Abstract
In this paper of the special issue dedicated for the Olympics 2020, we put the light on an exciting facet of exercise-oncology, which may still be unknown to some audience. Accumulating convincing evidences show that exercise reduces cancer progression and recurrence mainly in colon and breast cancer patients. Interestingly, the positive effects of exercise on cancer outcomes were mainly observed when patients practiced vigorous exercise of 6 METs or more. At the molecular level, experimental studies highlighted that regular vigorous exercise could reduce tumor growth by driving changes in immune system, metabolism, hormones, systemic inflammation, angiogenesis and redox status. In the present review, we describe the main redox-sensitive mechanisms mediated by exercise. These redox mechanisms are of particular therapeutic interest as they may explain the emerging preclinical findings proving that the association of vigorous exercise with chemotherapy or radiotherapy improves the anti-cancer responses of both interventions. Clinical and preclinical studies converge to support the practice of exercise as an adjuvant therapy that improves cancer outcomes. The understanding of the underpinning molecular mechanisms of exercise in cancer can open new avenues to improve cancer care in patients.
Keywords: Cancer, Exercise, Skeletal muscle, Redox signaling
1. Introduction
Cancer is a complex disease that affects physical performance of patients. Advanced stages of cancer are often associated with excessive loss of skeletal muscle mass, leading to functional impairment and high risk of inactivity [1,2]. In turn, physical inactivity is a deleterious factor that aggravates cancer outcomes such as, muscle weakness and fatigue, and cancer-related mortality, through increasing chronic systemic inflammation and oxidative stress [[3], [4], [5]]. There is clearly a vicious deconditioning circle between “cancer, muscle frailty and physical inactivity” deteriorating the quality of life in cancer patients; it is, therefore, needful to disrupt this circle.
In this light, physical exercise has been proposed as an effective strategy that could be prescribed immediately after the diagnosis of cancer and throughout the period of anticancer treatment [6]. During the treatment, exercise may preserve muscle strength, prevent skeletal muscle loss and reduce anxiety. Exercise could also reduce the adverse side effects in patients undergoing chemotherapy [7], which helps them to accomplish their treatment, without interruption or reduction of sessions that are usually due to the critical physical state and fatigue of patients. After completion of anticancer treatment, exercise could optimize recovery, reduce cancer relapse and prevent the appearance of late chronic complications, for which cancer survivors are at high risk (i.e. cardiovascular diseases) [8].
The impact of exercise on physical performance is an evidence so far. However, it turned out that exercise could decrease cancer incidence, with a linear dose-response relationship in breast and colon cancer [8]. Motivated by these observations, researchers aimed to investigate the impact of exercise in patients with diagnosed cancer [9]. Emerging data from breast, colon and prostate cancer indicate that physically active patients exhibited less recurrence and mortality, highlighting a possible role for exercise in the suppression of tumor growth [10,11]. This tumor-suppressive role of exercise is supported by 64% of preclinical studies [12]. These studies identified some of the anticancer mechanisms of exercise that are mainly driven by changes in systemic inflammation, immune responses, angiogenesis, hormones, insulin sensitivity and redox status [13]; the later will be the focus of our review. The ability of exercise to affect redox signaling in cancer seems to be particularly dependent on the chosen modality and intensity of training. From this perspective, here, we describe the impact of different endurance and resistance exercise modalities on tumor progression and recurrence. We discuss exercise conditions that must be brought together to induce a positive change in intra-tumor redox signaling. This review should help to design exercise protocols with respect to the type and intensity of exercise that are suitable to induce a significant biological changes in exercise-oncology studies.
2. Exercise recommendations in cancer patients
Frequency, intensity, time and type of exercise are determinant factors to produce an effective intervention in cancer patients. The American college of sports medicine (ACSM) recommends for healthy individuals: 20-to-60 min of aerobic exercise 3-to-5 times per week at 55% VO2R and 20 repetitions of resistance exercise with an intensity corresponding to 12-to-16 rate of exertion (20 is the maximum exertion rate) 2-to-3 times per week. Cancer patients may at least practice 150 min of moderate-intensity aerobic exercise or aerobic combined to resistance exercise [14]. Flexibility or stretching is a third type of exercise that helps to maintain balance and postural stability. A stretching time of 60 s per target muscle could be practiced 2-to-3 times per week [15]. Although flexibility exercise interventions are not extensively used and well documented in cancer patients population, they appear to be safe and feasible and to improve the capacity of patients to practice exercise during home stays [16,17]. The guidelines of ACSM are similar to those of the World Health Organization (WHO), recommending a combination between aerobic and resistance exercises. In the second roundtable of ACSM held in 2018, there was sufficient evidence to conclude that specific doses of aerobic exercise, resistance exercise and combination of both types improve common cancer-related health outcomes [18].
The adherence to these guidelines is low as 10% among cancer survivors, based on the findings of two independent research groups [19,20]. Patients identified as at high risk are obese patients, men with prostate or skin cancer, women receiving multimodal therapy for breast cancer, old patients and those professionally inactive in both men and women [20,21]. The adherence to the combined guidelines correlated with a higher level of education and the absence of children living at home [22]. Patients show more engagement in either aerobic (23%) or resistance (14.5%) exercise individually, than the combination of both types [20]. They seem to prefer aerobic exercise, possibly because it is more suitable to their physical situation where fatigue and muscle weakness are current [23]. However, we must not occlude that resistance exercise is especially important to improve muscle mass and strength and, subsequently, the ability of patients to train [24]. Therefore, combination of aerobic and resistance exercises could produce the best benefits for cancer patients and survivors. Programs aiming to improve the adherence of patients to exercise combination are of particular interest. A recent study suggested that individualization of exercise intensities could improve patients’ adherence to the official guidelines [24]. Also, strategies to help meet the combined guidelines may need to promote more motivational cues, favorable behavioral regulations and reflective process for both types of exercises rather than focusing on just the type of exercise in which patients are deficient [22].
3. Vigorous exercise reduces cancer progression and recurrence and improves survival
In cancer patients receiving chemotherapy, the combination of aerobic and resistance exercises resulted in a better long-term health-related fitness compared to aerobic exercise alone [25]. Despite the positive effects of aerobic and resistance exercise combination on social physical anxiety, fatigue, physical fitness, muscle strength and lean body mass [26,27], few is known about the impact of exercise type itself on cancer progression and related mortality. A solid information comes from a meta-analysis on 22 cohort studies indicating that the practice of post-diagnostic exercise meeting the physical activity guidelines was associated with reduction in breast cancer progression, new primaries and recurrence and breast-cancer specific-death [28]. Recently, a study from USA on 2863 patients found that resistance training alone was associated with 33% lower all-cause mortality among survivors of skin cancer [29]. Another study on a larger scale included more than 370 000 patients from 11 cohort and reported that resistance training was associated with 21% and 40% lower all-cause mortality alone and when combined with endurance exercise, respectively [30]. All these data support that combination of endurance and resistance exercise could exert a synergizing effect to reduce cancer progression and mortality. However, studies performed on athletes suggest that the concurrent practice of endurance with resistance exercise could reduce the improvement in muscle strength and mass stimulated by the later [31,32]. In the absence of research comparing physical adaptations between cancer patients and athletes, we suppose that the negative interference found in athletes would be similar or even worse in patients, given their lower physical status. Therefore, for cancer patients, it could be safer to practice endurance and resistance exercises in separated sessions during the same week rather than combining both exercises in a single session; this may minimize the risk of negative interference between both types of exercise.
