rajashekar C.B., Koiwa H., Nakata P.A., Cheng N., Hirschi K.D., et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2015;2:1–11. doi: 10.1038/hortres.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gang H., Liu G., Zhang M., Zhao Y., Jiang J., Chen S. Comprehensive characterization of T-DNA integration induced chromosomal rearrangement in a birch T-DNA mutant. BMC Genom. 2019;20:311. doi: 10.1186/s12864-019-5636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gang H., Li R., Zhao Y., Liu G., Chen S., Jiang J. Loss of GLK1 transcription factor function reveals new insights in chlorophyll biosynthesis and chloroplast development. J. Exp. Bot. 2019;70:3125–3138. doi: 10.1093/jxb/erz128. [DOI] [PubMed] [Google Scholar]
- 53.Chen S., Lin X., Zhang D., Li Q., Zhao X., Chen S. Genome-Wide Analysis of NAC Gene Family in Betula pendula. Forests. 2019;10:741. doi: 10.3390/f10090741. [DOI] [Google Scholar]
- 54.Wang F., Chen S., Liang D., Qu G.-Z., Chen S., Zhao X. Transcriptomic analyses of Pinus koraiensis under different cold stresses. BMC Genom. 2020;21:1–14. doi: 10.1186/s12864-019-6401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S., Huang H., Han R., Liu C., Qiu Z., Liu G., Chen S., Jiang J. Negative feedback loop between BpAP1 and BpPI/BpDEF heterodimer in Betula platyphylla× B. pendula. Plant Sci. 2019;289:110280. doi: 10.1016/j.plantsci.2019.110280. [DOI] [PubMed] [Google Scholar]
- 56.Jyoti A., Kaushik S., Srivastava V.K., Datta M., Kumar S., Yugandhar P., Kothari S.L., Rai V., Jain A. The potential application of genome editing by using CRISPR/Cas9, and its engineered and ortholog variants for studying the transcription factors involved in the maintenance of phosphate homeostasis in model plants. Semin. Cell Dev. Biol. 2019;96:77–90. doi: 10.1016/j.semcdb.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Yubing H., Min Z., Lihao W., Junhua W., Qiaoyan W., Rongchen W., Yunde Z. Improvements of TKC technology accelerate isolation of transgene-free CRISPR/Cas9-edited rice plants. Rice Sci. 2019;26:109–117. doi: 10.1016/j.rsci.2018.11.001. [DOI] [Google Scholar]
- 58.Abdelrahman M., Al-Sadi A.M., Pour-Aboughadareh A., Burritt D.J., Tran L.-S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol. Biochem. 2018;131:31–36. doi: 10.1016/j.plaphy.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Ma X., Zhu Q., Chen Y., Liu Y.-G. CRISPR/Cas9 platforms for genome editing in plants: Developments and applications. Mol. Plant. 2016;9:961–974. doi: 10.1016/j.molp.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Ma M., Shao W., Wang H., Fan R., Chen X., Wang X., Zhan Y., Zeng F. Molecular cloning and functional analysis of a UV-B photoreceptor gene, BpUVR8 (UV Resistance Locus 8), from birch and its role in ABA response. Plant Sci. 2018;274:294–308. doi: 10.1016/j.plantsci.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Shi H., Ye T., Zhong B., Liu X., Jin R., Chan Z. At HAP 5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 2014;203:554–567. doi: 10.1111/nph.12812. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Zeng W., Ding Y., Wang Y., Niu L., Yao J.-l., Pan L., Lu Z., Cui G., Li G., et al. Plant Science Peach ethylene response factor PpeERF2 represses the expression of ABA biosynthesis and cell wall degradation genes during fruit ripening. Plant Sci. 2019;283:116–126. doi: 10.1016/j.plantsci.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Ding C., Lei L., Yao L., Wang L., Hao X., Li N., Wang Y., Yin P., Guo G., Yang Y. The involvements of calcium-dependent protein kinases and catechins in tea plant [Camellia sinensis (L.) O. Kuntze] cold responses. Plant Physiol. Biochem. 2019;143:190–202. doi: 10.1016/j.plaphy.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Guo X., Zhang L., Dong G., Xu Z., Li G., Liu N., Wang A., Zhu J. A novel cold-regulated protein isolated from Saussurea involucrata confers cold and drought tolerance in transgenic tobacco (Nicotiana tabacum) Plant Sci. 2019;289:110246. doi: 10.1016/j.plantsci.2019.110246. [DOI] [PubMed] [Google Scholar]
