Abstract
Exercise imposes cellular stress on contracting skeletal muscle fibers, forcing them to complete molecular adaptations to maintain homeostasis. There is mounting evidence that redox signaling by reactive oxygen species (ROS) is vital for skeletal muscle exercise adaptations across many different exercise modalities. The study of redox signaling is moving towards a growing appreciation that these ROS do not signal in a global unspecific way, but rather elicit their effects in distinct subcellular compartments. This short review will first outline the sources of ROS in exercising skeletal muscle and then discuss some examples of exercise adaptations, which are evidenced to be regulated by compartmentalized redox signaling. We speculate that knowledge of these redox pathways might one day allow targeted manipulation to increase redox-signaling in specific compartments to augment the exercise-hormetic response in health and disease.
Keywords: Reactive oxygen species, Exercise, NADPH oxidase, Mitochondria, Skeletal muscle, Metabolism
1. Introduction
Acute and chronic exercise elicits a wide range of stress-adaptive responses in the working skeletal muscle, functioning to protect myocellular homeostasis and structural integrity. The type of exercise being performed can be simplistically viewed as a continuum ranging from a relatively low-mechanical load, prolonged repetitive endurance exercise to relatively high-load, short-duration resistance-type exercise, with an endless variety of permutations in-between these extremes. Focusing on the extremes, the myocellular adaptations are well-described to differ markedly with exercise modality, with mitochondrial biogenesis [1] and increased fat oxidation capacity [2] being hallmarks of endurance exercise training and myofibrillar hypertrophy and increased strength being hallmarks of resistance-type exercise training [3]. However, there are clearly exercise regimens such as high-intensity interval training (HIIT), eliciting potent mitochondrial adaptations despite being low-duration [4], and venous occlusion exercise, eliciting muscle hypertrophy despite being low-load [5], which suggest crosstalk between endurance and resistance-type exercise signals and challenge the dogmas of how skeletal muscle adaptations to exercise are orchestrated at the molecular level.
Across the continuum, exercise is believed to regulate the molecular stress-adaptation of skeletal muscle fibers by activating cellular signaling pathways. Phosphorylation-based-signaling is the most studied in this context with distinct kinases known being activated by endurance vs. resistance-type exercise, albeit with some overlap [6]. Relevant to this review, however, reactive oxygen species (ROS) eliciting specific redox signaling events, i.e. post-translational oxidation/reduction (redox) modifications of intracellular molecules by ROS, have also been linked to adaptations to both endurance [7] and resistance-type exercise [8]. The review will discuss some of the current evidence linking specific ROS to exercise adaptations in skeletal muscle, highlighting the likely role of compartmentalization. Due to space limitations, we will limit this discussion mainly to two of the best evidenced examples of compartment-specific ROS generation in skeletal muscle, NADPH oxidases (NOX), and mitochondria, considering antioxidant defense systems only to make specific points, although the antioxidant systems are obviously also highly compartmentalized [9]. Furthermore, we will focus on the myogenesis-independent muscle-fiber intrinsic redox signaling activated by non-damaging exercise. Many other aspects of exercise redox signaling have been covered by recent excellent reviews, and we refer the reader to these for more extensive background on skeletal muscle redox signaling [[10], [11], [12]].
2. Compartmentalization is a crucial regulator of exercise redox signaling in muscle
In fully differentiated skeletal muscle fibers, the majority of the cell interior is occupied by contractile myofibrils and a complex membrane system consisting of T-tubules and sarcoplasmic reticulum (SR). The remaining cytosolic space left in-between the myofibrils and beneath the sarcolemma is packed with the cytoskeleton, mitochondria networks, lysosomes, lipids and glycogen store particles, among other myocellular structures [[13], [14], [15]]. Due to the dimensions and packed environment of muscle fibers, the control of cellular function both at rest and during muscle contraction likely depends on both localized metabolic processes but also connectivity to distribute and coordinate metabolites such as ATP across different compartments via e.g. the mitochondrial reticulum [15]. It follows that the cell signaling mechanisms regulating muscle function must equally act locally and coordinated across large distances. Indeed, other signaling molecules in skeletal muscle are known to act locally and signal between different compartments, e.g. Ca2+ [16,17].
2.1. Compartmentalized ROS regulation in skeletal muscle – a brief summary
A large variety of reactive oxygen species are continuously produced in skeletal muscle during resting and contracting conditions [12,18,19]. Among these, superoxide anion (O2• −) is the primary oxidant molecule produced by adding an electron to molecular oxygen (O2). Multiple organelles are known to produce O2• − including peroxisomes, endoplasmic reticulum, and mitochondria [20]. In addition, multiple cytosolic enzymes can generate O2• − in a localized manner, such as NADPH oxidases [12], monoamine oxidases, and phospholipases [18]. O2• − is rapidly and spontaneously converted to hydrogen peroxide (H2O2), a process accelerated by compartmentalized superoxide dismutase (SOD) isoforms [18]. Due to its relatively lower reactivity, H2O2 is currently considered the primary redox signaling molecule [21]. H2O2 is converted to H2O and, depending on the pathway, O2 by compartmentalized peroxidases including catalase, peroxiredoxins (Prxs) and glutathione peroxidases (GPx) (Fig. 1). There is evidence in non-muscle cells to suggest that antioxidant-systems help to shape not only the effective radius of H2O2 signaling [9] but that Prxs in particular act as intermediates in transferring electrons to redox-target proteins, a process dubbed redox-relay [22]. Superoxide may also react with nitric oxide (NO) produced by compartmentalized nitric oxide synthase isoforms to form peroxynitrite (ONOO–). Thus, where ROS exert biological signaling effects in muscle cells is a function of their reactivity, the subcellular location of their source (s) and their localized removal by enzymatic and non-enzymatic antioxidant defense systems [23]. Fig. 1 depicts some of the systems contributing to the compartmentalized regulation of ROS generation in skeletal muscle.
