Abstract
Mistakenly thought to be the consequence of oxygen lack in contracting skeletal muscle we now know that the L-enantiomer of the lactate anion is formed under fully aerobic conditions and is utilized continuously in diverse cells, tissues, organs and at the whole-body level. By shuttling between producer (driver) and consumer (recipient) cells lactate fulfills at least three purposes: 1] a major energy source for mitochondrial respiration; 2] the major gluconeogenic precursor; and 3] a signaling molecule. Working by mass action, cell redox regulation, allosteric binding, and reprogramming of chromatin by lactylation of lysine residues on histones, lactate has major influences in energy substrate partitioning. The physiological range of tissue [lactate] is 0.5–20 mM and the cellular Lactate/Pyruvate ratio (L/P) can range from 10 to >500; these changes during exercise and other stress-strain responses dwarf other metabolic signals in magnitude and span. Hence, lactate dynamics have rapid and major short- and long-term effects on cell redox and other control systems. By inhibiting lipolysis in adipose via HCAR-1, and muscle mitochondrial fatty acid uptake via malonyl-CoA and CPT1, lactate controls energy substrate partitioning. Repeated lactate exposure from regular exercise results in major effects on the expression of regulatory enzymes of glycolysis and mitochondrial respiration. Lactate is the fulcrum of metabolic regulation in vivo.
Keywords: Glycolysis, Oxidative metabolism, Aerobic, Anaerobic, Mitochondrial biogenesis, Exercise, Gluconeogenesis, Energy substrate partitioning, Cell-cell signaling, SIRT activation, PPAR-γ, PGC-1α, HCAR1, Histone lactylation, TGFβ
1. Introduction'
Mistakenly thought to be the consequence of oxygen deficiency in contracting skeletal muscle, we now know that the L-enantiomer of the lactate anion is formed under fully aerobic conditions and is utilized continuously in diverse cells, tissues, organs and at the whole-body level. In contrast the D-enantiomer is not typical of mammalian metabolism and has negative consequences that have been described previously [22,49]. Because in mammalian systems L-lactate is the inexorable product of one metabolic pathway (glycolysis), and the substrate for another pathway (mitochondrial respiration), lactate is the link between glycolytic and aerobic pathways. In contrast to its early portrayal as a metabolic waste product and fatigue agent, lactate is the principal messenger in a complex feedback loop system. According to the Lactate Shuttle Hypothesis the linkage between driver cells of lactate formation and recipient cells of lactate use or signaling can transcend compartment barriers and occur within and among cells, tissues and organs [14,16,18,19,73,172] (Fig. 1). Challenges to adenosine triphosphate (ATP) supply stimulate lactate production, leading to immediate, short- and long-term cellular adaptions to support ATP homeostasis. The physiology and biochemistry of this topic was recently reviewed and should be consulted [22,49], but subsequently new information has become available, particularly with regard to the role the lactate shuttling in metabolic signaling [85,154,161,176].
Fig. 1.
Depiction of the Lactate Shuttle as it describes the roles of lactate in delivery of oxidative and gluconeogenic substrates as well as in cell signaling. Examples of the Cell-Cell Lactate Shuttles include lactate exchanges between producer (or driver) white-glycolytic (FW) and red-oxidative (SO) consumer (or recipient) fibers within a working muscle bed and between producer working skeletal muscle and consumer heart, brain, liver and kidneys. Examples of Intracellular Lactate Shuttles include cytosol-mitochondrial and cytosol-peroxisome exchanges. Indeed most, if not all, lactate shuttles are driven by a concentration or pH gradient, or by redox state. Symbols: G – Glucose and Glycogen, L – Lactate, and M – elements of the mitochondrial reticulum. Originally compiled from diverse sources (16, 18, 19); from (22).
As demonstrated on every mammalian model system studied (rats, dogs, mice), and mainly humans, at the whole-body level, lactate metabolism fulfills at least three functions: 1] lactate is a major energy source [14,37,45,86,105,158,159]; 2] lactate is the major gluconeogenic precursor [12,48,110,160]; and 3] lactate is a signaling molecule with autocrine-, paracrine- and endocrine-like effects and is referred to as a “lactormone” [16,19,75]. Lactate exchanges within and among cells are referred to as “Intracellular” and “Cell-Cell” lactate shuttles that describe the roles of lactate in delivery of oxidative and gluconeogenic substrates as well as a signaling moiety [16,19]. Examples of Intracellular Lactate Shuttles include cytosol-mitochondrial [23,32], and cytosol-peroxisome exchanges [106]. Examples of the Cell-Cell Lactate Shuttles include lactate exchanges between white-glycolytic and red-oxidative fibers within a muscle bed and between working skeletal muscle and heart [13,62,63], brain [65,132,164], liver and kidneys [12,48,111,173], astrocytes and neurons [126] and vice versa (i.e., neurons and astrocytes) [100]. Most, if not all Cell- Lactate Shuttles are driven by concentration or pH gradients, or by redox state. However, numerous body compartments and systems such as the interstitial space, vasculature and circulation contribute to lactate shuttling in vivo.
The role of lactate as the preferred fuel energy source for exercising muscles [14,105,108,158], and as the main gluconeogenic precursor [11,12,48,110,111] have recently been reviewed [22]. Hence, following a brief review of lactate shuttling [16,19], this paper explores aspects of lactate signaling in metabolic regulation (Fig. 2).
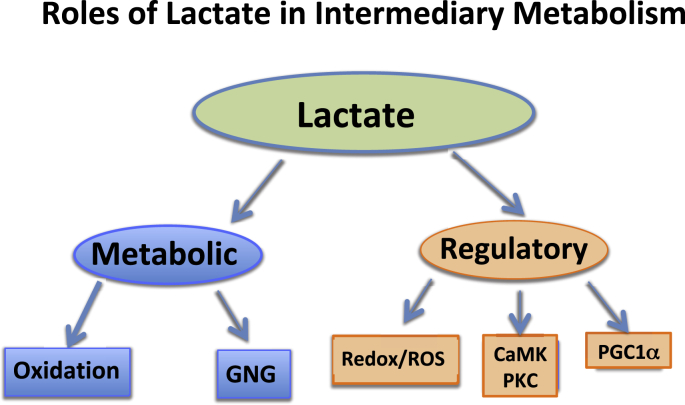
Fig. 2.
Depiction of the Lactate Shuttle as it fulfills three physiological Functions: 1] lactate is a major energy source; 2] lactate is the major gluconeogenic precursor; and 3] lactate is a signaling molecule with autocrine-, paracrine- and endocrine-like effects and has been called a “lactormone”. These functions can be subdivided to Metabolic (oxidative fuel) and gluconeogenic (GNG) functions, and Regulatory, or signaling functions.
1.1. Background
The purported roles of lactate shuttling in physiology and metabolism continue to gain support with ongoing research [37,86,91,161,176]. In functioning humans [112,113], working human skeletal muscles [14,159], as well as in the beating hearts of those individuals [13,62,63], lactate is preferred over glucose and fatty acids as a fuel. Lactate is preferred over glucose as a fuel in brain preparations [147,148,150] and, most importantly, in humans in vivo [76,164]. The Astrocyte-Neuron Lactate Shuttle (ANLS) posits that lactate is extruded by astrocytes and then actively consumed and oxidized by neurons involved in glutamatergic signaling [126]. Relevant to lactate shuttling in brain [4,6], neurons possess the cellular components necessary for glucose uptake and lactate production as well as direct arterial lactate uptake and use by an Intracellular Lactate Shuttle [74]. Moreover, a post-trauma neuro-protective role of lactate has been identified [83,149]. In fact, because lactate is the preferred brain fuel, normally, as well as post injury [66,67], lactate supplementation is being evaluated to hasten and improve outcomes following traumatic brain injury (TBI) and other conditions [103,116,174].
Aerobic Glycolysis and Lactate Production and Oxidation: Notwithstanding the Warburg Effect of aerobic glycolysis in cancer [170], vide infra, we now have good support showing that aerobic glycolysis leading to lactate production occurs continuously in resting humans as well as during stressful conditions such as exercise, high altitude exposure, trauma, pancreatitis, sepsis, myocardial infarction and heart failure. At the outset of this discussion it is important to note that lactate is the inexorable product of aerobic glycolysis (Fig. 3) [22,139]. Importantly and regrettably, there is no solid experimental support for the traditional view that glycolytic flux is directed to mitochondrial respiration solely through pyruvate uptake by the mitochondrial reticulum. In the past it was assumed by many that the rising lactate abundance in the body indicated anaerobic conditions at the cellular level, but our understanding of lactate as a metabolically valuable carbohydrate has now replaced this traditional view [16,49]. Not only is experimental support lacking for the notion that glycolytic flux is directed to lactate production only when oxygen is lacking, there is no evidence that the first step in lactate oxidation (i.e., conversion to pyruvate) occurs in the cytosol of any tissue, including the beating heart or working skeletal muscle that has a lactate to pyruvate concentration ratio (L/P) > 100 [80], and which are net glucose consumers [13]. In contrast, there is compelling evidence that glucose and glycogen catabolism proceed to lactate production under fully aerobic conditions [7,39,136,139].
Fig. 3.
There is strong evidence that glucose and glycogen catabolism proceed to lactate production under fully aerobic conditions in studies on intact animals, animal tissue preparations and healthy humans in vivo. In muscles and arterial blood of resting healthy humans lactate concentration approximates 1.0 mM, while pyruvate concentration approximates 0.1 mM, the Lactate/Pyruvate (L/P) being 10, with net lactate production and release from resting muscle of healthy individuals when intramuscular partial pressure of oxygen (PO2) approximates 40 Torr, well above the critical mitochondrial PO2 for maximal mitochondrial respiration (1–2 Torr) [7,39,140]. During exercise at about 65% of maximal oxygen consumption (VO2max) lactate production and net lactate release from working muscle beds rise and the L/P rises more than an order of magnitude (to ~500), but the intramuscular PO2 remains at 3–4 Torr, well above the critical mitochondrial O2 level. Hence, it is appropriate to conclude that in healthy humans glycolysis proceeds to lactate under fully aerobic conditions. Importantly, most (75–80%) of lactate is disposed of immediately within the tissue or subsequent to release and reuptake by working muscle, with significant uptake and oxidation by heart or oxidation and liver for gluconeogenesis. Adapted from diverse sources [14,39,80,136,159]; from Ref. [22].
