Abstract
Strenuous exercise is a potent stimulus to induce beneficial skeletal muscle adaptations, ranging from increased endurance due to mitochondrial biogenesis and angiogenesis, to increased strength from hypertrophy. While exercise is necessary to trigger and stimulate muscle adaptations, the post-exercise recovery period is equally critical in providing sufficient time for metabolic and structural adaptations to occur within skeletal muscle. These cyclical periods between exhausting exercise and recovery form the basis of any effective exercise training prescription to improve muscle endurance and strength. However, imbalance between the fatigue induced from intense training/competitions, and inadequate post-exercise/competition recovery periods can lead to a decline in physical performance. In fact, prolonged periods of this imbalance may eventually lead to extended periods of performance impairment, referred to as the state of overreaching that may progress into overtraining syndrome (OTS). OTS may have devastating implications on an athlete's career and the purpose of this review is to discuss potential underlying mechanisms that may contribute to exercise-induced OTS in skeletal muscle. First, we discuss the conditions that lead to OTS, and their potential contributions to impaired skeletal muscle function. Then we assess the evidence to support or refute the major proposed mechanisms underlying skeletal muscle weakness in OTS: 1) glycogen depletion hypothesis, 2) muscle damage hypothesis, 3) inflammation hypothesis, and 4) the oxidative stress hypothesis. Current data implicates reactive oxygen and nitrogen species (ROS) and inflammatory pathways as the most likely mechanisms contributing to OTS in skeletal muscle. Finally, we allude to potential interventions that can mitigate OTS in skeletal muscle.
1. Introduction
Exercise is arguably the most potent stimulus that triggers skeletal muscle adaptations during chronic endurance and resistance training. These adaptations range from increased endurance due to mitochondrial biogenesis and angiogenesis, to increased strength from hypertrophy in skeletal muscle. It is also well-known that the adaptation is dependent on exercise intensity, i.e. when exercise is performed to exhaustion, resulting in fatigue, it creates a metabolic drive that initiates a more powerful downstream activation of genes responsible for skeletal muscle remodeling than moderate exercise [1,2]. While exercise is necessary to trigger and stimulate muscle adaptations, the post-exercise recovery period is equally critical in providing sufficient time for metabolic and structural adaptations to occur within skeletal muscle [[3], [4], [5]]. These cyclical periods between fatigue and recovery form the basis of any effective exercise training prescription to improve muscle endurance and strength. However, we are currently lacking scientific knowledge of how long the recovery periods should be to receive optimal adaptation in skeletal muscle. Moreover, elite athletes and high-performance individuals might struggle to allow time for recovery between their exercise sessions and competitions where they, as required at the top level, are supposed to perform at their utmost capacity. Imbalance between the fatigue induced from intense training/competitions, and inadequate post-exercise/competition recovery periods can lead to a decline in physical performance. In fact, prolonged periods of this imbalance between fatigue and recovery may eventually lead to extended periods of performance impairment, referred to as the state of overreaching that may progress into overtraining syndrome (OTS). The prevalence of overreaching and OTS is difficult to establish as specific diagnostics are absent, but studies report that ~30% of both young athletes (<18 years) and elite athletes (>18 years old) have experienced overreaching/OTS at least once [[6], [7], [8], [9]]. However a prevalence of as high as ~60% in male and female elite runners have been described [10].
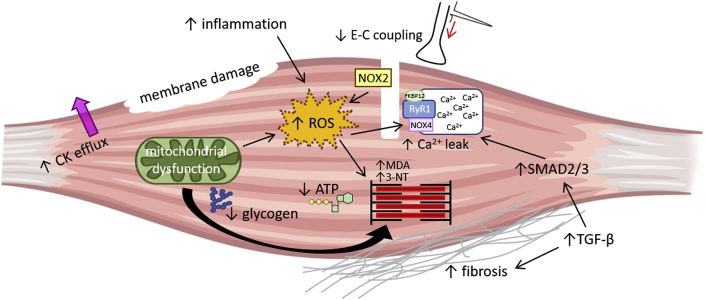
Performance decrements accompanying overreaching will require days to weeks for recovery, but appropriate rest will ultimately lead to performance increases. However, if the overreaching is extreme and/or combined with insufficient downtime (i.e. rest, recovery) it will advance into OTS [8,11]. OTS is defined by persistent underperformance despite >2 months of recovery, joined with changes in mood and absence of symptoms/diagnosis of other possible causes of underperformance [8,9,11,12]. OTS has been attributed to both central (psychological, neurological) and peripheral (intramuscular) mechanisms [8,9,11,12]. In this review, we will focus on intramuscular mechanisms that results in impaired skeletal muscle contractile function following exhaustive exercise and elucidate how these can lead to OTS (Fig. 1).
Fig. 1.
Illustration picturing potential intramuscular mechanisms of OTS, including glycogen depletion, membrane damage, creatine kinase efflux, reduced excitation-contraction (E–C) coupling, inflammation and cytokine signaling with e.g. enhanced TGF-β1 signaling, mitochondrial dysfunction and increased ROS signaling. Current data implicates ROS and inflammatory pathways as the most likely mechanisms contributing to OTS in skeletal muscle.
2. Prolonged low-frequency force depression, a potent contributor to OTS
Physiological assessments of OTS by coaches and athletes has been limited due to difficulties in employing practical tests to assess skeletal muscle performance in the field [13]. However, in studies where muscle function was investigated, muscle weakness was a defining symptom of overtraining in elite athletes [7,[14], [15], [16]]. The muscle force produced during an exercise session, which allows us to e.g. run, jump and breathe, is primarily muscle contractions carried out at the submaximal level. Prolonged low-frequency force depression (PLFFD) is defined as a persistent exercise-induced reduction in submaximal force that can last for several days or weeks during the post-exercise recovery period [[17], [18], [19], [20], [21]]. This means that PLFFD underlies the long-lasting sensation of muscle weakness during the post-exercise recovery period. Thus, one consequence of PLFFD is that depressed submaximal force will require greater perceived effort to perform any given exercise task, which implies that repeated periods of PLFFD without recovery could potentiate or even exacerbate OTS in skeletal muscle. This greater voluntary effort required to compensate for the depressed submaximal force associated with PLFFD may also accelerate muscle fatigability by requiring increased recruitment of muscle fibers and higher motor unit discharge rates to maintain a given force. Indeed, fatigue is a defining symptom of OTS and muscle weakness associated with PLFFD may underlie the impaired exercise capacity [8,22]. PLFFD was first described in a human exercise study by Edwards and colleagues (1977), but since then, accumulating evidence has shown that PLFFD following fatigue can be replicated in single muscle fibres [[23], [24], [25], [26]]. Thus, the primary cause of PLFFD appears to lie within muscle itself. Intriguingly, the prevailing hypotheses of underlying intramuscular causes of OTS [8] (Fig. 1) are also proposed causes of PLFFD [19,23,24,[26], [27], [28]], i.e. glycogen depletion, ultrastructural damage, inflammation, and oxidative stress, which will be discussed in more detail below.