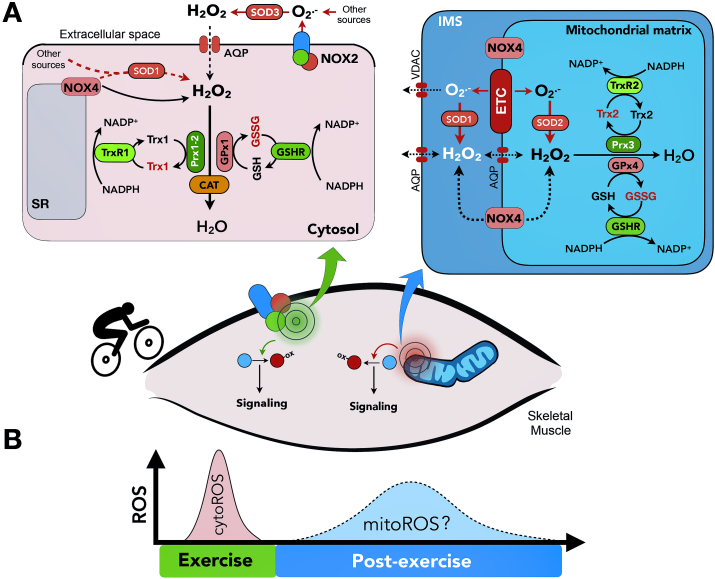
Fig. 1.
Overview of major oxidant and antioxidant systems contributing to compartmentalized reactive oxygen species (ROS) generation during skeletal muscle contraction. A) In the cytosol (top left), current evidence suggests that cytosolic H2O2 generated by NAPDH oxidase (NOX) 2 is a major regulator of exercise-stimulated ROS. NOX4, reported on the sarcoplasmic reticulum (SR) and mitochondrial intermembrane space (IMS in top left panel) has also been linked to several physiological endpoints in skeletal muscle. H2O2 generated by both sources are removed by an intricate antioxidant defense network which may itself be compartmentalized. In mitochondria, sources of ROS include the electron transport chain (ETC) and NOX4. Mitochondrial ROS may signal locally within their compartment of origin or traverse the mitochondrial membranes, likely as H2O2 assisted by various channels. Mitochondrial ROS is removed by mitochondria-specific antioxidant proteins. B) Cytosolic ROS increases during acute exercise-bouts and has been linked to multiple physiological adaptations. Mitochondrial ROS do not seem to increase during acute exercise-bouts, but have been suggested to increase post-exercise, where they may regulate processes such as mitophagy. IMS, intermembrane space; ETC, electron transport chain; AQP, aquaporin; VDAC, voltage-dependent anion channel; O2•−, superoxide anion; H2O2, hydrogen peroxide; NOX2, nicotinamide adenine dinucleotide phosphate oxidase 2; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; SOD3, superoxide dismutase 3; TrxR1, thioredoxin reductase; TrxR2, thioredoxin reductase 2; Trx1, thioredoxin 1; Trx2, thioredoxin 2; Prx1, peroxiredoxin 1; Prx2, peroxiredoxin 2; Prx3, peroxiredoxin 3; CAT, catalase; GSH, reduced glutathione; GSSG, glutathione disulfide; GPx1, glutathione peroxidase 1; GPx4, glutathione peroxidase 4; GSHR, glutathione disulfide reductase. Oxidized proteins are shown in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Focusing on exercise, recent work has studied mainly two compartments, mitochondria, and the cytosol. As discussed below, recent improvements in tools for studying sub-cellular redox-signaling, most notably compartment-targeted genetically encoded biosensors, have generated insight into the regulation of particularly H2O2 concentrations in mitochondria and cytosol under physiological exercise-conditions.
2.2. Mitochondrial ROS
In 1982, Davis et al. [24] were the first to report free radical production during treadmill exercise in rat skeletal muscle. They suggested mitochondria to be the main myocellular site of free radical production during muscular activity. Subsequently, the mitohormesis concept was proposed, positing that exercise-stimulated ROS generation by the mitochondria mediates some of the beneficial stress-adaptations to exercise [25]. The potential of mitochondrial ROS for mediating muscle training adaptation is discussed below.
The mitochondrial electron transport chain (ETC) can produce O2•− from at least 11 different sites at both sides of the inner mitochondrial membrane (for review see Ref. [26]). Among these, complex I and III are currently considered the major sites of O2• −-production under physiological conditions [27]. The generated O2• − may act locally to modulate mitochondrial function. O2• −, most likely after conversion to the less reactive H2O2 by SOD2 in the matrix or SOD1 in the intermembrane space (IMS), could also be released into the cytoplasm through mitochondrial membrane-specific channels via inner-membrane channels such the mitochondrial permeability transition pore (mPTP) during non-pathological “burst” activity and outer membrane channels including voltage-dependent anion channels (VDAC) [28], assisted by aquaporins (AQP) channels or via simple diffusion [29] (Fig. 1A). H2O2 can also be lowered by the mitochondrial Prx3/5 and GPx4 systems (Fig. 1A). Whether H2O2 -mediated signaling occurs inside of mitochondria or needs to diffuse to the cytoplasmatic compartment to exert biological effects, has not been studied in skeletal muscle.
