Abstract
The alcohol content in wine has increased due to external factors in recent decades. In recent reports, some non-Saccharomyces yeast species have been confirmed to reduce ethanol during the alcoholic fermentation process. Thus, an efficient screening of non-Saccharomyces yeasts with low ethanol yield is required due to the broad diversity of these yeasts. In this study, we proposed a rapid method for selecting strains with a low ethanol yield from forty-five non-Saccharomyces yeasts belonging to eighteen species. Single fermentations were carried out for this rapid selection. Then, sequential fermentations in synthetic and natural must were conducted with the selected strains to confirm their capacity to reduce ethanol compared with that of Saccharomyces cerevisiae. The results showed that ten non-Saccharomyces strains were able to reduce the ethanol content, namely, Hanseniaspora uvarum (2), Issatchenkia terricola (1), Metschnikowia pulcherrima (2), Lachancea thermotolerans (1), Saccharomycodes ludwigii (1), Torulaspora delbrueckii (2), and Zygosaccharomyces bailii (1). Compared with S. cerevisiae, the ethanol reduction of the selected strains ranged from 0.29 to 1.39% (v/v). Sequential inoculations of M. pulcherrima (Mp51 and Mp FA) and S. cerevisiae reduced the highest concentration of ethanol by 1.17 to 1.39% (v/v) in synthetic or natural must. Second, sequential fermentations with Z. bailii (Zb43) and T. delbrueckii (Td Pt) performed in natural must yielded ethanol reductions of 1.02 and 0.84% (v/v), respectively.
Keywords: alcoholic fermentation, wine yeast, sequential inoculation, ethanol reduction, Metschnikowia pulcherrima, Torulaspora delbrueckii, Zygosaccharomyces bailii
1. Introduction
Global climate change has caused an increase in the alcohol content of wines in recent decades [1,2,3]. Specifically, global warming has accelerated maturation, increased the total soluble solids content and pH, and unbalanced the maturation of phenolic compounds and the increase in sugar concentration [2]. If grapes are harvested when phenolic compounds are mature, the grape must will have high concentration of sugars and low acidity, which produces wines with a high ethanol content. Otherwise, if the harvest occurs before that point, when sugar accumulation and pH are appropriate, wines will present a reduction in several characteristics (aroma, taste, and astringency) due to insufficient phenolic maturation. Regarding alcoholic fermentation, a high concentration of ethanol may lead to sluggish and stuck fermentations [4,5,6]. In addition, it can break the balance among acids, sugars, and tannins and develop unpleasant characteristics due to the enhancement of bitterness and burning sensation during tasting [7]. There are other reasons to achieve a lower ethanol content in wines, from their reduction in aromatic profile to the tax increase that will impact the final price of wines.
Previous studies about the reduction of alcohol in wines have focused on viticulture management, prefermentation, and postfermentation treatments and microbiological strategies during fermentation [8,9,10,11]. Specifically, from the point of viticulture management, a reduction in leaf area and removal of functional leaves were tested to reduce sugar accumulation, which could lead to a reduction in anthocyanins and soluble solids, delay maturity and significantly reduce the yield of grapes [12]. Another strategy for viticulture management is sequential harvesting, which aside from influencing phenolic maturity, could also affect the balance between fruity and vegetal aromas [13]. As a prefermentation treatment, García-Martín et al. [8] and Mihnea et al. [14] used membrane technologies, especially nanofiltration, to remove sugars from grape must. However, this method led to a reduction in wine color and flavor compounds. In addition, the removal of ethanol in wine was mainly considered during the postfermentation process. Aguera et al. [15] reported that removing 2% (v/v) ethanol had a significant effect on the concentration of volatile compounds, such as fusel alcohol and esters, which were reduced by 25% and 45%, respectively.
In recent years, microbiological strategies have garnered interest as alternatives to reduce ethanol concentrations [16,17,18]. For instance, the selection of evolved or modified strains from Saccharomyces cerevisiae, as well as low-ethanol producer strains from non-Saccharomyces yeasts, have been considered. In terms of S. cerevisiae, non-GMO strategies, such as evolutionary engineering, including experimental evolution under selective cultivation conditions or quantitative trait loci (QTL) mapping followed by breeding, have been used to improve industrial yeasts [19,20]. However, evolutionary engineering could affect some strain features under the conditions of industrial production and fermentation and lead to a distinct response of evolved strains to environmental factors that is different than that of ancestral strains [21]. The other strategy was genetic modification (GM), which has focused on changing the carbon metabolic conversion of sugar into other byproducts [16,22,23]. However, the application of GM methods in food and beverage production is forbidden due to poor public acceptance and regulations. Based on this limitation, screening non-Saccharomyces yeasts with alcohol-lowering abilities has become a consistent proposal to maintain wine quality and reduce the ethanol content [24,25,26,27,28,29]. The use of some non-Saccharomyces strains from Candida, Hanseniaspora, Lachancea, Metschnikowia, Picha, Schizosaccharomyces, Starmerella, and Torulaspora species has been shown to reduce ethanol in wines [18].
Non-Saccharomyces yeasts have been used in fermentations to reduce ethanol as a single or mixed inoculation. For example, Candida sake H14Cs reduced 2.4% (v/v) ethanol in natural must fermentations with a single inoculation [30]. Varela et al. [28] reported that, compared with S. cerevisiae, single fermentation by Saccharomyces uvarum AWRI2846 reduced the ethanol content by 1.7% (v/v), and coinoculated fermentation by M. pulcherrima/S. cerevisiae (10:1) reduced the ethanol content by 1% (v/v) in Merlot wines. Strains from Hanseniaspora uvarum, Zygosaccharomyces sapae, Zygosaccharomyces bailii, and Zygosaccharomyces bisporus species used as pure cultures in fermentations also showed a significant ethanol reduction in ethanol yield compared with S. cerevisiae [31]. However, the growth and metabolism of non-Saccharomyces yeasts will be affected by the presence of S. cerevisiae, especially in simultaneous fermentations [32,33]. Thus, sequential inoculation strategies, where S. cerevisiae is inoculated 24 or 48 h after the beginning of fermentation with non-Saccharomyces yeast, have been adopted by researchers and wine producers [34,35,36]. Englezos et al. [37] proposed a protocol to reduce ethanol based on the sequential fermentation of S. bacillaris and S. cerevisiae, showing higher ethanol reduction inoculating S. cerevisiae at 48 h than at 24 h.
In the present work, we proposed a rapid method to select non-Saccharomyces strains with a low ethanol yield. The ethanol production and yield of 45 non-Saccharomyces yeasts, belonging to 18 species, were evaluated. After an initial screening on optimal medium for 3 days and on synthetic must for 48 h (to set the beginning of alcoholic fermentation), we reduced the number to 10 strains with a high potential to reduce the ethanol content. Afterwards, this ability was verified by complete fermentations in synthetic and natural must using sequential inoculations with a commercial S. cerevisiae wine yeast. In addition, all final samples were subjected to an in-depth chemical analysis to characterize the resulting wines.
2. Materials and Methods
2.1. Strains and Culture Conditions
One commercial wine yeast Saccharomyces cerevisiae (Lalvin QA23®, Lallemand Inc. Montreal, Canada, used as a control and referred to as Sc23) and forty-five non-Saccharomyces strains used in this study are listed in Table 1. Yeasts grew at 28 °C in YPD Agar (2% (w/v) glucose, 2% (w/v) yeast extract, 1% (w/v) peptone, and 1.7% (w/v) agar; Cultimed, Barcelona, Spain) and Wallerstein laboratory nutrient (WLN) agar (Becton, Dickinson and Company, Isère, France) from frozen stocks at −80 °C. Before starting fermentations, strains were identified at species level by PCR-RFLP analysis of 5.8S-ITS rDNA according to Esteve-Zarzoso et al. [38].
Table 1.
