Abstract
The HIV-1 transgenic (Tg) rat model is valuable for understanding HIV-associated neurocognitive disorders (HAND) and accompanying substance use and misuse. Tg and F344/NHsd wildtype (WT) rats were allowed to self-administer intrajugular cocaine. For the first seven sessions, neither genotype self-administered cocaine (0.1 mg/kg/infusion) on a fixed ratio 1 schedule. We thus implemented a lever–cocaine “autoshaping” session followed by a series of manipulations changing dose and reinforcement schedule. Tg rats self-administered much less cocaine than WT rats throughout the study. Five of eight Tg rats modestly increased self-administration from sessions 36–50. Of those, only three showed a lever discrimination. Eight of ten WT rats acquired robust self-administration by session 19; all WT rats self-administered cocaine by the end of the study. WT and Tg rats had similar baseline locomotor activity in the self-administration chamber suggesting that the low levels of cocaine intake in the Tg rats did not reflect a non-specific motor impairment in this rat strain. Concomitant measurement of activity with self-administration revealed activity increases that followed increased cocaine intake. That relation held in Tg rats. Therefore, the present study provides evidence that HIV-1 Tg rats are less sensitive to the reinforcing effects of cocaine than their F344 WT counterparts.
Keywords: HIV, transgenic rat, cocaine, self-administration
Substance abuse can be comorbid in individuals infected with human immunodeficiency virus (HIV-1), an infection that activates neuroinflammation and results in significant cognitive decline known as HIV-associated neurological disorders (HAND). People with HIV-1 infection use all classes of abused drugs at a higher rate than the general population. For cocaine, this elevated use is estimated at 3 to 6-fold more than uninfected individuals (Chang et al., 2014). Cocaine shows a particular bidirectional interaction with HIV-1 viral proteins and may act as a co-factor to influence HAND susceptibility and progression by upregulating HIV-1 expression in immune cells (Buch et al., 2011; Kim et al., 2013; Peterson et al., 1992). Notably, HIV-1 infection interacts with the neurocircuitry involved in the presumed reinforcing effects of drugs of abuse (Buch et al., 2011; Midde, Gomez, Harrod, & Zhu, 2011; Nath et al., 2002) in a manner that may alter drug sensitivity and abuse liability. For instance, administration of the HIV-1 viral protein Tat allosterically modulates dopamine transporter function (Zhu et al., 2011), but only produced an elevated basal dopaminergic tone in rats co-exposed to cocaine (Ferris et al., 2010). Interestingly, this elevated dopaminergic tone was not seen in rats treated with only cocaine or Tat alone, demonstrating the unique synergistic neurochemical action between cocaine and Tat (Ferris et al., 2010). Further, cocaine administration also potentiates the neurotoxicity wrought by the HIV-1 viral protein gp120, thereby aggravating HAND (Bagetta et al., 2004).
The development of the HIV-1 transgenic (Tg) rat, which expresses a non-infectious HIV-1 provirus (pEVd1143) and therefore produces similar neurological impairments, has been a valuable experimental tool for examining neural immune deficiencies (Chang et al., 2014; McLaurin et al., 2018; Reid et al., 2016; Reid et al., 2001). For these rats, the provirus contained the HIV-1 genome with a functional gag-pol-deletion, a truncation that prevents viral replication while maintaining viral protein expression (Vigorito et al., 2015). When mated with a WT Fisher/NHsd (F344) rat, the female transgene founder, which is hemizygous for the HIV-1 transgene, produce progeny that are either transgenic (Tg) or wildtype (WT) (McLaurin et al., 2018; Reid et al., 2001; Vigorito et al., 2015). In addition to its obvious utility for assessing the in vivo effects of toxic viral proteins (e.g., Tat and gp120), this transgenic organism also allows for further analysis of neurochemical and behavioral substrates of abused drugs such as cocaine.
The current published literature suggests that HIV-1 protein-induced neuroadaptations may potentially modulate the reinforcing effects of cocaine (Buch et al., 2012, 2011; Ferris et al., 2010; Paris et al., 2014). However, the findings are inconsistent from the few studies that have assessed the HIV-1 Tg rats in an operant cocaine self-administration task. In one such study by Wayman et al. (2016), Tg and WT rats had similar intake of cocaine at 0.1 mg/kg/infusion when analyzing the average number of infusions summed across the initial 14 sessions. In contrast, McIntosh, Sexton, Pattison, Childers, and Hemby (2015) reported that Tg rats required half the dose of WT rats to acquire stable cocaine self-administration – 0.083 mg/infusion versus 0.1666 mg/infusion with no statistical differences in the number of days to meet criterion. In a third report, Bertrand et al. (2018) used Tg and WT rats with an extensive history of lever pressing maintained by sucrose. In the five-day transition to cocaine self-administration (0.33 mg/kg/infusion) on a fixed ratio (FR) 1 schedule of reinforcement, Tg rats had substantially lower responding than their WT counterparts. This difference between strains was maintained when the schedule of reinforcement was switched to a progressive ratio and the cocaine dose was increased to 1 mg/kg/infusion.
To summarize, the current state of the cocaine self-administration literature with the HIV-1 Tg rats is that they are more (McIntosh et al., 2015), less (Bertrand et al., 2018), or similarly sensitive (Wayman et al., 2016) to the reinforcing effects of cocaine. In the present study, we initially sought to expand on this sparse literature by establishing cocaine self-administration without any initial lever press training and then investigate extinction and reinstatement. In doing so, we hypothesized that differences in persistence of responding in extinction and/or the extent that a priming dose of cocaine reinstated active (drug) lever pressing may help disambiguate these previous published reports comparing HIV-1 Tg and WT rats. However, we found little early self-administration behavior and then, as the study progressed, wide variation in cocaine self-administration between Tg and WT rats, as well as amongst the rats within each genotype. Therefore, our research goal shifted to examining factors that would encourage self-administration, especially in the Tg rats. Detailed acquisition data related to group or individual performance were not provided by the two studies that did not initially train responding with sucrose (i.e., McIntosh et al., 2015; Wayman et al., 2016). Accordingly, in the present paper we offer a full description of the acquisition of cocaine self-administration and associated manipulations in Tg and WT rats under the parameters we chose based on the available literature (see later).
Materials and Methods
Rats
Ten male F344/NHsd and ten male HIV-1 transgenic rats arrived from Envigo (Indianapolis, IN, USA) at 5 weeks of age. They were housed individually in clear polycarbonate tubs lined with TEK-Fresh bedding in a temperature- and humidity-controlled colony. Intravenous (IV) surgery was conducted in all rats at 9 weeks of age. All sessions were conducted during the light portion of a 12 h light:dark cycle (lights on at 6 AM). Figure 1 shows the individual body weights across the experiment. Rats were fed immediately following daily sessions a sufficient amount of chow to maintain them at no less than 95% of presurgical free-feeding body weights. In line with previous reports, Tg rats weighed less than WT rats (McLaurin et al., 2018; Moran, Booze, Webb, & Mactutus, 2013; Peng et al., 2010). This difference highlights the import of infusing a drug based on a rat’s body weight (see Drug section). Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Figure 1.
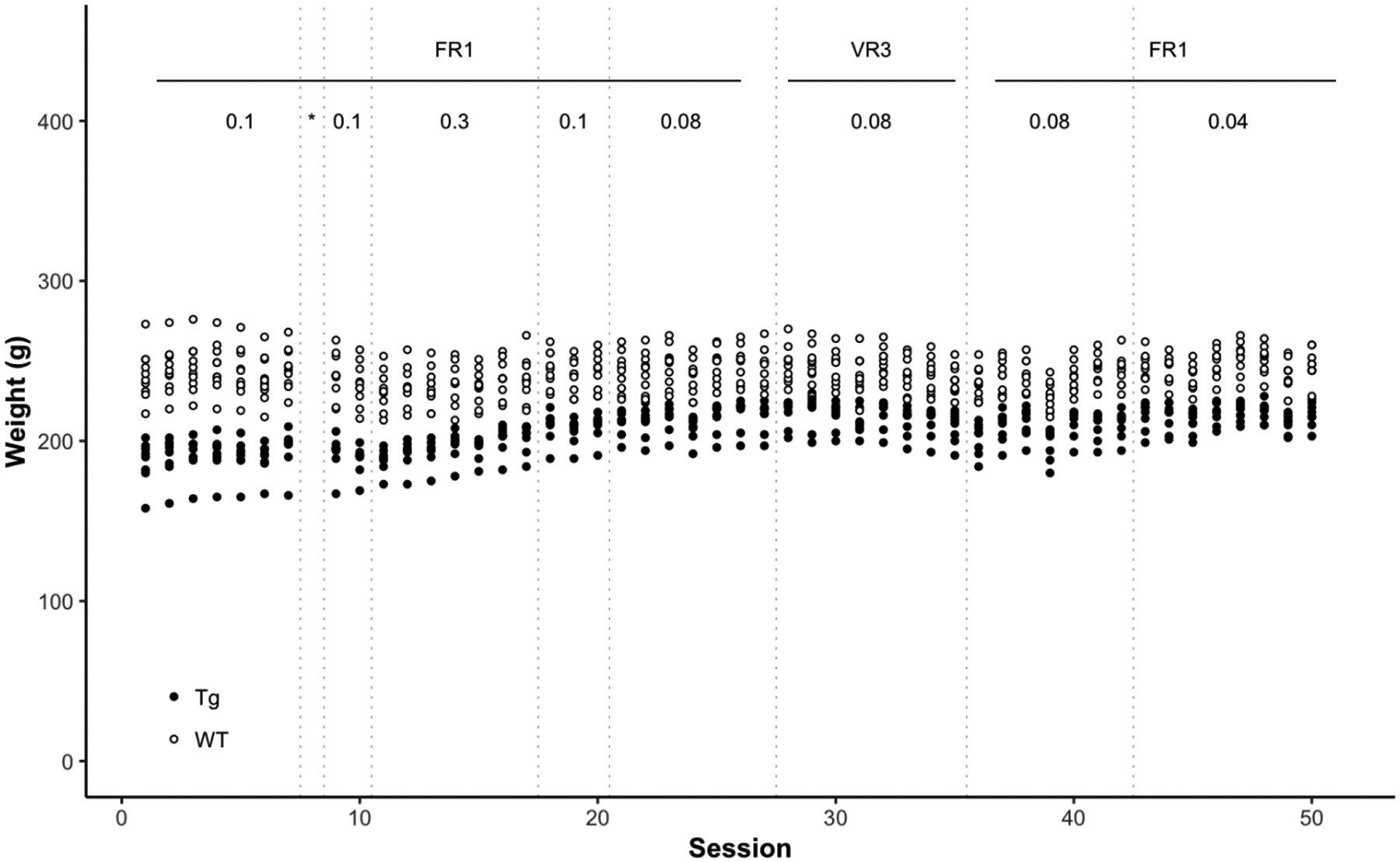
The daily weight in grams of each WT rat (empty symbol) and each Tg rat (filled symbols) across all stages of the experiment (see Figure 2 for a graphic of the timeline of the experiment). All cocaine doses are in units of mg/kg = milligrams per kilogram per intravenous infusion. * indicates the autoshaping session, FR = fixed ratio, VR = variable ratio, WT = wildtype, Tg = transgenic.