Pioneering work using mitochondria-specific redox probes in cultured intact mouse muscle fibers suggested that mitochondria were not the main site of ROS production during muscle contraction [30,31]. In our recent work, we extended these measurements to in vivo exercise conditions [32]. Taking advantage of a recently described N-ethylamide-based redox histology protocol [33] combined with transfection of redox-sensitive green fluorescent protein (roGFP) prior to in vivo treadmill exercise, Henriquez-Olguin et al. [32] showed a drop in the oxidation of the mitochondria-targeted H2O2-specific Orp1-roGFP biosensor during moderate-intensity in vivo treadmill running compared to resting levels. This suggests that exercise leads to either a decrease in H2O2 generation and/or increased H2O2 removal/buffering in the mitochondrial matrix [32]. A likely factor decreasing H2O2 production might be a decreased mitochondrial inner membrane potential due to increased ATP production, with the membrane potential being tightly coupled to O2• − production [34]. In terms of H2O2 removal, factors such as increased ADP and intracellular acidification may also shift mitochondria towards a more antioxidant rather than pro-oxidant state [35,36].
Although the behavior and role of mitochondrial ROS needs to be investigated across the exercise continuum and the current literature is limited to H2O2 measured in the mitochondrial matrix, the current evidence does not support that elevated mitochondrial H2O2 during exercise acts as a mito-hormetic signal transducer in muscle [25]. However, mitochondrial ROS have been suggested to increase in the post-exercise period in mice, where they might play a role in orchestrating exercise-adaptations such as mitophagy [37] (Fig. 1B).
2.3. Cytosolic ROS generation by NOX
NOX2 and 4 are likely the main NOX family isoforms expressed in skeletal muscle (for review [12]). The catalytic subunit of the O2• − -generating NOX2 has been reported to reside in the plasma membrane and transverse (T) tubules in skeletal muscle [38]. In non-muscle cell-types, NOX2 is internalized into so-called redox-active endosomes [39] but it is currently unclear if this occurs in skeletal muscle. Due to the orientation of NOX2 in the membrane, O2• − is likely produced outside the muscle fibers or inside redox-active endosomes [40] and must traverse the enter the cytosol, likely assisted by AQPs after conversion to H2O2 [41], to transduce signals in the cytosol. The NOX4 isoform has been reported to reside in multiple subcellular compartments including the sarcoplasmic reticulum in muscle and the mitochondrial IMS in non-muscle [30,42], likely in the inner mitochondrial membrane [43]. NOX4 is traditionally considered constitutively active, although this may still allow NOX4 to signal acutely in contracting muscle by responding to e.g. O2 levels [42]. In addition, mitochondrial NOX4 was reported to be inhibited by direct ATP binding in cancer cells, thereby acting as a sensor of low ATP which ultimately regulated pyruvate kinase acetylation and lysosomal degradation in the cytosol [43]. NOX4-derived ROS might obviously signal locally within mitochondria (IMS or matrix) as well.
In contrast to the unclear NOX4 regulation, NOX2 activity is regulated by many intracellular and extracellular signals which regulate the recruitment of cytosolic NOX2 regulatory subunits to the membrane-embedded catalytic NOX2 subunit to cause its activation [12]. NOX2 complex assembly was shown to be stimulated by electrically evoked muscle contraction [30] and endurance exercise in murine muscle [44], and increased NOX2 activity in mouse single muscle fibers in response to electrical stimulation and mechanical stretch has been measured using roGFP conjugated to the NOX2-regulatory subunit p47phox [45]. More recently, we demonstrated that the absence of NOX2 activity in transgenic mice lacking either of the NOX2-regulatory subunits p47phox or Rac1, prevented the moderate-intensity exercise-induced cytosolic H2O2 increase determined by the cytosolic Orp1-roGFP [32]. Furthermore, the ability for exercise to increase 2′,7′-dichlorodihydrofluorescein oxidation in muscle cryo-sections, was observed in wildtype mice but not in mice lacking active NOX2 [32]. This shows that the pro-oxidative shift observed with exercise in humans and mice likely depends on enzymatic O2• − -production by NOX2. Although the influence of exercise intensity on NOX2-dependent ROS generation has not been formally tested, high-intensity interval exercise in mice transiently increased NOX2 activity measured using the p47phox-roGFP probe, returning to the basal levels within 1 h post-exercise [46].
3. Adaptations controlled by compartmentalized exercise-stimulated ROS
This section will discuss three examples of acute physiological exercise adaptations across the exercise continuum that are likely regulated by compartmentalized redox-signaling, i.e. increased glucose uptake during moderate-intensity exercise, mechanical tension-induced activation of anabolic mechanistic Target of Rapamycin (mTORC1) signaling controlling muscle hypertrophy, and regulation of transcription factor translocation to the nucleus.
