Abstract
During exercise, muscle ATP demand increases with intensity, and at the highest power output, ATP consumption may increase more than 100-fold above the resting level. The rate of mitochondrial ATP production during exercise depends on the availability of O2, carbon substrates, reducing equivalents, ADP, Pi, free creatine, and Ca2+. It may also be modulated by acidosis, nitric oxide and reactive oxygen and nitrogen species (RONS). During fatiguing and repeated sprint exercise, RONS production may cause oxidative stress and damage to cellular structures and may reduce mitochondrial efficiency. Human studies indicate that the relatively low mitochondrial respiratory rates observed during sprint exercise are not due to lack of O2, or insufficient provision of Ca2+, reduced equivalents or carbon substrates, being a suboptimal stimulation by ADP the most plausible explanation. Recent in vitro studies with isolated skeletal muscle mitochondria, studied in conditions mimicking different exercise intensities, indicate that ROS production during aerobic exercise amounts to 1-2 orders of magnitude lower than previously thought. In this review, we will focus on the mechanisms regulating mitochondrial respiration, particularly during high-intensity exercise. We will analyze the factors that limit mitochondrial respiration and those that determine mitochondrial efficiency during exercise. Lastly, the differences in mitochondrial respiration between men and women will be addressed.
Keywords: Mitochondrial respiration, High-intensity exercise, Oxidative stress, Sprint performance, Fatigue
Graphical abstract
Abbreviations
- ΔΨ
Mitochondrial membrane potential
- Acetyl-CoA
Acetyl coenzyme A
- ADP
Adenosine diphosphate
- ANT
Adenine nucleotide translocase
- ATP
Adenosine triphosphate
- ATP-Mg/Pi carriers
ATP-Mg/inorganic phosphate (Pi) carriers
- AMPK
AMP-activated protein kinase
- AGC
aspartate/glutamate carriers
- Ca2+
Ion calcium
- CaMC
Ca2+ binding mitochondrial carriers
- CAMKKβ
Ca2+/calmodulin-dependent protein kinase kinase-β
- CaMKII
Calmodulin-dependent Protein Kinase II
- CD36
fatty acid transporter CD36
- CK
Creatine kinase
- COX
Cytochrome C Oxidase
- Cr
Creatine
- eNOS
endothelial nitric oxide synthase
- GSSG
Glutathione disulfide
- H2PO4
Phosphoric acid
- IMM
Inner mitochondrial membrane
- IMS
Intermembrane mitochondrial space
- MCU
Mitochondrial calcium uniporter
- MitoVD
Mitochondrial volume density
- MtCK
Mitochondrial creatine kinase
- NAD+
Nicotinamide adenine dinucleotide oxidized
- NADH+
Nicotinamide adenine dinucleotide reduced
- nNOS
neuronal nitric oxide synthase
- OXPHOS
Mitochondrial oxidative phosphorylation
- OMM
Outer mitochondrial membrane
- PaO2
Arterial O2 pressure
- PCr
Phosphocreatine
- PDH
Pyruvate dehydrogenase
- PDHC
Pyruvate dehydrogenase complex
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- Pi
Inorganic phosphate
- PIO2
Inspiratory O2 pressure
- PO2
O2 pressure
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- RONS
Reactive oxygen and nitrogen species
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase
- TRX
Thioredoxin
- TCA
Tricarboxylic acid cycle
- VDAC
voltage-dependent anion channel (or porin)
- VO2
Oxygen consumption
- VO2max
Maximal oxygen consumption
- VO2peak
Peak oxygen consumption
1. Introduction
Mitochondria originated nearly 2 billion years ago by the endosymbiosis of a specialized prokaryote [1]. The main function of mitochondria is the synthesis of ATP through oxidative phosphorylation, in which carbon substrates are oxidized with electrons flowing through the respiratory chain complexes to generate a proton gradient across the inner mitochondrial membrane (IMM). According to Mitchel's chemiosmotic theory [2], this proton gradient provides the energy necessary for the production of ATP. Besides, the proton gradient is the driving force for the mitochondrial uptake of positively charged ions and for the transport of ADP from the cytosol to the mitochondria [3]. During oxidative phosphorylation (OXPHOS), some reactive oxygen species (ROS), whose quantity in physiological conditions is 1-2 orders of magnitude lower than thought [5], are generated [4]. Although the rate of ROS production is low, oxidative stress has been detected during fatiguing and intense prolonged exercise [6]. Unchecked production of ROS and nitrogen reactive species (RNS) may be damaging [7] and could jeopardize cell survival [8]. The skeletal muscle and the mitochondria have redundant antioxidant systems and enzymes [6], with remarkable adaptive capacity to exercise training, which prevents oxidative damage by repeated exercise. ROS also play a critical signaling role, and partial counteraction of ROS production during exercise has been associated with incomplete adaptation to exercise training, blunting some of the beneficial adaptations of regular exercise [9,10]. In this review, we will focus on the mechanisms regulating mitochondrial respiration, particularly during high-intensity exercise. We will analyze the factors that limit mitochondrial respiration, with emphasis on the roles played by oxygen, reduced equivalents, carbon substrates, calcium, ROS, metabolites, and the factors that determine mitochondrial efficiency during exercise. Lastly, the differences in mitochondrial respiration between men and women will be addressed.
2. Regulation of mitochondrial respiration during exercise: some insights from sprint exercise in humans
During exercise, muscle ATP demand increases with exercise intensity and at the highest power output, ATP consumption may reach more than 100-fold the value observed at rest. ATP supply must match the ATP demand to maintain power output; otherwise power would progressively decline to a level at which it is possible to match energy demand and supply. These high rates of ATP output require a concerted contribution of both anaerobic (i.e., phosphocreatine and glycolysis), and aerobic ATP resynthesis mechanisms. At peak power output, as reached during maximal sprinting, the demand for ATP per second may exceed more than 10-fold the aerobic ATP resynthesis observed in the sprint [11]. Nonetheless, during a short sprint, the rate of O2 consumption is only 20–30% of VO2max [[11], [12], [13]]. Therefore, during maximal sprinting most of the ATP is resynthesized by the anaerobic metabolism, causing accumulation of end-products linked to muscle fatigue [14]. The strong activation of the anaerobic metabolism during exercise is connected to the production of reactive oxygen and nitrogen species (RONS), which may also contribute to muscle fatigue [15]. Aerobic ATP production results in the generation of H2O, CO2 and heat, as well as RONS [4] (see below RONS and mitochondrial respiration during exercise), with heat and RONS as potential contributors to fatigue. Heat accumulation is minimal during a short sprint in human skeletal muscle [16], and if anything, the moderate increase in muscle temperature may counteract fatigue [17]. The production of RONS is reduced with the increase in respiratory rate (O2 consumption, VO2), explained in part by lower accumulation of partially reduced intermediates capable of generating superoxide radicals, and due to a lower local oxygen availability in the microenvironment [3,18]. Thus, a lower RONS production from mitochondrial sources is expected as the VO2 increases during the sprint [16]. Production of RONS by mitochondrial dehydrogenases is stimulated by high NADH/NAD+ levels and inhibited by NAD+. The rate of NAD+ output is enhanced by high respiratory rates [3,[19], [20], [21]]. ADP plays a critical role in the regulation of energy metabolism during exercise since it regulates the rate of both the glycolytic flux [22] and OXPHOS [23].
2.1. Why do mitochondria do not respire at a higher rate during sprint exercise?
During a short sprint, the mitochondria are respiring at submaximal intensity. The question is then why mitochondria do not respire at a higher rate. We tried to answer this question by examining the different steps of the O2 cascade from the atmosphere to the skeletal muscles [16]. The energy necessary for the synthesis of ATP in the mitochondria is provided by the proton gradient across the inner mitochondrial membrane. This gradient is generated through electron transport reactions that are linked to the oxidation of carbon substrates. The rate of mitochondrial ATP production depends on the availability of O2, carbon substrates, reducing equivalents, ADP, Pi, Ca2+, and free creatine (Cr), as well as the stimulation by ADP [24].