Apparatus
Ten standard Med-Associates® self-administration chambers (Georgia, VT, USA) were used. Each chamber was enclosed in a sound- and light-attenuating cubicle fitted with a house light to provide general illumination and a ventilation fan. Chambers (30.5 cm × 24.1 cm × 21 cm; l × w × h) had aluminum sidewalls and clear polycarbonate ceiling, front, and back walls. Retractable levers were located on both sides of the right aluminum wall. Each lever required 0.25 N to close the circuit and record a lever press. A white cue light (2.54-cm diameter; 28 V, 100 mA) was centered 7 cm above each lever, 14.6 cm above the rod floor, and 3.5 cm from the nearest polycarbonate wall. An infrared emitter/detector unit bisected the chamber 14.5 cm from the sidewall containing the levers and was positioned 4 cm above the rod floor. This unit provided a measure of chamber activity that has been shown to be sensitive to the locomotor stimulant and depressant effects of drugs (e.g., Barrett et al., 2017; Murray et al., 2007). The outside of each chamber was fitted with a balanced metal arm and swivel. An attached spring leash hung into the center of the ceiling chamber. Tygon® tubing extended through the leash and was connected to a 20-ml syringe mounted on a precision-delivery infusion pump (Med Associates, PHM-111-EC) located outside of the sound-attenuating cubicle.
Drug
Cocaine hydrochloride was generously provided by the NIDA Drug Supply Program (RTI, Durham, NC, USA). We dissolved cocaine in 0.9% sterile saline (w/v) and infused it intravenously (IV) at a rate of 0.04 mL/second based on each rat’s weight measured <2 h before its self-administration session.
Surgery
All rats underwent jugular catheter surgery using our established protocol (Charntikov et al., 2013; Pittenger et al., 2018). Rats were anaesthetized with a 1 ml/kg intramuscular injection of a ketamine hydrochloride (100 mg/mL): xylazine hydrochloride (20 mg/mL) cocktail (Midwestern Veterinary Supply, Des Moines, IA, USA). Silastic® tubing (SAI Infusion Technologies, Lake Villa, IL, USA) of the catheter constructed in-house was implanted into the right external jugular vein. The tubing was threaded subcutaneously over the shoulder and connected to a metal cannula fitted within a polycarbonate back plate (Plastics One, Roanoke, VA, USA) implanted under the skin just below the scapula. Buprenorphine hydrochloride (0.1 mg/kg; Sigma, St. Louis, MO, USA) was injected subcutaneously immediately following surgery and once per day for two more days for pain management. Catheters were flushed daily with 0.2 mL of sterile saline mixed with heparin (30 U/mL; Midwest Veterinary Supply) to decrease clotting and Baytril® (5 mg/mL; Midwest Veterinary Supply) as a prophylactic against bacterial infection. Two of the Tg rats did not recover from anesthesia. The remaining rats were allowed 7 days of recovery before beginning the study. At the end of the experiment, catheter patency was confirmed with a 0.05-ml infusion of xylazine (20 mg/ml); rats with patent catheters displayed motor ataxia within 10 sec of the IV infusion. All rats had patent catheters at the end of the study – 8 Tg and 10 WT rats.
Behavioral Procedures
The timeline of behavioral procedures is illustrated in Figure 2. Sessions were conducted once per day. At the start of each 1-h session [training session length based on (Bertrand et al., 2018)], the house light was illuminated and both levers were available. Lever assignment of ‘active’ and ‘inactive’ was counterbalanced across rats and strain. Cocaine [0.1 mg/kg/infusion; dose chosen based on (Wayman et al., 2016) using the same training dose in the HIV-1 Tg rat model] was available under a continuous reinforcement schedule (fixed ratio 1; FR1) in which each active lever press was immediately followed by the cocaine infusion and initiated a 20-sec time out; inactive lever presses had no programmed consequence. During the time out, both levers were retracted, the house light was extinguished, and the cue light above the active lever location was illuminated. Rats received 7 consecutive daily sessions with the FR1 schedule at the 0.1 mg/kg/infusion dose. However, reliable cocaine self-administration did not emerge as expected (see Results). We then implemented several manipulations designed to encourage self-administration.
Figure 2.

Timeline of the reinforcement schedules and doses of cocaine used across the 50 sessions of self-administration. d = day, FR = fixed ratio, VR = variable ratio, mg/kg = milligrams of drug per kilogram body weight per intravenous infusion.
Session 8 was “auto-shaping” training. Upon the start of the session, the chamber house lights were illuminated, and the corresponding active lever assigned previously was inserted into the chamber for a maximum of 15 sec. After 15 sec, the lever retracted and a 0.1 mg/kg cocaine infusion (i.e., non-contingent) was delivered. If the lever was pressed within 15 sec, the lever was immediately retracted, and a cocaine infusion was delivered; the drug infusion delivery was not signaled by cue light illumination. The active lever was inserted 15 times in the session on a variable time (VT) 120-sec schedule.
Following the autoshaping session, rats received two sessions of cocaine (0.1 mg/kg/infusion) self-administration on an FR1 as described earlier. For sessions 11 to 17, we increased the dose of cocaine to 0.3 mg/kg/infusion [dose chosen based on initial training dose in (Bertrand et al., 2018; J. Zhu, Bertrand, Harrod, Mactutus, & Booze, 2015) using the HIV-1 Tg rat model]. Because that dose also failed to engender reliable self-administration, the cocaine dose in sessions 18 to 20 was returned to 0.1 mg/kg/infusion then subsequently decreased to 0.08 mg/kg/infusion during sessions 21 to 27. The 0.08 mg/kg/infusion dose was chosen based on McIntosh et al. (2015) finding that the highest self-administration in the dose effect curve for HIV-1 Tg rats was approximately ~0.06 mg/kg/infusion and ~0.13 for F344 rats. Therefore, this dose was selected as the mid-point between maximal responding on dose-effect curves of self-administration in Tg and F344 rats. We then shifted the schedule of reinforcement to a variable ratio 3 (VR3) as we have found better active versus inactive lever discrimination and increased drug intake (i.e., nicotine and methamphetamine) in some earlier self-administration studies (unpublished data). Rats remained on this schedule with 0.08 mg/kg/infusion of cocaine for sessions 28 to 35 and switched back to the FR1 schedule for sessions 36 to 42. Lastly, the dose of cocaine was shifted to 0.04 mg/kg/infusion for sessions 43 to 50; this dose was chosen to drive up response rates for self-titration.
Results
The number of cocaine infusions for the entire study is shown in Figure 3 for each WT rat and in Figure 4 for each Tg rat. Similarly, the number of active and inactive lever presses is displayed for WT (Figure 5) and Tg (Figure 6) rats. Overall, F344 WT rats more readily self-administered cocaine than their HIV-1 transgenic counterparts. More specifically, cocaine intake for all Tg rats remained low or absent for most sessions. Five out of eight Tg rats (R2, R8, R12, R14, and R18) began to increase cocaine intake in the final two stages of the experiment where the schedule of reinforcement was at a FR1 and the cocaine dose was 0.08 mg/kg/infusion followed by 0.04 mg/kg/infusion (see Figure 4). Of these five Tg rats, only R12, R14, and R18 reliably showed a lever discrimination in which active lever presses were higher than inactive lever presses during these later stages (see Figure 6). Thus, at best, only three of the eight HIV-1 Tg rats (37.5%) displayed any self-administration behavior that indicated sensitivity to which lever was associated with the cocaine.
Figure 3.
