Abstract
Frailty, a consequence of the interaction of the aging process and certain chronic diseases, compromises functional outcomes in the elderly and substantially increases their risk for developing disabilities and other adverse outcomes. Frailty follows from the combination of several impaired physiological mechanisms affecting multiple organs and systems. And, though frailty and sarcopenia are related, they are two different conditions. Thus, strategies to preserve or improve functional status should consider systemic function in addition to muscle conditioning. Physical activity/exercise is considered one of the main strategies to counteract frailty-related physical impairment in the elderly. Exercise reduces age-related oxidative damage and chronic inflammation, increases autophagy, and improves mitochondrial function, myokine profile, insulin-like growth factor-1 (IGF-1) signaling pathway, and insulin sensitivity. Exercise interventions target resistance (strength and power), aerobic, balance, and flexibility work. Each type improves different aspects of physical functioning, though they could be combined according to need and prescribed as a multicomponent intervention. Therefore, exercise intervention programs should be prescribed based on an individual's physical functioning and adapted to the ensuing response.
Keywords: Aging, Frailty, Physical activity, Exercise, Oxidative stress, Multicomponent intervention
Highlights
-
•
Intrinsic capacity close to disability threshold and low potential recovery due to low functional reserve lead to frailty.
-
•
Frailty follows from the combination of several impaired physiological mechanisms affecting multiple organs and systems.
-
•
Exercise reduces age-related oxidative damage and inflammation and improves mitochondrial function among other benefits.
-
•
Multicomponent intervention should be considered in order to impact different aspects of physical function in the elderly.
-
•
Exercise interventions should be prescribed based on individual’s physical functioning and adapted to the ensuing response.
1. Introduction
Increasing numbers of people reaching the ranks of the elderly is one of the achievements of the past century. Whereas the early years of the 20th Century saw a life expectancy of about 30 years, this statistic had more than doubled worlwide by the end of the century [1]. Further, this trend will continue at least until the middle of the current century. In 2015, there were 617 million people of the age of 65 or older, and the next 35 years will see a massive increase in the percentage of older people. The fastest growing age group will be those over the age of 80, reaching 447 million individuals by 2050, three times the current number [2]. Based on these predictions, millions of people will endure the aging process, with variations depending upon cultural and socio-economic conditions [3].
The aging process is a somewhat more complex issue than just the passing of time. Aging is characterized by several highly prevalent changes, including an increase in morbidity and a decrease in functional performance which, although linked, are two separate conditions [4,5]. In fact, as we age, functioning increasingly becomes the factor most strongly associated with quality of life and the risk for several adverse outcomes, including hospitalization, permanent institutionalization, use of health and social resources, and death [6].
In 2015, the World Health Organisation (WHO) released its first report on aging and health [7] in which it recognized the true relevance of function, the components involved in its preservation or deterioration, and how the relationship between what I am able to do and the challenges of the environment mark the presence of functional autonomy or disability. In that report, WHO defines healthy aging as “the process of developing and maintaining the functional ability that enables wellbeing in older ages.” According to this definition, older people with multiple diseases may enjoy a healthy aging process if they maintain functional ability, i.e., elderly's health status is defined by functional status rather than morbidity. This stance is a true milestone not only in its conception of health with its theoretical consequences but also in its approach to health, with its practical consequences [8,9]. The impact of the latter ones on the elderly is extremely important, as the possibility of prolonging life is dramatically reduced [10] and the potential for improving the quality of life reaches its maximal relevance.
Which factors are associated with function and, secondly, linked to personal autonomy? WHO (2015) distinguishes between two types of factors. The first type falls under what is called intrinsic capacity and the second type is environment. Intrinsic capacity is defined as “the composite of all the physical and mental capacities an individual can draw on” and is the final outcome caused by the joint effect of genetic background, chronic diseases, lifestyles, changes due to aging and broader geriatric syndromes.
Thus, in a stable environment, function is mainly determined by intrinsic capacity. However, intrinsic capacity starts declining at a quite constant rate (1% per year) as soon as the maturity process is completed (in human beings, around 20–25 years of age) [11]. When this declining intrinsic capacity reaches a certain threshold, challenges derived from the environment are no longer possible to be overcome and disability appears (Fig.1). Hence, in older people the transition from a robust state to disability is a long-lasting, continuous process which may take years [12]. Meanwhile, as intrinsic capacity declines an associated component also decreases: functional reserve (Fig. 2). Functional reserve is essential, among other reasons, to avoid stressors impacting function and, if impacted, to recover the affected function. Consequently, when intrinsic capacity and functional reserve are under minimums, the risk for additional disability is very high and the possibility of recovery is very low. This conceptual framework should include a stage in the pathway from robustness to disability where the intrinsic capacity is low, but still above the disability threshold, and the functional reserve is reduced, but still enough for total or partial function recovery. This stage in the pathway, characterized by high susceptibility to low power stressors and high risk for adverse outcomes (e.g., disability and its associated consequences like hospitalization, institutionalization, or death) while still maintaining potential for recovery, is what it is referred to as frailty [[13], [14], [15]].
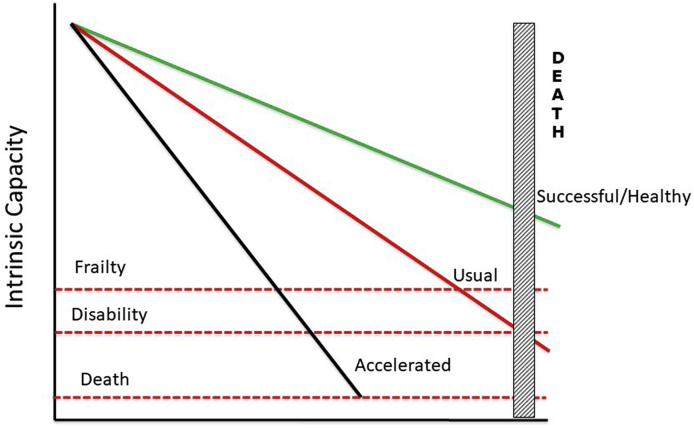
Fig. 1.
Three different trajectories of intrinsic capacity along the lifetime: Accelerated aging, usual aging, and successful/healthy aging. Trajectories are suitable for intervention, acting on the rate of decrease in intrinsic capacity.
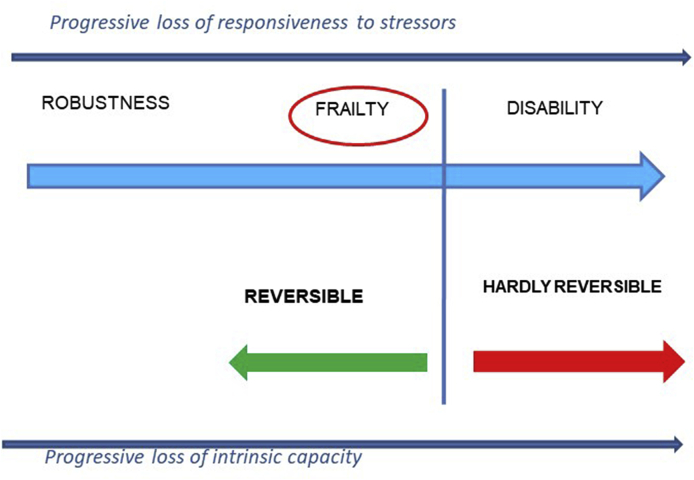
Fig. 2.
Shortening of intrinsic capacity and functional reserve leading to frailty and, if no intervention, to disability and death. As these shortages progress, the likelihood of reversing functional impairment decreases. When the threshold for disability is crossed, the possibility of restoring robustness is uncertain.
Prevention or delay of functional status decline, progression to disability, with its characteristic loss of personal self-sufficiency has been one of the main classical but also ongoing objectives of geriatric medicine [6,[16], [17], [18]]. Therefore, in the past decades frailty has received increasing attention as a window of opportunity to avoid or postpone disability. Frailty has been examined using two different approaches [15]. One of them, measuring deficit accumulation generates a frailty index; the other one is defining a particular phenotype (the frailty phenotype), which denotes the underlying processes where the effects of the aging process, subclinical and clinical diseases merge (Fig. 3). The frailty phenotype also captures the mechanisms shared by the aging process and certain chronic diseases which affect several organs and systems. These mechanisms are then phenotypically expressed as functional deterioration, including frailty [19]. By either approach, the roads leading to frailty are varied, with different trajectories and rates [20].
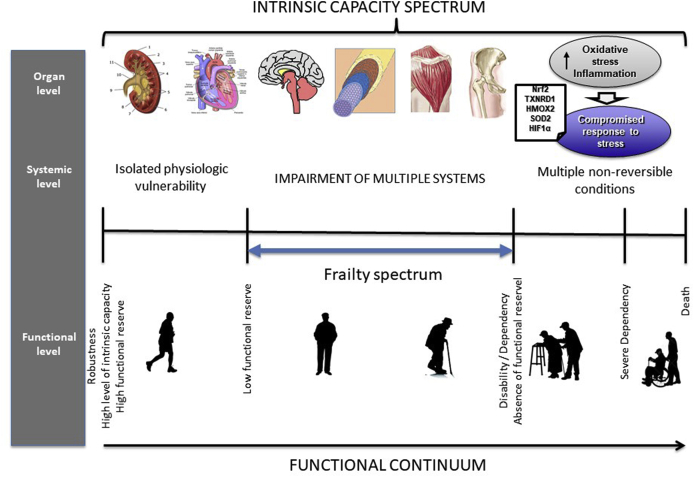
Fig. 3.
The spectrum of intrinsic capacity from the perspectives of organ and systemic level, and their clinical manifestations. In the first stages, there are changes in isolated organs that can still be reversed and do not impact function due to a high functional reserve. As the condition progresses, other organs start to deteriorate, due to the aging processes or to the accumulation of chronic diseases sharing similar mechanisms of damage. At this stage frailty status appears: there is still enough functional reserve to maintain functional autonomy, although some deterioration in performance-based tasks can be observed if carefully assessed. If the condition continues progressing, functional reserve is depleted and disability takes hold, with few chances for recovery.
Increasing evidence reports the benefits yielded by exercise and multimodal interventions on the functional status of older people, including frailty. Data clearly support early intervention in the pathway from robustness to disability in order to maximize potential benefits [21,22]. This evidence has fostered the assessment of pre-disability conditions, i.e., when individuals are still independent but their performance (as an indicator of the risk for developing disability) is impaired, as it is the case in frailty. Although there are over 50 frailty assessment tools, for the purpose of this review we will use the Frailty Phenotype as designed by Fried and colleagues and its three categories. The Frailty Phenotype evaluates five health- and function-related domains and classifies people into three categories accordingly: Robust (0 domains affected), pre-frail (1–2) and frail (≥3) [13].
This review has four main aims. First, to provide data and describe the mechanisms involved in frailty which emerge from the aging process itself and/or chronic diseases affecting certain organs and systems. Second, to show how physical activity and exercise hinder some of those harmful mechanisms, thus improving physical function and delaying or reversing frailty. Third, to analyse the individual components of the physical exercise programs and their best implementation to abate frailty. And, finally, to analyse current evidence and bases for designing, implementing, and evaluating a program of physical exercise for older frail people.
2. Pathophysiology of frailty
Frailty is a clinical syndrome that affects multiple key systems including the endocrine, respiratory, and cardiovascular systems as well as the skeletal muscle. This syndrome often marks the onset of the process known as “Cycle of Frailty” which leads to sarcopenia and other multisystemic failures [23] (Fig. 4). Close knowledge on how the aging process interacts with chronic diseases to impair organic systems function and bring about frailty is key for designing successful preventative strategies.
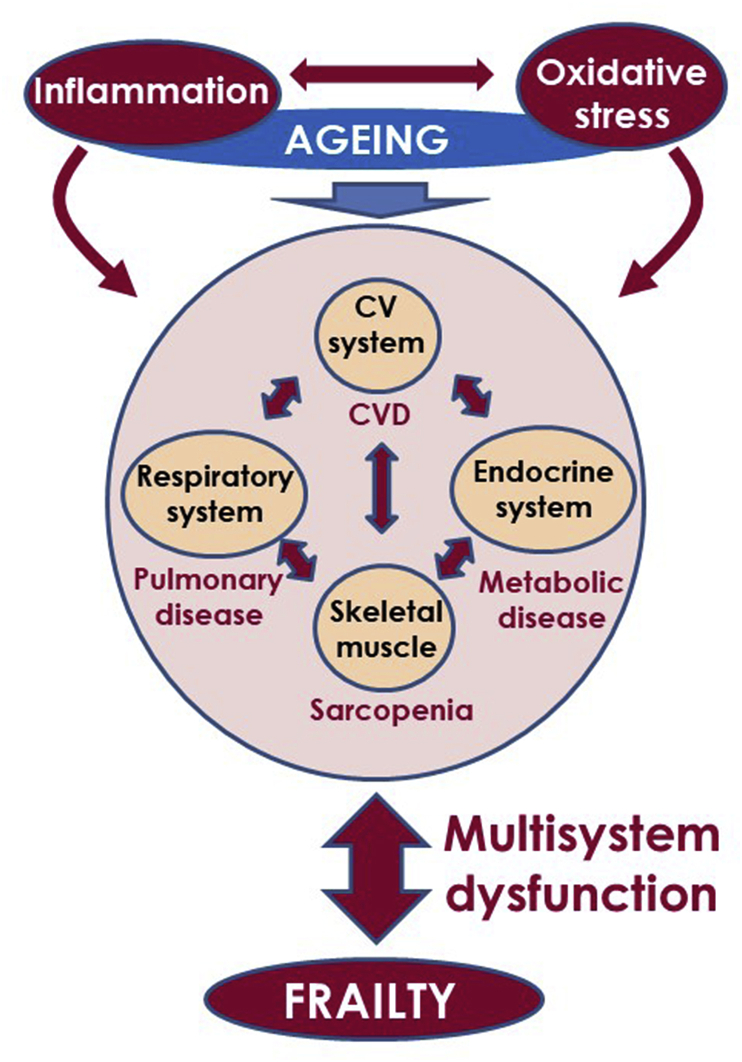
Fig. 4.
Aging together with increase in oxidative damage and chronic inflammation represent three interrelated age-dependent processes that provide a background prone to organic systems dysfunction and age-related chronic diseases. The interaction between age-related chronic diseases, aging process, oxidative stress, and inflammation may lead to multisystem dysfunction and frailty phenotype in the elderly.
2.1. Skeletal muscle
Evidence clearly establishes an association between frailty and the structure and function of skeletal muscle. However, although sarcopenia is one of the key risk factors for frailty syndrome, frailty and sarcopenia are distinct conditions [24]. In fact, muscle alterations are detected only in, approximately, 2/3 of frail individuals. This suggests that the frailty phenotype, albeit influenced by skeletal muscle function, is a clinical manifestation of a multisystemic functional impairment [25].
Originally conceptualized as the age-related loss of skeletal muscle mass, the concept of sarcopenia has evolved to stand for the decline of muscle mass and function among older adults [26]. In addition, the aging process compromises both muscle structure and function. The reduction in muscle mass causes an increase in the proportion of maximal power generating capacity of the remaining muscles performing the activities of daily living. This, let's say, overuse of the remaining muscles, leads to an earlier onset of fatigue which, in turn, hastens the shift from an independent to a dependent lifestyle [27].
Lean muscle mass suffers a considerable decline with age. On average, it makes up 50% of total body weight in young adults versus about 25% in 70 to 80 year-olds [28]. This decline, especially that of the lower limb, impacts mobility status significantly. Further, age-related muscle mass loss has been associated to a state of “anabolic resistance” where there is an imbalance of muscle protein synthesis and breakdown processes in response to stimuli, like exercise or nutrition [29]. In fact, reduced rates of cumulative muscle protein synthesis accompanied by blunted mass gains were observed in older adults in response to resistance exercise training when compared to young adults [30,31]. Both cross-sectional and longitudinal studies of older adults of both genders place the onset of age-related decline in muscle strength on the third or fourth decade of life [[32], [33], [34], [35]]. However, longitudinal studies reveal that muscle mass loss does not fully account for the loss of muscle strength and physical function in older adults [36,37]. In fact, muscle strength is lost much more rapidly than the concomitant muscle mass [38,39]. Thus, muscle strength, the best single measure of age-related muscle changes, is associated with physical disability in instrumental activities of daily living and functional limitation [40].
Additionally, changes in muscle quality (force corrected for size) also take place during aging [41,42]. Muscle power declines earlier and faster with age than strength does [43]. And, unfortunately, muscle power seems to have a stronger association to functional capacity among frail elderly populations than muscle strength [44].
Other authors have reported that age-related muscle power loss may be impacted by a reduced number of motor units, with the denervation of muscle preceding the loss of muscle axons and consecutively motor unit death [45,46]. As a compensatory mechanism, adjacent healthy motor neurons (often type I) reinnervate the denervated muscle fibers (often type II) through terminal axonal sprouting [47]. However, as this mechanism turns defective as we age, it actually triggers muscle loss by increasing myocytes apoptotic potential due to the loss of trophic factors [48]. It has been suggested that age-related changes in the grip and leg extensor strength are not primarily due to muscle atrophy but are reflective of declines in the integrity of the nervous system [26]. Interestingly, well-trained seniors who had exercised regularly in the previous 30 years had two advantages over healthy sedentary age-matched seniors; first, they had retained a greater maximal muscle force and function and, second, they had preserved muscle fibers size resulting from fiber rescue by reinnervation [49].
Further, research on satellite cells (SCs) reveals more potential links between exercise, physical functioning, and muscle health. SCs represent a population of muscle stem cells that reside within muscle tissues in defined niches and promote skeletal muscle repair [50]. Proliferation and differentiation of SCs during muscle regeneration is profoundly influenced by innervation, vasculature, hormones, nutrition, and the extent of tissue damage [51]. SCs may also play an important role in muscle maintenance and muscle growth in response to resistance exercise training [52]. Compared to younger muscle, aged muscle contains fewer SCs and a higher proportion of them display impaired proliferative capacity and regenerative potential, which may explain muscle's failure to regenerate successfully [53] and the inability to maintain its mass [52]. Fortunately, even though SCs density in type II muscle fibers and the size of the fibers themselves declined with age, both magnitudes increased after resistance exercise training for 12 weeks [54]. However, despite reports of a clear correlation between age and impaired regenerative potential of SCs, conflicting results arising from animal models [55,56] keep the debate on the contribution of SCs to sarcopenia alive.