3. Underlying intramuscular causes of OTS
3.1. The glycogen hypothesis
Excitation-contraction (E-C) coupling [29] and the force producing machinery (cross-bridge cycling) [30] are two energy-demanding processes in skeletal muscle, which are further potentiated by physical exercise. For instance, Ca2+ pumping by the sarcoplasmic reticulum Ca2+-ATPase (SERCA) is purportedly responsible for ~50–80% of the total energy cost in skeletal muscle [31,32]. Furthermore, one ATP molecule is required for each myosin head (myosin/myofibrillar ATPase) that will interact with actin to generate force in the cross-bridge cycling [30]. Glycogen is a multi-branched polymer of glucose molecules that serves as an energy storage form. Glycogen is found in a variety of tissues, but quantitatively high in skeletal muscles (and the liver). The large quantity of glycogen in skeletal muscle reflects its important role of rapidly providing muscle cells with ATP, which display a high and rapidly shifting energy turnover. Moreover, glycogen is located in close proximity to energy-consuming sites in skeletal muscle, e.g. SERCA and myofibrillar ATPase [33]. Thus, intramuscular glycogen depletion can be a significant contributor to fatigue and impaired post-exercise recovery [27,[34], [35], [36]]. The rate of muscle glycogen re-syntheses is slow and takes hours to several days to fully restore [[37], [38], [39], [40], [41]]. Nevertheless, intramuscular glycogen levels restore faster than the months-long duration of OTS.
The glycogen hypothesis of OTS states that exercise-induced muscle glycogen depletion is linked to decreased performance [42]. Based on this hypothesis, long-term carbohydrate supplementation was recently tested as an intervention to prevent or mitigate OTS in rodents [43]. Although a trend towards attenuated OTS-induced performance decrements in running and muscle atrophy with carbohydrate supplementation was observed, it did not reach statistical significance. Furthermore, glycogen supplementation was not able to protect against muscle damage in rodents [43], assessed by oxidative stress markers and creatine kinase (CK) levels [43]. On the other hand, a noticeable glycogen dependence has been observed in the post-exercise recovery of PLFFD. For instance, we have shown that submaximal force recovery is absent in muscles not provided with glucose, i.e. in muscles not able to resynthesize glycogen [27]. Thus, low intramuscular glycogen appears to contribute to PLFFD and OTS, but muscle glycogen content alone cannot explain the mechanism underlying PLFFD and/or OTS.
3.2. Exercise-induced muscle damage and OTS
Exercise-induced muscle damage is a condition characterized by e.g. loss of muscle strength, swelling, delayed onset muscle soreness (DOMS), ultrastructural myofibrillar disruption, systemic efflux of myocellular enzymes and proteins (e.g. CK), or a combination of these [[44], [45], [46]]. It is well recognized that muscle damage is pronounced after repeated eccentric contractions (i.e., lengthening) [[47], [48], [49]]. For instance, running downhill and limb deceleration (drop jumps) are two common movements of repeated eccentric muscle contractions that will induce muscle damage [19,21,49], which lead to both maximal as well as submaximal force depression (i.e. PLFFD) [19]. Moreover, there appears to be a temporal association between the extent of loss of muscle strength after exercise, and the time required to restore muscle strength back to normal, i.e. the more the muscle strength decreases immediately after exercise the longer it takes to recover [[49], [50], [51]] but if the next exercise bout takes place before full recovery, it could contribute to the negative performance spiral that can lead to overreaching and OTS. Overall, the multitude of symptoms initiated with muscle damage can last from weeks to over a month, including prolonged depression in muscle strength [21,44,46,48], hence the muscle damage-induced loss of muscle strength matches the duration of the performance decrements in OTS. However, what are the underlying cellular and molecular explanations for the loss of strength introduced by exercise-induced muscle damage and the recovery/regeneration that follows injury, and what is the evidence, if any, that these mechanisms are potential causes of OTS?
3.2.1. Mechanical damage not directly responsible for exercise-induced loss of force
Mechanical damage of the muscle fiber ultrastructure has been proposed to explain exercise-induced muscle damage and loss of muscle strength [19,52,53]. For instance, loss of z-disc integrity (i.e. z-disc streaming) and hence loss of the sarcomeric boundaries and the anchoring site for the contractile protein actin in skeletal muscle [19,52]. However, despite a wider z-disc, the force production of isolated myofibrils from human muscle biopsies were only marginally reduced following 100 repeated drop jumps [19]. Nonetheless, impaired shortening velocity following eccentric exercise could indicate dysfunction in crossbridge kinetics that could contribute to decreased muscle power generation [54].
Moreover, despite evidence of increased sarcolemmal membrane tearing and permeability as shown by elevated creatine kinase levels into blood plasma [47,55], measurements of M-wave properties (amplitude, duration, area) from surface electromyography shows that sarcolemmal excitability is unchanged under conditions that cause marked force depression and PLFFD [54,56]. The absence of a significant change in the M-wave suggests that failure in neuromuscular transmission and sarcolemmal excitation are not closely related to the force decrease, but instead imply that peripheral intramuscular mechanisms are responsible for the loss of force. Overall, accumulated data [19,44,54,[56], [57], [58]] suggests that the ultrastructural changes are signs of damage and/or remodeling, but are not directly responsible for the force decrements. Instead, intramuscular modifications targeting the E-C coupling and/or cross-bridges appear accountable for the weakness. Specifically, exercise-induced inflammation and oxidative stress targeting proteins involved in muscle contraction and force production are major candidates potentially responsible for the force and performance decrements in OTS [23,44,50,57,[59], [60], [61], [62], [63], [64], [65], [66]].