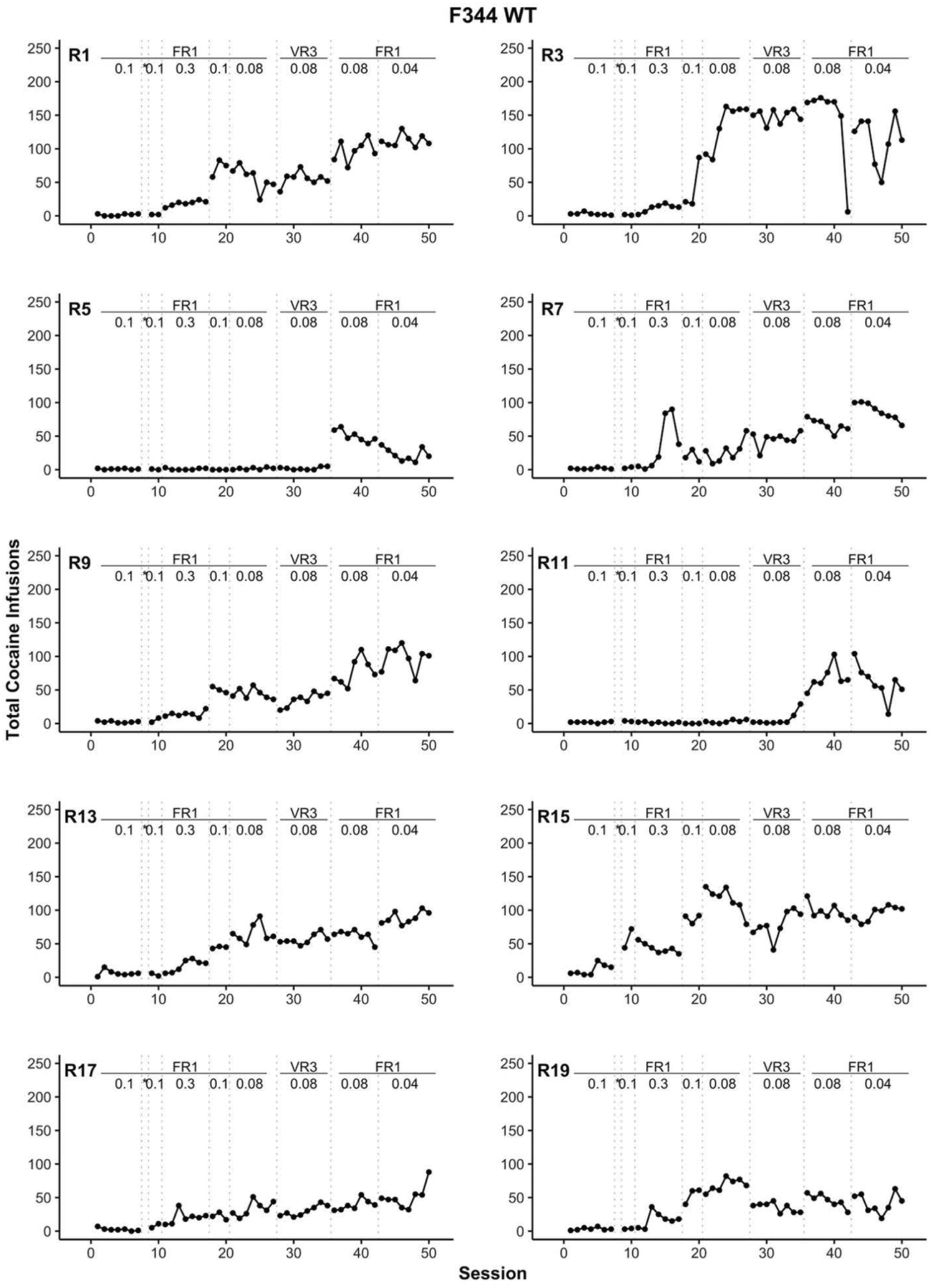
Individual rat (R) panels which display the total number of cocaine infusions across training for F344 WT rats. The transitions between each training stage are indicated with dotted vertical lines. The reinforcement schedule and cocaine dose for each training stage are positioned at the top of each panel. All cocaine doses are in units of mg/kg = milligrams per kilogram per intravenous infusion. * indicates the autoshaping session, FR = fixed ratio, VR = variable ratio, WT = wildtype.
Figure 4.
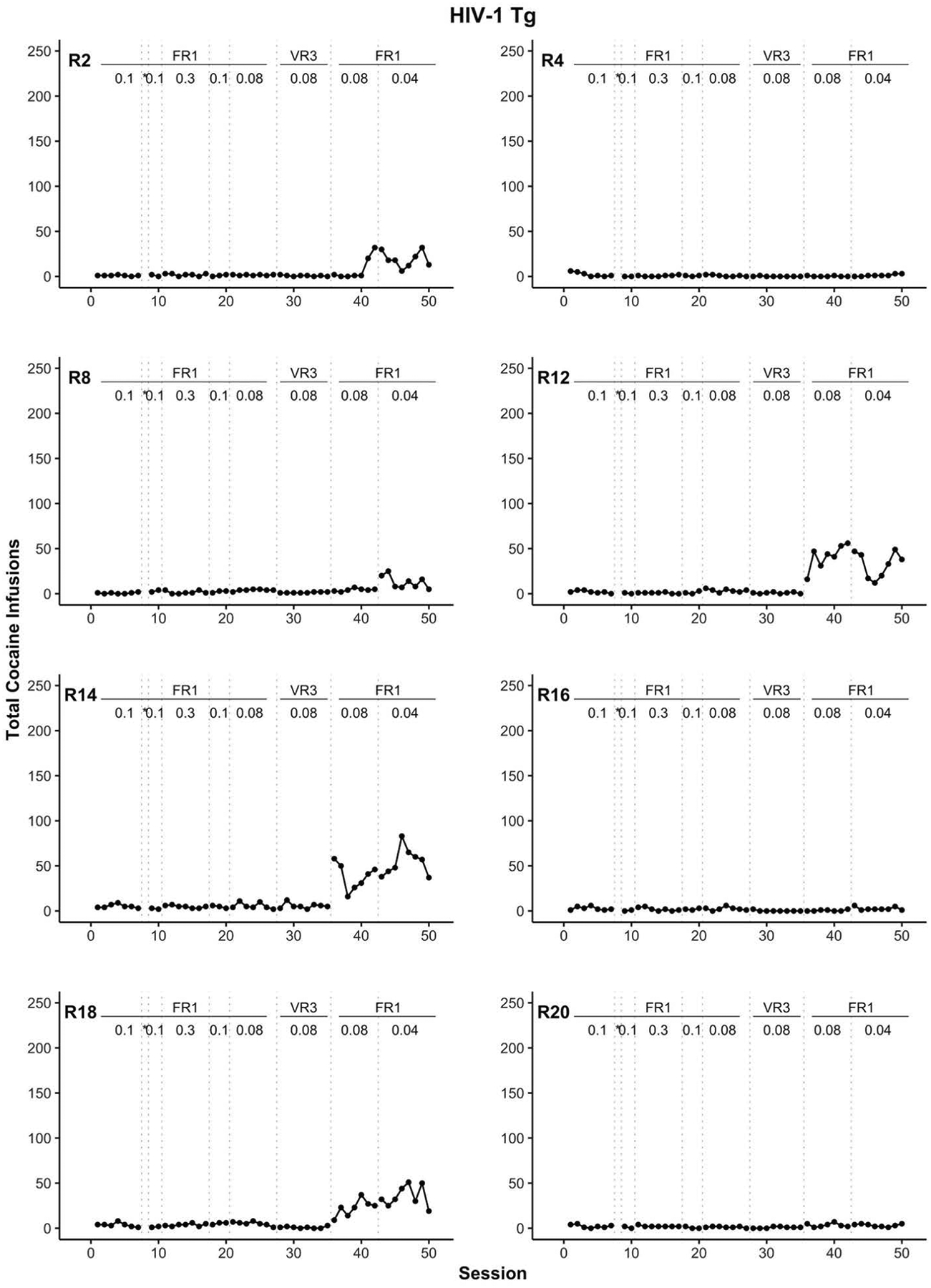
Individual rat (R) panels which display the total number of cocaine infusions across training for HIV-1 Tg rats. The transitions between each training stage are indicated with dotted vertical lines. The reinforcement schedule and cocaine dose for each training stage are positioned at the top of each panel. All cocaine doses are in units of mg/kg = milligrams per kilogram per intravenous infusion. * indicates the autoshaping session, FR = fixed ratio, VR = variable ratio, Tg = transgenic.
Figure 5.
