Abstract
Redox reactions control fundamental processes of human biology. Therefore, it is safe to assume that the responses and adaptations to exercise are, at least in part, mediated by redox reactions. In this review, we are trying to show that redox reactions are the basis of exercise physiology by outlining the redox signaling pathways that regulate four characteristic acute exercise-induced responses (muscle contractile function, glucose uptake, blood flow and bioenergetics) and four chronic exercise-induced adaptations (mitochondrial biogenesis, muscle hypertrophy, angiogenesis and redox homeostasis). Based on our analysis, we argue that redox regulation should be acknowledged as central to exercise physiology.
Keywords: Adaptations, Antioxidants, Exercise, Redox biology, Responses, Signaling
Graphical abstract
Highlights
-
•
Redox reactions are a fundamental part of human biology.
-
•
Exercise responses and adaptations are partially controlled by redox reactions.
-
•
Redox signaling should be acknowledged as central to exercise physiology.
Abbreviations
- Akt
protein kinase B
- AMPK
5′ AMP-activated protein kinase
- ARE
antioxidant response element
- c-Src
proto-oncogene tyrosine-protein kinase
- CaMK
Ca2+/calmodulin-dependent protein kinase
- cGMP
cyclic guanosine monophosphate
- ERK
extracellular signal-regulated kinase
- G6PD
glucose-6-phosphate dehydrogenase
- GAPDH
reduced glyceraldehyde 3-phosphate dehydrogenase
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia-inducible factor 1-alpha
- IGF-I
insulin-like growth factor 1
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinases
- Keap1
Kelch-like ECH-associated protein 1
- LKB1
liver kinase B1
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO•
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- O•-
superoxide radical
- OGG1
8-oxoguanine glycosylase
- OH•
hydroxyl radical
- ONOO-
peroxynitrite
- p70S6K
ribosomal protein S6 kinase beta-1
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K
phosphoinositide 3-kinases
- PPP
pentose phosphate pathway
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RONS
reactive oxygen and nitrogen species
- RYR
ryanodine receptors
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SOD
superoxide dismutase
- TnI
troponin I
- Trpv1
transient receptor potential cation channel subfamily V member 1
- VEGF
vascular endothelial growth factor.
1. Introduction
The multiple physical and mental health-related benefits of regular exercise have long been acknowledged and are nowadays undeniable [1,2]. As a logical consequence, researchers over the years commenced to search for the underlying molecular and biochemical mechanisms that drive these benefits. Since the first studies that implemented pioneering techniques (e.g., muscle biopsies) about 45 years ago [[3], [4], [5]], a great progress has been made in the field. For instance, it is currently well-established that exercise “releases” a set of local and systemic stressors that trigger integrated acute responses, which in the long-term result in phenotypic adaptations in all human body systems [[6], [7], [8]].
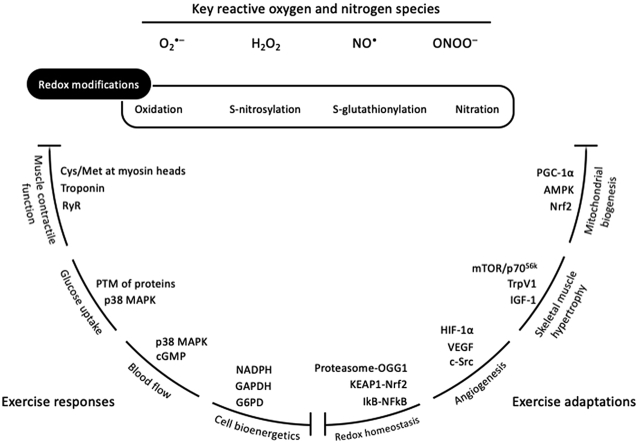
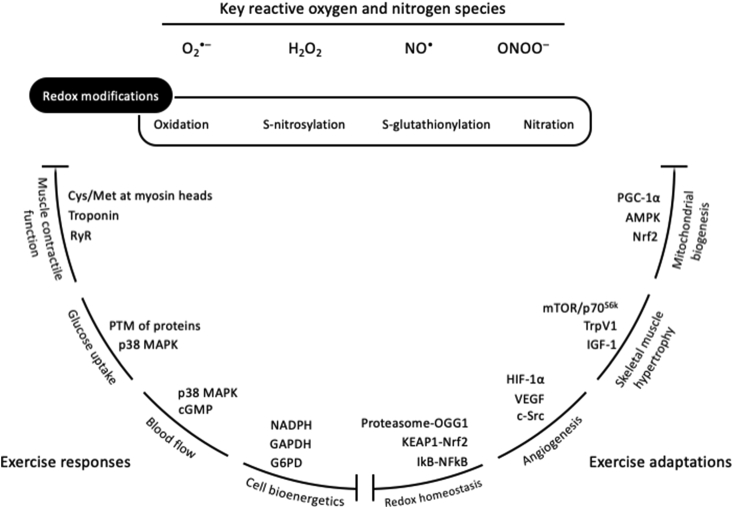
Redox processes are increasingly recognized as an integral part of the exercise-associated metabolism. Despite the traditional perception that reactive species are exclusively detrimental molecules, mounting recent evidence suggests that exercise-induced reactive species are essential upstream signals for the activation of redox-sensitive transcription factors and the induction of gene expression associated with exercise [9,10]. Terms such as “ROS”, “free radicals” and “oxidative stress” are more and more often included in leading exercise physiology reviews as regulators of responses and adaptations [[11], [12], [13]]. However, in most cases, the implication of redox processes in exercise physiology processes is either not supported by direct mechanistic evidence or is promoted as a subordinate element. Thus, the main purpose of the present review is to highlight the fundamental redox basis of exercise physiology, by outlining the contribution of redox network in 4 characteristic acute exercise-induced responses (i.e., muscle contractile function, glucose uptake, blood flow and cell bioenergetics) and 4 well-known training-induced adaptations (i.e., mitochondrial biogenesis, skeletal muscle hypertrophy, angiogenesis and redox homeostasis). We do not aim to comprehensively describe the underlying mechanisms for each of the 8 aforementioned exercise-related processes, but rather to concisely decipher the corresponding redox components probably involved.
Taking into account that the field of exercise redox biology is still in its infancy, the exercise responses and adaptations data presented in this review was selected irrespective of the exercise type (i.e., aerobic or anaerobic, endurance or resistance) or the experimental approach implemented in the original studies. Thus, exercise studies that utilized nutritional antioxidants (e.g., vitamin C) or reactive species source inhibitors (e.g., apocynin), transgenic rodent models over- or under-expressing redox related genes (e.g., G6PD-Tg mice), in-vitro, in-situ or ex-vivo set-ups as well as studies that implemented more invasive techniques, such as synergistic ablation or hindlimb ischemia, have been included. Moreover, the review is inevitably focused on skeletal muscle, due to lack of research in other tissues. Mechanistic information from other scientific fields (e.g., biochemistry, nutrition and clinical medicine [[14], [15], [16]] regarding well-established redox sensitive signaling pathways has been incorporated in particular cases as well. Collectively, our review aspires to tentatively translate molecular and biochemical redox data into physiological real-life exercise situations and, vice versa, to identify likely redox mechanisms underlying common physiological exercise responses and adaptations.
2. Redox-regulated exercise responses
2.1. Muscle contractile function
Redox regulation of muscle contractile function probably represents the “archetypal” example of how reactive oxygen and nitrogen species are implicated in exercise responses (the umbrella abbreviation RONS will be used throughout the paper, but the precise species will be named when available [17]. During the first half of the 1980's, two studies that used electron paramagnetic resonance spectroscopy demonstrated that the skeletal muscle tissue from rabbits [18] and humans [19] produces detectable amounts of RONS at rest, and this production greatly increases during contraction. These findings were further supported by more recent studies implementing elegant experimental approaches in exercising humans [20,21]. As a result, the key question about the potential biological role of RONS in skeletal muscle function sensibly emerged. Ever since this question was firstly placed, our understanding on the role of RONS on muscle contractile function has greatly progressed under both physiological (mild or transient RONS increase) and pathological (excessive or chronic RONS increase) conditions [22]. Most of the evidence however is inevitably based on studies using isolated muscle preparations and animal models due to limitations in existing analytical tools (i.e., volatile nature of RONS and low bioavailability of redox probes in humans).
An interesting series of studies were conducted in intact mouse fibers (i.e., fibers along with their entire redox environment consisting of RONS sources, antioxidants, and target molecules) [[23], [24], [25]]. In brief, transient exposure (i.e., 4 min) or low concentration (i.e., in the nM and pM range) of hydrogen peroxide (H2O2) or tert-butylhydroperoxide (t-BOOH) increased submaximal muscle force production in fast-twitch muscle fibers, while the addition of the antioxidant dithiothreitol (DTT) resulted in a progressive force decline. On the other hand, prolonged exposure (i.e., 8 min) or high concentration (i.e., in the mM range) of H2O2 decreased submaximal muscle force production (indication of muscle fatigue development) and this was reversed by DTT addition. Interestingly, transient exposure of unfatigued intact fast-twitch muscle fibers to DTT decreased submaximal force production and this was reversed after the treatment with the opposite redox stimulus, namely H2O2 [[23], [24], [25]]. Thus, an “optimal” cellular redox state seems to be the key determinant for normal muscle force production, whereas a more reduced (e.g., at rest) or oxidized state (e.g., during fatiguing protocols) negatively regulates this process (as illustrated in the classic biphasic model proposed by Reid [26]).
Similar studies using mechanically-skinned muscle fiber preparations showed that H2O2 per se, in concentrations as high as 10 mM, does not necessarily exert an effect in force production [27] yet in some cases comparable or even higher levels of H2O2 were found to negatively affect contractile function due to oxidation of specific cysteine and methionine residues at the actomyosin interface [[28], [29], [30]]. In a more physiological context, Murphy et al. [31] treated skinned muscle fibers with myoglobin and reduced glutathione along with low H2O2 concentrations (100–300 μM). They found that glutathione partially prevented the decline in muscle force production induced by the H2O2–Fe2+/myoglobin reaction in the slow-twitch muscle fibers, while in the fast-twitch muscle fibers, glutathione actually increased Ca2+ sensitivity (at least in the initial phase) [31]. This was subsequently explained by the interaction of glutathione with the oxidized cysteine residues on the fast isoform of troponin (TnI) that increased myofibrillar Ca2+ sensitivity [32]. Generally, in most of the aforementioned studies, it was demonstrated that H2O2 regulates muscle force production either positively or negatively mainly by altering myofibrillar Ca2+ sensitivity as indicated by the marginally affected free cytosolic Ca2+ concentration [33].
Beyond H2O2, the superoxide radical has been also demonstrated to affect muscle force production, however this was facilitated through a different mechanism, that is, an altered sarcoplasmic reticulum Ca2+ release (and not Ca2+ sensitivity) [34,35]. Regarding nitric oxide (the parent reactive nitrogen species) and its effects on force production, experiments on intact fast-twitch muscle fibers have shown that nitric oxide affects contractile function by altering the myofibrillar Ca2+ sensitivity [36]. Moreover, a study that used skinned muscle fibers treated with S-nitroso-N-acetylpenicillamine and nitrosoglutathione (two nitric oxide donors) reported decreased Ca2+ sensitivity in fast-twitch fibers, however maximum force was not affected [37]. In contrast to fast-twitch fibers, the same study showed that the slow-twitch muscle fibers treated with the two nitric oxide donors did not exhibit altered myofibrillar Ca2+ sensitivity. Other studies have demonstrated that, along with Ca2+ sensitivity, reactive nitrogen species may indeed affect muscle force production as well [38] and this may stem from the S-nitrosylation of the myosin heads by peroxynitrite, a highly reactive species produced by the reaction of superoxide radical with nitric oxide [[39], [40], [41]]. Finally, nitrosative modifications, along with other redox reactions, of calcium release-reuptake proteins, such as the ryanodine receptor/Ca2+ release channel (RyR1), have been also described. These structural alterations also affect muscle contractile function indicating that RONS regulate muscle force production and fatigue development by altering Ca2+ release [[42], [43], [44]].
In light of the above, several studies sought to investigate the effectiveness of acute antioxidant supplementation as an ergogenic strategy against muscle fatigue induced by RONS. Several nutritional and pharmaceutical compounds with purported antioxidant function (i.e., vitamins C and E, quercetin, resveratrol, coenzyme Q10, food-derived polyphenols, spirulina and N-acetylcysteine) have been utilized, typically in several-fold greater doses compared to the recommended dietary allowance guidelines. For most of them, the results are mixed but mostly disappointing [45]. Even among the studies that showed beneficial effects on performance, a certain antioxidant dose, time point of supplementation or exercise type and intensity could not be identified as key factors. N-acetylcysteine, as cysteine donor for the endogenous (re)synthesis of glutathione, seems to be the only antioxidant with promising ergogenic potential after acute supplementation that may offer a performance benefit around competition time [46] (although the antioxidant potential of N-acetylcysteine seems multifaceted from a mechanistic perspective [47,48]. This has been reported in many human exercise studies utilizing whole-body or specific muscle group performance tests, such as repeated bouts of running, electrical stimulation of the tibialis anterior muscle and isometric handgrip maneuvers [49,50,[52], [53], [54]]. Yet, it should be highlighted that in many studies NAC was delivered via infusion and not orally, as was the case for most of the other antioxidants. It is worth mentioning that antioxidant supplementation can also confer beneficial performance effects when reversing a particular antioxidant deficiency (e.g., Vitamin C supplementation only in vitamin C-deficient individuals) [55]. This is also in line with the recent consensus statement of the International Olympic Committee, which states that “supplements aimed at correcting nutrient deficiencies need to be judged on their ability to prevent or treat suboptimal nutrient status, with the benefit accruing from the removal of the associated impairment of health, training capacity or performance” [56].
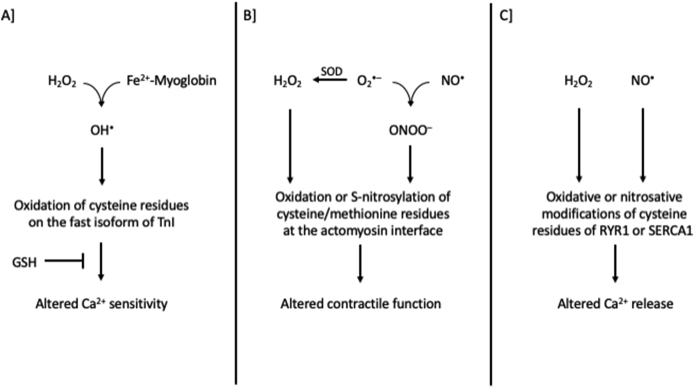
Collectively, RONS-regulation of muscle contractile function has been predominantly verified in fast-twitch muscle fibers rather than in slow-twitch muscle fibers and during submaximal rather than maximal intensities (e.g., during repeated tetanic stimulations). Moreover, RONS-induced alteration in Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release, rather than the impairment in membrane excitability, seem to be the main cellular processes regulating muscle contractile function; yet, the exact redox mechanisms still remain to be fully elucidated [e.g., oxidation, S-nitrosylation or S-glutathionylation of the skeletal muscle RyR1 and of the calcium binding subunit of troponin [57,58,405] (Fig. 1). For an in-depth analysis of the topic, the reader is referred to reviews about the redox-regulation of muscle contractile function and force production [59,33], the redox-mediated muscle fatigue [60,61] and the effectiveness of antioxidants as ergogenic aids [45,51,62,63].
Fig. 1.
Proposed mechanisms on how RONS regulate muscle contractile function. Panel A based on [31,32]; Panel B based on [28,29,39] and [30]; Panel C based on [[42], [43], [44]]. GSH, reduced glutathione; H2O2, hydrogen peroxide; NO•, nitric oxide; OH•, hydroxyl radical; ONOO−, peroxynitrite; RYR, ryanodine receptors; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; SOD, superoxide dismutase; TnI, troponin I.
2.2. Glucose uptake
During exercise, skeletal muscle tissue upregulates the utilization of substrates, predominantly carbohydrates and fats depending on the duration and intensity of exercise, to meet the increased energy demands. Diverse signaling transduction pathways have been described that coordinate the complex metabolic process of glucose uptake by skeletal muscle during exercise [64]. Up till now, none of these pathways appears to be the main governor orchestrating the whole process; instead, redundant mechanisms most probably account for the delivery, transport across the muscle membrane and intracellular metabolism of glucose [65]. Among these mechanisms, there is building evidence that reactive oxygen and nitrogen species, either individually or in combination play a key role in skeletal muscle glucose uptake during exercise (the peculiar role of RONS in insulin-mediated glucose uptake has been covered elsewhere [66,409]).
Regarding reactive oxygen species, Sandström and colleagues [68] treated an ex-vivo muscle preparation (i.e., extensor digitorum longus; EDL) with the non-specific antioxidant N-acetylcysteine or Ebselen (a glutathione peroxidase mimetic [67]; and demonstrated that both antioxidant interventions attenuated the increased muscle glucose uptake during contractions (but not resting uptake). Interestingly, this effect was accompanied by a decrease in CM-H2DCF fluorescence (a non-specific indicator of ROS production) pointing towards a redox implication in glucose uptake. In the same study, the EDL muscle from mice that over-expressed superoxide dismutase exhibited greater glucose uptake in response to ex vivo contractions compared to EDL from wild type mice. Given that increased superoxide dismutase activity normally results in increased H2O2 levels (due to dismutation-reduction of the superoxide radical) and Ebselen mimics glutathione peroxidase activity, it was concluded that H2O2 is the key oxygen species involved [68]. This idea was also supported by a study which showed that catalase, an enzyme that catalyzes the decomposition of H2O2 to water and oxygen, but not superoxide dismutase inhibits EDL muscle glucose uptake in response to a hypoxanthine-xanthine oxidase superoxide-generating system in rats [69].
In an effort to acquire a mechanistic perspective, the authors also evaluated AMPK activity and reported hampered activity after N-acetylcysteine treatment, indicating that RONS-regulation of glucose uptake is mediated by AMPK [68]. This scenario seemed to be biologically possible, since H2O2 has been shown to selectively activate the α1 isoform of AMPK via modifications of redox-sensitive residues in particular muscles, such as the epitrochlearis muscle [70,71]. However, Merry and colleagues [72,[78], [79], [80]] conducted a study to specifically examine whether AMPK indeed stimulates glucose uptake in response to exercise-induced reactive oxygen species by using AMPK kinase-dead mice and N-acetylcysteine administration during ex-vivo contractions of EDL and soleus muscles. Their findings did not verify the relationship between reactive oxygen species and AMPK activity in glucose uptake, since N-acetylcysteine hampered glucose uptake similarly in AMPK kinase-dead and wild type mice [72]. This notion was indirectly supported by the findings from Jensen et al. [73], who provided evidence for an essential role of α1 AMPK in contraction-stimulated EDL glucose uptake in a H2O2-independent manner. However, recent findings reconcile previous discrepancies and fundamentally redefine the role of AMPK activation in exercise, suggesting that AMPK is important for maintaining glucose permeability in skeletal muscle following exercise, but not during exercise in vivo [[74], [75], [76]]. In other mechanistic ex-vivo studies, p38 MAPK, the calmodulin family of kinases (CaMK) and phosphatidylinositol 3-kinase (PI3K) have been also directly or indirectly implicated in the reactive oxygen species regulation of muscle glucose uptake [69,77,410, 411]; yet mechanistic redox studies with a particular focus on exercise energy metabolism and muscle glucose uptake are scarce and, in some cases, data are controversial [78].
In a more physiological context, and in contrast to ex-vivo studies that suggested a role for reactive oxygen species in muscle glucose uptake, some in-vivo and in-situ data did not confirm this viewpoint. In particular, two studies from the same group, who administered N-acetylcysteine in humans during cycle ergometry (at about 60% of VO2max) and in rats’ hind-limb in-situ during repeated contractions, did not report any effect on muscle glucose uptake [79,80]. These contradictory findings may stem from the fact that in-vivo and in-situ set-ups maintained their circulating and local redox milieu and the contraction and exercise intensities applied were more physiologically relevant compared to the prolonged ex-vivo tetanic stimulations of isolated fibers (eliciting artificially high RONS production) and the supra-physiological doses of exogenously added oxidants (in the mM range) [81]. On the other hand, a very recent in-vivo study by Henríquez-Olguín and colleagues [82], who exploited the advantages of some modern and sophisticated analytical methods with genetically encoded biosensors, demonstrated that NADPH oxidase 2-originated reactive oxygen species play a pivotal role in muscle glucose uptake and GLUT4 translocation during moderate intensity exercise [82]. In addition, intravenous infusion of N-acetylcysteine attenuated thigh net glucose uptake during exercise after, but not prior to, blood flow restricted training in humans, which coincided with an elevated muscle abundance of nNOS and the antioxidant enzymes Cu/Zn-SOD and GPX-1. This finding indicated that the contribution from ROS to muscle glucose regulation in humans may depend on the trained state of skeletal muscle [83]. In sum, the role of reactive oxygen species in muscle glucose uptake remains, at least, elusive.
Regarding reactive nitrogen species, the case of muscle glucose uptake is slightly clearer at least in humans. More specifically, it has been demonstrated that local infusion of the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (L-NMMA) in the femoral artery of human subjects impaired the increase in leg glucose uptake by ~45% during cycling at 60% VO2max and this adverse effect was reversed by infusion of l-arginine (i.e., a key precursor for nitric oxide production by synthases) [84]. The effect of the L-NMMA administration was exacerbated in diabetic patients (i.e., up to 75% impaired glucose uptake during exercise [85], which is of utmost importance for this cohort taking into account the different mechanisms between insulin- and exercise-mediated glucose uptake [86]. Moreover, administration of l-arginine in healthy humans significantly increased glucose disposal during exercise as assessed via glucose disappearance rate (stable glucose tracer infusion method was used) and glucose clearance rate [87]. A point worth to mention is that nitric oxide synthase inhibition at this level may affect muscle glucose uptake without altering leg blood flow, blood pressure, or arterial plasma glucose content as indicated by a rat study [88]. In contrast to human studies, rodent studies show contradictory results with NO synthase inhibition yielding positive, negative or neutral effects. Although diverse methodological set-ups had been implemented, such as in-vivo l-NAME ingestion [89,90], in-situ sciatic nerve electrical stimulation followed by ex-vivo exposure to L-NMMA [91] and in-vitro muscle contractions undertaken in the presence of L-NMMA [92,93], the major difference compared to human studies is that, expect one [94], all other rodents studies evaluated muscle glucose uptake 20 or more min after the “exercise” stimulus and not during exercise. Thus, methodological practicalities may largely account for the discrepancy between human and rodent studies [95].
