Abstract
Objective
In contrast to acrometastasis, defined as bone metastasis to the hand or foot, the frequency and prognosis of bone metastasis of other limb segments remain unclear. To compare prognosis according to sites of bone metastasis, we defined two new terms in this study: ‘mesometastasis’ and ‘rhizometastasis’ as bone metastasis of ‘forearm or lower leg’ and ‘arm or thigh’, respectively.
Methods
A total of 539 patients who were registered to the bone metastasis database of The University of Tokyo Hospital from April 2012 to May 2016 were retrospectively surveyed. All patients who were diagnosed to have bone metastases in our hospital are registered to the database. Patients were categorized into four groups according to the most distal site of bone metastases: ‘acrometastasis’, ‘mesometastasis’, ‘rhizometastasis’ and ‘body trunk metastasis’.
Results
The frequency of rhizometastasis (22.5%) or body trunk metastasis (73.1%) was significantly higher than that of acrometastasis (2.0%) or mesometastasis (2.4%). The median survival time after diagnosis of bone metastases for each group was as follows: 6.5 months in acrometastasis, 4.0 months in mesometastasis, 16 months in rhizometastasis, 17 months in body trunk metastasis and 16 months overall. In survival curve, there was a statistically significant difference between mesometastasis and body trunk metastasis.
Conclusions
Our findings suggest that ‘mesometastasis’ could be another poor prognostic factor in cancer patients and that patients with mesometastasis should receive appropriate treatments according to their expected prognosis.
Keywords: neoplasm, metastasis, prognosis
We coined a new word mesometastasis: bone metastasis in the forearm or lower leg. Like acrometastasis, mesometastasis could be a poor prognostic factor in cancer patients.
Introduction
Filsakova first coined the term ‘acrometastasis’ as bone metastases of the hand and foot (1,2). ‘Acro’, ‘meso’ and ‘rhizo’ are anatomical words meaning distal, middle and proximal limb segments, respectively. These words are used, for example, as prefixes in such as acromelia, mesomelia and rhizomelia regarding limb shortenings.
In previous reports, acrometastasis only accounts for ~0.1% of all bone metastases (3,4), and its prognosis is ~6 months of life expectancy after diagnosis (3,5,6). Although acrometastasis is known for its rareness and poor prognosis, the frequency and prognosis of bone metastasis of other limb segments remain to be clarified.
In this study, we defined two new terms, ‘mesometastasis’ and ‘rhizometastasis’, as bone metastasis of ‘forearm or lower leg’ and ‘arm or thigh’, respectively. The frequency and prognosis of each metastasis group were surveyed.
Methods
A total of 539 patients who were registered to the bone metastasis database of The University of Tokyo Hospital from April 2012 to May 2016 were retrospectively surveyed. All patients diagnosed to have bone metastases in our hospital were automatically registered to the database without any exceptions. Patients with hematologic malignancy were excluded from this study.
For this study, all bone metastases were reconfirmed retrospectively by three to four orthopedic surgeons only based on either or both the reports from radiologists and the image examinations already performed: X-ray, computed tomography (CT) scan, magnetic resonance imaging (MRI), positron emission tomography (PET) scan or bone scintigraphy. All image examinations were performed by attending physicians or based on the advice from the bone metastasis board according to the necessity, which means not all examinations were routinely performed.
Patients were categorized into four groups according to the site of bone metastases: ‘acrometastasis: metastasis of hand or foot’, ‘mesometastasis: metastasis of forearm or lower leg’, ‘rhizometastasis: metastasis of upper arm or thigh’ and ‘body trunk metastasis’. If a patient had multiple bone metastasis, the patient was categorized according to the most distal site of bone metastases. Other bone metastases were counted as co-existence according to the category. For example, the patient who had both acrometastasis and rhizometastasis was categorized into acrometastasis group with a co-existence of rhizometastasis.
The following data were evaluated for each group: age, sex, primary tumor, sites of bone metastases, duration from the diagnosis of the primary tumor to the bone metastasis, follow-up period after diagnosis of bone metastases and prognosis. The prognosis was calculated by JMP Pro14 (SAS Institute Inc., Cary, NC, USA) as a median survival time (month) from Kaplan–Meier survival curve with data recorded by the end of January 2017. Log-rank test was used for comparison of survival time with Bonferroni correction. Other data of each group were compared by Welch’s t-test with Bonferroni correction by JMP Pro14. A multivariate analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). Data with continuous value as dependent variable were analyzed by multiple regression analysis, whereas Cox regression analysis was performed for survival analysis with forced entry method using dummy variables as objective variables: age, gender, acrometastasis, mesometastasis, rhizometastasis and body trunk metastasis. A P value <0.05 was considered statistically significant.