3.1. Exercise-stimulated glucose transport 4 (GLUT4) translocation
A process contributing to increased glucose availability as an energy substrate is the exercise intensity and duration-dependent stimulation of muscle glucose uptake [47]. This process is determined by a coordinated increase in muscle capillary perfusion, transport across the myofiber surface membrane by GLUT4, and intracellular metabolism [47]. Studies from the early 1990s and forward suggested that the glucose transport step, a process requiring redistribution (translocation) of GLUT4 from intracellular stores to the cell surface [48], was stimulated by pro-oxidants such as exogenous mM concentrations of H2O2 in incubated mouse muscle strips [[49], [50], [51]]. Conversely, non-specific antioxidants impaired the stimulation of muscle glucose uptake by electrical stimulation [49,52] and mechanical stress [53]. Using more physiologically relevant treadmill exercise, we identified the small GTPase Rac1 as being necessary for in vivo moderate-intensity exercise-stimulated glucose uptake in mice [54], a much greater effect than observed with ex vivo contraction [55]. Rac1 was previously linked to insulin-stimulated GLUT4 translocation in muscle cell culture via its well-established role as a regulator of the actin cytoskeleton [56] but is also an essential regulatory subunit of NOX2 but not NOX4 [12]. Therefore, we tested if Rac1 was essential for exercise-stimulated NOX2 activation and, as discussed above, found this to be so [32]. Furthermore, mice lacking another essential regulatory subunit of NOX2, p47phox, shared the impaired exercise-stimulated GLUT4 translocation and glucose uptake observed in Rac1 KO mice, suggesting that Rac1 is required for exercise-stimulated GLUT4 translocation because it regulates NOX2, the predominant source of cytosolic H2O2 [32]. The mechanism linking NOX2 to GLUT4 translocation is currently unknown, but NOX2-dependent ROS production presumably signals close to the surface membrane. There is evidence to suggest that contraction-stimulated GLUT4 translocation to the cell-surface is governed by decreased endocytosis rather than increased exocytosis of GLUT4 [47]. Interestingly, Thioredoxin (Trx)-interacting protein (TXNIP), a cytosolic Trx1-binding partner suggested to signal as part of a Trx1/TXNIP complex to regulate different biological endpoints [57], was found to regulate GLUT4 endocytosis [58], making redox-inactivation of TXNIP by NOX2 an attractive candidate (Fig. 2). Another attractive candidate is CaMKII, a requirement for muscle GLUT4 translocation and glucose uptake [59], which was recently suggested to be stimulated not only by Ca2+ via calmodulin but via NOX2-dependent cysteine-modifications in mouse skeletal muscle (Wang et al. Preprint available https://doi.org/10.1101/767525).
Fig. 2.
Examples of exercise-regulated endpoints linked to compartmentalized redox-signaling. Across the exercise-continuum, cytosolic redox-signaling has been described to regulate different processes. During endurance-type exercise, NOX2 activity-likely residing within or near the surface-membrane - is required for exercise-stimulated GLUT4 translocation to stimulate glucose uptake. We speculate in this review that this may involve TXNIP or CaMKII, two redox-sensitive proteins previously linked to GLUT4 translocation. NOX2 activity may also regulate translocation of transcription factors such as NF-κB to regulate antioxidant defense. This may involve redox-sensitive proteins such as p38 MAPK and IKKy. Prx2 may act as a cytosolic intermediate in redox signal transduction. In response to mechanical stress during resistance-type exercise, NOX4 and nNOS have, via their convergence product peroxinitrite (ONOO-), been proposed to regulate Trpv1-dependent Ca2+ release to activate mTORC1 and stimulate muscle hypertrophy. Mitochondria are unlikely to increase their net ROS levels during exercise but increased mitochondrial ROS post-exercise may signal to regulate e.g. mitophagy. NOX2, NADPH oxidase 2; NOX4, NADPH oxidase 4; SR, sarcoplasmic reticulum; nNOS (β), neuronal nitric oxide synthase β; nNOS (μ), neuronal nitric oxide synthase μ; O2•−, superoxide anion; H2O2, hydrogen peroxide; Prx2, peroxiredoxin 2; Trx1, thioredoxin 1; TXNIP; thioredoxin (Trx)-interacting protein; CaMKII, calcium/calmodulin-dependent protein kinase type II; p38 mitogen-activated protein (MAP) kinases; IKKγ, IκB kinase γ; NF-κB, Nuclear factor-κB; SOD2, superoxide dismutase 2; GPx, glutathione peroxidase; NO, nitric oxide; ONOO-, peroxynitrite; Trpv1, transient receptor potential vanilloid 1; Ca2+, calcium; mTORC1, mechanistic target of rapamycin complex 1.
3.2. mTORC1 signaling determining muscle hypertrophy
At the other end of the exercise-continuum, mTORC1-stimulated growth signaling has been proposed to be an important regulator of skeletal muscle hypertrophy and resistance exercise-induced hypertrophy [60,61]. The acute increase in pro-growth signaling by mTORC1 and other kinases after resistance-type exercise can be blocked by antioxidant ingestion in humans [62], as can muscle hypertrophy with resistance exercise training in older men [8]. A 2013 Nature Medicine-paper by Ito et al. [63] and their follow-up papers [64,65] presented evidence in a mouse synergist-ablation overload-induced hypertrophy model that the ~40% hypertrophy observed in control-mice required nNOS and NOX4-dependent ONOO- production to increase Trpv1 Ca2+ channel opening. The Trpv1-mediated Ca2+ release increased mTORC1-p70S6K1 signaling to orchestrate overload-induced muscle hypertrophy (Fig. 2). To our knowledge, no independent studies have so far scrutinized the Ito-model.
Worth mentioning, NOX2 activity is a known requirement for cardiac hypertrophy [66,67]. NOX2 activation is not confined to moderate-intensity exercise but is also activated by high-intensity treadmill-exercise [46] and isolated passive stretch [68]. Therefore, NOX2 activation by mechanical stress or other stimuli might also stimulate intracellular Ca2+ release to activate skeletal muscle anabolism in response to resistance-type exercise.
Thus, resistance exercise-stimulated anabolic signaling may depend on localized crosstalk between distinct ROS and RNS signaling domains, specific subcellular Ca2+ sources, and kinase-signaling [[68], [69], [70]]. The physiological relevance of these mechanisms to humans needs to be further studied.
3.3. Transcription factors regulating gene expression
Exercise training adaptations are elicited by repeated stimulation of gene transcription by exercise-responsive transcription factors (TFs) [71]. Redox-sensitive regulation have been demonstrated for many exercise-linked TFs, including PGC-1α, FOXO, Nuclear factor-κB, and Nrf2 [[72], [73], [74]]. There is most evidence in skeletal muscle suggesting exercise redox-regulation of the latter two, NF-κB and Nrf2. Since Nrf2 regulation has been amply reviewed elsewhere [75], this section will instead discuss NF-κB as an example of a redox-sensitive transcription factor regulated by localized compartmentalized redox-signaling.