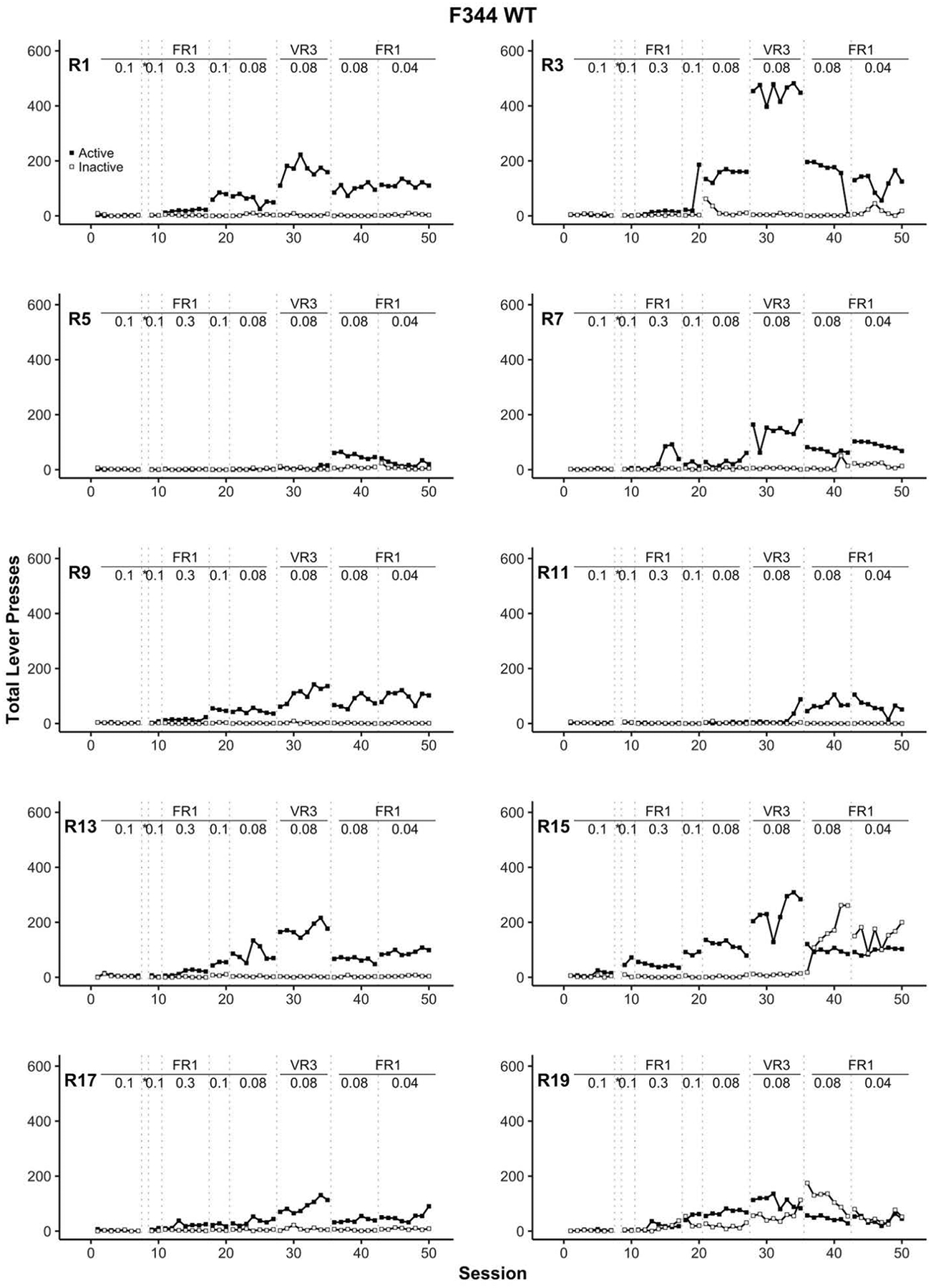
Individual rat (R) panels which display active (filled square) and inactive lever (open square) pressing across training for F344 WT rats. The transitions between each training stage are indicated with dotted vertical lines. The reinforcement schedule and cocaine dose for each training stage are positioned at the top of each panel. All cocaine doses are in units of mg/kg = milligrams per kilogram per intravenous infusion. * indicates the autoshaping session, FR = fixed ratio, VR = variable ratio, WT = wildtype.
Figure 6.
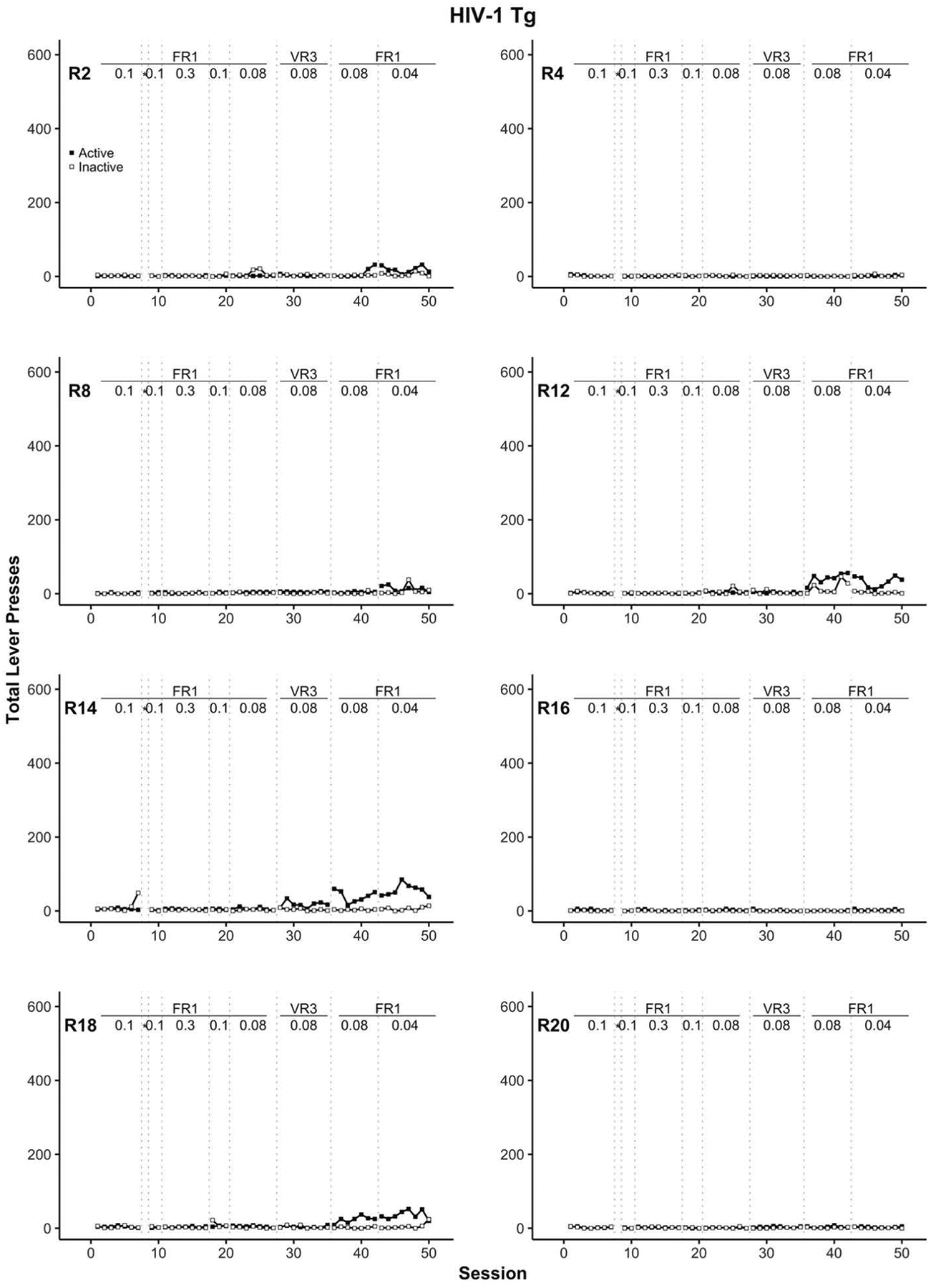
Individual rat (R) panels which display active (filled square) and inactive lever (open square) pressing across training for HIV-1 Tg rats. The transitions between each training stage are indicated with dotted vertical lines. The reinforcement schedule and cocaine dose for each training stage are positioned at the top of each panel. All cocaine doses are in units of mg/kg = milligrams per kilogram per intravenous infusion. * indicates the autoshaping session, FR = fixed ratio, VR = variable ratio, Tg = transgenic.
Comparatively, all WT rats eventually displayed more robust cocaine self-administration as demonstrated by the total number of infusions (Figure 3) and lever discrimination (Figure 5). None of the WT rats self-administered cocaine before the autoshaping session, suggesting that cocaine self-administration only emerged after additional training or procedural (i.e., dose and reinforcement schedule) manipulations. One rat (R15) began to self-administer cocaine the session immediately after the autoshaping session. Further, within nine days after the autoshaping session (i.e., the FR1 using 0.3 mg/kg/infusion of cocaine stage), seven additional WT rats began to self-administer cocaine. The remaining two WT rats, R5 and R11, eventually acquired cocaine self-administration; R11 during the VR3 stage and later R5 at the FR1 with 0.08 mg/kg/infusion stage. For WT rats, the discrimination between the active and inactive lever emerged with an increase in cocaine intake (Figure 5). For an unknown reason, inactive lever responding surpassed active lever responding for two WT rats (R15 and R19) during the last two stages of the study despite consistent lever discrimination in earlier sessions.