Lactate and The Mitochondrial Lactate Oxidation Complex (mLOC): A necessary inclusion on the mLOC (vide infra) is necessary because controversy surrounds the issue of mitochondrial lactate oxidation in intermediary metabolism. Some investigators have produced mitochondrial preparations that oxidize lactate [3,15,23,24,27,43,95,125], whereas some others [54,134,143,175] have failed in the attempt. Conceptually, the issue is resolved if one realizes that the cellular respiratory apparatus exists as an extensive network, the mitochondrial reticulum [93,94]. Hence, attempts to isolate “mitochondria” for ex vivo respiratory studies inevitably result in disruption of the mitochondrial reticulum and loss of functionality [64,93] because of labiality and fragility of mitochondrial constituents such as Cytochrome c and L-lactate dehydrogenase (LDH) in the face of severe homogenization and harsh detergents. Still, it is possible to show lactate oxidation in mitochondrial preparations from mammalian, including human skeletal muscle [91]. Importantly, the presence of muscle mitochondrial lactate oxidation can be demonstrated by several different methodologies such as magnetic resonance spectroscopy (MRS) [37,123]. Moreover, studies of muscle cells and tissues using confocal laser scanning microscopy, immunoprecipitation and immunohistochemistry show presence of LDH in the mitochondrial Lactate Oxidation Complex [73,74]. And finally on the matter of mitochondrial lactate oxidation ample studies on healthy human subjects show that the major route of lactate disposal is oxidation [14,47,105,108,[158], [159], [160]]. In retrospect, the inability of some investigators to produce mitochondrial preparations that respire lactate is reminiscent with previous studies in which mitochondrial preparations which failed to oxidize long-chain fatty acids. Those failed results were found to be attributable to action of the proteolytic enzyme Nagarse [122]. In that instance the inability of mitochondria preparations to recapitulate what is know to happen in vivo can be ascribed to isolation artifact. So it is with mitochondrial preparations that lose LDH during isolation and are unable to oxidize lactate; those failed results are due to isolation artifacts. Knocking out, or crippling mLDH in preparation only proves the essential role in mitochondrial lactate oxidation in vivo. And finally on this point, LDH is listed in mitochondrial constituent databases, the MitoCarta (https://www.broadinstitute.org/scientific-community/science/programs/metabolic-disease-program/publications/mitocarta/mitocarta-in-0), and MitoMiner (http://mitominer.mrc-mbu.cam.ac.uk/release-4.0/begin.do) [34,121].
Gluconeogenesis from Lactate: Because the majority of lactate disposal occurs via direct oxidation (≈50% at rest and 75–80% during sustained exercise) [14,47], the discussion above on lactate utilization centered on oxidative disposal. However, while approximately 25% of lactate disposal is via gluconeogenesis [12,48], lactate is by far the major gluconeogenic precursor [61,92]. First recognition of a metabolically beneficial use of lactate in normal physiology was discovery of the Cori Cycle with lactate as the major gluconeogenic precursor [41]. As typically rendered, the Cori Cycle is depicted as having Intracellular Lactate Shuttles in both muscle and liver, that are linked via a Cell-Cell, or rather an Organ-Organ (muscle to liver) Lactate Shuttle through the vasculature. Although developed on the basis of net metabolite balance studies on laboratory animals, due to technical limitations the Coris were unable to demonstrate the phenomenon in humans. Fortunately now with contemporary isotope tracer and arterial-venous difference measurements it is certain that glycemia is supported by gluconeogenesis from lactate during postabsorptive (fasted) rest and physical exercise [12,40,48,50,51,92,173], and that hepatic gluconeogenic function is augmented by the kidneys under diverse physiological conditions [110,111].
2. LACTATE, REDOX and ROS
Cell Work, Muscle Contraction and Mitochondrial Respiration: Processes of cellular energy transduction occur continuously, but in the case of muscle contraction metabolic rate is expanded by an order of magnitude or more [22]. Adenosine triphosphate (ATP) hydrolysis is immediately buffered by creatine phosphate acting through creatine phosphokinase and rates of glycolysis and glycogenolysis are affected by allosteric regulation of phosphofructokinase, other kinases and dehydrogenases. Nominal muscle lactate concentration ([lactate]) ranges from 0.5 to 1.0 mM, but the dynamic range in flux and concentration can be 20 to 30-fold [35] with a corresponding dynamic Lactate/Pyruvate (L/P) range of 10 to >500 [80]. Such dramatic and dynamic changes have corresponding effects on the cellular NAD+/NADH ratio. The rise in blood L/P during exercise is relatively larger than the relative rise in [lactate] because the relative and absolute increments in [pyruvate] are low compared the comparable changes in [lactate] [80,171]. Hence in terms of both its absolute dynamic range (mM), and relative effect on cell redox, cell work leading to lactate production has a huge effect on metabolic regulation in both driver and recipient cells. Other examples of the effects of lactate production on cell redox follow.
Aside from the major effects of muscle contraction on cell redox [68,165], L-lactate can effect cellular production of Reactive Oxygen Species (ROS) by both enzymatically and non-enzymatically catalyzed reactions. Perhaps, best known is the chemistry by which ROS are generated as the result of mitochondrial respiration [124,129]; less studied are lactate-iron interactions that are capable of generating ROS [168].
Because of the Intracellular Lactate Shuttle, lactate (not pyruvate) is the major fuel for mitochondrial respiration, always and particularly during exercise when the cellular L/P rises and order of magnitude or more (vide supra). Hence, as the major mitochondrial energy substrate, and electron donor, short-term cell respiration from lactate will raise mitochondrial ROS production via Electron Transport Chain (ETC) activity [75,128,129]. Importantly in terms of mitochondrial biogenesis and long-term metabolic adaptations to exercise, lactate exposure to L-6 cells up-regulated many genes [75] (vide infra).
A second way in which mitochondrial L‐lactate metabolism can generate ROS, specifically hydrogen peroxide (H2O2) was discovered by Passarella and colleagues [124]. Using rat liver mitochondria they showed that L‐LAC can generate H2O2 via a putative flavine‐dependent Lactate Oxidase (LOX) restricted to the mitochondrial intermembrane space [44].
Concepts that during exercise cell ROS production is quenched by and actions of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, other peroxidase and antioxidant enzymes are likely well covered in accompanying papers of this symposium volume. However, the idea of non-enzymatically mediated ROS production comes from T.K. Hunt and colleagues working in that area of wound healing [88,168]. Their findings are that iron-containing alkaline phosphate peroxidases of the PhoX family produce lactate as well as superoxide. The proposed mechanism is that lactate chelates with iron released from PhoX thus producing hydroxyl radicals that rapidly decay to superoxide. A favorable consequence is that lactate incites release of Vascular Endothelial Growth Factor (VEGF), Interlukin-1 (IL-1), and TGF-β stimulate angiogenesis and wound healing [88]. Thus, in the view of T.K. Hunt, lactate can function as a “pre-oxidant” at wound sites (personal communication).
Lactate, NAD+, and Sirtuin Activation: Sirtuins (SIRT) are a class of proteins that are thought to be much involved in cellular homeostasis including processes such as transcription, apoptosis, inflammation, stress resistance, mitochondrial biogenesis and energy efficiency following caloric restriction [85,154,161,176], and aging [36]. The name comes from studies of the yeast gene Silent mating-type Information Regulation 2. Seven sirtuin isoforms (SIRT1-7) are known and are deacetylases regulated by the equilibrium between nicotinamide (NAM) and NAD+. Little is known about the long-term effects of exercise on sirtuin regulation, but such studies have commenced [5].
Sirtuin activation is accomplished via changes in cell redox (i.e., the NAD+/NADH) through the concentration of NAM and the activity of enzyme NAM phosphoribosyl transferase (Nampt) that produces NAM. Subtle changes in cell redox affect cell homeostasis as occur in the above named situations. However, lacking are data on the change in Nampt activities in working muscle that drive increments in blood [lactate] as well as in non-working recipient tissues in which changes in NAD+ and NADH concentrations affect cell redox in vivo. However, as described above, changes in cell metabolic rate accompanying exercise result in similar, or greater effects on cellular NAD+/NADH than any other perturbation.
3. Lactate and allosteric binding
Mitochondrial Biogenesis and Lactate: Classic studies in exercise physiology and biochemistry have shown remarkable plasticity of the mitochondrial reticulum in response to regular physical activity that may double the mitochondrial mass [42,82,94,137]. Contemporary studies of physiology and biochemistry have also revealed the molecular signals for exercise-induced increases in mitochondrial protein expression. Among the cellular signaling candidates for transcriptional control of mitochondrial biogenesis are: Calcium ion [119], AMP-activated protein kinase (AMPK) [101,167], Sirtuin 1 (Sirt1) [46], Hypoxia Inducible Factor-1α (HIF-1α) [152], and the ‘master mitochondrial biogenesis activator,’ Peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) [72]. Downstream regulators of mitochondrial biogenesis are: Nuclear Respiratory Factors 1 and 2 (NRF-1 and NRF-2), and Mitochondrial Transcription Factor A (TFAM) [117]. What these signaling pathways appear to share in common are circumstances resulting from a challenge to ATP homeostasis [17,20,21]. While there has been major emphasis in studying downstream affectors of mitochondrial biogenesis (e.g., HIF-1α, AMPK, PGC-1α, and PPAR-γ activation), scarce attention has been placed on upstream affectors, such as lactate as described below.