- 65.Liu G., Khan N., Ma X., Hou X. Identification, Evolution, and Expression Profiling of Histone Lysine Methylation Moderators in Brassica rapa. Plants. 2019;8:526. doi: 10.3390/plants8120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X., Fu L., Qin P., Sun Y., Liu J., Wang X. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana. Gene. 2019;710:210–217. doi: 10.1016/j.gene.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Valitova J., Renkova A., Mukhitova F., Dmitrieva S., Beckett R.P., Minibayeva F.V. Membrane sterols and genes of sterol biosynthesis are involved in the response of Triticum aestivum seedlings to cold stress. Plant Physiol. Biochem. 2019;142:452–459. doi: 10.1016/j.plaphy.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 68.Visconti S., D’Ambrosio C., Fiorillo A., Arena S., Muzi C., Zottini M., Aducci P., Marra M., Scaloni A., Camoni L. Overexpression of 14-3-3 proteins enhances cold tolerance and increases levels of stress-responsive proteins of Arabidopsis plants. Plant Sci. 2019;289:110215. doi: 10.1016/j.plantsci.2019.110215. [DOI] [PubMed] [Google Scholar]
- 69.Wang W., Gao T., Chen J., Yang J., Huang H., Yu Y. The late embryogenesis abundant gene family in tea plant (Camellia sinensis): Genome-wide characterization and expression analysis in response to cold and dehydration stress. Plant Physiol. Biochem. 2019;135:277–286. doi: 10.1016/j.plaphy.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Mao Z., Jiang H., Zhang Z., Chen X. A feedback loop involving MdMYB108L and MdHY5 controls apple cold tolerance. Biochem. Biophys. Res. Commun. 2019;512:381–386. doi: 10.1016/j.bbrc.2019.03.101. [DOI] [PubMed] [Google Scholar]
- 71.Yang X., Wang R., Hu Q., Li S., Mao X., Jing H., Zhao J., Hu G., Fu J., Liu C. DlICE1, a stress-responsive gene from Dimocarpus longan, enhances cold tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2019;142:490–499. doi: 10.1016/j.plaphy.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Zuo Z.-F., Kang H.-G., Park M.-Y., Jeong H., Sun H.-J., Song P.-S., Lee H.-Y. Zoysia japonica MYC type transcription factor ZjICE1 regulates cold tolerance in transgenic Arabidopsis. Plant Sci. 2019;289:110254. doi: 10.1016/j.plantsci.2019.110254. [DOI] [PubMed] [Google Scholar]
- 73.Yu H., Kong X., Huang H., Wu W., Park J., Yun D.-J., Lee B.-h., Shi H., Zhu J.-K. STCH4/REIL2 Confers Cold Stress Tolerance in Arabidopsis by Promoting rRNA Processing and CBF Protein Translation. Cell Rep. 2020;30:229–242.e225. doi: 10.1016/j.celrep.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Puhakainen T. Short-Day Potentiation of Low Temperature-Induced Gene Expression of a C-Repeat-Binding Factor-Controlled Gene during Cold Acclimation in Silver Birch. Plant Physiol. 2004;136:4299–4307. doi: 10.1104/pp.104.047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demidchik V., Straltsova D., Medvedev S.S., Pozhvanov G.A., Sokolik A., Yurin V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014;65:1259–1270. doi: 10.1093/jxb/eru004. [DOI] [PubMed] [Google Scholar]
- 76.Khan H., Shah S.H., Uddin N., Azhar N., Asim M., Syed S., Ullah F., Tawab F., Inayat J. Biochemical and Physiological Changes of Different Plants Species in Response To Heat and Cold Stress. ARPN J. Agric. Biol. Sci. 2015;10:213–216. [Google Scholar]
- 77.Janská A., Maršík P., Zelenková S., Ovesná J. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010;12:395–405. doi: 10.1111/j.1438-8677.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- 78.Ding Y., Shi Y., Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–1704. doi: 10.1111/nph.15696. [DOI] [PubMed] [Google Scholar]
- 79.Abiri R., Azmi N., Maziah M., Norhana Z., Yusof B., Atabaki N., Sahebi M., Valdiani A., Kalhori N., Azizi P., et al. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017;134:33–44. doi: 10.1016/j.envexpbot.2016.10.015. [DOI] [Google Scholar]
- 80.Gupta K.J., Hincha D.K., Mur L.A. NO way to treat a cold. New Phytol. 2011;189:360–363. doi: 10.1111/j.1469-8137.2010.03586.x. [DOI] [PubMed] [Google Scholar]