This research has been approved by the institutional review board of authors’ affiliated institutions.
Results
There were 11 cases (2.0%) with acrometastasis, 13 cases (2.4%) with mesometastasis, 121 cases (22.5%) with rhizometastasis and 394 cases (73.1%) with body trunk metastasis. The frequency of rhizometastasis was significantly higher than that of acrometastasis or mesometastasis (P < 0.001) (Table 1).
Table 1.
Characteristics of cases in each metastasis group
| Acrometastasis | Mesometastasis | Rhizometastasis | Body trunk metastasis | Whole | |
|---|---|---|---|---|---|
| Cases, n (%) | 11 (2.0) | 13 (2.4) | 121 (22.5) | 394 (73.1) | 539 |
| Sex | |||||
| Male | 6 | 6 | 76 | 224 | 312 |
| Female | 5 | 7 | 45 | 170 | 227 |
| Mean age ± SD | 67.2 ± 10.7 | 69.0 ± 9.1 | 65.4 ± 13.2 | 64.5 ± 13.0 | 64.9 ± 12.9 |
| Prognosis (month) | 6.5 | 4.0 | 16 | 17 | 16 |
As to the primary tumor site, lung cancer was the most common overall (n = 94, 17.4%) followed by breast cancer (n = 61, 11.3%) and liver cancer (n = 46, 8.5%). In acrometastasis, kidney cancer was first (n = 4) followed by breast cancer (n = 2). In mesometastasis, lung cancer was first (n = 3) followed by prostate cancer (n = 2) and stomach cancer (n = 2). In rhizometastasis, lung cancer was first (n = 23) followed by prostate cancer (n = 19) and liver cancer (n = 11) (Table 2).
Table 2.
Primary tumor sites of each metastasis group
| Primary tumor | Acro | Meso | Rhizo | Body trunk | Total (% of total/whole) |
|---|---|---|---|---|---|
| Lung | 0 | 3 | 23 | 68 | 94 (17.4) |
| Breast | 2 | 1 | 10 | 48 | 61 (11.3) |
| Liver | 0 | 0 | 11 | 35 | 46 (8.5) |
| Prostate | 0 | 2 | 19 | 22 | 43 (8.0) |
| Colon | 1 | 0 | 4 | 35 | 40 (7.4) |
| Stomach | 1 | 2 | 5 | 23 | 31 (5.8) |
| Kidney | 4 | 1 | 4 | 19 | 28 (5.2) |
| Other | 3 | 4 | 45 | 144 | 196 (36.4) |
| Whole | 11 | 13 | 121 | 394 | 539 |
Acro, acrometastasis; meso, mesometastasis; rhizo, rhizometastasis; body trunk, body trunk metastasis.
The average time from the diagnosis of primary tumor sites to the bone metastasis was as follows: 62.8 months in acrometastasis, 28 months in mesometastasis, 24 months in rhizometastasis and 28.9 months in body trunk metastasis. In multiple regression analysis, age and acrometastasis were statistically significant variables with partial regression coefficient of 0.48 (P < 0.001) and 34.69 (P = 0.021), respectively, whereas mesometastasis was not statistically significant but had a tendency with partial regression coefficient of −19.41 (P = 0.085) (Table 3).
Table 3.
Result of multiple regression analysis
| Unstandardized coefficients | Standardized coefficients | P value | 95% confidence interval for B | |||
|---|---|---|---|---|---|---|
| B | SE | Beta | Lower bound | Upper bound | ||
| (Constant) | −11.72 | 14.91 | 0.432 | −41.01 | 17.56 | |
| Age | 0.48 | 0.14 | 0.15 | <0.001 | 0.20 | 0.75 |
| Gender | 6.62 | 3.68 | 0.08 | 0.073 | −0.61 | 13.85 |
| Acro | 34.69 | 14.94 | 0.11 | 0.021 | 5.33 | 64.05 |
| Meso | −19.41 | 11.26 | −0.09 | 0.085 | −41.52 | 2.70 |
| Rhizo | −4.45 | 4.34 | −0.05 | 0.305 | −12.97 | 4.07 |
| Trunk | −3.41 | 10.64 | −0.02 | 0.749 | −24.31 | 17.49 |
B, partial regression coefficient; SE, standard error; beta, standardized coefficients.
The median survival time after diagnosis of bone metastases for each group was as follows: 6.5 months in acrometastasis, 4.0 months in mesometastasis, 16 months in rhizometastasis, 17 months in body trunk metastasis and 16 months overall (Table 1).