As noted above, to explain the result of intramuscular and intracellular lactate oxidation observed in vivo we postulated the existence of the mLOC. In efforts to identify components of the mLOC we [75] determined genome-wide responses of L6 cells to elevated exogenous (10 and 20 mM) sodium-L-Lactate (Fig. 4). Lactate exposure increased Reactive Oxygen Species (ROS) production and up-regulated 673 genes, many known to be responsive to ROS and Ca++ (Fig. 5). The induction of genes encoding for components of the mLOC was confirmed by polymerase chain reaction (PCR) and electrophoretic mobility shift (EMSA) methods. Lactate increased Monocarboxylate Transporter-1 (MCT1) mRNA and protein expressions within 1 h and Cytochrome c Oxidase (COx) mRNA and protein expression in 6 h of incubation. Increases in COx coincided with increases in PGC1α expression and the DNA binding activity of nuclear NRF-2. Among the other genes upregulated by lactate were antioxidant enzymes, such as Glutathione Peroxidase (GPx), calcium (Ca++)-response genes including Calcineurin (CaN), slow type Troponin I (TnI), and myogenin that are also known to be responsive to CaN and Ca2+/Calmodulin-dependent protein kinase (CaMK). ROS can increase intracellular Ca++ that raises CaMK activity. As well, free Ca++ can also activate CaMK. Downstream of those signals are the transcription factor cyclic-AMP Response Element Binding protein (CREB), Nuclear Factor, Erythroid 2 (NF-E2), Nuclear Factor kappa light chain enhancer of activated B cells (NF-κB), and Activator Protein 1 (AP-1).
Fig. 4.
A schematic showing the putative mitochondrial lactate oxidation complex (mLOC): MCT1 is inserted into the mitochondrial inner membrane strongly interacting with its chaperone protein CD147, and is also associated with COX as well as mitochondrial LDH (mLDH) which could be located at the outer side of the inner membrane. Lactate, which is always produced in cytosol of muscle and other tissues because of the abundance, activity, and characteristics of cytosolic LDH, is oxidized to pyruvate via the lactate oxidation complex in mitochondria of the same cell. This endergonic lactate oxidation reaction is coupled to the exergonic redox change in COX during mitochondrial electron transport. Abbreviations: GP, glycerol phosphate; Mal-Asp, malate-aspartate; MCT, monocarboxylate (lactate) transporter, mPC, mitochondrial pyruvate carrier, ETC, electron transport chain; TCA, tricarboxylic acid; adapted from Ref. [73].
Fig. 5.
Schematic summarizing the effects of lactate on intracellular signaling in muscle. Contractions stimulate glycolysis and subsequent lactate production and accumulation. In combination, lactate accumulation and mitochondrial respiration induce ROS production. A ROS-sensitive transcriptome is activated, which elicits many cell responses seen in the response to exercise, including MCT1 expression, mitochondrial biogenesis, and the production of antioxidant enzymes (e.g., GPx). In this figure, novel signaling effects described in the present report (solid arrows) are shown. ROS generation (left side of figure) is responsible for regulating MCT1 expression. For mitochondrial biogenesis (right side), it is likely that the lactate-signaling pathway merges with calcium (Ca++) signaling as contractions increase cytosolic Ca++ flux. By itself, lactate increases expressions of slow type troponin I (TnI) and myogenin that are also known to be responsive to Ca++ flux via calcineurin (CaN). ROS can increase intracellular Ca++ that raises CaMK activity. As well, free Ca++ can also activate CaMK. Lactate elicits a large number of adaptive responses, which coordinate metabolism as a functional adaptation to exercise in skeletal muscle cells such as proliferation of the lactate oxidation complex; from Ref. [75].
Clearly, lactate can elicit a large number of adaptive responses, which coordinate metabolism as a functional adaptation to exercise in skeletal muscle cells such as proliferation of the mitochondrial reticulum and the lactate oxidation complex (Fig. 4). Consequently we concluded that the lactate signaling cascade involves ROS production, cytosolic Ca++ and other factors that converge on transcription factors affecting mitochondrial biogenesis. However, the upstream physiological signal is lactate that drives cellular adaptation by affecting expression of hundreds of genes.
3.1. Lactate controls fatty acid mobilization and oxidation by inhibition
Lactate And Lipolysis In Adipose: Inverse relationships between blood [La−] and plasma free fatty acid concentration [FFA] and oxidation during hard exercise when the ventilatory gas exchange ratio (R = VCO2/VO2 ≥ 1.0) have long been recognized [31], but mechanisms underlying the associations are under appreciated. In the 1960's Issekutz and colleagues noted the effect of lactacidemia on diminishing circulating [FFA] in dogs and humans engaged in hard exercise [90,138]. As well, lactate infusion into running dogs caused circulating [FFA] to decline [69,90,115]. In their work these investigators could clearly observe an effect of lactate on circulating [FFA], but whether the mechanism involved hydrogen ions or lactate anions, or represented an inhibition of lipolysis or a stimulation of fatty acid reesterification was not addressed.
The mechanism by which lactatemia suppresses circulating FFA is now know to be due to suppression of adipose lipolysis by lactate working through receptor binding [1,33,59,99]. Moreover, it is now known that, independent of pH or sodium ion, lactate inhibits lipolysis in fat cells through activation of an orphan G-protein coupled receptor (GPR81), now termed Hydroxycarboxylic acid receptor 1 (HCAR-1) (Fig. 6). The effect of lactate binding to HCAR-1 appears to operate through cyclic-AMP (cAMP) and CREB [9,84,98].
Fig. 6.
Illustration of how lactatemia affects blood [glucose] and peripheral glucose uptake as well as the production, uptake and oxidation of FFA giving rise to metabolic inflexibility in muscle. Lactate is the inevitable consequence of glycolysis [139], the minimal muscle L/P being 10 and rising to an L/P > 100 when glycolytic flux is high [80]. Lactate is the favored oxidizable substrate, provides product inhibition of glucose and FFA oxidation. As the products of glycolysis, lactate and pyruvate provide negative feedback inhibition of glucose disposal (blue dashed lines). Also as the predominant mitochondrial substrate, lactate gives rise to Acetyl-CoA, and in turn Malonyl-CoA. Acetyl-CoA inhibits β-ketothiolase, and hence β-oxidation, while Malonyl-CoA inhibits mitochondrial FFA-derivative uptake via CPT1 (T) [142]. Moreover, lactate is the main gluconeogenic precursor raising glucose production and blood [glucose] (red lines). Via GPR81 binding, lactate inhibits lipolysis in WAT (T) depressing circulating [FFA] [84,99]. This model explains the paradoxical presence of lactatemia in high intensity exercise and insulin resistant states with limited ability to oxidize fat (green lines). Modified from Hashimoto et al. [74]. Abbreviations: CPT1-Carnitine Palmitoyl Transporter-1, FFA-Free Fatty Acid, FAT-Fatty Acid Translocator comprised of CD36 and FABPc, GLUT-Glucose Transporter, s-sarcolemmal, m-mitochondrial, Malonyl-CoA formed from exported TCA citrate controlled by the interactions of Malonyl-CoA Decarboxylase (MCD) and Acetyl-CoA Carboxylase (ACC), MCT-Monocarboxylate Transporter, mPC-Mitochondrial Pyruvate Transporter, PDH-Pyruvate Dehydrogenase, WAT-White Adipose Tissue, T-Inhibition. Not shown is Fatty Acyl-Co (FA-CoA) that will accumulate if FFAs are taken up by myocytes, but blocked from mitochondrial entry by the effect of Malonyl-CoA on CPT1. Accumulated intracellular FA-CoA will give rise to Intramyocellular Triglyceride (IMTG) and the formulation of LC-FA, DAG and Ceramides via inhibition of PI3 Kinase (PI3-k) and reducing GLUT4 translocation; from Ref. [22]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Lactate and Pyruvate as Inhibitors of Mitochondrial β−Oxidation: As noted above, when glycolysis is accelerated during muscle contraction, lactate (L) and pyruvate (P) concentrations rise and raise the L/P because of the relatively greater effect on [lactate] than [pyruvate]. At rest, the L/P in muscle and venous effluent from a muscle bed approximates 10, but the ratio rises more than an order of magnitude during moderate intensity exercise [79]. By mass action the monocarboxylate pair floods into the mitochondrial reticulum [124,142], giving rise to acetyl-CoA and, thereby, malonyl-CoA formation (Fig. 6). The rise in malonyl-CoA inhibits the entry of activated FFAs into the mitochondrial matrix by inhibiting carnitine-palmitoyl transferase-1 (CPT1) [107,142] (Fig. 6). As well, the accumulation of acetyl-CoA down regulates β-ketothiolase, the terminal and rate-limiting enzyme of the mitochondrial β-oxidation pathway. Hence, by mass action, allosteric binding and effects on cell redox lactate acts to shut the gates of activated fatty acids into matrix of the mitochondrial reticulum.
Lactate and Transforming Growth Factor Beta2. Recently a large, international group of investigators have expanded knowledge of the role of lactate signaling via Transforming Growth Factor Beta2 (TGF-β2) secreted from adipose of exercising mice [161]. TGF-β is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β1 to −3). Because of its role in immune and stem cell regulation and differentiation, TGF-β2 is a highly researched cytokine in the fields of cancer, auto-immune and infectious diseases, and consequences of disruption of the blood-brain-barrier in epilepsy, aging and TBI [53]. While all TGF-β isoforms are known to be secreted by white blood cells, Takahashi et al. showed that after endurance training adipose of mice secreted TGF-β2 in response to lactate signaling. In turn, TGF-β2 improved glucose tolerance in mice leading the authors to conclude exercise training improves systemic metabolism through an interorgan (adipose to liver) communication via what the authors termed a “lactate-TGF-β2 signaling cycle” [161]. A role for lactate affecting TGF-β2 signaling is also mentioned below under the section on cancer biology.