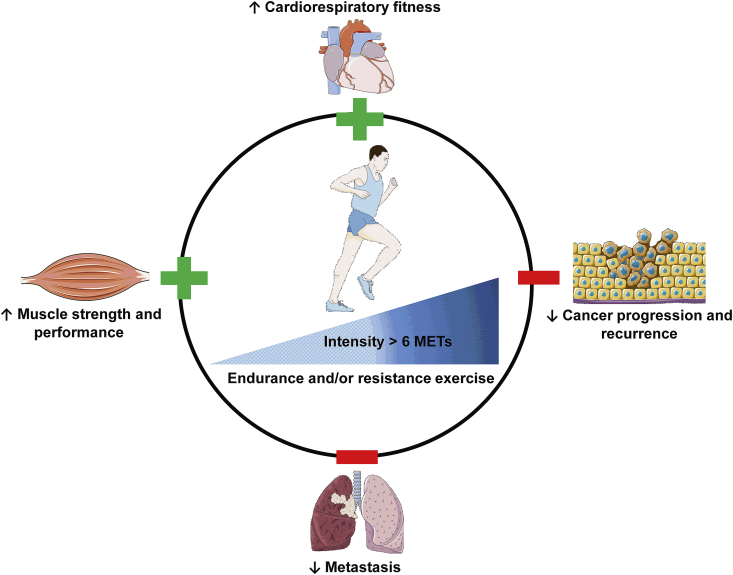
Literature abounds of studies addressing the impact of exercise volume or intensity, regardless exercise type, on cancer outcomes. The intensity of exercise is evaluated using metabolic equivalent task (MET), with one MET corresponds to the energy expended at rest. Moderate-intensity exercise is equivalent to 3-to-5.9 MET. High-intensity or vigorous exercise has a MET value superior to 6 and is defined as an activity that causes sweating and change in heart and respiratory rates. Early studies have shown that patients with colon cancer practicing 18-to-27 MET-hours/week or more exhibited an increase in survival-free recurrence [11]. Similarly, patients with diagnosed stage I and II colon cancer or with recurrent stage III colon cancer, exercise of 18-MET-hours/week was sufficient to prolong survival [33,34]. There was also a reduced breast cancer-specific death in patients practicing more than 9 MET-hours/week [35]. Even though there is a clear inverse correlation of higher MET values with cancer progression and mortality, the use of MET-hours/week as unit of measure could be confounding because the final MET value for each patient could be reached either by low-to-moderate intensity exercise during a long period or by the practice of high-intensity exercise for shorter periods. The unit of measure that could be privileged is MET-hours/day. However, new evidences suggest that the intensity of exercise has the greatest effect in terms of cancer progression and survival. For example, the volume of total no vigorous activity did not affect the risk of prostate cancer progression [36], while vigorous exercise, reduced 57% the risk of cancer progression [36]. The time spent in practicing vigorous exercise seems to be also important as reported in a study from Sweden demonstrating a lowered risk of prostate cancer-specific mortality for men who biked more than 20 min/day (>5 MET-hours/day) versus less than 20 min/day [37]. Vigorous exercise at least once per week was also associated with a less likelihood to progress into metastatic-lethal prostate cancer [38]. A recent study on ~65 000 adults showed that people practicing vigorous exercise have significantly reduced cancer-related mortality [39]. Intervention with exercise in lung cancer patients followed the same trend as in breast, prostate and colon cancer. Higher levels of physical activity were associated with lower cancer-related mortality in lung cancer patients [40] (Fig. 1). It seems that vigorous exercise might be practiced regularly and during enough time to get benefits in terms of cancer progression and survival; thus, the time and frequency of vigorous exercise are important parameters to take into account for the design of vigorous exercise programs. Taking together, there is convincing evidence about a protective role of vigorous exercise in terms of physical fitness, cancer progression and recurrence and cancer-specific mortality.
Fig. 1.
The benefits of vigorous exercise. Vigorous exercise of more than 6 MET improves cardiorespiratory fitness and muscle strength. Vigorous exercise reduces the risk of cancer progression and the evolution into lethal metastasis.
4. Vigorous exercise modulates intra-tumor redox homeostasis
The high potential of vigorous exercise in improving physical performance and cancer progression in patients could reflect its ability to modulate pro-oncogenic signaling pathways that are not affected by simply practicing recreational activities. One of the most important molecular hallmarks of cancer is oxidative stress (OS) [41]. OS is defined as a “disruption of the redox balance towards an increase in pro-oxidant over the capacity of antioxidants, leading to a perturbation of redox signaling and control and/or molecular damage” [42]. Oxidative damage in cancer patients are correlated with poorer survival or even with more advanced stages of cancer [43,44]. We and others, have previously documented numerous clinical and preclinical studies showing high levels of oxidative damage in multiple organs of both cancer patients and animal models of cancer [[45], [46], [47]]. Particularly, intra-muscular and intra-tumor OS contribute to cancer-related skeletal muscle atrophy and cancer growth [48,49], respectively, thereby controlling several aspects of cancer pathophysiology. Interestingly, emerging evidences indicate that combination of moderate-intensity endurance and resistance exercise reduced protein carbonyls and reactive oxygen and nitrogen species (RONS) levels in skeletal muscles of mice bearing colon 26 tumor [50]. These exercise-dependent redox changes are reproducible in patients with ovarian, pancreatic and breast cancer, showing that endurance and resistance exercise programs increased total antioxidant capacity and reduced levels of protein carbonyls in the blood [51]. The ability of exercise to correct redox imbalance at systemic and muscular levels, raised the question about its ability to induce equivalent changes in tumor tissue.
Our laboratory has previously shown that forced treadmill exercise reduced prostate cancer growth, in rats bearing subcutaneous AT-1 tumor, by reducing the intra-tumor levels of DNA oxidative damage, 8-oxoguanine (8-oxoG), and lipid peroxidation markers TBARS and 4-Hydroxynonenal (4-HNE) [52]. Contrariwise, less-vigorous training by voluntary wheel running failed to affect intra-tumor oxidative damage (i.e. 4-HNE and protein carbonyls) in different tumor types ([53] and unpublished data). Accordingly, voluntary running did not affect intra-tumor protein carbonyls levels and tumor growth in mice bearing subcutaneous PC-3 prostate tumor (unpublished data); these differences are not due to tumor location, as mice bearing orthotopic prostate cancer did not benefit from wheel running and developed similar tumors compared with untrained mice [54]. The comparison between these different studies is difficult due to protocols heterogeneity. However, four main factors could influence the ability of an exercise intervention to modulate tumor growth and intra-tumor OS: 1) exercise intensity (forced treadmill vs voluntary wheel running), 2) protocol length, 3) tumor type and 4) tumor stage. For the last factor, studies using mice models of cancer initiation showed that voluntary wheel running was sufficient to reduce tumor development; however, the impact on intra-tumor OS was not assessed [55]. Therefore, forced treadmill might be necessary to reduce the growth of already established tumors, which is more useful from a therapeutic point of view. These data strongly suggest that the anticancer benefits due to significant changes in intra-tumor redox balance could be mainly reached by high-intensity exercise. Although exercise alone seems to induce an antioxidant effect on tumor, its combination with radio- or chemotherapy induces rather a pro-oxidant response. This is an interesting aspect when we know that radiotherapy and many chemotherapeutic agents mediate an important part of their anticancer effects by increasing the intracellular levels of RONS [56]. Combination of radiotherapy with training on treadmill in mice bearing subcutaneous prostate tumor significantly reduced tumor growth compared to radiotherapy alone (in revision). Lemke and collaborators reported that treadmill training combined with temozolomide, a chemotherapeutic agent, reduced orthotopic glioblastoma growth compared to intervention with temozolomide alone [57]. These effects were attributed to an increase in intracellular levels of RONS in glioma cells [57]. Others have shown that exercise facilitated the accessibility of chemotherapeutic drugs into tumor by improving intra-tumor vascularization [58,59]. OS markers were not measured in these studies but the proposed mechanisms involved modulation in oxygen-sensing machinery and hypoxia, which are intimately linked to redox homeostasis.