NF-κB activation is, in itself, a good example of compartmentalized regulation. At rest, NF-κB is sequestered in the cytoplasm by IκBα, a regulatory protein, which upon IκB kinase (IKK)-dependent phosphorylation of Ser32 and Ser36 sites [76] is ubiquitinated and degraded by the proteasome [77], allowing translocation of active NF-κB to the nucleus to stimulate transcription of a range of genes, which include the well-described myokine IL-6 [78]. The exercise-induced translocation of NF-κB to the nucleus has been demonstrated in skeletal muscle across a range of exercise modalities, including low-intensity prolonged exercise in rats [79], high-intensity bicycling in humans [80] and 2 h post resistance-exercise in human muscle [81]. This process appears to be redox-regulated since the antioxidant pyrrolidine dithiocarbamate (PDCT) blunted the 1 h treadmill exercise-induced activation of NF-κB evaluated in different ways in rat skeletal muscle [44,82]. Furthermore, blockade of NOX2 activation using pharmacological inhibitors in mouse flexor digitorum longus muscle prior to electrical stimulation or swimming exercise strongly reduced measures of NF-κB activation [83]. Interestingly, dystrophic mdx mouse muscle was shown to display a phenotype of hyperactivated NOX2 [84] but a decreased ability to activate NF-κB in response to electrical stimulation and exercise [83]. NOX2 hyper-activation in mdx mice was also associated with Prx2 hyper-oxidation and degradation [85]. Based on these observations, it is tempting to speculate that NOX2 requires Prx2 to stimulate NF-κB transcription as part of a localized redox relay mechanism (Fig. 2). The exact redox-modified targets in the NF-κB pathway are currently unclear, but a study in macrophages suggested that mitochondrial ROS modified cysteine residues on the IKKγ subunit, specifically Cys54 and Cys347, to activate NF-κB [86].
4. What can athletes learn from cellular redox mechanisms?
Exercise training-responsiveness, measured as various performance and/or capacity markers, is known to differ markedly between individuals undertaking either endurance [87] and resistance-exercise [88]. Interestingly, some studies are suggestive of the concept that the responsiveness to endurance exercise training is, in part, determined by redox-status in humans [7,89]. Thus, Margaritelis and co-workers showed that various indicators of redox-status measured in plasma proteins, erythrocytes, and urine correlated negatively with the magnitude of adaptations in both exercise performance tests and redox-status indicators following 6 weeks of bicycle endurance training [7]. The source(s) of ROS modulating blood and urinary redox status are presently unclear, and the interaction between blood and tissue redox-status is likely complex, making the blood and urine redox-markers somewhat difficult to interpret. The stratification by redox-status might also be co-selecting other traits determining endurance-performance. For instance, muscle fiber types are known to differ in their expression in antioxidant proteins [90], and a high proportion of oxidative type I fibers is conducive to endurance performance [91]. Nonetheless, these studies suggest a redox-component to exercise responsiveness.
Consistent with the concept that perturbations in redox-status are inversely correlated to an individual's trainability, blood markers of oxidation increase proportional to training load - which determines the extent of endurance exercise adaptation - during training regimens utilizing either high intensity/volume [92] or high volume [93]. Conversely, elite athletes - who adapt less to a given accustomed exercise training regimen - exhibit higher resting antioxidant defense enzyme activities and lower oxidative products than healthy controls in blood [94]. Furthermore, exhaustive competitions acutely and transiently increased systemic oxidative markers in elite athletes [94,95]. Studies such as the above support that exercise trainability is influenced by a given individual's in-born and environmentally modified redox status.
If exercise-adaptability is indeed determined by redox-pertubations in one or more tissues, then one should in theory be able to enhance exercise performance using compartmentalized pro-oxidants. This could be accomplished by either increasing ROS generation by a specific source or by inhibiting a specific antioxidant process. Supporting the feasibility of using targeted pro-oxidants, knockout of the cytosolic antioxidant protein GPx1 in skeletal muscle was sufficient to sensitize a physiological process, specifically insulin-stimulated PI3K-Akt-signaling and glucose uptake [96]. However, an immediate challenge with this approach might be to target the correct subset of tissues, e.g. the exercising skeletal muscle, if using systemic supplementation. This is a known challenge with some antioxidants [97]. Another challenge is that oxidative stress might contribute to the overtraining syndrome, characterized by fatigue and decreased performance [98]. Thus, overtrained endurance athletes exhibit higher resting protein carbonyls and a blunted increase in post-exercise plasma antioxidant capacity [99,100]. Any targeted pro- or antioxidant would obviously have to overcome these challenges. Future animal studies combining exercise training with genetic or pharmacological augmentation of specific ROS sources could help to clarify if this approach has any merit.
5. Conclusions
The muscle redox-signaling field, like most other cell signaling field, is gradually moving towards the consideration of the biological importance of distinct subcellular signaling domains rather than global cell signaling. Currently, there is evidence that localized cytosolic ROS produced by NOX2 and NOX4 regulate acute exercise-adaptations, including glucose uptake, muscle hypertrophy signaling and transcriptional regulation. Mitochondrial ROS post-exercise may also regulate exercise adaptations. Harnessing and learning how to safely modulate localized ROS production and/or propagation might one day allow us to potentiate the redox-associated exercise hormetic responses to improve human muscle function in health and disease. Mechanistically, the contours of a complex connectivity between redox signaling, Ca2+ and in some cases NO is beginning to emerge, but more work in this field is clearly needed to define the regulation of each endpoint. Some of the major outstanding questions are highlighted in Table 1.
Table 1.