Authors have reported that muscle function alterations not only contribute to an individual's frailty status, but that reduced muscle strength, as measured by grip strength, is associated with an increased risk of mortality [57,58]. Recently, grip strength has revealed as a strong predictor of cardiovascular mortality and a moderately strong predictor of incident cardiovascular disease. These findings suggest muscle strength as a risk factor for incident cardiovascular disease and as a predictor of overall mortality [59]. In addition, regional muscle strength was shown as a predictor of medium-term mortality and hospitalization in older men and women [60].
2.2. Respiratory system
Pulmonary function also declines progressively with aging [61] due to fewer alveoli and capillaries or reduced diffusing capacity and increased residual volumes among other factors [62]. In addition, increased stiffness of chest wall combined with reduced respiratory muscle strength result in a shrunk forced expiratory volume [62]. These lung changes may, in turn, further contribute to loss in muscle strength and power as well as mobility. Further, the reverse has also been suggested, i.e., that the decline in muscle strength may lead to reduced pulmonary function, low physical performance, and mobility disability [63]. Although cross-sectional, a study in healthy older adults suggests that decline in mobility with aging may be due to a drop in both muscle strength and power and also mediated by decreases in spirometric pulmonary function [64]. In this sense, by limiting energy supply, lessened pulmonary function could result in impaired leg strength, contributing to the development of mobility disability [65]. Nevertheless, the observed association between mobility disability incidence, loss of ability to ambulate and pulmonary function in the elderly was relatively independent of respiratory muscle strength and leg strength. This suggests that physical activity and pulmonary function seem to contribute to the development of mobility disability through mechanisms other than the already known effects on muscle strength [66].
Findings regarding how frailty and exercise capacity were related to pulmonary function in community-dwelling disabled older women support the significance of respiratory function in physical performance. Further, the beneficial effects of pulmonary function on exercise capacity were only significant in the absence of frailty [67]. Along these lines, we may expect pulmonary/respiratory diseases to precipitate frailty and mobility disability. Supporting this idea, a higher frailty index was observed in a large sample of patients with chronic obstructive pulmonary disease (COPD) [68]. And indeed, a recent meta-analysis of observational studies concluded that older participants with COPD had a two-fold increased risk for frailty [69]. COPD patients exhibited lower physical function [70] and the risk for frailty with mobility disability increased in COPD patients as the disease grew in severity [71]. In fact, COPD was revealed as a main risk factor for frailty in a large sample of middle-aged and older subjects [72].
Finally, frailty has been reported as an independent predictor of all-cause mortality in patients with interstitial lung disease referred for lung transplantation [73] as well as associated with incident and longer-duration hospitalization and poor quality of life in COPD patients [74]. In fact, it has been proposed that frailty is an independent risk factor for the development and progression of COPD and, in turn, evidence supports that COPD can lead to frailty [75]. In fact, longitudinal studies have reported that the association between frailty and COPD is, indeed, bidirectional [76].
2.3. Cardiovascular system
The incidence and severity of subclinical and clinical manifestations of cardiovascular diseases (CVD) increase steeply with advanced age [77,78] even in the absence of traditional risk factors [79]. That is, even after accounting for the higher prevalence of cardiovascular risk factors in older people, the aging process, by its own nature, independently worsens cardiovascular health [80]. The impact of the passing of time on structure and function of the cardiovascular system leads not only to cardiovascular events and strokes, but also to frailty, functional decline, and cognitive impairment [81]. Aging-related alterations involve the endothelium, vascular smooth muscle cells, as well as the extracellular matrix of vessel wall [82,83]. Intima-media thickening (IMT), increased arterial stiffness, and dilation of central elastic arteries are key aspects of age-associated changes in vasculature resulting in reduced ability to expand and contract in response to pressure variations [84]. Further, reduced thigh muscle cross-sectional area as an indicator of sarcopenia was associated with increased carotid IMT and pulse wave velocity (PWV) as a measure of arterial stiffness in middle-aged to elderly men. Conversely, thigh skeletal muscle cross-sectional area was reported as an independent determinant of PWV, suggesting that sarcopenia and atherosclerosis may interact with each other [85].
As it is known, increased arterial stiffness is related to advanced age [86]. And, it has been proposed to contribute to the association between sarcopenia and cognitive impairment [87]. Furthermore, cross-sectional studies in community-dwelling older adults confirmed that frailty was associated with arterial stiffness [88,89]. Based on data from a sample of patients in a Chinese Hospital, frailty was also shown to be associated with increased carotid IMT as well as with arterial stiffness measured as cardio-ankle vascular index (CAVI). Likewise, CAVI was higher in the frail group than in the pre-frail or robust groups and was inversely correlated with grip strength and walking speed [90].
Arterial stiffness is linked to endothelial dysfunction as suggested by the impairment of endothelial vasodilation that always precedes arterial stiffness [91]. Endothelial dysfunction, manifested by a reduction of the endothelium-dependent vasodilation, is associated with the aging process in both the micro- and the macrovasculature of animal models [92] and humans [82,93,94]. Endothelial dysfunction plays a key role in the onset and persistence of atherosclerosis, and as an independent predictor of cardiovascular events [95]. Endothelial function is related to physical performance as indicated by the significant relationship between brachial artery flow-mediated dilation (FMD) and physical function in elderly men [96]. Indeed, the impairment of endothelial vasodilation induced by insulin in older adults relates to the defective anabolism of muscle proteins driven by this hormone in skeletal muscle [97]. In addition, the levels of asymmetric dimethylarginine (ADMA), a marker of endothelial dysfunction, increase the risk of frailty in older adults free of atherosclerotic disease [98]. Moreover, frailty was associated with worse endothelial function and mortality in patients with chronic kidney disease [99].
Along similar lines, the role of vascular health in motor function is supported by longitudinal studies results reporting associations between elevated cardiovascular risk in early midlife and poor motor function later in life [100]. In addition, carotid IMT at baseline was associated with lower isometric handgrip strength at follow-up in older men [101]. Also, cardiovascular risk scores at baseline in a cohort of CVD-free 45-69 year-olds were associated with future risk of incident frailty by the10-year follow-up [102]. A recent study in 392 older participants reported that those with CVD were more likely to experience a rapid functional decline than their CVD-free counterparts [103].
Thus, poor cardiovascular function in the elderly likely contributes to the onset of frailty but the relationship between CVD and frailty is likely bidirectional [104] as frailty is an adverse prognostic factor in cardiac patients [105]. Frailty and pre-frailty were associated cross-sectionally with higher risk of CVD and longitudinal studies concluded that pre-frailty and frailty increased the risk for incident CVD in 23% and 70%, respectively [106]. In fact, data from 2,825 participants aged 70–79 and residing in the USA showed that moderate and severe frailty were associated with increased incidence of heart failure [107]. And, similarly, pre-frailty and frailty were associated with higher risk of CVD in Italian older people who enjoyed no limitations in activities of daily living [108]. Finally, frailty increased the risk for CVD development independently from early atherosclerosis markers [106]. Among specific frailty domains, exhaustion, low physical activity, slow gait speed, and weak grip strength have been associated with increased risk for incident CVD [[109], [110], [111]]. Recent work concludes that frailty and CVD share main risk factors as well as oxidative stress and inflammatory background [105,112,113]. Thus, physical exercise represents an efficacious strategy for improving both conditions [114]. This supports the stance that both conditions are bidirectionally related as they both also uniquely contribute to unsuccessful aging, while not eliminating the possibility of being separate manifestations of an underlying common physiological pathology.
2.4. Endocrine system/metabolic diseases
Sex hormones decline progressively with age among healthy, non-obese men starting in their 30s whereas the sex hormone binding globulin (SHBG) increases during their 50s and beyond [115]. The combination of these two changes leads to a steep decrease in free testosterone levels in aging men [[116], [117], [118]]. Decline in androgen levels has been postulated as a potential mechanism contributing to frailty. In Spanish men aged ≥65 years, lower free and total testosterone serum levels were associated with frailty [119] supporting previous findings [[120], [121], [122]]. A significant increase in the risk for frailty was detected in men with SHBG serum concentrations ≥66 nmol/L or with free testosterone levels <243 pmol/L [123]. Moreover, prospective studies have shown that low levels of testosterone predict frailty onset or progression in men [120,122]. Although scarce, a handful of studies have also analysed the association of testosterone and frailty in women. In women aged 70–79 years of age, two or three deficiencies in hormonal levels of insulin-like growth factor 1 (IGF-1), free testosterone, or dihydroepiandrosterone sulphate (DHEAS) greatly increased the likelihood of being frail [124]. Older women presenting 4 or more Fried’s criteria for frailty displayed very low levels of free testosterone, although this association appeared confined to obese women [119]. Despite the clear relationship between low androgen levels and frailty, data provided by testosterone supplementation studies in older adults are not consistent. A recent review concluded that whereas testosterone modestly improves lean muscle mass in older men regardless of frailty status, the hormone's effect on physical function is weak [125]. However, more recent findings suggest that testosterone supplementation may facilitate the molecular changes produced by exercise in skeletal muscle so as to actually enhance resistance training's benefit on physical function [126].
Diabetes mellitus (DM), predominantly type 2 diabetes, is one of the most prevalent chronic diseases in older adults [127]. There is a clear relationship between diabetes and frailty [128] and the relationship seems to be bidirectional [129]. In fact, frailty and sarcopenia are emerging as new complications leading to disability in addition to traditional micro and macrovascular diseases [130]. In a cross-sectional study conducted in primary health care settings, older adults (65–74 years) with diabetes were more likely to perform poorly in physical tasks than their DM-free peers [131]. Longitudinal studies have reported that DM increases the risk for frailty in older adults (60 years or more) [132]. Multivariate analyses carried out in older diabetic participant data concluded that physical frailty was independently associated with a higher disability and mortality, suggesting that frailty in this population is an unfavorable and important prognostic factor [133,134]. The co-ocurrance of diabetes and frailty in older people is not surprising since these two age-related conditions share common underlying pathophysiological mechanisms. These mechanisms involve imbalances in endocrine, neurohormonal, vascular, and muscular function among others. The conditions also share key risk factors including impaired insulin resistance, glucose dysregulation, physical inactivity, and obesity [19].
In addition to the associated comorbidities, diabetes directly impacts skeletal muscle thus contributing to mobility reduction in older individuals [28]. The association of diabetes with low gait speed is potentially mediated by impaired muscle function [135]. In fact, loss of muscle mass and/or strength is greater in long-lasting diabetes or higher HbA1C, and the loss is attenuated by insulin sensitizers [28,136,137]. Muscle strength measured via handgrip test was inversely associated with fasting glucose, HbA1c, fasting insulin as well as with insulin resistance as determined by homeostasis model assessment of insulin resistance (HOMA) score [138]. Longitudinal study results concluded that older patients with diabetes and HbA1C level ≥8.5% had a higher risk of low muscle quality and performance status than control non-diabetic peers [139].
In addition to diabetes, other related metabolic alterations have been proposed to have influence on physical function in the elderly. For instance, metabolic syndrome [140] has been linked to an increased risk of frailty [[141], [142], [143]], but not in all studies [144]. On the other hand, among adults over 50, frail ones were more likely to have metabolic syndrome than the non-frail [145]. In a USA population, metabolic syndrome was correlated with frailty index in younger (≤65) and older subjects (>65), although the association was weaker in the latter. Frailty, but not metabolic syndrome, predicted mortality in both age groups [146], suggesting that the previously reported association of metabolic syndrome with mortality may have been influenced by the failure to adjust for frailty.
Insulin resistance seems to play a key role in the pathophysiological process underlying functional impairment related to diabetes in the elderly, even before the onset of diabetes. Supporting this, an inverse association was detected between quadriceps strength and insulin resistance in non-diabetic individuals 70 years and over [147]. Low insulin sensitivity has been associated with low lean muscle mass [148]. In fact, a positive association between HOMA-IR level and frailty in elderly adults has been recently reported whereas this association failed to reach significance in middle-aged participants. In this study, among the frailty components, weakness, exhaustion, and low physical activity were related to insulin resistance [149].
The direct impact of insulin resistance on skeletal muscle could explain the deterioration of physical function related to this condition [150]. In fact, altered muscle fiber type content and an increased accumulation of intramyocellular lipid droplets have been observed in muscle biopsies from older subjects with metabolic syndrome [151]. In this sense, non-diabetic insulin resistance exarcebates the decline in protein anabolic response in older adults and the decline only worsens in the presence of diabetes when glucose metabolism is further impaired [152].
Diabetes impacts vascular function in older adults. This is supported by the fact that heat-induced skin vasodilation was further impaired in the presence of type 2 diabetes in older subjects compared to their non-diabetic and younger counterparts [153], suggesting that, the impact of diabetes and aging on vascular function may be additive. Thus, evidence from epidemiological and functional studies strongly suggests that the co-presence of older age and diabetes increases vascular impairment to a greater extent than either condition separately. Although the underlying mechanisms are not fully understood, inflammation has been pointed out as a key factor in the exacerbated vascular damage produced when older age and diabetes coexist [154].
Additionally, diabetes and insulin resistance may interfere with other systemic functions that influence the risk of frailty. Hormonal alterations may represent one of them. In this sense, reduced plasma levels of free and total testosterone have been observed in men with type 2 diabetes and the prevalence of testosterone deficiency syndrome in type 2 diabetic patients is estimated at around one third [[155], [156], [157], [158], [159]]. The hormonal impact of diabetes was suggested to be sex specific since lower levels of testosterone were confirmed in diabetic men but not women [160]. Interestingly, low testosterone levels are associated with insulin resistance in both diabetic and non-diabetic men [[161], [162], [163]]. In contrast, insulin resistance is associated with increased levels of testosterone in women [161,164]. However, in both men and women, circulating sex hormone binding globulin (SHBG) is reduced in the presence of diabetes, inversely related to insulin resistance and predicts glucose intolerance [160,162,164,165].
3. Age-Related signaling pathways involved in physical dysfunction and frailty and its modulation by physical exercise/activty
Physical activity is known to preserve or improve the function of multiple key systems affected by age, including the endocrine, respiratory, and cardiovascular systems as well as skeletal muscle. Understanding the pathways through which exercise impacts physical function is important as it might facilitate the development of new therapeutic strategies to improve performance in the aged population and to prevent and reverse frailty. Below we discuss the most common age-related signaling pathways involved in physical dysfunction and how they are modulated by exercise (Fig. 5).
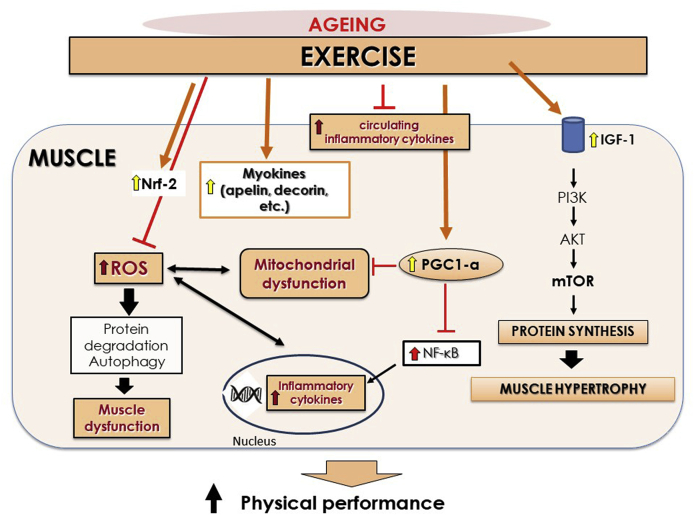
Fig. 5.
Aging process is associated with increased reactive oxygen species (ROS) and inflammation resulting in muscle dysfunction. Decreased levels of insulin-like growth factor (IGF-1) are related to aging with the subsequent diminished protein synthesis and muscle growth via phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway. Exercise may exert potent anti-inflammatory and anti-oxidative stress (i.e., through nuclear erythroid-2 like factor-2 (Nrf-2) activation) effects and consequently improve muscle function. In addition, exercise increases protein synthesis via activation of IGF-1 pathway and target myokines reducing protein degradation. An increased signaling of the transcription factor peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) is also induced by exercise, improving mitochondrial function and reducing inflammation mediated by nuclear factor κB (NF-κB).
3.1. Oxidative stress
The impact of oxidative stress on the functioning of different systems, tissues, and organs has been suggested as the main underlying link between aging and its associated chronic diseases and frailty [166]. In fact, both frailty and age-related chronic diseases were related to a common underlying oxidative damage [19].
Oxidative stress results from the imbalance between pro and antioxidant species. It is widely accepted that adequate efficiency of antioxidant response is compromised during aging [166]. The stimulation of transcription factor nuclear erythroid-2 like factor-2 (Nrf2)/antioxidant response element (ARE) pathway seems to play a prominent role in response to oxidative insult in order to induce antioxidant defense. Studies in rodents demonstrated that aging is associated with decreased expression of Nrf2 in skeletal muscle which is related to frailty phenotype in these animals [167]. In fact, Nrf2 deficiency exacerbates age-related unfavorable outcomes regarding loss of muscle mass and physical function together with increased oxidative stress and mitochondrial dysfunction [[167], [168], [169]]. Based on this evidence, it has been postulated that the defect in the Nrf2-mediated signaling during aging contributes to manifestation of chronic age-related diseases including frailty [19]. The observed association between frailty phenotype, reduced systemic mRNA expression of Nrf2, and three of its target genes (heme oxygenase-2, thioredoxin reductase-1 and superoxide dismutase-2) in community-dwelling older adults supports this hypothesis [170].
Despite their well-known deleterious role, ROS are key mediators in exercising muscle exerting an adaptive response that involves transcription of redox-sensitive factors which, in turn, promote the increase in cytoprotective proteins such as catalase, superoxide dismutases, and heat shock proteins that prevent oxidative damage. This response to ROS generation is severely diminished in aged muscle [171].
Although previous studies failed to demonstrate a reduction of oxidative stress with exercise in the elderly [172] despite improvement in their overall health condition [173], anti-oxidative effects of exercise training are widely accepted. But not all types of exercise produce the same effects. Single bouts of exercise exceeding certain intensity and duration have been shown to transiently increase ROS from the mitochondria and other oxidases which may actually harm the cell [174]. In contrast, chronic exercise showed benefits by attenuating age-related oxidative stress in different organs including skeletal muscle [175], and arteries [176] in animal models. Furthermore, muscle biopsies from lifelong trained older subjects showed decreased oxidative stress and increased catalase expression when compared to the untrained group [174]. In fact, increased antioxidant status has been closely related to age-associated skeletal muscle changes improvement [177].