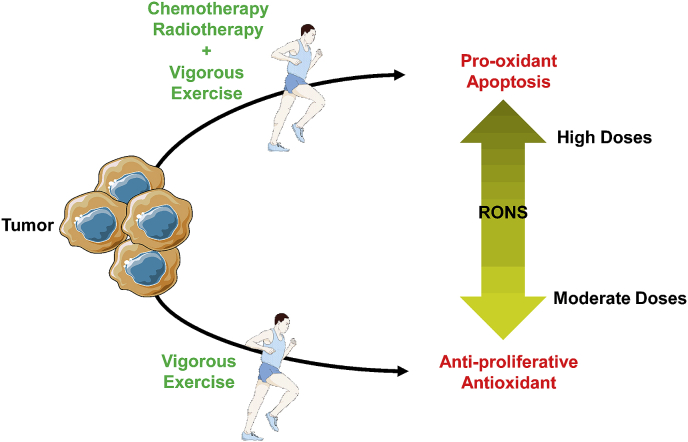
Based on the abovementioned studies we can conclude that exercise differentially modulate the intra-tumor redox signature. These differential responses could be explained by the paradoxical mechanisms through which moderate-to-high intensity exercise promotes the generation of moderate levels of RONS in tumor that activate the expression of numerous antioxidant genes [60] via the Keap-Nrf2 system and transcriptional factors like peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) (reviewed in Refs. [61,62]). Whereas, when exercise is combined to radio- or chemotherapy, RONS resulting from both interventions may provoke an additive effect that kills cancer cells. Additionally, if the tumor naturally exhibits high levels of RONS, intervention with exercise is expected to induce a pro-oxidant effect as well. Arguments in favor of these interpretations are well documented in the literature, for example, providing an extra source of RONS by overexpressing pro-oxidant enzymes improves the efficacy of chemotherapeutic drugs [63]; very high levels of intracellular RONS compromise DNA reparation mechanisms [64], which end by promoting cell death. Altogether, vigorous exercise alone or combined with conventional anticancer therapies could reduce tumor growth by differentially modulating intra-tumor redox homeostasis (Fig. 2).
Fig. 2.
A hypothetical scheme illustrating the differential impact of vigorous exercise on intra-tumor redox signature. Exercise alone is expected to produce an antioxidant response within tumor resulting in an anti-proliferative effect. While, the combination of exercise with radio/chemotherapy induces rather a pro-oxidant response promoting tumor cell death. More details about the mechanisms behind both responses are given in paragraph 4.
5. Exercise affects redox-sensitive pathways in tumor
After noticing that intra-tumor oxidative damage are modulated by exercise in a way slowing cancer cells growth or promoting their death, it becomes intuitive to question on How exercise could mechanistically affect intra-tumor redox signaling and homeostasis?
5.1. Production of skeletal muscle-specific myokines
To answer this question, many hypotheses can be proposed. For example, mechanical stimuli could highly affect tumor characteristics depending on tumor location, but this remains highly speculative. Nowadays, evidences from literature converge to support that the ability of exercise to affect intra-tumor oxidative balance is the result of a multi-step process that starts within skeletal muscles. In response to exercise, skeletal muscle is the first organ solicited and produces a cluster of cytokines and growth factors called myokines (reviewed in Ref. [65]). Skeletal muscle is considered as an endocrine organ given its ability to secrete biologically active myokines encapsulated into extracellular vesicles that influence the whole body [61,66]. Skeletal muscle cells can also secret microRNAs into circulation, which in turn are transported into tumor cells through exosomes, microvesicules or lipid-base complexes such as HDL [67]. Indeed, multiple microRNAs, like miR-221 and miR-133, are supposed to affect main signaling pathways including PI3K-Akt and MEK-ERK that regulate tumor cell proliferation and survival (reviewed in Ref. [67]). Other tissues could also secrete chemicals, like adipomyokines produced by adipose tissue, in response to exercise [68]. Myokines act in an autocrine manner on skeletal muscle to induce beneficial adaptations but could also travel the bloodstream to exert paracrine and endocrine effects on other organs [69]. In cancer, a crosstalk between skeletal muscle and tumor takes place. On the one hand, tumor-derived mediators induce skeletal muscle atrophy (reviewed in Refs. [45,70]). On the other hand, exercise-related myokines could reach tumor to affect its growth; this point will be discussed in the present paragraph. It is possible that the types of myokines and mechanisms controlling their production from muscles of tumor-bearing individuals could be different from those of normal skeletal muscles. However, for the moment, no evidence about such difference exists, which pushes us to suppose that mechanisms are quite similar.
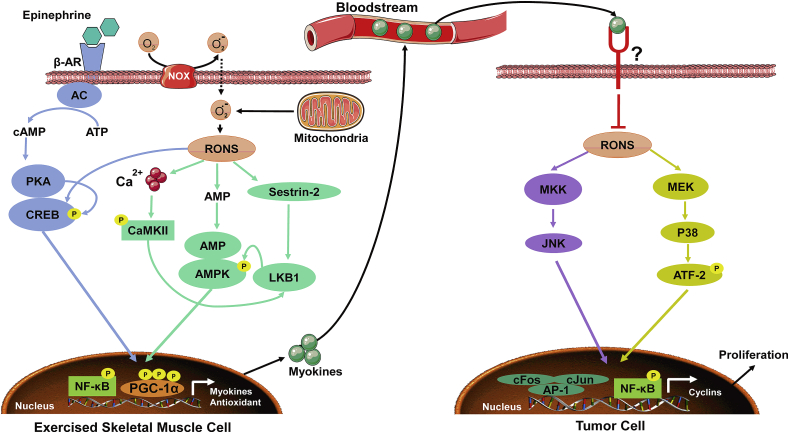
In skeletal muscles, the production of myokines is by itself a process regulated in a redox-dependent manner. The AMP-activated protein kinase (AMPK) is particularly activated in response to exercise [[71], [72], [73]]. Three main pathways are involved: (1) increase Ca2+ leakage from the sarcoplasmic reticulum, which in turn promotes the auto-phosphorylation of calmodulin-dependent kinase II (CaMKII) [74], (2) increase AMP:ATP ratio [73] and (3) activation of Sestrin-2 [75]. All these events lead to the activation and stabilization of LKB1 kinase that phosphorylates AMPK on the Thr172 site of the α-subunit. Activation of AMPK increases the mRNA expression of PGC-1α and can also directly phosphorylate PGC-1α and stabilize its expression in the nucleus [76]. The epinephrine/cAMP/PKA/CREB pathway could also induce the expression of PGC-1α after exercise [77,78]. The nuclear accumulation of PGC-1α promotes the expression of antioxidants, myokines, and other genes involved in angiogenesis and mitochondrial biogenesis [79,80] (Fig. 3). Indeed, RONS produced by xanthine oxidase and NADPH oxidase (NOX) [81] as well as mitochondria [82] stimulate the abovementioned mechanisms leading to AMPK phosphorylation at Thr172 residue and the subsequent activation of PGC-1α-derived myokines.