On first impression one might think that the effects lactate inhibiting lipolysis via HCAR-1 signaling and mitochondrial lactate oxidation via malonyl-CoA (Fig. 6) [99], on one hand, and increasing glucose tolerance via TGF-β2 signaling [161], on the other hand, are contradictory. However, a reasonable alternative explanation is that the effects of lactate on HCAR-1 in adipose are short-term, acute as occurs during hard exercise, and that the effects of TGF-β2 are long term as occurs during recovery from exercise when glucose tolerance and lipid oxidation are improved in men and women [[50], [51], [52],78]. While the purported effects of lactate signaling HCAR-1 and TGF-β2 observed in rodent models await validation in humans, for the present it is certain that short-term lactate both inhibits lipolysis and mitochondrial FFA oxidation, but long-term upregulates mitochondrial biogenesis, glucose tolerance and lipid oxidation in humans in vivo [10,14,51,52,112,113].
4. Gene regulation by lactylation of histones
In a recent report Zhang et al. reported regulation of gene expression by lactylation of 28 lysine residues on histones [176]. Histones are essential components of chromatin, a complex of DNA and proteins that regulate gene expression. As noted above with regard to redox regulation of sirtuins, histones were known to be regulated by cellular enzymes that add, or remove chemical tags such as methyl, acetyl or phosphate groups. Those epigenetic modifications to the genome were known to affect processes such as gene expression and DNA replication and repair. Now, based on the results of Zhang et al. the addition of lactate tags to histones is yet another, epigenetic, way by which the genome is regulated. And, as noted above, lactate release from driver cells into the circulation as occurs in physical exercise and other stressful conditions has the potential to affect gene regulation in diverse cells during and after physical exercise.
As acclaimed as the results of Zhang et al. are, in some ways the effects of lactate on gene expression were anticipated by results of previous investigations. For instance, addition of lactate decreases the content of Histone Deacetylase (HDAC) in the nucleus and HDAC activity [60], as well as chromatin methylation and compactness [97]. In aggregate, results of these studies that suggest a transcriptionally permissive chromatin conformation with rising lactate levels, could also be used to support the previous findings by Hashimoto and Brooks of increased DNA binding following the addition of lactate to L6 cells [75].
The work involving epigenetic regulation of genes by histone lactylation is in its infancy, but going forward it will be interesting to evaluate the short- and long-term effects of histone lactylation on mitochondrial biogenesis and other metabolic protein expression following exercise and exercise training regimens as well as in various acute and chronic diseases and conditions such as diabetes, sepsis and wound healing.
5. A new lactate shuttle: the gut-soma lactate shuttle?
Based on data from a variety of sources, the possibility of a Gut-Soma Lactate shuttle was previously proposed [22]. Support for the idea is to be found in the work of Scheiman et al. [146] who described the presence of “performance-enhancing” gut microbes, members of the genus Veillonella, in the stools of marathon runners. Their results help draw together evidence from diverse sets of experimental and epidemiological data relating gut and somatic health and athlete performance.
From nutrition science we know that pre- and pro-biotic dietary components favorably affect gut fermentation and health [81]. From epidemiology we know that regular physical exercise is beneficial for reducing risks of many common cancers including those of the colon [127]. Moreover, preliminary data indicate relationships between microbiota and the prevalence of insulin resistance and metabolic syndrome [166]. While enthusiasm is high, at present many aspects of the mechanisms of exercise-gut function and health remain to be explored.
From their studies Scheiman et al. [146] proposed that athletes' running performance benefitted because gut microbiota that took up lactate and disposed of it as propionate. However, their proposal is ‘old school’ and missing significance of lactate shuttling in vivo. Further, their proposed a mechanism is unlikely as the gut is under perfused during exercise [28] and the mechanism would be counterproductive because during exercise lactate is purposefully disposed of as a fuel energy source and gluconeogenic precursor [12]. More likely, the reverse is true, a scenario in which the gut supplies lactate, a fermentation product that is exported via sodium-mediated monocarboxylate transporters (sMCT) [38,162] thus supporting athletes' efforts.
6. Role of lactate in resuscitation and treatment of injuries and illnesses
Given the long-standing misunderstanding of lactate metabolism in basic science it was inevitable that misunderstanding would carry over to patient treatment in the clinical setting. Fortunately, now with ongoing revolution in understanding the role of lactate shuttling in metabolism, it is to be expected that practitioners would use new knowledge of lactate metabolism and signaling to improve outcomes in people suffering illnesses and injuries [29,31,58,104]; a summary of the conditions in which lactate treatment can be helpful is presented in (Table 1). Perhaps most remarkable in Table 1 are that L-Lactate treatment is proposed for the treatment of sepsis [58,102] and wound healing and muscle regeneration after injury [88,118,163].
Table 1.
Potential for lactate treatment for illness and injury.
| Resuscitation (Fluid, Electrolytes, Energy) [2,57,103] |
| Acidosis (Exogenous lactate infusion has an alkalotic effect) [103,114,174] |
| Regulation of Glycemia (Lactate is the major GNG precursor) [61,104,109,110] |
| Traumatic Brain Injury (Lactate is brain fuel and anti-inflammatory) [67] |
| Inflammation (via GPR81 binding down stream signaling lactate inhibit the inflammasome) [84] |
| Acute Pancreatitis and Hepatitis (Lactate is an energy substrate, a GNG precursor and anti-inflammatory agent) [84] |
| Myocardial Infarction, Cardiac Surgery and Acute Heart Failure (Lactate is heart fuel) [13,153] |
| Burns (Lactate is an energy substrate, a GNG precursor and anti-inflammatory agent) [157] |
| Sepsis (Lactate incorporation in a resuscitation fluids can support maintenance of blood pressure and circulation, help deliver antibiotics as well as energy substrate, a GNG precursor and have an anti-inflammatory effect) [55,103] |
| Dengue (Lactate is an energy substrate, a GNG precursor and anti-inflammatory agent) [155,174] |
| Cognition (Lactate readily crosses the Blood Brain Barrier, fuels neurons and stimulates secretion of BDNF, improves executive function and memory) [77,83,135] |
| Wound healing [88] and muscle regeneration after injury [118,163]. |
7. When lactate accumulation and shuttling may be maladaptive
While there is growing recognition of the role of lactate shuttling as part of normal physiology, and clinicians are using lactate therapy to improve patient outcomes (Table 1), there are cases in which lactate accumulation and shuttling can be problematic, or pathological. For instance, in cancer aggressiveness of the disease is associated with the extent of hyperlactatemia [71]. There is a long history of interest in lactate production and accumulation in cancer research (the “Warburg Effect”) and recently investigators have sought to inhibit lactate shuttling in tumors by blocking MCTs [156]. Recently also in the field of diabetes research investigators have sought to understand why MCT expression is silenced in pancreatic β-cells such that they do not participate in lactate shuttling and signaling [89,120]. Silencing of MCT1 expression and insertion into the plasma membrane is necessary to prevent hypersecretion of insulin and profound hypoglycemia when blood lactate is high and blood glucose is low as occurs in physical exercise. Dysregulation of lactate metabolism in cancer and in cases in which β-cell MCT protein expression is not silenced have been reviewed recently [22,144], but are presented in abbreviated form, below.
Lactate Shuttles in Cancer Metabolism: In 1923 Otto Warburg and Seigo Minami observed increased glucose uptake and excessive lactate formation even under fully oxygenated conditions [169]. The discovery of aerobic glycolysis was subsequently named the “Warburg Effect” by Efraim Racker [133] and today the high glucose uptake/lactate release phenotype remains a hallmark of cancer [96]. Still, even though lactate is a large part of cancer biology at present there is no consensus on meaning of the Warburg Effect. Again, rather than a byproduct of high glycolysis in cancer, could lactate production in cancer-susceptible cells be involved in transformation to cancer? [144].
In a previous review Iñigo San Millán and I described from the scientific literature how lactogenic cancers, oncogenes and mutated tumor suppressor factors appear to behave with the apparent purpose of reprogramming glycolysis for lactagenesis, thus creating concentration gradients for lactate exchange within, between and among cells [144]. The ways by which lactagenesis may support carcinogenesis; these are: 1) increased glucose uptake, 2) increased glycolytic enzyme expression and activity, 3) decreased mitochondrial function, 4) increased lactate production, accumulation and release, and 5) upregulation of monocarboxylate transporters MCT1 and MCT4 for lactate exchange (Fig. 7).
Fig. 7.
Lactate shuttling gone amiss. In cancer high glucose consumption leading to lactate production under fully aerobic conditions (i.e., the Warburg Effect) are features of cancer cells and tumors. Lactagenesis in cancer can be viewed as a highly orchestrated effort from oncogenes and tumor suppressor mutations for continuous and non-stop glucose utilization to produce lactate involving 5 major steps [1]: Increased glucose uptake through increased expression and translocation of glucose transporters GLUT by transcription factors Hypoxia-Inducible Factor 1α (HIF-1) and c-Myc oncogene as well as loss of expression of tumor suppression factor p53 [2]. Increased glycolytic enzyme expression and activity, especially Lactate Dehydrogenase A (LDHA) by HIF-1α, c-MYC and p53 dysregulation. 3) Decreased mitochondrial function mainly by p53 dysregulation [4]. Increased lactate production, accumulation and release due to mass effect of accelerated glycolysis, mitochondrial dysfunction and increased LDHA expression. 5) Upregulation of monocarboxylate transporters MTC1 and MCT4 and their plasma membrane chaperon, CD147, contributing to dysregulated lactate shuttling in support of carcinogenesis; from Ref. [144].
As a test of the Lactagenesis Hypothesis in cancer we decided to reproduce some of the early studies of Warburg and associates, but with modern genotyping technology. As a result, San Millán et al. provided experimental data in support of the Lactagenesis Hypothesis [145]. Using a human cancer cell line (MCF-7 cells) we found that both endogenously produced and exogenously provided lactate significantly affected the transcription of key oncogenes (MYC, RAS, and PI3KCA), transcription factors (HIF1α and E2F1), and tumor suppressors (BRCA1, BRCA2) as well as cell cycle and proliferation genes involved in breast cancer (AKT1, ATM, CCND1, CDK4, CDKN1A, CDK2B). Certainly results of those studies will need to be replicated on other types of cancer cells and tumor biopsies, but the results suggest the powerful, but potentially ‘double edged sword’ role of lactate in metabolic regulation.