2.2. Is oxygen delivery a limiting factor for mitochondrial respiration during sprint exercise in humans?
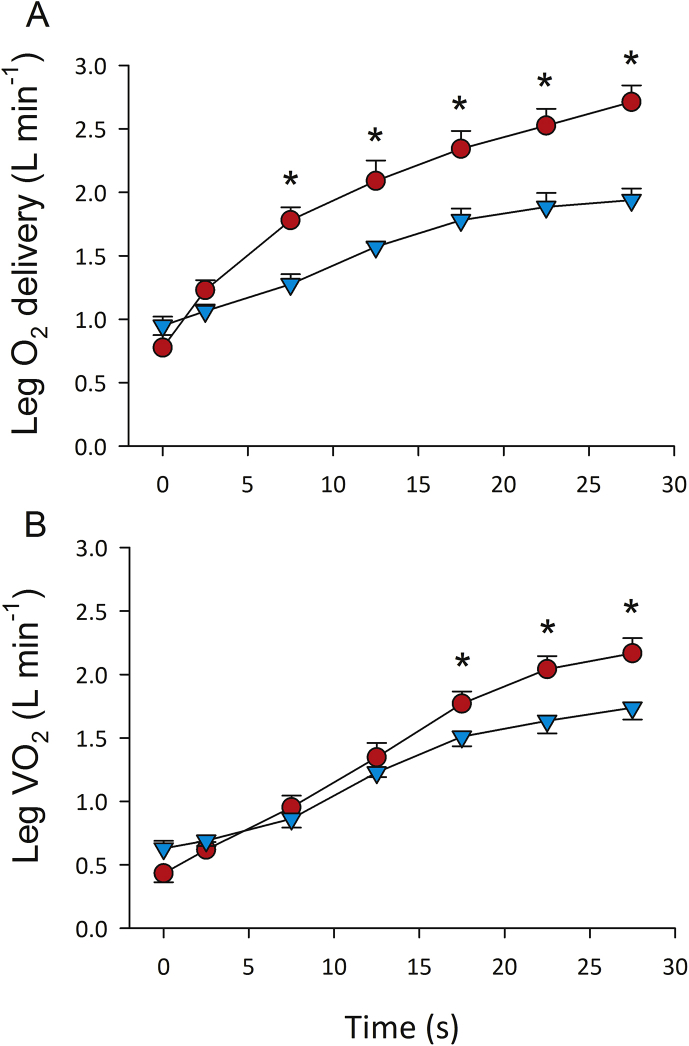
To determine why VO2 is so low despite a maximal ATP demand during sprint exercise, we asked some volunteers to perform maximal 10 or 30-s sprints in severe acute hypoxia (close to the level of human tolerance, PIO2 = 74 mmHg, PaO2 = 33 mmHg) and normoxia while measuring leg blood flow by thermodilution and the femoral's artery and vein O2 concentrations using the direct Fick method, which requires catheterization of the femoral artery and vein. During sprint exercise in normoxia and severe acute hypoxia, skeletal muscle VO2 is almost the same at the start of the sprint (first 10 s) and only slightly lower from the 15th to the 30th second, despite much higher oxygen delivery in normoxia (Fig. 1). These experiments demonstrated that muscle VO2 is not limited by O2 delivery during a maximal short sprint in normoxia [16]. This should not be surprising since in vitro experiments in cardiomyocytes have shown that mitochondria isolated in 20.9% O2 and 0.1% O2 both responded with significantly increased ATP levels to the stimulation of succinate and ADP, with greater production of ATP upon stimulation in the hypoxic isolated mitochondria compared to the normoxic isolated mitochondria [24]. Besides this, the Nanadikar et al. [24] experiment demonstrated that isolated mitochondria could operate at very low O2 tensions (i.e., PO2 < 1 mmHg).
Fig. 1.
Oxygen delivery (A) and consumption (VO2) (B) during sprint exercise in men. Oxygen delivery and VO2 were measured by the direct Fick method during 30-s all out sprints in normoxia (red circles) and severe acute hypoxia equivalent an altitude of 5300 m above sea level (light blue circles; PIO2 = 73 mmHg). The symbol (*) indicates significant differences between normoxia and hypoxia. Leg VO2 was similar in both conditions despite large differences in O2 delivery, indicating that at least during the first 15 s O2 delivery was not limiting mitochondrial respiration when the exercise was carried out in normoxia (modified from Calbet et al. [16]). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Is the provision of reduced equivalents and substrates limiting OXPHOS during sprint exercise?
During sprint exercise, the production of reduced equivalents (NADH.H+) is also in excess due to the high glycolytic rate elicited by sprint exercise [[25], [26], [27]]. Moreover, during sprint exercise in severe acute hypoxia, NADH.H+ is markedly higher and NAD+ lower than during the same sprints performed in normoxia [[25], [26], [27]]. Although the glycolysis produces NADH.H+ in the sarcoplasm, it can be rapidly transferred to the mitochondrial matrix by the malate-aspartate NADH shuttle. Thus, the low mitochondrial respiratory rate observed during sprint exercise in normoxia does not seem to be due to a lack of NADH.H+.
Insufficient provision of pyruvate can also be ruled out since the pyruvate dehydrogenase (PDH) is completely dephosphorylated (activated) during sprint exercise, both in normoxia and severe acute hypoxia [25,27]. Moreover, there is a substantial increase in muscle lactate, also reflecting the high glycolytic rate and production of pyruvate [25,27,28]. In theory, acetyl group availability may be a limiting factor for OXPHOS at the start of the sprint [29]. Nevertheless, it has been suggested that acetyl group deficit may occur only at moderate exercise intensities (65–90% VO2max) [30]. At higher exercise intensities (90–110% VO2max), activation of PDH with dichloroacetate (an inhibitor of PDH kinase) did not influence exercise performance, nor did it influence O2 utilization, despite the fact that acetyl-CoA was increased before the start of exercise and after the administration of dichloroacetate. Similar results were reported during sprint exercise, i.e., the administration before sprint exercise of either acetate to increase resting acetyl-CoA and acetyl-carnitine, or dichloroacetate to increase resting acetyl-CoA, acetyl-carnitine and PDH activation, did not affect non-oxidative ATP production nor performance [28]. Although Howlett et al. [28] did not directly measure muscle VO2, the fact that the anaerobic energy production was not affected strongly suggests a similar VO2, indicating that acetyl group availability is not a limiting factor for OXPHOS during sprint exercise. These experiments showed that the provision of acetyl-CoA groups is not a limiting factor for substrate oxidation during high-intensity exercise in humans [28,[31], [32], [33]].
A deficit of other carbon substrates for OXPHOS, downstream to acetyl-CoA, such as tricarboxylic cycle (TCA) intermediates, has also been suggested as potential limiting factors for aerobic energy production, at least during prolonged exercise to exhaustion [34]. There is no information regarding the muscle concentration of TCA intermediates during sprint exercise in humans. Skeletal muscle TCA intermediates, particularly succinate, malate, and fumarate, increase during the first minute of moderate-intensity exercise [35]. Supplementation with citrulline malate before repeated sprint exercise did not affect sprint performance [36], suggesting that the availability of malate is not limiting. However, without the measurement of muscle malate concentrates or VO2, no conclusion can be made regarding the effect of malate availability on mitochondrial respiration. The fact that blood lactate concentration is not altered by citrulline malate could indicate that malate is not limiting OXPHOS during sprint exercise in humans.
2.4. Calcium regulation of OXPHOS during sprint exercise
Sarcoplasmic calcium concentration ([Ca2+]) is presumably maximal during sprint exercise; otherwise power output will not be maximal, and therefore insufficient availability of Ca2+ is unlikely. Calcium may stimulate mitochondrial respiration by multiple mechanisms enhancing energy consumption such as providing reduced equivalents and substrates for OXPHOS, increasing the activity of several dehydrogenases and modulating F1-FO-ATPase and cytochrome oxidase [37]. Calcium triggers muscle contraction while simultaneously upregulating glycogenolysis, glycolysis, and glucose transport. Sarcoplasmic Ca2+ activates calmodulin-dependent protein kinase II (CaMKII), which in turn stimulates glycogenolysis and glycolysis by activating several of the critical enzymes involved (phosphorylate aldolase A, glyceraldehyde-3-phosphate dehydrogenase, enolase, lactate dehydrogenase, creatine kinase, pyruvate kinase, and phosphorylase b kinase) [[38], [39], [40]]. Besides, Ca2+ promotes the synthesis of fructose 2,6‐bisphosphate (F2,6BP), which in turn activates phosphofructokinase (PFK). In skeletal muscle, the bifunctional enzyme responsible for F2,6BP formation is the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) [41], which is activated via phosphorylation by AMPK [42]. AMPK is phosphorylated and activated by the Ca2+ sensitive CAMKKβ, an upstream kinase for AMPK [43]. Blunting CaMKII activation during sprint exercise reduces the glycolytic rate slightly without significantly altering performance [26,44]. This suggests that the provision of reduced equivalents and glycolytic substrates is in excess during sprint exercise.
Sarcoplasmic Ca2+ may act directly on Ca2+ binding mitochondrial carriers (CaMC), which host a specific binding site located on the outer side of the inner mitochondrial membrane, i.e., without need to penetrate in the mitochondrial matrix [45]. The main two CaMCs are the aspartate/glutamate carriers (AGC) [46], also called aralar1 (AGC1) and the ATP-Mg/inorganic phosphate (Pi) carriers (ATP-Mg/Pi carriers), also named SCaMC (for short CaMC) [47] (for review see Ref. [48]). Aralar is a member of the malate-aspartate NADH shuttle, present in skeletal muscle, which upon Ca2+ binding promotes the exchange of aspartate by glutamate and the transport of NADH into the matrix. Furthermore, an increase in mitochondrial glutamate stimulates state 3 respiration [49]. The malate-aspartate NADH shuttle activity is likely stimulated by small increases of sarcoplasmic Ca2+, as extramitochondrial Ca2+ does in the brain [50], i.e., below the Ca2+ concentrations at which the calcium uniporter (MCU) is activated. The role that AGC1 has on the activation of mitochondrial respiration at the start of intense exercise remains unknown.