Fig. 3.
Intra-muscular and intra-tumoral redox-sensitive mechanisms driven by exercise. Firstly, in exercised skeletal muscle cells, hormones like epinephrine and/or RONS activate a series of pathways such as, AMPK and cAMP/PKA/CREB, leading to the nuclear translocation of transcriptional factors like NF-κB and PGC-1α and transcription of genes encoding antioxidant enzymes and myokines. Secondly, skeletal muscle myokines are released into the bloodstream where they can reach tumor cells. We suppose that myokines will bind specific receptors on tumor cells and inhibit RONS-mediated activation of signaling pathways including MKK/JNK and MEK/p38 (basal levels of RONS are high in tumor cells given their accelerated metabolism), which results in less nuclear translocation of AP-1 and NF-κB transcriptional factors and less transcription of cyclin genes involved in cell proliferation. Some data indicate that myokines could also drive apoptosis of tumor cells but the mechanisms are still poorly elucidated.
5.2. Impact of myokines on tumor cells
Studies linking myokines to cancer used to treat cancer cells with serum from trained and untrained humans or animals. For example, treatment of LNCaP prostate cancer cells with serum from trained humans inhibited their growth by 31% and reduced their ability to form tumors in immune-deficient mice [83]. Secretome analysis revealed many myokines produced in mice and humans in response to exercise such as, secreted protein acidic and rich in cysteine (SPARC), leukemia inhibitory factor (LIF), myostatin, apelin, musclin, irisin, interleukin-6 (IL-6), IL-8, and IL-15 [84]. Hojman and collaborators identified oncostatin M (OSM) as a myokine produced after exercise and able to reduce the proliferation of MCF7 breast cancer cells in vitro [85]. Munoz et al. found similar results with MCF7 cell line after treatment with the muscle-isolated protein F-Substance [86]. Others have shown that irisin enhanced the cytotoxicity of doxorubicin in MDA-MB-231 breast cancer cells compared to non-malignant cells MCF-10a, indicating the presence of an interesting selectivity of myokines toward malignancy [87]. SPARC is one of the most studied and characterized myokines. In the early 2000s, SPARC was found to induce anti-proliferative effects on carcinomas through its receptors present on the surface of cancer cells [88,89]. More recently, it has been shown that SPARC can reduce the number of aberrant crypts and colon tumorigenesis in mice [90]. SPARC mediated its anti-cancer effects by attenuating intra-tumor oxidative damage [91] and production of H2O2 [92]; these effects could be due to a higher transcription of antioxidant genes thanks to PGC-1β [93]. The reduction in H2O2 levels resulted in a reduced expression of cyclins A1, D1 and E2 and cell cycle arrest of tumor cells [92]. Mechanistically, SPARC-mediated reduction in H2O2 decreased the activation of p38-MAPK and JNK pathways that converge to promote AP-1 and NF-κB transcriptional activities [92], which in turn could be responsible for the expression of cyclins, like cyclin D1 [94] (Fig. 3). Interestingly, SPARC also inhibited AP-1 and NF-κB activities in cancer-associated fibroblasts and tumor-associated macrophages, thereby inhibiting inflammation produced by both populations in tumor microenvironment to nourish tumorigenicity [92,95]. Altogether, these data indicate that redox-dependent processes tightly regulate both the production of myokines from skeletal muscle and myokines-mediated anti-cancer effects observed in different cancers.
6. Clinical implications and perspectives
Physically active cancer patients are more confident concerning their health status and express less fear of cancer recurrence comparing to less active or sedentary patients [96]. This could be due to the increased awareness and accumulating evidences about the beneficial effects of physical activity in reducing cancer progression and recurrence. In this review, we report a positive role for exercise on cancer progression in humans. Regular vigorous exercise of more than 6 MET reduced cancer progression and improved survival in breast, colon and prostate cancer, with emerging evidence in lung cancer as well. One recent study reported that exercise of at least 4.5 MET improves colorectal cancer-specific survival only in tumors with low immune cells infiltrate [97]; this could explain the heterogeneous responsiveness of patients to exercise depending on the sub-molecular features of tumor. Although exercise less than 6 MET could improve fatigue, physic-social anxiety and cardiorespiratory fitness, it seems not sufficient to affect cancer progression and survival. We conclude that vigorous exercise is needed to improve cancer outcomes. The combination of endurance and resistance exercises according to ACSM and WHO guidelines should produce the best benefit for cancer patients in terms of disease progression and disease-free survival. Strategies to augment the adherence of patients to international physical activity guidelines are under development. Preclinical findings show that the combination of vigorous exercise with radiotherapy or chemotherapy improved the anti-cancer response in rodents; future clinical studies can investigate this possibility in patients undergoing conventional therapies.
The downside of exercise practice is that patients exercising regularly are more prone to take antioxidant supplements [[98], [99], [100]]. The prevalence of supplements is high in educated and physically active cancer patients, because antioxidant supplementation refers to a healthy lifestyle [101]. Contrary to this imaginary, scientists found that antioxidants blunt the beneficial effect of exercise in humans [102,103]. In rodents, combination of antioxidants with exercise neutralized the beneficial effects of exercise alone on tumor growth [104]. As depicted in Fig. 3, many exercise benefits on skeletal muscle cells and tumor cells are mediated by RONS; supplementation with antioxidants is expected to block these mechanisms. Even though RONS play a key role in cancer development, it is becoming clear that we also need them to beat cancer. Antioxidants could be also deleterious for cancer patients especially those undergoing chemotherapy or radiotherapy [105]. At the molecular level, antioxidants may interfere with the RONS-generating mechanisms of radiotherapy or chemotherapy. Given the limited research supporting the use of antioxidants in cancer, physicians prohibit the use of antioxidants for patients during their anti-cancer treatment [105]. So, combination of antioxidants with exercise or with chemo/radiotherapy must be avoided as it reduces the anti-cancer potential of the mentioned interventions. Patients with specific antioxidant deficiencies could benefit from supplementation after completion of their anti-cancer treatment on a single-patient basis.