Some major outstanding questions in exercise and skeletal muscle redox signaling.
| Redox subject | Outstanding questions |
|---|---|
| Compartmentalized ROS sources |
|
| |
| |
| |
| |
| Signal transduction |
|
| |
| |
| Training adaptations |
|
|
Funding
C.H.-O. is supported by a research grant from the Danish Diabetes Academy, funded by the Novo Nordisk Foundation (Grant no. NNF17SA0031406) T.E.J. was supported by a Novo Nordisk Foundation Excellence project grant (no. 15182), Independent Research Fund Denmark grant (no. 9039-00029B) and a Lundbeck Foundation Ascending Investigator grant (no. R313-2019-643).
Declaration of competing interest
The authors have no conflicts to report.
References
- 1.Bishop D.J., Botella J., Genders A.J., Lee M.J., Saner N.J., Kuang J. High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiology. 2019;34(1):56–70. doi: 10.1152/physiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 2.Lundsgaard A.M., Fritzen A.M., Kiens B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metabol. 2018;29(1):18–30. doi: 10.1016/j.tem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Chrois K.M., Dohlmann T.L., Sogaard D., Hansen C.V., Dela F., Helge J.W. Mitochondrial adaptations to high intensity interval training in older females and males. Eur. J. Sport Sci. 2019:1–11. doi: 10.1080/17461391.2019.1615556. [DOI] [PubMed] [Google Scholar]
- 5.Lixandrao M.E., Ugrinowitsch C., Berton R., Vechin F.C., Conceicao M.S., Damas F. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48(2):361–378. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 6.Coffey V.G., Zhong Z., Shield A., Canny B.J., Chibalin A.V., Zierath J.R. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. Faseb. J. 2006;20(1):190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- 7.Margaritelis N.V., Theodorou A.A., Paschalis V., Veskoukis A.S., Dipla K., Zafeiridis A. Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability. Acta Physiol. 2018;222(2) doi: 10.1111/apha.12898. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsen T., Salvesen S., Berntsen S., Hetlelid K.J., Stea T.H., Lohne-Seiler H. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports. 2016;26(7):755–763. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 9.Mishina N.M., Bogdanova Y.A., Ermakova Y.G., Panova A.S., Kotova D.A., Bilan D.S. Which antioxidant system shapes intracellular H2O2 gradients? Antioxidants Redox Signal. 2019;31(9):664–670. doi: 10.1089/ars.2018.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobley J.N., Sakellariou G.K., Husi H., McDonagh B. Proteomic strategies to unravel age-related redox signalling defects in skeletal muscle. Free Radic. Biol. Med. 2019;132:24–32. doi: 10.1016/j.freeradbiomed.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 11.McArdle A., Pollock N., Staunton C.A., Jackson M.J. Aberrant redox signalling and stress response in age-related muscle decline: role in inter- and intra-cellular signalling. Free Radic. Biol. Med. 2019;132:50–57. doi: 10.1016/j.freeradbiomed.2018.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriquez-Olguin C., Boronat S., Cabello-Verrugio C., Jaimovich E., Hidalgo E., Jensen T.E. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxidants Redox Signal. 2019;31(18):1371–1410. doi: 10.1089/ars.2018.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortenblad N., Nielsen J. Muscle glycogen and cell function--Location, location, location. Scand. J. Med. Sci. Sports. 2015;25(Suppl 4):34–40. doi: 10.1111/sms.12599. [DOI] [PubMed] [Google Scholar]
- 14.Mukund K., Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020;12(1):e1462. doi: 10.1002/wsbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glancy B., Hartnell L.M., Malide D., Yu Z.X., Combs C.A., Connelly P.S. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523(7562):617–620. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiev T., Svirin M., Jaimovich E., Fink R.H. Localized nuclear and perinuclear Ca(2+) signals in intact mouse skeletal muscle fibers. Front. Physiol. 2015;6:263. doi: 10.3389/fphys.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherardi G., Di Marco G., Rizzuto R., Mammucari C. Crosstalk between mitochondrial Ca(2+) uptake and autophagy in skeletal muscle. Oxid. Med. Cell Longev. 2019:1845321. doi: 10.1155/2019/1845321. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakellariou G.K., Lightfoot A.P., Earl K.E., Stofanko M., McDonagh B. Redox homeostasis and age-related deficits in neuromuscular integrity and function. J. Cachexia Sarcopenia Muscle. 2017;8(6):881–906. doi: 10.1002/jcsm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson B.C., Chang C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011;7(8):504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veal E., Day A. Hydrogen peroxide as a signaling molecule. Antioxidants Redox Signal. 2011;15(1):147–151. doi: 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- 22.Stocker S., Maurer M., Ruppert T., Dick T.P. A role for 2-Cys peroxiredoxins in facilitating cytosolic protein thiol oxidation. Nat. Chem. Biol. 2018;14(2):148–155. doi: 10.1038/nchembio.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson M.J. Reactive oxygen species in sarcopenia: should we focus on excess oxidative damage or defective redox signalling? Mol. Aspect. Med. 2016;50:33–40. doi: 10.1016/j.mam.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Davies K.J., Quintanilha A.T., Brooks G.A., Packer L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 25.Merry T.L., Ristow M. Mitohormesis in exercise training. Free Radic. Biol. Med. 2016;98:123–130. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45(7–8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han D., Antunes F., Canali R., Rettori D., Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003;278(8):5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 29.Vieceli Dalla Sega F., Zambonin L., Fiorentini D., Rizzo B., Caliceti C., Landi L. Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta. 2014;1843(4):806–814. doi: 10.1016/j.bbamcr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Sakellariou G.K., Vasilaki A., Palomero J., Kayani A., Zibrik L., McArdle A. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants Redox Signal. 2013;18(6):603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaelson L.P., Shi G., Ward C.W., Rodney G.G. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42(4):522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriquez-Olguin C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019;10(1):4623. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujikawa Y., Roma L.P., Sobotta M.C., Rose A.J., Diaz M.B., Locatelli G. Mouse redox histology using genetically encoded probes. Sci. Signal. 2016;9(419):rs1. doi: 10.1126/scisignal.aad3895. [DOI] [PubMed] [Google Scholar]