Ca2+ stimulates oxidative phosphorylation by eliciting a drop in the ATP/ADP ratio through the stimulation of muscle contraction and SERCA, but also after its transport into the mitochondria through the MCU. Once in the matrix, Ca2+ activates pyruvate, isocitrate, and α-ketoglutarate dehydrogenases. The entry of Ca2+ may be facilitated by large increases of [Ca2+] in the microenvironment between sarcoplasmic reticulum and mitochondria [[51], [52], [53]].
Mitochondrial Ca2+ influx is driven by the membrane potential (ΔΨ). Since Ca2+ carries two positive charges, the entry of Ca2+ in the mitochondria entails a reduction of the chemical component (ΔpH) of the electrochemical potential due to charge compensation by the extrusion of 2H+, alkalinizing the mitochondrial matrix [54]. Therefore, the entry of Ca2+ reduces the ΔΨ, limiting the driving force for the influx of more Ca2+, unless Ca2+ transport occurs accompanied by anions in the protonated form, neutralizing the pH gradient and regenerating ΔΨ [55]. The most important of these anions accompanying the influx of Ca2+ is H3PO4, which is transported into the mitochondrial matrix by the Pi-H+ symporter [56], which likely plays a critical role in the stimulation of mitochondrial respiration at the start of exercise [57]. Ca2+ uptake and ADP phosphorylation compete for the electrochemical gradient generated by respiration [3]. Ca2+ uptake precedes ADP phosphorylation when they are simultaneously added to the reaction medium [58].
PDH activation can be used as a surrogate marker of matrix [Ca2+] [37]. Parolin et al. demonstrated that at 6th second of a 30-s all-out sprint (isokinetic Wingate test), the activation of PDH had increased from 14% at rest to 48%, to reach 95% activation at the 15th s [59]. Thus, the increase of Ca2+ in the matrix is fast during sprint exercise. Increased [Ca2+] may decrease the KM for ADP, facilitating the stimulation of mitochondrial respiration, as shown in skinned cardiomyocytes [60].
2.5. Regulation of mitochondrial respiration by reactive oxygen and nitrogen species
Skeletal muscle generates mostly superoxide (O2•-) and nitric oxide (•NO), which after reaction with other compounds form additional ROS and RNS, such as hydrogen peroxide (H2O2), hydroxyl radicals (•OH), singlet oxygen (an electronically excited form of O2, highly reactive but is not a radical), nitric oxide (•NO), and peroxynitrite (ONOO−, a strong oxidizing agent product of the reaction between superoxide and •NO) [61]. Eleven sites that produce O2·- and hydrogen peroxide H2O2 have been identified in mammalian mitochondria [62]. The contributing sites and the rate of ROS production vary with the type of substrate oxidized, the metabolic rate, and the nutritional state (caloric restriction, overfeeding, etc.) [62]. It remains unknown which sites are the primary producers of ROS during exercise in vivo [5].
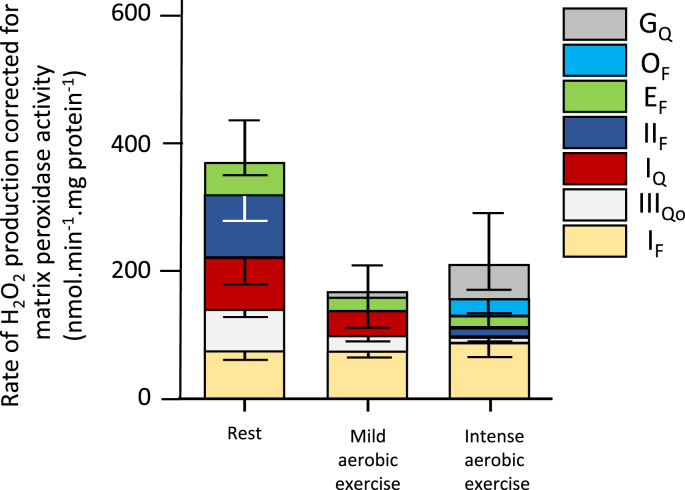
While at rest most ROS originate from the mitochondria, during intense exercise, the sarcoplasmic reticulum and transverse tubules or plasma membrane NAD(P)H oxidase (NOX) and, to a lesser extent, xanthine oxidase (XO) are considered the main sources [6,61,63]. During aerobic exercise mitochondrial ROS production is reduced [64] because skeletal muscle mitochondria are predominantly in state 3 respiration (ADP-stimulated) [65]. Gonsalves et al. [5] performed in vitro experiments with isolated skeletal muscle mitochondria reproducing physiological concentrations of substrates and effectors concentrations mimicking rest (10% VO2max), and low (22% VO2max) and intense (90% VO2max) aerobic exercise intensities. Electron leak was 0.35, 0.03, and 0.01% at 10, 22, and 90% of VO2max-simulated conditions, indicating that during exercise the mitochondria barely contribute to producing ROS, with a predominance of ROS originating in Complex I (site IF) during the “simulated” aerobic exercise [5] (Fig. 2).
Fig. 2.
Quantification of the relative contribution of several mitochondrial sites to superoxide and H2O2production in isolated rat skeletal muscle mitochondria assessed in a media mimicking conditions of rest, mild aerobic exercise, and intense aerobic exercise. The authors applied corrections for H2O2 losses due to degradation by mitochondrial matrix peroxidases. Error bars in inverted position denote the propagated errors for each site while regular error bars indicate the propagated sum of these errors. Values are means ± S.E. (error bars) (n = 3–20). Modified from Goncalves et al. [5].
Based on a complex model of muscle and whole-body metabolism in humans, Nilsson et al. [66] have proposed a bypass of the complex I during high-intensity exercise, as a mechanism to increase the catalytic capacity of the muscle (ATP production) at the expense of substrate efficiency. The model predicts that to bypass complex I, NADH is re-routed through the glycerol-3-phosphate shuttle, which transfers the NADH electrons directly to ubiquinone (QH2), entering the electron transport chain at complex III. Recent studies have shown that the proton-pumping step of complex I can be bypassed in vivo while retaining the NADH dehydrogenase activity [67]. This disengagement of the proton-pumping step of complex I is explained by a slow transition of the active (A) to the deactivated, dormant (D) form enzyme, as observed during ischemia in metabolically active organs such as the heart and brain [68]. With re-oxygenation, complex I reactivates increasing the production of ROS [68]. When the enzyme is in the D form is more susceptible to oxidation by reactive nitrogen species, resulting in nitrosylation of the enzyme. The latter has been proposed as an intrinsic protective mechanism that delays activation upon re-oxygenation, decreasing the production of ROS at the beginning of reperfusion [68]. It remains unknown, however, whether complex I can be bypassed during sprint exercise in human skeletal muscle. This could be the case with repeated prolonged sprints associated with oxidative stress [69,70], or when ischemia is applied at exhaustion followed after a few seconds by another bout of exercise with reperfusion [11,71].
As fatigue develops more RONS are produced, particularly if concurrent with the recruitment of anaerobic metabolism [6]. The fact that the high energy turnover achieved during sprint exercise elicits a greater production of ROS compared to low-intensity exercise or resting is supported by the observation of increased oxidative stress, the activation of ROS-induced signaling and the physiological adaptations increasing the antioxidant enzymes [61,69].
Current experimental evidence indicates that during fatiguing repeated sprint exercise, RONS production may cause oxidative stress and damage to cellular structures [69,70]. Therefore, to some extent, RONS-elicited fatigue [15] may contribute to avoid damage by ROS.
2.5.1. Pyruvate dehydrogenase complex is a source of ROS during sprint exercise
The pyruvate dehydrogenases complex (PDHC) catalyzes the irreversible conversion of pyruvate to acetyl-CoA [72]. PDHC is an important source of H2O2 in experiments with permeabilized human muscle fibers [73]. When pyruvate and NAD+ are abundant, PDHC produces H2O2 at a low rate. Nevertheless, when the NAD+/NADH+ ratio is lowered, the PDHC reactions release more H2O2 [73]. The H2O2 generated by PDHC is converted to H2O by reduced thioredoxin (TRx), while TRx becomes oxidized. Oxidized TRx is immediately reduced by glutathione disulfide (GSSG) through the glutathione reaction using NADPH+ as a cofactor (producing NADP+). NADPH+ should be resynthesized by nicotinamide nucleotide transhydrogenase (NNT) with consumption of NADH+ as shown the following reaction [74]:
| NADH+ + NADP+ ⇐ NNT ⇒ NAD+ + NADPH+ |
The latter agrees with the attenuation of the sprint exercise-elicited reduction of the NAD+/NADH+ ratio by the ingestion of an antioxidant cocktail (vitamin C, vitamin E, and alpha-lipoic acid) before de exercise [44]. In the latter study, the VO2 and the power output were similar in the sprints performed with placebo and antioxidants, while lactate accumulation was reduced by the ingestion of antioxidants [27,44]. These results indicate enhanced work efficiency during the sprints performed after the intake of antioxidants.