Yeast strains used in this study (CECT, Spanish Type Culture Collection; URV, our group yeast collection, some of them isolated in Priorat Appelation of origin [39]; UdlaR, Universidad de la República yeast collection, Uruguay; Agrovin S.A, Ciudad Real, Spain; Lallemand, Lallemand Inc. Montreal, Canada).
| Yeast Species | Strain Designation | Collections | Isolation Source | Abbreviations in This Paper |
|---|---|---|---|---|
| Saccharomyces cerevisiae | QA23 | Lallemand | Commercial | Sc23 |
| Candida boidinii | 10029 | CECT | Milk | Cb29 |
| 10035 | CECT | Frass on Amygdalus communis |
Cb35 | |
| 1014 T | CECT | Tanning fluid | Cb14 | |
| Candida mesenterica | 1025 | CECT | Brewery | Cm25 |
| Candida sake | 10034 | CECT | Feces of sheep | Cs34 |
| 1044 | CECT | Lambic beer | Cs44 | |
| Candida stellata | 11918T | CECT | Wine grape | Cs18 |
| Starmerella bacillaris | 4 | URV | Grape must (Priorat) | Sb4 |
| 11046 | CECT | Grape juice | Sb46 | |
| 11109 | CECT | Wine | Sb09 | |
| NS c | URV | Grape must | Sb Nc | |
| NS d | URV | Grape must | Sb Nd | |
| Hanseniaspora guilliermondii | 11027 | CECT | Grape must | Hg27 |
| 11029 T | CECT | Infected nail | Hg19 | |
| 11102 | CECT | Grape juice | Hg02 | |
| Hanseniaspora osmophila | 11206 | CECT | Ripe Riesling grape | Ho06 |
| 11207 | CECT | Grape | Ho07 | |
| Hanseniaspora uvarum | 11106 | CECT | Wine grape | Hu06 |
| 13130 | CECT | Grape must (Priorat) | Hu4 | |
| 3 | URV | Grape must (Priorat) | Hu3 | |
| 34 | URV | Grape must (Priorat) | Hu34 | |
| Hanseniaspora vineae | 11.24 | UdlaR | Grapes (Uruguay) | Hv24 |
| 12.219 | UdlaR | Grapes (Uruguay) | Hv19 | |
| 13714 | CECT | Nda | Hv14 | |
| 1471 | CECT | Grape juice | Hv71 | |
| Issatchenkia terricola | 11139 | CECT | Dregs of pressed grapes | It39 |
| 11176 | CECT | Soil | It76 | |
| Lachancea thermotolerans | 1 | Agrovin | Nd a | Lt1 |
| 2 | Agrovin | Nd a | Lt2 | |
| Meyerozyma guilliermondii | 1020 | CECT | Nd a | Mg20 |
| Metschnikowia pulcherrima | 51 | URV | Grape must (Priorat) | Mp51 |
| 52 | URV | Grape must (Priorat) | Mp52 | |
| FLAVIA | Lallemand | Commercial | Mp FA | |
| Saccharomycodes ludwigii | 1235 T | CECT | Nd a | Sl35 |
| 1371 | CECT | Nd a | Sl71 | |
| Schizosaccharomyces pombe | 1379 | CECT | Nd a | Sp79 |
| Torulaspora delbrueckii | 10558 | CECT | White wine | Td58 |
| 13135 | CECT | Grape must (Priorat) | Td35 | |
| 1880 | CECT | Wine of Airen grape | Td80 | |
| Priorat | URV | Grape must (Priorat) | Td Pt | |
| BIODIVA | Lallemand | Commercial | Td BA | |
| Zygosaccharomyces bailii | 11042 | CECT | Grape must | Zb42 |
| 11043 | CECT | Cloudy wine | Zb43 | |
| Zygosaccharomyces rouxii | 1230 | CECT | Honey | Zr30 |
| 1232 | CECT | Concentrate must | Zr32 |
T presents Type strain; a presents No description.
Propagation of strains was performed by picking a single colony from YPD plates. Strains grew in YPD liquid medium (2% (w/v) glucose, 2% (w/v) yeast extract, and 1% (w/v) peptone) for 24 h (Sc23) or 48 h (non-Saccharomyces strains) at 28 °C. After incubation, cells were counted in a Neubauer chamber (Leica Microsystems GMS QmbH, Leica, Germany), and 2 × 106 cells/mL were inoculated into the appropriate fermentation medium. In all cases, the identity at the species level was confirmed by growth on differential WLN medium, and molecular identification by PCR-RFLP of 5.8S-ITS rDNA [38] was used to distinguish the non-Saccharomyces yeasts that presented similar morphological profiles with Sc23 on the WLN medium.
2.2. Fermentations
Three different media were used in fermentations, namely, YPD liquid medium, synthetic must (SM), and natural must (NM). SM was prepared according to Beltran et al. [40] (200 g/L sugars), and NM was obtained from Muscat grapes from Finca Experimental Mas dels Frares of Rovira i Virgili University (Constantí, Spain) during the 2019 vintage (219.2 g/L sugars, 4.52 g/L total acidity (as tartaric acid), 77.8 mg/L assimilable nitrogen, and pH 3.27). The nitrogen concentration in NM was corrected with diammonium phosphate (Panreac Quimica SA, Barcelona, Spain) until a final concentration of 240 mg N/L. Before the start of fermentation, dimethyl dicarbonate (0.2 mL/L) (ChemCruzTM Biochemicals, Dallas, TX, USA) was added to NM, and kept at 4 °C for 24 h to eliminate the endogenous microorganisms. The absence of endogenous microorganisms was confirmed by plating a sample of the must on YPD Agar. Different fermentation procedures were performed in single and sequential fermentations. All fermentations were performed in triplicate, and single fermentations by Sc23 were used as a control.
In the first screening, strains were inoculated as single cultures in 5 mL YPD liquid medium in 12 mL tubes and incubated at 28 °C and 120 rpm for 3 days. Samples were taken daily to evaluate yeast growth, and after 3 days, extracellular media was kept to determine sugar and ethanol content. In the next step, strains were inoculated in 40 mL SM in 50 mL Falcon tubes, and fermentations were performed at 22 °C and 120 rpm and monitored over 48 h to evaluate yeast growth. Samples were taken at 48 h and centrifuged at 12000 rpm for 5 min, and the supernatant was kept at −20 °C until chemical compound analysis.
For sequential fermentations, experiments were carried out either in SM or NM. Non-Saccharomyces strains (2 × 106 cells/mL) were used to start the fermentation, and 48 h later, Sc23 was inoculated (2 × 106 cells/mL). Fermentations were conducted in 250 mL glass bottles with 230 mL of SM or NM (bottle caps were not tightly screwed in order to allow the release of CO2) and incubated at 22 °C with stirring at 120 rpm. SM and NM fermentations were monitored by evaluating yeast growth and must density which was determined with an electronic densitometer (Densito 30PX Portable Density Meter, Mettler Toledo, Hospitalet de Llobregat, Spain). The fermentation was considered finished when residual sugars were below 2 g/L, which was confirmed by enzymatic analysis in a Miura autoanalyzer (EE-MIURAONE Rev., I.S.E. S.r.l., Italy). Samples were centrifuged at 7800 rpm for 5 min, and the supernatants were frozen at −20 °C until analysis.
2.3. Population Dynamics
In single fermentation samples, the total population was assessed by microscope counting using a Neubauer chamber after 48 h of fermentation. Viability was also determined in sequential fermentations. Briefly, samples were serially diluted in sterilized Milli-Q water from a Milli-Q water purification system (Millipore S.A.S., Molsheim, France). The number of colony-forming units per milliliter (CFU/mL) was determined by plating 100 μL of three appropriately chosen dilutions on YPD, WLN, or lysine medium (11.75% (w/v) yeast carbon base, 2.5% (w/v) L-lysine monohydrochloride, and 20% (w/v) agar, Cultimed, Barcelona, Spain). Plates were incubated at 28 °C for 2 or 3 days.
2.4. Chemical Analysis
The glucose and ethanol contents of the samples from YPD cultures were determined with D-glucose and ethanol enzymatic bioanalysis kits (r-biopharm, Darmstadt, Germany), respectively. Residual sugars of samples at the end of fermentation in both SM and NM fermentations were quantified by D-glucose/D-fructose assays (Biosystems S.A., Barcelona, Spain).
Ethanol, glycerol, and organic acids (acetic acid, citric acid, malic acid, tartaric acid, lactic acid and succinic acid) in samples after 48 h of single fermentation and at the end of sequential fermentation and the sugars (glucose and fructose) after 48 h of single fermentation were determined by high-performance liquid chromatography (HPLC) using an Agilent 1100 HPLC (Agilent Technologies, Waldbronn, Germany) as previously described by Quirós et al. [41]. The HPLC was equipped with a Hi-Plex H column (300 mm × 7.7 mm) inside a 1260 MCT column compartment (Infinity II Multicolumn Thermostat) connected to MWD (G1365B multiwavelength detector) and RID detectors (1260 Infinity II refractive index detector) (Agilent Technologies, Waldbronn, Germany). The temperature of the column was maintained at 60 °C for a 30 min run time, and the mobile phase was 5 mM H2SO4 with a flow rate of 0.6 mL/min. The sample injection volume was 10 μL. Before injection, samples were filtered through 0.22 μm filters (Dominique Dutscher, Brumath, France). OpenLAB CDS (Agilent Technologies, Santa Clara, CA, USA) was used to analyze HPLC chromatographs.