3.3. Exercise-induced inflammation and cytokine production
The local classical signs of inflammation include pain, heat, redness, swelling and loss of function, i.e. it has many commonalities with symptoms of exercise-induced muscle damage. In fact, inflammation is an acknowledged key process in muscular repair and regeneration [67,68] and under non-pathophysiological conditions (e.g. after exercise-induced muscle damage) intramuscular inflammation is a tightly coordinated and dynamic process that eventually leads to adaptive remodeling, e.g. skeletal muscle hypertrophy [50,57,62,67,69]. Among the myeloid lineage cell types (including monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, and megakaryocytes to platelets) [70] that enter muscle following damage, macrophages are most clearly demonstrated as positive regulators of regeneration [44,50,63,71,72]. Macrophages demonstrate a wide continuum of phenotypic diversity, on one hand, macrophages can be activated to the M1 (F4/80+/CD68high/CD206-) phenotype by e.g. proinflammatory cytokines or other myeloid cells [73]. Interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) are well-characterized proinflammatory cytokines that activate macrophages to the M1 phenotype. At the other hand, macrophages can be activated to the M2 (F4/80+/CD68low/CD206+) phenotype by anti-inflammatory cytokines, including interleukin 4 (IL-4), IL-10 and IL-1373. Moreover, M1 and M2 macrophages appear functionally coupled to distinct stages of myogenesis in muscle regeneration. For instance, it has been shown that depletion of macrophages at the time of M1 to M2 transition reduced muscle growth, repair and regeneration, and perturbed the expression of the muscle-specific transcription factor MyoD in skeletal muscle from mice that had undergone hindlimb unloading and reloading as a model of muscle damage [74]. However, the spatial and temporal coordination of macrophage-mediated signaling of inflammation and muscle regeneration is not fully understood, but several cytokines, including TNFα, IFNγ, IL-6, and IL-10, appears to play key roles in muscle regeneration [44,50,63,64]. In accordance, non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to negatively impact satellite cell activity, translational signaling and protein synthesis in human biopsies after acute exercise and chronic resistance training (both concentric and eccentric contractions) [57,[59], [60], [61]]. Moreover, it was recently shown that in healthy young men and women which performed 8 weeks of supervised resistance training, the NSAID ibuprofen (1200 mg/day) compromised resistance exercise-induced muscle strength and muscle hypertrophic adaptations, which was accompanied by ibuprofen-induced downregulation of IL-6 expression [62].
Although there are beneficial effects of inflammation in the short term, a chronic inflammatory response will be deleterious ultimately resulting in decreased muscle function with reduced mitochondrial respiration and muscle weakness [[75], [76], [77], [78], [79], [80], [81]]. For instance, overexpression of IL-6 causing chronically elevated IL-6 levels in skeletal muscle, results in lowered force production, reduced expression of proteins in the mitochondrial electron transport chain, and diminished respiratory capacity [81]. Moreover, exercise-induced muscle damage can persist for weeks and trigger macrophage activation where several cytokines (incl. TNFα, IFNγ, IL-6, and IL-10), appear to be involved [44,50,63,64]. Thus, repeated strenuous exercise can induce a persistent intramuscular molecular cytokine signature, which shares commonalities with disease states of chronic inflammation (e.g. rheumatoid arthritis [80]) which is accompanied by muscle weakness [[76], [77], [78],80]. As a result, repeated strenuous physical activity with too short recovery periods that induces soluble factors which prolongs the duration of inflammation will most certainly lead to decreased muscle function and may well be a key component in OTS.
There are data from rodents that supports the link between cytokines and OTS, however, further experiments in humans are necessary to elucidate the relationship between inflammation and excessive exercise [82]. For example, in an experimental setting, overtraining was induced in rats by 11 weeks of motorized treadmill running, which resulted in decreased physical performance accompanied by increased cytokine levels of e.g. TNF-α, IL-4, IL-6, IL-10 as compared with a sedentary control group and a moderate trained control group [83]. Remarkably, the levels of TNF-α, IL-6, and IL-10 remained elevated even after a two week recovery period after the last exercise session [83]. Nevertheless, how can cytokines lead to decreased performance in skeletal muscle, i.e. decreased force production? Cytokines are known to increase the production of reactive oxygen species (ROS) and in turn, ROS can promote release of pro-inflammatory cytokines [67,80,[84], [85], [86]]. In the next chapter we will discuss how ROS can cause an imbalance in the redox state of the muscle, resulting in impaired exercise performance as evident in athletes with OTS [12,87].
3.4. Oxidative stress and decreased muscle function in OTS
Athletes with OTS exhibit exercise-induced oxidative stress [12,87], which is thought to be caused by an imbalance in the intramuscular redox state that triggers inflammatory signaling, resulting in impaired force production and exercise performance. In line with the data from human studies showing that the degree of exercise-induced muscle inflammation depends on the type of exercise and extent of loss of force [57,66,88], the amount and impact of the oxidative stress on muscle performance appears dependent on type of exercise and intensity [19,66]. The time course of exercise-induced oxidative stress in skeletal muscle is unclear, but transcriptional analyses of skeletal muscle biopsies after endurance exercise indicated transcriptional activity of oxidative-stress related genes (e.g. transforming growth factor β (TGF-β1), phospholipase A2) 96h post-exercise [66]. However, exercise-induced increases in ROS can also have direct effects on the force production in skeletal muscle [23,65].