- 81.Beck E.H., Heim R., Hansen J. Plant resistance to cold stress: Mechanisms and environmental signals triggering frost hardening and dehardening. J. Biosci. 2004;29:449–459. doi: 10.1007/BF02712118. [DOI] [PubMed] [Google Scholar]
- 82.Longo V., Kamran R.V., Michaletti A., Toorchi M., Zolla L., Rinalducci S. Proteomic and Physiological Response of Spring Barley Leaves to Cold Stress. Int. J. Plant Biol. Res. 2017;5:1–10. [Google Scholar]
- 83.Savitch L.V., Ivanov A.G., Gudynaite-Savitch L., Huner N.P.A., Simmonds J. Cold stress effects on PSI photochemistry in Zea mays: Differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol. 2011;52:1042–1054. doi: 10.1093/pcp/pcr056. [DOI] [PubMed] [Google Scholar]
- 84.Cantrel C., Vazquez T., Puyaubert J., Rezé N., Lesch M., Kaiser W.M., Dutilleul C., Guillas I., Zachowski A., Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189:415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 85.Laine P., Bigot J., Ourry A., Boucaud J. Effects of low temperature on nitrate uptake, and xylem and phloem flows of nitrogen, in Secale cereale L. and Brassica napus L. New Phytol. 1994;127:675–683. doi: 10.1111/j.1469-8137.1994.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 86.Sun J., Zheng T., Yu J., Wu T., Wang X., Chen G., Tian Y., Zhang H., Wang Y., Terzaghi W. TSV, a putative plastidic oxidoreductase, protects rice chloroplasts from cold stress during development by interacting with plastidic thioredoxin Z. New Phytol. 2017;215:240–255. doi: 10.1111/nph.14482. [DOI] [PubMed] [Google Scholar]
- 87.Cui X., Wang Y., Wu J., Han X., Gu X., Lu T., Zhang Z. The RNA editing factor DUA 1 is crucial to chloroplast development at low temperature in rice. New Phytol. 2019;221:834–849. doi: 10.1111/nph.15448. [DOI] [PubMed] [Google Scholar]
- 88.Garsed S., Davey H., Galley D. The Effects of Light and Temperature on the Growth of and Balances of Carbon, Nitrogen and Potassium between Vicia faba L. and Aphis fabae Scop. New Phytol. 1987;107:77–102. doi: 10.1111/j.1469-8137.1987.tb04884.x. [DOI] [Google Scholar]
- 89.Maibam P., Nawkar G.M., Park J.H., Sahi V.P., Lee S.Y., Kang C.H. The influence of light quality, circadian rhythm, and photoperiod on the CBF-mediated freezing tolerance. Int. J. Mol. Sci. 2013;14:11527–11543. doi: 10.3390/ijms140611527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shinozaki K., Kazuko Y. Molecular responses to drought and cold stress. Biotechnology. 1996;7:161–167. doi: 10.1016/S0958-1669(96)80007-3. [DOI] [PubMed] [Google Scholar]
- 91.Bigot J., Boucaud J. Effects of synthetic plant growth retardants and abscisic acid on root functions of Brassica rapa plants exposed to low root-zone temperature. New Phytol. 1998;139:255–265. doi: 10.1046/j.1469-8137.1998.00201.x. [DOI] [Google Scholar]
- 92.Zhou M., Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016;34:441–449. doi: 10.1016/j.biotechadv.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Müller M., Munné-Bosch S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015;169:32–41. doi: 10.1104/pp.15.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang G., Chen S., Jiang J. Transcriptome analysis reveals the role of BpGH3.5 in root elongation of Betula platyphylla × B. pendula. Plant CellTissue Organ Cult. 2015;121:605–617. doi: 10.1007/s11240-015-0731-5. [DOI] [Google Scholar]
- 95.Yang G., Chen S., Wang S., Liu G., Li H., Huang H., Jiang J. BpGH3.5, an early auxin-response gene, regulates root elongation in Betula platyphylla × Betula pendula. Plant CellTissue Organ Cult. 2015;120:239–250. doi: 10.1007/s11240-014-0599-9. [DOI] [Google Scholar]
- 96.Zhou B., Lin J., Peng W., Peng D., Zhuo Y., Zhu D., Huang X., Tang D., Guo M., He R., et al. Dwarfism in Brassica napus L. induced by the over-expression of a gibberellin 2-oxidase gene from Arabidopsis thaliana. Mol. Breed. 2012;29:115–127. doi: 10.1007/s11032-010-9530-1. [DOI] [Google Scholar]
- 97.Nohales M.A., Kay S.A. Molecular mechanisms at the core of the plant circadian oscillator. Nat. Struct. Mol. Mol. Biol. 2016;23:1061–1069. doi: 10.1038/nsmb.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]