2.5. Statistical Analysis
All graphs were generated using GraphPad Prism® version 8 (GraphPad Software, San Diego, CA, USA). The results are expressed as the mean ± standard deviation (SD). Statistically significant differences (one-way ANOVA) were analyzed by IBM SPSS Statistics version 23.0 (IBM, NY, USA). The ethanol yield was calculated with the formula “Ethanol yield (g/g) = ethanol production (g/L)/sugar consumption (g/L)”. Ethanol reduction was calculated by the formula “Δethanol (%, v/v) = Δethanol yield (g/g) × T sugars (g/L)/10 × 0.78924 (g/mL)”, where T sugars is the initial sugar concentration in the must and 0.78924 is the density of ethanol at room temperature.
3. Results
3.1. Rapid Screening of Non-Saccharomyces Strains with a Low Ethanol Yield
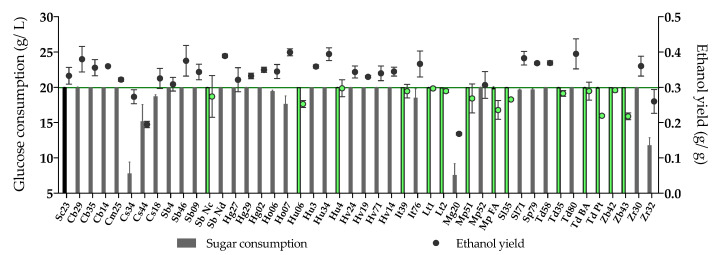
A first screening with forty-five non-Saccharomyces strains was performed under low sugar fermentation conditions (YPD medium), to evaluate the capacity of some yeast species and strains to consume sugars with a limited production of ethanol (fermentation vs. respiration capacity) (Figure 1, Table S1). Saccharomyces cerevisiae QA23 (Sc23), inoculated as a control, was able to consume all glucose (20 g/L) and produced 0.84% (v/v) ethanol, with an ethanol yield of 0.33 g ethanol/g glucose. The selection criteria for lower ethanol-producing yeast were established according to this result, taking into account their ability to consume glucose. Based on this, fourteen non-Saccharomyces strains were selected due to a high glucose consumption (> 19.90 g/L), and a lower ethanol production than that of the control with ethanol yields below 0.30 g/g (< 0.76%, v/v ethanol, 10% ethanol reduction compared with Sc23) (Figure 1). These strains belonged to the species Hanseniaspora uvarum (2), Issatchenkia terricola (1), Lachancea thermotolerans (2), Metschnikowia pulcherrima (2), Saccharomycodes ludwigii (1), Starmerella bacillaris (1), Torulaspora delbrueckii (3), and Zygosaccharomyces bailii (2).
Figure 1.
Glucose consumption (g/L) and ethanol yield (ethanol production (g/L)/sugar consumption (g/L), g/g) of 45 non-Saccharomyces yeasts and Sc23 (control yeast) after 3 days fermentation in YPD medium. The non-Saccharomyces yeasts selected for the next step are colored in green (glucose consumption > 19.90 g/L and ethanol yield ≤ 0.30 g/g). The value of the green line is 0.30 g/g (10% ethanol reduction of Sc23).
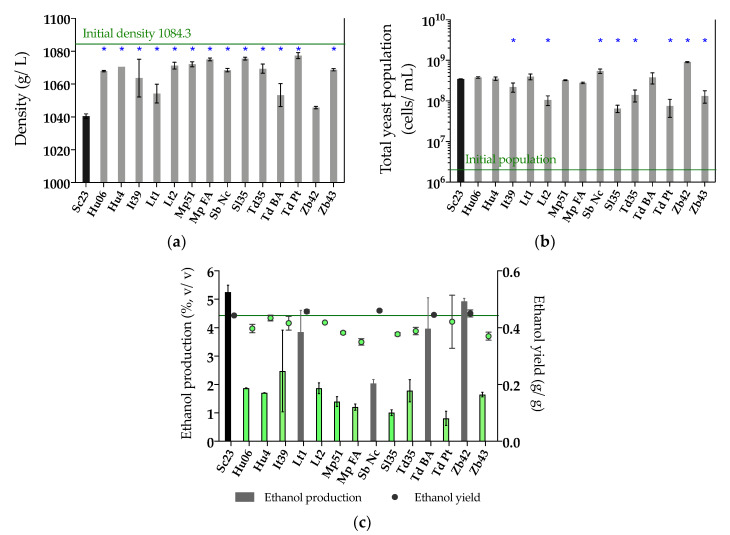
As non-Saccharomyces yeasts are commonly used in sequential fermentations, inoculating S. cerevisiae after 24-48 h, in the next step we analyzed the performance of the selected non-Saccharomyces strains during the first 48 h of fermentation. Therefore, we tested the 14 selected non-Saccharomyces strains on fermentation media (synthetic must) using Sc23 as a control. Must density, total yeast population, ethanol production, sugar consumption, and other main organic compounds were measured at 48 h of fermentation (Figure 2, Table S2). We observed that all selected strains were able to start fermentation in 48 h, consuming some of the sugars present in the must (with a corresponding decrease in must density, Figure 2a), although in a lesser amount than that of Sc23 (Table S2). The total yeast population showed that all strains were able to grow in fermentation media, and two of them, Sb Nc and Zb42, grew significantly higher than the control strain at 48 h (Figure 2b). The single fermentation with Sc23 was able to consume 47% (93.68 g/L) of total sugars in 48 h and produced the highest concentration of ethanol (5.26%, v/v), with an ethanol yield of 0.44 g/g (Table S2, Figure 2c). Most non-Saccharomyces strains consumed more glucose than fructose during 48 h, similar to the control Sc23 strain. However, three of the strains, Sb Nc, Sl35, and Zb43, consumed more fructose than glucose, and the two H. uvarum strains, Hu06 and Hu4, consumed equal quantities of glucose and fructose (Table S2). Ten out of 14 strains produced lower ethanol contents and lower ethanol yields than Sc23 (< 0.44 g/g), Hu06, Hu4, It39, Mp51, Mp FA, Lt2, Sl35, Td35, Td BA, and Zb43, and they were selected for subsequent experiments (Figure 2c).
Figure 2.
(a) Density (g/L); (b) Total yeast population (cells/mL); (c) Ethanol production (%, v/v) and ethanol yield (g/g) at 48 h of single fermentation in synthetic must. Non-Saccharomyces yeasts selected for the next step are colored in green, with the ethanol yield below that of Sc23 (0.44 g/g, the value of the green line in Figure (c)). Asterisk (*) means the significant difference compared with Sc23 (LSD, p < 0.05).
3.2. Sequential Inoculation in Synthetic Must (SM) and Natural Must (NM)
To verify the ability of the 10 selected strains to reduce ethanol, sequential fermentations were performed. In the sequential fermentations, Sc23 was inoculated at 48 h in both SM and NM fermentations.