Lack of MCT Pancreatic β-Cell MCT Silencing Can Interfere In the Regulation of Glycemia: Maintenance of blood [glucose] is one of the essential and tightly regulated parameters in human physiology. Glucose-Insulin interactions are complex and interference with glucoregulation, particularly something causing a sudden fall in blood [glucose] can be disastrous. Glucose and glycogen are the precursors to lactate formation [19,87], and lactate is the major gluconeogenic precursor [12,41,48,110,111]. However, whereas blood glucose level plays an important role in the regulation of its clearance rate via influencing the levels of insulin and counter-regulatory hormones, other than providing precursor material for gluconeogenesis, normally lactate is excluded from processes related to insulin secretion. However, if allowed to occur interference with glucose-insulin signaling lactate shuttling can be disruptive, perhaps lethal.
Monocarboxylate (lactate) transporters are ubiquitous, expressed in most tissues [16,56,130], including cancers [151,156] where they support lactate shuttling and are currently targets for cancer therapy. Above, importance of lactate in metabolic regulation was described in detail. However, another way to emphasize the importance of lactate in metabolic regulation is by exclusion. For instance, embryologic deletion of the lactate transporters (MCTs) is lethal. In terms of glucoregulation in vivo, the importance of lactate in glucoregulation is illustrated by exclusion of MCTs from insertion into pancreatic β-cell plasma membranes [131,141]. There, MCT expression is silenced to keep extracellular lactate from affecting intracellular redox and interfering with glucose sensing and insulin secretion [8]. The silencing of MCT1 in pancreatic β-cells is evolutionary proof of how lactate overrides glucose in regulating energy substrate partitioning in general, and insulin secretion in particular when the dominant role of lactate must be suppressed. Noteworthy in this regard is that individuals experiencing failed suppression of pancreatic β-cell MCT expression become hypoglycemic during hard exercise leading to lactatemia. This is because lactate gains entry to pancreatic β-cells and affects cell redox as if blood glucose was elevated. In those individuals aberrant entry of lactate into pancreatic β-cells causes insulin secretion during exercise. The combination of high insulin and increased glucose disposal through metabolism causes profound hypoglycemia [120].
8. Conclusion
Time is overdue to turn the page on understanding of lactate metabolism. Historically, when high lactate levels were seen in organs, tissues and cell compartments the observations were interpreted to represent a stress to those cells, tissues and organs. Regrettably, misinterpretation of early observations was inappropriate and unfortunate because the events observed were, in fact, strain responses to stresses. Hence, rather a waste product of anaerobic, oxygen-limited metabolism lactate is formed continuously under fully aerobic conditions. The velocity, magnitude and range of lactate production and accumulation rates, and perturbations to the L/P and cell redox are large and physiologically significant. Lactate shuttling between producer (driver) and consumer (recipient) cells fulfills at least three purposes: 1] a major energy source; 2] the major gluconeogenic precursor; and 3] a signaling molecule. As reviewed in this paper lactate is the inexorable product of glycolysis. In response to cell energy crisis, glycolysis results in ATP production by substrate-level phosphorylation of ADP. Importantly also, lactate (not pyruvate) enters the mitochondrial reticulum to support cell energy homeostasis by oxidative phosphorylation of ADP and creatine. When cell work rate is high, lactate produced by driver cells is secreted into the interstitium and circulation from where it can reach a variety of recipient cells such as in heart, liver, kidneys and brain. In diverse tissues lactate acts by mass action, cell redox regulation, ROS generation, allosteric binding and lactylation of histones. By inhibiting lipolysis in adipose, via HCAR-1 binding and CREB activation, and muscle mitochondrial fatty acid uptake, via malonyl-CoA and CPT1, lactate controls lipid oxidation and overall energy substrate partitioning. Repeated lactate exposure from regular exercise results in adaptive processes such as mitochondrial biogenesis and other healthful characteristics such as improved metabolic flexibility. The importance of lactate and lactate shuttling in healthful living is further emphasized when lactate signaling and shuttling are dysregulated as occur in cancer and other conditions. Lactate is the fulcrum of metabolic regulation in vivo.
Declaration of competing interest
The author has no competing interests to declare.
Acknowledgements
Supported by NIH 1 R01 AG059715-01, Pac-12 Conference Grant # 3-02-Brooks-17 and the UCB Center For Reseearch and Education On Aging (CREA).
Andrea Salvador Pascual, Casey Curl, Robert Leija and Adam Osmond are thanked for reading and commenting on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101454.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ahmed K., Tunaru S., Tang C., Muller M., Gille A., Sassmann A., Hanson J., Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metabol. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo J.L., Tietz E., Two-Feathers T., Paull J., Chapman K. Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PloS One. 2007;2:e927. doi: 10.1371/journal.pone.0000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba N., Sharma H.M. Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J. Cell Biol. 1971;51:621–635. doi: 10.1083/jcb.51.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barros L.F. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013;36:396–404. doi: 10.1016/j.tins.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Bayod S., Del Valle J., Lalanza J.F., Sanchez-Roige S., de Luxan-Delgado B., Coto-Montes A., Canudas A.M., Camins A., Escorihuela R.M., Pallas M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012;47:925–935. doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Bendahan D, Chatel B, and Jue T. Comparative NMR and NIRS analysis of oxygen dependent metabolism in exercising finger flexor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. ajpregu 00203 02017, 2017. [DOI] [PMC free article] [PubMed]
- 8.Bender K., Newsholme P., Brennan L., Maechler P. The importance of redox shuttles to pancreatic beta-cell energy metabolism and function. Biochem. Soc. Trans. 2006;34:811–814. doi: 10.1042/BST0340811. [DOI] [PubMed] [Google Scholar]
- 9.Bergersen L.H. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J. Cerebr. Blood Flow Metabol. 2015;35:176–185. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman B.C., Butterfield G.E., Wolfel E.E., Casazza G.A., Lopaschuk G.D., Brooks G.A. Evaluation of exercise and training on muscle lipid metabolism. Am. J. Physiol. 1999;276:E106–E117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- 11.Bergman B.C., Butterfield G.E., Wolfel E.E., Lopaschuk G.D., Casazza G.A., Horning M.A., Brooks G.A. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am. J. Physiol. 1999;277:E81–E92. doi: 10.1152/ajpendo.1999.277.1.E81. [DOI] [PubMed] [Google Scholar]
- 12.Bergman B.C., Horning M.A., Casazza G.A., Wolfel E.E., Butterfield G.E., Brooks G.A. Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metabol. 2000;278:E244–E251. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- 13.Bergman B.C., Tsvetkova T., Lowes B., Wolfel E.E. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J. Physiol. 2009;587:2087–2099. doi: 10.1113/jphysiol.2008.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman B.C., Wolfel E.E., Butterfield G.E., Lopaschuk G.D., Casazza G.A., Horning M.A., Brooks G.A. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 15.Brandt R.B., Laux J.E., Spainhour S.E., Kline E.S. Lactate dehydrogenase in rat mitochondria. Arch. Biochem. Biophys. 1987;259:412–422. doi: 10.1016/0003-9861(87)90507-8. [DOI] [PubMed] [Google Scholar]
- 16.Brooks G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009;587:5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks G.A. Energy flux, lactate shuttling, mitochondrial dynamics, and hypoxia. Adv. Exp. Med. Biol. 2016;903:439–455. doi: 10.1007/978-1-4899-7678-9_29. [DOI] [PubMed] [Google Scholar]
- 18.Brooks G.A. Glycolytic end product and oxidative substrate during sustained exercise in mammals--the "lactate shuttle. Comparative Physiology and Biochemistry - Current Topics and Trends, Volume A, Respiration - Metabolism - Circulation. 1984:208–218. [Google Scholar]
- 19.Brooks G.A. Lactate shuttles in nature. Biochem. Soc. Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 20.Brooks G.A. Master regulator or readout: the wisdom of distributed control. Focus on "Pyruvate suppresses PGC1alpha expression and substrate utilization despite increased respiratory chain content in C2C12 myotubes". Am. J. Physiol. Cell Physiol. 2010;299:C216–C217. doi: 10.1152/ajpcell.00212.2010. [DOI] [PubMed] [Google Scholar]
- 21.Brooks G.A. Metabolic systems: the formation and utilization of lactate. In: Tipton C.M., editor. History of Exercise Physiology. Human Kinetics; Champaign, IL: 2014. pp. 447–475. [Google Scholar]
- 22.Brooks G.A. The science and translation of lactate shuttle theory. Cell Metabol. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Brooks G.A., Brown M.A., Butz C.E., Sicurello J.P., Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J. Appl. Physiol. 1999;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. 1985. [DOI] [PubMed] [Google Scholar]
- 24.Brooks G.A., Brown M.A., Butz C.E., Sicurello J.P., Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J. Appl. Physiol. 1999;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- 27.Brooks G.A., Dubouchaud H., Brown M., Sicurello J.P., Butz C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks G.A., Fahey T.D., Baldwin K.M. Kindle Direct Publishing; 2019. EXERCISE PHYSIOLOGY: Human Bioenergetics and It's Applications. [Google Scholar]