Compared to a sedentary group, trained elderly presented lower plasmatic concentrations of oxidized LDL (marker of oxidative stress) and decreased expression of genes involved in oxidant production evaluated in peripheral blood mononuclear cells [178]. A recent study observed how 12-week exercise training produced a reduction in plasmatic levels of protein carbonylation in older subjects with COPD. Moreover, protein carbonylation was associated with variations in muscle size and pennation angle as well as with exercise-induced structural and functional adaptations [179]. Furthermore, Mota and collaborators reported the effectiveness of a combined exercise program in reducing oxidative stress including DNA damage and increased antioxidant capacity in women over 40, regardless of age, suggesting that physical exercise may help mitigate the aging processes associated with oxidative stress [180]. Supporting this work, Cobley and colleagues reported an attenuation of age-related macromolecules damage and an increase in HSP72 and catalase in muscle biopsies from lifelong trained individuals [177].
An increase in Nrf2 produced by exercise in the aged was proposed as one of the different mechanisms by which healthy lifestyle and dietary habits mediate the increase in longevity [23]. For instance, Nrf2 is proposed to be necessary for redox adaptations to exercise [181] and as one of mediators of physical activity-promoted protection against ROS-induced skeletal muscle damage [182]. Studies with knockout mice for Nrf2 supported the role of this factor in mediating exercise-induced antioxidant and functional actions in skeletal muscle [183,184].
Nrf2 activation in response to exercise is not limited to skeletal muscle level but also it occurs at the systemic level. In fact, exercise-induced Nrf2-signaling in blood mononuclear cells has been demonstrated to be impaired in older men [185]. Exercise also induces activation of Nrf2 in the cardiovascular system [186]. This is important since Nrf2 down-regulation seems to be related to age-related vascular dysfunction that improves after Nrf2 activation [187].
3.2. Inflammation
Low-grade chronic inflammation is one of the traits of the aging process. Substantial evidence shows that aging is characterized by a general increase in circulating levels and cell capability to produce pro-inflammatory cytokines (e.g. interleukin IL-6, tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP)). An increase in nuclear factor κB (NF-κB) signaling and a reduction in anti-inflammatory cytokines (e.g. interlukin 10, adiponectin, transforming growth factor- β) may also characterize the aging process [188,189]. Chronic inflammation, in turn, plays a role in the development of age-related diseases [112]. Moreover, elevated levels of pro-inflammatory cytokines correlate with increased risk of morbidity and mortality among the elderly, whether frail or not [23]. It is worth noting that several processes trigger these inflammatory pathways, including age-associated redox balance, senescence, and impaired autophagy [190].
Substantial evidence supports the relationship between chronic inflammation and physical performance in elderly subjects. Elevated levels of different cytokines such as IL-6, TNF-α and CRP as well as white blood cells (WBC) increase have been associated with poor function and mobility status [191,192]. A recent population-based cross-sectional study found an inverse and independent relationship between serum CRP levels in old males and handgrip strength and physical performance [193]. Likewise, abundant studies point to the association between IL-6 and frailty [194].
Again, how older people exercise matters. Acute bouts of exercise induce an inflammatory response which elevate pro-inflammatory cytokine levels which cause transient damage to contracting skeletal muscles [195]. Regular exercise, however, decreases the IL-6 and CRP levels according to a recent meta-analysis [196]. Additionally, increased levels of physical activity were also associated with higher levels of the anti-inflammatory mediators adiponectin and interleukin 10 (IL10) in addition to the reduction of pro-inflammatory cytokines [195].
Reduction in the expression of toll-like-receptor (TLR) on monocytes is one of the potential mechanisms through which physical activity brings about anti-inflammatory effects. Stewart and colleagues (2005) found that 10–12 weeks of moderate exercise lowered TLR4 expression and pro-inflammatory biomarkers in previously sedentary older participants to levels similar to those observed in young individuals [197]. This evidence suggests that inflammation in older subjects is reversible and, with the right intervention, it may be preventable [198].
It is worth noting that the effect of physical activity on inflammatory response varies by type, intensity, and frequency of the exercise as well as on participant's characteristics [195]. Resistance exercise appears to decrease TNF-α expression in aged skeletal muscle which, in turn, may attenuate age-associated muscle changes [174]. Furthermore, older diabetic patients saw a reduction in their CRP concentrations and an increase in adiponectin levels after 16 weeks of resistance training [199]. A recent study has provided evidence that aerobic exercise is more appropriate in modulating the immune system and inflammatory markers among the elderly population than resistance exercise [200]. Whereas there is overwhelming evidence pointing to the anti-inflammatory effect that exercise has on older people through its influence on cytokine levels, some studies have failed to reach such favourable conclusion [201]. In neither frail nor prefrail older adults did prolonged progressive resistance exercise training affect IL-6, IL-8 and TNF-α circulating levels. Moreover, the higher the levels of these markers the lower the strength gains were during resistance exercise intervention [202].
It has been proposed that age-related inflammation of adipose tissue is a key factor in skeletal muscle inflammation and muscle dysfunction [203]. As aforementioned, insulin resistance is a condition associated with physical impairment in the elderly. In fact, the relationship between insulin resistance and frailty could be mediated by inflammatory processes as inflammation downregulates IGF-1 which affects muscular regeneration [113]. In addition, inflammation causes insulin resistance via the TNF receptor superfamily member 1A and the TLR-induced activation of Janus kinase which, in turn, interferes with insulin signaling. Concurrently, insulin resistance causes inflammation via central obesity (adipocytes release the C-C motif chemokine 2 (CCL2)) and via M1 macrophages tissue accumulation [113,204].
3.3. Autophagy
Autophagy is one of the multiple pathways by which cellular content is degraded. Autophagy plays a key role in maintaining skeletal muscle homeostasis through the removal of damaged cell components [205] Thus, autophagy-deficient mice mimic several age-related characteristics including loss of muscle mass and quality [206] suggesting the main role of this process is protecting against aged-related cellular impairment. Evidence suggests that aging alters the autophagy process present in skeletal muscle, thus, contributing to sarcopenia [207]. There are two main markers of this important process. One, lipidated microtubule-associated protein 1 light chain 3 (LC3) II is often used as a marker of the number of autophagosomes; and two, elevated LC3 II/LC3I protein ratio in combination with decreased cargo protein Sequestosome-1 (p62) levels may indicate increased autophagy [205]. An increase, decrease, and non-change in LC3II/LC3I protein ratio have been observed in aged skeletal muscle of various species [208]. Recently, Aas and collaborators observed higher levels of LC3II, which may indicate increased levels of phagosomes, in older participants, across frailty status, compared to young participants, suggesting an age-related process. On the other hand, they found higher levels of LC3I, which may represent an attenuated phagosome formation, in frail old compared to young individuals. Furthermore, LC3I negatively correlated with specific strength. The correlations observed between specific strength and some markers of autophagy indicates a possible link between these two factors [206].
Exercise impacts on the autophagy implicated in skeletal muscle aging. Park and colleagues showed that activating autophagy through physical activity increases muscle functioning and improves the sarcopenic phenotype [209]. This could be related with the fact that induction of chronic contractile activities in skeletal muscle cells recovers LC3II levels and autophagy deficiency through decreasing oxidative stress, resulting in improvement of mitochondrial function [210]. In this sense, long term (34 weeks) voluntary resistance wheel exercise initiated in middle-aged mice (15 month-olds) has been shown to increase markers of mitochondrial density and activity and to increase LC3II/I ratios. This was related to causing soleus hypertrophy and to preventing sarcopenia in the hindlimb muscles of physically active aged mice when compared to sedentary controls [211]. Meanwhile, in human skeletal muscle, Dethlefsen and collaborators showed that age and training status only modestly affected autophagic and apoptotic markers. In this sense, a trend of higher LC3I, and LC3II levels were observed in both trained and untrained elderly men [208]. Nevertheless, endurance exercise training has been shown to increase the LC3II/LC3I ratio in skeletal muscle of aged rats [212] while lifelong endurance exercise training seems to prevent the ratio's age-related change in triceps muscle [213] and in humans' vastus lateralis [214]. These findings suggest that autophagy regulation is part of the metabolic adaptation to exercise training.
In addition to chronic exercise, acute exercise also activates autophagy and increases insulin sensitivity in skeletal muscle of older mice [215]. However, recent studies indicate that exercise-activated autophagy in skeletal muscle may depend on its intensity and duration [209]. Therefore, evidence relating autophagy and exercise is inconsistent and requires further study.
3.4. Mitochondrial dysfunction
Mitochondrial dysfunction, including alterations in proteostasis, biogenesis, dynamics, and mitophagy, is a key characteristic of aging [216]. In fact, impaired mitochondrial function and biogenesis, when observed in aged skeletal muscle, contributes to sarcopenia, poor physical performance, and chronic fatigue [217,218]. Not surprisingly, mitochondrial dysfunction seems to be a determinant factor in the association of frailty with other age-related diseases [219]. Probably due to the dysfunction's association to the highly prevalent age-related sedentarism which, in turn, is closely related to frailty and poor physical performance [220].
Mitochondria are not only a source of, but also a target of, oxidative stress resulting in mitochondrial dysfunction; thus, leading to a vicious cycle. Recent work identified alleles in mitochondrial DNA associated with unfavorable frailty and grip strength outcomes supporting the clinical relevance of mitochondrial DNA alterations [23].
Skeletal muscles activate different redox-sensitive pathways as an adaptive response to oxidative stress generated during contractile activity. In this activity, NF-κB, MAPK, and the peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), seem to be the most relevant to exercise physiology. PGC-1α is considered the master regulator of mitochondrial integrity, function, and biogenesis. Reduced PGC-1α in addition to an age-related lack of response mediated by this factor has been previously reported [19,221]. In addition, and if we take into account the implication of defective PGC-1α signaling in sarcopenia and age-related skeletal muscle alterations, PGC-1α has the potential of being a significant factor in the evolution of frailty phenotype [19].
Moreira and colleagues reviewed several studies reporting that chronic endurance exercise, regardless of time and intensity, induces PGC-1α gene expression in the skeletal muscle in animal and older human models [222]. Halling and collaborators provided evidence using mice that physical activity protects against age-related mitochondrial fragmentation in skeletal muscle by curbing mitochondrial fission protein expression in a PGC-1α dependent manner [213]. These findings support the important contribution of PGC-1α to the beneficial effects of exercise training at advanced age by maintaining mitochondrial metabolic and antioxidant capacity [223].
As discussed above, Nrf2 activation is key for adaptive antioxidant defense in response to exercise. In addition to its effects on oxidative damage, exercise-induced Nrf2 activation may exert beneficial effects on muscle performance by improving mitochondrial biogenesis and function [[167], [168], [169],224].
On the other hand, central to the maintenance of a healthy mitochondrial pool is the removal of dysfunctional organelles via mitophagy. Despite scarce data on how mitophagy is impacted by growing older and chronic exercise [225], mitophagy dysregulation has been denoted as a major pathogenic mechanism in muscle wasting [226]. In fact, reduced protein levels of LC3 II and Atg7 have been detected in muscle biopsies of older individuals [227]. Therefore, exercise-induced mitophagy might benefit aged muscle by preventing the accumulation of damaged mitochondria. Supporting this, 6-month weight loss in combination with a moderate intensity exercise program was reported to increase mRNA expression of LC3II, Atg, and lysosome-associated membrane protein 2 (LAMP-2) in the vastus lateralis of elderly overweight women [228]. Furthermore, lifelong exercise may preserve the expression of LC3II and Atg at the same levels as those observed in young controls [228].
3.5. IGF1/mTOR
As we age, insulin/IGF-1 signaling is impaired, mainly due to insulin resistance and lowered IGF-1 levels, with the subsequent diminished protein synthesis and muscle growth via phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway [23]. Previous work has reported an association between IGF-1 and frailty. Doi and colleagues concluded that lower serum IGF-1 levels were independently related to frailty in older adults [229]. Further, IGF-1 levels were lower in individuals with frail/low relative appendicular skeletal muscle mass (RASM) compared to their frail/normal RASM peers. IGF-1 levels were significantly associated with frail/low RASM status for the overall cohort [230].
Insulin-like growth factor cell signaling pathway, involving PI3K, AKT and mTOR, is crucial for post-exercise protein synthesis increase [221]. mTOR is one of the major pathways modulated by exercise, although different data indicated that the outcome varies depending on the type of training [231]. For instance, strength training activates IGF-1 and the mTOR pathway is stimulated resulting in muscle protein synthesis through mRNA translation and ribosomal biogenesis. The impact of aerobic exercise varies by age, however. In young animals, with a lower cell energy state, AMPK is activated, mTOR is inhibited, and PGC-1α is activated leading to mitochondrial biogenesis [232]. Among old animals, mitochondrial biogenesis activation by aerobic exercise is somehow inhibited [221].
In addition, studies involving resistance type of exercise have usually reported a weaker anabolic protein response in older individuals when compared to younger ones as a consequence of mTORC1 signalling pathway dysregulation. This may be indicative of the presence of higher levels of cellular stress markers in older individuals which, in turn, may impact the post-exercise remodeling responses [221].
It is worth underscoring that regular exercise augments skeletal muscle mass by increasing glucose levels. Higher glucose levels boost insulin action resulting in improved insulin resistance [233]. Furthermore, a recent study reported that a 12-week combined anaerobic and aerobic exercise program may improve insulin resistance, IGF-1, growth hormone (GH), and DHEA-S levels in elderly Korean women [234].
3.6. Myokines
Myokines are molecules, cytokines or signaling peptides (e.g., IL-6, IGF-1, irisin, myostatin, IL-15, fibroblast growth factor 21) expressed, synthesized, and released by skeletal myocytes in response to muscle contractions with pluripotent effects [235]. The expression of some of these myokines may vary with age in rodents and humans. Some such as apelin [236] may decline, some may remain unchanged such as myostatin, and still others may increase such as GDF-11 (a member of transforming growth factor TGF-β signaling pathway) [237].
Moreover, myokines seem to affect multiple processes associated with physical frailty, including muscle wasting, dynapenia, and slowness [238].
A recent review has reported that the benefits of exercise include estimulating a healthy anti-inflammatory environment, mainly via the release of muscle-derived myokines, and reducing age-related loss of muscle mass and function [239]. In humans, an increase in systemic concentration of decorin has been reported after acute and chronic exercise [240]. This myokine seems to play a role in inactivating myostatin, a known negative regulator of muscle mass, resulting in decreased muscle protein degradation [241]. The level of circulating irisin increased and presented a positive correlation with both grip and leg strength after an exercise program [242]. Apelin is another exercise-induced myokine reported to be involved in neutralizing age-associated muscle wasting [236]. Muscle wasting is offset by inducing mitochondriogenesis, fostering muscle's regenerative capacity, and mitigating muscle-related inflammation [237].
It is worth mentioning that myokines are likely to play an important role in whole body homeostasis. Thus, the shifts in their production/synthesis/downstream signaling may play a role in the onset of certain metabolic, cardiovascular, and kidney diseases among others [238].
This multisystemic effect, also attributable to the other mentioned signaling pathways, may contribute to the positive impact of physical activity on frailty, especially taking into account that this frailty results from a multisystemic dysfunction (Fig. 6).
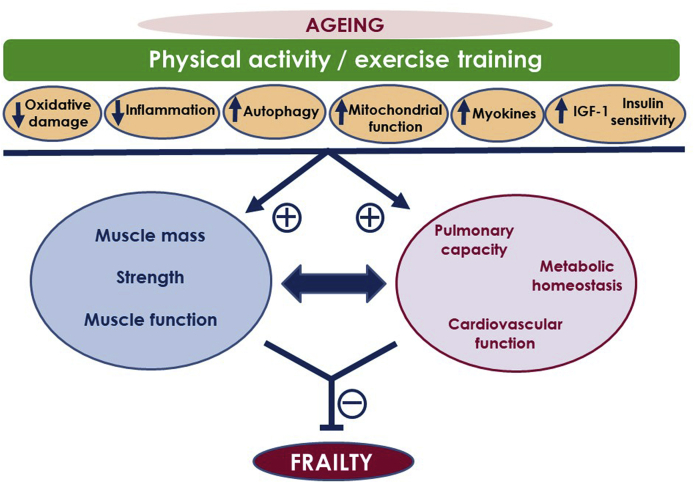
Fig. 6.
Physical activity/exercise training may influence the outcome of the aging process by modulating key signaling pathways. Exercising results in reduced age-related oxidative damage, reduced chronic inflammation, increased autophagy, improved mitochondrial function, improved myokine profile, augmented insulin-like growth factor-1 (IGF-1) signaling, and insulin sensitivity. These actions promote beneficial effects on skeletal muscle (muscle mass, strength, and function) but also at systemic level, inducing improvements in function of cardiovascular, respiratory and metabolic systems. Exercise-induced improvements in muscle function as well as systemic benefits and age-related chronic diseases alleviation are all related to the improvements in physical function and frailty improvement by exercise/physical activity.
4. Exercise training interventions
Several types of exercise programs have been described [243]. Every kind of intervention has its own characteristics and expected effects on skeletal muscle and function, as well as on other body systems.
4.1. Physical exercise
Physical activity is defined as any body movement produced by the skeletal system that increases energy expenditure. Physical exercise is physical activity carried out with a structured frequency and whose objective is to maintain or improve some component of an individual's physical fitness (muscular strength, flexibility, balance or cardiovascular endurance) [243]. Specificity is the fundamental principle of physical exercise. Hence, in order to generate muscular, cardiorespiratory, and central system adaptations, an exercise program that works on all these components should be proposed. When several adaptations are needed, as in the case of frail/prefrail people, multi-component exercise programs have been proposed as the most effective option [244,245].
The effectiveness of all these programs has been largely established (Table 1), although the ideal composition of the physical exercise program remains elusive [255]. Exercise training must be prescribed as a treatment for age-related frailty [256] and, like any treatment, must be adjusted to the right dose. Physical exercise prescribers must increase (or decrease) the dose based on individual progress. Prescribers should combine the main variables in training (duration, type, intensity and frequency) until they find the optimal dose for each individual. The goal should be to establish an evidence-based and feasible exercise protocol to be implemented in the clinic.
Table 1.