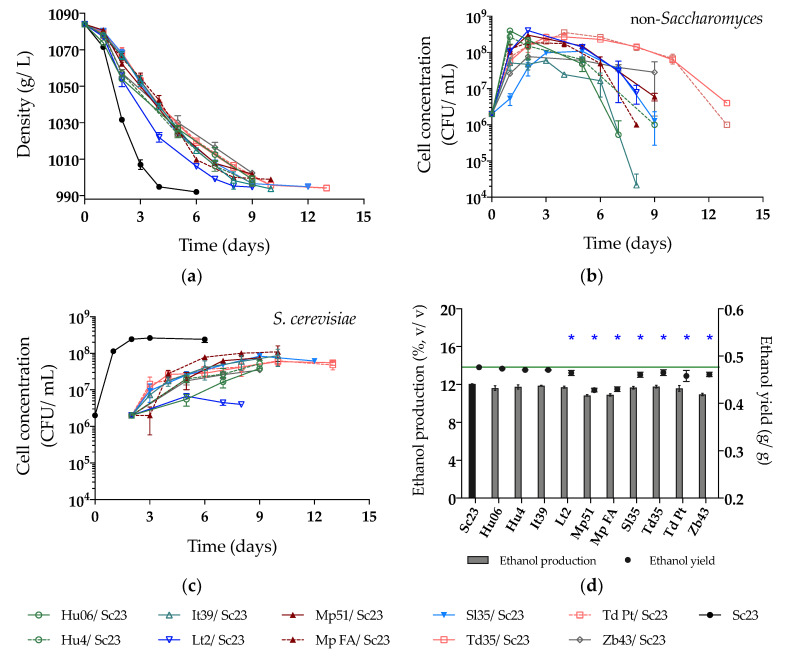
In the SM fermentation, Sc23 completed fermentation in 6 days, and the density of sequential fermentation trials with Lt2 showed the fastest reduction among the non-Saccharomyces strains. Nevertheless, more than 9 days were necessary to complete sequential fermentations by the other strains (Figure 3a). Interestingly, all non-Saccharomyces strains were detected during the fermentation process, with Hu06 and It39 being the strains with the fastest decrease in viability and Td Pt and Td35 maintaining relatively high viability until the end of fermentation (Figure 3b). Correspondingly, the Sc23 population reached a significant increase after inoculation at 48 h, with final viable populations between 3.5 × 107 and 1.1 × 108 CFU/mL, except in the Lt2/Sc23 fermentation, where Sc23 grew poorly (up to 6.7 × 106 CFU/mL) (Figure 3c). Ethanol production decreased by 0.08 to 1.23% (v/v) in all sequential fermentations compared with that of the single fermentation by Sc23 (Figure 3d, Table 2), although this decrease was significant only with 7 of the non-Saccharomyces strains (Lt2, Mp51, Mp FA, Sl35, Td35, Td Pt, and Zb43). Higher concentrations of residual sugars were observed in the fermentation of Zb43/Sc23 and Hu06/Sc23. Our results (Table 2) showed that the sequential fermentations with M. pulcherrima strains Mp51/Sc23 and Mp FA/Sc23 had the highest ethanol reduction with the lowest ethanol yields (both are 0.43g/g compared to 0.48 g/g for Sc23).
Figure 3.
(a) Density (g/L); (b) Yeast population of non-Saccharomyces; (c) Yeast population of Sc23 and (d) Ethanol production (%, v/v) and yield (g/g) during sequential fermentations in synthetic must. The value of the green line in Figure (d) is 0.48 g/g (ethanol yield of Sc23). Asterisks (*) show the significant difference of ethanol yield compared with Sc23 (LSD, p < 0.05).
Table 2.
Analysis of sugars, ethanol, organic acids, and glycerol from samples at the end of sequential fermentations.
| Residual Sugar | Sugar Consumption | Ethanol Production | Ethanol Yield | Ethanol Reduction | Succinic Acid | Lactic Acid | Acetic Acid | Glycerol | |
|---|---|---|---|---|---|---|---|---|---|
| (g/L) | (g/L) | % (v/v) | (g/g) | % (v/v) | (g/L) | (g/L) | (g/L) | (g/L) | |
| Synthetic must fermentation | |||||||||
| Sc23 | 0.12 ± 0.10 | 199.88 ± 0.10 | 12.07 ± 0.02 | 0.48 ± 0 | 0 ± 0 | 0.56 ± 0.04 | 0.23 ± 0 | 0.30 ± 0.03 | 5.76 ± 0.16 |
| Hu06 | 6.24 ± 3.83 * | 193.76 ± 3.83 * | 11.62 ± 0.25 * | 0.47 ± 0 | 0.08 ± 0.02 | 0.54 ± 0.08 | 0.18 ± 0.01 | 0.24 ± 0.04 | 6.60 ± 0.22 |
| Hu4 | 3 ± 3.94 | 197 ± 3.94 | 11.75 ± 0.23 | 0.47 ± 0 | 0.14 ± 0.01 | 0.41 ± 0.01 | 0.16 ± 0.01 | 0.28 ± 0.02 | 6.90 ± 0.03 |
| It39 | 0.71 ± 1 | 199.29 ± 1 | 11.89 ± 0.03 | 0.47 ± 0 | 0.15 ± 0.03 | 0.46 ± 0.06 | 0.23 ± 0.02 | 0.33 ± 0.01 | 7.65 ± 0.84 * |
| Lt2 | 0.82 ± 0.43 | 199.18 ± 0.43 | 11.72 ± 0.11 | 0.46 ± 0.01 * | 0.31 ± 0.14 * | 0.56 ± 0 | 0.55 ± 0.04 * | 0.27 ± 0.11 | 7.44 ± 0.33 * |
| Mp51 | 0.03 ± 0.04 | 199.97 ± 0.04 | 10.85 ± 0.09 * | 0.43 ± 0 * | 1.23 ± 0.10 * | 0.58 ± 0.01 | 0.21 ± 0.03 | 0.28 ± 0.04 | 10.30 ± 0.45 * |
| Mp FA | 0 ± 0 | 200 ± 0 | 10.90 ± 0.12 * | 0.43 ± 0 * | 1.17 ± 0.12 * | 0.59 ± 0.03 | 0.16 ± 0.01 | 0.16 ± 0.06 * | 9.83 ± 0.67 * |
| Sl35 | 0.12 ± 0.06 | 199.88 ± 0.06 | 11.67 ± 0.14 * | 0.46 ± 0.01 * | 0.40 ± 0.14 * | 0.65 ± 0.01 | 0.22 ± 0.11 | 0.17 ± 0.01 * | 7.76 ± 0.93 * |
| Td35 | 0.24 ± 0.05 | 199.77 ± 0.05 | 11.77 ± 0.15 | 0.47 ± 0.01 * | 0.29 ± 0.15 * | 0.53 ± 0.16 | 0.16 ± 0.04 | 0.26 ± 0.04 | 5.31 ± 0.12 |
| Td Pt | 0.40 ± 0.15 | 199.61 ± 0.15 | 11.59 ± 0.30 * | 0.46 ± 0.01 * | 0.47 ± 0.29 * | 0.53 ± 0.02 | 0.37 ± 0.05 * | 0.24 ± 0.05 | 5.36 ± 0.10 |
| Zb43 | 12.62 ± 2.58 * | 187.38 ± 2.58 * | 10.95 ± 0.12 * | 0.46 ± 0 * | 0.39 ± 0.11 * | 1.35 ± 0.52 * | 0.31 ± 0.12 | 0.12 ± 0.01 * | 8.61 ± 0.86 * |
| Natural must fermentation | |||||||||
| Sc23 | 0.76 ± 0.10 | 218.42 ± 0.10 | 13.48 ± 0.03 | 0.49 ± 0 | 0 ± 0 | 0.77 ± 0.08 | 0.16 ± 0.08 | 0.31 ± 0.04 | 5.56 ± 0.06 |
| Hu06 | 0.41 ± 0.16 | 218.76 ± 0.16 | 12.94 ± 0.16 * | 0.47 ± 0.01 * | 0.56 ± 0.17 * | 0.73 ± 0.04 | 0.15 ± 0.01 | 0.48 ± 0.17 * | 6.60 ± 0.75 * |
| Hu4 | 0.19 ± 0.14 | 218.98 ± 0.14 | 13.08 ± 0.11 * | 0.47 ± 0 * | 0.44 ± 0.11 * | 0.55 ± 0.04 | 0.13 ± 0.01 | 0.57 ± 0.04 * | 6.50 ± 0.08 * |
| It39 | 0.13 ± 0.03 | 218.91 ± 0.03 | 13.20 ± 0.10 | 0.47 ± 0 * | 0.37 ± 0 * | 0.52 ± 0.07 | 0.24 ± 0.04 | 0.26 ± 0.08 | 6.12 ± 0.37 |
| Lt2 | 0.24 ± 0.06 | 218.93 ± 0.06 | 13.15 ± 0.11 | 0.47 ± 0 * | 0.37 ± 0.10 * | 0.33 ± 0 * | 4.12 ± 0.06 * | 0.16 ± 0.01 | 8.48 ± 0.02 * |
| Mp51 | 0.70 ± 0.42 | 218.48 ± 0.42 | 12.10 ± 0.20 * | 0.44 ± 0.01 * | 1.39 ± 0.18 * | 0.80 ± 0.05 | 0.14 ± 0.02 | 0.20 ± 0.01 | 5.83 ± 0.31 |
| Mp FA | 0.75 ± 0.35 | 218.29 ± 0.35 | 12.74 ± 0.28 * | 0.46 ± 0.01 * | 0.74 ± 0.26 * | 0.70 ± 0.02 | 0.25 ± 0.02 | 0.12 ± 0.02 * | 6.71 ± 0.33 * |
| Sl35 | 0.65 ± 0.52 | 218.39 ± 0.52 | 12.97 ± 0.15 * | 0.47 ± 0 * | 0.51 ± 0.12 * | 0.72 ± 0.10 | 0.25 ± 0.06 | 0.20 ± 0.06 | 6.18 ± 0.08 |
| Td35 | 0.87 ± 0.48 | 218.17 ± 0.48 | 13.12 ± 0.25 * | 0.47 ± 0.01 * | 0.34 ± 0.28 * | 0.56 ± 0 | 0.33 ± 0.14 * | 0.26 ± 0.01 | 5.14 ± 0.33 |
| Td Pt | 1.01 ± 0.04 | 218.03 ± 0.04 | 12.62 ± 0.06 * | 0.46 ± 0 * | 0.84 ± 0.06 * | 0.95 ± 0.43 | 0.50 ± 0.01 * | 0.31 ± 0.09 | 6.37 ± 0.61 * |
| Zb43 | 0.37 ± 0.50 | 218.80 ± 0.50 | 12.61 ± 0.21 * | 0.45 ± 0 * | 1.02 ± 0.05 * | 0.83 ± 0.13 | 0.07 ± 0.04 | 0.30 ± 0.04 | 4.67 ± 0.09 * |
Values are mean ± standard deviation of three independent replicates; The initial sugar concentration of synthetic and natural must was 200 and 219.2 g/L, respectively; * means statistically significant differences from the control sample in the same column (LSD test, p < 0.05).