In survival curve, there was a statistically significant difference among four groups (P = 0.024) and between mesometastasis and body trunk metastasis (P = 0.041 with Bonferroni correction) at log-rank test (Fig. 1). Another statistically significant difference was only seen between the distal metastasis (acrometastasis and mesometastasis) and the proximal metastasis (rhizometastasis and body trunk metastasis) (P = 0.014 with Bonferroni correction) (Fig. 2).
Figure 1.
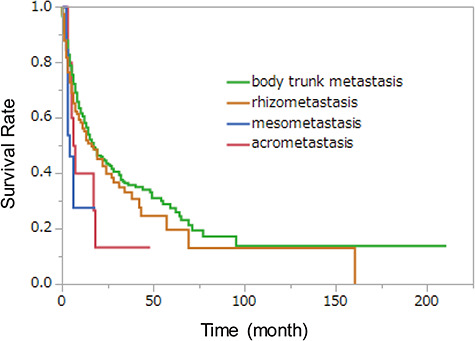
Kaplan–Meier survival curves of each metastasis group. There was a statistically significant difference among four groups (P = 0.024) and between mesometastasis and body trunk metastasis (P = 0.041 with Bonferroni correction) at log-rank test.
Figure 2.
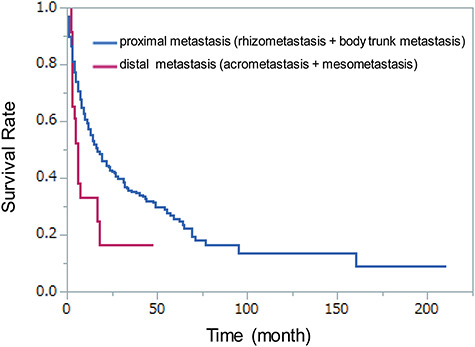
Kaplan–Meier survival curves of the distal and proximal metastasis group. There was a statistically significant difference between the distal and proximal metastasis groups (P = 0.014). Distal metastasis, acrometastasis and mesometastasis; proximal metastasis, rhizometastasis and body trunk metastasis.
On the other hand, the median survival times of major primary tumor sites were as follows: 15 months in lung cancer, 59 months in breast cancer, 19 months in liver cancer, 43 months in prostate cancer, 13 months in colon cancer, 5 months in stomach cancer and 31 months in kidney cancer. However, the mean times of the available median survival times of primary tumor sites in each group were similar: 25.4 months in acrometastasis, 21.1 months in mesometastasis, 24.3 months in rhizometastasis and 21.4 months in body trunk metastasis.
We also calculated the co-existence rate of bone metastases for further investigations of the relationship between bone metastasis sites and prognosis. The co-existence rates were higher in more proximal region. Acrometastasis was co-existed with 36.4% (n = 4) of acrometastasis (which means two or more acrometastases); mesometastasis was co-existed with 45.5% (n = 5) of acrometastasis and 15.4% (n = 2) of mesometastasis; rhizometastasis was co-existed with 81.8% (n = 9) of acrometastasis, 46.2% (n = 6) of mesometastasis and 16.5% (n = 20) of rhizometastasis; body trunk metastasis was co-existed with 81.8% (n = 9) of acrometastasis, 69.2% (n = 9) of mesometastasis, 89.3% (n = 108) of rhizometastasis and 75.9% (n = 299) of body trunk metastasis (Table 4). Anyway, in Cox regression analysis, gender and mesometastasis were statistically significant variables with the hazard ratio of 1.47 (P = 0.002) and 0.41 (P = 0.004), respectively (Table 5).
Table 4.
Co-existence rate (%) of bone metastasis with each other
| Acrometastasis | Mesometastasis | Rhizometastasis | Body trunk metastasis | |
|---|---|---|---|---|
| Acrometastasis | 36.4 | 45.5 | 81.8 | 81.8 |
| Mesometastasis | 15.4 | 46.2 | 69.2 | |
| Rhizometastasis | 16.5 | 89.3 | ||
| Body trunk metastasis | 75.9 |
Table 5.
Result of Cox regression analysis
| B | SE | Wald | P value | Exp(B) | |
|---|---|---|---|---|---|
| Age | 0.003 | 0.004 | 0.53 | 0.469 | 1.003 |
| Gender | 0.39 | 0.12 | 10.03 | 0.002 | 1.47 |
| Acro | −0.11 | 0.38 | 0.09 | 0.770 | 0.89 |
| Meso | −0.88 | 0.31 | 8.26 | 0.004 | 0.41 |
| Rhizo | −0.12 | 0.14 | 0.84 | 0.360 | 0.88 |
| Trunk | 0.17 | 0.30 | 0.30 | 0.582 | 1.18 |
Exp(B), hazard ratio.