2.7. Role of metabolite accumulation in the regulation of mitochondrial respiration during sprint exercise
We hypothesized that during short sprints, the mitochondria do not generate more ATP, likely due to insufficient stimulation of the respiratory rate [11,16]. Some exercise is required for ADP and Pi to increase and accumulate to stimulate mitochondrial respiration [33]. However, during 30-s all-out sprints metabolite accumulation, including ADP, pyruvate, lactate, H+, and Pi approaches maximal levels [11,75]. Sarcoplasmic ADP should be transported to the mitochondrial matrix in a process linked to the proton gradient. Although adenine nucleotide transport is rapid, it may be the rate-limiting step in cellular respiration [76].
Despite the abundance of ADP, VO2 only reaches 80% of VO2max during the sprint [13,25,26]. Besides, the high glycolytic rate reached during sprint exercise [25,59,77] and the subsequent acidification of the muscle fibers could limit OXPHOS [78,79].
Acidification is expected to reduce the glycolytic rate by slowing glycogenolysis at the level of the glycogen phosphorylase a (active form) and glycolysis at the level of phosphofructokinase [80]. Although lactate is a potent antioxidant at physiological pH, due to its scavenging activity toward both superoxide and •OH [81], the accompanying H+ accelerates the rate of dismutation of O2−• to H2O2, which can react with Fe2+ to produce •OH. In addition, acidosis facilitates the conversion of O2−• to the more reactive and more liposoluble hydroperoxyl radical HO2• and facilitates the dissociation of protein-bound iron [79] (iron is also a strong oxidant). Nevertheless, the administration of antioxidants has no effect on VO2 during high-intensity exercise [82].
To find out whether acidosis and metabolite accumulation limits OXPHOS we asked some volunteers to perform an incremental exercise to exhaustion. Right at exhaustion, we occluded the circulation of both legs during 10-s or 60-s in separate trials. At the end of the occlusion period, the circulation was re-established, and the subjects performed a 10-s all-out sprint, with VO2 reaching values 2 to 3-fold higher in these two sprints than observed in a control sprint in the rested condition [11]. During the occlusion, muscle O2 stores were depleted, phosphocreatine (PCr) further reduced, while the glycolysis remained active eliciting a progressive accumulation of lactate and further acidification [11]. Actually, after 1 min of occlusion, muscle lactate was increased by 25% and pH further reduced. Despite the increased acidification, VO2 was much larger than observed when the sprint was performed in optimal conditions, i.e., 5 min after a standardized warm-up [11]. In follow-up experiments, we have seen that VO2max can be reached when supramaximal exercise is performed right after a cycle of exhaustion followed by 10–60 s of occlusion of the circulation. This indicates that acidosis and sarcoplasmic Pi accumulation to levels above those that can be reached at exhaustion under physiological conditions, do not prevent the attainment of VO2max [71,83]. Consequently, the potential in vivo inhibitory effect of acidosis or sarcoplasmic Pi accumulation on OXPHOS in skeletal is likely counteracted by other mechanisms such as increased ADP stimulation and increased muscle temperature.
Moreover, the combination of fatiguing sprinting exercise followed by occlusion and reperfusion is expected to cause substantial RONS production [71,83]. Nevertheless, even in these conditions, skeletal muscles can reach VO2max. Therefore, these experiments in humans demonstrate that OXPHOS is not inhibited by the level of acidification, Pi accumulation, and RONS production seen in working human muscles, or at least the inhibition caused is not sufficient to negatively influence VO2max. This is possible because the human skeletal muscle has a large functional reserve in mitochondrial respiratory capacity [84] or other mechanisms compensate for the potential adverse effects of acidosis, Pi, and RONS.
2.8. Role of ADP transport from the sarcoplasm to the mitochondrial matrix
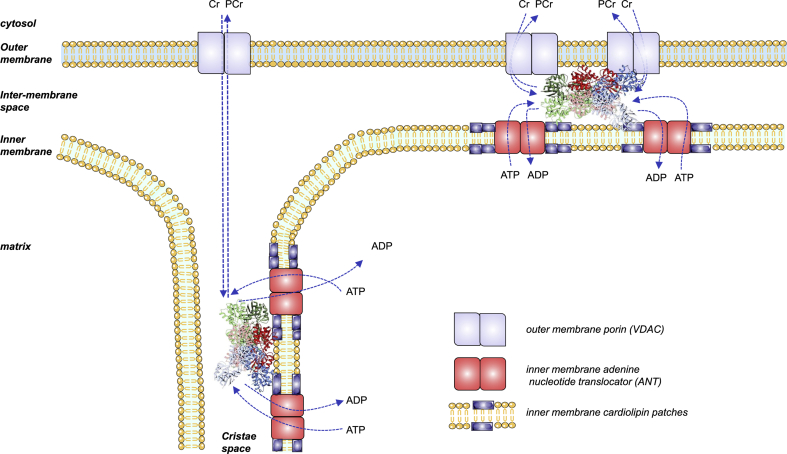
ADP, being the substrate for the synthesis of ATP, stimulates both the glycolysis [22] and OXPHOS [23]. Therefore, the transport of ADP from the sarcoplasm to the mitochondrial matrix is likely to determine the relative contribution of glycolytic and aerobic energy pathways to the resynthesis of ATP during high-intensity exercise. Three carrier protein complexes transport ADP from the sarcoplasm to the mitochondrial matrix: 1) the voltage-dependent anion channel (VDAC or porin) located on the outer mitochondrial membrane (OMM) [85]; 2) the mitochondrial creatine kinase (MtCK) [85]; and the adenine nucleotide translocase (ANT) [[86], [87], [88]] localized in cristae and intermembrane space [89]. MtCK increases the concentration of ADP within the intermembrane mitochondrial space (IMS) [90], where it is exchanged with ATP by the ANT. Since the OMM has a high permeability to PCr and Cr [91], both become rapidly available to the MtCK, which can use the ATP received from the ANT and the Cr diffusing from the sarcoplasm [92,93]. The rate of Cr and Pi supply is maximal during all-out sprints, however PCr is depleted within a few seconds after the start of the sprint [75]. This is due to the limitation imposed by the rate of ATP supply by the ANT since during high glycolytic rates, or when O2 is not available, there is no anaerobic resynthesis of PCr [11,94,95]. Due to spatial constraints, ADP and ATP tend to accumulate in IMS of the cristae in the vicinity of MtCK, facilitating the rapid resynthesis of ATP [96]. The ATP flowing through ANT is rapidly hydrolyzed to ADP, which is introduced in the matrix by ANT. At the same time, MtCK catalyzes the synthesis of PCr with the ATP provided by ANT, using the Pi and Cr available in the IMS. The resulting PCr is exported to the sarcoplasm by VDAC (Fig. 3).
Fig. 3.
ADP/ATP exchange in the mitochondria. Mitochondrial creatine kinase (MtCK) forms proteolipid complexes with VDAC and ANT (‘‘contact site complexes’’, top right) or with ANT alone (‘‘cristae complexes’’, bottom left). ADP and ATP tend to accumulate in the intermembrane mitochondrial space (IMS) of the cristae in the vicinity of MtCK, facilitating the rapid resynthesis of ATP. The ATP flowing through ANT is rapidly hydrolyzed to ADP, which is sent back to the matrix by ANT. MtCK catalyzes the synthesis of PCr with the ATP provided by ANT, using the Pi and Cr available in IMS. The resulting PCr is exported to the sarcoplasm by VDAC. Modified from Schlattner et al. [96].
VDAC forms an unspecific channel whose permeability is regulated by Ca2+ [97] and ROS [98,99]. VDAC can be found in contact sites between the inner and outer mitochondrial membranes forming proteolipid complexes containing adenine nucleotide translocase in the IMM, and VDAC in the OMM [100], which also can recruit additional proteins such as kinases that preferentially use mitochondrial ATP, including MtCK [96]. The interactions between MtCK and VDAC are stimulated by Ca2+, which interacts with VDAC in OMM [97], facilitating metabolite channeling and energy provision when sarcoplasmic Ca2+ increases, as during high-intensity exercise. Mitochondrial creatine kinase is particularly sensitive to oxidative damage, resulting in an impairment of metabolite channeling through the creatine phosphate shuttle [101]. The latter could, in theory, limit the aerobic contribution to the overall energy production [102]. Although the physiological relevance of this mechanism during exercise remains unknown, it is clinically meaningful, because ischemic preconditioning exerts its protective effects by preserving mitochondrial function and functional coupling between adenine nucleotide translocase and creatine kinase [103,104].
ANT activity can be regulated through tyrosine phosphorylation [105], lysine acetylation [106], glutathionylation [107], carbonylation [108], and nitrosylation [99], although the role of these modifications in vivo has not been established. For example, ANT acetylation is reduced after high-intensity exercise, which may facilitate the exchange flux of ADP/ATP [106]. Thus, most scientific evidence supports a crucial role of ANT in regulating mitochondrial respiration during exercise, and especially during sprint exercise, by establishing the flux rate of ADP from the sarcoplasm to the matrix.