Study findings of the effect of physical exercise interventions on physical function in older people across frailty status.
| Author, year | Sample | Intervention | Main findings |
|---|---|---|---|
| Fiatarone et al., 1994 [246] | 100 older adults Frail 72-98 year-olds Nursing home |
10 weeks MCI (3 types: ET, multinutrient supplementation, or both (group B)) |
|
| Pahor et al., 2006 [247] | 424 older adults SPPB ≤ 9 70-89 year-olds Community dwellers |
24 weeks MCI (aerobic, strength, balance and flexibility) |
|
| Cameron et al., 2013 [248] | 216 older adults Frail (Fried phenotype) ≥70 year-olds Community dwellers |
12 months multifactorial individually according the Fried criteria met: If weight loss, a dietician evaluated nutritional intake If exhaustion and Geriatric Depression Scale score was high, study team considered referral to a psychologist or psychiatrist If weakness, slowness or low energy expenditure, patient received 10 home-based physiotherapy sessions |
|
| Cadore et al., 2014 [249] | 24 older adults Frail and prefrail (Fried phenotype) ≥90 year-olds Nursing home |
12 weeks MCI (muscle power training, balance and gait retrain) |
|
| Pahor et al., 2014 [21] | 818 older adults 70 - 89 year-olds Community-dwellers |
|
|
| Kwon et al., 2015 [250] | 89 older adults Prefrail (Fried criteria, although considered frail if met 2 criteria at the time of enrolment) 65–91 year-olds Community-dwellers |
12 weeks. Three groups: one MCI (Warm-up, strength training, balance training and cool-down), MCI plus nutritional program (cooking class) (MCI-NP); and control |
|
| Tarazona-Santabalbina et al., 2016 [251] | 100 frail subjects (Fried phenotype) ≥70 year-olds Community-dwellers |
|
|
| Losa-Reyna et al., 2019 [252] | 20 frail and prefrail (Fried phenotype) 77.2–95.8 year-olds Community-dwellers |
|
|
| Martínez-Velilla et al., 2019 [253] | 370 older adults >75 year-olds Acute care hospitalization |
|
|
| Rodríguez-Mañas et al. et al., 2019 [22] | 964 prefrail and frail older adults (Fried phenotype) with type 2 diabetes >70 year-olds Community-dwellers |
|
|
| Yu et al., 2019 [254] | 127 prefrail subjects (FRAIL scale) ≥50 year-olds Community- dwellers |
|
|
ET: Exercise Training; HIIT: High Interval Intensity Training; MCI: Multicomponent Intervention; MCI-NP: Multicomponent Intervention plus Nutritional Program; SPPB: Short Physical Performance Battery; TUG: Timed Up and Go.
4.2. Aerobic exercise
Aerobic capacity, i.e., the heart and lungs ability to oxygenate muscles, has been strongly associated with mortality, CVD, mobility limitations, and disability [58]. Aerobic capacity is usually measured via VO2 maximum or peak. After the third decade of age, this capacity progressively declines, decreasing the ability to perform activities of daily living (ADLs). One of the most notable effects of endurance training is on increasing VO2 peak, an important determinant of frailty in older adults [257]. Moreover, as the muscle adapts to endurance exercise training, its oxidative capacity increases resulting in higher fatigue resistance or increased muscle endurance [246].
Several ways of assessing cardiopulmonary condition are currently available. Based on exercise gas exchange variables, cardiopulmonary exercise testing (CPET) is the best evaluation tool for determining exercise intolerance, or dyspnea on exercise, and estimating the cardiorespiratory fitness. Despite being limited by the setting and by the expensive equipment required, CPET is most commonly used for disease management such as heart failure and pulmonary hypertension. Further, it has potential diagnostic utility for older adults with decline in exercise levels [258].
Two of the conventional submaximal exercise tests commonly used in elderly as a measure of walking speed and mobility performance are the 6-min walking test and the 400 m walking test. These tests only require a long corridor (≥30 m recommended) and a stopwatch. Additionally, the placement of chairs along the corridor and having a trained professional walk closely behind the patient are suggested. In addition, the 400 m test has been shown as a prognostic factor and a predictor for CVD, mobility limitation, and mobility disability in older persons reporting no walking difficulties [58]. Additional, valid measures of health outcomes using shorter distances have been established. A well-validated protocol entails a 4 m course from a standing start [259]. A gait speed of at least 1.4 m/s indicates patients are likely to be able to perform their ADLs independently, whereas a gait speed of <0.8 m/s suggests patients are likely to be frail, to be at increased risk of falls, and to be dependent regarding their ADLs and their Instrumental Activities of Daily Living (IADLs) [260].
Guidelines from the American College of Sports Medicine and the American Heart Association for older adults recommend a minimum of 150 min per week of moderate intensity aerobic training (30 min on five out of seven days per week) or a minimum of 60 min (20 min on three days per week) of vigorous activity [261]. Although frail individuals may not be able to meet this recommendation, modest increases in activity and strenghtening exercise could impact positively in the progression of functional improvement [246,262].
Many activities have been proposed for increasing aerobic capacity such as brisk walking, jogging, water aerobics, swimming, dancing, or bicycle riding. In addition to these classical approaches, a recent paper has shown benefits after implementing a High Interval Intensity Training- HIIT program [252]. Among them, walking is the easiest, cheapest, most feasible, most relevant to ADLs, and the easiest to be assessed in the clinic. Although there are different approaches according to the functional status of the person [253,263,264] there are not standardized and generally accepted rules about the prescription of aerobic exercise to frail people.
4.3. Strength exercise
Muscle strength is the force or tension that a muscle or muscle group generates [265]. The extent of muscle strength loss with age, inactivity, injury, and immobilization depends on impaired neuromuscular activation and reduced muscle volumen [266]. Strength workout seems to be the key element in preventing sarcopenia and falls, while keeping functional capacity [249,267], also in older people [246].
There are some well-validated techniques to measure muscle strength. Handgrip strength (on dominant arm) is the most common, and it is used in research and clinical settings. The most common dynamometer used for this test is JAMAR Hydraulic Hand Dynamometer. Test should be repeated ideally twice, recording the best one [268].
In order to measure intensity (percentage of the maximal strength that an individual can develop), the one-repetition maximum (1-RM) is commonly used. It is defined as the maximal weight an individual can lift for just one repetition with correct technique, and it is considered the gold standard for assessing muscle strength [269]. Depending on the intensity, strength training should be prescribed every other day, especially in the initial workout stages [270]. Simple instructions should be provided, and both haptic support and mirror techniques are recommended [271].
4.4. Power exercise
Musculoskeletal power can be defined as the product of the force of a muscular contraction and its velocity. After the age of 50, around 3% of muscle power is lost every year, three times faster than strength [272]. Leg power is highly related to physical performance in older people [273].
Muscle power can be measured in any exercise or movement. Mostly common muscle lower exercises, leg press and knee extension, show good reliability and validity [274]. The 5 times sit-to-stand (5STS) test is an easy and inexpensive procedure in which only a chair and a stopwatch are required. It has shown to be a valid, clinically relevant tool [275].
Power training intensity should range from 30% to 60% of 1-RM load because maximal power output is maximized at these intensities [276]. Participants should perform concentric (shortening) phase exercises as fast as possible.
Power training should be prioritized in elderly, because it improves gait speed, chair stand test and stair climb time [270]. In a recent meta-analysis, high speed training resulted in improvements in the Short Physical Performance Battery (SPPB), although not in other functional capacity tools [270]. Furthermore, lower training volumes have been reported to be associated with greater improvements in muscle power [277].
4.5. Flexibility exercise
Flexibility is the range of motion (ROM) of one or several joints. Flexibility is scarcely measured in studies, and no scale has achieved wide acceptance. Probably, measuring flexibility with a goniometer on each specific joint could be the best option to assess ROM changes. A stretching or flexibility program is aimed at greater ROM by rising both static and dynamic stretching tolerance [278]. Static stretching refers to the ability to maintain the position at the end of the ROM. Dynamic stretching refers to achieving, on a repeated gradual transition on any part of the body, a progressive increase in ROM [279]. Dynamic stretching can be included in warm-up [266], whereas static stretching exercises could be performed at the end, as part of the cool-down phase [278].
4.6. Balance exercise
Neuromuscular systems, sensory systems (i.e., vestibular, visual, somatosensory) and cognitive systems (i.e., cerebellum, hippocampus, prefrontal and parietal cortices) have an important role in balance. With aging, all these systems deteriorate, increasing the risk of falling [280]. Inter-joint coordination and the appropriate timing of muscle action is also affected.
Berg Balance test is probably the most common test to evaluate balance in the elderly, assessing risk for falls in older community-dwelling adults through direct observation of their performance [281]. However, this test is time-consuming and balance assessment by SPPB is much faster, representing a more convenient tool for clinical practice.
There is evidence showing that balance and functional exercises reduce the rate of falls by 24% and by 13% the number of older people who experience one or more falls. Moreover, multi-component exercise programs (balance and functional exercises plus resistance exercises) reduce the rate of falls by 34% and by 22% the number of people experiencing one or more falls [282]. Further, supervised balance exercise programs seem to get better results than unsupervised ones [283]. Balance exercises are recommended three days per week, with at least two of them being supervised. Both dynamic and static exercises should be performed [283]. Patients with poor scores in the progressive Romberg test of the SPPB could benefit from a higher frequency of balance training [284].
There are many exercises to improve balance depending on which system (sensory, cognitive, or musculoskeletal system) needs to be worked on. They include single leg stances, semi-tandem and tandem stance, toe walking, heel walking, tandem gait, walking on a balance board, and eye-hand or eye-leg coordination. The possibility of making each of them with open or closed eyes, joining the arms to the body or opening them, doing them on unstable surfaces or adding cognitive components such as specific orders, music or dual-task exercise, makes it almost impossible to monitor the workload. The program should include static vs. dynamic task, changes in the base of support, variations in the center of gravity height, and distinct standing surfaces [280]. Exercise difficulty should progressively increase involving both motor and cognitive tasks (dual- and multi-task activities) [280].
4.7. Non-physical training exercises
Recently Marusic and Grosprêtre reviewed one of the proposed tools, the non-physical training exercises, to improve balance and strength in older people, including those who are frail. These exercises include activities such as motor imagery (MI) and action observation (AO) [285,286]. These tools have been gaining relevance because normal aging is also associated with a progressive reduction in central and peripheral neural function. MI stands for the mental simulation of an action without any corresponding motor output, but still causing neuronal activation [286]. Meanwhile, AO, a tool grounded in basic neuroscience and the mirror neuron system [287], has been proposed to intensify neural activation induced by mental practice [286]. These types of interventions are deemed safe and efficient and do not add additional neuromuscular fatigue when incorporated into any type of intervention, something relevant in frail people [286]. Although the results are very promising, their effectiveness is still awaiting supporting evidence.
4.8. Adherence
The best physical exercise is the one that is actually carried out. Adherence rates to exercise programs are usually suboptimal, although comparisons across studies are often hampered due to differences in reporting [288]. Adherence may be improved by following certain tips. Before starting the exercise program, patients must be informed about the possible risks and benefits of the exercise program. A clear description of the program may increase motivation and adherence [289]. In addition, physical exercise professionals must inform about the importance of physical exercise in physical functioning, well-being, and quality of life [290], and attend to patient preferences and acceptance [291]. It is also desirable to create a friendly and familiar training atmosphere with the patients and; finally, the proper execution of the exercises must be closely monitored [271].
Other barriers to overcome are socioeconomic factors. Participation in exercise programs may be reduced by lack of transportation, fixed incomes, or inclement weather [292]. Thus, although supervised exercise programs seem to have better results in terms of strength and balance [283], home exercises [293] or video games-based interventions [294] could be a good option for some patients. Notwithstanding, as the impact of social isolation on frailty is well established, group exercise enjoys the additional benefit of socialization [295].
4.9. Adverse events
In contrast to the usual risks associated with poli-pharmacy, physical exercise administration is safe, relatively free of potential unwanted side effects [296], and adverse events are rare [297]. The use of a multidisciplinary team (e.g., geriatricians, nurses, physiotherapists) seems to be the best option in order to avoid unnecessary risks, especially regarding the most vulnerable participants.
4.10. Adapting exercise intervention to individual functional status
4.10.1. Robust subjects
Older people who perform more than 180 min of weekly exercise report better health-related quality of life [298]. A substantial increase in physical activity level regardless of the baseline level (i.e., from sedentarism to moderate activity or from moderate to vigorous activity) has been associated with a reduction in sarcopenia prevalence and better performance of muscle mass and function [299]. More specifically, strength training seems fundamental, since decline in strength levels in older adults compromises overall function and it is predictive of future functional decline and higher incidence of frailty, disability, and mortality [58,300].
4.10.2. Prefrail older adults
The population of prefail adults is one of the main targets of exercise interventions since it includes between 35% and 50% of individuals over 65 [108,301] and it is the subpopulation most likely to benefit from an exercise program [302]. Again, strength training is key since muscle weakness seems to be the most prevalent of the Fried components of frailty among the prefrail [303,304]. Kwon et al. reported that with just one weekly training, prefrail women could improve their strength. However, one day a week was not enough to achieve improvements in physical function, and any improvements in strength were lost three months after the end of the intervention [250]. In support of more frequent training, the MID-FRAIL study, which included pre-frail older people with type 2 diabetes mellitus participating in a multimodal intervention (detailed description of the study is provided below), reported improvements in functioning (as assessed by SPPB) among those participating in a strength training program twice weekly for 16 weeks [22].
4.10.3. Frail patients
Several training programs in different care settings (ambulatory or in-hospital) have been designed for frail people. A well-designed multicomponent exercise program showed improvements in gait speed, balance, SPPB functioning scores, ADL performance, as well as in frailty status [251]. The program was more intensive than most. It included a high training frequency (5 days per week) and relatively long sessions (65 min each). The intervention included strength exercises with elastic bands, stretching, and aerobic exercises including walking, arm movements and stair climbing. Attendance of at least 50% of the programmed sessions was associated with a substantially higher likelihood of reversing frailty to the point of robustness [251]. There is also evidence of improvements in leg strength and in SPPB functioning score obtained with a two-day/week supervised lower and upper body strength training program. Further, greater gains in muscle mass were obtained in the group receiving protein supplements [305,306].
Results from the “Sarcopenia and Physical fRailty IN older people: multi-componenT
Treatment strategies (SPRINTT)” study are expected to clarify the association between physical exercise and physical frailty and their relationship with sarcopenia. This randomized controlled trial follows 1500 community dwelling older persons aged 70 years and older with sarcopenia and physical function impairment (SPPB ≤ 9) for up to 36 months. The efficacy of a MultiComponent Intervention (MCI), based on long-term planned exercise program and dietary guidance /intervention for preventing mobility disability is tested in comparison with a Healthy Aging Lifestyle Education (HALE) [307].
Among the many comorbidities present in frail and prefrail individuals, one of the most common is diabetes. In the MID-Frail study, 964 frail and prefrail elderly with diabetes were included according to the Fried phenotype. In this study, the effect of a three-pronged intervention, including a resistance exercise program, a nutritional and educational program, and a standardized across sites researcher-enhanced training for best practices in diabetes care, was compared with a usual care group. The physical exercise intervention was a supervised individualized resistance exercise program consisting of a 2-week pre-training and learning phase followed by a 16-week program with participants undergoing two 45-min exercise sessions per week. Selected exercises were leg presses and bilateral knee extensions carried out in two to three sets of 8–10 repetitions each with a load equivalent to 40–80% of 1-RM. The intervention not only improved the functional capacity of the treatment group compared to the usual care group but also it proved to be cost-efficient with average savings of 428.02 euros per participant per year [22].
A recent systematic review found strength muscle improvements in frail older adults with very different protocols [308]. Training frequencies ranged from 1 to 6 times per week, including from 1 to 3 exercise series, and series including from 6 to 15 repetitions. Intensity was controlled by the rate of perceived effort (RPE) or by the percentage of 1-RM (from 30 to 100%). The review reported several findings. The main ones being that strength exercise, either performed in isolation or in combination with other training modalities, did improve muscle mass, strength and power, and/or functional capacity (not all studies saw improvements in all aspects). It is worth mentioning that using RPE to control exercise intensity was associated with no muscle strength improvement, suggesting that RPE underestimates exercise-related effort in this population of individuals.
4.10.4. Hospitalized older adults
Being immobilized, a usual condition in institutionalized and hospitalized older people [309], reduces muscle mass by 0.5% and muscle strength by 0.3–4.2% per day [310]. These losses often result in a significant deterioration in functional status with the corresponding decline in quality of life [311].
Improving the functional capacity of hospitalized oldest old patients through an MCI has proven feasible [253]. Due to the high levels of sedentarism these patients present, high frequency interventions (i.e., 2 daily exercise sessions for 5–7 consecutive days) seem to have better results than lower intensity interventions. The first session of the day was supervised, lasted about 20 min, and included balance, gait retraining, and resistance exercise. The second one was unsupervised and consisted of strenght exercises using 0.5–1.0 kg anklets and a handgrip ball. This intervention improved SPPB score, Barthel Index, and cognition [253].
Different strategies have been proposed to succesfully improve strength in these patients [312]. Nevertheless, resistance training seems to be the best exercise to improve strength and other outcomes, such as muscle mass, neural impulse, or mitigated bone loss. The effects of resistance training could be optimized if combined with vibration (general or local), blood flow restriction, and especially with neuromuscular electrical stimulation [312,313] Restriction of blood flow and vibration can be combined with aerobic training and with low intensity strength training (<20%), having special relevance in situations where high intensity exercise is not possible.
4.11. From research to clinical setting
Due to the heterogeneity of the population of older adults, there is a lack of consensus regarding a gold standard measure of functional capacity. This, combined with the differences among exercise programs, makes the translating of results from research settings to clinical practice difficult to say the least.
Numerous scales and tools have been tested as measures for physical capabilities of older adults and to detect clinically significant changes. Subjective scales are important to epidemiologic and qualitative research, as well as for learning what patients feel or think about their household or recreational activity functional level.
However, objective scales are more sensitive to changes, and can be standardized and reproduced. For instance, gait speed is a key element in the physical independence of a person and, thus, frailty, and, consequently, it has been repeatedly evaluated in different clinical settings.
Another test used and validated to evaluate physical function in older adults is the SPPB [314]. In addition to assessing gait speed at the individual's usual pace, this test measures balance (using the Romberg progressive test) and strength in lower limbs (with the 5STS test). The SPPB has been correlated with ADLs loss [315] and it is a good tool to identify patients who may benefit from an exercise intervention [302].
The prescription of different physical exercise programs in frail and prefrail patients based on the baseline physical performance of the patient is becoming a well-established strategy. Although the main evidence comes from the SPPB-based prescription, other instruments to assess frailty status, like the Frailty Trait Scale (FTS) [316] or its short-form FTS5 [317], could be theoretically used for the same purpose [248].
In the LIFE study, the prescription of physical activity-based interventions depending upon the SPPB score improved subsequent health outcomes [21,247,314]. Similarly, exercise prescription based on functional capacity as revealed by SPPB score could be optimal for using in physical exercise programs such as Vivifrail [263].
Using this approach may improve the prescription of physical exercise programs, thus optimizing their effects in the following ways (Fig. 7):
-
1
Emphasizing strength interventions could be useful in those patients who have low knee strength, low grip strength, and poor 5STS as well as people with higher scores in the Brachial/Ankle Index (BAI) which could be compatible with peripheral artery disease [318].
-
2
Emphasizing power interventions for patients with low gait speed, poor 5STS and balance test scores.