- 29.Brooks G.A., Martin N.A. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front. Neurosci. 2014;8:408. doi: 10.3389/fnins.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks G.A., Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J. Appl. Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- 32.Butz C.E., McClelland G.B., Brooks G.A. MCT1 confirmed in rat striated muscle mitochondria. J. Appl. Physiol. 2004;97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 33.Cai T.Q., Ren N., Jin L., Cheng K., Kash S., Chen R., Wright S.D., Taggart A.K., Waters M.G. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 2008;377:987–991. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 34.Calvo S.E., Clauser K.R., Mootha V.K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheetham M.E., Boobis L.H., Brooks S., Williams C. Human muscle metabolism during sprint running. J. Appl. Physiol. 1986;61:54–60. doi: 10.1152/jappl.1986.61.1.54. 1985. [DOI] [PubMed] [Google Scholar]
- 36.Chen D., Kerr C. The epigenetics of stem cell aging comes of age. Trends Cell Biol. 2019;29:563–568. doi: 10.1016/j.tcb.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y.J., Mahieu N.G., Huang X., Singh M., Crawford P.A., Johnson S.L., Gross R.W., Schaefer J., Patti G.J. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 2016;12:937–943. doi: 10.1038/nchembio.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coady M.J., Chang M.H., Charron F.M., Plata C., Wallendorff B., Sah J.F., Markowitz S.D., Romero M.F., Lapointe J.Y. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J. Physiol. 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connett R.J., Honig C.R., Gayeski T.E., Brooks G.A. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J. Appl. Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. 1985. [DOI] [PubMed] [Google Scholar]
- 40.Consoli A., Nurjhan N., Reilly J.J., Jr., Bier D.M., Gerich J.E. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am. J. Physiol. 1990;259:E677–E684. doi: 10.1152/ajpendo.1990.259.5.E677. [DOI] [PubMed] [Google Scholar]
- 41.Cori C.F., Cori G.T. Carbohydrate metabolism. Annu. Rev. Biochem. 1946;15:193–218. doi: 10.1146/annurev.bi.15.070146.001205. [DOI] [PubMed] [Google Scholar]
- 42.Davies K.J., Packer L., Brooks G.A. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch. Biochem. Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- 43.De Bari L., Atlante A., Valenti D., Passarella S. Partial reconstruction of in vitro gluconeogenesis arising from mitochondrial l-lactate uptake/metabolism and oxaloacetate export via novel L-lactate translocators. Biochem. J. 2004;380:231–242. doi: 10.1042/BJ20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bari L., Valenti D., Atlante A., Passarella S. L-lactate generates hydrogen peroxide in purified rat liver mitochondria due to the putative L-lactate oxidase localized in the intermembrane space. FEBS Lett. 2010;584:2285–2290. doi: 10.1016/j.febslet.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 45.Depocas F., Minaire Y., Chatonnet J. Rates of formation and oxidation of lactic acid in dogs at rest and during moderate exercise. Can. J. Physiol. Pharmacol. 1969;47:603–610. doi: 10.1139/y69-106. [DOI] [PubMed] [Google Scholar]
- 46.Dumke C.L., Mark Davis J., Angela Murphy E., Nieman D.C., Carmichael M.D., Quindry J.C., Travis Triplett N., Utter A.C., Gross Gowin S.J., Henson D.A., McAnulty S.R., McAnulty L.S. Successive bouts of cycling stimulates genes associated with mitochondrial biogenesis. Eur. J. Appl. Physiol. 2009;107:419–427. doi: 10.1007/s00421-009-1143-1. [DOI] [PubMed] [Google Scholar]
- 47.Emhoff C.A., Messonnier L.A., Horning M.A., Fattor J.A., Carlson T.J., Brooks G.A. Direct and indirect lactate oxidation in trained and untrained men. J. Appl. Physiol. 2013;115:829–838. doi: 10.1152/japplphysiol.00538.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emhoff C.A., Messonnier L.A., Horning M.A., Fattor J.A., Carlson T.J., Brooks G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 2013;114:297–306. doi: 10.1152/japplphysiol.01202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson B.S., Rogatzki M.J., Goodwin M.L., Kane D.A., Rightmire Z., Gladden L.B. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018;118:691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 50.Friedlander A.L., Casazza G.A., Horning M.A., Huie M.J., Brooks G.A. Training-induced alterations of glucose flux in men. J. Appl. Physiol. 1997;82:1360–1369. doi: 10.1152/jappl.1997.82.4.1360. [DOI] [PubMed] [Google Scholar]
- 51.Friedlander A.L., Casazza G.A., Horning M.A., Huie M.J., Piacentini M.F., Trimmer J.K., Brooks G.A. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J. Appl. Physiol. 1998;85:1175–1186. doi: 10.1152/jappl.1998.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 52.Friedlander A.L., Casazza G.A., Horning M.A., Usaj A., Brooks G.A. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J. Appl. Physiol. 1999;86:2097–2105. doi: 10.1152/jappl.1999.86.6.2097. [DOI] [PubMed] [Google Scholar]
- 53.Friedman A., Kaufer D., Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fulghum K.L., Rood B.R., Shang V.O., McNally L.A., Riggs D.W., Zheng Y.T., Hill B.G. Mitochondria-associated lactate dehydrogenase is not a biologically significant contributor to bioenergetic function in murine striated muscle. Redox Biol. 2019;24:101177. doi: 10.1016/j.redox.2019.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia C.K., Brown M.S., Pathak R.K., Goldstein J.L. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J. Biol. Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 56.Garcia C.K., Goldstein J.L., Pathak R.K., Anderson R.G., Brown M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Alvarez M., Marik P., Bellomo R. Sepsis-associated hyperlactatemia. Crit. Care. 2014;18:503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Alvarez M., Marik P., Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol. 2014;2:339–347. doi: 10.1016/S2213-8587(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 59.Ge H., Weiszmann J., Reagan J.D., Gupte J., Baribault H., Gyuris T., Chen J.L., Tian H., Li Y. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J. Lipid Res. 2008;49:797–803. doi: 10.1194/jlr.M700513-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Genders A.J., Martin S.D., McGee S.L., Bishop D.J. A physiological drop in pH decreases mitochondrial respiration, and HDAC and Akt signaling, in L6 myocytes. Am. J. Physiol. Cell Physiol. 2019;316:C404–C414. doi: 10.1152/ajpcell.00214.2018. [DOI] [PubMed] [Google Scholar]
- 61.Gerich J.E., Meyer C., Woerle H.J., Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 62.Gertz E.W., Wisneski J.A., Neese R., Bristow J.D., Searle G.L., Hanlon J.T. Myocardial lactate metabolism: evidence of lactate release during net chemical extraction in man. Circulation. 1981;63:1273–1279. doi: 10.1161/01.cir.63.6.1273. [DOI] [PubMed] [Google Scholar]
- 63.Gertz E.W., Wisneski J.A., Stanley W.C., Neese R.A. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J. Clin. Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glancy B., Hartnell L.M., Combs C.A., Fenmou A., Sun J., Murphy E., Subramaniam S., Balaban R.S. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 2017;19:487–496. doi: 10.1016/j.celrep.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glenn T.C., Martin N.A., Horning M.A., McArthur D.L., Hovda D.A., Vespa P., Brooks G.A. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J. Neurotrauma. 2015;32:820–832. doi: 10.1089/neu.2014.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glenn T.C., Martin N.A., McArthur D.L., Hovda D.A., Vespa P., Johnson M.L., Horning M.A., Brooks G.A. Endogenous Nutritive Support after traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma. 2015;32:811–819. doi: 10.1089/neu.2014.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glenn T.C., Martin N.A., Hovda D.A., Vespa P., Johnson M.L., Horning M.A., MacArthur D.L., Brooks G.A. 2014. Lactate; Brain Fuel Following Traumatic Brain Injury. submitted for publication. [Google Scholar]
- 68.Gohil K., Viguie C., Stanley W.C., Brooks G.A., Packer L. Blood glutathione oxidation during human exercise. J. Appl. Physiol. 1988;64:115–119. doi: 10.1152/jappl.1988.64.1.115. 1985. [DOI] [PubMed] [Google Scholar]
- 69.Gold M., Miller H.I., Issekutz B., Jr., Spitzer J.J. Effect of exercise and lactic acid infusion on individual free fatty acids of plasma. Am. J. Physiol. 1963;205:902–904. doi: 10.1152/ajplegacy.1963.205.5.902. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Handschin C., Spiegelman B.M. PGC-1 coactivators and the regulation of skeletal muscle fiber-type determination. Cell Metabol. 2011;13:351. doi: 10.1016/j.cmet.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Hashimoto T., Hussien R., Brooks G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metabol. 2006;290:E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto T., Hussien R., Cho H.S., Kaufer D., Brooks G.A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PloS One. 2008;3 doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto T., Hussien R., Oommen S., Gohil K., Brooks G.A. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. Faseb. J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto T., Tsukamoto H., Takenaka S., olesen N.D., Petersen L.G., Sorensen H., Nielsen H.B., Secher N.B., Ogoh S. Exercise improves brain executive function related to cerebral lactate metabolism in men. Faseb J. 2017 doi: 10.1096/fj.201700381RR. In Press. [DOI] [PubMed] [Google Scholar]
- 77.Hashimoto T., Tsukamoto H., Takenaka S., Olesen N.D., Petersen L.G., Sorensen H., Nielsen H.B., Secher N.H., Ogoh S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. Faseb. J. 2018;32:1417–1427. doi: 10.1096/fj.201700381RR. [DOI] [PubMed] [Google Scholar]