3. Regulation of mitochondrial efficiency
Mitochondrial efficiency is defined here as the P/O ratio (ATP produced per O2 atom reduced by the respiratory chain), whose value may vary between tissues and whose calculation is extremely complex and prone to methodological errors [109]. The P/O ratio may be reduced by proton leak, proton slip, energy expended in substrate transport, and physiological mechanisms of uncoupling (see Hinkle et al. [109] for a review). The only substrates that use the ΔΨ during mitochondrial uptake are ADP plus Pi (in exchange for ATP) and glutamate (in exchange for aspartate) [109]. The single supplement for which there is experimental evidence indicating an improvement of the P/O ratio in humans is supplementation with nitrate, which is reduced to nitrite by commensal bacteria in the oral cavity [110]. Nitrite is then absorbed and further reduced to nitric oxide by nitrite reductases [111].
In skeletal muscle, NO is produced by nitric oxide synthases (NOS) that catalyze the conversion of l-arginine to l-citrulline and have an essential role in exercise-induced muscle signaling [112] and fatigue [113]. Neuronal (nNOS or NOS-1) and endothelial (eNOS or NOS-3) NOS are activated by the interaction of Ca2+ with calmodulin, and the expression of both NOS enzymes is upregulated by exercise training [[114], [116], [115]]. Nitric oxide may cause reversible inhibition of cytochrome c oxidase [117,118]. However, the physiological relevance in vivo remains unclear [119], since humans reach similar whole-body VO2peak values and performance during supramaximal exercise (105% VO2max) after the infusion of -L-NAME (a NOS inhibitor) [120].
Dietary nitrates, abundant in leafy vegetables, have been shown to increase the P/O ratio during state 4 respiration in isolated mitochondria, as well as the maximal rate of ATP production in human skeletal muscle fibers, although without effects during state 3 respiration [111]. Larsen et al. tested the same subjects during submaximal exercise and found a 3% lower VO2 (from 1.95 to 1.89 mL min−1) after three days on nitrate supplementation. Nevertheless, about one-third of the effects on VO2 could be attributed to a shift in substrate oxidation to carbohydrates, since RER was increased (placebo 0.883 ± 0.01 versus nitrate 0.914 ± 0.01) [111]. The improved mitochondrial efficiency was explained by a reduced leakage or slippage of protons across the inner mitochondrial membrane in part due to the reduced expression of ANT, which is considered a significant contributor to proton leak [87]. Nitrate supplementation has also been reported to reduce VO2max by 2.7% [121], an effect that could be explained by the inhibitory actions of NO on cytochrome c [117,118].
3.1. Cytochrome C oxidase (COX) and mitochondrial efficiency
Cytochrome C Oxidase (COX), also called Complex IV, is a transmembrane protein complex of the inner mitochondrial membrane [122]. It is the last step in the electron transport chain, and its principal function is to catalyze the reduction of O2 to water. Every COX protein is composed of fourteen subunits that combine differently to conform distinct isoforms of the enzyme [123], whose distribution has tissue specificity. COX IV-1 is ubiquitously expressed and is regulated by allosteric inhibition by a high ATP/ADP ratio [123]. COX IV-2 is expressed mainly in the lungs, fetal skeletal muscle, and neurons [123]. COX IV-2 expression decreases cell respiration and enhances metabolic efficiency [124]. Overexpression of COX IV-2 in primary human myotubes reduces respiration and H2O2 production [124]. In humans, COX IV-2 protein expression in mitochondrial skeletal muscle correlates negatively with the resting metabolic rate [124]. Little is known regarding the effects that training may have on the regulation of COX isoforms.
4. Sexual dysmorphism in mitochondrial respiratory control
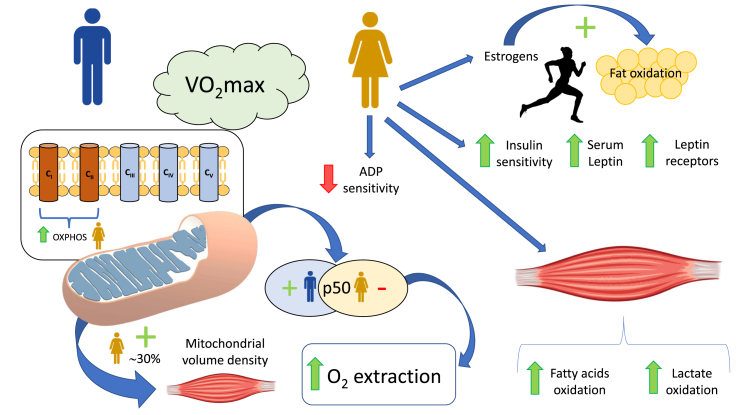
In resting conditions and during the post-absorptive state, women tend to incorporate non-esterified fatty acids (NEFAs) into triacylglycerols to store fat. In contrast, men are more prone to produce energy through plasma NEFA oxidation [125]. As a consequence, women display increased intramyocellular lipid content [126]. During exercise, estrogens promote fat oxidation [127], and at the same relative exercise intensity, women obtain a higher fraction of the energy from the oxidation of fatty acids than men [[128], [129], [130], [131]]. Several structural, functional, and endocrine factors explain this sexual dimorphism in energy metabolism [125,132,133]. In addition to the higher circulating estrogens, women have higher serum leptin concentrations and more leptin receptors in their skeletal muscles [134] and higher insulin sensitivity [125].
A recent study using permeabilized fibers from eight men and women has found that women have similar maximal respiration rates and abundance of ANT, but lower mitochondrial ADP sensitivity (+30% apparent Km) and absolute respiration rates at a physiologically relevant ADP concentration (100 μM) than men [133]. A ~3-fold reduction of skeletal muscle ADP sensitivity has been observed in endurance trained humans [135], and by analogy the lower ADP sensitivity observed in women compared to men could be advantageous for prolonged exercise. Miotto et al. [133] found higher sensitivity to malonyl-CoA-mediated respiratory inhibition and more abundance of the fatty acid transporter CD36 in women. At the same time, no sex differences in carnitine palmitoyl transferase-I protein content- and palmitoyl-CoA-supported respiration were observed.
In 12 men and 12 women matched by VO2max and exercise performance, a ~30% higher mitochondrial volume density (MitoVD) was observed in female vastus lateralis muscle [132]. The latter was accompanied by increased whole-body fat utilization during submaximal exercise compared with males, which was associated with an increased oxidative capacity for fatty acids and lactate in the permeabilized fibers. However, these differences in whole-body fat oxidation and skeletal muscle variables disappeared when adjusted for body mass or leg mass, which were lower in females [132]. More recently, Cardinale et al. [136] found higher CI, CII, and OXPHOS (CI + CII) respiratory capacity (normalized to the amount of mitochondrial protein content) in women than men also matched by VO2max. In the same study, women displayed lower mitochondrial p50 (higher oxygen affinity) than men [136], which has been associated with enhanced O2 extraction capacity [137] (Fig. 4).
Fig. 4.
Schematic representation of sexual dysmorphisms in mitochondrial function. Higher circulating estrogens promote a greater contribution from fat oxidation to the overall energy expenditure in women than in men at the same relative exercise intensity. Compared to men, women exhibit higher serum leptin concentrations, higher insulin sensitivity, and more leptin receptors in muscle, but less ADP sensitivity. Experiments with permeabilized fiber indicate that women skeletal muscle fibers have increased maximal capacity to oxidize fatty acids and lactate. Women display lower mitochondrial p50 (increased mitochondrial oxygen affinity), and greater mitochondrial volume density, respiratory capacity (OXPHOS) and enhanced O2 extraction capacity when compared with men matched by VO2max. ADP = adenosine diphosphate; O2 = oxygen; OXPHOS = mitochondrial oxidative phosphorylation system; p50 = calculated oxygen pressure at 50% of the maximum flux; VO2max = maximal oxygen uptake.
5. Conclusions
At low exercise intensities, ATP demand is matched by aerobic ATP resynthesis via oxidative phosphorylation. However, during sprint exercise, muscle ATP demand may increase more than 100-fold the resting level, exceeding the maximal in vivo mitochondrial ATP production by 2-3-fold in humans. The rate of mitochondrial respiration depends on the availability of O2, carbon substrates, reducing equivalents, ADP, Pi and Ca2+, and may be modulated by acidosis, nitric oxide, and reactive oxygen and nitrogen species. The relatively low mitochondrial respiratory rates observed during sprint exercise in humans are likely due to suboptimal stimulation by ADP, linked to insufficient ADP/ATP ANT antiport activity. Recent in vitro studies with isolated skeletal muscle mitochondria tested in conditions mimicking different exercise intensities indicate that ROS production during aerobic exercise is 1-2 orders of magnitude lower than previously thought. Mitochondrial efficiency is impaired by ROS and improved by nitrate supplementation, through the reduction of leakage or slippage of protons across the inner mitochondrial membrane. The latter has been attributed to a lower expression of the adenine nucleotide translocase and some reversible inhibition of cytochrome c oxidase. This may cause a small reduction (2–3%) of submaximal and maximal VO2. A sexual dimorphism exists in mitochondrial respiratory control with women displaying larger mitochondrial volume density, higher capacity to oxidize fat and sensitivity to malonyl-CoA-mediated respiratory inhibition, more abundance of the fatty acid transporter CD36, and lower mitochondrial ADP sensitivity. The mitochondrial p50 is also lower (increased affinity for O2) in women contributing to an enlarged O2 extraction capacity compared to men.