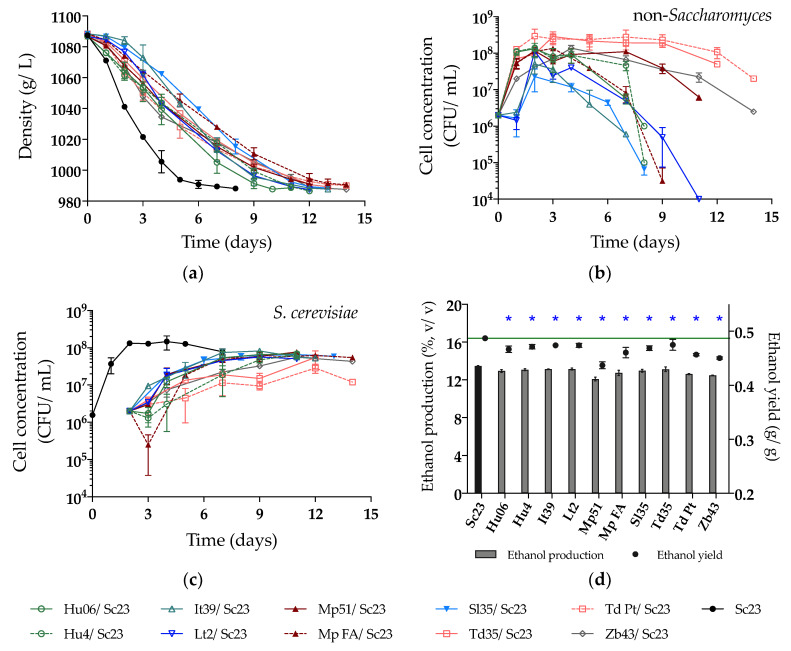
In the NM fermentation, all fermentations were delayed, probably due to the higher concentration of sugars in the natural must (219.2 g/L), especially fermentations that involved non-Saccharomyces strains, with Mp FA, Td Pt, and Zb43 taking the longest time, up to 14 days (Figure 4a). Noteworthy, the fermentation with Lt2/Sc23 was slower in NM, differing from the behavior observed in SM. In NM, the growth of Sc23 in sequential fermentations (Figure 4c) was higher than in SM (Figure 3c), and consequently, the growth of some non-Saccharomyces was hampered (Figure 4b). Only five non-Saccharomyces strains could be counted on WLN at the end of NM fermentations (Lt2, Mp51, Td35, Td Pt and Zb43) (Figure 4b), which were also the ones observed at the end of SM fermentations (Figure 3b). The ethanol production of all selected strains was reduced compared to the control fermentation with Sc23 (13.48%, v/v). The sequential fermentation by Mp51/Sc23 again showed the highest ethanol reduction, followed by Zb43/Sc23, Td Pt/Sc23, and Mp FA/Sc23 (Figure 4d, Table 2).
Figure 4.
(a) Density (g/L); (b) Yeast population of non-Saccharomyces; (c) Yeast population of Sc23 and (d) Ethanol production (%, v/v) and yield (g/g) during sequential fermentations in natural must. The value of the green line in Figure (d) is 0.49 g/g (ethanol yield of Sc23). Asterisks (*) show the significant difference of ethanol yield compared with Sc23 (LSD, p < 0.05).
The production of glycerol differed significantly among the different sequential fermentations (Table 2), with Mp51/Sc23 and Mp FA/Sc23 fermentations having the highest glycerol levels in SM (10.3 and 9.83 g/L, respectively) and Lt2/Sc23 fermentations in NM (8.48 g/L). Indeed, the increase in glycerol of Mp FA/Sc23 and Lt/Sc23 fermentations, compared to single Sc23 fermentation, was significant both in SM and NM. The concentration of acetic acid remained under the recommended values for wines, below 0.35 g/L in SM and below 0.6 g/L in NM (the highest values were for Hu06 and Hu4 strains, 0.48 and 0.57 g/L, respectively). On the other hand, a significant increase in lactic acid was observed in the sequential fermentations performed with the Lt2 strain, both in SM and NM. Noteworthily, the concentration of succinic acid was significantly higher (1.35 g/L) in SM fermentation with Zb43.
4. Discussion
The selection of non-Saccharomyces yeasts to be used as fermentation starters, usually in combination with S. cerevisiae, has been mainly focused on improving the aromatic characteristics of wines [28,42] and reproducing the microbiota of vineyard or grapes [43]. In recent years, another reason for screening non-Saccharomyces yeasts has been the ability of some species to reduce ethanol content. Researchers have applied different combinations of non-Saccharomyces and S. cerevisiae yeasts to achieve this goal [24,27,44]. In this study, we focused on the selection of non-Saccharomyces yeasts with low ethanol yield by performing two short-term trials in 5 days. In the first selection step, we used YPD medium, which contains a low concentration of sugar, and analyzed ethanol yield and sugar consumption of the different strains. The metabolic characteristics of non-Saccharomyces yeasts will determine ethanol reduction, which implies that their metabolic footprints should be introduced before the inoculation of S. cerevisiae [26]. Therefore, the second selection step was performed in synthetic must for 48 h, in order to detect their ability to reduce ethanol before the inoculation of S. cerevisiae. With the selected strains, two sequential fermentation trials were performed, in synthetic and natural must, in which S. cerevisiae was inoculated after 48h. Simultaneous inoculations could reduce the contribution of non-Saccharomyces yeast in the fermentation process, and periods of longer than two days could jeopardize the imposition of S. cerevisiae and, as a consequence, the development of fermentation [33].
Regarding non-Saccharomyces screening strategies to achieve wines with low ethanol concentrations, Contreras et al. [25] used the fermentation of single yeast species in a defined medium for 4 days under anaerobic conditions to select strains from 50 non-Saccharomyces yeasts, followed by a second step with sequential fermentation for 7 days. After 11 days, eleven strains showed lower ethanol yields than S. cerevisiae. Another study reported by Contreras et al. [44] used a similar methodology over 11 days, and the difference was the use of semi-aerobic conditions of the initial fermentation. They selected seven strains out of 48 non-Saccharomyces yeasts with lower ethanol yield than S. cerevisiae. Quirós et al. [24] selected fifteen yeasts from 63 non-Saccharomyces strains by determining the respiratory quotient under fully aerobic conditions in 4 days, followed by the performance of selected strains in synthetic must for 4 days. However, after 8 days of analysis, several of the selected strains showed a higher ethanol yield than that of S. cerevisiae. Thus, compared to previous selection trials, the screening process applied in our study was equally rapid, but the pre-selection of strains was more reliable, and we included an important screening criterion to be considered, that is, the selected non-Saccharomyces yeasts were able to finish fermentations under low-sugar conditions.
The ethanol yields of S. cerevisiae in YPD medium (0.33 g/g) were lower than those in semi-anaerobic fermentative conditions (approximately 0.48 g/g), which agrees with the results of Quirós et al. [24] in fully aerobic conditions, where the ethanol yield of S. cerevisiae was approximately 0.25–0.30 g/g. Instead, when fermentative conditions in synthetic or natural must were used, the ethanol yields for S. cerevisiae were close to the expected values (i.e., 0.44–0.48 g/g) [25]. The differences could be due to the importance of respiratory metabolism in YPD medium, where the sugar concentration was low (20 g/L), whereas in synthetic or natural must, with high sugar concentrations (≥ 200 g/L), glucose repression occurred [45].