- 34.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banh S., Treberg J.R. The pH sensitivity of H2O2 metabolism in skeletal muscle mitochondria. FEBS Lett. 2013;587(12):1799–1804. doi: 10.1016/j.febslet.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Munro D., Treberg J.R. A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017;220(Pt 7):1170–1180. doi: 10.1242/jeb.132142. [DOI] [PubMed] [Google Scholar]
- 37.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017;8(1):548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidalgo C., Sanchez G., Barrientos G., Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J. Biol. Chem. 2006;281(36):26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 39.Oakley F.D., Abbott D., Li Q., Engelhardt J.F. Signaling components of redox active endosomes: the redoxosomes. Antioxidants Redox Signal. 2009;11(6):1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hervera A., De Virgiliis F., Palmisano I., Zhou L., Tantardini E., Kong G. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018;20(3):307–319. doi: 10.1038/s41556-018-0039-x. [DOI] [PubMed] [Google Scholar]
- 41.Nordzieke D.E., Medrano-Fernandez I. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants. 2018;7(11) doi: 10.3390/antiox7110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Q.A., Hess D.T., Nogueira L., Yong S., Bowles D.E., Eu J. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanmugasundaram K., Nayak B.K., Friedrichs W.E., Kaushik D., Rodriguez R., Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017;8(1):997. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriquez-Olguin C., Diaz-Vegas A., Utreras-Mendoza Y., Campos C., Arias-Calderon M., Llanos P. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front. Physiol. 2016;7:282. doi: 10.3389/fphys.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal R., Basu Thakur P., Li S., Minard C., Rodney G.G. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henriquez-Olguin C., Renani L.B., Arab-Ceschia L., Raun S.H., Bhatia A., Li Z. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019;24:101188. doi: 10.1016/j.redox.2019.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017;13(3):133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 48.Zisman A., Peroni O.D., Abel E.D., Michael M.D., Mauvais-Jarvis F., Lowell B.B. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000;6(8):924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 49.Sandstrom M.E., Zhang S.J., Bruton J., Silva J.P., Reid M.B., Westerblad H. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2006;575(Pt 1):251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen T.E., Schjerling P., Viollet B., Wojtaszewski J.F., Richter E.A. AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PloS One. 2008;3(5) doi: 10.1371/journal.pone.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cartee G.D., Holloszy J.O. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am. J. Physiol. 1990;258(2 Pt 1):E390–E393. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- 52.Merry T.L., Steinberg G.R., Lynch G.S., McConell G.K. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 2010;298(3):E577–E585. doi: 10.1152/ajpendo.00239.2009. [DOI] [PubMed] [Google Scholar]
- 53.Chambers M.A., Moylan J.S., Smith J.D., Goodyear L.J., Reid M.B. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J. Physiol. 2009;587(Pt 13):3363–3373. doi: 10.1113/jphysiol.2008.165639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sylow L., Nielsen I.L., Kleinert M., Moller L.L., Ploug T., Schjerling P. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J. Physiol. 2016;594(17):4997–5008. doi: 10.1113/JP272039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sylow L., Jensen T.E., Kleinert M., Mouatt J.R., Maarbjerg S.J., Jeppesen J. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes. 2013;62(4):1139–1151. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khayat Z.A., Tong P., Yaworsky K., Bloch R.J., Klip A. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J. Cell Sci. 2000;113(Pt 2):279–290. doi: 10.1242/jcs.113.2.279. [DOI] [PubMed] [Google Scholar]
- 57.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldhart A.N., Dykstra H., Peck A.S., Boguslawski E.A., Madaj Z.B., Wen J. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep. 2017;19(10):2005–2013. doi: 10.1016/j.celrep.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witczak C.A., Jessen N., Warro D.M., Toyoda T., Fujii N., Anderson M.E. CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010;298(6):E1150–E1160. doi: 10.1152/ajpendo.00659.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 61.Ogasawara R., Jensen T.E., Goodman C.A., Hornberger T.A. Resistance exercise-induced hypertrophy: a potential role for rapamycin-insensitive mTOR. Exerc. Sport Sci. Rev. 2019;47(3):188–194. doi: 10.1249/JES.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulsen G., Hamarsland H., Cumming K.T., Johansen R.E., Hulmi J.J., Borsheim E. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014;592(24):5391–5408. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito N., Ruegg U.T., Kudo A., Miyagoe-Suzuki Y., Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013;19(1):101–106. doi: 10.1038/nm.3019. [DOI] [PubMed] [Google Scholar]