Patients with advanced stages of cancer can suffer from excessive weight loss, muscle wasting, fatigue, anemia and cardiorespiratory troubles [1,106]. Applying moderate-to-vigorous endurance and resistance exercise in these conditions could be limited [107]. The evaluation of feasibility and safety of such exercise interventions in patients with advanced stages of cancer could be unallowable for ethical or physical reasons. In the absence of clear directive concerning physical activity, patients and their families will turn more easily to risky alternatives by fear from cancer progression or recurrence. From there, one of the remained unanswered questions in the field is How to make exercise benefits accessible for patients with advanced cancer and functional impairment? The answer could come from the recent technological advances achieved in the last few years. High throughput analysis of proteome, phospho-proteome and secretome will allow the identification of new myokines and the understanding of regulatory mechanisms of new or already known myokines. As a first approach, analysis could be performed on a simple culture system consisting of skeletal muscle cells subjected to electrical pulse stimulation in vitro, which mimics skeletal muscle contraction in vivo. A list of the most abundant myokines could be then established and validated in exercised animals and humans. Recombinant form of selected myokines could be tested at different doses in animals bearing the most frequent malignancies (i.e. lungs, colon, pancreas, liver, prostate and breast cancer). The combination of myokines that have the widest anti-cancer response on different cancer types could be considered as clinically relevant. In addition, myokines with selective but powerful anti-cancer effect on one cancer type are interesting candidates. Finally, a combination of myokines endowed with high anticancer activity and low side effect could be tested in cancer patient trials. We speculate that the administration of myokines directly would produce a more rapid and effective anticancer effect compared to existing exercise mimicking-strategies. Actually, ideas to emulate exercise effects have been proposed in the early 2000s, by administrating multiple agonists that could stimulate some of the signaling pathways activated by exercise itself, into individuals unable to exercise [108]. For example, AICAR that activate the AMPK-PGC-1α signaling, involved in myokines production, has been proposed as an effective exercise mimetic that recapitulates exercise-induced genes expression in rodents’ skeletal muscles, without exercising [109]. Reports show that the use of exercise mimetics, in the form of pills, improves survival and cardiovascular complications [110,111]; the majority of the research was performed in link with cardiovascular diseases or muscle endurance and confirmations are still needed for cancer. Nonetheless, we are not promoting in any way the use of exercise mimetics to replace physical activity or to treat the syndrome of inactivity. The effects of exercise touch many organs and are very complex to be simply reproduced by using a combination of pills. The best way to get complete health benefits is to practice “real” exercise [112]. However, in case where advanced cancer patients cannot exercise correctly, exercise-mimicking strategies (agonists for exercise signaling or myokines) become an essential and tolerated alternative. As low-intensity exercise could be feasible in advanced cancer patients [113], the combination of low-intensity exercise with low doses of exercise mimetics is expected to provide the best exercise-related benefits.
Author contributions
MA and AR conceived and designed the paper. MA wrote the paper. SD collected data mentioned in the text as “unpublished” or “in revision”. MA and AR edited and revised the manuscript. MA, SD and AR approved the final version of manuscript.
Declaration of competing interest
The authors of the present manuscript declare no conflict of interest.
Acknowledgement
Mohamad Assi is a postdoctoral researcher at the National Fund for Scientific Research (FRS-F.N.R.S), Belgium. Amélie Rébillard is a Full Professor at Université Rennes 2, France.
Contributor Information
Mohamad Assi, Email: m.assi@uclouvain.be.
Amélie Rébillard, Email: amelie.rebillard@univ-rennes2.fr.
References
- 1.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., Davis M., Muscaritoli M., Ottery F., Radbruch L., Ravasco P., Walsh D., Wilcock A., Kaasa S., Baracos V.E. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Fouladiun M., Korner U., Gunnebo L., Sixt-Ammilon P., Bosaeus I., Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2007;13:6379–6385. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 3.Morrison D.S., Parr C.L., Lam T.H., Ueshima H., Kim H.C., Jee S.H., Murakami Y., Giles G., Fang X., Barzi F., Batty G.D., Huxley R.R., Woodward M. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: analysis of data from the Asia-Pacific Cohort Studies Collaboration. Asian Pac. J. Cancer Prev. APJCP : Asian Pac. J. Cancer Prev. APJCP. 2013;14:1083–1087. doi: 10.7314/apjcp.2013.14.2.1083. [DOI] [PubMed] [Google Scholar]
- 4.Chambers M.A., Moylan J.S., Reid M.B. Physical inactivity and muscle weakness in the critically ill. Crit. Care Med. 2009;37:S337–S346. doi: 10.1097/CCM.0b013e3181b6e974. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen B.K. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J. Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashcraft K.A., Warner A.B., Jones L.W., Dewhirst M.W. Exercise as adjunct therapy in cancer. Semin. Radiat. Oncol. 2019;29:16–24. doi: 10.1016/j.semradonc.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnsson A., Demmelmaier I., Sjovall K., Wagner P., Olsson H., Tornberg A.B. A single exercise session improves side-effects of chemotherapy in women with breast cancer: an observational study. BMC Canc. 2019;19:1073. doi: 10.1186/s12885-019-6310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenreich C.M. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 9.Ballard-Barbash R., Friedenreich C.M., Courneya K.S., Siddiqi S.M., McTiernan A., Alfano C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J. Natl. Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedenreich C.M., Neilson H.K., Farris M.S., Courneya K.S. Physical activity and cancer outcomes: a precision medicine approach. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2016;22(19):4766–4775. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhardt J.A., Giovannucci E.L., Holmes M.D., Chan A.T., Chan J.A., Colditz G.A., Fuchs C.S. Physical activity and survival after colorectal cancer diagnosis. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 12.Ashcraft K.A., Peace R.M., Betof A.S., Dewhirst M.W., Jones L.W. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 2016;76:4032–4050. doi: 10.1158/0008-5472.CAN-16-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McTiernan A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz K.H., Courneya K.S., Matthews C., Demark-Wahnefried W., Galvao D.A., Pinto B.M., Irwin M.L., Wolin K.Y., Segal R.J., Lucia A., Schneider C.M., von Gruenigen V.E., Schwartz A.L., American College of Sports M. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sport. Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 15.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P., American College of Sports M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sport. Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 16.Winter C.C. The assessment of physical activity in children undergoing cancer treatment. Leuk. Res. 2013;37:243–244. doi: 10.1016/j.leukres.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Reis A.D., Pereira P., Diniz R.R., de Castro Filha J.G.L., Dos Santos A.M., Ramallo B.T., Filho F.A.A., Navarro F., Garcia J.B.S. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual. Life Outcomes. 2018;16:58. doi: 10.1186/s12955-018-0882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell K.L., Winters-Stone K.M., Wiskemann J., May A.M., Schwartz A.L., Courneya K.S., Zucker D.S., Matthews C.E., Ligibel J.A., Gerber L.H., Morris G.S., Patel A.V., Hue T.F., Perna F.M., Schmitz K.H. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sport. Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinh L., Strom D.A., Wong J.N., Courneya K.S. Modality-specific exercise guidelines and quality of life in kidney cancer survivors: a cross-sectional study. Psycho Oncol. 2018;27:2419–2426. doi: 10.1002/pon.4844. [DOI] [PubMed] [Google Scholar]