The mechanisms underlying the reactive nitrogen species regulation of muscle glucose uptake during exercise were for a long time based on the combination of two distinct hypotheses. In particular, it has been previously demonstrated that nitric oxide production induced by a nitric oxide donor (i.e., spermine NONOate) triggered glucose uptake in isolated human skeletal muscle and L6 skeletal muscle cells at rest and this was mediated by the typical cyclic guanosine monophosphate (cGMP)-cGMP-dependent protein kinase (PKG) pathway and AMPK [96]. In addition, Lau and colleagues [97] demonstrated that contracting fast-twitch skeletal muscles increase nitric oxide production, which subsequently increases cGMP concentration [97]. By linking the findings from these two conditions (one at resting state and the other during contraction), it was frequently argued that the nitric oxide/cGMP/PKG pathway also regulates muscle glucose uptake during exercise. However, this was not verified in a study by Merry and colleagues [72,[78], [79], [80]] who reported that skeletal muscle glucose uptake during ex vivo contractions takes place via a cGMP/PKG-independent pathway [78]. Other mechanisms that can been proposed for the reactive nitrogen species-mediated insulin signaling and glucose uptake include specific redox post-translational modifications, such as S-nitrosylation, S-glutathionylation and tyrosine nitration of target proteins, such as p21ras and ERK [[98], [99], [100], [101]]. However, and to the best of our knowledge, studies to validate these scenarios in an exercise setting are lacking [78]. Interestingly, Hong and colleagues [103] recently reported that nitric oxide regulates skeletal muscle glucose uptake during ex-vivo contractions of the EDL muscles independently of nNOSμ (the primary isoform of nitric oxide synthase expressed in skeletal muscle following exercise [102], adding further complexity to the field [103].
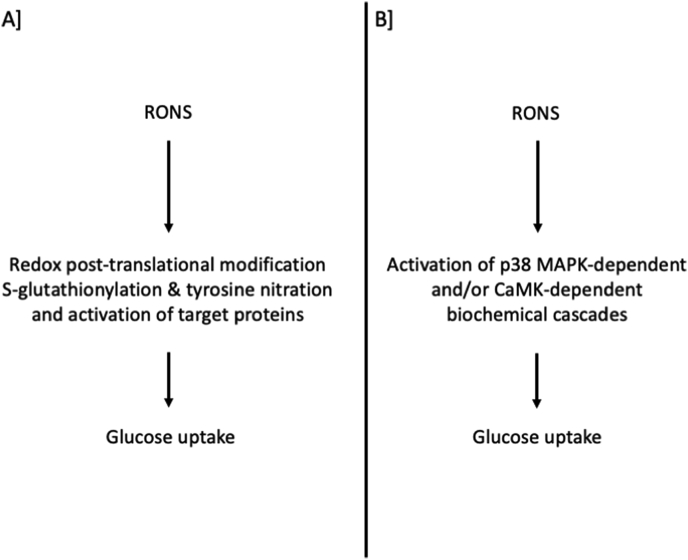
Collectively, mounting evidence suggests that RONS production represents one of the multiple and redundant mechanisms that mediate muscle glucose uptake during exercise [64,104] (Fig. 2). However, further work is needed, especially for the reactive oxygen species, to reveal the potential underlying mechanisms involved. We specifically focused on insulin-independent glucose uptake during exercise and not on the enhanced insulin-stimulated glucose uptake following exercise (i.e., insulin sensitivity [105,106]. For an in-depth analysis of the topic, the reader is referred to reviews about the oxidative [81,107] or nitrosative [95,108] regulation of muscle glucose uptake during exercise.
Fig. 2.
Proposed mechanisms on how RONS regulate tissue glucose uptake. Panel A based on [98,101,108]; Panel B based on [[69], [77], [410]]. CaMK, Ca2+/calmodulin-dependent protein kinase; MAPK, mitogen-activated protein kinase; RONS, reactive oxygen and nitrogen species.
2.3. Blood flow
One of the most well-known physiological changes that immediately occur during exercise is the hemodynamic reorganization of blood flow to organs. For instance, blood flow in skeletal muscles may increase up to 100-fold in order to match the oxygen and energy substrate demands of exercise and to remove metabolic by-products [109,110]. Diverse central (e.g., increased cardiac output) and peripheral (i.e., vascular smooth muscle relaxation) mechanisms have been implicated in the regulation of blood flow during exercise, and although none of them seems to be clearly dominating, nitric oxide appears as the central node molecule of most pathways [111]. Optimal exercise performance dictates a physiological-based “hierarchical” redistribution of blood flow in almost all human organs and tissues, such as brain [112], skin [113] and liver [114], however in the present section we will focus on the role of RONS in vascular function and in blood flow to active skeletal muscles.
Both the vasculature and the endothelium are tissues that encompass many typical RONS sources, such as NADPH oxidases, xanthine oxidases and nitric oxide synthases [115,116]. The potential role of RONS in vascular function during exercise, along with their interaction (e.g., superoxide reduces the bioavailability of nitric oxide [117], as well as their synergistic or antagonistic effects with other vasoactive molecules (e.g., acetylcholine) remain to a large extend unknown, at least from a mechanistic perspective [118]. As already stated, nitric oxide is regarded a potent inherently vasoactive RONS under diverse physiological conditions including exercise [119,120]. A series of exercise studies performed in humans during the ‘90s demonstrated that nitric oxide positively regulates vascular tone, whilst interesting findings were reported about the time course of this effect, with some studies arguing that nitric oxide is predominantly implicated at rest and during recovery, and not at the initiation or maintenance of active hyperemia [121,122]. Partially contradictory findings were observed regarding the role of nitric oxide in exercise-induced skeletal muscle hyperemia, possibly stemming from the different limbs involved in the original studies (i.e., lower limbs compared to upper limbs) or from the complex redundancy in this process (e.g., simultaneous activity of prostanoids and endothelial derived hyperpolarizing factors) [84,[123], [124], [125], [126], [127]]. In general, these classic exercise physiology studies indicated that nitric oxide is implicated in vascular function during exercise and especially in the peri-exercise period, while its role in muscle blood flow is probably compensatory to other factors.
In addition to nitric oxide, two more recent redox-oriented studies employed a generic antioxidant treatment, consisting of Vitamin C, vitamin E and alpha lipoic acid, and demonstrated that in healthy young males the antioxidant scheme impaired handgrip exercise-induced brachial artery vasodilation (assessed via Doppler ultrasound) and this was accompanied by a remarkable reduction in RONS production (assessed via electron paramagnetic resonance spectroscopy; EPR) [128,129]. Given that the use of generic antioxidants and the probes of EPR do not always provide insights about the precise reactive species involved in the process, it is reasonable to argue that RONS, other than nitric oxide, may also be implicated. For instance, H2O2 [130], peroxynitrite [131] and peroxy-radicals [132] have been reported to exert vasodilatory effects under particular, yet non-exercising, conditions. Interestingly, an opposing outcome was observed when the same exercise and antioxidant treatment was applied to aged individuals. In fact, antioxidants restored the impaired vasodilation in this population during an acute exercise bout; however, after a training period the old subjects “mimicked” their young counterparts exhibiting normal resting redox status and impaired exercise-induced vasodilation after antioxidant supplementation [128,133]. In another elegant study, Sindler and colleagues [134] treated soleus muscle resistance arterioles from rats habituated to treadmill running with Tempol (an “antioxidant” redox cycling nitroxide) and/or catalase and reported that both treatments eliminated flow-induced vasodilation indicating a key role of RONS in this process [134]. In light of the above, it seems that under physiological conditions an exercise-induced pro-oxidant environment is necessary for vasodilation, which should not be disturbed with exogenous antioxidants. On the other hand, under pro-oxidative conditions (e.g., ageing or disease) exogenous antioxidants “normalize” resting redox balance facilitating optimal vasodilation both at rest [135] and in response to exercise [133].
Regarding muscle-specific blood flow during exercise, it seems that the regulation of the microvascular tone, coordinated by resistance arteries, arterioles and capillaries, is probably the key biological component and that the tissue blood flow is not a matter of the macrovascular tone (i.e., larger conduit vessels) [136]. This is exemplified by the vasoactive role of RONS (assessed using the spin trap α-phenyl-tert-butylnitrone and EPR) in a conduit vessel (i.e., brachial artery) during handgrip exercise in humans without a concomitant increase in forearm muscle blood flow [129]. Similarly, hyperemia in the lower limbs of young males during passive knee extension was impaired after L-NMMA infusion (a nitric oxide synthase inhibitor) without a concomitant decrease in femoral artery diameter [137]. Interestingly, the findings by Ref. [128,129] that the antioxidant cocktail, consisting of generic antioxidants, did not exert any significant effect on muscle blood flow questioned the role of reactive oxygen species in this process. On the other hand, some studies have favored the role of H2O2 in muscle blood flow during exercise. For instance, Copp and colleagues (2009) reported reduced blood flow in young rat spinotrapezius muscle both at rest (−61%) and during contractions (−40%) after acute antioxidant infusion with Tempol [412]. Similar findings were also reported by Hirai and colleagues [138], who reported increased mean spinotrapezius muscle blood flow at rest and during contractions after H2O2 treatment compared to placebo [138]. Thus, in young individuals, the role of oxygen species in muscle blood flow remains equivocal. Similar to vascular function, studies conducted under pro-oxidative conditions, such as in aged [139] and diseased populations [140] or after exposure to hyperoxia [141], described a beneficial effect of antioxidant treatments (either generic or mitochondria-targeted [142]; in skeletal muscle blood flow. This emphasizes the importance of the resting redox status when evaluating the likely “biphasic” regulatory potential of RONS on skeletal muscle microvascular tone and tissue blood flow [143].
Collectively, most of the available evidence supports a clear role of RONS in vascular function during exercise, while their contribution in skeletal muscle blood flow remains partially obscure due to complex interaction with other local vasoactive molecules (Fig. 3). In addition, it seems that the background redox status (i.e., physiological or pathological) largely affects the magnitude and the biological impact of the exercise-induced RONS production in blood flow. For an in-depth analysis of the topic, the reader is referred to reviews about the regulation of vascular tone and blood flow to skeletal muscle during exercise [114,119] and the role of free radicals in this process [111,144].
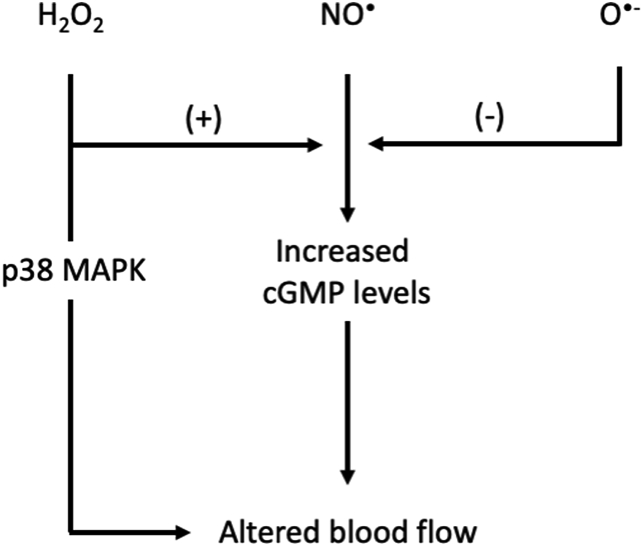
Fig. 3.
Proposed mechanisms on how RONS regulate blood flow. Based on [[117], [138], [412]] and [111]. cGMP, cyclic guanosine monophosphate; H2O2, hydrogen peroxide; MAPK, mitogen-activated protein kinase; NO•, nitric oxide; O•-, superoxide radical.
2.4. Cell bioenergetics
Redox-regulation of cell bioenergetics (also termed “redox bioenergetics”) represents an intriguing concept and a recently developed area of redox biology [145]. The interplay between cellular metabolic processes and certain delicate redox pathways is increasingly recognized as a fundamental feature of the cell, transducing signals that fine-tune a variety of responses, such as cell proliferation, differentiation and apoptotic procedures [146,147]. The fact that the mitochondrion is a key source, regulator and at the same time target of many RONS, which control both mitochondrial and distinct nucleic and cytosolic signaling pathways as second messengers (e.g., the “retrograde” signaling pathway [148], is a first clear indication regarding the involvement of redox processes in cell energetic metabolism [149]. A second indication is that diverse redox reactions modulate the function of many phosphatases, kinases and transcription factors strongly associated with exercise metabolism and energy production [e.g., glyceraldehyde 3-phosphate dehydrogenase (GAPDH), creatine kinase (CK), pyruvate dehydrogenase kinase-4, phosphatase and tensin homolog (PTEN) and nuclear factor erythroid 2-related factor 2 (Nrf2)] [66,[150], [151], [152], [153]]. Given that the studies in redox bioenergetics specifically focused on physical activity are scarce [154], selected mechanistic information is derived from other scientific fields.
It is nowadays well-established that mitochondria, along with their fundamental duty to generate ATP, are also critically involved in redox-sensitive cellular pathways either by directly producing RONS or by regulating NAD+/NADH and NADP+/NADPH homeostasis [155,156]. Although our knowledge about the mitochondrial topology and contribution of the various sites of superoxide and hydrogen peroxide production have rapidly expanded [157], other cellular RONS sources are also considered major contributors (i.e., NADPH oxidases, nitric oxide synthases, xanthine oxidases and cytochrome P450). Especially regarding exercise, a study by Sakellariou and colleagues [158], [196] examined the mitochondrial and cytosolic sites that contribute to superoxide production in mature single muscle fibers at rest and during contractile activity and reported that NADPH oxidase is the major contributor under both conditions [158]. In another elegant study, Frasier and colleagues [159] demonstrated that NADPH oxidase-originated RONS mediate the cardioprotective effects of exercise (i.e., isolated hearts from trained rats were exposed to an ischemia/reperfusion challenge). In fact, this beneficial effect was abolished in the presence of apocynin or Vas2870 (i.e., NADPH oxidase inhibitors), whereas mitoTEMPO or Bendavia (i.e., mitochondria-targeted antioxidants) did not exert any significant impact [159]. A potential mechanism that has been previously proposed about the cardioprotective effects of NADPH oxidase-originated RONS during exercise includes the S-glutathionylation of the ryanodine receptor-2 (RyR2), which leads to increased calcium release rates in isolated sarcoplasmic reticulum vesicles [160]. In addition, Pearson and colleagues [161] have shown that cytosolic superoxide levels increased in response to muscle contractions, but this was not accompanied by a similar change in mitochondrial superoxide availability and that inhibition of nitric oxide synthases influenced mitochondrial superoxide availability but not cytosolic [161].
Two more recent in vivo studies provided additional evidence to support this notion. In particular, one study demonstrated that apocynin injected intraperitoneally for 3 days in mice impaired early muscle gene expression (i.e., p38 MAP kinase, ERK1/2, and NFκB signaling pathways) induced by a single swimming exercise bout [162]. The second study reported that three weeks of MitoQ supplementation in humans (i.e., the antioxidant coenzyme Q10 is conjugated to the lipophilic cation triphenyl phosphonium, which accumulates selectively in mitochondria driven by the plasma membrane potential [163]; did not affect blood and skeletal muscle adaptations induced by endurance exercise training in healthy men and did not affect performance as assessed via a VO2max test [164]. Nevertheless, allopurinol administration (a xanthine oxidase inhibitor), akin to NAPDH oxidase inhibitors, adversely affected cell signaling pathways in gastrocnemius muscle from rats submitted to exhaustive exercise resulting in suppressed MnSOD mRNA increases [165]. The latter finding stresses the need to reveal the potential mechanisms underlying the crosstalk between subcellular compartments and organelles generating RONS [155].
The mitochondrial RONS production at rest and during exercise has been greatly revised throughout the years. In particular, it was for a long time reported that at rest 1–3% of electron flow during mitochondrial respiration produces reactive species. However, these values most probably overestimated the free radical leak in mitochondria, with studies that implemented more advanced techniques and physiologically relevant substrate concentrations reporting significantly lower values as much as 0.15% of the electron flow [166,167]. With respect to exercise, the increased mitochondrial respiration during physical activity was also incorrectly translated into increased electron leakage and reactive species production. In fact, Goncalves and colleagues [154] investigated the superoxide and hydrogen peroxide production by isolated muscle mitochondria ex vivo under conditions mimicking rest and exercise (i.e., incubated in media containing the cytoplasmic substrate and effector mix of skeletal muscle during rest and exercise) and demonstrated a steep decline in total mitochondrial superoxide/H2O2 production during “exercise” conditions [154]. Interestingly, the more increased the mitochondrial respiration rate between “rest”, “mild exercise” and “intense exercise” conditions the lower the superoxide/H2O2 production, which imitates the rate of state 3 respiration. This was further supported recently by an in vivo study that reported lower mitochondrial H2O2 production during exercise [82]. In another study by Quinlan and colleagues [168] it was demonstrated that the contribution of each mitochondrial site to the total RONS production in rat skeletal muscle is largely dependent on the available oxidizable substrates (i.e., succinate, glycerol 3-phosphate, palmitoyl-carnitine plus carnitine and glutamate plus malate) [168]. This is of utmost importance taking into account that the exercise- and redox-sensitive Nrf2 impacts cellular bioenergetics by controlling the substrate availability for mitochondrial respiration [151] and provides a redox perspective on the health-promoting effects of regular exercise and calorie restriction (nicely summarized by Ref. [169]. However, whether the improved cellular redox status after these situations is exclusively due to a decreased mitochondrial superoxide/H2O2 production or also mediated by the more reduced glutathione pool remains a matter of debate [170]. For instance, it was shown that both pharmacological (i.e., treatment of permeabilized myofibers with the glutathione-depleting agent 1-chloro-2,4-dinitrobenzene) and physiological oxidation of the cellular glutathione pool (i.e., high fat feeding in rodents) elevates pyruvate dehydrogenase-induced H2O2 emission [171].
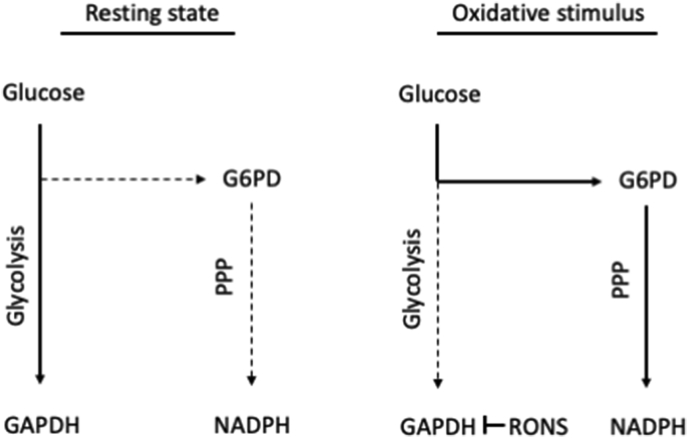
The NAD+/NADH and NADP+/NADPH couples orchestrate many catabolic and anabolic processes, respectively, to conserve cell survival at the interface between energy metabolism and redox signaling. Of note, these molecules synchronize complex cellular pathways, such as the energy-consuming redox circuit between the pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase, to defend homeostasis during periods of positive and negative energy balance [172]. From a redox standpoint, NAPDH serves as substrate for NADPH oxidases generating RONS, but at the same time it represents the most important reductive substrate for major antioxidant enzymes, such as peroxiredoxins and glutathione peroxidases [173,174]. The dominant role of NADPH in maintaining redox homeostasis is best exemplified by studies that reported a dynamic rerouting of the carbohydrate flux and acute activation (within seconds [175]) of the pentose phosphate pathway (PPP) as a first-line (i.e., transcription-independent) metabolic response to oxidative insults [[176], [177], [178], [179]]. Several mechanisms have been proposed regarding how this immediate PPP activation is achieved and include the highly conserved H2O2-induced reversible inactivation of GAPDH (via mechanisms distinct from those relating to the reaction between its active site cysteine and the glycolytic substrate [180]; and the enhancement of G6PDH activity [181,182]. Based on the above, exogenous supplementation with NADPH precursors, such as nicotinamide riboside which is currently regarded the most efficient molecule [183], has been utilized in conditions with increased underlying oxidative stress (e.g., disease and ageing) and have yielded promising results [[184], [185], [186]]. However, when similar treatments were used in young male humans or rats either neutral [185] or negative outcomes [187] were observed, respectively, in exercise performance.
Collectively, a strong interplay exists between cellular energetic processes and redox reactions, since energy metabolism is both a source and target of RONS (Fig. 4). In fact, reactions of catabolic pathways generate RONS, which subsequently regulate (structurally or functionally) selective target-molecules of metabolic cascades, while the antioxidant mechanisms coordinate this process. Considering that redox reactions have been linked, at least in part, to some central metabolic molecules strongly associated with exercise metabolism, such as AMPK [188], TCA enzymes [189], succinate dehydrogenase [190], ATP synthase [191], creatine kinase [192], cytochrome c oxidase [193], SIRT3 [194], G6PD [195] and GAPDH [180], it becomes evident that the field of redox bioenergetics is one of the most attractive, yet unexplored, areas of exercise physiology. For an in-depth analysis of the topic, the reader is referred to reviews about the spatiotemporal production of RONS at rest and in response to exercise [157,196], the role of RONS as cellular messengers regulating kinases and phosphatases [150] and the emerging field of redox bioenergetics [145].