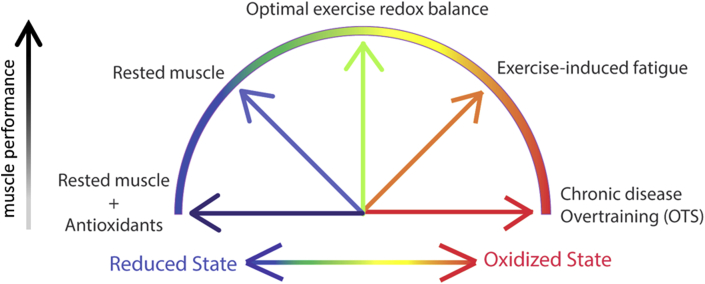
Exercise-induced increases in ROS has been associated with muscle fatigue and impaired post-exercise recovery [89]. For instance, Reid et al. showed already 25 years ago that the general antioxidant N-acetylcysteine (NAC) alleviated the fatigue-related force decline when human subjects performed repeated submaximal contractions [90]. This finding established the mechanism that ROS production increases in skeletal muscle during physical exercise and ameliorating the resulting oxidative stress with antioxidants lessens the force decrease. Another study that investigated the direct effect of ROS on muscle force generation in unfatigued muscle, showed via exposure of isolated muscle fibers to oxidizing (i.e. H2O2) and reducing agents (i.e. DTT) that shifting the redox state of the muscle also had implications on the muscle force generation [91]. Specifically, these findings revealed that an unfatigued muscle is mostly in a reduced state and upon exposure to mild oxidation, ROS increases contractile force to a state considered “optimal redox balance” [91]. However, continued exposure of the muscle fiber to ROS caused force depression due to excessive oxidation, which may represent the state of severe fatigue and OTS [91] (Fig. 2). Since these landmark studies though, the role of ROS and oxidative stress in fatigue and force depression has been a subject of intense debate. For instance, it is known that ROS are produced to the greatest extent during metabolically demanding high-intensity exercise [92]. However, it was recently shown that treating muscle with potent antioxidants that inhibited ROS production from the major cellular sites (e.g. mitochondria and NADPH oxidase 2 (NOX2)) during one session of high-intensity stimulation did not mitigate the fatigue-induced decline in contractile force [23]. One plausible explanation why no effect was seen with antioxidants in direct conjunction with one session of fatigue-induced decline in contractile force [23] is that the ROS produced during the contractions was transient which shifted it to an “optimal redox balance” that was beneficial for the force generation [24,25,91,93], and direct application of antioxidants could not reduce the redox state of the fiber and thus no altered muscle performance was observed (Fig. 2). However, several human and animal studies show that chronic treatment with antioxidants to remove ROS can hamper the beneficial effects of endurance training [[94], [95], [96]], probably because continuous antioxidants intake/application neutralizes the oxidative stress and also the beneficial effects of ROS on e.g. force generation and signaling leading to mitochondrial biogenesis. In comparison to acute and brief increases in ROS with exercise in healthy skeletal muscle, several chronic diseases showing symptoms of skeletal muscle dysfunction and muscle weakness, including rheumatoid arthritis (RA) [[76], [77], [78]], Duchenne muscle dystrophy [97], malignant hyperthermia [98], and even in normal ageing [99] show chronic intramuscular increases in ROS and oxidative stress (Fig. 2). Similar to chronic disease, OTS may represent a state of chronic oxidative stress. For instance, blood markers of oxidative stress (e.g. depletion of reduced glutathione (GSH)) can persist for longer than a month following an ultra-endurance running event [100]. Athletes categorized as chronically suffering from OTS (>6 months) show increased levels of the oxidative stress marker malondialdehyde (MDA) adducts at baseline and reduced blood plasma antioxidant capacity (i.e., oxygen radical absorbance capacity) [101]. In chronic conditions with oxidative stress and muscle weakness, antioxidant treatment has been shown to be beneficial in restoring muscle force production [75,77,102]. Thus, increases in ROS can have both good and bad effects on skeletal muscle contractile function and fitness, and the outcome probably depends on a combination of factors, e.g. the type of ROS, the size of ROS increase, the duration of ROS/RNS elevation (e.g. milliseconds vs hours and months), as well as the site of ROS production and accumulation [[103], [104], [105]].
Fig. 2.
Cartoon illustrating the proposed bell-shaped relationship between redox state and performance in skeletal muscle. In the rested state, muscle fibers appear in a semi-reduced redox state and can become oxidized during exercise to an “optimal exercise redox balance” at which the muscle can reach peak performance. Muscle fibers can become overly oxidized during fatiguing exercise and even further in OTS and chronic disease which leads to a reduced muscle performance. On the opposite end, an exceedingly reduced fiber will also result in lower muscle performance.
Furthermore, oxidative stress contributes to PLFFD in skeletal muscle and both could act as potent promoters of OTS. Within skeletal muscle, ROS appears to promote PLFFD by either reducing Ca2+ release from the sarcoplasmic reticulum (SR) mediated by the ryanodine receptor (RyR1) or myofibrillar Ca2+ sensitivity, and this appears to depend on the major origin of the ROS-producing cellular site (i.e., mitochondria, cytosol), and on the ROS species interacting with various intracellular proteins (i.e., superoxide (O2●-), hydrogen peroxide (H2O2), hydroxyl radicals (●OH), nitric oxide (NO), peroxynitrite (ONOO●-)) [23,24,26,28]. In a state potentially representing chronically elevated oxidative stress in OTS, H2O2 treatment of rat skinned muscle fibers decreased myofibrillar Ca2+ sensitivity and cross-bridge force [106]. A mechanism was revealed whereby prolonged and elevated [H2O2] interacts with the Fe2+ on myoglobin, to generate hydroxyl radicals, which then oxidizes the major cytosolic antioxidant enzyme, glutathione, to decrease myofibrillar Ca2+ sensitivity via irreversible oxidation on the contractile apparatus [106].
Another intramuscular mechanism by which oxidative stress interferes with cross-bridge cycling and force production is by oxidative post-translational modifications (PTMs) on actin [76] (Fig. 1). Using mass spectrometry we recently identified a specific set of oxidative MDA and 3-nitrotyrosine (3-NT) PTMs on skeletal muscle actin from mice and humans with chronic inflammation (i.e. RA), which caused impaired actin polymerization, reduced myofibrillar force production and muscle weakness [76].
Animal models might have been criticized for not completely mimicking OTS in humans, however, the multifunctional cytokine TGF-β1 has been found in transcriptional analyses of skeletal muscle from both animal models and humans with OTS [66,107]. Thus, animal models might be a promising tool for future mechanistic studies in order to further understand cellular and molecular aspects of intramuscular OTS. TGF-β1 belongs to the transforming growth factor superfamily together with myostatin and activin A, and known to have catabolic effects on skeletal muscle [108,109]. TGF-β1 can be secreted by macrophages and is known to induce pathological fibrosis [110,111], but other cells e.g. microvascular smooth muscle cells have also been reported to release TGF-β1 and contribute to fibrosis [108,112,113]. For instance, TGF-β1 can activate myofibroblasts to deposit extracellular matrix, of which a major component is collagen and fibrosis formation [[109], [110], [111]]. Furthermore, patients with peripheral artery disease (PAD) exhibit decreased muscle function that is associated with oxidative stress and mitochondrial defects [114], i.e. skeletal muscle biopsies (gastrocnemius) from PAD patients that exhibit increased levels of oxidative stress markers (e.g. carbonylation (DNP), and lipid hydroperoxides) [114] also have higher TGF-β1 expression which correlates with increasing collagen deposition [108]. Moreover, TGF-β1 has been shown to provoke skeletal muscle weakness by phosphorylation and activation of the SMAD2/3 transcription factor, leading to NOX4 transcription which in turn induces oxidative PTMs on the Ca2+ release channel RyR1 [102]. Oxidative PTMs (e.g. DNP [102,115]) of RyR1 can lead to reduced binding of the 12-kDa FK506-binding protein (FKBP12) to the channel which contributes to SR Ca2+ leak that is considered an underlying mechanism of muscle weakness in several pathological conditions, including diaphragm weakness during mechanical ventilation and in bone metastases [102,115]. Moreover, increased SMAD2/3 signaling and decreased running performance and grip strength have been observed in muscle from mice where overtraining was induced by running [116].