Sometimes genotypic differences between transgenic and wild-type animals induce non-specific behavioral effects that can alter basal activity levels. To assess this possibility, we examined the chamber photobeam breaks in the first seven sessions; before self-administration developed in either Tg or WT rats. Figure 7 shows the average number of beam breaks across the first seven sessions for each rat. Except for one highly active HIV-1 Tg rat, the baseline level of activity was quite similar in average and range. From this comparable baseline, it is notable that increased cocaine intake for Tg and WT rats was clearly reflected in an increase in general chamber activity. For example, the average number of beam breaks in the chamber across all 49 self-administration sessions was correlated with the average number of cocaine infusions [r(16) = 0.649, p = 0.0036]. We also conducted a separate Pearson product-moment correlation between activity and total cocaine infusions for each of the final three stages of this study separately for Tg and WT rats. We selected these stages as this is when most to all of the WT rats were self-administering cocaine and also when the five Tg rats began to increase their cocaine intake. In the top panel of Figure 8, we plotted the number of infusions in the last session of the VR3 stage as a function of the number of beam breaks for each rat along with the line of best fit (see Table 1 for descriptive statistics). There was a significant positive correlation between locomotor activity and cocaine infusions for this VR3 stage for Tg rats [r(6)=0.72, p=0.045] but not WT rats [r(8)=−0.0626, p=0.86]. The center panel of Figure 8 shows the penultimate FR1 stage (0.08 mg/kg/infusion cocaine) when a subset of Tg rats began to display some cocaine intake. Again, there was a significant positive correlation between activity and infusions for Tg rats [r(6)=0.96, p=0.00022] but not for WT rats [r(8)=0.60, p=0.066] on this last session of training. Note that the activity of three of these Tg rats (R12, R14, and R18) was now intermixed with that of the WT rats, indicating that Tg rats were sensitive to the locomotor stimulant effects of intravenous cocaine. For the final session of the FR1 stage with 0.04 mg/kg/infusion of cocaine (bottom panel of Figure 8), the positive correlation was not significant for Tg rats [r(6)=0.69, p = 0.059] but was significant for WT rats [r(8)=0.67, p=0.033].
Figure 7.
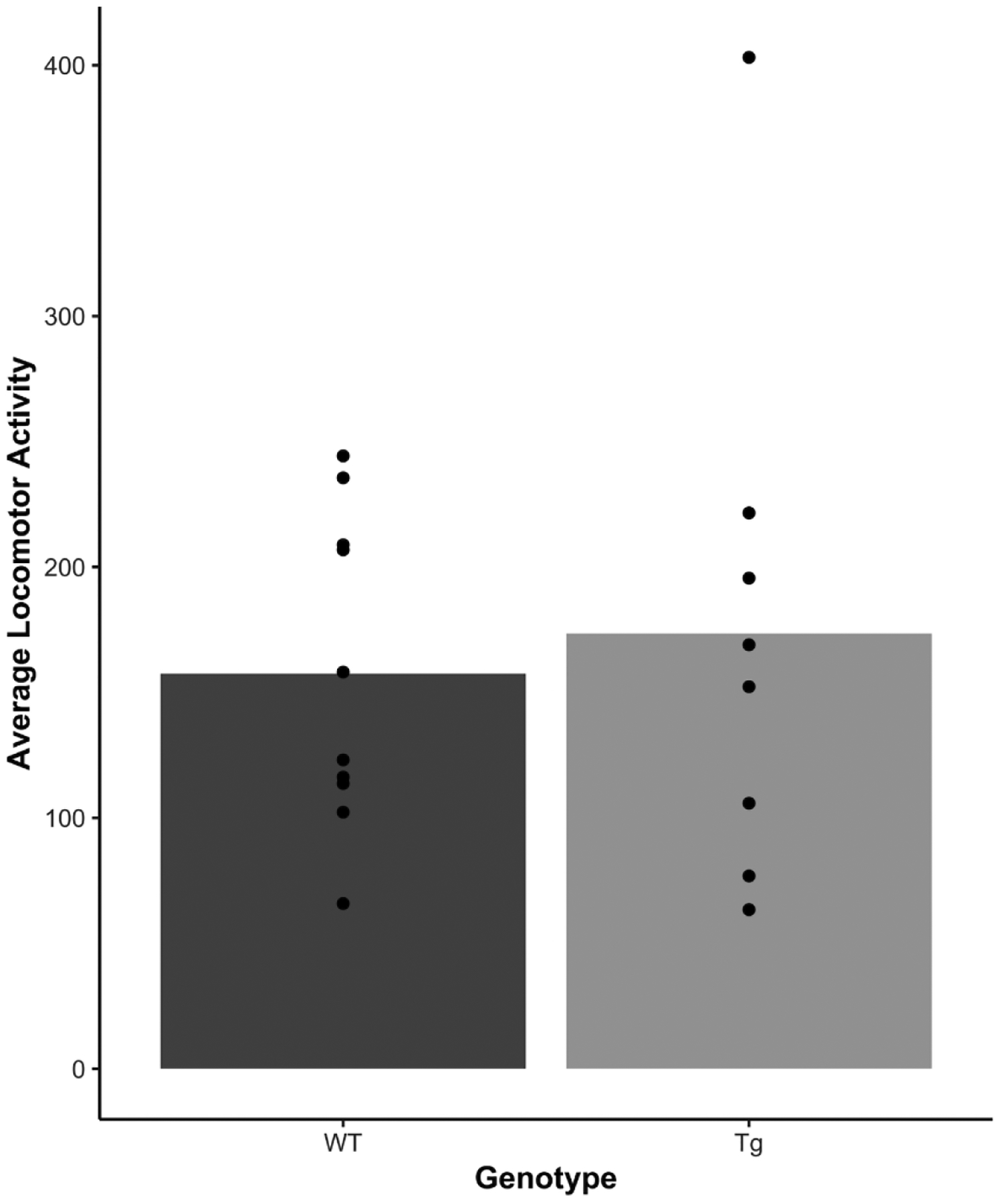
A measure of locomotor activity (i.e., infrared beam breaks) between the HIV-Tg (light grey) and F344 WT (dark grey) rats during the first stage of the study (fixed ratio 1, 0.1 mg/kg/infusion cocaine). The averaged locomotor activity for each subject is plotted as individual data points per the respective genotype (filled circles). WT = wildtype, Tg = transgenic.
Figure 8.
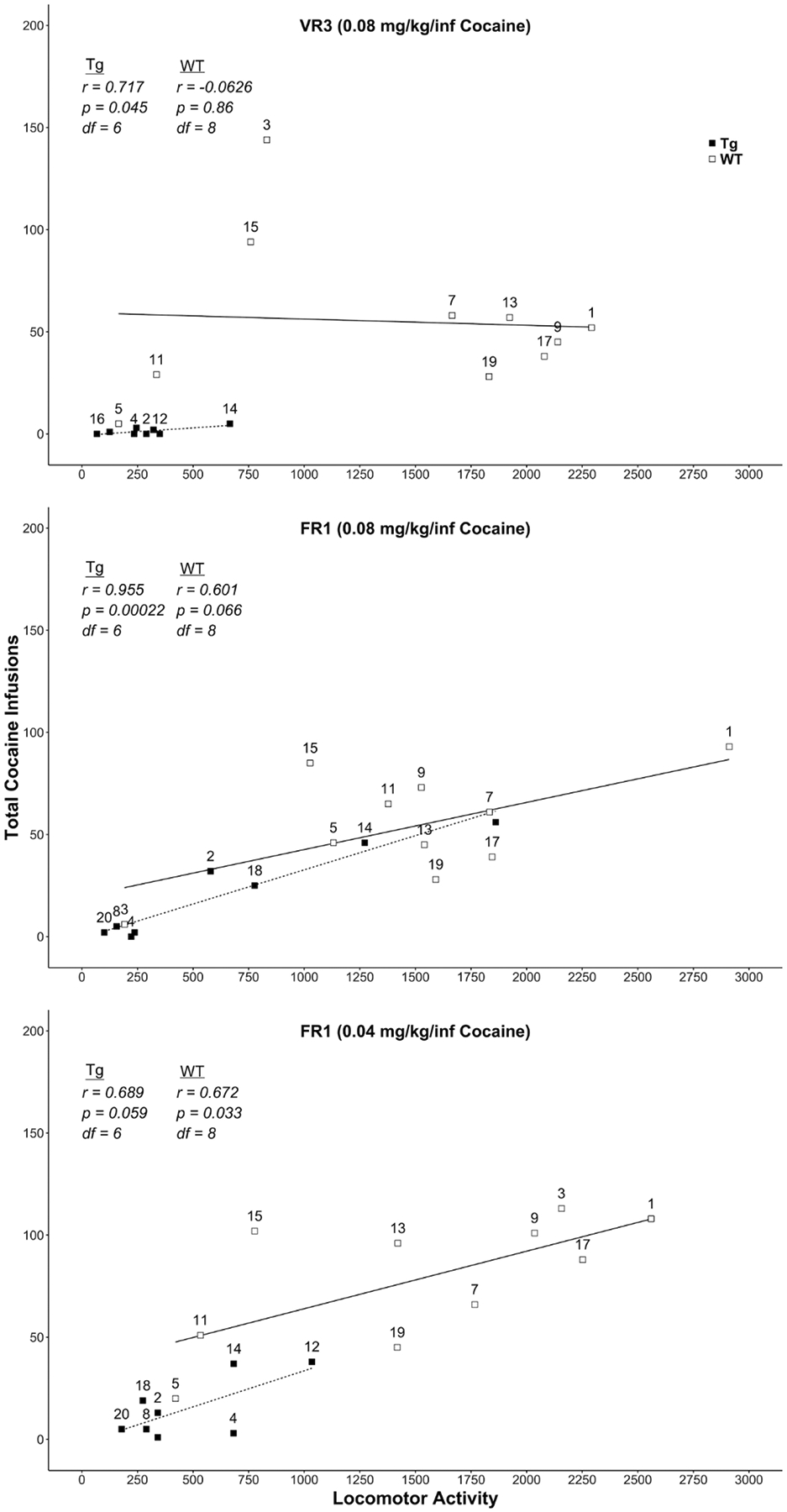
Scatterplot which depicts the relation between locomotor activity and cocaine infusions. Each panel depicts the final session of the last three training stages for HIV-1 Tg rats (filled symbol) and F344 WT rats (open symbol). Statistical results from Pearson’s correlations are displayed in each panel for both rat strains. Rat identification numbers are labeled above their respective data points where possible. The line of best fit for each training stage was plotted using a linear model. All cocaine doses are in units of mg/kg = milligram per kilogram per intravenous infusion. VR = variable ratio, FR = fixed ratio, WT = wildtype, Tg = transgenic.