- 20.Coletta A.M., Marquez G., Thomas P., Thoman W., Bevers T., Brewster A.M., Hawk E., Basen-Engquist K., Gilchrist S.C. Clinical factors associated with adherence to aerobic and resistance physical activity guidelines among cancer prevention patients and survivors. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fassier P., Zelek L., Partula V., Srour B., Bachmann P., Touillaud M., Druesne-Pecollo N., Galan P., Cohen P., Hoarau H., Latino-Martel P., Menai M., Oppert J.M., Hercberg S., Deschasaux M., Touvier M. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: results from the prospective population-based NutriNet-Sante cohort. Medicine. 2016;95 doi: 10.1097/MD.0000000000004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallerand J.R., Rhodes R.E., Walker G.J., Courneya K.S. Correlates of meeting the combined and independent aerobic and strength exercise guidelines in hematologic cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2017;14:44. doi: 10.1186/s12966-017-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santa Mina D., Alibhai S.M., Matthew A.G., Guglietti C.L., Pirbaglou M., Trachtenberg J., Ritvo P. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J. Aging Phys. Act. 2013;21:455–478. doi: 10.1123/japa.21.4.455. [DOI] [PubMed] [Google Scholar]
- 24.Quevedo-Jerez K., Gil-Rey E., Maldonado-Martin S., Herrero-Roman F. Exercise-intensity adherence during aerobic training and cardiovascular response during resistance training in cancer survivors. J. Strength Cond. Res. 2019 doi: 10.1519/JSC.0000000000003144. [DOI] [PubMed] [Google Scholar]
- 25.An K.Y., Morielli A.R., Kang D.W., Friedenreich C.M., McKenzie D.C., Gelmon K., Mackey J.R., Reid R.D., Courneya K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: a randomized controlled trial. Int. J. Cancer. 2019;146(1):150–160. doi: 10.1002/ijc.32493. [DOI] [PubMed] [Google Scholar]
- 26.Galvao D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J. Clin. Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 27.Milne H.M., Wallman K.E., Gordon S., Courneya K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Canc. Res. Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 28.Lahart I.M., Metsios G.S., Nevill A.M., Carmichael A.R. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 29.Hardee J.P., Counts B.R., Carson J.A. Understanding the role of exercise in cancer cachexia therapy. Am. J. Lifestyle Med. 2019;13:46–60. doi: 10.1177/1559827617725283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeidifard F., Medina-Inojosa J.R., West C.P., Olson T.P., Somers V.K., Bonikowske A.R., Prokop L.J., Vinciguerra M., Lopez-Jimenez F. The association of resistance training with mortality: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019;26:1647–1665. doi: 10.1177/2047487319850718. [DOI] [PubMed] [Google Scholar]
- 31.Fyfe J.J., Bishop D.J., Stepto N.K. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sport. Med. 2014;44:743–762. doi: 10.1007/s40279-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 32.Coffey V.G., Hawley J.A. Concurrent exercise training: do opposites distract? J. Physiol. 2017;595:2883–2896. doi: 10.1113/JP272270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon J., Sato K., Niedzwiecki D., Ye X., Saltz L.B., Mayer R.J., Mowat R.B., Whittom R., Hantel A., Benson A., Wigler D.S., Atienza D., Messino M., Kindler H., Venook A., Fuchs C.S., Meyerhardt J.A. Impact of physical activity after cancer diagnosis on survival in patients with recurrent colon cancer: findings from CALGB 89803/Alliance. Clin. Colorectal Cancer. 2013;12:233–238. doi: 10.1016/j.clcc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyerhardt J.A., Heseltine D., Niedzwiecki D., Hollis D., Saltz L.B., Mayer R.J., Thomas J., Nelson H., Whittom R., Hantel A., Schilsky R.L., Fuchs C.S. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 35.Holmes M.D., Chen W.Y., Feskanich D., Kroenke C.H., Colditz G.A. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 36.Richman E.L., Kenfield S.A., Stampfer M.J., Paciorek A., Carroll P.R., Chan J.M. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonn S.E., Sjolander A., Lagerros Y.T., Wiklund F., Stattin P., Holmberg E., Gronberg H., Balter K. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol. Biomarkers Prev.: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:57–64. doi: 10.1158/1055-9965.EPI-14-0707. [DOI] [PubMed] [Google Scholar]
- 38.Dai J.Y., Wang B., Wang X., Cheng A., Kolb S., Stanford J.L., Wright J.L. Vigorous physical activity is associated with lower risk of metastatic-lethal progression in prostate cancer and hypomethylation in the CRACR2A gene. Cancer Epidemiol. Biomarkers Prev.: a publication of the American Association for cancer research, cosponsored by the American Society of Preventive Oncology. 2019;28:258–264. doi: 10.1158/1055-9965.EPI-18-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rey Lopez J.P., Gebel K., Chia D., Stamatakis E. Associations of vigorous physical activity with all-cause, cardiovascular and cancer mortality among 64 913 adults. BMJ Open Sport Exer. Med. 2019;5 doi: 10.1136/bmjsem-2019-000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sui X., Lee D.C., Matthews C.E., Adams S.A., Hebert J.R., Church T.S., Lee C.D., Blair S.N. Influence of cardiorespiratory fitness on lung cancer mortality. Med. Sci. Sport. Exerc. 2010;42:872–878. doi: 10.1249/MSS.0b013e3181c47b65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J., Solimini N.L., Elledge S.J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones D.P. Redefining oxidative stress. Antioxidants Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 43.Crohns M., Saarelainen S., Erhola M., Alho H., Kellokumpu-Lehtinen P. Impact of radiotherapy and chemotherapy on biomarkers of oxidative DNA damage in lung cancer patients. Clin. Biochem. 2009;42:1082–1090. doi: 10.1016/j.clinbiochem.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Dziaman T., Banaszkiewicz Z., Roszkowski K., Gackowski D., Wisniewska E., Rozalski R., Foksinski M., Siomek A., Speina E., Winczura A., Marszalek A., Tudek B., Olinski R. 8-Oxo-7,8-dihydroguanine and uric acid as efficient predictors of survival in colon cancer patients. Int. J. Cancer. 2014;134:376–383. doi: 10.1002/ijc.28374. [DOI] [PubMed] [Google Scholar]