Oxidative PTMs on proteins involved in excitation-contraction coupling (e.g. RyR1) or cross-bridge cycling (e.g. actin, myosin) has, to our knowledge, not been investigated in muscle samples from OTS subjects. However, exhaustive endurance exercise ranging from days to weeks in mice (swimming, running) and human (cycling) have shown a progressive increase in PTM on RyR1 (nitrosylation and phosphorylation) which correlated inversely with FKBP12 binding to RyR1 and increased open probability of RyR1, indicative of enhanced Ca2+ leak [117]. These results suggest that during exercise, remodeling of the RyR1 macromolecular complex with FKBP12 dissociation results in leaky channels that play a role in limiting exercise capacity. The same physiological mechanisms that impair exercise capacity during chronic exercise are likely beneficial during acute exercise, but with repeated exhaustive exercise without recovery period, this may contribute to OTS (Fig. 1). Thus, given that OTS mimics a state of chronic inflammation and oxidative stress, oxidative PTMs modifications of contractile proteins [65,76,106,117,118] are a potential intramuscular mechanism of the decreased force production in OTS.
3.5. Reduced mitochondrial capacity in skeletal muscle from subjects with OTS?
As mitochondria are the cellular powerhouse that generates ATP to fuel muscle force production, reduced oxidative phosphorylation will directly limit exercise performance and thus an obvious player that could contribute to OTS. Athletes with OTS exhibit performance decrements, reduced ability to perform high intensity exercise and persistent high fatigue ratings [9,22,119], which all can be linked to reduced mitochondrial capacity and a reduced maximum oxygen consumption (VO2max). However, understandably, results from VO2max tests are not a reliable physiological indicator of OTS, partly because one might not have a ‘baseline’ value to compare with, but more importantly the listed symptoms of OTS makes it impossible for the subject to perform at maximum capacity in a physiological test. Instead, ex vivo cellular respiration (using e.g. Seahorse or Oroboros instruments) [120,121] of muscle fibers would be a more direct, controlled and repeatable procedure to assess oxygen consumption and energy production rates. To our knowledge, no cellular respiration analyses of skeletal muscle are currently available, but mitochondrial respiration have been analyzed in skinned myofibers from rat myocardium in response to chronic exhaustive exercise [121]. The overtraining resulted in a reduced oxidative phosphorylation rate in myofibers from the overtrained group [121]. In skeletal muscle, lower levels of the mitochondrial oxidative enzyme citrate synthase have been reported in rats with OTS [122], indicative of reduced mitochondrial respiration that might contribute to the impaired performance of skeletal muscle in OTS.
4. Prevention and possible treatment options
OTS may have devastating effects on an athlete's career and thus prevention is of importance. Prevention includes carefully planned training programs that include regular monitoring by coaches and the athletes themselves to assess adaptation to training over both the short and long term. Measures suggested to prevent overtraining include minimizing abrupt increases in training loads, monitoring inadequate dietary intake and too frequent competition, individualizing and periodizing training plans, as well as allowing adequate post-exercise recovery and rest days into the training/competition program. Despite careful prevention, it is possible that athletes develop OTS anyways, and except providing adequate rest, no pharmacological treatment strategies are currently available. However, based on the intramuscular processes that appears to contribute to OTS, one obvious solution to mitigate the syndrome could be the use of antioxidants to alleviate the oxidative stress. We acknowledge that antioxidants (e.g., vitamin C and E) given to healthy individuals can have detrimental effects on endurance training adaptations [[94], [95], [96]]. However, here we imply that OTS more closely resembles a state of chronically elevated oxidative stress, such as in chronic disease, rather than exercise adaptation. Moreover, we have previously shown that the SOD/catalase mimetic EUK-134 is an antioxidant that can counteract muscle weakness induced by oxidative stress and thus could prove useful in improving muscle performance in athletes with OTS [75,77]. Using the same argument but instead that OTS mimics a state of chronic inflammation, an anti-inflammatory treatment option could be an alternative. However, in any case, more research is needed before giving any specific recommendations with doses and length of treatment.
5. Conclusion
OTS has severe implications on training/competition performance and hence may have devastating effects on an athlete's career. OTS is caused by chronic imbalances between exercise-induced fatigue and provision of sufficient post-exercise rest. Skeletal muscle accounts for approximately 40% of your body weight and is essential for our ability to move and breath. Here we showed that skeletal muscle is an important contributor to OTS with the long-lasting prolonged low-frequency force depression developed following exhaustive exercise as a potentially potent inducer of skeletal muscle weakness in OTS. Exercise causes an increase in pro-inflammatory cytokines, which in turn can increase muscle oxidative stress that results in a vicious cycle to further elevate inflammation. Although the effects of post-translational modifications of proteins involved in muscle force generation have not been examined in athletes with OTS, pathological conditions of chronic inflammation and increased oxidative stress have shown that oxidation of calcium-handling and contractile proteins can cause long-term skeletal muscle weakness and exacerbated fatigue. Other than providing adequate rest, there is no effective pharmacological treatment to counteract OTS and accelerate recovery. However, antioxidants and anti-inflammatory compounds may show promise in neutralizing the elevated oxidative stress and chronic inflammation in muscles of athletes with OTS, although further research is required to determine the effectiveness of these pharmacological strategies to treat OTS. Finally, novel animal models that mimic the progression from overreaching to OTS is needed to achieve further mechanistic understanding of this physical impairment, including deciphering the interaction between inflammation and oxidative stress in the development of the decreased muscle performance in OTS.
Author contributions
All authors contributed equally to the writing of this Review and all authors approved the final version of the manuscript.