In general, most non-Saccharomyces yeasts present weak fermentation capacity and grow slower than S. cerevisiae [46,47]. Similar results were observed in the current study, where all non-Saccharomyces yeasts started fermentations slower than Sc23, and eight of the strains had poorer growth than Sc23 during the first 48 h. In the present work, Sc23 consumed almost half of the sugars at 48 h and presented the highest sugar consumption among all fermentations. This is supported by previous studies in which different non-Saccharomyces strains, M. pulcherrima, S. bombicola, H. uvarum, T. delbrueckii, and Z. bailii consumed less sugar than S. cerevisiae in a single fermentation before 48 h [24,34,44]. On the other hand, four of the strains had faster growth and higher ethanol yields than Sc23 (Lt1, Sb Nc, Td BA, and Zb42), and three of them (Lt1, Td BA, and Zb42) also had higher sugar consumption at 48 h in SM. Thus, during alcoholic fermentation in synthetic must, growth seemed to be positively correlated with sugar consumption and ethanol yield. In fully aerobic conditions, Quirós et al. [24] also observed a positive correlation between ethanol yield and sugar consumption in non-Saccharomyces strains but a negative correlation with biomass, which may be due to the higher growth capacity in respiratory conditions.
After the proposed screening, we demonstrated that the ten selected non-Saccharomyces yeasts reduced the ethanol content, in both synthetic and natural musts, by sequential fermentations. Therefore, the strategy of two short-term trials in 5 days to select the non-Saccharomyces strains was appropriate, as the ethanol reduction was confirmed for most strains. Moreover, the timing of S. cerevisiae inoculation in the sequential fermentations (48 h) was also appropriate, as most non-Saccharomyces species could persist until mid-end of the fermentation, showing an impact on the ethanol content and the final product.
Non-Saccharomyces yeasts lose viability during alcoholic fermentation and are soon replaced by S. cerevisiae. This may be due to several factors, such as low resistance to ethanol [48], nutrient competition [33,49,50], or microbial interactions, either by cell-to-cell contact [51,52,53] or the secretion of antimicrobial compounds by different yeasts (mainly S. cerevisiae) [54,55,56]. The populations of Lt2, Mp51, Td Pt, Td35, and Zb43 were found viable until the end of fermentation (cultivating on WLN), although they showed different performance in SM and NM fermentations. This persistence seems to be inconsistent with previous studies that claimed that most non-Saccharomyces species cannot tolerate ethanol concentrations above 5–7% (v/v) [26,52,55]. However, we have recently shown that L. thermotolerans and T. delbrueckii, used as a single culture, are able to finish a fermentation with 200 g/L of sugars, producing up to 9–10% (v/v) of ethanol [57]. Even if the presence of S. cerevisiae in mixed fermentations can induce the death of other yeast species [58], other studies have shown that the presence of both Saccharomyces and non-Saccharomyces yeasts increased the persistence of non-Saccharomyces yeasts during the fermentation process [47,59]. Indeed, interactions between Saccharomyces and non-Saccharomyces wine yeasts have an effect not only on the persistence of the non-Saccharomyces yeasts but also on the behavior of the Saccharomyces wine strains [32]. Thus, the survival of these non-Saccharomyces yeasts until the end of fermentation in the current study might be a result of possible synergistic interactions between yeasts, and also due to their tolerance to a higher alcohol content, although this fact needs to be confirmed by further research.
Our results also showed that yeast performance and survival was influenced by the type of must (SM and NM), which could be due to the different nutrient composition. Indeed, we have previously observed that different sugar and nitrogen concentrations on the must have a clear effect on the evolution of mixed fermentations done with H. uvarum, S. bacillaris, and T. delbrueckii species, both on sugar consumption and population dynamics [33]. Another study showed different sugar consumption profiles between Chardonnay and Shiraz grape must (with 240 and 210 g/L sugars, respectively) in mixed fermentations with M. pulcherrima/S. cerevisiae [27]. Moreover, in a previous study we observed that changes in the concentration of some fermentation metabolites had an effect on the culturability of some non-Saccharomyces strains (H. uvarum, S. bacillaris and M. pulcherrima) when used in mixed fermentations [53].
In the present work, Mp51/Sc23 fermentation demonstrated the highest ethanol reduction of 1.23% (v/v) in SM and 1.39% (v/v) in NM. The other strain belonging to the M. pulcherrima species, Mp FA, reduced the ethanol content by 1.17% (v/v) in SM fermentation. Similar to previous studies, M. pulcherrima has been recognized as a strain with a relatively high capacity to reduce ethanol in sequential fermentation with S. cerevisiae and had exhibited ethanol reductions by 0.9 to 3.6% (v/v) [25,60,61]. In addition, fermentation by Zb43/Sc23 and Td Pt/Sc23 reduced the ethanol content by 1.02 and 0.84% (v/v) in NM fermentation, respectively. In agreement with previous research, Z. bailli and T. delbrueckii in sequential fermentation reduced the ethanol content by 1.0 to 1.6% (v/v) [31,44].
During the fermentation process, the reduction of the ethanol concentration by non-Saccharomyces yeasts could be explained not only by their greater accumulation of yeast biomass but also by other byproducts produced after consuming sugars [26]. Under sufficient oxygen availability, carbon from sugar metabolism can be diverted towards organic acids and glycerol, resulting in low ethanol production [62,63]. As the present study aims to be a method for screening non-Saccharomyces, we only evaluated the concentration of main by-products after alcoholic fermentation. Interestingly, the content of byproducts was influenced by the type of must used. In the current study, the highest concentration of glycerol was achieved in fermentations with Mp51 and Mp FA but only in SM. Furthermore, the production of glycerol in Mp51/Sc23 fermentation was affected by the type of must, as no significant increase was observed in NM fermentation, even if the highest ethanol reduction was obtained in this condition. As discussed before, the different nutrient composition of the must could affect the viability and metabolism of some non-Saccharomyces strains [27,53]. Thus, the current study reveals that the glycerol production should not be the only metabolic pathway to reduce ethanol content. On the contrary, the highest concentration of glycerol was observed in NM fermentation with Lt2. This was consistent with the results from Gobbi et al. [61] when fermentations with L. thermotolerans generated higher concentrations of glycerol (more than 7 g/L) in natural must. Associated with the overproduction of glycerol caused by ethanol reduction, the concentration of acetic acid might be increased, mainly in aerobic conditions [64,65]. However, in the present work, performed in semi-anaerobic conditions, the fermentation with Mp FA/Sc23 in SM and NM significantly reduced the concentration of acetic acid, although increased the glycerol content, when achieving an ethanol reduction. The same performance was also observed in sequential fermentations with Sl35 and Zb43 in SM. These results supported those of Morales et al. [65], where M. pulcherrima was able to reduce the concentration of acetic acid while increased glycerol and reduced ethanol content in mixed fermentations, compared with single S. cerevisiae inoculation. In the current study, the concentration of acetic acid remained below 0.8 g/L, considered the level when acetic acid may confer unpleasant acidic taste to wine [46]. Nevertheless, our results showed that both H. uvarum strains (Hu4 and Hu06) have significantly increased the acetic acid content in NM wines, confirming its higher production of negative byproducts and its poor oenological performance [66]. Previous studies have shown that in T. delbrueckii and H. uvarum species acetic acid production was unrelated to ethanol formation, being T. delbruecki a low and constant acetic acid producer, and H. uvarum a high acetic acid producer species [66]. Additionally, fermentation by L. thermotolerans (Lt2/Sc23) produced the highest concentration of lactic acid in SM and NM fermentations, especially in NM fermentation. Strains of L. thermotolerans are frequently used for the acidification of low-acidity wines due to their ability of producing lactic acid during wine fermentations [67,68]. Our results also agreed with Binati et al. [69], who reported that sequential fermentation with L. thermotolerans followed by inoculation of S. cerevisiae at 48 h produced a high concentration of lactic acid and reduced the ethanol content by 0.35% (v/v) in Pinot Grigio must. In our study, Lt2 reduced approximately 0.35% (v/v) ethanol in SM and NM fermentations. The highest content of succinic acid was produced in SM fermentation with Zb43. Likewise, sequential fermentation by Z. bailli increased the concentration of succinic acid in defined grape must [44].