- 45.Assi M., Rebillard A. The Janus-faced role of antioxidants in cancer cachexia: new insights on the established concepts. Oxidative Med. Cell. Longevity. 2016;2016 doi: 10.1155/2016/9579868. 9579868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 47.De Santis M.C., Porporato P.E., Martini M., Morandi A. Signaling pathways regulating redox balance in cancer metabolism. Front. Oncol. 2018;8:126. doi: 10.3389/fonc.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puig-Vilanova E., Rodriguez D.A., Lloreta J., Ausin P., Pascual-Guardia S., Broquetas J., Roca J., Gea J., Barreiro E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015;79:91–108. doi: 10.1016/j.freeradbiomed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Assi M., Derbre F., Lefeuvre-Orfila L., Rebillard A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Radic. Biol. Med. 2016;91:204–214. doi: 10.1016/j.freeradbiomed.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 50.Ballaro R., Penna F., Pin F., Gomez-Cabrera M.C., Vina J., Costelli P. Moderate exercise improves experimental cancer cachexia by modulating the redox homeostasis. Cancers. 2019;11 doi: 10.3390/cancers11030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Repka C.P., Hayward R. Oxidative stress and fitness changes in cancer patients after exercise training. Med. Sci. Sport. Exerc. 2016;48:607–614. doi: 10.1249/MSS.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gueritat J., Lefeuvre-Orfila L., Vincent S., Cretual A., Ravanat J.L., Gratas-Delamarche A., Rannou-Bekono F., Rebillard A. Exercise training combined with antioxidant supplementation prevents the antiproliferative activity of their single treatment in prostate cancer through inhibition of redox adaptation. Free Radical Biol. Med. 2014;77:95–105. doi: 10.1016/j.freeradbiomed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Assi M., Derbre F., Lefeuvre-Orfila L., Saligaut D., Stock N., Ropars M., Rebillard A. Maintaining a regular physical activity aggravates intramuscular tumor growth in an orthotopic liposarcoma model. Am. J. Cancer Res. 2017;7:1037–1053. [PMC free article] [PubMed] [Google Scholar]
- 54.Jones L.W., Antonelli J., Masko E.M., Broadwater G., Lascola C.D., Fels D., Dewhirst M.W., Dyck J.R., Nagendran J., Flores C.T., Betof A.S., Nelson E.R., Pollak M., Dash R.C., Young M.E., Freedland S.J. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 2012;113:263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen L., Idorn M., Olofsson G.H., Lauenborg B., Nookaew I., Hansen R.H., Johannesen H.H., Becker J.C., Pedersen K.S., Dethlefsen C., Nielsen J., Gehl J., Pedersen B.K., Thor Straten P., Hojman P. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metabol. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Coia L.R., Minsky B.D., John M.J., Haller D., Landry J., Pisansky T.M., Willet C.G., Mahon I., Owen J., Hanks G.E. Patterns of care study decision tree and management guidelines for esophageal cancer. American College of Radiology. Radiat. Med. 1998;16:321–327. [PubMed] [Google Scholar]
- 57.Lemke D., Pledl H.W., Zorn M., Jugold M., Green E., Blaes J., Low S., Hertenstein A., Ott M., Sahm F., Steffen A.C., Weiler M., Winkler F., Platten M., Dong Z., Wick W. Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget. 2016;7(35):56713–56725. doi: 10.18632/oncotarget.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrell M.B.G., Alvarez-Florez C., Zhang A., Kleinerman E.S., Savage H., Marmonti E., Park M., Shaw A., Schadler K.L. Vascular modulation through exercise improves chemotherapy efficacy in Ewing sarcoma. Pediatr. Blood Cancer. 2019;66 doi: 10.1002/pbc.27835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Florez Bedoya C.A., Cardoso A.C.F., Parker N., Ngo-Huang A., Petzel M.Q., Kim M.P., Fogelman D., Romero S.G., Wang H., Park M., Katz M.H.G., Schadler K.L. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci. Rep. 2019;9:13966. doi: 10.1038/s41598-019-49582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higuchi M., Cartier L.J., Chen M., Holloszy J.O. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J. Gerontol. 1985;40:281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- 61.Schnyder S., Handschin C. Skeletal muscle as an endocrine organ: PGC-1alpha, myokines and exercise. Bone. 2015;80:115–125. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Done A.J., Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams C., McCarthy H.O., Coulter J.A., Worthington J., Murphy C., Robson T., Hirst D.G. Nitric oxide synthase gene therapy enhances the toxicity of cisplatin in cancer cells. J. Gene Med. 2009;11:160–168. doi: 10.1002/jgm.1280. [DOI] [PubMed] [Google Scholar]
- 64.Feng Z., Hu W., Tang M.S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019;10:287. doi: 10.3389/fphys.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trovato E., Di Felice V., Barone R. Extracellular vesicles: delivery vehicles of myokines. Front. Physiol. 2019;10:522. doi: 10.3389/fphys.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dufresne S., Rebillard A., Muti P., Friedenreich C.M., Brenner D.R. A review of physical activity and circulating miRNA expression: implications in cancer risk and progression. Cancer Epidemiol. Biomarkers Prev.: a publication of the American Association for cancer research, cosponsored by the American Society of Preventive Oncology. 2018;27:11–24. doi: 10.1158/1055-9965.EPI-16-0969. [DOI] [PubMed] [Google Scholar]
- 68.Trayhurn P., Drevon C.A., Eckel J. Secreted proteins from adipose tissue and skeletal muscle - adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem. 2011;117:47–56. doi: 10.3109/13813455.2010.535835. [DOI] [PubMed] [Google Scholar]
- 69.Pedersen L., Hojman P. Muscle-to-organ cross talk mediated by myokines. Adipocyte. 2012;1:164–167. doi: 10.4161/adip.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mantovani G., Madeddu C., Maccio A. Cachexia and oxidative stress in cancer: an innovative therapeutic management. Curr. Pharmaceut. Des. 2012;18:4813–4818. doi: 10.2174/138161212803216889. [DOI] [PubMed] [Google Scholar]
- 71.Garcia D., Shaw R.J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M., Hardie D.G. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metabol. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi S.L., Kim S.J., Lee K.T., Kim J., Mu J., Birnbaum M.J., Soo Kim S., Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem. Biophys. Res. Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 74.Hudmon A., Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morrison A., Chen L., Wang J., Zhang M., Yang H., Ma Y., Budanov A., Lee J.H., Karin M., Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 2015;29:408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puigserver P., Rhee J., Lin J., Wu Z., Yoon J.C., Zhang C.Y., Krauss S., Mootha V.K., Lowell B.B., Spiegelman B.M. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol. Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 77.Hartley L.H., Mason J.W., Hogan R.P., Jones L.G., Kotchen T.A., Mougey E.H., Wherry F.E., Pennington L.L., Ricketts P.T. Multiple hormonal responses to prolonged exercise in relation to physical training. J. Appl. Physiol. 1972;33:607–610. doi: 10.1152/jappl.1972.33.5.607. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez G.A., Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 79.Shin K.O., Bae J.Y., Woo J., Jang K.S., Kim K.S., Park J.S., Kim I.K., Kang S. The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J. Exer. Nutr. Biochem. 2015;19:91–98. doi: 10.5717/jenb.2015.15061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang C., O'Moore K.M., Dickman J.R., Ji L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic. Biol. Med.Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Takamasa Tsuzuki A.G., Toshinori Yoshihara. NAPDH oxidase and xanthine oxidase inhibition attenuates the activation of AMPK signaling after a single bout of endurance exercise in mouse skeletal muscle Juntendo. Med. J. 2018:107–113. [Google Scholar]