Funding
Baptiste Jude is supported by a postdoctoral fellowship from the Wenner-Gren Foundation. Johanna Lanner acknowledge support from the Swedish Research Council, the Strategic Research Program in Diabetes and Diabetes Foundation Sweden.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Burd N.A., Mitchell C.J., Churchward-Venne T.A., Phillips S.M. Bigger weights may not beget bigger muscles: evidence from acute muscle protein synthetic responses after resistance exercise. Appl. Physiol. Nutr. Metabol. 2012;37:551–554. doi: 10.1139/h2012-022. [DOI] [PubMed] [Google Scholar]
- 2.Gibala M.J., McGee S.L. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008;36:58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 3.Barnett A. Using recovery modalities between training sessions in elite athletes: does it help? Sports Med. 2006;36:781–796. doi: 10.2165/00007256-200636090-00005. [DOI] [PubMed] [Google Scholar]
- 4.Figueiredo V.C. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiol. Rep. 2016;4 doi: 10.14814/phy2.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood D.A. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 1985;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. 2001. [DOI] [PubMed] [Google Scholar]
- 6.Matos N.F., Winsley R.J., Williams C.A. Prevalence of nonfunctional overreaching/overtraining in young English athletes. Med. Sci. Sports Exerc. 2011;43:1287–1294. doi: 10.1249/MSS.0b013e318207f87b. [DOI] [PubMed] [Google Scholar]
- 7.Koutedakis Y., Sharp N.C. Seasonal variations of injury and overtraining in elite athletes. Clin. J. Sport Med. 1998;8:18–21. doi: 10.1097/00042752-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kreher J.B., Schwartz J.B. Overtraining syndrome: a practical guide. Sport Health. 2012;4:128–138. doi: 10.1177/1941738111434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacKinnon L.T. Special feature for the Olympics: effects of exercise on the immune system: overtraining effects on immunity and performance in athletes. Immunol. Cell Biol. 2000;78:502–509. doi: 10.1111/j.1440-1711.2000.t01-7-.x. [DOI] [PubMed] [Google Scholar]
- 10.Morgan W.P., O'Connor P.J., Sparling P.B., Pate R.R. Psychological characterization of the elite female distance runner. Int. J. Sports Med. 1987;8(Suppl 2):124–131. doi: 10.1055/s-2008-1025717. [DOI] [PubMed] [Google Scholar]
- 11.Meeusen R. Diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013;45:186–205. doi: 10.1249/MSS.0b013e318279a10a. [DOI] [PubMed] [Google Scholar]
- 12.Margonis K. Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radic. Biol. Med. 2007;43:901–910. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Taylor K.L., Chapman D.W., Cronin J.B., Newton M.J., Gill N. Fatigue monitoring in high performance sport: a survey of current trends. J. Aust. Strength Cond. 2012;20:12–23. [Google Scholar]
- 14.Callister R., Callister R.J., Fleck S.J., Dudley G.A. Physiological and performance responses to overtraining in elite judo athletes. Med. Sci. Sports Exerc. 1990;22:816–824. doi: 10.1249/00005768-199012000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Coutts A., Reaburn P., Piva T.J., Murphy A. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int. J. Sports Med. 2007;28:116–124. doi: 10.1055/s-2006-924145. [DOI] [PubMed] [Google Scholar]
- 16.Koutedakis Y., Frischknecht R., Vrbova G., Sharp N.C., Budgett R. Maximal voluntary quadriceps strength patterns in Olympic overtrained athletes. Med. Sci. Sports Exerc. 1995;27:566–572. [PubMed] [Google Scholar]
- 17.Edwards R.H., Hill D.K., Jones D.A., Merton P.A. Fatigue of long duration in human skeletal muscle after exercise. J. Physiol. (Lond.) 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allman B.L., Rice C.L. Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve. 2001;24:1156–1167. doi: 10.1002/mus.1127. [DOI] [PubMed] [Google Scholar]
- 19.Kamandulis S. Prolonged force depression after mechanically demanding contractions is largely independent of Ca(2+) and reactive oxygen species. Faseb. J. 2017;31:4809–4820. doi: 10.1096/fj.201700019R. [DOI] [PubMed] [Google Scholar]
- 20.Place N. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. Unit. States Am. 2015 doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayers S.P., Clarkson P.M. Force recovery after eccentric exercise in males and females. Eur. J. Appl. Physiol. 2001;84:122–126. doi: 10.1007/s004210000346. [DOI] [PubMed] [Google Scholar]
- 22.Urhausen A., Gabriel H.H., Weiler B., Kindermann W. Ergometric and psychological findings during overtraining: a long-term follow-up study in endurance athletes. Int. J. Sports Med. 1998;19:114–120. doi: 10.1055/s-2007-971892. [DOI] [PubMed] [Google Scholar]
- 23.Cheng A.J., Bruton J.D., Lanner J.T., Westerblad H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015;593:457–472. doi: 10.1113/jphysiol.2014.279398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe D., Aibara C., Wada M. Treatment with EUK-134 improves sarcoplasmic reticulum Ca(2+) release but not myofibrillar Ca(2+) sensitivity after fatiguing contraction of rat fast-twitch muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;316:R543–r551. doi: 10.1152/ajpregu.00387.2018. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe D. Contribution of impaired myofibril and ryanodine receptor function to prolonged low-frequency force depression after in situ stimulation in rat skeletal muscle. J. Muscle Res. Cell Motil. 2015;36:275–286. doi: 10.1007/s10974-015-9409-1. [DOI] [PubMed] [Google Scholar]
- 26.Bruton J.D. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J. Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng A.J. Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. J. Physiol. 2017;595:7413–7426. doi: 10.1113/jp274870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandra P.G., Shiah A.A., Nogueira L., Hogan M.C. A mitochondrial-targeted antioxidant improves myofilament Ca2+ sensitivity during prolonged low frequency force depression at low. J. Physiol. 2018;596:1079–1089. doi: 10.1113/jp275470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a003996. cshperspect.a003996 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 31.Smith I.C., Bombardier E., Vigna C., Tupling A.R. ATP consumption by sarcoplasmic reticulum Ca(2)(+) pumps accounts for 40-50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PloS One. 2013;8 doi: 10.1371/journal.pone.0068924. e68924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S.J., Andersson D.C., Sandström M.E., Westerblad H., Katz A. Cross-bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am. J. Physiol. Cell Physiol. 2006;291:C147–C154. doi: 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ortenblad N., Nielsen J. Muscle glycogen and cell function--Location, location, location. Scand. J. Med. Sci. Sports. 2015;25(Suppl 4):34–40. doi: 10.1111/sms.12599. [DOI] [PubMed] [Google Scholar]