Disclosure summary
The authors have nothing to disclose.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financed by grants from the Ministerio de Economía y Competitividad (PI14/01509, DEP2017-86409-C2-1-P, and DEP2015-71171-R and FEDER) and ACIISI (ProID2017010106). The authors would like to express their gratitude to Tobias Lopez Jessen for proofreading the final version of the manuscript.
References
- 1.Gray M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012;4(9) doi: 10.1101/cshperspect.a011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 3.Vercesi A.E., Castilho R.F., Kowaltowski A.J., de Oliveira H.C.F., de Souza-Pinto N.C., Figueira T.R. Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radic. Biol. Med. 2018;129:1–24. doi: 10.1016/j.freeradbiomed.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves R.L., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015;290(1):209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sastre J., Asensi M., Gasco E., Pallardo F.V., Ferrero J.A., Furukawa T. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am. J. Physiol. 1992;263(5 Pt 2):R992–R995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- 8.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C., Pallardo F.V. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Alamo D., Losa-Reyna J., Torres-Peralta R., Martin-Rincon M., Perez-Valera M., Curtelin D. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015;593(20):4631–4648. doi: 10.1113/JP270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calbet J.A., Chavarren J., Dorado C. Fractional use of anaerobic capacity during a 30- and a 45-s Wingate test. Eur. J. Appl. Physiol. 1997;76(4):308–313. doi: 10.1007/s004210050253. [DOI] [PubMed] [Google Scholar]
- 13.Calbet J.A., De Paz J.A., Garatachea N., Cabeza De Vaca S., Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. 2003;94(2):668–676. doi: 10.1152/japplphysiol.00128.2002. [DOI] [PubMed] [Google Scholar]
- 14.Allen D.G., Lamb G.D., Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 15.Westerblad H., Allen D.G. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxidants Redox Signal. 2011;15(9):2487–2499. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 16.Calbet J.A., Losa-Reyna J., Torres-Peralta R., Rasmussen P., Ponce-Gonzalez J.G., Sheel A.W. Limitations to oxygen transport and utilization during sprint exercise in humans: evidence for a functional reserve in muscle O2 diffusing capacity. J. Physiol. 2015;593(20):4649–4664. doi: 10.1113/JP270408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen T.H., Clausen T., Nielsen O.B. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and beta2-agonist. J. Physiol. 2003;551(Pt 1):277–286. doi: 10.1113/jphysiol.2003.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starkov A.A., Fiskum G., Chinopoulos C., Lorenzo B.J., Browne S.E., Patel M.S. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tretter L., Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24(36):7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakimoto P.A., Tamaki F.K., Cardoso A.R., Marana S.R., Kowaltowski A.J. H2O2 release from the very long chain acyl-CoA dehydrogenase. Redox Biol. 2015;4:375–380. doi: 10.1016/j.redox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T.F., Davis E.J. Regulation of glycolytic flux in an energetically controlled cell-free system: the effects of adenine nucleotide ratios, inorganic phosphate, pH, and citrate. Arch. Biochem. Biophys. 1981;209(1):85–99. doi: 10.1016/0003-9861(81)90260-5. [DOI] [PubMed] [Google Scholar]
- 23.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 1955;217(1):383–393. [PubMed] [Google Scholar]
- 24.Nanadikar M.S., Vergel Leon A.M., Borowik S., Hillemann A., Zieseniss A., Belousov V.V. O2 affects mitochondrial functionality ex vivo. Redox Biol. 2019;22 doi: 10.1016/j.redox.2019.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales-Alamo D., Ponce-Gonzalez J.G., Guadalupe-Grau A., Rodriguez-Garcia L., Santana A., Cusso M.R. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKalpha phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J. Appl. Physiol. 2012;113(6):917–928. doi: 10.1152/japplphysiol.00415.2012. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Alamo D., Ponce-Gonzalez J.G., Guadalupe-Grau A., Rodriguez-Garcia L., Santana A., Cusso R. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKalpha phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013;114(5):566–577. doi: 10.1152/japplphysiol.01246.2012. [DOI] [PubMed] [Google Scholar]
- 27.Morales-Alamo D., Guerra B., Santana A., Martin-Rincon M., Gelabert-Rebato M., Dorado C. Skeletal muscle pyruvate dehydrogenase phosphorylation and lactate accumulation during sprint exercise in normoxia and severe acute hypoxia: effects of antioxidants. Front. Physiol. 2018;9:188. doi: 10.3389/fphys.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howlett R.A., Heigenhauser G.J., Spriet L.L. Skeletal muscle metabolism during high-intensity sprint exercise is unaffected by dichloroacetate or acetate infusion. J. Appl. Physiol. 1999;87(5):1747–1751. doi: 10.1152/jappl.1999.87.5.1747. [DOI] [PubMed] [Google Scholar]
- 29.Greenhaff P.L., Campbell-O'Sullivan S.P., Constantin-Teodosiu D., Poucher S.M., Roberts P.A., Timmons J.A. An acetyl group deficit limits mitochondrial ATP production at the onset of exercise. Biochem. Soc. Trans. 2002;30(2):275–280. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 30.Roberts P.A., Loxham S.J., Poucher S.M., Constantin-Teodosiu D., Greenhaff P.L. The acetyl group deficit at the onset of contraction in ischaemic canine skeletal muscle. J. Physiol. 2002;544(2):591–602. doi: 10.1113/jphysiol.2002.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marwood S., Constantin-Teodosiu D., Casey E., Whyte M., Boobis L., Bowtell J. No acetyl group deficit is evident at the onset of exercise at 90% of maximal oxygen uptake in humans. J. Sports Sci. 2010;28(3):267–279. doi: 10.1080/02640410903440884. [DOI] [PubMed] [Google Scholar]
- 32.Bangsbo J., Gibala M.J., Krustrup P., Gonzalez-Alonso J., Saltin B. Enhanced pyruvate dehydrogenase activity does not affect muscle O2 uptake at onset of intense exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282(1):R273–R280. doi: 10.1152/ajpregu.2002.282.1.R273. [DOI] [PubMed] [Google Scholar]
- 33.Savasi I., Evans M.K., Heigenhauser G.J., Spriet L.L. Skeletal muscle metabolism is unaffected by DCA infusion and hyperoxia after onset of intense aerobic exercise. Am. J. Physiol. Endocrinol. Metab. 2002;283(1):E108–E115. doi: 10.1152/ajpendo.00337.2001. [DOI] [PubMed] [Google Scholar]
- 34.Sahlin K., Katz A., Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 1990;259(5 Pt 1):C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- 35.Gibala M.J., MacLean D.A., Graham T.E., Saltin B. Anaplerotic processes in human skeletal muscle during brief dynamic exercise. J. Physiol. 1997;502(Pt 3):703–713. doi: 10.1111/j.1469-7793.1997.703bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunniffe B., Papageorgiou M., O'Brien B., Davies N.A., Grimble G.K., Cardinale M. Acute citrulline-malate supplementation and high-intensity cycling performance. J. Strength Condit Res. 2016;30(9):2638–2647. doi: 10.1519/JSC.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 37.Balaban R.S. The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta. 2009;1787(11):1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozcan L., Wong C.C., Li G., Xu T., Pajvani U., Park S.K. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metabol. 2012;15(5):739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacchetto R., Bovo E., Salviati L., Damiani E., Margreth A. Glycogen synthase binds to sarcoplasmic reticulum and is phosphorylated by CaMKII in fast-twitch skeletal muscle. Arch. Biochem. Biophys. 2007;459(1):115–121. doi: 10.1016/j.abb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Singh P., Salih M., Leddy J.J., Tuana B.S. The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J. Biol. Chem. 2004;279(34):35176–35182. doi: 10.1074/jbc.M402282200. [DOI] [PubMed] [Google Scholar]
- 41.Minchenko O., Opentanova I., Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1-4) expression in vivo. FEBS Lett. 2003;554(3):264–270. doi: 10.1016/s0014-5793(03)01179-7. [DOI] [PubMed] [Google Scholar]
- 42.Almeida A., Moncada S., Bolanos J.P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6(1):45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 43.Sanders M.J., Grondin P.O., Hegarty B.D., Snowden M.A., Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales-Alamo D., Guerra B., Ponce-Gonzalez J.G., Guadalupe-Grau A., Santana A., Martin-Rincon M. Skeletal muscle signaling, metabolism, and performance during sprint exercise in severe acute hypoxia after the ingestion of antioxidants. J. Appl. Physiol. 2017;123(5):1235–1245. doi: 10.1152/japplphysiol.00384.2017. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri L., Pardo B., Lasorsa F.M., del Arco A., Kobayashi K., Iijima M. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20(18):5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.del Arco A., Satrustegui J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J. Biol. Chem. 1998;273(36):23327–23334. doi: 10.1074/jbc.273.36.23327. [DOI] [PubMed] [Google Scholar]