Discussion
As current advances in cancer therapy increase patients’ life expectancies, the role of orthopedic management of bone metastases is increasing. To meet the accompanying social needs, a bone metastasis board was established in our hospital in 2012. Four years after its establishment, ~200 patients suspected of bone metastasis are referred to our board every year. As patients with bone metastases are automatically registered to the board, the comprehensive analysis is possible.
Compared with previous reports (3,4), the ratio of acrometastasis in this study was higher. One possible reason is prolonged life expectancy of cancer patients (5,7). As patients live longer, the chance of having acrometastasis in their lifetime may be increased. Another possible reason may be the prevalence of systemic surveillance for bone metastases by the bone metastasis board. As patients consulted to our bone metastasis board were well screened for bone metastases with advanced radiological surveys such as CT scan, MRI, PET scan or bone scintigraphy, the detection rate might be much higher to the previous studies held in the late 1990s.
While acrometastasis showed poorer prognosis, but not significantly, compared with body trunk metastasis, mesometastasis showed significantly poorer prognosis compared with body trunk metastasis. This can be the first report showing the poor prognosis of bone metastases in the specific regions other than acrometastases.
In our study, the average time from the primary site diagnosis to the bone metastasis was significantly longer in the acrometastasis group compared with the other groups. We assume that as the acrometastases occurred in the later or terminal period, they usually showed worse prognosis.
On the other hand, mesometastasis, rhizometastasis and body trunk metastasis were diagnosed earlier. It suggests that these metastases occur earlier in the disease time course than acrometastasis and could result in lead time bias. But even with the possibility of lead time bias, mesometastasis showed poorer prognosis than rhizometastasis and body trunk metastasis. This result strongly suggests that mesometastasis is a sign of the disease progression even it seems to occur in early period of the disease. Taken together, our results suggest that mesometastasis may be regarded as a new ‘red flag’ poor prognostic factor in cancer patients. When we see patients with mesometastases, we may have to consider the prognosis more negatively than before and have to take proper managements for them rather palliative than invasive.
Limitations of this study were as follows. First, as we did not survey the symptom, we cannot tell whether the metastasis was symptomatic even when it was diagnosed. Second, some distal metastases, such as acrometastases and mesometastases, may have been overlooked, because CT imaging and PET scan did not routinely cover the hand and foot. In our hospital, PET scan includes distal to forearms but does not include distal to the midthigh routinely. To reduce the bias, routine PET scan and/or bone scintigraphy including whole bodies for patients with bone metastases may be one of the supportive ways for future studies. Third, a greater number of severe patients were included in this study, because our hospital is designated as an advanced treatment hospital by the Japanese Health Ministry Committee.
Conclusion
Although acrometastasis showed poor prognosis similarly to the previous studies (3,5,6), the ratio of acrometastasis was higher than expected. ‘Mesometastasis’, a newly defined term for bone metastasis of the forearm or lower leg, could be another poor prognostic factor in cancer patients. When we see patients with mesometastases, we may have to consider the prognosis more negatively than before.
Conflict of interest
None declared.
References
- 1. Filsakova E, Foit R, Machartova J, Zahor Z. Acrometastases of the bronchogenic carcinoma. Radiol Diagn (Berl) 1962;3:193–200. [PubMed] [Google Scholar]
- 2. Bricout PB. Acrometastases. J Nat Med Assn 1981;73:325–9. [PMC free article] [PubMed] [Google Scholar]
- 3. Stomeo D, Tulli A, Ziranu A, Perisano C, Maccauro VS. Acrometastasis: a literature review. Eur Rev Med Pharmacol Sci 2015;19:2906–15. [PubMed] [Google Scholar]
- 4. Kerin R. Metastatic tumors of the hand. J Bone Joint Surg Am 1983;65:1331–5. [PubMed] [Google Scholar]
- 5. Mavrogenis AF, Mimidis G, Kokkalis ZT, et al. . Acrometastases. Eur J Orthop Surg Traumatol 2014;24:279–83. [DOI] [PubMed] [Google Scholar]
- 6. Flynn CJ, Danjoux C, Wong J, et al. . Two cases of acrometastasis to the hands and review of the literature. Curr Oncol 2008;15:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am 1986;68:743–6. [PubMed] [Google Scholar]