-
3
Emphasizing balance intervention for patients with low Romberg progressive test scores.
-
4
Verbal fluency could be improved with dual tasks and strength training [249] and multicomponent exercise programs [254,319].
-
5
Emphasizing aerobic training in patients with sedentary behavior, low gait speed, or low physical condition. It could be also recommended in patients with higher BAI scores [320].
Fig. 7.
Distribution of the type of exercises in a standard training session according to patient frailty status. Intensity should generally increase as the frailty status improves, although High Interval Intensity Training-HIIT can be used also in frail patients.
Additional research is needed to further contribute to our knowledge about the optimal dose of individually fitted exercise programs by frailty status. In fact, not prescribing exercise programs to older populations may be considered unethical, in light of the substantial evidence showing health benefits [321]. Finally, evidence-based effective physical exercise intervention protocols should be translated to clinical practice.
Funding
This work has received funding from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement n°305483, Frailomic project, and was also supported by a grant from the Innovative Medicines Initiative - Joint Undertaking (IMI-JU 115621), and by grants from the Ministry of Economy and Competitiveness and co-financed by FEDER funds (Instituto de Salud Carlos III, PI15/00674, PI15/01160 and CIBERFES (CB16/10/00464), Spanish Government.
Declaration of competing interest
No Conflicts Of Interest.
References
- 1.Dzau V.J., Inouye S.K., Rowe J.W., Finkelman E., Yamada T. Enabling healthful aging for all. The National Academy of Medicine grand challenge in health longevity. N. Engl. J. Med. 2019;381:1699–1701. doi: 10.1056/NEJMp1912298. [DOI] [PubMed] [Google Scholar]
- 2.He W., Goodkind D., Kowal P. U.S. Government Publishing Office; Washington, DC: 2016. U.S. Census Bureau, International Population Reports, P95/16-1, an Aging World: 2015. [Google Scholar]
- 3.Chen C., Goldman D.P., Zissimopoulos J., Rowe J.W. Research Network on an Aging Society. Multidimensional comparison of countries’ adaptation to societal aging. Proc. Natl. Acad. Sci. U. S. A. 2018;115:9169–9174. doi: 10.1073/pnas.1806260115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayflick L. The not-so-close relationship between biological aging and age-associated pathologies in humans. J Gerontol A Biol Sci Med Sci. 2004;59:B547–B550. doi: 10.1093/gerona/59.6.b547. [DOI] [PubMed] [Google Scholar]
- 5.Hayflick L. Biological aging is no longer an unsolved problem. Ann. N. Y. Acad. Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Mañas L., Rodríguez-Artalejo F., Sinclair A.J. The third transition: the clinical evolution oriented to the contemporary older patient. J. Am. Med. Dir. Assoc. 2017;18:8–9. doi: 10.1016/j.jamda.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . 2015. World Report on Ageing and Health. [Google Scholar]
- 8.Beard J.R., Officer A., Araujo de Carvalho I., Sadana R. Pot AM, Michel J-P et al., the World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurice J. WHO puts healthy aging on the front burner. Lancet. 2016;387:109–110. doi: 10.1016/S0140-6736(15)01365-3. [DOI] [PubMed] [Google Scholar]
- 10.Dong X., Milholland B., Vijg J. Evidence for a limit to human lifespan. Nature. 2016;538:257–259. doi: 10.1038/nature19793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Study on global AGEing and adult health (SAGE), World Health Organization, Health Statistics and Information Systems [website] http://www.who.int/healthinfo/sage/en/ Geneva, World Health Organization.
- 12.Cieza A., Sabariego C., Bickenbach J., Chatterji S. Rethinking disability. BMC Med. 2018;16:14. doi: 10.1186/s12916-017-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., McBurnie M.A. & cardiovascular health study collaborative research group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Mañas L., Féart C., Mann G., Viña J., Chatterji S., Chodzko-Zajko W., Gonzalez-Colaço Harmand M., Bergman H., Carcaillon L., Nicholson C., Scuteri A., Sinclair A., Pelaez M., Van der Cammen T., Beland F., Bickenbach J., Delamarche P., Ferrucci L., Fried L.P., Gutiérrez-Robledo L.M., Rockwood K., Rodríguez Artalejo F., Serviddio G., Vega E., FOD-CC group Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent E., Martin F.C., Bergman H., Woo J., Romero-Ortuño R., Walston J.D. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 16.Warren M.W. Activity in advancing age. Br. Med. J. 1950;2:921–924. doi: 10.1136/bmj.2.4685.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez Mañas L., García-Sánchez I., Hendry A., Bernabei R., Roller-Wirnsberger R., Gabrovec B., Liew A., Carriazo A.M., Redon J., Galluzzo L., Viña J., Antoniadou E., Targowski T., Di Furia L., Lattanzio F., Bozdog E., Telo M. Key messages for a frailty prevention and management policy in Europe from the ADVANTAGE Joint Action consortium. J. Nutr. Health Aging. 2018;22:892–897. doi: 10.1007/s12603-018-1064-y. [DOI] [PubMed] [Google Scholar]
- 18.Hoogendijk E.O., Afilalo J., Ensrud K.E., Kowal P., Onder G., Fried L.P. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 19.El Assar M., Angulo J., Rodríguez-Mañas L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2019 doi: 10.1016/j.freeradbiomed.2019.08.011. pii: S0891-5849(19)31163-3, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Partridge L., Deelen J., Slagboom P.E. Facing up to the global challenges of aging. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8. [DOI] [PubMed] [Google Scholar]
- 21.Pahor M., Guralnik J.M., Ambrosius W.T., Blair S., Bonds D.E., Church T.S., Espeland M.A., Fielding R.A., Gill T.M., Groessl E.J., King A.C., Kritchevsky S.B., Manini T.M., McDermott M.M., Miller M.E., Newman A.B., Rejeski W.J., Sink K.M., Williamson J.D. LIFE study investigators.Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez‐Mañas L., Laosa O., Vellas B., Paolisso G., Topinkova E., Oliva‐Moreno J., Bourdel‐Marchasson I., Izquierdo M., Hood K., Zeyfang A., Gambassi G., Petrovic M., Hardman T.C., Kelson M.J., Bautmans I., Abellan G., Barbieri M., Peña‐Longobardo L.M., Regueme S.C.…Sinclair A.J. European MID-Frail Consortium. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10(4):721–733. doi: 10.1002/jcsm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo J., El Assar M., Rodriguez-Mañas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Aspect. Med. 2016;50:1–32. doi: 10.1016/j.mam.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Mori H., Tokuda Y. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac. J. Clin. Nutr. 2019;28:157–165. doi: 10.6133/apjcn.201903_28(1).0021. [DOI] [PubMed] [Google Scholar]
- 25.Davies B., García F., Ara I., Rodríguez-Artalejo F., Rodriguez-Mañas L., Walter S. Relationship between sarcopenia and frailty in the toledo study of healthy aging: a population based cross-sectional study. J. Am. Med. Dir. Assoc. 2018;19:282–286. doi: 10.1016/j.jamda.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Clark B.C. Neuromuscular changes with aging and sarcopenia. J Frailty Aging. 2019;8:7–9. doi: 10.14283/jfa.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand. J. Med. Sci. Sports. 2010;20:28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalyani R.R., Corriere M., Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson D.J., Piasecki M., Atherton P.J. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brook M.S., Wilkinson D.J., Mitchell W.K., Lund J.N., Phillips B.E., Szewczyk N.J., Greenhaff P.L., Smith K., Atherton P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016;594:7399–7417. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brook M.S., Wilkinson D.J., Phillips B.E., Perez-Schindler J., Philp A., Smith K., Atherton P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol. 2016;216:15–41. doi: 10.1111/apha.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 1985;89:81–88. doi: 10.1152/jappl.2000.89.1.81. 2000. [DOI] [PubMed] [Google Scholar]
- 33.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J. Appl. Physiol. 1985;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hughes V.A., Frontera W.R., Wood M., Evans W.J., Dallal G.E., Roubenoff R., Fiatarone Singh M.A. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 35.McGregor R.A., Cameron-Smith D., Poppitt S.D. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Heal. 2014;3:9. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaap L.A., Koster A., Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 37.Auyeung T.W., Lee S.W., Leung J., Kwok T., Woo J. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 2014;14(Suppl 1):76–84. doi: 10.1111/ggi.12213. [DOI] [PubMed] [Google Scholar]
- 38.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., Simonsick E.M., Tylavsky F.A., Visser M., Newman A.B. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hairi N.N., Cumming R.G., Naganathan V., Handelsman D.J., Le Couteur D.G., Creasey H., Waite L.M., Seibel M.J., Sambrook P.N. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010;58(11):2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 41.Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011;4(3):248–259. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostek M.C., Delmonico M.J. Age-related changes in adult muscle morphology. Curr. Aging Sci. 2011;4(3):221–233. doi: 10.2174/1874609811104030221. [DOI] [PubMed] [Google Scholar]
- 43.Reid K.F., Fielding R.A. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casas Herrero A., Cadore E.L., Martinez Velilla N., Izquierdo Redin M. Physical exercise in the frail elderly: an update. Rev. Esp. Geriatr. Gerontol. 2015;50(2):74–81. doi: 10.1016/j.regg.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Clark D.J., Patten C., Reid K.F., Carabello R.J., Phillips E.M., Fielding R.A. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J. Gerontol. A Biol Sci Med Sci. 2010;65(5):495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deschenes M.R. Motor unit and neuromuscular junction remodeling with aging. Curr. Aging Sci. 2011;4(3):209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- 47.Gordon T., Hegedus J., Tam S.L. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol. Res. 2004;26(2):174–185. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- 48.Budui S.L., Rossi A.P., Zamboni M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015;12(1):22–26. doi: 10.11138/ccmbm/2015.12.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zampieri S., Pietrangelo L., Loefler S., Fruhmann H., Vogelauer M., Burggraf S., Pond A., Grim-Stieger M., Cvecka J., Sedliak M., Tirpáková V., Mayr W., Sarabon N., Rossini K., Barberi L., De Rossi M., Romanello V., Boncompagni S., Musarò A., Sandri M., Protasi F., Carraro U., Kern H. Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci. 2015;70:163–173. doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- 50.Blau H.M., Cosgrove B.D., Ho A.T. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015;21(8):854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snijders T., Parise G. Role of muscle stem cells in sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:186–190. doi: 10.1097/MCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 53.Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardí M., Ballestar E., González S., Serrano A.L., Perdiguero E., Muñoz-Cánoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506(7488):316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 54.Verdijk L.B., Snijders T., Drost M., Delhaas T., Kadi F., van Loon L.J.C. Satellite cells in human skeletal muscle; from birth to old age. Age. 2014;36:545–557. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blaauw B., Reggiani C. The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 2014;35:3–10. doi: 10.1007/s10974-014-9376-y. [DOI] [PubMed] [Google Scholar]
- 56.Fry C.S., Lee J.D., Mula J., Kirby T.J., Jackson J.R., Liu F., Yang L., Mendias C.L., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015;21(1):76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gale C.R., Martyn C.N., Cooper C., Sayer A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007;36(1):228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 58.Newman A.B., Kupelian V., Visser M., Simonsick E.M., Goodpaster B.H., Kritchevsky S.B., Tylavsky F.A., Rubin S.M., Harris T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 59.Leong D.P., Teo K.K., Rangarajan S., Lopez-Jaramillo P., Avezum A., Jr., Orlandini A., Seron P., Ahmed S.H., Rosengren A., Kelishadi R., Rahman O., Swaminathan S., Iqbal R., Gupta R., Lear S.A., Oguz A., Yusoff K., Zatonska K., Chifamba J., Igumbor E., Mohan V., Anjana R.M., Gu H., Li W., Yusuf S. Prospective urban rural epidemiology study, investigators. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 60.Guadalupe-Grau A., Carnicero J.A., Gomez-Cabello A., Gutierrez Avila G., Humanes S., Alegre L.M., Castro M., Rodríguez-Mañas L., Garcia-Garcia F.J. Association of regional muscle strength with mortality and hospitalisation in older people. Age Ageing. 2015;44(5):790–795. doi: 10.1093/ageing/afv080. [DOI] [PubMed] [Google Scholar]
- 61.Lowery E.M., Brubaker A.L., Kuhlmann E., Kovacs E.J. The aging lung. Clin. Interv. Aging. 2013;8:1489–1496. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skloot G.S. The effects of aging on lung structure and function. Clin. Geriatr. Med. 2017;33:447–457. doi: 10.1016/j.cger.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Buchman A.S., Boyle P.A., Wilson R.S., Gu L., Bienias J.L., Bennett D.A. Pulmonary function, muscle strength and mortality in old age. Mech. Ageing Dev. 2008;129:625–631. doi: 10.1016/j.mad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sillanpaa E., Stenroth L., Bijlsma A.Y., Rantanen T., McPhee J.S., Maden-Wilkinson T.M., Jones D.A., Narici M.V., Gapeyeva H., Paasuke M., Barnouin Y., Hogrel J.Y., Butler-Browne G.S., Meskers C.G., Maier A.B., Tormakangas T., Sipila S. Associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults. Age. 2014;36:9667. doi: 10.1007/s11357-014-9667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aliverti A., Macklem P.T. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J. Appl. Physiol. 2008;105:749–751. doi: 10.1152/japplphysiol.90336.2008. [DOI] [PubMed] [Google Scholar]
- 66.Buchman A.S., Boyle P.A., Leurgans S.E., Evans D.A., Bennett D.A. Pulmonary function, muscle strength, and incident mobility disability in elders. Proc. Am. Thorac. Soc. 2009;6:581–587. doi: 10.1513/pats.200905-030RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss C.O., Hoenig H.H., Varadhan R., Simonsick E.M., Fried L.P. Relationships of cardiac, pulmonary, and muscle reserves and frailty to exercise capacity in older women. J Gerontol A Biol Sci Med Sci. 2010;65:287–294. doi: 10.1093/gerona/glp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gale N.S., Albarrati A.M., Munnery M.M., Hubbard R.E., Tal-Singer R., Cockcroft J.R., Shale D.J. Frailty: a global measure of the multisystem impact of COPD. Chron. Respir. Dis. 2018;15:347–355. doi: 10.1177/1479972317752763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marengoni A., Vetrano D.L., Manes-Gravina E., Bernabei R., Onder G., Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154:21–40. doi: 10.1016/j.chest.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Navarro-Cruz R., Alcazar J., Rodriguez-Lopez C., Losa-Reyna J., Alfaro-Acha A., Ara I., García-García F.J., Alegre L.M. The effect of the stretch-shortening cycle in the force-velocity relationship and its association with physical function in older adults with COPD. Front. Physiol. 2019;10:316. doi: 10.3389/fphys.2019.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ierodiakonou D., Kampouraki M., Poulonirakis I., Papadokostakis P., Lintovoi E., Karanassos D., Maltezis K., Chorti M., Petrovitsos E., Dimopoulou S., Hamind S., Gialamas I., Athanasiou P., Bempi V., Lambraki I., Tsiligianni I. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm. Med. 2019;19:63. doi: 10.1186/s12890-019-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanlon P., Nicholl B.I., Jani B.D., Lee D., McQueenie R., Mair F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montgomery E., Macdonald P.S., Newton P.J., Chang S., Jha S.R., Hannu M.K., Thomson C., Havryk A., Malouf M. Frailty as a predictor of mortality in patients with interstitial lung disease referred for lung transplantation. Transplantation. 2019 doi: 10.1097/TP.0000000000002901. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Kennedy C.C., Novotny P.J., LeBrasseur N.K., Wise R.A., Sciurba F.C., Benzo R.P. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16:217–224. doi: 10.1513/AnnalsATS.201803-175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan C., Niu H. Frailty assessment in older adults with chronic obstructive respiratory diseases. Clin. Interv. Aging. 2018;13:1513–1524. doi: 10.2147/CIA.S173239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marengoni A., Vetrano D.L., Manes-Gravina E., Bernabei R., Onder G., Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154(1):21–40. doi: 10.1016/j.chest.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 77.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 78.Paneni F., Costantino S., Cosentino F. Molecular pathways of arterial aging. Clin. Sci. 2015;128:69–79. doi: 10.1042/CS20140302. [DOI] [PubMed] [Google Scholar]
- 79.Wu J., Xia S., Kalionis B., Wan W., Sun T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res. Int. 2014;2014:615312. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pepe S., Lakatta E.G. Aging hearts and vessels: masters of adaptation and survival. Cardiovasc. Res. 2005;66:190–193. doi: 10.1016/j.cardiores.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Heckman G.A., McKelvie R.S. Cardiovascular aging and exercise in healthy older adults. Clin. J. Sport Med. 2008;18:479–485. doi: 10.1097/JSM.0b013e3181865f03. [DOI] [PubMed] [Google Scholar]
- 82.Rodríguez-Mañas L., El-Assar M., Vallejo S., López-Dóriga P., Solís J., Petidier R., Montes M., Nevado J., Castro M., Gómez-Guerrero C., Peiró C., Sánchez-Ferrer C.F. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 83.Rubio-Ruiz M.E., Pérez-Torres I., Soto M.E., Pastelín G., Guarner-Lans V. Aging in blood vessels. Medicinal agents for systemic arterial hypertension in the elderly. Ageing Res. Rev. 2014;18:132–147. doi: 10.1016/j.arr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Kotsis V., Stabouli S., Karafillis I., Nilsson P. Early vascular aging and the role of central blood pressure. J. Hypertens. 2011;29:1847–1853. doi: 10.1097/HJH.0b013e32834a4d9f. [DOI] [PubMed] [Google Scholar]
- 85.Ochi M., Kohara K., Tabara Y., Kido T., Uetani E., Ochi N., Igase M., Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 86.Wen W., Luo R., Tang X., Tang L., Huang H.X., Wen X., Hu S., Peng B. Age-related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis. 2015;238:147–152. doi: 10.1016/j.atherosclerosis.2014.10.089. [DOI] [PubMed] [Google Scholar]