Table 1.
Descriptive statistics for the cocaine infusions, locomotor activity (infrared beam breaks), active lever presses, and inactive lever presses for the final session during each of the three training stages and for each genotype (HIV-Tg and F344 WT). The values represent the mean and standard error of the mean (in parentheses). VR = variable ratio, FR = fixed ratio, WT = wildtype, Tg = transgenic, mg/kg = milligrams per kilogram per intravenous infusion.
| Descriptive Statistics (N=18) | ||
|---|---|---|
| HIV-1 Tg (n=8) | F344 WT (n=10) | |
| Infusions | ||
| VR3 (0.08 mg/kg) | 1.38 (0.65) | 55.0 (12.4) |
| FR1 (0.08 mg/kg) | 21.0 (7.80) | 54.1 (8.41) |
| FR1 (0.04 mg/kg) | 15.1 (5.30) | 79.0 (10.0) |
| Locomotor Activity | ||
| VR3 (0.08 mg/kg) | 288 (63.7) | 1402 (252) |
| FR1 (0.08 mg/kg) | 651 (223) | 1497 (219) |
| FR1 (0.04 mg/kg) | 478 (103) | 1534 (238) |
| Active Lever Presses | ||
| VR3 (0.08 mg/kg) | 5.13 (1.98) | 168 (38.4) |
| FR1 (0.08 mg/kg) | 21.6 (8.10) | 54.9 (8.52) |
| FR1 (0.04 mg/kg) | 15.44 (5.39) | 81.3 (10.6) |
| Inactive Lever Presses | ||
| VR3 (0.08 mg/kg) | 1.38 (0.38) | 15.2 (10.9) |
| FR1 (0.08 mg/kg) | 5.50 (3.28) | 35.0 (25.6) |
| FR1 (0.04 mg/kg) | 6.75 (3.06) | 30.5 (19.4) |
In order to capture cross-sessional behavior patterns, we also examined the total number of infusions and total locomotor activity for each rat. Specifically, both measures were plotted in 5-min bins for each of the last training sessions of the final three stages for WT rats (see Figure 9) and Tg rats (see Figure 10). These graphs also show that locomotor activity tends to relate to infusions across time within session for both WT and Tg rats.
Figure 9.
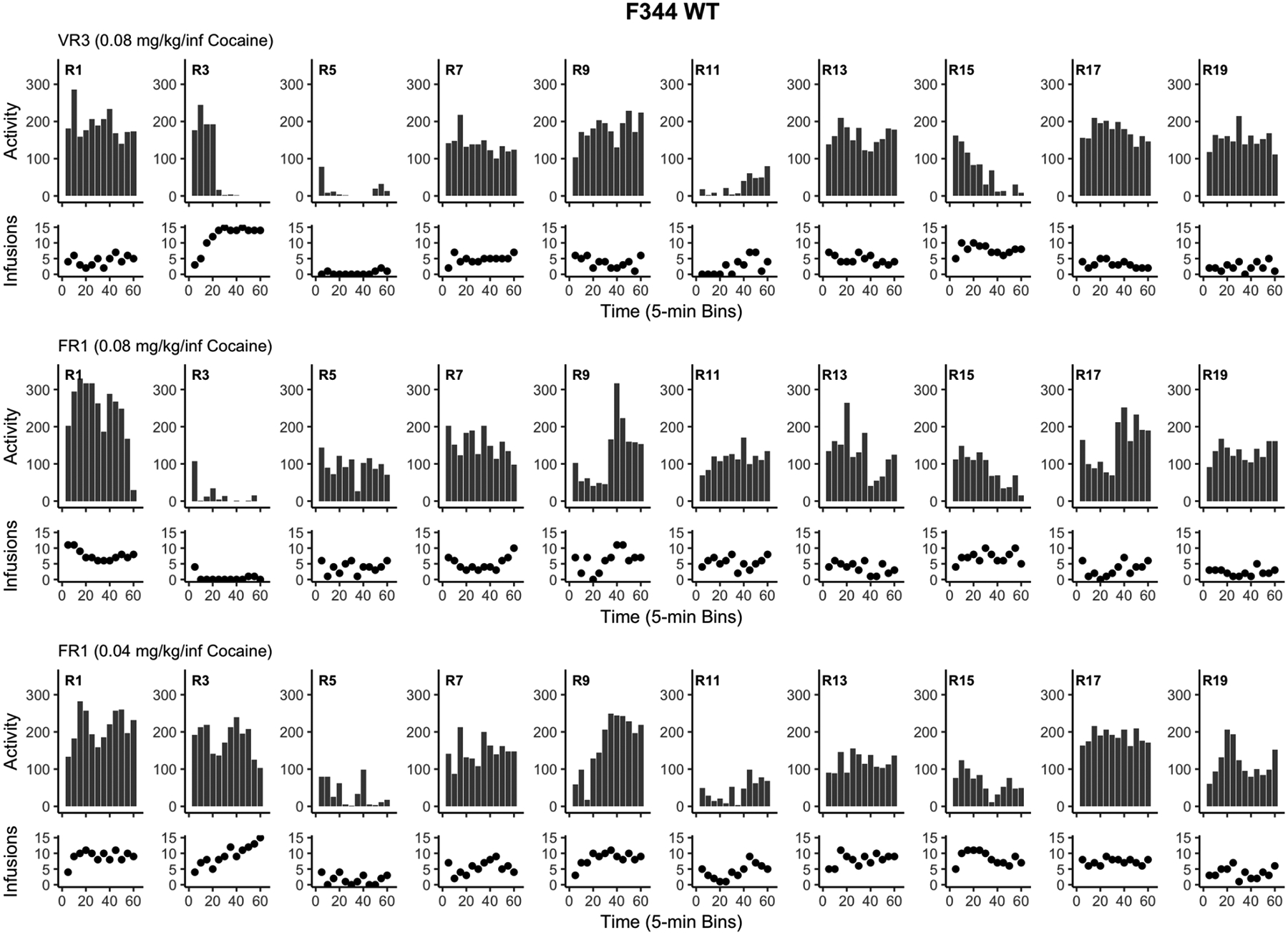
Displays the total locomotor activity (infrared beam breaks; bar graphs) and total cocaine infusions (scatter plots) for each F344 WT rat (R). Each panel depicts the final session of the last three training stages in 5-min bins. VR = variable ratio, FR = fixed ratio, WT = wildtype.
Figure 10.
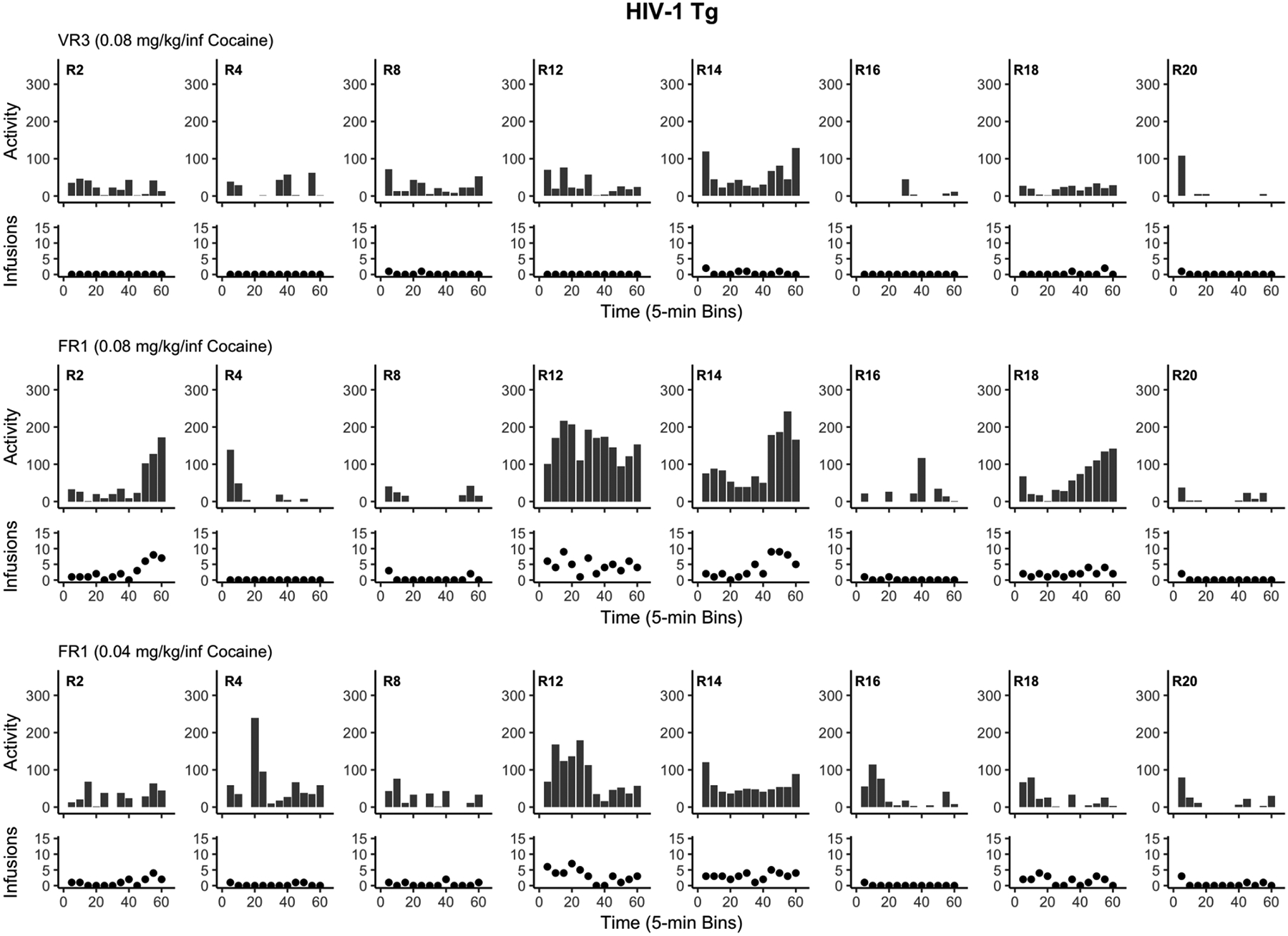
Displays the total locomotor activity (infrared beam breaks; bar graphs) and total cocaine infusions (scatter plots) for each HIV-1 Tg rat (R). Each panel depicts the final session of the last three training stages in 5-min bins. VR = variable ratio, FR = fixed ratio, Tg = transgenic.