In conclusion, this was a rapid method for screening yeasts with low ethanol yields. M. pulcherrima Mp51 and Mp FA are two appropriate wine yeasts for reducing ethanol in sequential fermentation trials. The potential of Z. bailii Zb43 and T. delbrueckii Td Pt to reduce ethanol concentrations needs to be explored. In addition, a complete analysis of the aromatic compounds should be analyzed to determine the impact of those sequential fermentations and ethanol reduction on wine quality and flavor. Thus, further research should focus on optimizing the inoculation time of non-Saccharomyces strains in sequential fermentation, as well as on the chemical and sensory analysis of the resulting wines. However, the application at the industrial scale is still a challenge to be addressed in the future.
Acknowledgments
The authors would like to thank Braulio Esteve-Zarzoso and Rosa Pastor for their help at the laboratory, as well as Francisco Carrau and Ramon González, for providing some of the strains.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/5/658/s1, Table S1: Glucose consumption (g/L), ethanol production (%, v/v) and ethanol yield (g/g) after 3 d in 5 mL YPD medium, Table S2: Analysis of sugars, ethanol, organic acids and glycerol from samples after 48 h of single fermentation.
Author Contributions
Conceptualization, A.M., G.B. and M.-J.T.; Methodology, X.Z., Y.N. and G.B.; Formal analysis, X.Z. and Y.N.; Investigation, X.Z. and Y.N.; Resources, A.M., G.B. and M.-J.T.; Data curation, X.Z., Y.N. and G.B.; Writing—original draft preparation, X.Z. and Y.N.; Writing—review and editing, A.M., G.B. and M.-J.T.; Supervision, A.M., Y.N. and G.B.; Project administration, A.M. and G.B.; Funding acquisition, A.M. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Innovation and Universities, Spain (Project CoolWine, PCI2018-092962), under the call ERA-NET ERA COBIOTECH. Y.N. is a postdoctoral Fellow of this project. X. Z. has a Fellowship from the Oenological Biotechnology group (URV).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Godden P. Persistent wine instability issues. Aust. Grapegrow. Winemak. 2000;443:10–14. [Google Scholar]
- 2.Jones G.V., White M.A., Cooper O.R., Storchmann K. Climate change and global wine quality. Clim. Chang. 2005;73:319–343. doi: 10.1007/s10584-005-4704-2. [DOI] [Google Scholar]
- 3.Alston J.M., Fuller K.B., Lapsley J.T., Soleas G. Too much of a good thing? Causes and consequences of increases in sugar content of California wine grapes. J. Wine Econ. 2011;6:135–159. doi: 10.1017/S1931436100001565. [DOI] [Google Scholar]
- 4.Bisson L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999;50:107–119. [Google Scholar]
- 5.Buescher W.A., Siler C.E., Morris J.R., Threlfall R.T., Main G.L., Cone G.C. High alcohol wine production from grape juice concentrates. Am. J. Enol. Vitic. 2001;52:345–351. [Google Scholar]
- 6.Coulter A.D., Henschke P.A., Simos C.A., Pretorius I.S. When the heat is on, yeast fermentation runs out of puff. Aust. N. Z. Wine Ind. 2008;23:26–30. [Google Scholar]
- 7.Escudero A., Campo E., Fariña L., Cacho J., Ferreira V. Analytical characterization of the aroma of five premium red wines. Insight into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007;55:4501–4510. doi: 10.1021/jf0636418. [DOI] [PubMed] [Google Scholar]
- 8.García-Martín N., Perez-Magariño S., Ortega-Heras M., González-Huerta C., Mihnea M., González-Sanjosé M.L., Palacio L., Prádanos P., Hernández A. Sugar reduction in musts with nanofiltration membranes to obtain low alcohol-content wines. Sep. Purif. Technol. 2010;76:158–170. doi: 10.1016/j.seppur.2010.10.002. [DOI] [Google Scholar]
- 9.Kontoudakis N., Esteruelas M., Fort F., Canals J.M., Zamora F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine R. 2011;17:230–238. doi: 10.1111/j.1755-0238.2011.00142.x. [DOI] [Google Scholar]
- 10.Diban N., Arruti A., Barcelo A., Puxeu M., Urtiaga A., Ortiz I. Membrane dealcoholization of different wine varieties reducing aroma losses. Modeling and experimental validation. Innov. Food Sci. Emerg. 2013;20:259–268. doi: 10.1016/j.ifset.2013.05.011. [DOI] [Google Scholar]
- 11.Varela C., Dry P.R., Kutyna D.R., Francis I.L., Henschke P.A., Curtin C.D., Chambers P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine R. 2015;21:670–679. doi: 10.1111/ajgw.12187. [DOI] [Google Scholar]
- 12.De Toda F.M., Sancha J.C., Balda P. Reducing the sugar and pH of the grape (Vitis vinifera L. cvs. ‘Grenache’ and ‘Tempranillo’) through a single shoot trimming. S. Afr. J. Enol. Vitic. 2013;34:246–251. doi: 10.21548/34-2-1101. [DOI] [Google Scholar]
- 13.Bindon K., Varela C., Kennedy J., Holt H., Herderich M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. cabernet sauvignon 1. Grape and wine chemistry. Food Chem. 2013;138:1696–1705. doi: 10.1016/j.foodchem.2012.09.146. [DOI] [PubMed] [Google Scholar]
- 14.Mihnea M., González-SanJosé M.L., Ortega-Heras M., Pérez-Magariño S., García-Martin N., Palacio L., Prádanos P., Hernández A. Impact of must sugar reduction by membrane applications on volatile composition of verdejo wines. J. Agric. Food Chem. 2012;60:7050–7063. doi: 10.1021/jf301433j. [DOI] [PubMed] [Google Scholar]
- 15.Aguera E., Bes M., Roy A., Camarasa C., Sablayrolles J.M. Partial removal of ethanol during fermentation to obtain reduced-alcohol wines. Am. J. Enol. Vitic. 2010;61:53–60. [Google Scholar]
- 16.Kutyna D.R., Varela C., Henschke P.A., Chambers P.J., Stanley G.A. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Tech. 2010;21:293–302. doi: 10.1016/j.tifs.2010.03.004. [DOI] [Google Scholar]
- 17.Gonzalez R., Quirós M., Morales P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Tech. 2013;29:55–61. doi: 10.1016/j.tifs.2012.06.015. [DOI] [Google Scholar]
- 18.Varela J., Varela C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019;56:88–96. doi: 10.1016/j.copbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen S., Thevelein J.M., Nevoigt E. Genetic mapping of quantitative phenotypic traits in Saccharomyces cerevisiae. FEMS Yeast Res. 2013;12:215–227. doi: 10.1111/j.1567-1364.2011.00777.x. [DOI] [PubMed] [Google Scholar]
- 20.Steensels J., Snoek T., Meersman E., Nicolino M.P., Voordeckers K., Verstrepen K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014;38:947–995. doi: 10.1111/1574-6976.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollero S., Bloem A., Camarasa C., Sanchez I., Ortiz-Julien A., Sablayrolles J.M., Dequin S., Mouret J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015;99:2291–2304. doi: 10.1007/s00253-014-6210-9. [DOI] [PubMed] [Google Scholar]
- 22.Varela C., Kutyna D.R., Solomon M.R., Black C.A., Borneman A., Henschke P.A., Pretorius I.S., Chambers P.J. Evaluation of gene modification strategies for the development of low-alcohol wine yeasts. Appl. Environ. Microbiol. 2012;78:6068–6077. doi: 10.1128/AEM.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossouw D., Heyns E.H., Setati M.E., Bosch S., Bauer F.F. Adjustment of trehalose metabolism in wine Saccharomyces cerevisiae strains to modify ethanol yields. Appl. Environ. Microbiol. 2013;79:5197–5207. doi: 10.1128/AEM.00964-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quirós M., Rojas V., Gonzalez R., Morales P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014;181:85–91. doi: 10.1016/j.ijfoodmicro.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Contreras A., Hidalgo C., Henschke P.A., Chambers P.J., Curtin C., Varela C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014;80:1670–1678. doi: 10.1128/AEM.03780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciani M., Morales P., Comitini F., Tronchoni J., Canonico L., Curiel J.A., Oro L., Rodrigues A.J., Gonzalez R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016;7:642. doi: 10.3389/fmicb.2016.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varela C., Sengler F., Solomon M., Curtin C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016;209:57–64. doi: 10.1016/j.foodchem.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Varela C., Barker A., Tran T., Borneman A., Curtin C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017;252:1–9. doi: 10.1016/j.ijfoodmicro.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Canonico L., Solomonb M., Comitinia F., Ciani M., Varela C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019;84:103247. doi: 10.1016/j.fm.2019.103247. [DOI] [PubMed] [Google Scholar]