- 78.Henderson G.C., Fattor J.A., Horning M.A., Faghihnia N., Johnson M.L., Mau T.L., Luke-Zeitoun M., Brooks G.A. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 2007;584:963–981. doi: 10.1113/jphysiol.2007.137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson G.C., Horning M.A., Lehman S.L., Wolfel E.E., Bergman B.C., Brooks G.A. Pyruvate shuttling during rest and exercise before and after endurance training in men. J. Appl. Physiol. 2004;97:317–325. doi: 10.1152/japplphysiol.01367.2003. [DOI] [PubMed] [Google Scholar]
- 80.Henderson G.C., Horning M.A., Wallis G.A., Brooks G.A. Pyruvate metabolism in working human skeletal muscle. Am. J. Physiol. Endocrinol. Metabol. 2007;292:E366. doi: 10.1152/ajpendo.00363.2006. [DOI] [PubMed] [Google Scholar]
- 81.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 82.Holloszy J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 83.Holloway R., Zhou Z., Harvey H.B., Levasseur J.E., Rice A.C., Sun D., Hamm R.J., Bullock M.R. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. 2007;149:919–927. doi: 10.1007/s00701-007-1241-y. discussion 927. [DOI] [PubMed] [Google Scholar]
- 84.Hoque R., Farooq A., Ghani A., Gorelick F., Mehal W.Z. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hui S., Ghergurovich J.M., Morscher R.J., Jang C., Teng X., Lu W., Esparza L.A., Reya T., Le Z., Yanxiang Guo J., White E., Rabinowitz J.D. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hultman E.A. Physiological role of muscle glycogen in man. Physiol. Muscular Exerc. 1967;I99-I112 [Google Scholar]
- 88.Hunt T.K., Aslam R.S., Beckert S., Wagner S., Ghani Q.P., Hussain M.Z., Roy S., Sen C.K. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxidants Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishihara H., Wang H., Drewes L.R., Wollheim C.B. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J. Clin. Invest. 1999;104:1621–1629. doi: 10.1172/JCI7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Issekutz B.J., Miller H. Plasma free fatty acids during exercise and the effect of lactic acid. Proc. Soc. Exp. Biol. Med. 1962;110:237–239. [Google Scholar]
- 91.Jacobs R.A., Meinild A.K., Nordsborg N.B., Lundby C. Lactate oxidation in human skeletal muscle mitochondria. Am. J. Physiol. Endocrinol. Metabol. 2013;304:E686–E694. doi: 10.1152/ajpendo.00476.2012. [DOI] [PubMed] [Google Scholar]
- 92.Jenssen T., Nurjhan N., Consoli A., Gerich J.E. Dose-response effects of lactate infusions on gluconeogenesis from lactate in normal man. Eur. J. Clin. Invest. 1993;23:448–454. doi: 10.1111/j.1365-2362.1993.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 93.Kirkwood S.P., Munn E.A., Brooks G.A. Mitochondrial reticulum in limb skeletal muscle. Am. J. Physiol. 1986;251:C395–C402. doi: 10.1152/ajpcell.1986.251.3.C395. [DOI] [PubMed] [Google Scholar]
- 94.Kirkwood S.P., Packer L., Brooks G.A. Effects of endurance training on a mitochondrial reticulum in limb skeletal muscle. Arch. Biochem. Biophys. 1987;255:80–88. doi: 10.1016/0003-9861(87)90296-7. [DOI] [PubMed] [Google Scholar]
- 95.Kline E.S., Brandt R.B., Laux J.E., Spainhour S.E., Higgins E.S., Rogers K.S., Tinsley S.B., Waters M.G. Localization of L-lactate dehydrogenase in mitochondria. Arch. Biochem. Biophys. 1986;246:673–680. doi: 10.1016/0003-9861(86)90323-1. [DOI] [PubMed] [Google Scholar]
- 96.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Canc. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 97.Latham T., Mackay L., Sproul D., Karim M., Culley J., Harrison D.J., Hayward L., Langridge-Smith P., Gilbert N., Ramsahoye B.H. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lauritzen K.H., Morland C., Puchades M., Holm-Hansen S., Hagelin E.M., Lauritzen F., Attramadal H., Storm-Mathisen J., Gjedde A., Bergersen L.H. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cerebr. Cortex. 2014;24:2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 99.Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J., Sutton S.W., Li X., Yun S.J., Mirzadegan T., Mazur C., Kamme F., Lovenberg T.W. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 100.Liu L., MacKenzie K.R., Putluri N., Maletic-Savatic M., Bellen H.J. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metabol. 2017;26:719–737 e716. doi: 10.1016/j.cmet.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo Z., Saha A.K., Xiang X., Ruderman N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 102.Marik P., Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Crit. Care. 2013;1:3–5. [Google Scholar]
- 103.Marik P., Bellomo R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth. 2016;116:339–349. doi: 10.1093/bja/aev349. [DOI] [PubMed] [Google Scholar]
- 104.Marik P.E. Glycemic control in critically ill patients: what to do post NICE-SUGAR? World J. Gastrointest. Surg. 2009;1:3–5. doi: 10.4240/wjgs.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mazzeo R.S., Brooks G.A., Schoeller D.A., Budinger T.F. Disposal of blood [1-13C]lactate in humans during rest and exercise. J. Appl. Physiol. 1986;60:232–241. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- 106.McClelland G.B., Khanna S., Gonzalez G.F., Butz C.E., Brooks G.A. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem. Biophys. Res. Commun. 2003;304:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- 107.McGarry J.D., Mannaerts G.P., Foster D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Messonnier A.L., Emhoff C.W., Fattor J.A., Horning M.A., C T.J., Brooks G.A. Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol. 2013;114 doi: 10.1152/japplphysiol.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyer C., Dostou J., Nadkarni V., Gerich J. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am. J. Physiol. 1998;275:F915–F921. doi: 10.1152/ajprenal.1998.275.6.F915. [DOI] [PubMed] [Google Scholar]
- 110.Meyer C., Dostou J.M., Welle S.L., Gerich J.E. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am. J. Physiol. Endocrinol. Metabol. 2002;282:E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 111.Meyer C., Stumvoll M., Dostou J., Welle S., Haymond M., Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am. J. Physiol. Endocrinol. Metabol. 2002;282:E428–E434. doi: 10.1152/ajpendo.00116.2001. [DOI] [PubMed] [Google Scholar]
- 112.Miller B.F., Fattor J.A., Jacobs K.A., Horning M.A., Navazio F., Lindinger M.I., Brooks G.A. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J. Physiol. 2002;544:963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller B.F., Fattor J.A., Jacobs K.A., Horning M.A., Suh S.H., Navazio F., Brooks G.A. Metabolic and cardiorespiratory responses to "the lactate clamp. Am. J. Physiol. Endocrinol. Metabol. 2002;283:E889–E898. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]
- 114.Miller B.F., Lindinger M.I., Fattor J.A., Jacobs K.A., Leblanc P.J., Duong M., Heigenhauser G.J., Brooks G.A. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J. Appl. Physiol. 2005;98:856–865. doi: 10.1152/japplphysiol.00753.2004. [DOI] [PubMed] [Google Scholar]
- 115.Miller H.I., Issekutz B., Jr., Rodahl K., Paul P. Effect of lactic acid on plasma free fatty acids in pancreatectomized dogs. Am. J. Physiol. 1964;207:1226–1230. doi: 10.1152/ajplegacy.1964.207.6.1226. [DOI] [PubMed] [Google Scholar]
- 116.Murphy N.D., Kodakat S.K., Wendon J.A., Jooste C.A., Muiesan P., Rela M., Heaton N.D. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit. Care Med. 2001;29:2111–2118. doi: 10.1097/00003246-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 117.Nirwane A., Majumdar A. Understanding mitochondrial biogenesis through energy sensing pathways and its translation in cardio-metabolic health. Arch. Physiol. Biochem. 2017;1–13 doi: 10.1080/13813455.2017.1391847. [DOI] [PubMed] [Google Scholar]
- 118.Ohno Y., Ando K., Ito T., Suda Y., Matsui Y., Oyama A., Kaneko H., Yokoyama S., Egawa T., Goto K. Lactate stimulates a potential for hypertrophy and regeneration of mouse skeletal muscle. Nutrients. 2019;11 doi: 10.3390/nu11040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ojuka E.O., Jones T.E., Han D.H., Chen M., Wamhoff B.R., Sturek M., Holloszy J.O. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1040–E1045. doi: 10.1152/ajpendo.00242.2002. [DOI] [PubMed] [Google Scholar]
- 120.Otonkoski T., Jiao H., Kaminen-Ahola N., Tapia-Paez I., Ullah M.S., Parton L.E., Schuit F., Quintens R., Sipila I., Mayatepek E., Meissner T., Halestrap A.P., Rutter G.A., Kere J. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. Am. J. Hum. Genet. 2007;81:467–474. doi: 10.1086/520960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.E., Walford G.A., Sugiana C., Boneh A., Chen W.K., Hill D.E., Vidal M., Evans J.G., Thorburn D.R., Carr S.A., Mootha V.K. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pande S.V., Blanchaer M.C. Preferential loss of ATP-dependent long-chain fatty acid activating enzyme in mitochondria prepared using Nagarse. Biochim. Biophys. Acta. 1970:43–48. doi: 10.1016/0005-2760(70)90216-x. 202. [DOI] [PubMed] [Google Scholar]
- 123.Park J.M., Josan S., Mayer D., Hurd R.E., Chung Y., Bendahan D., Spielman D.M., Jue T. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J. Exp. Biol. 2015;218:3308–3318. doi: 10.1242/jeb.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Passarella S., de Bari L., Valenti D., Pizzuto R., Paventi G., Atlante A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008;582:3569–3576. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 125.Passarella S., Paventi G., Pizzuto R. The mitochondrial L-lactate dehydrogenase affair. Front. Neurosci. 2014;8:407. doi: 10.3389/fnins.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pellerin L., Pellegri G., Bittar P.G., Charnay Y., Bouras C., Martin J.L., Stella N., Magistretti P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 127.Powell K.E., King A.C., Buchner D.M., Campbell W.W., DiPietro L., Erickson K.I., Hillman C.H., Jakicic J.M., Janz K.F., Katzmarzyk P.T., Kraus W.E., Macko R.F., Marquez D.X., McTiernan A., Pate R.R., Pescatello L.S., Whitt-Glover M.C. The scientific foundation for the physical activity guidelines for Americans. J. Phys. Activ. Health. 2018;1–11 doi: 10.1123/jpah.2018-0618. 2nd Edition. [DOI] [PubMed] [Google Scholar]