Fig. 4.
A representative example of the interplay between cellular energy metabolism and redox homeostasis. At rest, glucose is directed mainly towards glycolysis, whereas under increased oxidative stress conditions, ROS inactivate GAPDH, as a first-line metabolic response, and carbohydrate flux is dynamically rerouted to the pentose phosphate pathway to produce NADPH (the major reductant of key antioxidant enzymes). Based on [[177], [178], [179]]. G6PD, glucose-6-phosphate dehydrogenase; GAPDH, reduced glyceraldehyde 3-phosphate dehydrogenase; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PPP, pentose phosphate pathway; RONS, reactive oxygen and nitrogen species.
3. Redox-regulated exercise adaptations
3.1. Mitochondrial biogenesis
If we accept muscle contractile function as the archetypal example of redox-regulated exercise responses, mitochondrial biogenesis represents the respective archetypal example in the category of redox-regulated exercise adaptations [197]. Endurance exercise training increases skeletal muscle mitochondrial number and volume density leading to improved oxidative capacity [198]. Since cells do not produce mitochondria de novo, mitochondrial content is determined by the dynamic balance between the synthesis and degradation (also known as mitophagy) of these organelles. In particular, already existing highly functional mitochondria become segregated from the defective ones, which are disposed, and are subsequently set for proliferation [199]. This highly complex process is accomplished by a coordinated bigenomic network. In fact, proteomic analyses revealed that almost 1500 proteins are present in mitochondria and the vast majority of them are encoded inside nucleus and not within the mitochondrion (i.e., only 13 polypeptides are encoded by mtDNA and synthesized there) [[200], [201], [202]].
The key factors that orchestrate the communication between the mitochondrial and the nuclear genome are the mitochondrial transcription factor A (mtTFA) and the nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2). However, almost all upstream signals that stimulate muscle contraction-induced mitochondrial biogenesis (e.g., AMPK, CaMK, mitogen-activated protein kinases p38 and p53) converge to the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which is therefore regarded the major governor of the specialized regulatory proteins involved in mitochondrial biogenesis [203,403]. Several studies in rodent (mainly) and human skeletal muscles have underlined the key role of PGC-1α in exercise-induced mitochondrial biogenesis. It has been demonstrated that an acute exercise bout can impact PGC-1α at both transcriptional and post-translational level regulating its expression and activity, respectively [[204], [205], [206], [207], [208], [209]]. In addition, overexpression of PGC-1α was found to significantly enhance the oxidative phenotype of muscle fibers, as indicated by increases in the activity and content of many enzymes involved in oxidative metabolism, such as cytochrome oxidases and citrate synthase [210]. Our knowledge about how redox processes are implicated in exercise-induced mitochondrial biogenesis comes from studies that utilized redox treatments along with an exercise protocol and measured markers of mitochondrial biogenesis (e.g., PGC-1α mRNA and COX4 content) as well as from mechanistic studies that investigated the role of RONS in the regulation of the upstream molecules, such as AMPK.
Gomez-Cabrera and colleagues [211] were the first to report that daily vitamin C administration in rats blunted the exercise-induced expression of key transcription factors involved in mitochondrial biogenesis (i.e., PGC-1α, mtTFA and NRF-1), while it also prevented the exercise-induced expression of cytochrome c [211]. One year later, another study reported that an antioxidant scheme consisting of vitamin C and E impaired mRNA responses in several markers of mitochondrial biogenesis, including PGC-1α, in healthy young men subjected to a training intervention [212]. The adverse effect of antioxidant supplementation on cellular exercise-induced adaptations was further supported by the findings of Strobel and colleagues [213] who reported that an antioxidant scheme consisting of vitamin E and α-lipoic acid negatively affected several markers of skeletal muscle mitochondrial biogenesis in rats after treadmill training (i.e., PGC-1α mRNA and protein concentration, COX4 protein content and citrate synthase activity) [213]. In the same line are generally the findings from 5 other studies performed in rats [214,215], mice [216] and humans [217,218], which used generic antioxidants in combination or separately, and reported blunted increases in the mRNA levels and protein content of some (but not all) factors related to mitochondrial biogenesis (i.e., COX4, PGC-1α, mtTFA, NRF-1 and NRF-2). Finally, two studies on the topic followed alternative approaches. The first was conducted by Strobel and colleagues (2014), who treated rats with diethyl maleate in order to deplete skeletal muscle glutathione, and showed that exercise induced greater oxidative stress and augmented muscle PGC-1α gene expression [219]. In the second study, the authors used allopurinol along with exercise to selectively inhibit xanthine oxidase and found lower PGC-1α, NRF-1, and mtTFA levels compared to the exercise only group [220].
Certainly, opposing findings also exist. Two studies that used antioxidant schemes consisting of vitamin C and E did not report adverse effects on mitochondrial protein content in rat triceps muscle [221] or on the activity of citrate synthase in human vastus lateralis [222]. Likewise, Petersen and colleagues [223] reported that N-acetylcysteine infusion in humans impaired many early adaptive responses to exercise, however, PGC-1α was not included in the hindered factors [223]. Interestingly, two other studies, which utilized generic [216] or targeted antioxidants [224], demonstrated that although some acute exercise-induced changes in mitochondrial markers were disturbed (e.g., mtTFA mRNA levels), training-induced adaptations were not affected in terms of mitochondrial biogenesis (e.g., normal mitochondrial mass). Hence, it should be cautioned that mitochondrional biogenesis signaling (i.e., gene expression following an acute about of exercise) is not always linked to mitochondrial biogenesis per se (i.e., changes in protein/enzyme content after exercise training). Even more important is the fact that, in some cases, the molecular and biochemical impairments observed after antioxidant supplementation did not translate into worse performance. For instance Ref. [211], reported impaired increases in endurance capacity in rats after the supplementation period, but this was not statistically verified in humans despite the large difference in VO2max improvement between groups after the training protocol (i.e., 22 vs. 10.8%) [211]. Similarly, the hampered cellular adaptations observed after vitamin C and E supplementation by Paulsen and colleagues [218] were not projected in VO2max and running performance. This issue emphasizes the need to combine molecular and biochemical data with physiological and/or performance outcomes in order to support the translational potential of cellular findings [10,225].
Regarding reactive nitrogen species, it is nowadays beyond doubt that nitric oxide promotes mitochondrial biogenesis at rest via canonical (i.e., cGMP) and non-canonical (i.e., S-glutathionylation) pathways [226,227]. However, the data about the role of nitric oxide in exercise-induced mitochondrial biogenesis are scarce and, thus, this scenario remains far less clear [228]. In fact, few studies that used either the nitric oxide synthase inhibitor l-NAME [229,230] or recruited mice deficient in endothelial or neuronal nitric oxide synthase (eNOS−/- and nNOS−/-) have yielded both positive and negative results in mitochondrial biogenesis markers after exercise, with some of them (i.e., mtTFA) being affected only in particular muscles. As a result, the contribution of nitric oxide in mitochondrial biogenesis in response to exercise remains, at least, equivocal. Another interesting case regarding mitochondrial biogenesis is polyphenols and especially resveratrol supplementation [231]. Many studies that used resveratrol along with exercise (nicely summarized in Ref. [58]) have yielded promising results; however, whether the effects are mediated by redox mechanisms cannot be safely argued due to the multiple and pleiotropic biological properties of resveratrol (e.g., AMPK activator and Sirt1 agonist) and its poor bioavailability and, consequently, low antioxidant potential of this polyphenol in humans [232,233].
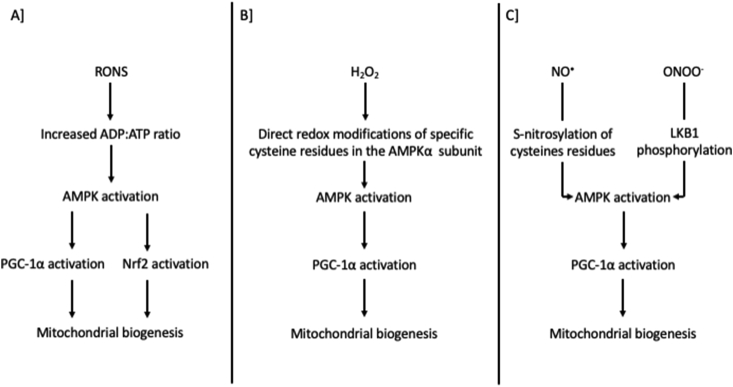
From a mechanistic point of view, PGC-1α regulation at rest and during exercise depends on several (most probably redundant) factors. There is nowadays considerable evidence that AMPK, Nrf2 and p38 serve as the principal intermediate molecules connecting RONS with PGC-1α activation and/or mitochondrial biogenesis markers [165,220,224,[234], [235], [236], [237]]. Regarding AMPK, Choi and colleagues [238] demonstrated that AMPK becomes activated under oxidative stress conditions induced by H2O2 and this is mediated by energy disturbance (AMP:ATP ratio) [238]. In the same vein, another study that used AMP-insensitive cell lines demonstrated that AMPK signaling was abolished under oxidative stress conditions, pointing towards an energy-mediated redox regulation of AMPK [239]. Likewise, a study that also used AMP-insensitive mutant cultured cells showed that changes in adenine nucleotides content, rather than a direct oxidative modification, is the major driver of AMPK activation during oxidative stress [240].
On the other hand, some studies have shown that reactive oxygen species and especially H2O2 may also promote AMPK activation via direct redox modifications (i.e., oxidation and S-glutathionylation) of specific cysteine residues (i.e., 299 and 304) independent of energy imbalances [[241], [242], [243]]. Interestingly, Shao and colleagues [244] have shown that oxidation of other cysteine residues (i.e., 130 and 174) in the AMPKα subunit leads to disulphide bonds formation and aggregation of AMPK [244]. Thus, oxygen species may both directly activate or deactivate AMPK by modifying redox-sensitive residues at the α subunit. Overall, the predominant idea suggests that energy imbalance derived from pro-oxidant conditions is the central means of redox-mediated AMPK activation compared to the possible direct oxidative modifications of target cysteine residues at AMPK subunits [70,245]. Reactive nitrogen species have been also implicated in the regulation of AMPK. Similar to oxygen species, the most common pathway that has been described is the modulation of the ATP production in mitochondria via inhibition of the cytochrome c oxidase [406]. In addition, nitric oxide is known to activate soluble guanylyl cyclase, which leads to intracellular Ca2+ release and CaMKKb-mediated activation of AMPK [246]. Direct regulatory nitrosative modifications of AMPK have been also described, such as the S-nitrosylation of target cysteines (i.e., 299 and 304) and nitration of tyrosine residues, as well as the peroxynitrite-mediated activation of AMPK via LKB1 phosphorylation [245,247].
p38 MAPK and particularly the p38γ isoform mediates PGC-1α phosphorylation and translocation to nucleus to trigger mitochondrial biogenesis [220,248,249]. It is well-established that diverse redox processes, especially in response to stress stimuli, activate p38 which subsequently facilitates cell signaling events [250,251]. Redox activation of p38 takes place indirectly through activation of upstream redox-sensitive kinases (e.g., ASK1 [252]); or inactivation of redox-sensitive inhibitory phosphatases (JNK-inactivating phosphatases; [253]). However, data from Galli and colleagues [254].provided supporting evidence for dose-dependent direct activation of p38 by the oxidation of the Cys162 within the docking domain of p38, affecting thereby its interaction with other upstream MAPKs [254]. Finally, regarding Nrf2 it has been suggested that both H2O2 and NO-induced increases in mitochondrial biogenesis markers are dependent on Nrf2 and NRF-1, yet only NO-mediated mitochondrial biogenesis gene expression requires PGC1α [236]. However, since most of the available mechanistic data presented above come from studies outside the exercise field, it is still an active debate whether these mechanisms also orchestrate exercise-induced AMPK-, Nrf2 and p38-mediated activation of PGC-1α and mitochondrial biogenesis.
Collectively, mounting evidence supports the key role of RONS in mitochondrial biogenesis at rest and in response to exercise (Fig. 5). More specifically, the majority of exercise studies that used antioxidants or RONS inhibitors substantiate the role of reactive species in mitochondrial biogenesis, however, the mechanistic basis (especially for reactive nitrogen species) warrants further investigation. For an in-depth analysis of the topic, the reader is referred to reviews about the redox-regulation of exercise-induced mitochondrial biogenesis [197] and the role of RONS in the activity of upstream molecules, such as PGC-1α, AMPK and Nrf2 [70,245,255].
Fig. 5.
Proposed mechanisms on how RONS regulate mitochondrial biogenesis. Panel A based on [188,[238], [239], [240]]; Panel B based on [[241], [242], [243]]; Panel C based on [245,247]. AMPK, 5′ AMP-activated protein kinase; cGMP, cyclic guanosine monophosphate; H2O2, hydrogen peroxide; LKB1, liver kinase B1; NO•, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; ONOO−, peroxynitrite; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; RONS, reactive oxygen and nitrogen species.
3.2. Skeletal muscle hypertrophy
Skeletal muscle hypertrophy, as the phenotypic result of net increase in protein synthesis relative to protein degradation, is the most common adaptation observed in response to resistance exercise training. Diverse mechanical (e.g., loading) and chemical (e.g., Ca2+ influx) processes have been identified as signals that trigger anabolic cellular transduction pathways leading to muscle hypertrophy, including the insulin/IGF-1-PI3K pathway, mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (p70S6k), eukaryotic translation initiation factor 4E (eIF4E) and ERK1/2 [256,257]. Although the vast majority of redox exercise studies have focused on endurance training adaptations (e.g., mitochondrial biogenesis; [197]), increasing evidence now suggests that redox processes may also regulate complex resistance training-mediated adaptations and we will present the existing literature regarding the role of reactive oxygen and nitrogen species in muscle hypertrophy.
The findings from 5 studies that used generic antioxidants (i.e., vitamin C only or in combination with vitamin E) along with a muscle hypertrophic stimulus yielded mixed results. In particular, Makanae and colleagues [258] demonstrated that the antioxidant treatment impaired overload-induced (i.e., gastrocnemius and soleus ablation) skeletal muscle hypertrophy in the plantaris muscle of rats [258]. This effect was partially supported by the data regarding the phosphorylated form of ERK1/2 and p70S6k as well as by the expression of atrogin-1. That is, although antioxidant group was not different compared to placebo, it was also not different compared to the sham group, indicating a tendency for blunted anabolic hypertrophy signaling and potent catabolic signaling. Another study showed that in healthy young men and women, subjected to a heavy-load resistance exercise protocol, antioxidant supplementation did not impair lean body mass gains (via DXA), cross-sectional areas of several muscle groups (via ultrasound imaging) or the acute changes in protein synthesis in the vastus lateralis muscle. However, the increased phosphorylation of ERK1/2 and p70S6k induced by training was blunted by the antioxidant scheme [259]. A recent human study by Dutra and colleagues [260] demonstrated that antioxidant supplementation did not affect quadriceps muscle thickness assessed via ultrasound after a strength training program. However, performance measurements (i.e., peak torque and total work assessed via isokinetic dynamometer) were adversely affected by supplementation [260]. The rest two studies were conducted in aged populations and reported contradictory findings as well. In particular, Bjørnsen and colleagues [261] reported greater total lean mass gains and rectus femoris thickness after a strength training program in the placebo group compared to the antioxidant group (although other muscles groups exhibited similar increases) [261]. On the other hand, Bobeuf and colleagues [262] reported no difference in fat free mass after a resistance training protocol between placebo and antioxidant groups of older individuals [262].
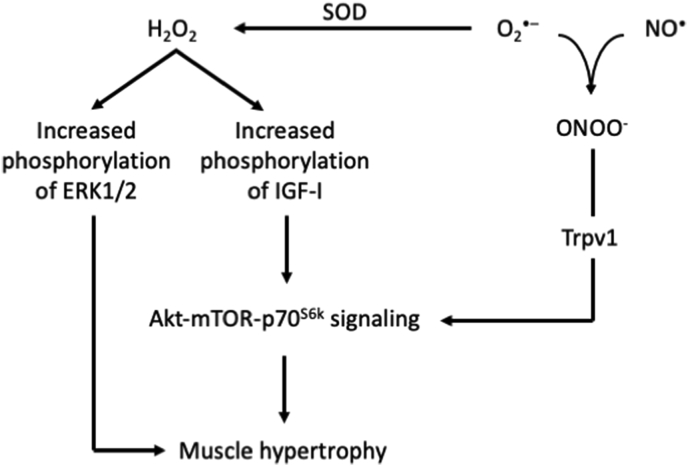
Handayaningsih and colleagues [263] investigated the role of RONS in IGF-1 signaling in C2C12 myocytes and used both generic (i.e., N-acetylcysteine) and targeted antioxidants (i.e., Tempol and Tiron as superoxide dismutase mimetics). The authors reported that both endogenous and exogenous H2O2 increased phosphorylation of the IGF-I receptor and subsequently up-regulated the downstream signaling pathway (i.e., Akt-mTOR-p70S6k). However, this effect was abolished when cells were treated with all antioxidants. These signaling warnings were further supported by the impaired increase in myotube diameter after N-acetylcysteine treatment [263]. Two rat studies aimed to investigate the role of nitric oxide in muscle hypertrophy and used targeted antioxidant treatments [i.e., l-NAME and 1-(2-trifluoromethyl-phenyl)-imidazole (TRIM)] followed by synergistic ablation (i.e., removal of gastrocnemius and soleus) to induce hypertrophy in the plantaris muscle unilaterally. The first study demonstrated that l-NAME treatment partially impaired the hypertrophic adaptation induced by synergistic ablation; however, the effect was present only in specific muscle fiber types (i.e., Type IIA) [264]. The second study reported up-regulation of skeletal α-actin and type I Myosin Heavy Chain mRNA after ablation; however, this effect was abolished after l-NAME and TRIM treatment. On the other, antioxidant treatments did not affect induction of the growth factors VEGF and IGF-1 as well as the expression of total p70S6k (instead the ratio of phosphorylated to total p70S6k was significantly higher in the antioxidant groups) [265].
Zhang and colleagues [266] recruited a skeletal muscle-specific Sod1-knockout mouse model to scrutinize the role of RONS in muscle mass and contractile force loss during aging. The knockout mice did not exhibit evidence of muscle fiber loss, instead they partially showed increased muscle mass compared to wild-type mice (however gastrocnemius muscle isometric force was compromised). In addition, Akt-mTOR signaling was not disturbed in knockout mice, which suggested enhanced regenerative pathways after CuZnSOD loss [266]. The most comprehensive original study about the role of reactive nitrogen species on muscle hypertrophy was performed by Ito and colleagues [267] who conducted a series of experiments including synergistic ablation in nNOS null (nNOS−/-) mice, nitric oxide synthase inhibitors and peroxynitrite selective scavengers. Ablation increased plantaris muscle weight, fiber size and total protein contents, whereas no such substantial increases were observed in the nNOS-null mice. Administration of l-NAME prevented the increase in muscle weight and fiber size, whereas its inactive enantiomer did not affect hypertrophy indices, indicating a role of nitric oxide in this process especially during the first day after ablation. Interestingly, the canonical nitric oxide-activated soluble guanylate cyclase and cyclic GMP pathway was found not to be involved in this process. Additional measurements using the specific peroxynitrite catalyst and scavenger FeTPPS, the nitric oxide and peroxynitrite donors molsidomine or SIN-1 and the NOX4-specific inhibitor GKT136901 were performed. Collectively, the authors suggested that peroxynitrite was the reactive nitrogen species that promoted normal hypertrophic responses in response to ablation in the nNOS null mice. These findings were also supported by signaling data; that is, activated mTOR signaling and increased phosphorylation of p70S6k were found after synergistic ablation, however this effect was absent in nNOS null mice and those treated with peroxynitrite scavengers. Finally, the authors proposed that peroxynitrite regulated muscle hypertrophy through the transient receptor potential cation channel V1-mediated mTOR activation and not through the insulin/IGF-1-P13K pathway [267].
Collectively, despite the fact that the available literature is limited and to some extend controversial [[258], [259], [260]], RONS are increasingly recognized as factors regulating muscle hypertrophic adaptation in response to resistance training (Fig. 6). Studies that implemented targeted redox interventions [265] or multifaceted methodological approaches [267] have shed light on the potential underlying redox mechanisms. Certainly, many aspects still remain unanswered. For instance, although it is clear that skeletal muscle regeneration is controlled by redox processes at several different steps of the process [268], two studies that evaluated overload-induced activation of satellite cells did not report differences between wild-type and nNOS-null mice [267] or after l-NAME treatment [265]. Thus, further work is needed for this type of exercise training and the corresponding adaptations from a redox perspective. For an in-depth analysis of the topic, the reader is referred to a very recent systematic review and meta-analysis about the effect of antioxidants on muscle hypertrophy after strength training [269].
Fig. 6.
Proposed mechanisms on how RONS regulate muscle hypertrophy. Based on [258,263,267] and [259]. Akt, protein kinase B; ERK, extracellular signal-regulated kinase; H2O2, hydrogen peroxide; IGF-I, insulin-like growth factor 1; mTOR, mammalian target of rapamycin; NO•, nitric oxide; O•-, superoxide radical; ONOO−, peroxynitrite; p70S6K, ribosomal protein S6 kinase beta-1; SOD, superoxide dismutase; Trpv1, transient receptor potential cation channel subfamily V member 1.
3.3. Angiogenesis
Angiogenesis is the physiological process of the formation of new blood vessels from the pre-existing vasculature. The two types of angiogenesis that have been identified during postnatal development are sprouting and intussusceptive (or splitting) angiogenesis. Under conditions of increased demands, such as during exercise, any blood, nutrient or metabolite demand-to-supply mismatch triggers a highly dynamic adaptive response that results in an extended vascular network in the tissue under stress (e.g., skeletal and cardiac muscle). Although several kinds of vascular growth or remodeling have been described, and the term ‘neovascularization’ is frequently used as a collective term, angiogenesis in the context of exercise is most commonly used to describe the formation of new capillaries [270]. The advantages stemming from the increased muscle capillarity are multiple and can be summarized as follows: i) the surface area for diffusion is augmented, ii) the average diffusion path length within the muscle shortens and iii) the length of time for diffusive exchange between blood and tissue increases [408].