Discussion
Drugs of abuse interact with HIV-1 viral proteins to exacerbate the neuroinflammation and cognitive decline associated with the infection (Buch et al., 2011; Kim et al., 2013; Midde et al., 2011; Nath et al., 2002; Peterson et al., 1992; Vigorito et al., 2015). However, most of the research investigating such interactions uses experimenter-administered drug rather than rodent self-administered drug. As detailed in the Introduction, of those studies using self-administration of cocaine, the outcome has been quite mixed regarding intake by the WT versus Tg rats. In contrast to Wayman et al. (2016), who reported no statistical difference between genotypes for cocaine self-administration, we found that while a majority of WT rats acquired cocaine self-administration within 9 days after the autoshaping session, the majority of Tg rats (5 of the 8) never increased cocaine intake with an accompanying discrimination between the active and inactive lever. That is, our HIV-1 Tg rats were highly resistant to acquiring cocaine self-administration (see later). This outcome is also in contrast to McIntosh et al. (2015) who reported that cocaine self-administration in Tg rats stabilized at a dose half that of the WT rats. Our findings are more in line with those reported by (Bertrand et al., 2018; McIntosh et al., 2015) that Tg rats consistently consumed substantially less cocaine than the WT rats on an FR1 schedule of reinforcement. This difference persisted into the next phase when those rats were moved to a PR schedule. Notably, the experimental approaches were different across these cocaine self-administration studies, including whether nose-poke (Wayman et al., 2016) or lever-press (Bertrand et al., 2018; McIntosh et al., 2015) was the response operandum and whether only visual (Bertrand et al., 2018) or visual plus auditory (McIntosh et al., 2015; Wayman et al., 2016) stimuli were paired with the infusion. Thus, at this point, it is unclear what experimental factors may be at play to account for such differences.
In the current study, individual WT rats can be categorized into two groups: early acquisition or late acquisition. Eight of the ten WT rats showed active lever discrimination within nine sessions after the cocaine autoshaping session and the remaining two WT rats showed high, consistent rates of active lever pressing beginning around session 36 (recall Figure 5). In stark contrast, individual Tg rats can be categorized as late acquisition or no acquisition. Beginning on session 36, five of the eight HIV-1 Tg rats showed an increase in cocaine consumption (recall Figure 4). Upon examination of active and inactive lever data (see Figure 6), just three of these five rats discriminated between the two levers. That is, 38.5% of Tg rats were sensitive at some point in the experiment to the active lever – cocaine relation; contrast this to 100% of the WT rats. One possible account we considered was that expression of the genetic mutation in HIV-1 Tg rats translated functionally into a learning impairment in our task. Indeed, relative to WT rats, Tg rats show behavioral deficits in such tasks as pre-pulse inhibition (McLaurin et al., 2018; Moran, Booze, & Mactutus, 2013), Morris Water Maze (Vigorito et al., 2007), and fear conditioning (Nesil et al., 2015). However, our enthusiasm for this possibility is diminished by the demonstration of cocaine self-administration by McIntosh et al. (2015) and Wayman et al. (2016) without any reported preliminary training phase in which responding (lever-press or nose-poke) was first trained with food reinforcement.
At the start of the experiment, before cocaine self-administration developed in any of the rats, there was no discernable difference between Tg and WT rats in baseline chamber activity. Although past work has reported some locomotor impairment in HIV-1 Tg rats over the WT rats (e.g., June et al., 2009; McLaurin et al., 2018), this finding is not universal (e.g., Kaas et al., 2010; Liu et al., 2009). Though a systematic experiment has not been conducted, our read of the literature suggests that size and familiarity of the environment may be important factors. For example, June et al. (2009) found motor impairment in an acute open field test; chamber dimensions were 42 × 42 × 30 cm, a larger apparatus than our operant chambers. Similarly, Moran, Booze, Webb, and Mactutus (2013) reported fewer beam breaks in Tg rats early in a 1-hour test session (i.e., especially first 10 min) when compared to WT rats. This early locomotor difference persisted in the acute open field tests conducted at 6, 7, and then 11 months of age. Note that their apparatus was also larger than our operant chambers at 40 × 40 cm square and the start of a session is arguably the most novel (least familiar) part of test session (Burstein, 1967; Skinner 1950 [see p. 205]). In contrast, Kass et al. (2010) assessed baseline locomotor activity using each rat’s home cage and found no difference between Tg and WT rats with their measure of activity (i.e., live observation of head movements). Although we did not find the home-cage dimensions reported in that manuscript, we assume that it is more similar to our standard housing which is as long but not as wide as the open field apparatuses – 48.3 × 26.7 × 20.3 cm (l × w × h). Notably, the self-administration chambers (30.5 × 24.1 × 21 cm; l × w × h) used in the present study were even smaller than the home cages. Although we reported the average activity for each rat across the first seven days of the study as basal activity in the Results section, we did not see a difference in activity even on the first day of the experiment: mean number of beam breaks for WT rats = 257.3 (median = 271.5; range = 103 to 409) versus the mean for Tg rats = 251 (median = 287; range = 21 to 439).
We are the first to concomitantly measure chamber activity while WT and HIV-1 Tg rats are intravenously self-administering cocaine. In the final three segments of training when cocaine was being self-administered by most to all WT rats and by some of the Tg rats, there was a positive correlation between number of cocaine infusions and the number of beam breaks in the self-administration chamber. That is, an increase in cocaine intake was accompanied by an increase in chamber activity. In general, this finding is unsurprising given that cocaine is a well-documented potent locomotor stimulant (Pinkston & Branch, 2010; Segal & Kuzcensk, 1992), including in the HIV-1 Tg and F344/NHsd WT rats used in the present study. Indeed, Tg and F344 rats show sensitization (i.e., increased locomotor behavior) with repeated cocaine treatment (e.g., Moran, Booze, Webb, & Mactutus, 2013; Ortiz et al., 1995). Although we did not design the present study to explicitly examine locomotor sensitization, what is notable is that when the three Tg rats (R12, R14, R18) in the final stage of the study began to increase their cocaine consumption, chamber activity increased similar to their WT counterparts and correlations between intake and locomotor activity for Tg rats emerge once they begin self-administering.
In sum, the present study adds supporting behavioral evidence for the conclusion of Bertrand et al. (2018) that Tg rats are less sensitive to the reinforcing effects of cocaine as measured in a cocaine self-administration task and to neurochemical findings that Tg rats show striatal dopamine transporter dysfunction compared to WT (Javadi-Paydar, Roscoe, Denton, Mactutus, & Booze, 2017). This effect is unlikely reflecting a decreased sensitivity to reward in general, as Tg and WT rats show equivalent sucrose preference (Bertrand et al., 2018). However, Tg rats took longer to reach the acquisition criterion than WT rats when sucrose was the reinforcer. This distinction demonstrates that response rate was lower in Tg rats even though the reinforcement value of sucrose was similar between Tg and Wt rats (Bertrand et al., 2018). Based on the self-administration findings, those authors do conclude that the Tg rats may be displaying diminished motor planning, habituation learning, or greater difficulty in learning complex tasks that may influence the motivation for operant reinforcement (Bertrand et al., 2018). However, this potential for learning deficit in Tg rats does not appear to be reflected in the establishment of a morphine place preference where Tg and WT rats acquired a morphine-conditioned place preference at the same dose (Homji, Vigorito, & Chang, 2012) indicating capacity for associative learning. And, as discussed earlier, reduced cocaine sensitivity observed in the current report does not seem to reflect a non-specific locomotor impairment in the Tg rats. Furthermore, the other two cocaine self-administration studies (McIntosh et al., 2015; Wayman et al., 2016) reporting equivalent intake diminish enthusiasm for an account based on impaired learning of an operant response.