- 87.Kohara K., Okada Y., Ochi M., Ohara M., Nagai T., Tabara Y., Igase M. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J Cachexia Sarcopenia Muscle. 2017;8:557–566. doi: 10.1002/jcsm.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Iorio A., Di Blasio A., Napolitano G., Ripari P., Paganelli R., Cipollone F. High fat mass, low muscle mass, and arterial stiffness in a population of free-living healthy subjects: the "al passo con la tua salute" project. Medicine (Baltim.) 2019;98 doi: 10.1097/MD.0000000000016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orkaby A.R., Lunetta K.L., Sun F.J., Driver J.A., Benjamin E.J., Hamburg N.M., Mitchell G.F., Vasan R.S., Murabito J.M. Cross-sectional association of frailty and arterial stiffness in community-dwelling older adults: the framingham heart study. J Gerontol A Biol Sci Med Sci. 2019;74:373–379. doi: 10.1093/gerona/gly134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue Q., Qin M.Z., Jia J., Liu J.P., Wang Y. Association between frailty and the cardio-ankle vascular index. Clin Invest Aging. 2019;14:735–742. doi: 10.2147/CIA.S195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scuteri A., Tesauro M., Rizza S., Iantorno M., Federici M., Lauro D., Campia U., Turriziani M., Fusco A., Cocciolillo G., Lauro R. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr. Metabol. Cardiovasc. Dis. 2008;18:349–356. doi: 10.1016/j.numecd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 92.El Assar M., Fernández A., Sánchez-Ferrer A., Angulo J., Rodríguez-Mañas L. Multivessel analysis of progressive vascular aging in the rat: asynchronous vulnerability among vascular territories. Mech. Ageing Dev. 2018;173:39–49. doi: 10.1016/j.mad.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther. 2012;133:159–176. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Walker A.E., Kaplon R.E., Pierce G.L., Nowlan M.J., Seals D.R. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin. Sci. (Lond.) 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steyers C.M., 3rd, Miller F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsch M.A., Dobrosielski D.A., Arce-Esquivel A.A., Wood R.H., Ravussin E., Rowley C., Jazwinski S.M. The association between flow-mediated dilation and physical function in older men. Med. Sci. Sports Exerc. 2008;40:1237–1243. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Timmerman K.L., Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr. Metabol. Cardiovasc. Dis. 2013;23(Suppl 1):S44–S50. doi: 10.1016/j.numecd.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alonso-Bouzón C., Carcaillon L., García-García F.J., Amor-Andrés M.S., El Assar M., Rodríguez-Mañas L. Association between endothelial dysfunction and frailty: the toledo study for healthy aging. Age (Dordr.) 2014;36:495–505. doi: 10.1007/s11357-013-9576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mansur H.N., Lovisi J.C., Colugnati F.A., Raposo N.R., Fernandes N.M., Bastos M.G. Association of frailty with endothelial dysfunction and its possible impact on negative outcomes in Brazilian predialysis patients with chronic kidney disease. BMC Nephrol. 2015;16:157. doi: 10.1186/s12882-015-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elbaz A., Shipley M.J., Nabi H., Brunner E.J., Kivimaki M., Singh-Manoux A. Trajectories of the Framingham general cardiovascular risk profile in midlife and poor motor function later in life: the Whitehall II study. Int. J. Cardiol. 2014;172:96–102. doi: 10.1016/j.ijcard.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.den Ouden M.E., Schuurmans M.J., Arts I.E., Grobbee D.E., Bots M.L., van den Beld A.W., Lamberts S.W., van der Schouw Y.T. J. Nutr. Health Aging. 2013;17:97–104. doi: 10.1007/s12603-012-0424-2. [DOI] [PubMed] [Google Scholar]
- 102.Bouillon K., Batty G.D., Hamer M., Sabia S., Shipley M.J., Britton A., Singh-Manoux A., Kivimäki M. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart. 2013;99:737–742. doi: 10.1136/heartjnl-2012-302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keeney T., Fox A.B., Jette D.U., Jette A. Functional trajectories of persons with cardiovascular disease in late life. J. Am. Geriatr. Soc. 2019;67:37–42. doi: 10.1111/jgs.15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strandberg T.E., Nieminen T. Future perspectives on the role of frailty in cardiovascular diseases. Adv. Exp. Med. Biol. 2020;1216:149–152. doi: 10.1007/978-3-030-33330-0_14. [DOI] [PubMed] [Google Scholar]
- 105.Uchmanowicz I. Oxidative stress, frailty and cardiovascular diseases: current evidence. Adv. Exp. Med. Biol. 2020;1216:65–77. doi: 10.1007/978-3-030-33330-0_8. [DOI] [PubMed] [Google Scholar]
- 106.Veronese N., Sigeirsdottir K., Eiriksdottir G., Marques E.A., Chalhoub D., Phillips C.L., Launer L.J., Maggi S., Gudnason V., Harris T.B. Frailty and risk of cardiovascular diseases in older persons: the age gene/environment susceptibility-Reykjavik Study. Rejuvenation Res. 2017;20:517–524. doi: 10.1089/rej.2016.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan H., Kalogeropoulos A.P., Georgiopoulou V.V., Newman A.B., Harris T.B., Rodondi N., Bauer D.C., Kritchevsky S.B., Butler J. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am. Heart J. 2013;166:887–894. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sergi G., Veronese N., Fontana L., De Rui M., Bolzetta F., Zambon S., Corti M.C., Baggio G., Toffanello E.D., Crepaldi G., Perissinotto E., Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J. Am. Coll. Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 109.Celis-Morales C.A., Welsh P., Lyall D.M., Steell L., Petermann F., Anderson J., Iliodromiti S., Sillars A., Graham N., Mackay D.F., Pell J.P., Gill J.M.R., Sattar N., Gray S.R. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all-cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Veronese N., Stubbs B., Volpato S., Zuliani G., Maggi S., Cesari M., Lipnicki D.M., Smith L., Schofield P., Firth J., Vancampfort D., Koyanagi A., Pilotto A., Cereda E. Association between gait speed with mortality, cardiovascular disease and cancer: a systematic review and meta-analysis of prospective cohort studies. J. Am. Med. Dir. Assoc. 2018;19:981–988. doi: 10.1016/j.jamda.2018.06.007. e7. [DOI] [PubMed] [Google Scholar]
- 111.Veronese N. Frailty as cardiovascular risk factor (and vice versa) Adv. Exp. Med. Biol. 2020;1216:51–54. doi: 10.1007/978-3-030-33330-0_6. [DOI] [PubMed] [Google Scholar]
- 112.Soysal P., Arik F., Smith L., Jackson S.E., Isik A.T. Inflammation, frailty and cardiovascular disease. Adv. Exp. Med. Biol. 2020;1216:55–64. doi: 10.1007/978-3-030-33330-0_7. [DOI] [PubMed] [Google Scholar]
- 113.Piotrowicz K., Gasowski J. Risk factors for frailty and cardiovascular diseases: are they the same? Adv. Exp. Med. Biol. 2020;1216:39–50. doi: 10.1007/978-3-030-33330-0_5. [DOI] [PubMed] [Google Scholar]
- 114.Ricci N.A., Cunha A.I.L. Physical Exercise for frailty and cardiovascular diseases. Adv. Exp. Med. Biol. 2020;1216:115–129. doi: 10.1007/978-3-030-33330-0_12. [DOI] [PubMed] [Google Scholar]
- 115.Leifke E., Gorenoi V., Wichers C., Von Zur Mühlen A., Von Büren E., Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin. Endocrinol. 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 116.Yeap B.B., Almeida O.P., Hyde Z., Norman P.E., Chubb S.A., Jamrozik K., Flicker L. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur. J. Endocrinol. 2007;156:585–594. doi: 10.1530/EJE-06-0714. [DOI] [PubMed] [Google Scholar]
- 117.Wu F.C., Tajar A., Pye S.R., Silman A.J., Finn J.D., O'Neill T.W., Bartfai G., Casanueva F., Forti G., Giwercman A., Huhtaniemi I.T., Kula K., Punab M., Boonen S., Vanderschueren D. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J. Clin. Endocrinol. Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 118.Camacho E.M., Huhtaniemi I.T., O'Neill T.W., Finn J.D., Pye S.R., Lee D.M., Tajar A., Bartfai G., Boonen S., Casanueva F.F., Forti G., Giwercman A., Han T.S., Kula K., Keevil B., Lean M.E., Pendleton N., Punab M., Vanderschueren D., Wu F.C., EMAS Group Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur. J. Endocrinol. 2013;168:445–455. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 119.Carcaillon L., Blanco C., Alonso-Bouzón C., Alfaro-Acha A., García-García F.J., Rodríguez-Mañas L. Sex differences in the association of testosterona and frailty in an elderly population: the Toledo Study for Healthy aging. PloS One. 2012;7 doi: 10.1371/journal.pone.0032401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Travison T.G., Nguyen A.H., Naganathan V., Stanaway F.F., Blyth F.M., Cumming R.G., Le Couteur D.G., Sambrook P.N., Handelsman D.J. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. J. Clin. Endocrinol. Metab. 2011;96:2464–2474. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 121.Tajar A., O’Connell M.D., Mitnitski A.B., O’Neill T.W., Searle S.D., Huhtaniemi I.T., Finn J.D., Bartfai G., Boonen S., Casanueva F.F., Forti G., Giwercman A., Han T.S., Kula K., Labrie F., Lean M.E., Pendleton N., Punab M., Silman A.J., Vanderschueren D., Rockwood K., Wu F.C. European Male Aging Study Group. Frailty in relations to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J. Am. Geriatr. Soc. 2011;59:814–821. doi: 10.1111/j.1532-5415.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 122.Hyde Z., Flicker L., Almeida O.P., Hankey G.J., McCaul K.A., Chubb S.A., Yeap B.B. Low free testosterone predicts frailty in older men: the health in men study. J. Clin. Endocrinol. Metab. 2010;95:3165–3172. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- 123.Eichholzer M., Barbir A., Basaria S., Dobs A.S., Feinleib M., Guallar E., Menke A., Nelson W.G., Rifai N., Platz E.A., Rohrmann S. Serum sex steroid hormones and frailty in older American men of the third national health and nutrition examination survey (NHANES III) Aging Male. 2012;15:208–215. doi: 10.3109/13685538.2012.705366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cappola A.R., Xue Q.L., Fried L.P. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu B., Cumming R.G., Handelsman D.J. Testosterone, frailty and physical function in older men. Expet Rev. Endocrinol. Metabol. 2018;13:159–165. doi: 10.1080/17446651.2018.1475227. [DOI] [PubMed] [Google Scholar]
- 126.Gharahdaghi N., Rudrappa S., Brook M.S., Idris I., Crossland H., Hamrock C., Abdul Aziz M.H., Kadi F., Tarum J., Greenhaff P.L., Constantin-Teodosiu D., Cegielski J., Phillips B.E., Wilkinson D.J., Szewczyk N.J., Smith K., Atherton P.J. Testosterone therapy induces molecular programming augmenting physiological adaptations to resistance exercise in older men. J Cachexia Sarcopenia Muscle. 2019;10:1276–1294. doi: 10.1002/jcsm.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 128.Yanase T., Yanagita I., Muta K., Nawatta H. Frailty in elderly diabetes patients. Endocr. J. 2018;65:1–11. doi: 10.1507/endocrj.EJ17-0390. [DOI] [PubMed] [Google Scholar]
- 129.Dimitriadis G., Mitrou P., Lambadiari V., Maratou E. Raptis SA Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011;93(Suppl 1):S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 130.Sinclair A.J., Abdelhafiz A.H., Rodríguez-Mañas L. Frailty and sarcopenia - newly emerging and high impact complications of diabetes. J. Diabet. Complicat. 2017;31:1465–1473. doi: 10.1016/j.jdiacomp.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 131.Kotsani M., Chatziadamidou T., Economides D., Benetos A. Higher prevalence and earlier appearance of geriatric phenotypes in old adults with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018;135:206–217. doi: 10.1016/j.diabres.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 132.García-Esquinas E., Graciani A., Guallar-Castillón P., López-García E., Rodríguez-Mañas L., Rodríguez-Artalejo F. Diabetes and risk of frailty and its potential mechanisms: a prospective cohort study of older adults. J. Am. Med. Dir. Assoc. 2015;16:748–754. doi: 10.1016/j.jamda.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 133.Castro-Rodriguez M., Carnicero J.A., Garcia-Garcia F.J. Frailty as a major factor in the increased risk of death and disability in older people with diabetes. J. Am. Med. Dir. Assoc. 2016;17(10):949–955. doi: 10.1016/j.jamda.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 134.Thein F.S., Li Y., Nyunt M.S.Z., Gao Q., Wee S.L., Ng T.P. Physical frailty and cognitive impairment is associated with diabetes and adversely impact functional status and mortality. Postgrad. Med. 2018;130:561–567. doi: 10.1080/00325481.2018.1491779. [DOI] [PubMed] [Google Scholar]
- 135.Volpato S., Bianchi L., Lauretani F., Lauretani F., Bandinelli S., Guralnik J.M., Zuliani G., Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park S.W., Goodpaster B.H., Lee J.S., Kuller L.H., Boudreau R., de Rekeneire N., Harris T.B., Kritchevsky S., Tylavsky F.A., Nevitt M., Cho Y.W., Newman A.B., Health, Aging, and Body Composition Study Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee C.G., Boyko E.J., Barrett-Connor E., Miljkovic I., Hoffman A.R., Everson-Rose S.A., Lewis C.E., Cawthon P.M., Strotmeyer E.S., Orwoll E.S. Osteoporotic Fractures in Men (MrOS) Study Research Group. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee M.R., Jung S.M., Bang H., Kim H.S., Kim Y.B. Association between muscle strength and type 2 diabetes mellitus in adults in Korea: data from the Korea national health and nutrition examination survey (KNHANES) VI. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000010984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon J.W., Ha Y.C., Kim K.M., Moon J.H., Choi S.H., Lim S., Park Y.J., Lim J.Y., Kim K.W., Park K.S., Jang H.C. Hyperglycemia is associated with impaired muscle quality in older men with diabetes: the Korean longitudinal study on health and aging. Diabetes Metab. J. 2016;40:140–146. doi: 10.4093/dmj.2016.40.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alberti K.G.M.M., Zimmet P., Shaw J. Metabolic syndrome – a new world wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 141.Tang Z., Wang C., Song X., Shi J., Mitnitski A., Fang X., Yu P., Rockwood K. Co-occurrence of cardiometabolic diseases and frailty in older Chinese adults in the Beijing Longitudinal Study of Ageing. Age Ageing. 2013;42:346–351. doi: 10.1093/ageing/aft004. [DOI] [PubMed] [Google Scholar]
- 142.Lin F., Roiland R., Chen D., Qiu C. Linking cognition and frailty in middle and old age: metabolic syndrome matters. Int. J. Geriatr. Psychiatr. 2015;30:64–71. doi: 10.1002/gps.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pérez-Tasigchana R.F., León-Muñoz L.M., Lopez-Garcia E., Gutierrez-Fisac J.L., Laclaustra M., Rodríguez-Artalejo F., Guallar-Castillón P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing. 2017;46:807–812. doi: 10.1093/ageing/afx023. [DOI] [PubMed] [Google Scholar]
- 144.Hao Q., Song X., Yang M., Dong B., Rockwood K. Understanding risk in the oldest old: frailty and the metabolic syndrome in a Chinese community sample aged 90+ years. J. Nutr. Health Aging. 2016;20:82–88. doi: 10.1007/s12603-016-0680-7. [DOI] [PubMed] [Google Scholar]
- 145.Viscogliosi G. The metabolic syndrome: a risk factor for the frailty syndrome? J. Am. Med. Dir. Assoc. 2016;17:364–366. doi: 10.1016/j.jamda.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 146.Kane A.E., Gregson E., Theou O., Rockwood K., Howlett S.E. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience. 2017;39:221–229. doi: 10.1007/s11357-017-9967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barzilay J.I., Blaum C., Moore T., Xue Q.L., Hirsch C.H., Walston J.D., Fried L.P. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch. Intern. Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 148.Yang C.W., Li C.I., Li T.C., Liu C.S., Lin C.H., Lin W.Y., Lin C.C. The joint association of insulin sensitivity and physical activity on the skeletal muscle mass and performance in community-dwelling older adults. Exp. Gerontol. 2017;95:34–38. doi: 10.1016/j.exger.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 149.Peng P.S., Kao T.W., Chang P.K., Chen W.L., Peng P.J., Wu L.W. Association between HOMA-IR and frailty among U.S. middle-aged and elderly population. Sci. Rep. 2019;9:4238. doi: 10.1038/s41598-019-40902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cleasby M.E., Jamieson P.M., Atherton P.J. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J. Endocrinol. 2016;229:R67–R81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 151.Gueugneau M., Coudy-Gandilhon C., Théron L., Meunier B., Barboiron C., Combaret L., Taillandier D., Polge C., Attaix D., Picard B., Verney J., Roche F., Féasson L., Barthélémy J.C., Béchet D. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci. 2015;70:566–576. doi: 10.1093/gerona/glu086. [DOI] [PubMed] [Google Scholar]
- 152.Morais J.A., Jacob K.W., Chevalier S. Effects of aging and insulin resistant states on protein anabolic responses in older adults. Exp. Gerontol. 2018;108:262–268. doi: 10.1016/j.exger.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 153.Petrofsky J.S., Alshammari F., Bains G.S., Khowailed I.A., Lee H., Kuderu Y.N., Lodha R.D., Rodrigues S., Nguyen D., Potnis P.A., Deshpande P.P., Yim J.E., Berk L. What is more damaging to vascular endothelial function: diabetes, age, high BMI, or all of the above? Med Sci Monit. 2013;19:257–263. doi: 10.12659/MSM.883878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.El Assar M., Angulo J., Rodríguez-Mañas L. Diabetes and ageing-induced vascular inflammation. J. Physiol. 2016;594:2125–2146. doi: 10.1113/JP270841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann. Intern. Med. 1992;117:807–811. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 156.Corona G., Monami M., Rastrelli G., Aversa A., Sforza A., Lenzi A., Forti G., Mannucci E., Maggi M. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int. J. Androl. 2011;34:528–540. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 157.Ogbera O.A., Sonny C., Olufemi F., Wale A. Hypogonadism and subnormal btotal testosterone levels in men with type 2 diabetes mellitus. J Coll Physicians Surg Pak. 2011;21:517–521. [PubMed] [Google Scholar]
- 158.Al Hayek A.A., Khader Y.S., Jafal S., Khawaja N., Robert A.A., Ajlouni K. Prevalence of low testosterone levels in men with type 2 diabetes mellitus: a cross-sectional study. J Family Community Med. 2013;20:179–186. doi: 10.4103/2230-8229.122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gibbs F.W., Strachan M.W. Androgen deficiency and type 2 diabetes mellitus. Clin. Biochem. 2014;47:940–949. doi: 10.1016/j.clinbiochem.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Ding E.L., Song Y., Malik V.S., Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 161.Goodman-Gruen D., Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–918. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 162.Muller M., Grobbee D.E., den Tokelaar J., Lamberts S.W., van der Schouw Y.T. Endogenous sex hormones and metabolic syndrome in aging men. J. Clin. Endocrinol. Metab. 2005;90:2618–2623. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 163.Grossmann M., Thomas M.C., Panagiotopoulos S., Sharpe K., Macisaac R.J., Clarke S., Zajac J.D., Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 164.Needham B.L., Kim C., Mukherjee B., Bagchi P., Stanczyk F.Z., Kanaya A.M. Endogenous sex steroid hormones and glucose in a South-Asian population without diabetes: the Metabolic Syndrome and Atherosclerosis in South-Asians Living in America pilot study. Diabet. Med. 2015;32:1193–1200. doi: 10.1111/dme.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Birkeland K.I., Hanssen K.F., Torjesen P.A., Vaaler S. Levels of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J. Clin. Endocrinol. Metab. 1993;76:275–278. doi: 10.1210/jcem.76.2.8432768. [DOI] [PubMed] [Google Scholar]