Exercise as a potent physiological stimulus integrates a multiset of key angiogenic mechanisms that initiate the enrichment of working skeletal muscle capillarity, such as mechanical forces (e.g., vascular wall tension, shear stress and strain), tissue hypoxia and cycling hypoxia-reoxygenation phenomena as well as humoral biochemical factors and accumulation of metabolic by-products [[271], [272], [273]]. The central factor that largely orchestrates the process of angiogenesis is the vascular endothelial growth factor (VEGF), while a key role for angiopoietins and platelet-derived growth factor-B has been also reported [274]. Several human and animal studies have demonstrated that exercise elicits both acute (i.e., immediately after and up to several hours post-exercise) and chronic increases in the mRNA levels and activity of VEGF and its receptor tyrosine kinases (VEGFR1 and VEGFR2) [275,276], [277], [408]. The downstream molecules of VEGF/VEGFR pathway that control the process of angiogenesis include a wide variety of redox-sensitive kinases, phosphatases, cytokines and transcription factors, such as c-Src, ERK1/2, JNK, NFkB, PTP1B, PTEN, p38 MAP kinase, protein kinase C and PI3K-Akt [[278], [279], [280], [281]]. In fact, the direct (e.g., oxidative activation of transcription factors) or indirect (e.g., modulation of intermediary regulatory molecules) implication of redox processes in angiogenesis is delicately co-regulated by RONS production and scavenging enzymes or non-enzymatic antioxidants [[282], [283], [284]]. In the next paragraphs we will try to synopsize the evidence suggesting a role for redox molecules in the angiogenic process and to recontextualize current knowledge in an exercise setting, focusing on VEGF. Aberrant angiogenesis, namely excessive or insufficient, which is linked to many pathologies (e.g., cancer and hypertension), has been also associated with RONS production [285,286]. However, such conditions are typically characterized by chronic and uncontrollable over-production of RONS, denoting a different pattern compared to exercise which is of transient nature.
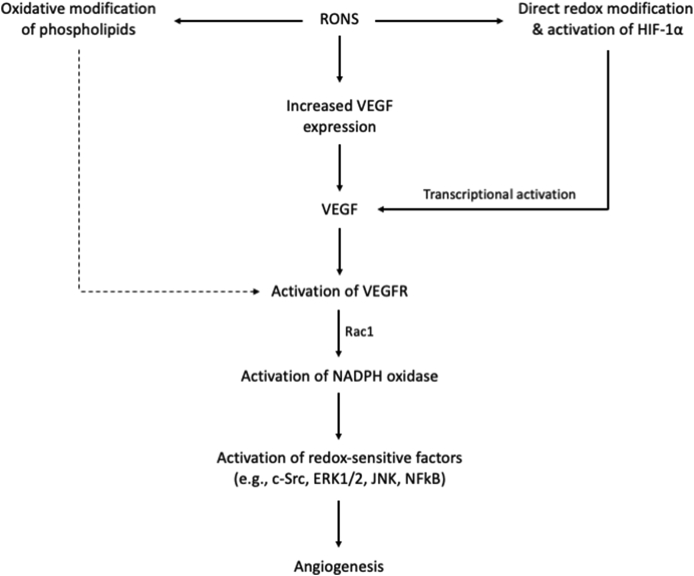
Although angiogenesis is a complex process necessitating the activity of multiple cell types, including endothelial, smooth muscle and blood cells, it predominantly depends on the recruitment, proliferation and differentiation of endothelial cells. The two principal endogenous RONS sources in endothelial cells are the mitochondrial electron transport chain and the NADPH oxidases, with some particular NOX isoforms (i.e., 1,2 and 4) being strongly associated with the angiogenic process [287]. Redox processes regulate VEGF fate and metabolism at both transcriptional and signaling level acting as second messengers. In particular, RONS and especially superoxide, H2O2 and nitric oxide produced endogenously or added exogenously in low amounts in cell cultures, have been found to directly up-regulate VEGF expression in diverse cell types [[288], [289], [290]], [[291], [292], [293]], [410, 411]. Thus, RONS with signaling properties and adequate diffusion distances, such as H2O2, produced during exercise even from extracellular sources (e.g., blood cells) could theoretically induce VEGF expression in endothelial cells and trigger angiogenesis [280]. Although RONS seem to serve mainly as upstream VEGF inductors, intracellular VEGF-related angiogenesis pathways also involve RONS production and redox signaling [[294], [295], [296]]. In particular, VEGF binding to VEGFR2 activates and translocates the small GTPase Rac1 to the plasma membrane, which stimulates RONS production by NADPH oxidase in endothelial cells. RONS subsequently oxidatively inactivate protein tyrosine phosphatases enhancing thereby VEGFR2 autophosphorylation and redox signaling linked to endothelial cell proliferation and migration [295]. Taking into account that exercise increases VEGF content, it seems reasonable to speculate that RONS originated from the VEGF-stimulated NADPH oxidases are involved in exercise-induced angiogenesis. In light of the above, RONS seem to act both as upstream and downstream mediators of VEGF/VEGFR2 signaling and angiogenesis.
Hypoxia is a key physiological characteristic that may also explain the contribution of RONS in exercise-induced angiogenesis. Low oxygen levels in working skeletal muscle is a common characteristic as a consequence of increased oxygen consumption and leads to activation of the hypoxia-sensitive transcription factor hypoxia-inducible factor-1 alpha (HIF-1α) [297]. In fact, a series of studies have shown that acute exercise increases HIF-1α mRNA expression, while chronic exercise moderates this increase as an adaptation [[298], [299], [300]]. Although the exact molecular mechanisms by which cells sense hypoxia and activate HIF-1α have not been fully unraveled, redox chemistry appears as a competent candidate [301]. In particular, HIF-1α activity can by upregulated through reactive oxygen and nitrogen species-induced redox modifications of specific cysteine residues [[302], [303], [304]]. Moreover, several genes have been identified which are transcriptionally activated by HIF-1α and encode proteins that increase oxygen delivery in hypoxic cells. These genes include, among others, VEGF and nitric oxide synthase [296,305]. The dependence of VEGF expression on HIF-1α was strongly supported by a study using HIF-1α−/- cells, which demonstrated that VEGF mRNA levels did not increase in response to hypoxia in these cells [306]. Based on the above, it could be hypothesized that RONS produced during exercise may also activate VEGF and promote angiogenesis in a HIF-1α-dependent manner.
Additional indirect evidence about the implication of RONS in VEGF metabolism and/or angiogenesis comes from studies that used experimental models that under- or over-expressed either RONS sources (e.g., NAPDH oxidases and nitric oxide synthases [[307], [308], [309]]; or antioxidant enzymes (e.g., mnSOD and catalase [310], [410, 411]), as well as from studies that utilized generic (e.g., vitamins C and E and N-acetylcysteine [311,312]); or targeted antioxidants (gp91ds-tat, l-NAME and Tempol [288], [407]) and nutritional compounds with purported antioxidant properties (e.g., resveratrol and catechins [313,314]). Most of these studies report increased VEGF expression and angiogenesis after RONS production and blunted responses after RONS source inhibition or antioxidant administration. Nevertheless, since the aforementioned studies are outside the exercise field, it is important to clarify that increased levels of angiogenesis is not always a beneficial effect of RONS (e.g., in tumor growth); thus, antioxidants act in essence as homeostatic regulators of the pro-angiogenic activity of RONS, guaranteeing optimal levels of angiogenesis.
Collectively, our knowledge on the role of RONS in exercise-induced angiogenesis is by some means fragmented. In fact, we now have clear evidence that RONS: i) regulate the expression of the central angiogen VEGF, ii) fine-tune the VEGF/VEGFR2 signaling cascade, iii) activate HIF-1α, which in turn participates in VEGF regulation and iv) cooperate with endogenous antioxidant enzymes to secure optimal levels of angiogenesis. Based on the above, and taking into account that exercise (especially in acute form) upregulates VEGF and HIF-1α mRNA expression, it seems realistic to argue that RONS produced during exercise partially contribute to the VEGF expression and angiogenesis observed after exercise training either directly or via HIF-1α (Fig. 7). Other redox mechanisms regarding angiogenesis have been also described, yet available evidence is much lesser especially in a physiological context. These mechanisms involve mainly oxidized phospholipids, which can stimulate angiogenesis via both VEGF/VEGFR2-dependent and independent mechanisms (i.e., via Src and Toll-like receptor activation, respectively) [315,316]. For an in-depth analysis of the topic, the reader is referred to reviews about the redox-regulation of angiogenesis in response to exercise training [280], the central role of endothelial NADPH oxidases [287,317] and the key action of antioxidant enzymes [282,283] in this process.
Fig. 7.
Proposed mechanisms on how RONS regulate angiogenesis. Based on [280,282,296,315,317] and [287]. c-Src, proto-oncogene tyrosine-protein kinase; ERK, extracellular signal-regulated kinase; HIF-1α, hypoxia-inducible factor 1-alpha; JNK, c-Jun N-terminal kinases; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; Rac1, Ras-related C3 botulinum toxin substrate 1; RONS, reactive oxygen and nitrogen species; VEGF, vascular endothelial growth factor.
3.4. Redox homeostasis
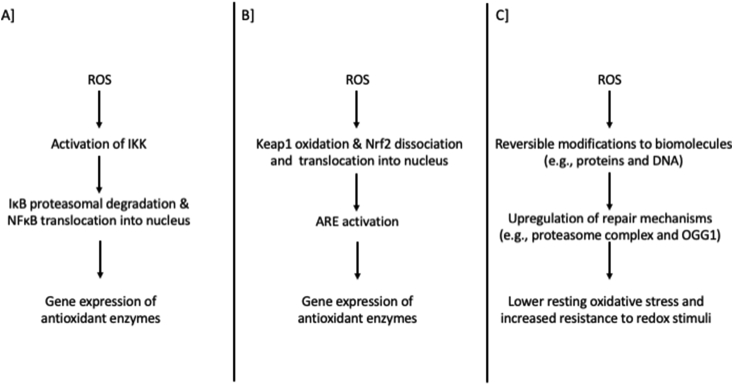
Probably the most idiosyncratic exercise adaptation mediated by RONS is the “upgraded” redox homeostasis. This is generally represented by the following indices: i) increased gene expression, protein content and activity of antioxidant enzymes, ii) higher resting levels of low molecular weight antioxidants, iii) lower resting levels of oxidative stress biomarkers, iv) increased resistance to oxidative insults and v) improved repair mechanisms for oxidatively modified proteins and DNA. Although for a long time this adaptation was regarded an unpolished stress-response paradigm to RONS production, it is nowadays clear that this revised redox profile is orchestrated by well-organized redox signaling processes [9,255,318]. Moreover, this adaptation is frequently referred to as “improved antioxidant defense”; however, taking into account the prevailing concept that antioxidants fine-tune redox signaling [319,320], we preferred the broader idea of “upgraded redox homeostasis”.
Quintanilha & Packer first reported that endurance training increases the levels of antioxidant enzymes in skeletal muscle of rats [321]. Afterwards, an abundant literature has verified that exercise training increases the activity of superoxide dismutase (especially the MnSOD isoform) [322],[323], glutathione peroxidase (GPx) [324,325] and catalase [322,326]. Along with activity, gene expression and protein content of these enzymes have been also shown to become upregulated with regular exercise [[327], [328], [329], [330]]. Of note, several early studies have demonstrated that these adaptations were fiber type specific [[331], [332], [333], [334]]. The first indication that these adaptive responses were triggered by the exercise-induced redox alterations derived from studies that used antioxidants, RONS source inhibitors or mouse models along with an exercise training protocol. In particular, most studies reported detrimental effects of supplementation in the acute antioxidant responses after an exercise bout (i.e., mRNA levels of enzymes), which subsequently led to blunted long-term improvements in enzyme activity and protein content [211,212,216,335,336]. In line with these findings, a recent study that used Ncf1-deficient mice (i.e., NOX2 deficiency) reported impaired catalase and SOD2 expression after high intensity interval exercise [337]. It should however be mentioned that other studies reported either no long-term effect of supplementation in antioxidant enzyme activity and protein levels (despite a blunted acute signal the hours after exercise) or even no impact in any parameter related to antioxidant enzymes [221,222,338]. In sum, antioxidant treatments seem to blunt the acute redox signals that initiate the antioxidant adaptations after exercise, however the long-term effects on activity and protein concentration are not fully supported.
The underlying mechanisms that largely account for the upgraded redox homeostasis after exercise training include several redox-sensitive factors, such NFκB and Nrf2 which will be briefly described next. NFκB belongs to the "rapid-acting" primary transcription factors and is normally sequestered in an inactive state in the cytoplasm by the inhibitory IkB proteins. RONS (e.g., H2O2), among other stimuli, activate IκB kinase (IKK) which drives IκB ubiquitination and its proteolytic degradation by the 26S proteasome. Subsequently, the P50/P65 subunits of NFκB translocate into the nucleus and bind the DNA sequence of target genes related to antioxidant enzymes (e.g., MnSOD and GPx), while the whole process is controlled by glutathione and thioredoxin [339,340]. Some studies reported increased NFκB binding after an acute exercise bout accompanied by increased IKK activity, IκBα phosphorylation followed by degradation and P50 accumulation in the nucleus [341,342]. The study by Gomez-Cabrera and colleagues [165] demonstrated that exercise increased reactive species production and NFkB binding in muscle nuclear extracts and this was accompanied by increased mRNA levels of MnSOD and iNOS. However, allopurinol administration blunted both NFkB binding and mRNA levels of antioxidants strongly supporting the idea that RONS production triggers the expression of antioxidant enzymes [165].
The transcription factor Nrf2 is a member of the basic leucine zipper family of transcription factors and is found inactive in the cytosol through binding to the homodimeric protein Kelch-like ECH-associated protein 1 (Keap1). In this condition, Keap1 facilitates the degradation of Nrf2 by promoting its ubiquitination and subsequent degradation by the 26S proteasome. RONS do oxidize specific cysteine residues on Keap1 promoting either the stabilization of the Keap1-Nrf2 complex (slowing thereby the degradation process at the proteasome) or the dissociation of Nrf2 from the complex. In the latter case, Nrf2 is translocated into the nucleus where it has the capacity to heterodimerize with small MAF proteins and activate the antioxidant response element (ARE) [343]. Done & Traustadóttir have comprehensively reviewed exercise studies that implemented either acute or chronic protocols and evaluated the effects on Nfr2 signaling and its downstream consequences (i.e., expression of SOD, GPx and catalase), as well as the impact of antioxidant supplementation on these procedures [344]. In brief, both acute and chronic exercise has been consistently shown to activate Nrf2 signaling across multiple tissues and species, whereas this effect was suppressed by generic antioxidants [344]. However, some phytonutrients, that are widely portrayed to feature antioxidant properties, serve in essence as Nrf2 and ARE activators providing synergistic effects with exercise [345].
The redox-mediated upregulation of antioxidant enzymes after exercise training is accompanied by a higher content of non-enzymatic antioxidants. The most representative example is the increased glutathione content after training [[346], [347], [348]]. This is particularly important since glutathione serves both as a direct nucleophile against electrophiles and as reducing agent for key antioxidant enzymes (i.e., glutathione peroxidases, glutaredoxins) and low molecular weight antioxidants (e.g., vitamin C) [349,350]. The improved antioxidant network is represented by the lower resting oxidative stress levels and the increased resistance against a subsequent oxidative stress-inducing stimulus. For instance, it has been recently demonstrated that human individuals who experience larger increases in RONS production after an acute exercise bout exhibit greater redox adaptations (i.e., in F2-isoprostanes and protein carbonyls) in the long-term compared to their counterparts who experienced low or minimal increases in acute RONS production [351]. In the same context, repeated bouts of aerobic exercise were found to lead to moderate free radical generation in skeletal muscle, while exercise has been reported to act as a redox preconditioning measure against subsequent hydrogen peroxide-induced oxidative damage in proteins of rat myocardium [352,353]. Two hypothetical yet realistic scenarios about this “paradoxical” redox effect of exercise have been recently described by Ref. [354,355]. The first scenario is called “adaptive homeostasis” and refers to the transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events (in our case exercise-induced RONS production) and the removal or cessation of them [354]. The second scenario refers to the ability of an organism to extend the peak and/or the optimal physiological zones after exercise training in order to better tolerate an upcoming “greater dose” of a redox stimulus [355].
Along with the increased resistance to subsequent oxidative stimuli, the repair mechanisms of redox modifications are also upregulated with training. This is of utmost importance, since oxidized macromolecules are not chemically and biologically inert [[356], [357], [358]]. These molecules rather modulate normal cell signaling either through direct reaction with other functional macromolecules (e.g., reactive lipid species) or by affecting intracellular RONS fate by feedback of their repair mechanisms (e.g., 8-oxoguanine-DNA glycosylase; OGG1). Exercise training has been repeatedly shown to improve these repair mechanisms, especially regarding protein and DNA oxidation adducts [255]. Regarding proteins, it has been shown that the activity of the proteasome complex is increased in response to aerobic training leading to higher protein turnover and lower levels of reactive carbonyl derivatives [359,360]. Of note, some studies have demonstrated that the activity of the proteasome complex and the degradation of oxidatively modified proteins can be induced by RONS, such as H2O2 [353,361], and this effect seems to be ubiquitination-independent [362,363]. Likewise, regular exercise also upregulates the expression of OGG1, reducing thereby oxidative damage to nuclear and mitochondrial DNA [364,365]. Acute RONS production has been reported to activate the DNA repair pathway of OGG1 in order to maintain genomic stability, highlighting the active crosstalk between redox processes and DNA damage in response to diverse cellular stress stimuli [[366], [367], [368], [369]]. In light of the above, the redox-mediated improvement in the repair mechanisms of oxidation products represents a major component of the improved redox metabolism after exercise training.
Collectively, it is clear that the upgraded redox homeostasis after exercise training is mediated by delicate redox processes (Fig. 8). In particular, RONS activate a series of transcription factors that rapidly turn on gene coding for the expression of antioxidant enzymes. The improved repair mechanisms that facilitate more rapid and efficient turnover of oxidatively modified proteins and DNA, as well as the increased resistance to redox modifications in response to a following stimulus, corroborate also towards a more solid redox homeostasis. For an in-depth analysis of the topic, the reader is referred to reviews about the upregulation of antioxidant genes by exercise training [9,318], the oxidative stress biomarkers and the oxidation repair mechanisms [255,370,371] and the regulation of the two central redox-sensitive transcription factors, NFkB and Nrf2 [340,344,372].
Fig. 8.
Proposed mechanisms on how RONS regulate redox homeostasis. Panel A based on [9,318]; Panel B based on [344,372]; Panel C based on [255,370]. ARE, antioxidant response element; DNA, deoxyribonucleic acid; IKK, IκB kinase; Keap1, Kelch-like ECH-associated protein 1; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor erythroid 2-related factor 2; OGG1, 8-oxoguanine glycosylase; ROS, reactive oxygen species.
4. Some underappreciated issues in (exercise) redox biology research
In this section we will try to highlight some frequently neglected issues in the field of exercise redox biology that relate predominantly with the interpretational and translational potential of the current literature, and not with analytical issues which have been covered comprehensively elsewhere [[373], [374], [375], [376], [377], [378]]. Our intention is by no means to set certain “methodological-guidelines-for-redox-research”, but rather to present some features that may have led to contradictions (as is the case frequently in redox biology), especially when there is inevitable absence of clear evidence to assess the truth about the role of RONS in a particular phenotype. Taking into account the extreme nature of the reactive species and the immature state of the exercise redox biology field (e.g., many fundamental questions remain still unanswered), we argue that researchers should realize that the redox stories of the literature may have most probably loose ends [379].
4.1. Exercise-induced redox metabolism: more than a stress-response
In the conclusion of their pioneering study, Davies and colleagues [18] proposed that “exercise-induced free radicals may cause limited damage to mitochondrial membranes which, in a chronic training situation, may be the initiating stimulus to mitochondrial biogenesis” [18]. This inspirational statement was actually one of the first tentative efforts to negate the very bad reputation of free radicals those days in both academic and mass culture settings. Despite the fact that important advances in our understanding about the biology of RONS have been achieved since then, exercise-induced RONS production is still widely regarded, especially by exercise physiologists, merely as a stress- or damage-response signal, analogous to the idea of mithridatism. However, this is not the entire truth, or may even describe only a small part of the adaptive response (e.g., via the repair mechanisms or oxidation products themselves [370]. In fact, we now know that RONS directly (and not necessarily as a response) regulate molecular and biochemical transduction pathways (e.g., via oxidation, S-nitrosylation or S-glutathionylation) both at rest and in response to several stimuli including exercise. This is accomplished through firmly controlled redox signaling cascades coordinated by RONS and antioxidants. Thus, the “damaging” part of the process may not be an essential component or, in many cases, could well refer to transient and reversible redox modifications. For instance, H2O2 produced during exercise by NADPH oxidases can directly activate AMPK via selective oxidation of cysteine residues in the α subunit independent of energy imbalance and this may lead to PGC-1α activation and mitochondrial biogenesis [70].