- 82.Rabinovitch R.C., Samborska B., Faubert B., Ma E.H., Gravel S.P., Andrzejewski S., Raissi T.C., Pause A., St-Pierre J., Jones R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 83.Rundqvist H., Augsten M., Stromberg A., Rullman E., Mijwel S., Kharaziha P., Panaretakis T., Gustafsson T., Ostman A. Effect of acute exercise on prostate cancer cell growth. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Catoire M., Kersten S. The search for exercise factors in humans. FASEB J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 2015;29:1615–1628. doi: 10.1096/fj.14-263699. [DOI] [PubMed] [Google Scholar]
- 85.Hojman P., Dethlefsen C., Brandt C., Hansen J., Pedersen L., Pedersen B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 2011;301:E504–E510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 86.Munoz R.M., Han H., Tegeler T., Petritis K., Von Hoff D.D., Hoffman S.A. Isolation and characterization of muscle fatigue substance with anti-tumor activities. J. Cancer. 2013;4:343–349. doi: 10.7150/jca.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gannon N.P., Vaughan R.A., Garcia-Smith R., Bisoffi M., Trujillo K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer. 2015;136:E197–E202. doi: 10.1002/ijc.29142. [DOI] [PubMed] [Google Scholar]
- 88.Yiu G.K., Chan W.Y., Ng S.W., Chan P.S., Cheung K.K., Berkowitz R.S., Mok S.C. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am. J. Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhanesuan N., Sharp J.A., Blick T., Price J.T., Thompson E.W. Doxycycline-inducible expression of SPARC/Osteonectin/BM40 in MDA-MB-231 human breast cancer cells results in growth inhibition. Breast Canc. Res. Treat. 2002;75:73–85. doi: 10.1023/a:1016536725958. [DOI] [PubMed] [Google Scholar]
- 90.Aoi W., Naito Y., Takagi T., Tanimura Y., Takanami Y., Kawai Y., Sakuma K., Hang L.P., Mizushima K., Hirai Y., Koyama R., Wada S., Higashi A., Kokura S., Ichikawa H., Yoshikawa T. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 91.Naczki C., John B., Patel C., Lafferty A., Ghoneum A., Afify H., White M., Davis A., Jin G., Kridel S., Said N. SPARC inhibits metabolic plasticity in ovarian cancer. Cancers. 2018;10 doi: 10.3390/cancers10100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Said N., Frierson H.F., Sanchez-Carbayo M., Brekken R.A., Theodorescu D. Loss of SPARC in bladder cancer enhances carcinogenesis and progression. J. Clin. Investig. 2013;123:751–766. doi: 10.1172/JCI64782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawada M., Yamamoto H., Ogasahara A., Tanaka Y., Kihara S. beta-aminoisobutyric acid protects against vascular inflammation through PGC-1beta-induced antioxidative properties. Biochem. Biophys. Res. Commun. 2019;516:963–968. doi: 10.1016/j.bbrc.2019.06.141. [DOI] [PubMed] [Google Scholar]
- 94.Klein E.A., Assoian R.K. Transcriptional regulation of the cyclin D1 gene at a glance. J. Cell Sci. 2008;121:3853–3857. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Said N. Roles of SPARC in urothelial carcinogenesis, progression and metastasis. Oncotarget. 2016;7:67574–67585. doi: 10.18632/oncotarget.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher A., Beeken R.J., Heinrich M., Williams K., Wardle J. Health behaviours and fear of cancer recurrence in 10 969 colorectal cancer (CRC) patients. Psycho-Oncol. 2016 doi: 10.1002/pon.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koh H., Hamada T., Song M., Liu L., Cao Y., Nowak J.A., da Silva A., Twombly T., Morikawa T., Kim S.A., Masugi Y., Kosumi K., Shi Y., Gu M., Li W., Du C., Chen Y., Li W., Liu H., Li C., Wu K., Nosho K., Inamura K., Hanyuda A., Zhang X., Giannakis M., Chan A.T., Fuchs C.S., Nishihara R., Meyerhardt J.A., Ogino S. Physical activity and colorectal cancer prognosis according to tumor-infiltrating T cells. JNCI Cancer Spectr. 2018;2:pky058. doi: 10.1093/jncics/pky058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandler R.S., Halabi S., Kaplan E.B., Baron J.A., Paskett E., Petrelli N.J. Use of vitamins, minerals, and nutritional supplements by participants in a chemoprevention trial. Cancer. 2001;91:1040–1045. [PubMed] [Google Scholar]
- 99.Hall J.D., Bissonette E.A., Boyd J.C., Theodorescu D. Motivations and influences on the use of complementary medicine in patients with localized prostate cancer treated with curative intent: results of a pilot study. BJU Int. 2003;91:603–607. doi: 10.1046/j.1464-410x.2003.04181.x. [DOI] [PubMed] [Google Scholar]
- 100.Jatoi A., Williams B., Nichols F., Marks R., Aubry M.C., Wampfler J., Finke E.E., Yang P. Is voluntary vitamin and mineral supplementation associated with better outcome in non-small cell lung cancer patients? Results from the Mayo Clinic lung cancer cohort. Lung Cancer. 2005;49:77–84. doi: 10.1016/j.lungcan.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 101.Velicer C.M., Ulrich C.M. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J. Clin. Oncol. 2008;26:665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 102.Ristow M., Zarse K., Oberbach A., Kloting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C.R., Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomez-Cabrera M.C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Gueritat J., Lefeuvre-Orfila L., Vincent S., Cretual A., Ravanat J.L., Gratas-Delamarche A., Rannou-Bekono F., Rebillard A. Exercise training combined with antioxidant supplementation prevents the antiproliferative activity of their single treatment in prostate cancer through inhibition of redox adaptation. Free Radic. Biol. Med. 2014;77:95–105. doi: 10.1016/j.freeradbiomed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book. 2014:e478–486. doi: 10.14694/EdBook_AM.2014.34.e478. [DOI] [PubMed] [Google Scholar]
- 106.Argiles J.M., Busquets S., Lopez-Soriano F.J., Costelli P., Penna F. Are there any benefits of exercise training in cancer cachexia? J. Cachexia Sarcopenia Muscle. 2012;3:73–76. doi: 10.1007/s13539-012-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grande A.J., Silva V., Riera R., Medeiros A., Vitoriano S.G., Peccin M.S., Maddocks M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD010804.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Wald N.J., Law M.R. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Narkar V.A., Downes M., Yu R.T., Embler E., Wang Y.X., Banayo E., Mihaylova M.M., Nelson M.C., Zou Y., Juguilon H., Kang H., Shaw R.J., Evans R.M. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wald D.S., Morris J.K., Wald N.J. Randomized Polypill crossover trial in people aged 50 and over. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roshandel G., Khoshnia M., Poustchi H., Hemming K., Kamangar F., Gharavi A., Ostovaneh M.R., Nateghi A., Majed M., Navabakhsh B., Merat S., Pourshams A., Nalini M., Malekzadeh F., Sadeghi M., Mohammadifard N., Sarrafzadegan N., Naemi-Tabiei M., Fazel A., Brennan P., Etemadi A., Boffetta P., Thomas N., Marshall T., Cheng K.K., Malekzadeh R. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394:672–683. doi: 10.1016/S0140-6736(19)31791-X. [DOI] [PubMed] [Google Scholar]
- 112.Hawley J.A., Joyner M.J., Green D.J. Mimicking exercise: what matters most and where to next? J. Physiol. 2019 doi: 10.1113/JP278761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kisner C., Colby L.A., Borstad J. F.A. Davis Company; Philadelphia, USA: 2018. Therapeutic Exercise: Foundations and Techniques. [Google Scholar]