- 166.Tan B.L., Norhaizan M.E., Liew W.P., Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 2018;9:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang D.D., Fan S.D., Chen X.Y., Yan X.L., Zhang X.Z., Ma B.W., Yu D.Y., Xiao W.Y., Zhuang C.L., Yu Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 2019;119:61–73. doi: 10.1016/j.exger.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 168.Ahn B., Pharaoh G., Premkumar P., Huseman K., Ranjit R., Kinter M., Szweda L., Kiss T., Fulop G., Tarantini S., Csiszar A., Ungvari Z., Van Remmen H. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018;17:47–58. doi: 10.1016/j.redox.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kitaoka Y., Tamura Y., Takahashi K., Takeda K., Takemasa T., Hatta H. Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle. Phys. Rep. 2019;7 doi: 10.14814/phy2.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.El Assar M., Angulo J., Carnicero J.A., Walter S., García-García F.J., López-Hernández E., Sánchez-Puelles J.M., Rodríguez-Mañas L. Frailty is associated with lower expression of genes involved in cellular response to stress: results from the toledo study for healthy aging. J. Am. Med. Dir. Assoc. 2017;18:734.e1–734.e7. doi: 10.1016/j.jamda.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 171.McArdle A., Pollock N., Staunton C.A., Jackson M.J. Aberrant redox signalling and stress response in age-related muscle decline: role in inter- and intra-cellular signalling. Free Radic. Biol. Med. 2019;132:50–57. doi: 10.1016/j.freeradbiomed.2018.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aldred S., Rohalu M. A moderate intensity exercise program did not increase the oxidative stress in older adults. Arch. Gerontol. Geriatr. 2011;53:350–353. doi: 10.1016/j.archger.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 173.de Gonzalo-Calvo D., Fernandez-Garcia B., de Luxan-Delgado B., Rodriguez-Gonzalez S., Garcia-Macia M., Suarez F.M., Solano J.J., Rodriguez-Colunga M.J., Coto-Montes A. Chronic training increases blood oxidative damage but promotes health in elderly men. Age. 2013;35:407–417. doi: 10.1007/s11357-011-9358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gomes M.J., Martinez P.F., Pagan L.U., Damatto R.L., Cezar M.D.M., Lima A.R.R., Okoshi K., Okoshi M.P. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8:20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Joseph A.M., Adhihetty P.J., Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. 2016;594:5105–5123. doi: 10.1113/JP270659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gu Q., Wang B., Zhang X.F., Ma Y.P., Liu J.D., Wang X.Z. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp. Gerontol. 2014;56:37–44. doi: 10.1016/j.exger.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 177.Cobley J.N., Sakellariou G.K., Owens D.J., Murray S., Waldron S., Gregson W., Fraser W.D., Burniston J.G., Iwanejko L.A., McArdle A., Morton J.P., Jackson M.J., Close G.L. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014;70:23–32. doi: 10.1016/j.freeradbiomed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 178.Seals D.R., Nagy E.E., Moreau K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019;597:4901–4914. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alcazar J., Losa-Reyna J., Rodriguez-Lopez C., Navarro-Cruz R., Alfaro-Acha A., Ara I., Garcia-Garcia F.J., Alegre L.M., Guadalupe-Grau A. Effects of concurrent exercise training on muscle dysfunction and systemic oxidative stress in older people with COPD. Scand. J. Med. Sci. Sports. 2019;29:1591–1603. doi: 10.1111/sms.13494. [DOI] [PubMed] [Google Scholar]
- 180.Mota M.P., Dos Santos Z.A., Soares J.F.P., de Fatima Pereira A., Joao P.V., O'Neil Gaivao I., Oliveira M.M. Intervention with a combined physical exercise training to reduce oxidative stress of women over 40years of age. Exp. Gerontol. 2019;123:1–9. doi: 10.1016/j.exger.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 181.Done A.J., Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Di Meo S., Napolitano G., Venditti P. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int. J. Mol. Sci. 2019;20:E3024. doi: 10.3390/ijms20123024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oh S., Komine S., Warabi E., Akiyama K., Ishii A., Ishige K., Mizokami Y., Kuga K., Horie M., Miwa Y., Iwawaki T., Yamamoto M., Shoda J. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017;7:12902. doi: 10.1038/s41598-017-12926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yamada M., Iwata M., Warabi E., Oishi H., Lira V.A., Okutsu M. p62/SQSTM1 and Nrf2 are essential for exercise-mediated enhancement of antioxidant protein expression in oxidative muscle. Faseb. J. 2019;33:8022–8032. doi: 10.1096/fj.201900133R. [DOI] [PubMed] [Google Scholar]
- 185.Done A.J., Gage M.J., Nieto N.C., Traustadottir T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radic. Biol. Med. 2016;96:130–138. doi: 10.1016/j.freeradbiomed.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 186.Narasimhan M., Rajasekaran N.S. Cardiac aging - benefits of exercise, Nrf2 activation and antioxidant signaling. Adv. Exp. Med. Biol. 2017;999:231–255. doi: 10.1007/978-981-10-4307-9_13. [DOI] [PubMed] [Google Scholar]
- 187.Angulo J., El Assar M., Sevilleja-Ortiz A., Fernández A., Sánchez-Ferrer A., Romero-Otero J., Martínez-Salamanca J.I., La Fuente J.M., Rodríguez-Mañas L. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. 2019;26:101271. doi: 10.1016/j.redox.2019.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.El Assar M., Angulo J., Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 189.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 190.Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Verghese J., Holtzer R., Oh-Park M., Derby C.A., Lipton R.B., Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1083–1089. doi: 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Adriaensen W., Mathei C., van Pottelbergh G., Vaes B., Legrand D., Wallemacq P., Degryse J.M. Significance of serum immune markers in identification of global functional impairment in the oldest old: cross-sectional results from the BELFRAIL study. Age. 2014;36:457–467. doi: 10.1007/s11357-013-9558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meng Y., Wu H., Yang Y., Du H., Xia Y., Guo X., Liu X., Li C., Niu K. Relationship of anabolic and catabolic biomarkers with muscle strength and physical performance in older adults: a population-based cross-sectional study. BMC Muscoskel. Disord. 2015;16:202. doi: 10.1186/s12891-015-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Velissaris D., Pantzaris N., Koniari I., Koutsogiannis N., Karamouzos V., Kotroni I., Skroumpelou A., Ellul J. C-reactive protein and frailty in the elderly: a literature review. J. Clin. Med. Res. 2017;9:461–465. doi: 10.14740/jocmr2959w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sallam N., Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid Med Cell Longev. 2016;2016:7239639. doi: 10.1155/2016/7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Monteiro-Junior R.S., de Tarso Maciel-Pinheiro P., da Matta Mello Portugal E., da Silva Figueiredo L.F., Terra R., Carneiro L.S.F., Rodrigues V.D., Nascimento O.J.M., Deslandes A.C., Laks J. Effect of exercise on inflammatory profile of older persons: systematic review and meta-analyses. J. Phys. Activ. Health. 2018;15:64–71. doi: 10.1123/jpah.2016-0735. [DOI] [PubMed] [Google Scholar]
- 197.Stewart L.K., Flynn M.G., Campbell W.W., Craig B.A., Robinson J.P., McFarlin B.K. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav. Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 198.Flynn M.G., Markofski M.M., Carrillo A.E. Elevated inflammatory status and increased risk of chronic disease in chronological aging: inflamm-aging or inflamm-inactivity? Aging Dis. 2019;10:147–156. doi: 10.14336/AD.2018.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brooks N., Layne J.E., Gordon P.L., Roubenoff R., Nelson M.E., Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int. J. Med. Sci. 2006;4:19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Abd El-Kader S.M., Al-Shreef F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018;18:120–131. doi: 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zheng G., Qiu P., Xia R., Lin H., Ye B., Tao J., Chen L. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front. Aging Neurosci. 2019;11:98. doi: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hangelbroek R.W.J., Knuiman P., Tieland M., de Groot L. Attenuated strength gains during prolonged resistance exercise training in older adults with high inflammatory status. Exp. Gerontol. 2018;106:154–158. doi: 10.1016/j.exger.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 203.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 204.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., Adhihetty P.J., Adler S.G., Agam G., Agarwal R., Aghi M.K., Agnello M., Agostinis P., Aguilar P.V.…Zughaier S.M. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12 doi: 10.1080/15548627.2015.1100356. 1-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aas S.N., Hamarsland H., Cumming K.T., Rognlien S.H., Aase O.J., Nordseth M., Karsrud S., Godager S., Tommerbakke D., Handegard V., Raastad T. The impact of age and frailty on skeletal muscle autophagy markers and specific strength: a cross-sectional comparison. Exp. Gerontol. 2019;125:110687. doi: 10.1016/j.exger.2019.110687. [DOI] [PubMed] [Google Scholar]
- 207.Fan J., Kou X., Jia S., Yang X., Yang Y., Chen N. Autophagy as a potential target for sarcopenia. J. Cell. Physiol. 2016;231:1450–1459. doi: 10.1002/jcp.25260. [DOI] [PubMed] [Google Scholar]
- 208.Dethlefsen M.M., Halling J.F., Moller H.D., Plomgaard P., Regenberg B., Ringholm S., Pilegaard H. Regulation of apoptosis and autophagy in mouse and human skeletal muscle with aging and lifelong exercise training. Exp. Gerontol. 2018;111:141–153. doi: 10.1016/j.exger.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 209.Park S.S., Seo Y.K., Kwon K.S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep. 2019;52:64–69. doi: 10.5483/BMBRep.2019.52.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Parousis A., Carter H.N., Tran C., Erlich A.T., Mesbah Moosavi Z.S., Pauly M., Hood D.A. Contractile activity attenuates autophagy suppression and reverses mitochondrial defects in skeletal muscle cells. Autophagy. 2018;14:1886–1897. doi: 10.1080/15548627.2018.1491488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.White Z., Terrill J., White R.B., McMahon C., Sheard P., Grounds M.D., Shavlakadze T. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skeletal Muscle. 2016;6(1):45. doi: 10.1186/s13395-016-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luo L., Lu A.M., Wang Y., Hong A., Chen Y., Hu J., Li X., Qin Z.H. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp. Gerontol. 2013;48:427–436. doi: 10.1016/j.exger.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 213.Halling J.F., Ringholm S., Olesen J., Prats C., Pilegaard H. Exercise training protects against aging-induced mitochondrial fragmentation in mouse skeletal muscle in a PGC-1alpha dependent manner. Exp. Gerontol. 2017;96:1–6. doi: 10.1016/j.exger.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 214.Carnio S., LoVerso F., Baraibar M.A., Longa E., Khan M.M., Maffei M., Reischl M., Canepari M., Loefler S., Kern H., Blaauw B., Friguet B., Bottinelli R., Rudolf R., Sandri M. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014;8:1509–1521. doi: 10.1016/j.celrep.2014.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lenhare L., Crisol B.M., Silva V.R.R., Katashima C.K., Cordeiro A.V., Pereira K.D., Luchessi A.D., da Silva A.S.R., Cintra D.E., Moura L.P., Pauli J.R., Ropelle E.R. Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp. Gerontol. 2017;97:17–21. doi: 10.1016/j.exger.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 216.Picca A., Calvani R., Bossola M., Allocca E., Menghi A., Pesce V., Lezza A.M.S., Bernabei R., Landi F., Marzetti E. Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia. Biol. Chem. 2018;399:421–436. doi: 10.1515/hsz-2017-0331. [DOI] [PubMed] [Google Scholar]
- 217.Wawrzyniak N.R., Joseph A.M., Levin D.G., Gundermann D.M., Leeuwenburgh C., Sandesara B., Manini T.M., Adhihetty P.J. Idiopathic chronic fatigue in older adults is linked to impaired mitochondrial content and biogenesis signaling in skeletal muscle. Oncotarget. 2016;7:52695–52709. doi: 10.18632/oncotarget.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Picca A., Calvani R., Leeuwenburgh C., Coelho-Junior H.J., Bernabei R., Landi F., Marzetti E. Targeting mitochondrial quality control for treating sarcopenia: lessons from physical exercise. Expert Opin. Ther. Targets. 2019;23:153–160. doi: 10.1080/14728222.2019.1559827. [DOI] [PubMed] [Google Scholar]
- 219.Gamboa J.L., FTt Billings, Bojanowski M.T., Gilliam L.A., Yu C., Roshanravan B., Roberts L.J., 2nd, Himmelfarb J., Ikizler T.A., Brown N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Phys. Rep. 2016;4(9) doi: 10.14814/phy2.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kehler D.S. The impact of sedentary and physical activity behaviour on frailty in middle-aged and older adults. Appl. Physiol. Nutr. Metabol. 2018;43:638. doi: 10.1139/apnm-2018-0092. [DOI] [PubMed] [Google Scholar]
- 221.Viña J., Rodriguez-Mañas L., Salvador-Pascual A., Tarazona-Santabalbina F.J., Gomez-Cabrera M.C. Exercise: the lifelong supplement for healthy ageing and slowing down the onset of frailty. J. Physiol. 2016;594:1989–1999. doi: 10.1113/JP270536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moreira O.C., Estebanez B., Martinez-Florez S., de Paz J.A., Cuevas M.J., Gonzalez-Gallego J. Mitochondrial function and mitophagy in the elderly: effects of exercise. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/2012798. 2012798.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Leick L., Lyngby S.S., Wojtaszewski J.F., Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp. Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 224.Islam H., Bonafiglia J.T., Turnbull P.C., Simpson C.A., Perry C.G.R., Gurd B.J. The impact of acute and chronic exercise on Nrf2 expression in relation to markers of mitochondrial biogenesis in human skeletal muscle. Eur. J. Appl. Physiol. 2019 doi: 10.1007/s00421-019-04259-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 225.Carter H.N., Kim Y., Erlich A.T., Zarrin-Khat D., Hood D.A. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 2018;596:3567–3584. doi: 10.1113/JP275998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marzetti E., Calvani R., Cesari M., Buford T.W., Lorenzi M., Behnke B.J., Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wohlgemuth S.E., Lees H.A., Marzetti E., Manini T.M., Aranda J.M., Daniels M.J., Pahor M., Perri M.G., Leeuwenburgh C., Anton S.D. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 2011;14:315–324. doi: 10.1089/rej.2010.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wohlgemuth S.E., Lees H.A., Marzetti E., Manini T.M., Aranda J.M., Daniels M.J. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 2011;14:315–324. doi: 10.1089/rej.2010.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Doi T., Makizako H., Tsutsumimoto K., Hotta R., Nakakubo S., Makino K., Suzuki T., Shimada H. Association between insulin-like growth factor-1 and frailty among older adults. J. Nutr. Health Aging. 2018;22:68–72. doi: 10.1007/s12603-017-0916-1. [DOI] [PubMed] [Google Scholar]
- 230.Chew J., Tay L., Lim J.P., Leung B.P., Yeo A., Yew S., Ding Y.Y., Lim W.S. Serum myostatin and IGF-1 as gender-specific biomarkers of frailty and low muscle mass in community-dwelling older adults. J. Nutr. Health Aging. 2019;23:979–986. doi: 10.1007/s12603-019-1255-1. [DOI] [PubMed] [Google Scholar]
- 231.Song Z., Moore D.R., Hodson N., Ward C., Dent J.R., O'Leary M.F., Shaw A.M., Hamilton D.L., Sarkar S., Gangloff Y.G., Hornberger T.A., Spriet L.L., Heigenhauser G.J., Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci. Rep. 2017;7:5028. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 233.Horowitz J.F. Exercise-induced alterations in muscle lipid metabolism improve insulin sensitivity. Exerc. Sport Sci. Rev. 2007;35:192–196. doi: 10.1097/jes.0b013e318156e084. [DOI] [PubMed] [Google Scholar]
- 234.Ha M.S., Son W.M. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly Korean women. Exp. Gerontol. 2018;114:13–18. doi: 10.1016/j.exger.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 235.Lee J.H., Jun H.S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 2019;10:42. doi: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vinel C., Lukjanenko L., Batut A., Deleruyelle S., Pradere J.P., Le Gonidec S., Dortignac A., Geoffre N., Pereira O., Karaz S., Lee U., Camus M., Chaoui K., Mouisel E., Bigot A., Mouly V., Vigneau M., Pagano A.F., Chopard A., Pillard F., Guyonnet S., Cesari M., Burlet-Schiltz O., Pahor M., Feige J.N., Vellas B., Valet P., Dray C. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 237.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019;10:287. doi: 10.3389/fphys.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Coelho-Junior H.J., Picca A., Calvani R., Uchida M.C., Marzetti E. If my muscle could talk: myokines as a biomarker of frailty. Exp. Gerontol. 2019;127:110715. doi: 10.1016/j.exger.2019.110715. Epub 2019 Aug 29. [DOI] [PubMed] [Google Scholar]