4.2. Studies with generic antioxidants: interpretational difficulties
A large part of the literature that aimed to investigate the role of RONS in a particular exercise response or adaptation is based on studies with generic antioxidants. Irrespective of the outcomes, the interpretation of such studies needs caution. If antioxidants indeed affect the response/adaptation of interest (either negatively or positively), we cannot be entirely sure that this effect is mediated by redox processes. This is because most (if not all) generic antioxidants are pleiotropic molecules exerting also multiple redox-independent biological effects (e.g., vitamin E as signaling molecule [380]. In addition, these antioxidants are non-selective against a certain reactive species and, at the same time, are unevenly distributed in various cells, tissues and organelles of the human body. Thus, the final read-outs of these studies lack redox mechanistic evidence and additional insights should be gained from in-vitro, in-situ or ex-vivo experiments. On the other, the failure of generic antioxidants as modulators of a specific exercise response/adaptation (e.g., to reduce muscle fatigue) does not necessarily imply that RONS are not implicated in this process. In fact, even the most well-known dietary antioxidants (vitamin C and E) cannot, based on available kinetic data, outcompete antioxidant enzymes in order to appreciably react with redox signaling molecules [381]. Thus, it is probably our inability to exogenously regulate the fate of key RONS in this process and not the real absence of a redox implication. Obviously, we do not disregard this kind of studies, which are the most practical and realistic approach in an in-vivo setting and actually represent the largest part of the present review, especially after taking into account that even the selective and targeted antioxidants may have non-selective or off-target effects [382].
4.3. Antioxidant supplementation: should we identify and target particular deficiencies?
There is an active and polarized debate in the literature about the effectiveness of antioxidants to enhance performance and improve health. Of course, several factors related to the methodological choices of each study can partially explain some discrepancies in the literature (e.g., type, dose and duration of antioxidant supplementation, type of exercise etc.). Halliwell in his viewpoint in Lancet stressed a different concern in antioxidant research by arguing that we should test the effects of antioxidants on the most “rancid” people, who may be those at greatest risk [383]. Put differently, akin to the case of drugs that are administered only in patients with specific needs, antioxidants should be administered only in individuals with specific redox needs. By exploiting this idea, we followed a “targeted” supplementation strategy (instead of administering antioxidants indiscriminately) and found that vitamin C and N-acetylcysteine exert the ergogenic potential only in individuals with vitamin C and glutathione insufficiency, respectively [384,385]. As an additional verification of our concept, we recently examined the effect of both targeted and non-targeted supplementation in deficient individuals and found that only the antioxidant in deficiency yielded ergogenic effects after supplementation (unpublished data). In light of the above, we recently proposed the idea of personalized treatments according to the antioxidant profile of each individual, a concept we called “redox phenotyping” [55]. The redox background could also rationally explain the differential effect of antioxidants between young and old or diseased populations. For instance, we and others have shown that a detrimental impact of a chronic antioxidant protocol in young individuals (e.g., nicotinamide riboside, vitamin C and E) can be of great importance for older individuals or under a disease state (e.g., chronic obstructive pulmonary disease, diabetes mellitus) [85,185,187,[386], [387], [388]]. Thus, it seems that the basic redox background largely affects the impact of both exercise training and antioxidant supplementation [389], and based on this notion, we tried to refine the original idea of [383] by stating that it is more than necessary to identify the precise antioxidant deficiency that contributed to the increased levels of oxidative stress [55].
4.4. Linking molecular and biochemical data with physiological read-outs
When a specific molecular or biochemical pathway has been found to regulate an exercise response/adaptation, it is very common to examine the role of RONS on molecules involved in this pathway in order to extend the conclusions also in the physiological phenotype of interest. However, if the molecular and biochemical data are not linked with measurements of the investigated physiological phenotype, no safe conclusions can be drawn. For instance, two studies by Paulsen and colleagues reported blunted cellular adaptations (i.e., COX4 and PGC-1α) and protein signaling (i.e., ERK1/2 and p70S6k) after antioxidant supplementation with vitamin C and E, however the improvements in VO2max and running performance and in muscle mass, respectively, were not different between groups [218,259]. If the performance tests were not performed, a completely different outcome would have been drawn in both studies. The indefinite communication between the distinct layers of biological organization (from molecular and cellular to organism and whole-body level) remains still a subject of intense debate in redox biology [10,390], exercise physiology [225,391] and biology in general [392,393].
5. Conclusion
Since the first studies more than 35 years ago that reported increased free radical production during exercise, our understanding on the biology of RONS has greatly advanced in terms of identification (e.g., what kind of RONS are produced during exercise and from which source), quantification (e.g., orders of magnitude change in RONS production after a redox treatment) and mechanistic impact (e.g., structural and functional changes of redox-sensitive transcription factors) [10]. Nowadays, redox reactions are increasingly recognized as a fundamental element of the cell signaling machinery along with other well-established types of biochemical reactions which fine-tune human metabolism (e.g., phosphorylation and ubiquitination). This is best exemplified by the strong interaction between RONS and classic metabolic molecules, such as protein kinases and phosphatases [66,394]. In the present review we provided an overview of how RONS and antioxidants are integrated into molecular and biochemical pathways that regulate 4 exercise responses and 4 exercise adaptations (summarized in Fig. 9). Apparently, there are many other examples of responses and adaptations linked to exercise metabolism that are controlled, at least in part, by redox reactions. Such examples are neuroprotection and cognitive function [395], mechanotransduction [396], muscle regeneration [268], autophagy [397], insulin sensitivity and glycemic control [398,399], heat shock proteins metabolism [400] and nerve-muscle interactions [401]. Taking into account that redox biology research is currently in a key turning point, characterized by more targeted methodological approaches and sophisticated analytical tools, we believe that the “unconventional” relationship between exercise and redox homeostasis (i.e., redox cascades triggered by exercise result in oxidative eustress and well-being [402]) represents a fascinating translational research area, with many challenging questions still unanswered.
Fig. 9.
A summary figure illustrating the central RONS, types of redox modifications and molecules involved in the 4 acute exercise-induced responses (muscle contractile function, glucose uptake, blood flow and cell bioenergetics) and the 4 training-induced adaptations described in the present review. AMPK, 5′ AMP-activated protein kinase; c-Src, proto-oncogene tyrosine-protein kinase; cGMP, cyclic guanosine monophosphate; G6PD, glucose-6-phosphate dehydrogenase; GAPDH, reduced glyceraldehyde 3-phosphate dehydrogenase; H2O2, hydrogen peroxide; HIF-1α, hypoxia-inducible factor 1-alpha; IGF-I, insulin-like growth factor 1; IKK, IκB kinase; Keap1, Kelch-like ECH-associated protein 1; LKB1, liver kinase B1; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO•, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; O•-, superoxide radical; OGG1, 8-oxoguanine glycosylase; ONOO−, peroxynitrite; p70S6K, ribosomal protein S6 kinase beta-1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PTM, post-translational modifications; RYR, ryanodine receptors; Trpv1, transient receptor potential cation channel subfamily V member 1; VEGF, vascular endothelial growth factor.
Funding
None.
Declaration of competing interest
None to declare.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101499.
Contributor Information
N.V. Margaritelis, Email: nvmargar@auth.gr.
M.G. Nikolaidis, Email: nikolaidis@auth.gr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Penedo F.J., Dahn J.R. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatr. 2005 Mar;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: the evidence. CMAJ (Can. Med. Assoc. J.) 2006 Mar 14;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gollnick P.D., Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin. Physiol. 1982 Feb;2(1):1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Holloszy J.O. Adaptation of skeletal muscle to endurance exercise. Med. Sci. Sports. 1975;7(3):155–164. [PubMed] [Google Scholar]
- 5.Holloszy J.O., Booth F.W. Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 6.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014 Nov 6;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Neufer P.D., Bamman M.M., Muoio D.M., Bouchard C., Cooper D.M., Goodpaster B.H., Booth F.W., Kohrt W.M., Gerszten R.E., Mattson M.P., Hepple R.T., Kraus W.E., Reid M.B., Bodine S.C., Jakicic J.M., Fleg J.L., Williams J.P., Joseph L., Evans M., Maruvada P., Rodgers M., Roary M., Boyce A.T., Drugan J.K., Koenig J.I., Ingraham R.H., Krotoski D., Garcia-Cazarin M., McGowan J.A., Laughlin M.R. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabol. 2015 Jul 7;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Ruegsegger G.N., Booth F.W. Health benefits of exercise. Cold Spring Harb. Perspect. Med. 2018 Jul 2;8(7):a029694. doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Cabrera M.C., Domenech E., Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008 Jan 15;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Principles for integrating reactive species into in vivo biological processes: examples from exercise physiology. Cell. Signal. 2016 Apr;28(4):256–271. doi: 10.1016/j.cellsig.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013 Feb 5;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Hoppeler H., Baum O., Lurman G., Mueller M. Molecular mechanisms of muscle plasticity with exercise. Comp. Physiol. 2011 Jul;1(3):1383–1412. doi: 10.1002/cphy.c100042. [DOI] [PubMed] [Google Scholar]
- 13.Sarzynski M.A., Ghosh S., Bouchard C. Genomic and transcriptomic predictors of response levels to endurance exercise training. J. Physiol. 2017 May 1;595(9):2931–2939. doi: 10.1113/JP272559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brigelius-Flohé R., Flohé L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxidants Redox Signal. 2019 Dec 10 doi: 10.1089/ars.2019.7905. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 15.Dennis K.K., Go Y.M., Jones D.P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 2019 Apr 1;149(4):553–565. doi: 10.1093/jn/nxy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheehan D., McDonagh B. The clinical potential of thiol redox proteomics. Expert Rev. Proteomics. 2019 Dec 25:1–8. doi: 10.1080/14789450.2020.1704260. [DOI] [PubMed] [Google Scholar]
- 17.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015 Jan;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 18.Davies K.J., Quintanilha A.T., Brooks G.A., Packer L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982 Aug 31;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 19.Jackson M.J., Edwards R.H., Symons M.C. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim. Biophys. Acta. 1985 Nov 20;847(2):185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 20.Bailey D.M., Young I.S., McEneny J., Lawrenson L., Kim J., Barden J., Richardson R.S. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am. J. Physiol. Heart Circ. Physiol. 2004 Oct;287(4):H1689–H1699. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 21.Davison G.W., Ashton T., George L., Young I.S., McEneny J., Davies B., Jackson S.K., Peters J.R., Bailey D.M. Molecular detection of exercise-induced free radicals following ascorbate prophylaxis in type 1 diabetes mellitus: a randomised controlled trial. Diabetologia. 2008 Nov;51(11):2049–2059. doi: 10.1007/s00125-008-1101-1. [DOI] [PubMed] [Google Scholar]
- 22.Powers S.K., Smuder A.J., Criswell D.S. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxidants Redox Signal. 2011 Nov 1;15(9):2519–2528. doi: 10.1089/ars.2011.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998 Jun 1;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade F.H., Reid M.B., Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. Faseb. J. 2001 Feb;15(2):309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A.J., Bruton J.D., Lanner J.T., Westerblad H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015 Jan 15;593(2):457–472. doi: 10.1113/jphysiol.2014.279398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid M.B. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J. Appl. Physiol. 2001 Feb;90(2):724–731. doi: 10.1152/jappl.2001.90.2.724. 1985. [DOI] [PubMed] [Google Scholar]
- 27.Lamb G.D., Posterino G.S. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J. Physiol. 2003 Jan 1;546(Pt 1):149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein J.C., Moen R.J., Smith E.A., Titus M.A., Thomas D.D. Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry. 2011 Nov 29;50(47):10318–10327. doi: 10.1021/bi201279u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moen R.J., Klein J.C., Thomas D.D. Electron paramagnetic resonance resolves effects of oxidative stress on muscle proteins. Exerc. Sport Sci. Rev. 2014 Jan;42(1):30–36. doi: 10.1249/JES.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prochniewicz E., Spakowicz D., Thomas D.D. Changes in actin structural transitions associated with oxidative inhibition of muscle contraction. Biochemistry. 2008 Nov 11;47(45):11811–11817. doi: 10.1021/bi801080x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy R.M., Dutka T.L., Lamb G.D. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J. Physiol. 2008 Apr 15;586(8):2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollica J.P., Dutka T.L., Merry T.L., Lamboley C.R., McConell G.K., McKenna M.J., Murphy R.M., Lamb G.D. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012 Mar 15;590(6):1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb G.D., Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011 May 1;589(Pt 9):2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruton J.D., Place N., Yamada T., Silva J.P., Andrade F.H., Dahlstedt A.J., Zhang S.J., Katz A., Larsson N.G., Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J. Physiol. 2008 Jan 1;586(1):175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami M., Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol. Pharmacol. 1998 Mar;53(3):497–503. doi: 10.1124/mol.53.3.497. [DOI] [PubMed] [Google Scholar]
- 36.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J. Physiol. 1998 Jun 1;509(Pt 2):577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer T., Posterino G.S. Sequential effects of GSNO and H2O2 on the Ca2+ sensitivity of the contractile apparatus of fast- and slow-twitch skeletal muscle fibers from the rat. Am. J. Physiol. Cell Physiol. 2009 May;296(5):C1015–C1023. doi: 10.1152/ajpcell.00251.2008. [DOI] [PubMed] [Google Scholar]
- 38.Perkins W.J., Han Y.S., Sieck G.C. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J. Appl. Physiol. 1997 Oct;83(4):1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- 39.Dutka T.L., Mollica J.P., Lamb G.D. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J. Appl. Physiol. 2011 Mar;110(3):705–716. doi: 10.1152/japplphysiol.00739.2010. 1985. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira L., Figueiredo-Freitas C., Casimiro-Lopes G., Magdesian M.H., Assreuy J., Sorenson M.M. Myosin is reversibly inhibited by S-nitrosylation. Biochem. J. 2009 Nov 11;424(2):221–231. doi: 10.1042/BJ20091144. [DOI] [PubMed] [Google Scholar]
- 41.Tiago T., Simão S., Aureliano M., Martín-Romero F.J., Gutiérrez-Merino C. Inhibition of skeletal muscle S1-myosin ATPase by peroxynitrite. Biochemistry. 2006 Mar 21;45(11):3794–3804. doi: 10.1021/bi0518500. [DOI] [PubMed] [Google Scholar]
- 42.Place N., Ivarsson N., Venckunas T., Neyroud D., Brazaitis M., Cheng A.J., Ochala J., Kamandulis S., Girard S., Volungevičius G., Paužas H., Mekideche A., Kayser B., Martinez-Redondo V., Ruas J.L., Bruton J., Truffert A., Lanner J.T., Skurvydas A., Westerblad H. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. U. S. A. 2015 Dec 15;112(50):15492–15497. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salanova M., Schiffl G., Gutsmann M., Felsenberg D., Furlan S., Volpe P., Clarke A., Blottner D. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol. 2013 Oct 28;1:514–526. doi: 10.1016/j.redox.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Q.A., Wang B., Miyagi M., Hess D.T., Stamler J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor/Ca2+ release channel (RyR1): sites and nature of oxidative modification. J. Biol. Chem. 2013 Aug 9;288(32):22961–22971. doi: 10.1074/jbc.M113.480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braakhuis A.J., Hopkins W.G. Impact of dietary antioxidants on sport performance: a review. Sports Med. 2015 Jul;45(7):939–955. doi: 10.1007/s40279-015-0323-x. [DOI] [PubMed] [Google Scholar]
- 46.Reid M.B. Redox interventions to increase exercise performance. J. Physiol. 2016 Sep 15;594(18):5125–5133. doi: 10.1113/JP270653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 2018 Jul;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 48.Ezeriņa D., Takano Y., Hanaoka K., Urano Y., Dick T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol. 2018 Apr 19;25(4):447–459.e4. doi: 10.1016/j.chembiol.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobley J.N., McGlory C., Morton J.P., Close G.L. N-Acetylcysteine's attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int. J. Sport Nutr. Exerc. Metabol. 2011 Dec;21(6):451–461. doi: 10.1123/ijsnem.21.6.451. [DOI] [PubMed] [Google Scholar]
- 50.Matuszczak Y., Farid M., Jones J., Lansdowne S., Smith M.A., Taylor A.A., Reid M.B. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005 Nov;32(5):633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- 51.Clifford T., Jeffries O., Stevenson E.J., Davies K.A.B. The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019 Dec 18:1–11. doi: 10.1080/10408398.2019.1703642. [DOI] [PubMed] [Google Scholar]
- 52.McKenna M.J., Medved I., Goodman C.A., Brown M.J., Bjorksten A.R., Murphy K.T., Petersen A.C., Sostaric S., Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J. Physiol. 2006 Oct 1;576(Pt 1):279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medved I., Brown M.J., Bjorksten A.R., Murphy K.T., Petersen A.C., Sostaric S., Gong X., McKenna M.J. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 2004 Oct;97(4):1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 54.Reid M.B., Stokić D.S., Koch S.M., Khawli F.A., Leis A.A. N-acetylcysteine inhibits muscle fatigue in humans. J. Clin. Invest. 1994 Dec;94(6):2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Margaritelis N.V., Paschalis V., Theodorou A.A., Kyparos A., Nikolaidis M.G. Antioxidants in personalized nutrition and exercise. Adv. Nutr. 2018 Nov 1;9(6):813–823. doi: 10.1093/advances/nmy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maughan R.J., Burke L.M., Dvorak J., Larson-Meyer D.E., Peeling P., Phillips S.M., Rawson E.S., Walsh N.P., Garthe I., Geyer H., Meeusen R., van Loon L.J.C., Shirreffs S.M., Spriet L.L., Stuart M., Vernec A., Currell K., Ali V.M., Budgett R.G., Ljungqvist A., Mountjoy M., Pitsiladis Y.P., Soligard T., Erdener U., Engebretsen L. IOC consensus statement: dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018 Apr;52(7):439–455. doi: 10.1136/bjsports-2018-099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Affourtit C., Bailey S.J., Jones A.M., Smallwood M.J., Winyard P.G. On the mechanism by which dietary nitrate improves human skeletal muscle function. Front. Physiol. 2015 Jul 29;6:211. doi: 10.3389/fphys.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason S.A., Morrison D., McConell G.K., Wadley G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016 Sep;98:29–45. doi: 10.1016/j.freeradbiomed.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 59.Cheng A.J., Yamada T., Rassier D.E., Andersson D.C., Westerblad H., Lanner J.T. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016 Sep 15;594(18):5149–5160. doi: 10.1113/JP270650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Debold E.P. Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species. Front. Physiol. 2015 Sep 1;6:239. doi: 10.3389/fphys.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westerblad H., Allen D.G. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxidants Redox Signal. 2011 Nov 1;15(9):2487–2499. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 62.Clifford T., Jeffries O., Stevenson E.J., Davies K.A.B. The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019 Dec 18:1–11. doi: 10.1080/10408398.2019.1703642. [DOI] [PubMed] [Google Scholar]
- 63.Peternelj T.T., Coombes J.S. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011 Dec 1;41(12):1043–1069. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013 Jul;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 65.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017 Mar;13(3):133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 66.Fisher-Wellman K.H., Neufer P.D. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metabol. 2012 Mar;23(3):142–153. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noguchi N. Ebselen, a useful tool for understanding cellular redox biology and a promising drug candidate for use in human diseases. Arch. Biochem. Biophys. 2016 Apr 1;595:109–112. doi: 10.1016/j.abb.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 68.Sandström M.E., Zhang S.J., Bruton J., Silva J.P., Reid M.B., Westerblad H., Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2006 Aug 15;575(Pt 1):251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higaki Y., Mikami T., Fujii N., Hirshman M.F., Koyama K., Seino T., Tanaka K., Goodyear L.J. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2008 May;294(5):E889–E897. doi: 10.1152/ajpendo.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morales-Alamo D., Calbet J.A.L. AMPK signaling in skeletal muscle during exercise: role of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2016 Sep;98:68–77. doi: 10.1016/j.freeradbiomed.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Toyoda T., Hayashi T., Miyamoto L., Yonemitsu S., Nakano M., Tanaka S., Ebihara K., Masuzaki H., Hosoda K., Inoue G., Otaka A., Sato K., Fushiki T., Nakao K. Possible involvement of the alpha1 isoform of 5'AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287(1):E166–E173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- 72.Merry T.L., Steinberg G.R., Lynch G.S., McConell G.K. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 2010 Mar;298(3):E577–E585. doi: 10.1152/ajpendo.00239.2009. [DOI] [PubMed] [Google Scholar]
- 73.Jensen T.E., Schjerling P., Viollet B., Wojtaszewski J.F., Richter E.A. AMPK alpha1 activation is required for stimulation of glucose uptake by twitch contraction, but not by H2O2, in mouse skeletal muscle. PloS One. 2008 May 7;3(5):e2102. doi: 10.1371/journal.pone.0002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kjøbsted R., Roll J.L.W., Jørgensen N.O., Birk J.B., Foretz M., Viollet B., Chadt A., Al-Hasani H., Wojtaszewski J.F.P. AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes. 2019 Jul;68(7):1427–1440. doi: 10.2337/db19-0050. [DOI] [PubMed] [Google Scholar]