- 34.Chin E.R., Allen D.G. Effects of reduced muscle glycogen concentration on force, Ca 2+ release and contractile protein function in intact mouse skeletal muscle. J. Physiol. (Lond.) 1997;498:17–29. doi: 10.1113/jphysiol.1997.sp021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen J., Schroder H.D., Rix C.G., Ortenblad N. Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J. Physiol. 2009;587:3679–3690. doi: 10.1113/jphysiol.2009.174862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortenblad N., Nielsen J., Saltin B., Holmberg H.C. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J. Physiol. 2011;589:711–725. doi: 10.1113/jphysiol.2010.195982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piehl K., Adolfsson S., Nazar K. Glycogen storage and glycogen synthetase activity in trained and untrained muscle of man. Acta Physiol. Scand. 1974;90:779–788. doi: 10.1111/j.1748-1716.1974.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiens B., Richter E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am. J. Physiol. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 39.Jentjens R., Jeukendrup A. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003;33:117–144. doi: 10.2165/00007256-200333020-00004. [DOI] [PubMed] [Google Scholar]
- 40.Pilegaard H. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism. 2005;54:1048–1055. doi: 10.1016/j.metabol.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Krustrup P. Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Eur. J. Appl. Physiol. 2011;111:2987–2995. doi: 10.1007/s00421-011-1919-y. [DOI] [PubMed] [Google Scholar]
- 42.Kirwan J.P. Carbohydrate balance in competitive runners during successive days of intense training. J. Appl. Physiol. 1985;65:2601–2606. doi: 10.1152/jappl.1988.65.6.2601. 1988. [DOI] [PubMed] [Google Scholar]
- 43.Coutinho de Oliveira C.V. Carbohydrate supplementation attenuates decrement in performance in overtrained rats. Appl. Physiol. Nutr. Metabol. 2016;41:76–82. doi: 10.1139/apnm-2015-0393. [DOI] [PubMed] [Google Scholar]
- 44.Peake J.M., Neubauer O., Della Gatta P.A., Nosaka K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 1985;122:559–570. doi: 10.1152/japplphysiol.00971.2016. 2017. [DOI] [PubMed] [Google Scholar]
- 45.Chen T.C. Damage and the repeated bout effect of arm, leg, and trunk muscles induced by eccentric resistance exercises. Scand. J. Med. Sci. Sports. 2019;29:725–735. doi: 10.1111/sms.13388. [DOI] [PubMed] [Google Scholar]
- 46.Nosaka K., Clarkson P.M. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med. Sci. Sports Exerc. 1996;28:953–961. doi: 10.1097/00005768-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Newham D.J., Jones D.A., Edwards R.H. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983;6:380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- 48.Kamandulis S. The contribution of low-frequency fatigue to the loss of quadriceps contractile function following repeated drop jumps. Exp. Physiol. 2019;104:1701–1710. doi: 10.1113/ep087914. [DOI] [PubMed] [Google Scholar]
- 49.Malm C. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004;556:983–1000. doi: 10.1113/jphysiol.2003.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulsen G. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med. Sci. Sports Exerc. 2010;42:75–85. doi: 10.1249/MSS.0b013e3181ac7adb. [DOI] [PubMed] [Google Scholar]
- 51.Paulsen G. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand. J. Med. Sci. Sports. 2010;20:e195–e207. doi: 10.1111/j.1600-0838.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 52.Lauritzen F., Paulsen G., Raastad T., Bergersen L.H., Owe S.G. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J. Appl. Physiol. 1985;107:1923–1934. doi: 10.1152/japplphysiol.00148.2009. 2009. [DOI] [PubMed] [Google Scholar]
- 53.Newham D.J., McPhail G., Mills K.R., Edwards R.H.T. Ultrastructural changes after concentric and eccentric contractions of human muscle. J. Neurol. Sci. 1983;61:109–122. doi: 10.1016/0022-510X(83)90058-8. [DOI] [PubMed] [Google Scholar]
- 54.Power G.A., Dalton B.H., Rice C.L., Vandervoort A.A. Delayed recovery of velocity-dependent power loss following eccentric actions of the ankle dorsiflexors. J. Appl. Physiol. 1985;109:669–676. doi: 10.1152/japplphysiol.01254.2009. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newham D.J., Jones D.A., Edwards R.H. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve. 1986;9:59–63. doi: 10.1002/mus.880090109. [DOI] [PubMed] [Google Scholar]
- 56.Pasquet B., Carpentier A., Duchateau J., Hainaut K. Muscle fatigue during concentric and eccentric contractions. Muscle Nerve. 2000;23:1727–1735. doi: 10.1002/1097-4598(200011)23:11<1727::aid-mus9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 57.Paulsen G., Mikkelsen U.R., Raastad T., Peake J. M. Leucocytes. Cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 58.Yu J.-G., Carlsson L., Thornell L.-E. Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: an ultrastructural and immunoelectron microscopic study. Histochem. Cell Biol. 2004;121:219–227. doi: 10.1007/s00418-004-0625-9. [DOI] [PubMed] [Google Scholar]
- 59.Mackey A.L. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J. Appl. Physiol. 1985;103:425–431. doi: 10.1152/japplphysiol.00157.2007. 2007. [DOI] [PubMed] [Google Scholar]
- 60.Mikkelsen U.R. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J. Appl. Physiol. 1985;107:1600–1611. doi: 10.1152/japplphysiol.00707.2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trappe T.A. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2002;282:E551–E556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 62.Lilja M. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2018;222 doi: 10.1111/apha.12948. [DOI] [PubMed] [Google Scholar]
- 63.Tidball J.G., Dorshkind K., Wehling-Henricks M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development. 2014;141:1184. doi: 10.1242/dev.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Summan M. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 65.Persson M., Steinz M.M., Westerblad H., Lanner J.T., Rassier D.E. Force generated by myosin cross-bridges is reduced in myofibrils exposed to ROS/RNS. Am. J. Physiol. Cell Physiol. 2019 doi: 10.1152/ajpcell.00272.2019. [DOI] [PubMed] [Google Scholar]
- 66.Neubauer O. Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: from inflammation to adaptive remodeling. J. Appl. Physiol. 1985;116:274–287. doi: 10.1152/japplphysiol.00909.2013. 2014. [DOI] [PubMed] [Google Scholar]
- 67.Tidball J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017;17:165–178. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tidball J.G., Villalta S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardi M., Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metabol. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Iwasaki H., Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Arnold L. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun D. Bone marrow-derived cell regulation of skeletal muscle regeneration. Faseb. J. 2008;23:382–395. doi: 10.1096/fj.07-095901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 74.Tidball J.G., Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Himori K. Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension. PloS One. 2017;12 doi: 10.1371/journal.pone.0169146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinz M.M. Oxidative hotspots on actin promote skeletal muscle weakness in rheumatoid arthritis. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamada T. Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment. Skeletal Muscle. 