- 128.Powers S.K., Ji L.L., Kavazis A.N., Jackson M.J. Reactive oxygen species: impact on skeletal muscle. Comp. Physiol. 2011;1:941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Powers S.K., Ji L.L., Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med. Sci. Sports Exerc. 1999;31:987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 130.Price N.T., Jackson V.N., Halestrap A.P. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem. J. 1998;329(Pt 2):321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pullen T.J., Khan A.M., Barton G., Butcher S.A., Sun G., Rutter G.A. Identification of genes selectively disallowed in the pancreatic islet. Islets. 2010;2:89–95. doi: 10.4161/isl.2.2.11025. [DOI] [PubMed] [Google Scholar]
- 132.Quistorff B., Secher N.H., Van Lieshout J.J. Lactate fuels the human brain during exercise. Faseb. J. 2008;22:3443–3449. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- 133.Racker E. Bioenergetics and the problem of tumor growth. Am. Sci. 1972;60:56–63. [PubMed] [Google Scholar]
- 134.Rasmussen H.N., van Hall G., Rasmussen U.F. Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J. Physiol. 2002;541:575–580. doi: 10.1113/jphysiol.2002.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rice A.C., Zsoldos R., Chen T., Wilson M.S., Alessandri B., Hamm R.J., Bullock M.R. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–159. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- 136.Richardson R.S., Noyszewski E.A., Leigh J.S., Wagner P.D. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J. Appl. Physiol. 1998;85:627–634. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- 137.Robinson M.M., Dasari S., Konopka A.R., Johnson M.L., Manjunatha S., Esponda R.R., Carter R.E., Lanza I.R., Nair K.S. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metabol. 2017;25:581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodahl K., Miller H.I., Issekutz B., Jr. Plasma free fatty acids in exercise. J. Appl. Physiol. 1964;19:489–492. doi: 10.1152/jappl.1964.19.3.489. [DOI] [PubMed] [Google Scholar]
- 139.Rogatzki M.J., Ferguson B.S., Goodwin M.L., Gladden L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015;9(22) doi: 10.3389/fnins.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rumsey W.L., Schlosser C., Nuutinen E.M., Robiolio M., Wilson D.F. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J. Biol. Chem. 1990;265:15392–15402. [PubMed] [Google Scholar]
- 141.Rutter G.A., Pullen T.J., Hodson D.J., Martinez-Sanchez A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 142.Saddik M., Gamble J., Witters L.A., Lopaschuk G.D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 143.Sahlin K., Fernstrom M., Svensson M., Tonkonogi M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J. Physiol. 2002;541:569–574. doi: 10.1113/jphysiol.2002.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.San-Millan I., Brooks G.A. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38:119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.San-Millan I., Julian C.G., Matarazzo C., Brooks G.A. Is lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Front. Oncol. - Canc. Metabol. 2020;9:1–10. doi: 10.3389/fonc.2019.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheiman J., Luber J.M., Chavkin T.A., MacDonald T., Tung A., Pham L.D., Wibowo M.C., Wurth R.C., Punthambaker S., Tierney B.T., Yang Z., Hattab M.W., Avila-Pacheco J., Clish C.B., Lessard S., Church G.M., Kostic A.D. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schurr A. Lactate: a major and crucial player in normal function of both muscle and brain. J. Physiol. 2008;586:2665–2666. doi: 10.1113/jphysiol.2008.155416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J. Cerebr. Blood Flow Metabol. : Off. J. Int. Soc. Cerebr. Blood Flow Metabol. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- 149.Schurr A., Gozal E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2011;2:96. doi: 10.3389/fphar.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schurr A., Payne R.S. Lactate, not pyruvate, is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience. 2007;147:613–619. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 151.Semenza G.L. Tumor metabolism: cancer cells give and take lactate. J. Clin. Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shapiro N.I., Howell M.D., Talmor D., Nathanson L.A., Lisbon A., Wolfe R.E., Weiss J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 154.Shin J., Kim J., Park H., Kim J. Investigating the role of Sirtuins in cell reprogramming. BMB Rep. 2018;51:500–507. doi: 10.5483/BMBRep.2018.51.10.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Somasetia D.H., Setiati T.E., Sjahrodji A.M., Idjradinata P.S., Setiabudi D., Roth H., Ichai C., Fontaine E., Leverve X.M. Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium-lactate: a randomized single-blind clinical trial of efficacy and safety. Crit. Care. 2014;18:466. doi: 10.1186/s13054-014-0466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sonveaux P., Vegran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F., Kelley M.J., Gallez B., Wahl M.L., Feron O., Dewhirst M.W. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spitzer J.J. Gluconeogenesis in the burned patient. J. Trauma. 1979;19:899–900. [PubMed] [Google Scholar]
- 158.Stanley W.C., Gertz E.W., Wisneski J.A., Morris D.L., Neese R.A., Brooks G.A. Systemic lactate kinetics during graded exercise in man. Am. J. Physiol. 1985;249:E595–E602. doi: 10.1152/ajpendo.1985.249.6.E595. [DOI] [PubMed] [Google Scholar]
- 159.Stanley W.C., Gertz E.W., Wisneski J.A., Neese R.A., Morris D.L., Brooks G.A. Lactate extraction during net lactate release in legs of humans during exercise. J. Appl. Physiol. 1986;60:1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- 160.Stanley W.C., Wisneski J.A., Gertz E.W., Neese R.A., Brooks G.A. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metab., Clin. Exp. 1988;37:850–858. doi: 10.1016/0026-0495(88)90119-9. [DOI] [PubMed] [Google Scholar]
- 161.Takahashi H., Alves C.R.R., Stanford K.I., Middelbeek R.J.W., Pasquale N., Ryan R.E., Xue R., Sakaguchi M., Lynes M.D., So K., Mul J.D., Lee M.Y., Balan E., Pan H., Dreyfuss J.M., Hirshman M.F., Azhar M., Hannukainen J.C., Nuutila P., Kalliokoski K.K., Nielsen S., Pedersen B.K., Kahn C.R., Tseng Y.H., Goodyear L.J. TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019;1:291–303. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teramae H., Yoshikawa T., Inoue R., Ushida K., Takebe K., Nio-Kobayashi J., Iwanaga T. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed. Res. 2010;31:239–249. doi: 10.2220/biomedres.31.239. [DOI] [PubMed] [Google Scholar]
- 163.Tsukamoto S., Shibasaki A., Naka A., Saito H., Iida K. Lactate promotes myoblast differentiation and myotube hypertrophy via a pathway involving MyoD in vitro and enhances muscle regeneration in vivo. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Hall G., Stromstad M., Rasmussen P., Jans O., Zaar M., Gam C., Quistorff B., Secher N.H., Nielsen H.B. Blood lactate is an important energy source for the human brain. J. Cerebr. Blood Flow Metabol. : Off. J. Int. Soc. Cerebr. Blood Flow. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 165.Viguie C.A., Frei B., Shigenaga M.K., Ames B.N., Packer L., Brooks G.A. Antioxidant status and indexes of oxidative stress during consecutive days of exercise. J. Appl. Physiol. 1993;75:566–572. doi: 10.1152/jappl.1993.75.2.566. 1985. [DOI] [PubMed] [Google Scholar]
- 166.Vrieze A., Van Nood E., Holleman F., Salojarvi J., Kootte R.S., Bartelsman J.F., Dallinga-Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R., Derrien M., Druesne A., Van Hylckama Vlieg J.E., Bloks V.W., Groen A.K., Heilig H.G., Zoetendal E.G., Stroes E.S., de Vos W.M., Hoekstra J.B., Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. e917. [DOI] [PubMed] [Google Scholar]
- 167.Wadley G.D. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am. J. Physiol. Endocrinol. Metababol. 2006;290:E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- 168.Wagner S., Hussain M.Z., Hunt T.K., Bacic B., Becker H.D. Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Repair Regen. 2004;12:368–373. doi: 10.1111/j.1067-1927.2004.012315.x. [DOI] [PubMed] [Google Scholar]
- 169.Warburg O., Minami S. Versuche an überlebendem carcinom-gewebe. Klin. Wochenschr. 1923;2:776–777. [Google Scholar]
- 170.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wasserman K., Beaver W.L., Davis J.A., Pu J.Z., Heber D., Whipp B.J. Lactate, pyruvate, and lactate-to-pyruvate ratio during exercise and recovery. J. Appl. Physiol. 1985;59:935–940. doi: 10.1152/jappl.1985.59.3.935. 1985. [DOI] [PubMed] [Google Scholar]
- 172.Watt P.W., MacLennan P.A., Hundal H.S., Kuret C.M., Rennie M.J. L(+)-lactate transport in perfused rat skeletal muscle: kinetic characteristics and sensitivity to pH and transport inhibitors. Biochim. Biophys. Acta. 1988;944:213–222. doi: 10.1016/0005-2736(88)90434-8. [DOI] [PubMed] [Google Scholar]
- 173.Woerle H.J., Meyer C., Dostou J.M., Gosmanov N.R., Islam N., Popa E., Wittlin S.D., Welle S.L., Gerich J.E. Pathways for glucose disposal after meal ingestion in humans. Am. J. Physiol. Endocrinol. Metab. 2003;284:E716–E725. doi: 10.1152/ajpendo.00365.2002. [DOI] [PubMed] [Google Scholar]
- 174.Wu B.U., Hwang J.Q., Gardner T.H., Repas K., Delee R., Yu S., Smith B., Banks P.A., Conwell D.L. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011;9:710–717 e711. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 175.Yoshida Y., Holloway G.P., Ljubicic V., Hatta H., Spriet L.L., Hood D.A., Bonen A. Negligible direct lactate oxidation in subsarcolemmal and inter myofibrillar mitochondria obtained from red and white rat skeletal muscle. J. Physiol. 2007;582:1317–1335. doi: 10.1113/jphysiol.2007.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang D., Tang Z., Huang H., Zhou G., Cui C., Weng Y., Liu W., Kim S., Lee S., Perez-Neut M., Ding J., Czyz D., Hu R., Ye Z., He M., Zheng Y.G., Shuman H.A., Dai L., Ren B., Roeder R.G., Becker L., Zhao Y. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.