- 47.del Arco A., Satrustegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004;279(23):24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 48.Satrustegui J., Pardo B., Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 2007;87(1):29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 49.Gellerich F.N., Gizatullina Z., Trumbeckaite S., Nguyen H.P., Pallas T., Arandarcikaite O. The regulation of OXPHOS by extramitochondrial calcium. Biochim. Biophys. Acta. 2010;1797(6–7):1018–1027. doi: 10.1016/j.bbabio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Pardo B., Contreras L., Serrano A., Ramos M., Kobayashi K., Iijima M. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J. Biol. Chem. 2006;281(2):1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86(1):369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 52.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R., Cavagna D. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brand M.D., Chen C.H., Lehninger A.L. Stoichiometry of H+ ejection during respiration-dependent accumulation of Ca2+ by rat liver mitochondria. J. Biol. Chem. 1976;251(4):968–974. [PubMed] [Google Scholar]
- 55.Lehninger A.L., Reynafarje B., Vercesi A., Tew W.P. Transport and accumulation of calcium in mitochondria. Ann. N. Y. Acad. Sci. 1978;307:160–176. doi: 10.1111/j.1749-6632.1978.tb41941.x. [DOI] [PubMed] [Google Scholar]
- 56.Lehninger A.L. Role of phosphate and other proton-donating anions in respiration-coupled transport of Ca2+ by mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1974;71(4):1520–1524. doi: 10.1073/pnas.71.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connett R.J. Why is there a delay in the increased oxygen consumption during the rest-work transition in skeletal muscle? Adv. Exp. Med. Biol. 1986;194:271–281. doi: 10.1007/978-1-4684-5107-8_20. [DOI] [PubMed] [Google Scholar]
- 58.Vercesi A., Reynafarje B., Lehninger A.L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J. Biol. Chem. 1978;253(18):6379–6385. [PubMed] [Google Scholar]
- 59.Parolin M.L., Chesley A., Matsos M.P., Spriet L.L., Jones N.L., Heigenhauser G.J. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. 1999;277(5 Pt 1):E890–E900. doi: 10.1152/ajpendo.1999.277.5.E890. [DOI] [PubMed] [Google Scholar]
- 60.Anmann T., Guzun R., Beraud N., Pelloux S., Kuznetsov A.V., Kogerman L. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim. Biophys. Acta. 2006;1757(12):1597–1606. doi: 10.1016/j.bbabio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Morales-Alamo D., Calbet J.A. Free radicals and sprint exercise in humans. Free Radic. Res. 2014;48(1):30–42. doi: 10.3109/10715762.2013.825043. [DOI] [PubMed] [Google Scholar]
- 62.Wong H.S., Dighe P.A., Mezera V., Monternier P.A., Brand M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017;292(41):16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Cabrera M.C., Borras C., Pallardo F.V., Sastre J., Ji L.L., Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005;567(Pt 1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson T., Kabayo T., Ng R., Chamberlain J., McArdle A., Jackson M.J. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0096378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Meo S., Venditti P. Mitochondria in exercise-induced oxidative stress. Biol. Signals Recept. 2001;10(1–2):125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 66.Nilsson A., Bjornson E., Flockhart M., Larsen F.J., Nielsen J. Complex I is bypassed during high intensity exercise. Nat. Commun. 2019;10(1):5072. doi: 10.1038/s41467-019-12934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabrera-Orefice A., Yoga E.G., Wirth C., Siegmund K., Zwicker K., Guerrero-Castillo S. Locking loop movement in the ubiquinone pocket of complex I disengages the proton pumps. Nat. Commun. 2018;9(1):4500. doi: 10.1038/s41467-018-06955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drose S., Stepanova A., Galkin A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta. 2016;1857(7):946–957. doi: 10.1016/j.bbabio.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsen F.J., Schiffer T.A., Ortenblad N., Zinner C., Morales-Alamo D., Willis S.J. High-intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. Faseb. J. 2016;30:417–427. doi: 10.1096/fj.15-276857. [DOI] [PubMed] [Google Scholar]
- 70.Place N., Ivarsson N., Venckunas T., Neyroud D., Brazaitis M., Cheng A.J. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. U. S. A. 2015;112(50):15492–15497. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gelabert-Rebato M., Wiebe J.C., Martin-Rincon M., Gericke N., Perez-Valera M., Curtelin D. Mangifera indica L. Leaf extract in combination with Luteolin or Quercetin enhances VO2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018;9:740. doi: 10.3389/fphys.2018.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constantin-Teodosiu D. Regulation of muscle pyruvate dehydrogenase complex in insulin resistance: effects of exercise and dichloroacetate. Diabetes Metabol. J. 2013;37(5):301–314. doi: 10.4093/dmj.2013.37.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher-Wellman K.H., Gilliam L.A.A., Lin C.T., Cathey B.L., Lark D.S., Darrell Neufer P. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic. Biol. Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher-Wellman K.H., Lin C.T., Ryan T.E., Reese L.R., Gilliam L.A., Cathey B.L. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem. J. 2015;467(2):271–280. doi: 10.1042/BJ20141447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bogdanis G.C., Nevill M.E., Boobis L.H., Lakomy H.K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J. Appl. Physiol. 1996;80(3):876–884. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 76.LaNoue K.F., Schoolwerth A.C. Metabolite transport in mitochondria. Annu. Rev. Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- 77.Siesjo B.K., Bendek G., Koide T., Westerberg E., Wieloch T. Influence of acidosis on lipid peroxidation in brain tissues in vitro. J. Cerebr. Blood Flow Metabol. 1985;5(2):253–258. doi: 10.1038/jcbfm.1985.32. [DOI] [PubMed] [Google Scholar]
- 78.Dean J.B. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J. Appl. Physiol. 2010;108(6):1786–1795. doi: 10.1152/japplphysiol.01337.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernheim F. Biochemical implications of pro-oxidants and antioxidants. Radiat. Res. 1963;(Suppl 3):17–32. [PubMed] [Google Scholar]
- 80.Hollidge-Horvat M.G., Parolin M.L., Wong D., Jones N.L., Heigenhauser G.J. Effect of induced metabolic acidosis on human skeletal muscle metabolism during exercise. Am. J. Physiol. 1999;277(4 Pt 1):E647–E658. doi: 10.1152/ajpendo.1999.277.4.E647. [DOI] [PubMed] [Google Scholar]
- 81.Groussard C., Morel I., Chevanne M., Monnier M., Cillard J., Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J. Appl. Physiol. 2000;89(1):169–175. doi: 10.1152/jappl.2000.89.1.169. [DOI] [PubMed] [Google Scholar]
- 82.Bailey S.J., Winyard P.G., Blackwell J.R., Vanhatalo A., Lansley K.E., Dimenna F.J. Influence of N-acetylcysteine administration on pulmonary O(2) uptake kinetics and exercise tolerance in humans. Respir. Physiol. Neurobiol. 2011;175(1):121–129. doi: 10.1016/j.resp.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Gelabert-Rebato M., Wiebe J.C., Martin-Rincon M., Galvan-Alvarez V., Curtelin D., Perez-Valera M. Enhancement of exercise performance by 48 hours, and 15-day supplementation with Mangiferin and Luteolin in men. Nutrients. 2019;11(2):344. doi: 10.3390/nu11020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boushel R., Gnaiger E., Calbet J.A., Gonzalez-Alonso J., Wright-Paradis C., Sondergaard H. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion. 2011;11(2):303–307. doi: 10.1016/j.mito.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Guzun R., Gonzalez-Granillo M., Karu-Varikmaa M., Grichine A., Usson Y., Kaambre T. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within Mitochondrial Interactosome. Biochim. Biophys. Acta. 2012;1818(6):1545–1554. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 86.Miotto P.M., Holloway G.P. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport. Biochem. J. 2016;473(18):2831–2843. doi: 10.1042/BCJ20160373. [DOI] [PubMed] [Google Scholar]
- 87.Brand M.D., Pakay J.L., Ocloo A., Kokoszka J., Wallace D.C., Brookes P.S. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005;392(Pt 2):353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dummler K., Muller S., Seitz H.J. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem. J. 1996;317(Pt 3):913–918. doi: 10.1042/bj3170913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kottke M., Wallimann T., Brdiczka D. Dual electron microscopic localization of mitochondrial creatine kinase in brain mitochondria. Biochem. Med. Metab. Biol. 1994;51(2):105–117. doi: 10.1006/bmmb.1994.1015. [DOI] [PubMed] [Google Scholar]
- 90.Saks V.A., Kuznetsov A.V., Kupriyanov V.V., Miceli M.V., Jacobus W.E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J. Biol. Chem. 1985;260(12):7757–7764. [PubMed] [Google Scholar]
- 91.Wallimann T., Tokarska-Schlattner M., Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bessman S.P., Geiger P.J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 93.Jacobus W.E., Lehninger A.L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J. Biol. Chem. 1973;248(13):4803–4810. [PubMed] [Google Scholar]
- 94.Yoshida T., Watari H. Effect of circulatory occlusion on human muscle metabolism during exercise and recovery. Eur. J. Appl. Physiol. Occup. Physiol. 1997;75(3):200–205. doi: 10.1007/s004210050148. [DOI] [PubMed] [Google Scholar]