- 30.Ballester-Tomás L., Prieto J.A., Gil J.V., Baeza M., Randez-Gil F. The Antarctic yeast Candida sake: Understanding cold metabolism impact on wine. Int. J. Food Microbiol. 2017;245:59–65. doi: 10.1016/j.ijfoodmicro.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Gobbi M., De Vero L., Solieri L., Comitini F., Oro L., Giudici P., Ciani M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014;239:41–48. doi: 10.1007/s00217-014-2187-y. [DOI] [Google Scholar]
- 32.Ciani M., Comitini F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015;1:1–6. doi: 10.1016/j.cofs.2014.07.001. [DOI] [Google Scholar]
- 33.Lleixà J., Manzano M., Mas A., Portillo M.C. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016;7:1959. doi: 10.3389/fmicb.2016.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canonico L., Comitini F., Oro L., Ciani M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016;7:278. doi: 10.3389/fmicb.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padilla B., Zulian L., Ferreres À., Pastor R., Esteve-Zarzoso B., Beltran G., Mas A. Sequential inoculation of native non-Saccharomyces and Saccharomyces cerevisiae strains for wine making. Front. Microbiol. 2017;8:1293. doi: 10.3389/fmicb.2017.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maturano Y.P., Mestre M.V., Kuchen B., Toro M.E., Mercado L.A., Vazquez F., Combina M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019;289:40–48. doi: 10.1016/j.ijfoodmicro.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Englezos V., Rantsiou K., Cravero F., Torchio F., Ortiz-Julien A., Vincenzo Gerbi V., Rolle L., Cocolin L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016;100:5515–5526. doi: 10.1007/s00253-016-7413-z. [DOI] [PubMed] [Google Scholar]
- 38.Esteve-Zarzoso B., Belloch C., Uruburu F., Querol A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999;49:329–337. doi: 10.1099/00207713-49-1-329. [DOI] [PubMed] [Google Scholar]
- 39.Padilla B., García-Fernández D., González B., Izidoro I., Esteve-Zarzoso B., Beltran G., Mas A. Yeast biodiversity from DOQ priorat uninoculated fermentations. Front. Microbiol. 2016;7:930. doi: 10.3389/fmicb.2016.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beltran G., Novo M., Rozès N., Mas A., Guillamón J.M. Nitrogen catabolite repression in during wine fermentations. FEMS Yeast Res. 2004;4:625–632. doi: 10.1016/j.femsyr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Quirós M., Gonzalez-Ramos D., Tabera L., Gonzalez R. A new methodology to obtain wine yeast strains overproducing mannoproteins. Int. J. Food Microbiol. 2010;139:9–14. doi: 10.1016/j.ijfoodmicro.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Jolly N.P., Augustyn O.H.P., Pretorius I.S. The role and use of non-Saccharomyces yeasts in wine production. S. Afr. J. Enol. Vitic. 2006;27:15–39. doi: 10.21548/27-1-1475. [DOI] [Google Scholar]
- 43.Mas A., Padilla B., Esteve-Zarzoso B., Beltran G., Reguant C., Bordons A. Taking advantage of natural biodiversity for wine making: The WILDWINE project. Agric. Agric. Sci. Procedia. 2016;8:4–9. doi: 10.1016/j.aaspro.2016.02.002. [DOI] [Google Scholar]
- 44.Contreras A., Hidalgo C., Schmidt S., Henschke P.A., Curtin C., Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Barnett J.A., Entian K.D. A history of research on yeasts 9: Regulation of sugar metabolism. Yeast. 2005;22:835–894. doi: 10.1002/yea.1249. [DOI] [PubMed] [Google Scholar]
- 46.Fleet G.H., Heard G. Yeasts-growth during fermentation. In: Fleet G.H., editor. Wine Microbiology and Biotechnology. Harwood Academic Publishers; Chur, Switzerland: 1993. pp. 27–54. [Google Scholar]
- 47.Ciani M., Beco L., Comitini F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006;108:239–245. doi: 10.1016/j.ijfoodmicro.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Ribéreau-Gayon P., Dubourdieu D., Donèche B., Lonvaud A. Handbook of Enology: The Microbiology of Wine and Vinifications. 2nd ed. John Wiley & Sons, Ltd.; Chichester, UK: 2006. pp. 22–53. [Google Scholar]
- 49.Albergaria H., Torrão A.R., Hogg T., Gírio F.M. Physiological behaviour of Hanseniaspora guilliermondii in aerobic glucose-limited continuous cultures. FEMS Yeast Res. 2003;3:211–216. doi: 10.1016/S1567-1356(02)00187-3. [DOI] [PubMed] [Google Scholar]
- 50.Andorrà I., Berradre M., Mas A., Esteve-Zarzoso B., Guillamón J.M. 2012. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT Food Sci. Technol. 2012;49:8–13. doi: 10.1016/j.lwt.2012.04.008. [DOI] [Google Scholar]
- 51.Nissen P., Nielsen D., Arneborg N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell–cell contact-mediated mechanism. Yeast. 2003;20:331–341. doi: 10.1002/yea.965. [DOI] [PubMed] [Google Scholar]
- 52.Arneborg N., Siegumfeldt H., Andersen G.H., Nissen P., Daria V.R., Rodrigo P.J., Glückstad J. Interactive optical trapping shows that confinement is a determinant of growth in a mixed yeast culture. FEMS Microbiol. Lett. 2005;245:155–159. doi: 10.1016/j.femsle.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Wang C., Mas A., Esteve-Zarzoso B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016;7:502. doi: 10.3389/fmicb.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albergaria H., Francisco D., Gori K., Arneborg N., Gírio F.M. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010;86:965–972. doi: 10.1007/s00253-009-2409-6. [DOI] [PubMed] [Google Scholar]
- 55.Branco P., Francisco D., Chambon C., Hébraud M., Arneborg N., Almeida M.G., Caldeira J., Albergaria H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014;98:843–853. doi: 10.1007/s00253-013-5411-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Mas A., Esteve-Zarzoso B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015;206:67–74. doi: 10.1016/j.ijfoodmicro.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Roca-mesa H., Sendra S., Mas A., Beltran G., Torija M.J. Nitrogen preferences during alcoholic fermentation of different non-Saccharomyces yeasts of oenological interest. Microorganisms. 2020;8:157. doi: 10.3390/microorganisms8020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albergaria H., Arneborg N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016;100:2035–2046. doi: 10.1007/s00253-015-7255-0. [DOI] [PubMed] [Google Scholar]
- 59.Mendoza L.M., De Nadra M.C.M., Farías M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007;29:1057–1063. doi: 10.1007/s10529-007-9355-0. [DOI] [PubMed] [Google Scholar]
- 60.Röcker J., Strub S., Ebert K., Grossmann M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016;242:2051–2070. doi: 10.1007/s00217-016-2703-3. [DOI] [Google Scholar]
- 61.Gobbi M., Comitini F., Domizio P., Romani C., Lencioni L., Mannazzu I., Ciani M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013;33:271–281. doi: 10.1016/j.fm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Giovanelli G., Peri C., Parravicini E. Kinetics of grape juice fermentation under aerobic and anaerobic conditions. Am. J. Enol. Vitic. 1996;47:429–434. [Google Scholar]
- 63.Rodrigues A.J., Raimbourg T., Gonzalez R., Morales P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT Food Sci Tech. 2016;65:1038–1043. doi: 10.1016/j.lwt.2015.09.046. [DOI] [Google Scholar]
- 64.Ciani M., Rosini G. Validity of Genevois’ equation in wine yeast selection. Ann. Microbiol. Enzim. 1995;45:201–207. [Google Scholar]
- 65.Morales P., Rojas V., Quirós M., Gonzalez R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotech. 2015;99:3993–4003. doi: 10.1007/s00253-014-6321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciani M., Maccarelli F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998;14:199–203. doi: 10.1023/A:1008825928354. [DOI] [Google Scholar]
- 67.Kapsopoulou K., Mourtzini A., Anthoulas M., Nerantzis E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microb. Biot. 2007;23:735–739. doi: 10.1007/s11274-006-9283-5. [DOI] [Google Scholar]
- 68.Benito A., Calderón F., Palomero F.E., Benito S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules. 2015;20:9510–9523. doi: 10.3390/molecules20069510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binati R.L., Lemos Junior W.J.F., Luzzini G., Slaghenaufi D., Ugliano M., Torriani S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020;318:108470. doi: 10.1016/j.ijfoodmicro.2019.108470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.