- 239.Fiuza-Luces C., Santos-Lozano A., Joyner M., Carrera-Bastos P., Picazo O., Zugaza J.L., Izquierdo M., Ruilope L.M., Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018;15:731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 240.Kanzleiter T., Rath M., Gorgens S.W., Jensen J., Tangen D.S., Kolnes A.J., Kolnes K.J., Lee S., Eckel J., Schurmann A., Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014;450:1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 241.El Shafey N., Guesnon M., Simon F., Deprez E., Cosette J., Stockholm D., Scherman D., Bigey P., Kichler A. Inhibition of the myostatin/Smad signaling pathway by short decorin-derived peptides. Exp. Cell Res. 2016;341:187–195. doi: 10.1016/j.yexcr.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 242.Kim H.J., So B., Choi M., Kang D., Song W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015;70:11–17. doi: 10.1016/j.exger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 243.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Publ. Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 244.Cadore E.L., Rodríguez-Mañas L., Sinclair A., Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–114. doi: 10.1089/rej.2012.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Aguirre L.E., Villareal D.T. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser. 2015;83:83–92. doi: 10.1159/000382065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fiatarone M.A., O'Neill E.F., Ryan N.D., Clements K.M., Solares G.R., Nelson M.E., Roberts S.B., Kehayias J.J., Lipsitz L.A., Evans W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 247.LIFE Study Investigators. Pahor M., Blair S.N., Espeland M., Fielding R., Gill T.M., Guralnik J.M., Hadley E.C., King A.C., Kritchevsky S.B., Maraldi C., Miller M.E., Newman A.B., Rejeski W.J., Romashkan S., Studenski S. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 248.Cameron I.D., Fairhall N., Langron C., Lockwood K., Monaghan N., Aggar C., Sherrington C., Lord S.R., Kurrle S.E. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cadore E.L., Casas-Herrero A., Zambom-Ferraresi F., Idoate F., Millor N., Gómez M., Rodriguez-Mañas L., Izquierdo M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordrecht, Netherlands) 2014;36(2):773–785. doi: 10.1007/s11357-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kwon J., Yoshida Y., Yoshida H., Kim H., Suzuki T., Lee Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: a randomized controlled trial. J. Am. Med. Dir. Assoc. 2015;16(3):263.e1–263.e8. doi: 10.1016/j.jamda.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 251.Tarazona-Santabalbina F.J., Gómez-Cabrera M.C., Pérez-Ros P., Martínez-Arnau F.M., Cabo H., Tsaparas K., Salvador-Pascual A., Rodriguez-Mañas L., Viña J.A. Multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J. Am. Med. Dir. Assoc. 2016;17(5):426–433. doi: 10.1016/j.jamda.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 252.Losa-Reyna J., Baltasar-Fernandez I., Alcazar J., Navarro-Cruz R., Garcia-Garcia F.J., Alegre L.M., Alfaro-Acha A. Effect of a short multicomponent exercise intervention focused on muscle power in frail and pre frail elderly: a pilot trial. Exp. Gerontol. 2019;115:114–121. doi: 10.1016/j.exger.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 253.Martínez-Velilla N., Casas-Herrero A., Zambom-Ferraresi F., Sáez de Asteasu M.L., Lucia A., Galbete A., García-Baztán A., Alonso-Renedo J., González-Glaría B., Gonzalo-Lázaro M., Apezteguía Iráizoz I., Gutiérrez-Valencia M., Rodríguez-Mañas L., Izquierdo M. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization. JAMA Intern Med. 2019;179(1):28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yu R., Tong C., Ho F., Woo J. Effects of a multicomponent frailty prevention program in prefrail community-dwelling older persons: a randomized controlled trial. J. Am. Med. Dir. Assoc. 2019;(19):30640–30641. doi: 10.1016/j.jamda.2019.08.024. pii: S1525-8610, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 255.Giné-Garriga M., Roqué-Fíguls M., Coll-Planas L., Sitjà-Rabert M., Salvà A. Physical exercise interventions for improving performance-based measures of physical function in community-dwelling, frail older adults: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2014;95:753–769. doi: 10.1016/j.apmr.2013.11.007. e3. [DOI] [PubMed] [Google Scholar]
- 256.Viña J., Salvador-Pascual A., Tarazona-Santabalbina F.J., Rodriguez-Mañas L., Gomez-Cabrera M.C. Exercise training as a drug to treat age associated frailty. Free Radic. Biol. Med. 2016;98:159–164. doi: 10.1016/j.freeradbiomed.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 257.Villareal D.T., Banks M., Siener C., Sinacore D.R., Klein S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 2004;12(6):913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 258.Guazzi M., Bandera F., Ozemek C., Systrom D., Arena R. Cardiopulmonary exercise testing: what is its value? J. Am. Coll. Cardiol. 2017;70(13):1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 259.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M., Brach J., Chandler J., Cawthon P., Connor E.B., Nevitt M., Visser M., Kritchevsky S., Badinelli S., Harris T., Newman A.B., Cauley J., Ferrucci L., Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Abellan van Kan G., Rolland Y., Andrieu S., Bauer J., Beauchet O., Bonnefoy M., Cesari M., Donini L.M., Gillette Guyonnet S., Inzitari M., Nourhashemi F., Onder G., Ritz P., Salva A., Visser M., Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 261.Nelson M.E., Rejeski W.J., Blair S.N., Duncan P.W., Judge J.O., King A.C., Macera C.A., Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports medicine and the American heart association. Med. Sci. Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 262.Fielding R.A., Guralnik J.M., King A.C., Pahor M., McDermott M.M., Tudor-Locke C., Manini T.M., Glynn N.W., Marsh A.P., Axtell R.S., Hsu F.C., Rejeski W.J. LIFE study group. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0182155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Izquierdo M., Casas-Herrero A., Martínez-Velilla N., Alonso-Bouzón C., Rodríguez-Mañas L. En representación del Grupo de Investigadores. An example of cooperation for implementing programs associated with the promotion of exercise in the frail elderly. European Erasmus + «Vivifrail» program. Rev. Esp. Geriatr. Gerontol. 2017;52:110–111. doi: 10.1016/j.regg.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 264.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A., Broccatelli M., Savera G., D'Elia M., Pahor M., Bernabei R., Landi F., SPRINTT Consortium Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017;29(1):35–42. doi: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 265.Kell R.T., Bell G., Quinney A. Musculoskeletal fitness, health outcomes and quality of life. Sports Med. 2001;31(12):863–873. doi: 10.2165/00007256-200131120-00003. [DOI] [PubMed] [Google Scholar]
- 266.Micheo W., Baerga L., Miranda G. Basic principles regarding strength, flexibility, and stability exercises. PM R. 2012;4(11):805–811. doi: 10.1016/j.pmrj.2012.09.583. [DOI] [PubMed] [Google Scholar]
- 267.Bosaeus I., Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc. Nutr. Soc. 2016;75(2):174–180. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 268.Roberts H.C.1, Denison H.J., Martin H.J., Patel H.P., Syddall H., Cooper C., Sayer A.A. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 269.Levinger I., Goodman C., Hare D.L., Jerums G., Toia D., Selig S. The reliability of the 1RM strength test for untrained middle-aged individuals. J. Sci. Med. Sport. 2009;12(2):310–316. doi: 10.1016/j.jsams.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 270.Orssatto LB. da R., Cadore E.L., Andersen L.L., Diefenthaeler F. Why fast velocity resistance training should Be prioritized for elderly people. J. Strength Condit Res. 2019;41(1):105. [Google Scholar]
- 271.Cadore E.L., Izquierdo M. Muscle power training: a hallmark for muscle function retaining in frail clinical setting. J. Am. Med. Dir. Assoc. 2018;19(3):190–192. doi: 10.1016/j.jamda.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 272.Skelton D.A., Greig C.A., Davies J.M., Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23(5):371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 273.Bean J.F., Kiely D.K., Herman S., Leveille S.G., Mizer K., Frontera W.R., Fielding R.A. The relationship between leg power and physical performance in mobility-limited older people. J. Am. Geriatr. Soc. 2002;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 274.Mijnarends D.M., Meijers J.M., Halfens R.J., ter Borg S., Luiking Y.C., Verlaan S., Schoberer D., Cruz Jentoft A.J., van Loon L.J., Schols J.M. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J. Am. Med. Dir. Assoc. 2013;14(3):170–178. doi: 10.1016/j.jamda.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 275.Bohannon R.W., Bubela D.J., Magasi S.R., Wang Y.C., Gershon R.C. Sit-to-stand test: performance and determinants across the age-span. Isokinet. Exerc. Sci. 2010;18(4):235–240. doi: 10.3233/IES-2010-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Izquierdo M., Ibañez J., Gorostiaga E., Garrues M., Zúñiga A., Antón A., Larrión J.L., Häkkinen K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol. Scand. 1999;167(1):57–68. doi: 10.1046/j.1365-201x.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 277.Straight C.R., Lindheimer J.B., Brady A.O., Dishman R.K., Evans E.M. Effects of resistance training on lower-extremity muscle power in middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med (Auckland, N.Z.) 2016;46(3):353–364. doi: 10.1007/s40279-015-0418-4. [DOI] [PubMed] [Google Scholar]
- 278.Schwellnus M. John Wiley & Sons, Ltd; 2008. Flexibility and Joint Range of Motion. En Rehabilitation of Sports Injuries: Scientific Basis; pp. 232–257. [Google Scholar]
- 279.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P., American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 280.Dunsky A. The effect of balance and coordination exercises on quality of life in older adults: a mini-review. Front. Aging Neurosci. 2019;11:318. doi: 10.3389/fnagi.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Berg K.O., Wood-Dauphinee S.L., Williams J.I., Maki B. Measuring balance in the elderly: validation of an instrument. Can. J. Public Health. 1992;83(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 282.Sherrington C., Fairhall N.J., Wallbank G.K., Tiedemann A., Michaleff Z.A., Howard K., Clemson L., Hopewell S., Lamb S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lacroix A., Hortobágyi T., Beurskens R., Granacher U. Effects of supervised vs. Unsupervised training programs on balance and muscle strength in older adults: a systematic review and meta-analysis. Sports Med. 2017;47(11):2341–2361. doi: 10.1007/s40279-017-0747-6. [DOI] [PubMed] [Google Scholar]
- 284.Karahan A.Y., Tok F., Taşkın H., Kuçuksaraç S., Başaran A., Yıldırım P. Effects of exergames on balance, functional mobility, and quality of life of geriatrics versus home exercise Programme: randomized controlled study. Cent. Eur. J. Publ. Health. 2015;23(Suppl):S14–S18. doi: 10.21101/cejph.a4081. [DOI] [PubMed] [Google Scholar]
- 285.Mouthon A.A., Ruffieux J., Keller M., Taube W. Age-related differences in corticospinal excitability during observation and motor imagery of balance tasks. Front. Aging Neurosci. 2016;8:317. doi: 10.3389/fnagi.2016.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Marusic U., Grosprêtre S. Non-physical approaches to counteract age-related functional deterioration: applications for rehabilitation and neural mechanisms. Eur. J. Sport Sci. 2018;18(5):639–649. doi: 10.1080/17461391.2018.1447018. [DOI] [PubMed] [Google Scholar]
- 287.Shih T.Y., Wu C.Y., Lin K.C., Cheng C.H., Hsieh Y.W., Chen C.L., Lai C.J., Chen C.C. Effects of action observation therapy and mirror therapy after stroke on rehabilitation outcomes and neural mechanisms by MEG: study protocol for a randomized controlled trial. Trials. 2017;18(1):459. doi: 10.1186/s13063-017-2205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Picorelli A.M., Pereira L.S., Pereira D.S., Felício D., Sherrington C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J. Physiother. 2014;60(3):151–156. doi: 10.1016/j.jphys.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 289.Dalle Grave R., Calugi S., Centis E., El Ghoch M., Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2011;2011:348293. doi: 10.1155/2011/348293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Forkan R., Pumper B., Smyth N., Wirkkala H., Ciol M.A., Shumway-Cook A. Exercise adherence following physical therapy intervention in older adults with impaired balance. Phys. Ther. 2006;86:401–410. [PubMed] [Google Scholar]
- 291.Rivera-Torres S., Fahey T.D., Rivera M.A. Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med. 2019;5 doi: 10.1177/2333721418823604. 2333721418823604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.O’Neill D., Forman D.E. The importance of physical function as a clinical outcome: assessment and enhancement. Clin. Cardiol. 2019 doi: 10.1002/clc.23311. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Buford T.W., Anton S.D., Clark D.J., Higgins T.J., Cooke M.B. Optimizing the benefits of exercise on physical function in older adults. PM R. 2014;6(6):528–543. doi: 10.1016/j.pmrj.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Vázquez F.L., Otero P., García-Casal J.A., Blanco V., Torres Á.J., Arrojo M. Efficacy of video game-based interventions for active aging. A systematic literature review and meta-analysis. PloS One. 2018;13(12) doi: 10.1371/journal.pone.0208192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Makizako H., Shimada H., Doi T., Tsutsumimoto K., Hotta R., Nakakubo S., Makino K., Lee S. Social frailty leads to the development of physical frailty among physically non-frail adults: a four-year follow-up longitudinal cohort study. Int. J. Environ. Res. Publ. Health. 2018;15(3):E490. doi: 10.3390/ijerph15030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cadore E.L., Izquierdo M. Exercise interventions in polypathological aging patients that coexist with diabetes mellitus: improving functional status and quality of life. Age (Dordrecht, Netherlands) 2015;37(3):64. doi: 10.1007/s11357-015-9800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liu C.J., Latham N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kaushal N., Langlois F., Desjardins-Crépeau L., Hagger M.S., Bherer L. Investigating dose–response effects of multimodal exercise programs on health-related quality of life in older adults. Clin. Interv. Aging. 2019;14:209–217. doi: 10.2147/CIA.S187534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sánchez‐Sánchez J.L., Mañas A., García‐García F.J., Ara I., Carnicero J.A., Walter S., Rodríguez‐Mañas L. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle. 2019;10(1):188–198. doi: 10.1002/jcsm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Legrand D., Vaes B., Matheï C., Adriaensen W., Van Pottelbergh G., Degryse J.M. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J. Am. Geriatr. Soc. 2014;62(6):1030–1038. doi: 10.1111/jgs.12840. [DOI] [PubMed] [Google Scholar]
- 301.Fernández-Garrido J., Ruiz-Ros V., Buigues C., Navarro-Martinez R., Cauli O. Clinical features of prefrail older individuals and emerging peripheral biomarkers: a systematic review. Arch. Gerontol. Geriatr. 2014;59(1):7–17. doi: 10.1016/j.archger.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 302.Vasunilashorn S., Coppin A.K., Patel K.V., Lauretani F., Ferrucci L., Bandinelli S., Guralnik J.M. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64:223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Xue Q.L., Bandeen-Roche K., Varadhan R., Zhou J., Fried L.P. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 304.Danon-Hersch N., Rodondi N., Spagnoli J., Santos-Eggimann B. Prefrailty and chronic morbidity in the youngest old: an insight from the Lausanne cohort Lc65+ J. Am. Geriatr. Soc. 2012;60(9):1687–1694. doi: 10.1111/j.1532-5415.2012.04113.x. [DOI] [PubMed] [Google Scholar]
- 305.Tieland M., Dirks M.L., van der Zwaluw N., Verdijk L.B., van de Rest O., de Groot L.C., van Loon L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 306.Tieland M., van de Rest O., Dirks M.L., van der Zwaluw N., Mensink M., van Loon L.J., de Groot L.C. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 307.Landi F., Cesari M., Calvani R., Cherubini A., Di Bari M., Bejuit R., Mshid J., Andrieu S., Sinclair A.J., Sieber C.C., Vellas B., Topinkova E., Strandberg T., Rodriguez-Manas L., Lattanzio F., Pahor M., Roubenoff R., Cruz-Jentoft A.J., Bernabei R.…SPRINTT Consortium The «Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies» (SPRINTT) randomized controlled trial: design and methods. Aging Clin. Exp. Res. 2017;29(1):89–100. doi: 10.1007/s40520-016-0715-2. [DOI] [PubMed] [Google Scholar]
- 308.Lopez P., Izquierdo M., Radaelli R., Sbruzzi G., Grazioli R., Pinto R.S., Cadore E.L. Effectiveness of multimodal training on functional capacity in frail older people: a meta-analysis of randomized controlled trials. J. Aging Phys. Activ. 2018;26(3):407–418. doi: 10.1123/japa.2017-0188. [DOI] [PubMed] [Google Scholar]
- 309.Brown C.J., Redden D.T., Flood K.L., Allman R.M. The underrecognized epidemic of low mobility during hospitalization of older adults. J. Am. Geriatr. Soc. 2009;57(9):1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 310.Wall B.T., van Loon L.J.C. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013;71(4):195–208. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 311.Blain H., Masud T., Dargent-Molina P., Martin F.C., Rosendahl E., van der Velde N., Bousquet J., Benetos A., Cooper C., Kanis J.A., Reginster J.Y., Rizzoli R., Cortet B., Barbagallo M., Dreinhöfer K.E., Vellas B., Maggi S., Strandberg T. EUGMS falls and fracture interest group,; international association of gerontology and geriatrics for the European region (IAGG-ER),; European union of medical specialists (EUMS),; fragility fracture network (FFN),; European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO), and; international osteoporosis foundation (IOF) Aging Clin. Exp. Res. 2016;28(4):797–803. doi: 10.1007/s40520-016-0588-4. [DOI] [PubMed] [Google Scholar]
- 312.Valenzuela P.L., Castillo-García A., Morales J.S., Izquierdo M., Serra-Rexach J.A., Santos-Lozano A., Lucia A. Physical exercise in the oldest old. Comp. Physiol. 2019;9(4):1281–1304. doi: 10.1002/cphy.c190002. [DOI] [PubMed] [Google Scholar]
- 313.Jones S., Man W.D., Gao W., Higginson I.J., Wilcock A., Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016;10:CD009419. doi: 10.1002/14651858.CD009419.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., Scherr P.A., Wallace R.B. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 315.Chang M., Cohen-Mansfield J., Ferrucci L., Leveille S., Volpato S., de Rekeneire N., Guralnik J.M. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J. Am. Geriatr. Soc. 2004;52:2094–2098. doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 316.García-García F.J., Carcaillon L., Fernandez-Tresguerres J., Alfaro A., Larrion J.L., Castillo C., Rodriguez-Mañas L. A new operational definition of frailty: the Frailty Trait Scale. J. Am. Med. Dir. Assoc. 2014;15(5) doi: 10.1016/j.jamda.2014.01.004. 371.e7-371.e13. [DOI] [PubMed] [Google Scholar]
- 317.García-García F.J., Carnicero J.A., Losa-Reyna J., Alfaro-Acha A., Castillo-Gallego C., Rosado- Artalejo C., Gutiérrrez-Ávila G., Rodriguez-Mañas L. Frailty Trait Scale short form: a frailty instrument for clinical practice. J. Am. Med. Dir. Assoc. 2020 doi: 10.1016/j.jamda.2019.12.008. S1525-8610(19)30868-0, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 318.Parmenter B.J., Mavros Y., Ritti Dias R., King S., Fiatarone Singh M. Resistance training as a treatment for older persons with peripheral artery disease: a systematic review and meta-analysis. Br. J. Sports Med. 2019 Apr 12 doi: 10.1136/bjsports-2018-100205. pii: bjsports-2018-100205, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 319.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hamburg N.M., Balady G.J. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123(1):87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Izquierdo M., Rodriguez-Mañas L., Casas-Herrero A., Martinez-Velilla N., Cadore E.L., Sinclair A.J. Is it ethical not to precribe physical activity for the elderly frail? J. Am. Med. Dir. Assoc. 2016;17:779–781. doi: 10.1016/j.jamda.2016.06.015. [DOI] [PubMed] [Google Scholar]