- 75.McConell G.K. It's well and truly time to stop stating that AMPK regulates glucose uptake and fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2020 Feb 4 doi: 10.1152/ajpendo.00511.2019. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 76.Sylow L., Møller L.L.V., Kleinert M., D'Hulst G., De Groote E., Schjerling P., Steinberg G.R., Jensen T.E., Richter E.A. Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes. 2017 Jun;66(6):1548–1559. doi: 10.2337/db16-1138. [DOI] [PubMed] [Google Scholar]
- 77.Chambers M.A., Moylan J.S., Smith J.D., Goodyear L.J., Reid M.B. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J. Physiol. 2009 Jul 1;587(Pt 13):3363–3373. doi: 10.1113/jphysiol.2008.165639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merry T.L., Lynch G.S., McConell G.K. Downstream mechanisms of nitric oxide-mediated skeletal muscle glucose uptake during contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010 Dec;299(6):R1656–R1665. doi: 10.1152/ajpregu.00433.2010. [DOI] [PubMed] [Google Scholar]
- 79.Merry T.L., Dywer R.M., Bradley E.A., Rattigan S., McConell G.K. Local hindlimb antioxidant infusion does not affect muscle glucose uptake during in situ contractions in rat. J. Appl. Physiol. 2010 May;108(5):1275–1283. doi: 10.1152/japplphysiol.01335.2009. [DOI] [PubMed] [Google Scholar]
- 80.Merry T.L., Wadley G.D., Stathis C.G., Garnham A.P., Rattigan S., Hargreaves M., McConell G.K. N-Acetylcysteine infusion does not affect glucose disposal during prolonged moderate-intensity exercise in humans. J. Physiol. 2010 May 1;588(Pt 9):1623–1634. doi: 10.1113/jphysiol.2009.184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merry T.L., McConell G.K. Do reactive oxygen species regulate skeletal muscle glucose uptake during contraction? Exerc. Sport Sci. Rev. 2012 Apr;40(2):102–105. doi: 10.1097/JES.0b013e318245837b. [DOI] [PubMed] [Google Scholar]
- 82.Henríquez-Olguín C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T., Sylow L., Holmdahl R., Richter E.A., Jaimovich E., Jensen T.E. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019 Oct 11;10(1):4623. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christiansen D., Eibye K.H., Hostrup M., Bangsbo J. Blood flow-restricted training enhances thigh glucose uptake during exercise and muscle antioxidant function in humans. Metabolism. 2019 Sep;98:1–15. doi: 10.1016/j.metabol.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Bradley S.J., Kingwell B.A., McConell G.K. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999 Sep;48(9):1815–1821. doi: 10.2337/diabetes.48.9.1815. [DOI] [PubMed] [Google Scholar]
- 85.Kingwell B.A., Formosa M., Muhlmann M., Bradley S.J., McConell G.K. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes. 2002 Aug;51(8):2572–2580. doi: 10.2337/diabetes.51.8.2572. [DOI] [PubMed] [Google Scholar]
- 86.Ploug T., Galbo H., Richter E.A. Increased muscle glucose uptake during contractions: no need for insulin. Am. J. Physiol. 1984 Dec;247(6 Pt 1):E726–E731. doi: 10.1152/ajpendo.1984.247.6.E726. [DOI] [PubMed] [Google Scholar]
- 87.McConell G.K., Huynh N.N., Lee-Young R.S., Canny B.J., Wadley G.D. L-Arginine infusion increases glucose clearance during prolonged exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2006 Jan;290(1):E60–E66. doi: 10.1152/ajpendo.00263.2005. [DOI] [PubMed] [Google Scholar]
- 88.Ross R.M., Wadley G.D., Clark M.G., Rattigan S., McConell G.K. Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes. 2007 Dec;56(12):2885–2892. doi: 10.2337/db07-0745. [DOI] [PubMed] [Google Scholar]
- 89.Higaki Y., Hirshman M.F., Fujii N., Goodyear L.J. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001 Feb;50(2):241–247. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- 90.Roberts C.K., Barnard R.J., Scheck S.H., Balon T.W. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am. J. Physiol. 1997 Jul;273(1 Pt 1):E220–E225. doi: 10.1152/ajpendo.1997.273.1.E220. [DOI] [PubMed] [Google Scholar]
- 91.Balon T.W., Nadler J.L. Evidence that nitric oxide increases glucose transport in skeletal muscle. J. Appl. Physiol. 1997 Jan;82(1):359–363. doi: 10.1152/jappl.1997.82.1.359. 1985. [DOI] [PubMed] [Google Scholar]
- 92.Etgen G.J., Jr., Fryburg D.A., Gibbs E.M. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997 Nov;46(11):1915–1919. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- 93.Stephens T.J., Canny B.J., Snow R.J., McConell G.K. 5'-aminoimidazole-4-carboxyamide-ribonucleoside-activated glucose transport is not prevented by nitric oxide synthase inhibition in rat isolated skeletal muscle. Clin. Exp. Pharmacol. Physiol. 2004 Jul;31(7):419–423. doi: 10.1111/j.1440-1681.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 94.Rottman J.N., Bracy D., Malabanan C., Yue Z., Clanton J., Wasserman D.H. Contrasting effects of exercise and NOS inhibition on tissue-specific fatty acid and glucose uptake in mice. Am. J. Physiol. Endocrinol. Metab. 2002 Jul;283(1):E116–E123. doi: 10.1152/ajpendo.00545.2001. [DOI] [PubMed] [Google Scholar]
- 95.McConell G.K., Kingwell B.A. Does nitric oxide regulate skeletal muscle glucose uptake during exercise? Exerc. Sport Sci. Rev. 2006 Jan;34(1):36–41. doi: 10.1097/00003677-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Deshmukh A.S., Long Y.C., de Castro Barbosa T., Karlsson H.K., Glund S., Zavadoski W.J., Gibbs E.M., Koistinen H.A., Wallberg-Henriksson H., Zierath J.R. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle. Diabetologia. 2010 Jun;53(6):1142–1150. doi: 10.1007/s00125-010-1716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau K.S., Grange R.W., Isotani E., Sarelius I.H., Kamm K.E., Huang P.L., Stull J.T. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol. Genom. 2000 Jan 24;2(1):21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- 98.Clavreul N., Bachschmid M.M., Hou X., Shi C., Idrizovic A., Ido Y., Pimentel D., Cohen R.A. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2006 Nov;26(11):2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 99.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007 Sep 15;43(6):883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. Epub 2007 Jun 15. [DOI] [PubMed] [Google Scholar]
- 100.Hayashi T., Hirshman M.F., Kurth E.J., Winder W.W., Goodyear L.J. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998 Aug;47(8):1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 101.Stamler J.S., Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001 Jan;81(1):209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 102.Rudnick J., Püttmann B., Tesch P.A., Alkner B., Schoser B.G., Salanova M., Kirsch K., Gunga H.C., Schiffl G., Lück G., Blottner D. Differential expression of nitric oxide synthases (NOS 1-3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. Faseb. J. 2004 Aug;18(11):1228–1230. doi: 10.1096/fj.03-0792fje. [DOI] [PubMed] [Google Scholar]
- 103.Hong Y.H., Frugier T., Zhang X., Murphy R.M., Lynch G.S., Betik A.C., Rattigan S., McConell G.K. Glucose uptake during contraction in isolated skeletal muscles from neuronal nitric oxide synthase μ knockout mice. J. Appl. Physiol. 2015 May 1;118(9):1113–1121. doi: 10.1152/japplphysiol.00056.2015. [DOI] [PubMed] [Google Scholar]
- 104.Henríquez-Olguín C., Boronat S., Cabello-Verrugio C., Jaimovich E., Hidalgo E., Jensen T.E. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxidants Redox Signal. 2019 Dec 20;31(18):1371–1410. doi: 10.1089/ars.2018.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S., Bruce C., Shields B.J., Skiba B., Ooms L.M., Stepto N., Wu B., Mitchell C.A., Tonks N.K., Watt M.J., Febbraio M.A., Crack P.J., Andrikopoulos S., Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metabol. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sjøberg K.A., Frøsig C., Kjøbsted R., Sylow L., Kleinert M., Betik A.C., Shaw C.S., Kiens B., Wojtaszewski J.F.P., Rattigan S., Richter E.A., McConell G.K. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 2017 Jun;66(6):1501–1510. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 107.Katz A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J. Physiol. 2016 Jun 1;594(11):2787–2794. doi: 10.1113/JP271665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McConell G.K., Rattigan S., Lee-Young R.S., Wadley G.D., Merry T.L. Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2012 Aug 1;303(3):E301–E307. doi: 10.1152/ajpendo.00667.2011. [DOI] [PubMed] [Google Scholar]
- 109.Rowell L.B. Ideas about control of skeletal and cardiac muscle blood flow (1876-2003): cycles of revision and new vision. J. Appl. Physiol. 2004 Jul;97(1):384–392. doi: 10.1152/japplphysiol.01220.2003. 1985. [DOI] [PubMed] [Google Scholar]
- 110.Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J. Physiol. 2007 Sep 15;583(Pt 3):819–823. doi: 10.1113/jphysiol.2007.136309. Epub 2007 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trinity J.D., Broxterman R.M., Richardson R.S.3. Regulation of exercise blood flow: role of free radicals. Free Radic. Biol. Med. 2016 Sep;98:90–102. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Querido J.S., Sheel A.W. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37(9):765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 113.Simmons G.H., Wong B.J., Holowatz L.A., Kenney W.L. Changes in the control of skin blood flow with exercise training: where do cutaneous vascular adaptations fit in? Exp. Physiol. 2011 Sep;96(9):822–828. doi: 10.1113/expphysiol.2010.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joyner M.J., Casey D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol. Rev. 2015 Apr;95(2):549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Antoniades C., Antonopoulos A.S., Bendall J.K., Channon K.M. Targeting redox signaling in the vascular wall: from basic science to clinical practice. Curr. Pharmaceut. Des. 2009;15(3):329–342. doi: 10.2174/138161209787354230. [DOI] [PubMed] [Google Scholar]
- 116.Tejero J., Shiva S., Gladwin M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019 Jan 1;99(1):311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gliemann L., Nyberg M., Hellsten Y. Nitric oxide and reactive oxygen species in limb vascular function: what is the effect of physical activity? Free Radic. Res. 2014 Jan;48(1):71–83. doi: 10.3109/10715762.2013.835045. [DOI] [PubMed] [Google Scholar]
- 118.Hellsten Y., Nyberg M., Jensen L.G., Mortensen S.P. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012 Dec 15;590(24):6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gliemann L., Hansen C.V., Rytter N., Hellsten Y. Regulation of skeletal muscle blood flow during exercise. Curr. Opin. Physiol. 2019 Aug;10:146–155. [Google Scholar]
- 120.Tschakovsky M.E., Joyner M.J. Nitric oxide and muscle blood flow in exercise. Appl. Physiol. Nutr. Metabol. 2008 Feb;33(1):151–161. doi: 10.1139/H07-148. [DOI] [PubMed] [Google Scholar]
- 121.Gilligan D.M., Panza J.A., Kilcoyne C.M., Waclawiw M.A., Casino P.R., Quyyumi A.A. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994 Dec;90(6):2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- 122.Rådegran G., Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am. J. Physiol. 1999 Jun;276(6):H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- 123.Brock R.W., Tschakovsky M.E., Shoemaker J.K., Halliwill J.R., Joyner M.J., Hughson R.L. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J. Appl. Physiol. 1998 Dec;85(6):2249–2254. doi: 10.1152/jappl.1998.85.6.2249. 1985. [DOI] [PubMed] [Google Scholar]
- 124.Hickner R.C., Fisher J.S., Ehsani A.A., Kohrt W.M. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am. J. Physiol. 1997 Jul;273(1 Pt 2):H405–H410. doi: 10.1152/ajpheart.1997.273.1.H405. [DOI] [PubMed] [Google Scholar]
- 125.Mortensen S.P., González-Alonso J., Damsgaard R., Saltin B., Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007 Jun 1;581(Pt 2):853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schrage W.G., Joyner M.J., Dinenno F.A. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J. Physiol. 2004 Jun 1;557(Pt 2):599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shoemaker J.K., Halliwill J.R., Hughson R.L., Joyner M.J. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am. J. Physiol. 1997 Nov;273(5):H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- 128.Donato A.J., Uberoi A., Bailey D.M., Wray D.W., Richardson R.S. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010 Feb;298(2):H671–H678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richardson R.S., Donato A.J., Uberoi A., Wray D.W., Lawrenson L., Nishiyama S., Bailey D.M. Exercise-induced brachial artery vasodilation: role of free radicals. Am. J. Physiol. Heart Circ. Physiol. 2007 Mar;292(3):H1516–H1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 130.Lucchesi P.A., Belmadani S., Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J. Hypertens. 2005 Mar;23(3):571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 131.Nossaman B.D., Bivalacqua T.J., Champion H.C., Baber S.R., Kadowitz P.J. Analysis of vasodilator responses to peroxynitrite in the hindlimb vascular bed of the cat. J. Cardiovasc. Pharmacol. 2007 Oct;50(4):358–366. doi: 10.1097/FJC.0b013e31811242cd. [DOI] [PubMed] [Google Scholar]
- 132.Peluso I., Serafini M., Campolongo P., Palmery M. Effect on rat arterial blood pressure of chemically generated peroxyl radicals and protection by antioxidants. J. Nutr. Biochem. 2004 Jun;15(6):323–327. doi: 10.1016/j.jnutbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 133.Wray D.W., Uberoi A., Lawrenson L., Bailey D.M., Richardson R.S. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin. Sci. (Lond.) 2009 Mar;116(5):433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 134.Sindler A.L., Delp M.D., Reyes R., Wu G., Muller-Delp J.M. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J. Physiol. 2009 Aug 1;587(Pt 15):3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jablonski K.L., Seals D.R., Eskurza I., Monahan K.D., Donato A.J. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J. Appl. Physiol. 2007 Nov;103(5):1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 136.Bagher P., Segal S.S. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol. 2011;202(3):271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Trinity J.D., Groot H.J., Layec G., Rossman M.J., Ives S.J., Runnels S., Gmelch B., Bledsoe A., Richardson R.S. Nitric oxide and passive limb movement: a new approach to assess vascular function. J. Physiol. 2012 Mar 15;590(6):1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirai D.M., Copp S.W., Schwagerl P.J., Musch T.I., Poole D.C. Acute effects of hydrogen peroxide on skeletal muscle microvascular oxygenation from rest to contractions. J. Appl. Physiol. 2011 May;110(5):1290–1298. doi: 10.1152/japplphysiol.01489.2010. [DOI] [PubMed] [Google Scholar]
- 139.Richards J.C., Crecelius A.R., Larson D.G., Dinenno F.A. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am. J. Physiol. Heart Circ. Physiol. 2015 Jul 15;309(2):H360–H368. doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rossman M.J., Trinity J.D., Garten R.S., Ives S.J., Conklin J.D., Barrett-O'Keefe Z., Witman M.A., Bledsoe A.D., Morgan D.E., Runnels S., Reese V.R., Zhao J., Amann M., Wray D.W., Richardson R.S. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am. J. Physiol. Heart Circ. Physiol. 2015 Sep;309(5):H977–H985. doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ranadive S.M., Joyner M.J., Walker B.G., Taylor J.L., Casey D.P. Effect of vitamin C on hyperoxia-induced vasoconstriction in exercising skeletal muscle. J. Appl. Physiol. 2014 Nov 15;117(10):1207–1211. doi: 10.1152/japplphysiol.00073.2014. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miura S., Saitoh S.I., Kokubun T., Owada T., Yamauchi H., Machii H., Takeishi Y. Mitochondrial-targeted antioxidant maintains blood flow, mitochondrial function, and redox balance in old mice following prolonged limb ischemia. Int. J. Mol. Sci. 2017 Sep 4;18(9) doi: 10.3390/ijms18091897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cseko C., Bagi Z., Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J. Appl. Physiol. 2004 Sep;97(3):1130–1137. doi: 10.1152/japplphysiol.00106.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 144.Kadlec A.O., Gutterman D.D. Redox regulation of the microcirculation. Comp. Physiol. 2019 Dec 18;10(1):229–259. doi: 10.1002/cphy.c180039. [DOI] [PubMed] [Google Scholar]
- 145.Kramer P.A., Darley-Usmar V.M. The emerging theme of redox bioenergetics in health and disease. Biomed. J. 2015 Jul-Aug;38(4):294–300. doi: 10.4103/2319-4170.155591. [DOI] [PubMed] [Google Scholar]
- 146.Dodson M., Darley-Usmar V., Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 2013 Oct;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yin F., Sancheti H., Cadenas E. Mitochondrial thiols in the regulation of cell death pathways. Antioxidants Redox Signal. 2012 Dec 15;17(12):1714–1727. doi: 10.1089/ars.2012.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cadenas E. Mitochondrial free radical production and cell signaling. Mol. Aspect. Med. 2004 Feb-Apr;25(1–2):17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 149.Wende A.R., Young M.E., Chatham J., Zhang J., Rajasekaran N.S., Darley-Usmar V.M. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic. Biol. Med. 2016 Nov;100:94–107. doi: 10.1016/j.freeradbiomed.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic. Res. 2005 Apr;39(4):353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 151.Holmström K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013 Jun 20;2(8):761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Townsend L.K., Weber A.J., Barbeau P.A., Holloway G.P., Wright D.C. Reactive oxygen species-dependent regulation of pyruvate dehydrogenase kinase-4 in white adipose tissue. Am. J. Physiol. Cell Physiol. 2020;318(1):C137–C149. doi: 10.1152/ajpcell.00313.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013 Jul-Aug;48(4):332–356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goncalves R.L., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015 Jan 2;290(1):209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011 Oct 1;51(7):1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009 Jan 1;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016 Nov;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 158.Sakellariou G.K., Vasilaki A., Palomero J., Kayani A., Zibrik L., McArdle A., Jackson M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants Redox Signal. 2013 Feb 20;18(6):603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frasier C.R., Moukdar F., Patel H.D., Sloan R.C., Stewart L.M., Alleman R.J., La Favor J.D., Brown D.A. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc. Res. 2013 Apr 1;98(1):47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 160.Sánchez G., Escobar M., Pedrozo Z., Macho P., Domenech R., Härtel S., Hidalgo C., Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc. Res. 2008 Jan 15;77(2):380–386. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- 161.Pearson T., Kabayo T., Ng R., Chamberlain J., McArdle A., Jackson M.J. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PloS One. 2014 May 29;9(5) doi: 10.1371/journal.pone.0096378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Henríquez-Olguín C., Díaz-Vegas A., Utreras-Mendoza Y., Campos C., Arias-Calderón M., Llanos P., Contreras-Ferrat A., Espinosa A., Altamirano F., Jaimovich E., Valladares D.M. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front. Physiol. 2016 Jul 14;7:282. doi: 10.3389/fphys.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smith R.A., Murphy M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010 Jul;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 164.Shill D.D., Southern W.M., Willingham T.B., Lansford K.A., McCully K.K., Jenkins N.T. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J. Physiol. 2016 Dec 1;594(23):7005–7014. doi: 10.1113/JP272491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gomez-Cabrera M.C., Borrás C., Pallardó F.V., Sastre J., Ji L.L., Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005 Aug 15;567(Pt 1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hansford R.G., Hogue B.A., Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997 Feb;29(1):89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 167.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002 Nov 22;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 168.Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013 May 23;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wong H.S., Dighe P.A., Mezera V., Monternier P.A., Brand M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017 Oct 13;292(41):16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Walsh M.E., Shi Y., Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014 Jan;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fisher-Wellman K.H., Gilliam L.A.A., Lin C.T., Cathey B.L., Lark D.S., Darrell Neufer P. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic. Biol. Med. 2013 Dec;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fisher-Wellman K.H., Lin C.T., Ryan T.E., Reese L.R., Gilliam L.A., Cathey B.L., Lark D.S., Smith C.D., Muoio D.M., Neufer P.D. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem. J. 2015 Apr 15;467(2):271–280. doi: 10.1042/BJ20141447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pollak N., Niere M., Ziegler M. NAD kinase levels control the NADPH concentration in human cells. J. Biol. Chem. 2007 Nov 16;282(46):33562–33571. doi: 10.1074/jbc.M704442200. [DOI] [PubMed] [Google Scholar]
- 174.Wadley A.J., Aldred S., Coles S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016 Aug;8:51–58. doi: 10.1016/j.redox.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ralser M., Wamelink M.M., Latkolik S., Jansen E.E., Lehrach H., Jakobs C. Metabolic reconfiguration precedes transcriptional regulation in the antioxidant response. Nat. Biotechnol. 2009 Jul;27(7):604–605. doi: 10.1038/nbt0709-604. [DOI] [PubMed] [Google Scholar]
- 176.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., Thomas C.J., Vander Heiden M.G., Cantley L.C. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011 Dec 2;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Colussi C., Albertini M.C., Coppola S., Rovidati S., Galli F., Ghibelli L. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. Faseb. J. 2000 Nov;14(14):2266–2276. doi: 10.1096/fj.00-0074com. [DOI] [PubMed] [Google Scholar]
- 178.Kuehne A., Emmert H., Soehle J., Winnefeld M., Fischer F., Wenck H., Gallinat S., Terstegen L., Lucius R., Hildebrand J., Zamboni N. Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol. Cell. 2015 Aug 6;59(3):359–371. doi: 10.1016/j.molcel.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 179.Ralser M., Wamelink M.M., Kowald A., Gerisch B., Heeren G., Struys E.A., Klipp E., Jakobs C., Breitenbach M., Lehrach H., Krobitsch S. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007 Dec 21;6(4):10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peralta D., Bronowska A.K., Morgan B., Dóka É., Van Laer K., Nagy P., Gräter F., Dick T.P. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat. Chem. Biol. 2015 Feb;11(2):156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 181.Dick T.P., Ralser M. Metabolic remodeling in times of stress: who shoots faster than his shadow? Mol. Cell. 2015 Aug 20;59(4):519–521. doi: 10.1016/j.molcel.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 182.Stincone A., Prigione A., Cramer T., Wamelink M.M., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.M., Krüger A., Tauqeer Alam M., Keller M.A., Breitenbach M., Brindle K.M., Rabinowitz J.D., Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Phil. Soc. 2015 Aug;90(3):927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trammell S.A., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cantó C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A.A., Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabol. 2012 Jun 6;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dolopikou C.F., Kourtzidis I.A., Margaritelis N.V., Vrabas I.S., Koidou I., Kyparos A., Theodorou A.A., Paschalis V., Nikolaidis M.G. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur. J. Nutr. 2020;59(2):505–515. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 186.Vaur P., Brugg B., Mericskay M., Li Z., Schmidt M.S., Vivien D., Orset C., Jacotot E., Brenner C., Duplus E. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. Faseb. J. 2017 Dec;31(12):5440–5452. doi: 10.1096/fj.201700221RR. [DOI] [PubMed] [Google Scholar]