- 64.Ito N., Ruegg U.T., Kudo A., Miyagoe-Suzuki Y., Takeda S. Capsaicin mimics mechanical load-induced intracellular signaling events: involvement of TRPV1-mediated calcium signaling in induction of skeletal muscle hypertrophy. Channels. 2013;7(3):221–224. doi: 10.4161/chan.24583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito N., Ruegg U.T., Takeda S. ATP-induced increase in intracellular calcium levels and subsequent activation of mTOR as regulators of skeletal muscle hypertrophy. Int. J. Mol. Sci. 2018;19(9) doi: 10.3390/ijms19092804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bendall J.K., Cave A.C., Heymes C., Gall N., Shah A.M. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105(3):293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 67.Satoh M., Ogita H., Takeshita K., Mukai Y., Kwiatkowski D.J., Liao J.K. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2006;103(19):7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333(6048):1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 69.Daiber A., Di Lisa F., Oelze M., Kroller-Schon S., Steven S., Schulz E. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174(12):1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pal R., Palmieri M., Loehr J.A., Li S., Abo-Zahrah R., Monroe T.O. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat. Commun. 2014;5:4425. doi: 10.1038/ncomms5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Popov D.V., Makhnovskii P.A., Shagimardanova E.I., Gazizova G.R., Lysenko E.A., Gusev O.A. Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019;316(4):E605–E614. doi: 10.1152/ajpendo.00449.2018. [DOI] [PubMed] [Google Scholar]
- 72.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003;546(Pt 3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merry T.L., Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016;594(18):5195–5207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji L.L., Gomez-Cabrera M.C., Steinhafel N., Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. Faseb. J. 2004;18(13):1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 75.Done A.J., Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arkan M.C., Hevener A.L., Greten F.R., Maeda S., Li Z.W., Long J.M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11(2):191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 77.Li Y.P., Reid M.B. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279(4):R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 78.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 79.Hollander J., Fiebig R., Gore M., Ookawara T., Ohno H., Ji L.L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Archiv. 2001;442(3):426–434. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- 80.Cuevas M.J., Almar M., Garcia-Glez J.C., Garcia-Lopez D., De Paz J.A., Alvear-Ordenes I. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic. Res. 2005;39(4):431–439. doi: 10.1080/10715760500072149. [DOI] [PubMed] [Google Scholar]
- 81.Vella L., Caldow M.K., Larsen A.E., Tassoni D., Della Gatta P.A., Gran P. Resistance exercise increases NF-kappaB activity in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(6):R667–R673. doi: 10.1152/ajpregu.00336.2011. [DOI] [PubMed] [Google Scholar]
- 82.Ho R.C., Hirshman M.F., Li Y., Cai D., Farmer J.R., Aschenbach W.G. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2005;289(4):C794–C801. doi: 10.1152/ajpcell.00632.2004. [DOI] [PubMed] [Google Scholar]
- 83.Henriquez-Olguin C., Altamirano F., Valladares D., Lopez J.R., Allen P.D., Jaimovich E. Altered ROS production, NF-kappaB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta. 2015;1852(7):1410–1419. doi: 10.1016/j.bbadis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loehr J.A., Wang S., Cully T.R., Pal R., Larina I.V., Larin K.V. NADPH oxidase mediates microtubule alterations and diaphragm dysfunction in dystrophic mice. Elife. 2018;7 doi: 10.7554/eLife.31732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olthoff J.T., Lindsay A., Abo-Zahrah R., Baltgalvis K.A., Patrinostro X., Belanto J.J. Loss of peroxiredoxin-2 exacerbates eccentric contraction-induced force loss in dystrophin-deficient muscle. Nat. Commun. 2018;9(1):5104. doi: 10.1038/s41467-018-07639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herb M., Gluschko A., Wiegmann K., Farid A., Wolf A., Utermohlen O. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. 2019;12(568) doi: 10.1126/scisignal.aar5926. [DOI] [PubMed] [Google Scholar]
- 87.Prud'homme D., Bouchard C., Leblanc C., Landry F., Fontaine E. Sensitivity of maximal aerobic power to training is genotype-dependent. Med. Sci. Sports Exerc. 1984;16(5):489–493. doi: 10.1249/00005768-198410000-00012. [DOI] [PubMed] [Google Scholar]
- 88.Ahtiainen J.P., Walker S., Peltonen H., Holviala J., Sillanpaa E., Karavirta L. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age. 2016;38(1):10. doi: 10.1007/s11357-015-9870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Margaritelis N.V., Kyparos A., Paschalis V., Theodorou A.A., Panayiotou G., Zafeiridis A. Reductive stress after exercise: the issue of redox individuality. Redox Biol. 2014;2:520–528. doi: 10.1016/j.redox.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Powers S.K., Criswell D., Lawler J., Ji L.L., Martin D., Herb R.A. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994;266(2 Pt 2):R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 91.Horowitz J.F., Sidossis L.S., Coyle E.F. High efficiency of type I muscle fibers improves performance. Int. J. Sports Med. 1994;15(3):152–157. doi: 10.1055/s-2007-1021038. [DOI] [PubMed] [Google Scholar]
- 92.Margonis K., Fatouros I.G., Jamurtas A.Z., Nikolaidis M.G., Douroudos I., Chatzinikolaou A. Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radic. Biol. Med. 2007;43(6):901–910. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 93.Knez W.L., Jenkins D.G., Coombes J.S. The effect of an increased training volume on oxidative stress. Int. J. Sports Med. 2014;35(1):8–13. doi: 10.1055/s-0033-1333746. [DOI] [PubMed] [Google Scholar]
- 94.Knez W.L., Jenkins D.G., Coombes J.S. Oxidative stress in half and full Ironman triathletes. Med. Sci. Sports Exerc. 2007;39(2):283–288. doi: 10.1249/01.mss.0000246999.09718.0c. [DOI] [PubMed] [Google Scholar]
- 95.Neubauer O., Konig D., Kern N., Nics L., Wagner K.H. No indications of persistent oxidative stress in response to an ironman triathlon. Med. Sci. Sports Exerc. 2008;40(12):2119–2128. doi: 10.1249/MSS.0b013e3181824dab. [DOI] [PubMed] [Google Scholar]
- 96.Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S. Reactive oxygen species enhance insulin sensitivity. Cell Metabol. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halson S.L., Jeukendrup A.E. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004;34(14):967–981. doi: 10.2165/00007256-200434140-00003. [DOI] [PubMed] [Google Scholar]
- 99.Tanskanen M., Atalay M., Uusitalo A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010;28(3):309–317. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- 100.Palazzetti S., Richard M.J., Favier A., Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can. J. Appl. Physiol. 2003;28(4):588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]