2015;5:20. doi: 10.1186/s13395-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada T. Nitrosative modifications of the Ca2+ release complex and actin underlie arthritis-induced muscle weakness. Ann. Rheum. Dis. 2015;74:1907–1914. doi: 10.1136/annrheumdis-2013-205007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamada T. Electrical stimulation prevents preferential skeletal muscle myosin loss in steroid-denervation rats. Front. Physiol. 2018;9:1111. doi: 10.3389/fphys.2018.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamada T., Steinz M.M., Kenne E., Lanner J.T. Muscle weakness in rheumatoid arthritis: the role of Ca2+ and free radical signaling. EBioMedicine. 2017;23:12–19. doi: 10.1016/j.ebiom.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.VanderVeen B.N. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp. Physiol. 2019;104:385–397. doi: 10.1113/ep087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.da Rocha A.L. The proinflammatory effects of chronic excessive exercise. Cytokine. 2019;119:57–61. doi: 10.1016/j.cyto.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 83.Gholamnezhad Z., Boskabady M.H., Hosseini M., Sankian M., Khajavi Rad A. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran. J. Basic Med. Sci. 2014;17:1–8. [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph J. Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium. Nat. Commun. 2016;7 doi: 10.1038/ncomms12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parsa R. TGFbeta regulates persistent neuroinflammation by controlling Th1 polarization and ROS production via monocyte-derived dendritic cells. Glia. 2016;64:1925–1937. doi: 10.1002/glia.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bala A., Mondal C., Haldar P.K., Khandelwal B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: clinical efficacy of dietary antioxidants. Inflammopharmacology. 2017;25:595–607. doi: 10.1007/s10787-017-0397-1. [DOI] [PubMed] [Google Scholar]
- 87.Palazzetti S., Richard M.J., Favier A., Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can. J. Appl. Physiol. 2003;28:588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- 88.Hyldahl R.D., Olson T., Welling T., Groscost L., Parcell A.C. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol. 2014;5 doi: 10.3389/fphys.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng A.J. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016;594:5149–5160. doi: 10.1113/jp270650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reid M.B., Stokic D.S., Koch S.M., Khawli F.A., Leis A.A. N-acetylcysteine inhibits muscle fatigue in humans. J. Clin. Invest. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. (Lond.) 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakellariou G.K. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gejl K.D. Repeated high-intensity exercise modulates Ca(2+) sensitivity of human skeletal muscle fibers. Scand. J. Med. Sci. Sports. 2016;26:488–497. doi: 10.1111/sms.12483. [DOI] [PubMed] [Google Scholar]
- 94.Gomez-Cabrera M.C. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. 87/1/142 [pii] [DOI] [PubMed] [Google Scholar]
- 95.Ristow M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paulsen G. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014;592:1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khairallah R.J. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci. Signal. 2012;5:ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lanner J.T. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat. Med. 2012;18:244–251. doi: 10.1038/nm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andersson D.C. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metabol. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turner J.E., Hodges N.J., Bosch J.A., Aldred S. Prolonged depletion of antioxidant capacity after ultraendurance exercise. Med. Sci. Sports Exerc. 2011;43:1770–1776. doi: 10.1249/MSS.0b013e31821240bb. [DOI] [PubMed] [Google Scholar]
- 101.Tanskanen M., Atalay M., Uusitalo A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010;28:309–317. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- 102.Waning D.L. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015;21:1262–1271. doi: 10.1038/nm.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 104.Westerblad H., Allen D.G. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxidants Redox Signal. 2011;15:2487–2499. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 105.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 106.Murphy R.M., Dutka T.L., Lamb G.D. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J. Physiol. (Lond.) 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao W., Chen P., Dong J. Effects of overtraining on skeletal muscle growth and gene expression. Int. J. Sports Med. 2012;33:846–853. doi: 10.1055/s-0032-1311585. [DOI] [PubMed] [Google Scholar]
- 108.Ha D.M. Transforming growth factor-beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J. Transl. Med. 2016;14:39. doi: 10.1186/s12967-016-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mendias C.L. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45:55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mann C.J. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gibbons G.H., Pratt R.E., Dzau V.J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J. Clin. Invest. 1992;90:456–461. doi: 10.1172/jci115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ostriker A., Horita H.N., Poczobutt J., Weiser-Evans M.C., Nemenoff R.A. Vascular smooth muscle cell-derived transforming growth factor-beta promotes maturation of activated, neointima lesion-like macrophages. Arterioscler. Thromb. Va c. Biol. 2014;34:877–886. doi: 10.1161/atvbaha.114.303214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pipinos I.I. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic. Biol. Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 115.Matecki S. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc. Natl. Acad. Sci. U.S.A. 2016;113:9069–9074. doi: 10.1073/pnas.1609707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.da Rocha A.L. Downhill running excessive training inhibits hypertrophy in mice skeletal muscles with different fiber type composition. J. Cell. Physiol. 2016;231:1045–1056. doi: 10.1002/jcp.25197. [DOI] [PubMed] [Google Scholar]
- 117.Bellinger A.M. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mollica J.P. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012;590:1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hooper S.L., Mackinnon L.T., Howard A., Gordon R.D., Bachmann A.W. Markers for monitoring overtraining and recovery. Med. Sci. Sports Exerc. 1995;27:106–112. [PubMed] [Google Scholar]
- 120.Agudelo L.Z. Skeletal muscle PGC-1alpha1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019;10:2767. doi: 10.1038/s41467-019-10712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kadaja L. Impaired oxidative phosphorylation in overtrained rat myocardium. Exp. Clin. Cardiol. 2010;15:e116–127. [PMC free article] [PubMed] [Google Scholar]
- 122.Ferraresso R.L. Interaction between overtraining and the interindividual variability may (not) trigger muscle oxidative stress and cardiomyocyte apoptosis in rats. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/935483. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]