- 187.Kourtzidis I.A., Dolopikou C.F., Tsiftsis A.N., Margaritelis N.V., Theodorou A.A., Zervos I.A., Tsantarliotou M.P., Veskoukis A.S., Vrabas I.S., Paschalis V., Kyparos A., Nikolaidis M.G. Nicotinamide riboside supplementation dysregulates redox and energy metabolism in rats: implications for exercise performance. Exp. Physiol. 2018 Oct;103(10):1357–1366. doi: 10.1113/EP086964. [DOI] [PubMed] [Google Scholar]
- 188.Matzinger M., Fischhuber K., Pölöske D., Mechtler K., Heiss E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020;29:101393. doi: 10.1016/j.redox.2019.101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Quijano C., Trujillo M., Castro L., Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016 Aug;8:28–42. doi: 10.1016/j.redox.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Radi R., Cassina A., Hodara R., Quijano C., Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 2002 Dec 1;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 191.Lokuta A.J., Maertz N.A., Meethal S.V., Potter K.T., Kamp T.J., Valdivia H.H., Haworth R.A. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005 Mar 1;111(8):988–995. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- 192.Konorev E.A., Hogg N., Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 1998 May 8;427(2):171–174. doi: 10.1016/s0014-5793(98)00413-x. [DOI] [PubMed] [Google Scholar]
- 193.Palacios-Callender M., Hollis V., Frakich N., Mateo J., Moncada S. Cytochrome c oxidase maintains mitochondrial respiration during partial inhibition by nitric oxide. J. Cell Sci. 2007 Jan 1;120(Pt 1):160–165. doi: 10.1242/jcs.03308. [DOI] [PubMed] [Google Scholar]
- 194.Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S., Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011 Jun;12(6):534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.García-Nogales P., Almeida A., Bolaños J.P. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J. Biol. Chem. 2003 Jan 10;278(2):864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- 196.Sakellariou G.K., Jackson M.J., Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014 Jan;48(1):12–29. doi: 10.3109/10715762.2013.830718. [DOI] [PubMed] [Google Scholar]
- 197.Gomez-Cabrera M.C., Salvador-Pascual A., Cabo H., Ferrando B., Viña J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015 Sep;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 198.Hood D.A. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001 Mar;90(3):1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 199.Kowald A., Kirkwood T.B. Evolution of the mitochondrial fusion-fission cycle and its role in aging. Proc. Natl. Acad. Sci. U. S. A. 2011 Jun 21;108(25):10237–10242. doi: 10.1073/pnas.1101604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Christian B.E., Spremulli L.L. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta. 2012 Sep-Oct;1819(9–10):1035–1054. doi: 10.1016/j.bbagrm.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.E., Walford G.A., Sugiana C., Boneh A., Chen W.K., Hill D.E., Vidal M., Evans J.G., Thorburn D.R., Carr S.A., Mootha V.K. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008 Jul 11;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Scarpulla R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta. 2011 Jul;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Erlich A.T., Tryon L.D., Crilly M.J., Memme J.M., Moosavi Z.S.M., Oliveira A.N., Beyfuss K., Hood D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr. Med. Res. 2016 Sep;5(3):187–197. doi: 10.1016/j.imr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M., Kelly D.P., Holloszy J.O. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb. J. 2002 Dec;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 205.Goto M., Terada S., Kato M., Katoh M., Yokozeki T., Tabata I., Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem. Biophys. Res. Commun. 2000 Aug 2;274(2):350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 206.Norrbom J., Sundberg C.J., Ameln H., Kraus W.E., Jansson E., Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J. Appl. Physiol. 2004 Jan;96(1):189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 207.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003 Feb 1;546(Pt 3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Terada S., Goto M., Kato M., Kawanaka K., Shimokawa T., Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem. Biophys. Res. Commun. 2002 Aug 16;296(2):350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 209.Zechner C., Lai L., Zechner J.F., Geng T., Yan Z., Rumsey J.W., Collia D., Chen Z., Wozniak D.F., Leone T.C., Kelly D.P. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metabol. 2010 Dec 1;12(6):633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O., Michael L.F., Puigserver P., Isotani E., Olson E.N., Lowell B.B., Bassel-Duby R., Spiegelman B.M. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002 Aug 15;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 211.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C., Pallardo F.V., Sastre J., Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008 Jan;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 212.Ristow M., Zarse K., Oberbach A., Klöting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C.R., Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U. S. A. 2009 May 26;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Strobel N.A., Peake J.M., Matsumoto A., Marsh S.A., Coombes J.S., Wadley G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011 Jun;43(6):1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 214.Silveira L.R., Pilegaard H., Kusuhara K., Curi R., Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim. Biophys. Acta. 2006 Sep;1763(9):969–976. doi: 10.1016/j.bbamcr.2006.06.010. Epub 2006 Jul 7. [DOI] [PubMed] [Google Scholar]
- 215.Venditti P., Napolitano G., Barone D., Di Meo S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic. Res. 2014 Oct;48(10):1179–1189. doi: 10.3109/10715762.2014.937341. [DOI] [PubMed] [Google Scholar]
- 216.Meier P., Renga M., Hoppeler H., Baum O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem. Funct. 2013 Jan;31(1):51–59. doi: 10.1002/cbf.2859. [DOI] [PubMed] [Google Scholar]
- 217.Morrison D., Hughes J., Della Gatta P.A., Mason S., Lamon S., Russell A.P., Wadley G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015 Dec;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 218.Paulsen G., Cumming K.T., Holden G., Hallén J., Rønnestad B.R., Sveen O., Skaug A., Paur I., Bastani N.E., Østgaard H.N., Buer C., Midttun M., Freuchen F., Wiig H., Ulseth E.T., Garthe I., Blomhoff R., Benestad H.B., Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014 Apr 15;592(8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Strobel N.A., Matsumoto A., Peake J.M., Marsh S.A., Peternelj T.T., Briskey D., Fassett R.G., Coombes J.S., Wadley G.D. Altering the redox state of skeletal muscle by glutathione depletion increases the exercise-activation of PGC-1α. Phys. Rep. 2014 Dec 23;2(12) doi: 10.14814/phy2.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kang C., O'Moore K.M., Dickman J.R., Ji L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic. Biol. Med. 2009 Nov 15;47(10):1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 221.Higashida K., Kim S.H., Higuchi M., Holloszy J.O., Han D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011 Nov;301(5):E779–E784. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yfanti C., Akerström T., Nielsen S., Nielsen A.R., Mounier R., Mortensen O.H., Lykkesfeldt J., Rose A.J., Fischer C.P., Pedersen B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010 Jul;42(7):1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 223.Petersen A.C., McKenna M.J., Medved I., Murphy K.T., Brown M.J., Della Gatta P., Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol. 2012 Mar;204(3):382–392. doi: 10.1111/j.1748-1716.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 224.Wadley G.D., Nicolas M.A., Hiam D.S., McConell G.K. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am. J. Physiol. Endocrinol. Metab. 2013 Apr 15;304(8):E853–E862. doi: 10.1152/ajpendo.00568.2012. [DOI] [PubMed] [Google Scholar]
- 225.Miller B.F., Konopka A.R., Hamilton K.L. The rigorous study of exercise adaptations: why mRNA might not be enough. J. Appl. Physiol. 1985;121(2):594–596. doi: 10.1152/japplphysiol.00137.2016. 2016 Aug 1. [DOI] [PubMed] [Google Scholar]
- 226.Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C., Bracale R., Valerio A., Francolini M., Moncada S., Carruba M.O. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003 Feb 7;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 227.Tengan C.H., Rodrigues G.S., Godinho R.O. Nitric oxide in skeletal muscle: role on mitochondrial biogenesis and function. Int. J. Mol. Sci. 2012 Dec 14;13(12):17160–17184. doi: 10.3390/ijms131217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Piantadosi C.A., Suliman H.B. Redox regulation of mitochondrial biogenesis. Free Radic. Biol. Med. 2012 Dec 1;53(11):2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McConell G.K., Ng G.P., Phillips M., Ruan Z., Macaulay S.L., Wadley G.D. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J. Appl. Physiol. 1985;108(3):589–595. doi: 10.1152/japplphysiol.00377.2009. 2010 Mar. [DOI] [PubMed] [Google Scholar]
- 230.Wadley G.D., McConell G.K. Effect of nitric oxide synthase inhibition on mitochondrial biogenesis in rat skeletal muscle. J. Appl. Physiol. 2007 Jan;102(1):314–320. doi: 10.1152/japplphysiol.00549.2006. 1985. [DOI] [PubMed] [Google Scholar]
- 231.Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr. Pharmaceut. Des. 2014;20(35):5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 232.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 2007 Jan 15;73(2):341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 233.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004 Dec;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 234.Crilly M.J., Tryon L.D., Erlich A.T., Hood D.A. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J. Appl. Physiol. 2016 Sep 1;121(3):730–740. doi: 10.1152/japplphysiol.00042.2016. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Irrcher I., Ljubicic V., Hood D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009 Jan;296(1):C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 236.Merry T.L., Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016 Sep 15;594(18):5195–5207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Y., Uguccioni G., Ljubicic V., Irrcher I., Iqbal S., Singh K., Ding S., Hood D.A. Multiple signaling pathways regulate contractile activity-mediated PGC-1α gene expression and activity in skeletal muscle cells. Phys. Rep. 2014 May 19;2(5) doi: 10.14814/phy2.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Choi S.L., Kim S.J., Lee K.T., Kim J., Mu J., Birnbaum M.J., Soo Kim S., Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochem. Biophys. Res. Commun. 2001 Sep 14;287(1):92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 239.Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M., Hardie D.G. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metabol. 2010 Jun 9;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Auciello F.R., Ross F.A., Ikematsu N., Hardie D.G. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014 Sep 17;588(18):3361–3366. doi: 10.1016/j.febslet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Emerling B.M., Weinberg F., Snyder C., Burgess Z., Mutlu G.M., Viollet B., Budinger G.R., Chandel N.S. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 2009 May 15;46(10):1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morales-Alamo D., Ponce-González J.G., Guadalupe-Grau A., Rodríguez-García L., Santana A., Cusso R., Guerrero M., Dorado C., Guerra B., Calbet J.A. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013 Mar 1;114(5):566–577. doi: 10.1152/japplphysiol.01246.2012. [DOI] [PubMed] [Google Scholar]
- 243.Zmijewski J.W., Banerjee S., Bae H., Friggeri A., Lazarowski E.R., Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010 Oct 22;285(43):33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shao D., Oka S., Liu T., Zhai P., Ago T., Sciarretta S., Li H., Sadoshima J. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metabol. 2014 Feb 4;19(2):232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cardaci S., Filomeni G., Ciriolo M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012 May 1;125(Pt 9):2115–2125. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- 246.Zhang J., Xie Z., Dong Y., Wang S., Liu C., Zou M.H. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J. Biol. Chem. 2008 Oct 10;283(41):27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 247.Xie Z., Dong Y., Zhang M., Cui M.Z., Cohen R.A., Riek U., Neumann D., Schlattner U., Zou M.H. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J. Biol. Chem. 2006 Mar 10;281(10):6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 248.Akimoto T., Pohnert S.C., Li P., Zhang M., Gumbs C., Rosenberg P.B., Williams R.S., Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005 May 20;280(20):19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 249.Lira V.A., Benton C.R., Yan Z., Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010 Aug;299(2):E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McCubrey J.A., Lahair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxidants Redox Signal. 2006 Sep-Oct;8(9–10):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 251.Torres M., Forman H.J. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17(1–4):287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 252.Matsuzawa A., Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim. Biophys. Acta. 2008 Nov;1780(11):1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 253.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005 Mar 11;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 254.Galli S., Antico Arciuch V.G., Poderoso C., Converso D.P., Zhou Q., Bal de Kier Joffé E., Cadenas E., Boczkowski J., Carreras M.C., Poderoso J.J. Tumor cell phenotype is sustained by selective MAPK oxidation in mitochondria. PloS One. 2008 Jun 11;3(6):e2379. doi: 10.1371/journal.pone.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants Redox Signal. 2013 Apr 1;18(10):1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014 Jan-Feb;49(1):59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013 Sep;280(17):4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 258.Makanae Y., Kawada S., Sasaki K., Nakazato K., Ishii N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiol. 2013 May;208(1):57–65. doi: 10.1111/apha.12042. [DOI] [PubMed] [Google Scholar]
- 259.Paulsen G., Hamarsland H., Cumming K.T., Johansen R.E., Hulmi J.J., Børsheim E., Wiig H., Garthe I., Raastad T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014 Dec 15;592(24):5391–5408. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dutra M.T., Alex S., Mota M.R., Sales N.B., Brown L.E., Bottaro M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl. Physiol. Nutr. Metabol. 2018 Aug;43(8):775–781. doi: 10.1139/apnm-2017-0866. [DOI] [PubMed] [Google Scholar]
- 261.Bjørnsen T., Salvesen S., Berntsen S., Hetlelid K.J., Stea T.H., Lohne-Seiler H., Rohde G., Haraldstad K., Raastad T., Køpp U., Haugeberg G., Mansoor M.A., Bastani N.E., Blomhoff R., Stølevik S.B., Seynnes O.R., Paulsen G. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports. 2016 Jul;26(7):755–763. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 262.Bobeuf F., Labonte M., Dionne I.J., Khalil A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J. Nutr. Health Aging. 2011 Dec;15(10):883–889. doi: 10.1007/s12603-011-0097-2. [DOI] [PubMed] [Google Scholar]
- 263.Handayaningsih A.E., Iguchi G., Fukuoka H., Nishizawa H., Takahashi M., Yamamoto M., Herningtyas E.H., Okimura Y., Kaji H., Chihara K., Seino S., Takahashi Y. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011 Mar;152(3):912–921. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 264.Smith L.W., Smith J.D., Criswell D.S. Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J. Appl. Physiol. 2002 May;92(5):2005–2011. doi: 10.1152/japplphysiol.00950.2001. 1985. [DOI] [PubMed] [Google Scholar]
- 265.Sellman J.E., DeRuisseau K.C., Betters J.L., Lira V.A., Soltow Q.A., Selsby J.T., Criswell D.S. In vivo inhibition of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J. Appl. Physiol. 2006 Jan;100(1):258–265. doi: 10.1152/japplphysiol.00936.2005. 1985. [DOI] [PubMed] [Google Scholar]
- 266.Zhang Y., Davis C., Sakellariou G.K., Shi Y., Kayani A.C., Pulliam D., Bhattacharya A., Richardson A., Jackson M.J., McArdle A., Brooks S.V., Van Remmen H. CuZnSOD gene deletion targeted to skeletal muscle leads to loss of contractile force but does not cause muscle atrophy in adult mice. Faseb. J. 2013 Sep;27(9):3536–3548. doi: 10.1096/fj.13-228130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ito N., Ruegg U.T., Kudo A., Miyagoe-Suzuki Y., Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013 Jan;19(1):101–106. doi: 10.1038/nm.3019. [DOI] [PubMed] [Google Scholar]
- 268.Le Moal E., Pialoux V., Juban G., Groussard C., Zouhal H., Chazaud B., Mounier R. Redox control of skeletal muscle regeneration. Antioxidants Redox Signal. 2017 Aug 10;27(5):276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Dutra M.T., Martins W.R., Ribeiro A.L.A., Bottaro M. The effects of strength training combined with vitamin C and E supplementation on skeletal muscle mass and strength: a systematic review and meta-analysis. J. Sports Med. 2020;2020:3505209. doi: 10.1155/2020/3505209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kissane R.W.P., Egginton S. Exercise-mediated angiogenesis. Curr Opin. Physiol. 2019 Aug;10:193–201. [Google Scholar]
- 271.Bloor C.M. Angiogenesis during exercise and training. Angiogenesis. 2005;8(3):263–271. doi: 10.1007/s10456-005-9013-x. [DOI] [PubMed] [Google Scholar]
- 272.Gustafsson T. Vascular remodelling in human skeletal muscle. Biochem. Soc. Trans. 2011 Dec;39(6):1628–1632. doi: 10.1042/BST20110720. [DOI] [PubMed] [Google Scholar]
- 273.Prior B.M., Lloyd P.G., Yang H.T., Terjung R.L. Exercise-induced vascular remodeling. Exerc. Sport Sci. Rev. 2003 Jan;31(1):26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 274.Chung A.S., Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 275.Brown M.D., Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6(1):1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- 276.Gustafsson T., Ameln H., Fischer H., Sundberg C.J., Timmons J.A., Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J. Appl. Physiol. 2005 Jun;98(6):2137–2146. doi: 10.1152/japplphysiol.01402.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 277.Yao Z., Lafage-Proust M.H., Plouët J., Bloomfield S., Alexandre C., Vico L. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J. Bone Miner. Res. 2004 Sep;19(9):1471–1480. doi: 10.1359/JBMR.040517. [DOI] [PubMed] [Google Scholar]
- 278.Abid M.R., Tsai J.C., Spokes K.C., Deshpande S.S., Irani K., Aird W.C. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. Faseb. J. 2001 Nov;15(13):2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 279.Gavin T.P. Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc. Sport Sci. Rev. 2009 Apr;37(2):86–92. doi: 10.1097/JES.0b013e31819c2e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Roy S., Khanna S., Sen C.K. Redox regulation of the VEGF signaling path and tissue vascularization: hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic. Biol. Med. 2008 Jan 15;44(2):180–192. doi: 10.1016/j.freeradbiomed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 281.Stone J.R., Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 282.Bir S.C., Kolluru G.K., Fang K., Kevil C.G. Redox balance dynamically regulates vascular growth and remodeling. Semin. Cell Dev. Biol. 2012 Sep;23(7):745–757. doi: 10.1016/j.semcdb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chen C., Li L., Zhou H.J., Min W. The role of NOX4 and TRX2 in angiogenesis and their potential cross-talk. Antioxidants (Basel) 2017 Jun 8;6(2) doi: 10.3390/antiox6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Prasai P.K., Shrestha B., Orr A.W., Pattillo C.B. Decreases in GSH:GSSG activate vascular endothelial growth factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox Biol. 2018 Oct;19:22–27. doi: 10.1016/j.redox.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kerr B.A., Byzova T.V. The dark side of the oxidative force in angiogenesis. Nat. Med. 2012 Aug;18(8):1184–1185. doi: 10.1038/nm.2881. [DOI] [PubMed] [Google Scholar]
- 286.Knock G.A. NADPH oxidase in the vasculature: expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 2019 Dec;145:385–427. doi: 10.1016/j.freeradbiomed.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 287.Ushio-Fukai M., Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxidants Redox Signal. 2009 Oct;11(10):2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Al-Shabrawey M., Bartoli M., El-Remessy A.B., Platt D.H., Matragoon S., Behzadian M.A., Caldwell R.W., Caldwell R.B. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am. J. Pathol. 2005 Aug;167(2):599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chua C.C., Hamdy R.C., Chua B.H. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic. Biol. Med. 1998 Nov 15;25(8):891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 290.Feliers D., Gorin Y., Ghosh-Choudhury G., Abboud H.E., Kasinath B.S. Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2006 Apr;290(4):F927–F936. doi: 10.1152/ajprenal.00331.2005. [DOI] [PubMed] [Google Scholar]
- 291.Sen C.K., Khanna S., Babior B.M., Hunt T.K., Ellison E.C., Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 2002 Sep 6;277(36):33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- 292.Suzuki J. L-arginine supplementation causes additional effects on exercise-induced angiogenesis and VEGF expression in the heart and hind-leg muscles of middle-aged rats. J. Physiol. Sci. 2006 Feb;56(1):39–44. doi: 10.2170/physiolsci.rp000505. [DOI] [PubMed] [Google Scholar]
- 293.Wang Y., Zang Q.S., Liu Z., Wu Q., Maass D., Dulan G., Shaul P.W., Melito L., Frantz D.E., Kilgore J.A., Williams N.S., Terada L.S., Nwariaku F.E. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. Cell Physiol. 2011 Sep;301(3):C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Colavitti R., Pani G., Bedogni B., Anzevino R., Borrello S., Waltenberger J., Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002 Feb 1;277(5):3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 295.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc. Res. 2006 Jul 15;71(2):226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 296.Maulik N. Redox signaling of angiogenesis. Antioxidants Redox Signal. 2002 Oct;4(5):805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 297.Lindholm M.E., Rundqvist H. Skeletal muscle hypoxia-inducible factor-1 and exercise. Exp. Physiol. 2016 Jan;101(1):28–32. doi: 10.1113/EP085318. [DOI] [PubMed] [Google Scholar]
- 298.Lundby C., Gassmann M., Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur. J. Appl. Physiol. 2006 Mar;96(4):363–369. doi: 10.1007/s00421-005-0085-5. [DOI] [PubMed] [Google Scholar]
- 299.Vogt M., Puntschart A., Geiser J., Zuleger C., Billeter R., Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J. Appl. Physiol. 2001 Jul;91(1):173–182. doi: 10.1152/jappl.2001.91.1.173. 1985. [DOI] [PubMed] [Google Scholar]
- 300.Zoll J., Ponsot E., Dufour S., Doutreleau S., Ventura-Clapier R., Vogt M., Hoppeler H., Richard R., Flück M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J. Appl. Physiol. 2006 Apr;100(4):1258–1266. doi: 10.1152/japplphysiol.00359.2005. 1985. [DOI] [PubMed] [Google Scholar]