- 95.Harris R.C., Hultman E., Kaijser L., Nordesjo L.O. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand. J. Clin. Lab. Invest. 1975;35(1):87–95. [PubMed] [Google Scholar]
- 96.Schlattner U., Tokarska-Schlattner M., Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta. 2006;1762(2):164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Gincel D., Zaid H., Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358(Pt 1):147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martel C., Wang Z., Brenner C. VDAC phosphorylation, a lipid sensor influencing the cell fate. Mitochondrion. 2014;19(Pt A):69–77. doi: 10.1016/j.mito.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 99.Chang A.H., Sancheti H., Garcia J., Kaplowitz N., Cadenas E., Han D. Respiratory substrates regulate S-nitrosylation of mitochondrial proteins through a thiol-dependent pathway. Chem. Res. Toxicol. 2014;27(5):794–804. doi: 10.1021/tx400462r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beutner G., Ruck A., Riede B., Brdiczka D. Complexes between hexokinase, mitochondrial porin and adenylate translocator in brain: regulation of hexokinase, oxidative phosphorylation and permeability transition pore. Biochem. Soc. Trans. 1997;25(1):151–157. doi: 10.1042/bst0250151. [DOI] [PubMed] [Google Scholar]
- 101.Koufen P., Stark G. Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim. Biophys. Acta. 2000;1501(1):44–50. doi: 10.1016/s0925-4439(00)00005-3. [DOI] [PubMed] [Google Scholar]
- 102.Steeghs K., Benders A., Oerlemans F., de Haan A., Heerschap A., Ruitenbeek W. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89(1):93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 103.Kancirova I., Jasova M., Murarikova M., Sumbalova Z., Ulicna O., Ravingerova T. Cardioprotection induced by remote ischemic preconditioning preserves the mitochondrial respiratory function in acute diabetic myocardium. Physiol. Res. 2016;65(Supplementum 5):S611–S619. doi: 10.33549/physiolres.933533. [DOI] [PubMed] [Google Scholar]
- 104.Laclau M.N., Boudina S., Thambo J.B., Tariosse L., Gouverneur G., Bonoron-Adele S. Cardioprotection by ischemic preconditioning preserves mitochondrial function and functional coupling between adenine nucleotide translocase and creatine kinase. J. Mol. Cell. Cardiol. 2001;33(5):947–956. doi: 10.1006/jmcc.2001.1357. [DOI] [PubMed] [Google Scholar]
- 105.Feng J., Zhu M., Schaub M.C., Gehrig P., Roschitzki B., Lucchinetti E. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc. Res. 2008;80(1):20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- 106.Mielke C., Lefort N., McLean C.G., Cordova J.M., Langlais P.R., Bordner A.J. Adenine nucleotide translocase is acetylated in vivo in human muscle: modeling predicts a decreased ADP affinity and altered control of oxidative phosphorylation. Biochemistry. 2014;53(23):3817–3829. doi: 10.1021/bi401651e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aniya Y., Imaizumi N. Mitochondrial glutathione transferases involving a new function for membrane permeability transition pore regulation. Drug Metab. Rev. 2011;43(2):292–299. doi: 10.3109/03602532.2011.552913. [DOI] [PubMed] [Google Scholar]
- 108.Yan L.J., Sohal R.S. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. U. S. A. 1998;95(22):12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hinkle P.C. P/O ratios of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta. 2005;1706(1–2):1–11. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Lundberg J.O., Weitzberg E., Cole J.A., Benjamin N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004;2(7):593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 111.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabol. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 112.Steensberg A., Keller C., Hillig T., Frosig C., Wojtaszewski J.F., Pedersen B.K. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. Faseb. J. 2007;21(11):2683–2694. doi: 10.1096/fj.06-7477com. [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi Y.M., Rader E.P., Crawford R.W., Iyengar N.K., Thedens D.R., Faulkner J.A. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cocks M., Shaw C.S., Shepherd S.O., Fisher J.P., Ranasinghe A.M., Barker T.A. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J. Physiol. 2013;591(3):641–656. doi: 10.1113/jphysiol.2012.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoier B., Passos M., Bangsbo J., Hellsten Y. Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp. Physiol. 2013;98(2):585–597. doi: 10.1113/expphysiol.2012.067967. [DOI] [PubMed] [Google Scholar]
- 116.Huber-Abel F.A., Gerber M., Hoppeler H., Baum O. Exercise-induced angiogenesis correlates with the up-regulated expression of neuronal nitric oxide synthase (nNOS) in human skeletal muscle. Eur. J. Appl. Physiol. 2012;112(1):155–162. doi: 10.1007/s00421-011-1960-x. [DOI] [PubMed] [Google Scholar]
- 117.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 118.Poderoso J.J., Helfenberger K., Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. 2019;88:61–72. doi: 10.1016/j.niox.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 119.Grassi B., Hogan M.C., Kelley K.M., Howlett R.A., Gladden L.B. Effects of nitric oxide synthase inhibition by L-NAME on oxygen uptake kinetics in isolated canine muscle in situ. J. Physiol. 2005;568(Pt 3):1021–1033. doi: 10.1113/jphysiol.2005.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilkerson D.P., Campbell I.T., Jones A.M. Influence of nitric oxide synthase inhibition on pulmonary O2 uptake kinetics during supra-maximal exercise in humans. J. Physiol. 2004;561(Pt 2):623–635. doi: 10.1113/jphysiol.2004.071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010;48(2):342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 122.Castresana J., Lubben M., Saraste M., Higgins D.G. Evolution of cytochrome oxidase, an enzyme older than atmospheric oxygen. EMBO J. 1994;13(11):2516–2525. doi: 10.1002/j.1460-2075.1994.tb06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sinkler C.A., Kalpage H., Shay J., Lee I., Malek M.H., Grossman L.I. Tissue- and condition-specific isoforms of mammalian cytochrome c oxidase subunits: from function to human disease. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1534056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schiffer T.A., Peleli M., Sundqvist M.L., Ekblom B., Lundberg J.O., Weitzberg E. Control of human energy expenditure by cytochrome c oxidase subunit IV-2. Am. J. Physiol. Cell Physiol. 2016;311(3):C452–C461. doi: 10.1152/ajpcell.00099.2016. [DOI] [PubMed] [Google Scholar]
- 125.Tramunt B., Smati S., Grandgeorge N., Lenfant F., Arnal J.F., Montagner A. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steffensen C.H., Roepstorff C., Madsen M., Kiens B. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am. J. Physiol. Endocrinol. Metab. 2002;282(3):E634–E642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- 127.Hamadeh M.J., Devries M.C., Tarnopolsky M.A. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J. Clin. Endocrinol. Metab. 2005;90(6):3592–3599. doi: 10.1210/jc.2004-1743. [DOI] [PubMed] [Google Scholar]
- 128.Horton T.J., Pagliassotti M.J., Hobbs K., Hill J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. 1998;85(5):1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- 129.Phillips S.M., Atkinson S.A., Tarnopolsky M.A., MacDougall J.D. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol. 1993;75(5):2134–2141. doi: 10.1152/jappl.1993.75.5.2134. [DOI] [PubMed] [Google Scholar]
- 130.Blatchford F.K., Knowlton R.G., Schneider D.A. Plasma FFA responses to prolonged walking in untrained men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1985;53(4):343–347. doi: 10.1007/BF00422851. [DOI] [PubMed] [Google Scholar]
- 131.Roepstorff C., Steffensen C.H., Madsen M., Stallknecht B., Kanstrup I.L., Richter E.A. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am. J. Physiol. Endocrinol. Metab. 2002;282(2):E435–E447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- 132.Montero D., Madsen K., Meinild-Lundby A.K., Edin F., Lundby C. Sexual dimorphism of substrate utilization: differences in skeletal muscle mitochondrial volume density and function. Exp. Physiol. 2018;103(6):851–859. doi: 10.1113/EP087007. [DOI] [PubMed] [Google Scholar]
- 133.Miotto P.M., McGlory C., Holloway T.M., Phillips S.M., Holloway G.P. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314(6):R909–R915. doi: 10.1152/ajpregu.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guerra B., Fuentes T., Delgado-Guerra S., Guadalupe-Grau A., Olmedillas H., Santana A. Gender dimorphism in skeletal muscle leptin receptors, serum leptin and insulin sensitivity. PloS One. 2008;3(10) doi: 10.1371/journal.pone.0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zoll J., Sanchez H., N'Guessan B., Ribera F., Lampert E., Bigard X. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 2002;543(Pt 1):191–200. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cardinale D.A., Larsen F.J., Schiffer T.A., Morales-Alamo D., Ekblom B., Calbet J.A.L. Superior intrinsic mitochondrial respiration in women than in men. Front. Physiol. 2018;9:1133. doi: 10.3389/fphys.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cardinale D.A., Larsen F.J., Jensen-Urstad M., Rullman E., Sondergaard H., Morales-Alamo D. Muscle mass and inspired oxygen influence oxygen extraction at maximal exercise: role of mitochondrial oxygen affinity. Acta Physiol. 2019;225(1) doi: 10.1111/apha.13110. [DOI] [PubMed] [Google Scholar]