- 301.Bunn H.F., Poyton R.O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996 Jul;76(3):839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 302.Kietzmann T., Görlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin. Cell Dev. Biol. 2005 Aug-Oct;16(4–5):474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 303.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007 Apr 13;26(1):63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sumbayev V.V., Budde A., Zhou J., Brüne B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003 Jan 30;535(1–3):106–112. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 305.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D., Semenza G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996 Sep;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Iyer N.V., Kotch L.E., Agani F., Leung S.W., Laughner E., Wenger R.H., Gassmann M., Gearhart J.D., Lawler A.M., Yu A.Y., Semenza G.L. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998 Jan 15;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C., Shibata R., Sato K., Walsh K., Keaney J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011 Aug 9;124(6):731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tojo T., Ushio-Fukai M., Yamaoka-Tojo M., Ikeda S., Patrushev N., Alexander R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005 May 10;111(18):2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 309.Vallet P., Charnay Y., Steger K., Ogier-Denis E., Kovari E., Herrmann F., Michel J.P., Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132(2):233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 310.Connor K.M., Subbaram S., Regan K.J., Nelson K.K., Mazurkiewicz J.E., Bartholomew P.J., Aplin A.E., Tai Y.T., Aguirre-Ghiso J., Flores S.C., Melendez J.A. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J. Biol. Chem. 2005 Apr 29;280(17):16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 311.Nespereira B., Pérez-Ilzarbe M., Fernández P., Fuentes A.M., Páramo J.A., Rodríguez J.A. Vitamins C and E downregulate vascular VEGF and VEGFR-2 expression in apolipoprotein-E-deficient mice. Atherosclerosis. 2003 Nov;171(1):67–73. doi: 10.1016/j.atherosclerosis.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 312.Woodson K., Triantos S., Hartman T., Taylor P.R., Virtamo J., Albanes D. Long-term alpha-tocopherol supplementation is associated with lower serum vascular endothelial growth factor levels. Anticancer Res. 2002 Jan-Feb;22(1A):375–378. [PubMed] [Google Scholar]
- 313.Lin M.T., Yen M.L., Lin C.Y., Kuo M.L. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol. Pharmacol. 2003 Nov;64(5):1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 314.Oak M.H., El Bedoui J., Schini-Kerth V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005 Jan;16(1):1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 315.Birukova A.A., Lee S., Starosta V., Wu T., Ho T., Kim J., Berliner J.A., Birukov K.G. A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.West X.Z., Malinin N.L., Merkulova A.A., Tischenko M., Kerr B.A., Borden E.C., Podrez E.A., Salomon R.G., Byzova T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010 Oct 21;467(7318):972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kim Y.W., Byzova T.V. Oxidative stress in angiogenesis and vascular disease. Blood. 2014 Jan 30;123(5):625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ji L.L., Kang C., Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016 Sep;98:113–122. doi: 10.1016/j.freeradbiomed.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 319.Forman H.J., Ursini F., Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014 Aug;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008 Jul 1;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Quintanilha A.T., Packer L. Vitamin E, physical exercise and tissue oxidative damage. Ciba Found. Symp. 1983;101:56–69. doi: 10.1002/9780470720820.ch5. [DOI] [PubMed] [Google Scholar]
- 322.Higuchi M., Cartier L.J., Chen M., Holloszy J.O. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J. Gerontol. 1985 May;40(3):281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- 323.Leeuwenburgh C., Fiebig R., Chandwaney R., Ji L.L. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am. J. Physiol. 1994 Aug;267(2 Pt 2):R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 324.Ji L.L., Stratman F.W., Lardy H.A. Enzymatic down regulation with exercise in rat skeletal muscle. Arch. Biochem. Biophys. 1988 May 15;263(1):137–149. doi: 10.1016/0003-9861(88)90622-4. [DOI] [PubMed] [Google Scholar]
- 325.Laughlin M.H., Simpson T., Sexton W.L., Brown O.R., Smith J.K., Korthuis R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990;68(6):2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 326.Vincent H.K., Powers S.K., Stewart D.J., Demirel H.A., Shanely R.A., Naito H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000;81(1–2):67–74. doi: 10.1007/PL00013799. [DOI] [PubMed] [Google Scholar]
- 327.Gore M., Fiebig R., Hollander J., Leeuwenburgh C., Ohno H., Ji L.L. Endurance training alters antioxidant enzyme gene expression in rat skeletal muscle. Can. J. Physiol. Pharmacol. 1998 Dec;76(12):1139–1145. doi: 10.1139/cjpp-76-12-1139. [DOI] [PubMed] [Google Scholar]
- 328.Hollander J., Fiebig R., Gore M., Ookawara T., Ohno H., Ji L.L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Archiv. 2001 Jun;442(3):426–434. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- 329.Oh-ishi S., Kizaki T., Nagasawa J., Izawa T., Komabayashi T., Nagata N., Suzuki K., Taniguchi N., Ohno H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin. Exp. Pharmacol. Physiol. 1997 May;24(5):326–332. doi: 10.1111/j.1440-1681.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 330.Wittwer M., Billeter R., Hoppeler H., Flück M. Regulatory gene expression in skeletal muscle of highly endurance-trained humans. Acta Physiol. Scand. 2004 Feb;180(2):217–227. doi: 10.1046/j.0001-6772.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- 331.Gonchar O. Muscle fiber specific antioxidative system adaptation to swim training in rats: influence of intermittent hypoxia. J. Sports Sci. Med. 2005 Jun 1;4(2):160–169. [PMC free article] [PubMed] [Google Scholar]
- 332.Hollander J., Fiebig R., Gore M., Bejma J., Ookawara T., Ohno H., Ji L.L. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. Am. J. Physiol. 1999 Sep;277(3):R856–R862. doi: 10.1152/ajpregu.1999.277.3.R856. [DOI] [PubMed] [Google Scholar]
- 333.Ji L.L., Fu R., Mitchell E.W. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J. Appl. Physiol. 1992;73(5):1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 334.Powers S.K., Criswell D., Lawler J., Ji L.L., Martin D., Herb R.A., Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994 Feb;266(2 Pt 2):R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 335.Chang C.K., Huang H.Y., Tseng H.F., Hsuuw Y.D., Tso T.K. Interaction of vitamin E and exercise training on oxidative stress and antioxidant enzyme activities in rat skeletal muscles. J. Nutr. Biochem. 2007 Jan;18(1):39–45. doi: 10.1016/j.jnutbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 336.Khassaf M., McArdle A., Esanu C., Vasilaki A., McArdle F., Griffiths R.D., Brodie D.A., Jackson M.J. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J. Physiol. 2003 Jun 1;549(Pt 2):645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Henríquez-Olguín C., Renani L.B., Arab-Ceschia L., Raun S.H., Bhatia A., Li Z., Knudsen J.R., Holmdahl R., Jensen T.E. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019 Jun;24:101188. doi: 10.1016/j.redox.2019.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Cumming K.T., Raastad T., Holden G., Bastani N.E., Schneeberger D., Paronetto M.P., Mercatelli N., Ostgaard H.N., Ugelstad I., Caporossi D., Blomhoff R., Paulsen G. Effects of vitamin C and E supplementation on endogenous antioxidant systems and heat shock proteins in response to endurance training. Phys. Rep. 2014 Oct 7;2(10) doi: 10.14814/phy2.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Draganidis D., Karagounis L.G., Athanailidis I., Chatzinikolaou A., Jamurtas A.Z., Fatouros I.G. Inflammaging and skeletal muscle: can protein intake make a difference? J. Nutr. 2016 Oct;146(10):1940–1952. doi: 10.3945/jn.116.230912. [DOI] [PubMed] [Google Scholar]
- 340.Ji L.L. Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic. Biol. Med. 2008 Jan 15;44(2):142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 341.Ho R.C., Hirshman M.F., Li Y., Cai D., Farmer J.R., Aschenbach W.G., Witczak C.A., Shoelson S.E., Goodyear L.J. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2005 Oct;289(4):C794–C801. doi: 10.1152/ajpcell.00632.2004. [DOI] [PubMed] [Google Scholar]
- 342.Ji L.L., Gomez-Cabrera M.C., Steinhafel N., Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. Faseb. J. 2004 Oct;18(13):1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 343.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T., Hayes J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88(Pt B):108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Done A.J., Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016 Dec;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bowtell J., Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019 Feb;49(Suppl 1):3–23. doi: 10.1007/s40279-018-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Elokda A.S., Nielsen D.H. Effects of exercise training on the glutathione antioxidant system. Eur. J. Cardiovasc. Prev. Rehabil. 2007 Oct;14(5):630–637. doi: 10.1097/HJR.0b013e32828622d7. [DOI] [PubMed] [Google Scholar]
- 347.Ferreira J.C., Bacurau A.V., Bueno C.R., Jr., Cunha T.C., Tanaka L.Y., Jardim M.A., Ramires P.R., Brum P.C. Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice. Exp. Biol. Med. 2010 Apr;235(4):497–505. doi: 10.1258/ebm.2009.009165. [DOI] [PubMed] [Google Scholar]
- 348.Pittaluga M., Sgadari A., Dimauro I., Tavazzi B., Parisi P., Caporossi D. Physical exercise and redox balance in type 2 diabetics: effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxid. Med. Cell Longev. 2015;2015:981242. doi: 10.1155/2015/981242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. 2013 May;1830(5):3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 350.May J.M., Qu Z.C., Neel D.R., Li X. Recycling of vitamin C from its oxidized forms by human endothelial cells. Biochim. Biophys. Acta. 2003 May 12;1640(2–3):153–161. doi: 10.1016/s0167-4889(03)00043-0. [DOI] [PubMed] [Google Scholar]
- 351.Margaritelis N.V., Theodorou A.A., Paschalis V., Veskoukis A.S., Dipla K., Zafeiridis A., Panayiotou G., Vrabas I.S., Kyparos A., Nikolaidis M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability. Acta Physiol. 2018 Feb;222(2) doi: 10.1111/apha.12898. [DOI] [PubMed] [Google Scholar]
- 352.Brooks S.V., Vasilaki A., Larkin L.M., McArdle A., Jackson M.J. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J. Physiol. 2008 Aug 15;586(16):3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Radák Z., Sasvári M., Nyakas C., Pucsok J., Nakamoto H., Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch. Biochem. Biophys. 2000 Apr 15;376(2):248–251. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- 354.Davies K.J.A. Cardiovascular adaptive homeostasis in exercise. Front. Physiol. 2018 May 1;9:369. doi: 10.3389/fphys.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Radak Z., Ishihara K., Tekus E., Varga C., Posa A., Balogh L., Boldogh I., Koltai E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017 Aug;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Brigelius-Flohé R., Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants Redox Signal. 2011 Oct 15;5(8):2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Dizdaroglu M. Oxidatively induced DNA damage: mechanisms, repair and disease. Canc. Lett. 2012 Dec 31;327(1–2):26–47. doi: 10.1016/j.canlet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 358.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009 Sep 1;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 359.Pikosky M.A., Gaine P.C., Martin W.F., Grabarz K.C., Ferrando A.A., Wolfe R.R., Rodriguez N.R. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J. Nutr. 2006 Feb;136(2):379–383. doi: 10.1093/jn/136.2.379. [DOI] [PubMed] [Google Scholar]
- 360.Radák Z., Sasvári M., Nyakas C., Taylor A.W., Ohno H., Nakamoto H., Goto S. Regular training modulates the accumulation of reactive carbonyl derivatives in mitochondrial and cytosolic fractions of rat skeletal muscle. Arch. Biochem. Biophys. 2000 Nov 1;383(1):114–118. doi: 10.1006/abbi.2000.2042. [DOI] [PubMed] [Google Scholar]
- 361.Sitte N., Merker K., Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998 Dec 4;440(3):399–402. doi: 10.1016/s0014-5793(98)01495-1. [DOI] [PubMed] [Google Scholar]
- 362.Grune T., Davies K.J. The proteasomal system and HNE-modified proteins. Mol. Aspect. Med. 2003 Aug-Oct;24(4–5):195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 363.Shringarpure R., Grune T., Mehlhase J., Davies K.J. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J. Biol. Chem. 2003 Jan 3;278(1):311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 364.Nakamoto H., Kaneko T., Tahara S., Hayashi E., Naito H., Radak Z., Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp. Gerontol. 2007 Apr;42(4):287–295. doi: 10.1016/j.exger.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 365.Radak Z., Bori Z., Koltai E., Fatouros I.G., Jamurtas A.Z., Douroudos, Terzis G., Nikolaidis M.G., Chatzinikolaou A., Sovatzidis A., Kumagai S., Naito H., Boldogh I. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic. Biol. Med. 2011 Jul 15;51(2):417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bravard A., Vacher M., Gouget B., Coutant A., de Boisferon F.H., Marsin S., Chevillard S., Radicella J.P. Redox regulation of human OGG1 activity in response to cellular oxidative stress. Mol. Cell Biol. 2006 Oct;26(20):7430–7436. doi: 10.1128/MCB.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Davison G.W. Exercise and oxidative damage in nucleoid DNA quantified using single cell gel electrophoresis: present and future application. Front. Physiol. 2016 Jun 22;7:249. doi: 10.3389/fphys.2016.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Li T.S., Marbán E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cell. 2010 Jul;28(7):1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Tryfidou D.V., McClean C., Nikolaidis M.G., Davison G.W. DNA damage following acute aerobic exercise: a systematic review and meta-analysis. Sports Med. 2020;50(1):103–127. doi: 10.1007/s40279-019-01181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Cobley J.N., Margaritelis N.V., Morton J.P., Close G.L., Nikolaidis M.G., Malone J.K. The basic chemistry of exercise-induced DNA oxidation: oxidative damage, redox signaling, and their interplay. Front. Physiol. 2015 Jun 17;6:182. doi: 10.3389/fphys.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Margaritelis N.V., Cobley J.N., Paschalis V., Veskoukis A.S., Theodorou A.A., Kyparos A., Nikolaidis M.G. Going retro: oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016 Sep;98:2–12. doi: 10.1016/j.freeradbiomed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 372.Vargas-Mendoza N., Morales-González Á, Madrigal-Santillán E.O., Madrigal-Bujaidar E., Álvarez-González I., García-Melo L.F., Anguiano-Robledo L., Fregoso-Aguilar T., Morales-Gonzalez J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants (Basel) 2019 Jun 25;8(6):E196. doi: 10.3390/antiox8060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012 Jan 1;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Dikalov S.I., Harrison D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxidants Redox Signal. 2014 Jan 10;20(2):372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Meyer A.J., Dick T.P. Fluorescent protein-based redox probes. Antioxidants Redox Signal. 2010 Sep 1;13(5):621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 376.Pouvreau S. Genetically encoded reactive oxygen species (ROS) and redox indicators. Biotechnol. J. 2014 Feb;9(2):282–293. doi: 10.1002/biot.201300199. [DOI] [PubMed] [Google Scholar]
- 377.Winterbourn C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta. 2014 Feb;1840(2):730–738. doi: 10.1016/j.bbagen.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 378.Zhang X., Gao F. Imaging mitochondrial reactive oxygen species with fluorescent probes: current applications and challenges. Free Radic. Res. 2015 Apr;49(4):374–382. doi: 10.3109/10715762.2015.1014813. [DOI] [PubMed] [Google Scholar]
- 379.Nikolaidis M.G., Margaritelis N.V. Same redox evidence but different physiological "stories": the rashomon effect in biology. Bioessays. 2018 Sep;40(9) doi: 10.1002/bies.201800041. [DOI] [PubMed] [Google Scholar]
- 380.Azzi A., Meydani S.N., Meydani M., Zingg J.M. The rise, the fall and the renaissance of vitamin E. Arch. Biochem. Biophys. 2016 Apr 1;595:100–108. doi: 10.1016/j.abb.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 381.Cobley J.N., McHardy H., Morton J.P., Nikolaidis M.G., Close G.L. Influence of vitamin C and vitamin E on redox signaling: implications for exercise adaptations. Free Radic. Biol. Med. 2015 Jul;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 382.Heumüller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schröder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008 Feb;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 383.Halliwell B. The antioxidant paradox. Lancet. 2000 Apr 1;355(9210):1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 384.Paschalis V., Theodorou A.A., Kyparos A., Dipla K., Zafeiridis A., Panayiotou G., Vrabas I.S., Nikolaidis M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur. J. Nutr. 2016 Feb;55(1):45–53. doi: 10.1007/s00394-014-0821-x. [DOI] [PubMed] [Google Scholar]
- 385.Paschalis V., Theodorou A.A., Margaritelis N.V., Kyparos A., Nikolaidis M.G. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 2018 Feb 1;115:288–297. doi: 10.1016/j.freeradbiomed.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 386.Ives S.J., Harris R.A., Witman M.A., Fjeldstad A.S., Garten R.S., McDaniel J., Wray D.W., Richardson R.S. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension. 2014 Mar;63(3):459–467. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Margaritelis N.V., Paschalis V., Theodorou A.A., Vassiliou V., Kyparos A., Nikolaidis M.G. Rapid decreases of key antioxidant molecules in critically ill patients: a personalized approach. Clin. Nutr. 2019 Apr 29;S0261–5614(19):30205. doi: 10.1016/j.clnu.2019.04.029. 5. [DOI] [PubMed] [Google Scholar]
- 388.Ting H.H., Timimi F.K., Boles K.S., Creager S.J., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1996 Jan 1;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Theodorou A.A., Paschalis V., Kyparos A., Panayiotou G., Nikolaidis M.G. Passive smoking reduces and vitamin C increases exercise-induced oxidative stress: does this make passive smoking an anti-oxidant and vitamin C a pro-oxidant stimulus? Biochem. Biophys. Res. Commun. 2014 Nov 7;454(1):131–136. doi: 10.1016/j.bbrc.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 390.Close G.L., Jackson M.J. Antioxidants and exercise: a tale of the complexities of relating signalling processes to physiological function? J. Physiol. 2014 Apr 15;592(8):1721–1722. doi: 10.1113/jphysiol.2014.272294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Impey S.G., Hearris M.A., Hammond K.M., Bartlett J.D., Louis J., Close G.L., Morton J.P. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018 May;48(5):1031–1048. doi: 10.1007/s40279-018-0867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Dalziel A.C., Rogers S.M., Schulte P.M. Linking genotypes to phenotypes and fitness: how mechanistic biology can inform molecular ecology. Mol. Ecol. 2009;18(24):4997–5017. doi: 10.1111/j.1365-294X.2009.04427.x. [DOI] [PubMed] [Google Scholar]
- 393.Pigliucci M. From molecules to phenotypes? The promise and limits of integrative biology. Basic Appl. Ecol. 2003;4:297–306. [Google Scholar]
- 394.van der Vliet A., Dustin C.M., Heppner D.E. Redox regulation of protein kinase signalling. In: Sies H., editor. Oxidative Stress: Eustress and Distress. Academic Press; Cambridge, Massachusetts: 2020. pp. 287–313. [Google Scholar]
- 395.Radak Z., Suzuki K., Higuchi M., Balogh L., Boldogh I., Koltai E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016 Sep;98:187–196. doi: 10.1016/j.freeradbiomed.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 396.Chatterjee S., Fisher A.B. Mechanotransduction: forces, sensors, and redox signaling. Antioxidants Redox Signal. 2014 Feb 20;20(6):868–871. doi: 10.1089/ars.2013.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Rodney G.G., Pal R., Abo-Zahrah R. Redox regulation of autophagy in skeletal muscle. Free Radic. Biol. Med. 2016 Sep;98:103–112. doi: 10.1016/j.freeradbiomed.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Merry T.L., MacRae C., Pham T., Hedges C.P., Ristow M. Deficiency in ROS-sensing nuclear factor erythroid 2-like 2 causes altered glucose and lipid homeostasis following exercise training. Am. J. Physiol. Cell Physiol. 2020 Feb 1;318(2):C337–C345. doi: 10.1152/ajpcell.00426.2019. [DOI] [PubMed] [Google Scholar]
- 399.Parker L., Shaw C.S., Stepto N.K., Levinger I. Exercise and glycemic control: focus on redox homeostasis and redox-sensitive protein signaling. Front. Endocrinol. 2017 May 5;8:87. doi: 10.3389/fendo.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Dimauro I., Mercatelli N., Caporossi D. Exercise-induced ROS in heat shock proteins response. Free Radic. Biol. Med. 2016 Sep;98:46–55. doi: 10.1016/j.freeradbiomed.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 401.Vasilaki A., Richardson A., Van Remmen H., Brooks S.V., Larkin L., McArdle A., Jackson M.J. Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J. Physiol. 2017 Oct 15;595(20):6409–6415. doi: 10.1113/JP274336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Cobley J.N. How exercise induces oxidative eustress. In: Sies H., editor. Oxidative Stress: Eustress and Distress. Academic Press; Cambridge, Massachusetts: 2020. pp. 447–462. [Google Scholar]
- 403.Drake J.C., Wilson R.J., Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. Faseb. J. 2016 Jan;30(1):13–22. doi: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Mészáros L.G., Minarovic I., Zahradnikova A. Inhibition of the skeletal muscle ryanodine receptor calcium release channel by nitric oxide. FEBS Lett. 1996 Feb 12;380(1–2):49–52. doi: 10.1016/0014-5793(96)00003-8. [DOI] [PubMed] [Google Scholar]
- 406.Piantadosi C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta. 2012 Jun;1820(6):712–721. doi: 10.1016/j.bbagen.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Polytarchou C., Papadimitriou E. Antioxidants inhibit human endothelial cell functions through down-regulation of endothelial nitric oxide synthase activity. Eur. J. Pharmacol. 2005 Mar 7;510(1–2):31–38. doi: 10.1016/j.ejphar.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 408.Prior B.M., Yang H.T., Terjung R.L. What makes vessels grow with exercise training? J. Appl. Physiol. 2004 Sep;97(3):1119–1128. doi: 10.1152/japplphysiol.00035.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 409.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol. Sci. 2011 Feb;32(2):82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 410.Kim J.S., Saengsirisuwan V., Sloniger J.A., Teachey M.K., Henriksen E.J. Oxidant stress and skeletal muscle glucose transport: roles of insulin signaling and p38 MAPK. Free Radic. Biol. Med. 2006 Sep 1;41(5):818–824. doi: 10.1016/j.freeradbiomed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 411.Kim Y.M., Kim K.E., Koh G.Y., Ho Y.S., Lee K.J. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Canc. Res. 2006 Jun 15;66(12):6167–6174. doi: 10.1158/0008-5472.CAN-05-3640. [DOI] [PubMed] [Google Scholar]
- 412.Copp The effects of antioxidants on microvascular oxygenation and blood flow in skeletal muscle of young rats. Exp. Physiol. 2009;94(9):961–971. doi: 10.1113/expphysiol.2009.048223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.