The reduced cocaine sensitivity in Tg compared to WT rats emerged even with the relatively low cocaine self-administration doses used in the current study and in some of the related literature (Bertrand et al., 2018; McIntosh et al., 2015; Wayman et al., 2016; J. Zhu et al., 2015). Indeed, research investigating this transgenic model should use the higher doses more common in cocaine self-administration studies (Miguéns et al., 2015; Morgan, Liu, & Roberts, 2006) and provide a comparative background across strains. Further, the time taken for dopamine reuptake in striatal slices in HIV-1 Tg rats is extended compared to WT controls (Javadi-Paydar et al., 2017). Given the finding that the addition of cocaine to these in vitro striatal slice preparations does not further reduce the dopamine reuptake impairment observed in HIV-1 Tg rat tissue (Javadi-Paydar et al., 2017), a full in vivo assessment of dose-responsiveness to cocaine sensitivity is warranted. Finally, the wide variability in self-administration that eventually develops in the Tg rats opens a path for the study of inter-individual differences, rather than group analysis, in investigating the interactions between drug consumption, HIV-1 viral proteins effects on neural tissue, and unique environmental factors that may differentially impact Tg rats and gradually increase cocaine intake. Recall that consumption of cocaine among people with HIV-1 infection is higher than uninfected individuals (Chang et al., 2014), possibly reflecting dysfunction in dopamine transporter activity (Javadi-Paydar et al., 2017). Refining our models to parallel this observation may lead to novel insights into behavioral and pharmacological approaches to treating this at-risk population.
Acknowledgements
Funding for the rodent procurement was supported by DA044586 and DA043138 awarded to MG and SB. Personnel costs and consumables were provided for by DA034389 and DA039356 awarded to RAB. RAB was partially supported by GM130461 and JEM was partially supported by DA045740 while preparing this manuscript for publication. Cocaine was generously supplied by the NIDA Drug Supply Program. All MedPC programs are available upon request from Rick Bevins at rbevins1@unl.edu. These data in their raw form were made freely available on the Open Science Framework osf.io/xtygc/ DOI 10.17605/OSF.IO/XTYGC
Contributor Information
Y. Wendy Huynh, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE, USA.
Brady M. Thompson, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE, USA
Christopher E. Larsen, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE, USA
Shilpa Buch, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha NE, USA.
Ming-Lei Guo, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha NE, USA.
Rick A. Bevins, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE, USA.
Jennifer E. Murray, Department of Psychology, University of Guelph, Guelph ON, Canada.
References
- Bagetta G, Piccirilli S, Del Duca C, Morrone LA, Rombolà L, Nappi G, … Corasaniti MT (2004). Inducible nitric oxide synthase is involved in the mechanisms of cocaine enhanced neuronal apoptosis induced by HIV-1 gp120 in theneocortex of rat. Neuroscience Letters, 356(3), 183–186. 10.1016/J.NEULET.2003.11.065 [DOI] [PubMed] [Google Scholar]
- Barrett ST, Geary TN, Steiner AN, & Bevins RA (2017). Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology, 234(2), 187–198. 10.1007/s00213-016-4448-x [DOI] [PubMed] [Google Scholar]
- Bernstein N (1967). The Co-ordination and Regulations of Movements. Oxford: Pergamon Press. [Google Scholar]
- Bertrand SJ, Mactutus CF, Harrod SB, Moran LM, & Booze RM (2018). HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Scientific Reports, 8(1), 7869 10.1038/s41598-018-25109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, … Su T-P (2012). Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Current HIV Research, 10(5), 425–428. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22591366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Su T-P, & Wang J (2011). Cocaine and HIV-1 Interplay: Molecular Mechanisms of Action and Addiction. Journal of Neuroimmune Pharmacology, 6(4), 503–515. 10.1007/s11481-011-9297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Connaghan KP, Wei Y, & Li MD (2014). NeuroHIV and Use of Addictive Substances. International Review of Neurobiology, 118, 403–440. 10.1016/B978-0-12-801284-0.00013-0 [DOI] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger ST, Fink K, Schepers S, Hadlock GC, … Bevins RA (2013). Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology, 75, 138–144. 10.1016/j.neuropharm.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, & Booze RM (2010). Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. Journal of Neurochemistry, 115(4), 885–896. 10.1111/j.1471-4159.2010.06968.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homji NF, Vigorito M, & Chang SL (2012). Morphine-induced conditioned place preference and associated behavioural plasticity in HIV-1 transgenic rats. International Journal of Clinical and Experimental Medicine, 5(2), 105–123. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22567172 [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Roscoe RF, Denton AR, Mactutus CF, & Booze RM (2017). HIV-1 and cocaine disrupt dopamine reuptake and medium spiny neurons in female rat striatum. PloS One, 12(11), e0188404 10.1371/journal.pone.0188404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Tzeng Yang ARS, Bryant JL, Jones O, & Royal W (2009). Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat. Journal of Neurovirology, 15(5–6), 380–389. 10.3109/13550280903350200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, & Chang SL (2010). Methamphetamine-Induced Behavioral and Physiological Effects in Adolescent and Adult HIV-1 Transgenic Rats. Journal of Neuroimmune Pharmacology, 5(4), 566–573. 10.1007/s11481-010-9221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Jung JB, Dixit D, Rovner R, Zack JA, Baldwin GC, & Vatakis DN (2013). Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. Journal of Leukocyte Biology, 94(4), 835–843. 10.1189/jlb.1112566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh S, Sexton T, Pattison LP, Childers SR, & Hemby SE (2015). Increased Sensitivity to Cocaine Self-Administration in HIV-1 Transgenic Rats is Associated with Changes in Striatal Dopamine Transporter Binding. Journal of Neuroimmune Pharmacology, 10(3), 493–505. 10.1007/s11481-015-9594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, & Mactutus CF (2017). Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. Journal of NeuroVirology, 1–17. 10.1007/s13365-017-0544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, & Zhu J (2011). Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacology, Biochemistry, and Behavior, 98(4), 587–597. 10.1016/j.pbb.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguéns M, Kastanauskaite A, Coria SM, Selvas A, Ballesteros-Yañez I, DeFelipe J, & Ambrosio E (2015). The Effects of Cocaine Self-Administration on Dendritic Spine Density in the Rat Hippocampus Are Dependent on Genetic Background. Cerebral Cortex, 25(1), 56–65. 10.1093/cercor/bht200 [DOI] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, & Mactutus CF (2013). Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Experimental Neurology, 239, 139–147. 10.1016/j.expneurol.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Landhing M., Booze RM, & Mactutus CF (2013). Time and Time Again: Temporal Processing Demands Implicate Perceptual and Gating Deficits in the HIV-1 Transgenic Rat. Journal of Neuroimmune Pharmacology, 8(4), 988–997. 10.1007/s11481-013-9472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Liu Y, & Roberts DCS (2006). Rapid and Persistent Sensitization to the Reinforcing Effects of Cocaine. Neuropsychopharmacology, 31(1), 121–128. 10.1038/sj.npp.1300773 [DOI] [PubMed] [Google Scholar]
- Murray JE, Li C, Palmatier MI, & Bevins RA (2007). The interoceptive Pavlovian stimulus effects of caffeine. Pharmacology Biochemistry and Behavior, 86(4), 838–846. 10.1016/j.pbb.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, … Turchan JT (2002). Molecular basis for interactions of HIV and drugs of abuse. Journal of Acquired Immune Deficiency Syndromes, 31, S62–9. [DOI] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, & Li MD (2015). Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Molecular Brain, 8(1), 43 10.1186/s13041-015-0134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, DeCarpio JL, Kosten TA, & Nestler EJ (1995). Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience, 67(2), 383–397. 10.1016/0306-4522(95)00018-E [DOI] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, & McLaughlin JP (2014). Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 39(2), 380–388. 10.1038/npp.2013.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, & Chang SL (2010). The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. Journal of Neuroimmunology, 218(1–2), 94–101. 10.1016/j.jneuroim.2009.09.014 [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Chao CC, Schut R, Verhoef J, Edelman CK, … Balfour HH (1992). Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. The Journal of Immunology, 149(2), 676 LP–680. [PubMed] [Google Scholar]
- Pinkston JW, & Branch MN (2010). Acute and chronic effects of cocaine on the spontaneous behavior of pigeons. Journal of the Experimental Analysis of Behavior, 94(1), 25–36. 10.1901/jeab.2010.94-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Schaal VL, Moore D, Guda RS, Koul S, Yelamanchili SV, … Pendyala G (2018). MicroRNA cluster miR199a/214 are differentially expressed in female and male rats following nicotine self-administration. Scientific Reports, 8(1), 1–10. 10.1038/s41598-018-35747-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, … Hammoud DA (2016). Neurobehavioral Abnormalities in the HIV-1 Transgenic Rat Do Not Correspond to Neuronal Hypometabolism on 18F-FDG-PET. PLOS ONE, 11(3), e0152265 10.1371/journal.pone.0152265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, … Bryant J (2001). An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proceedings of the National Academy of Sciences of the United States of America, 98(16), 9271–9276. 10.1073/pnas.161290298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, & Kuczenski R (1992). Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain Research, 577(2), 351–355. 10.1016/0006-8993(92)90297-M [DOI] [PubMed] [Google Scholar]
- Skinner BF (1950). Are theories of learning necessary? Psychological Review, 57(4), 193–216. 10.1037/h0054367 [DOI] [PubMed] [Google Scholar]
- Vigorito M, Connaghan KP, & Chang SL (2015). The HIV-1 transgenic rat model of neuroHIV. Brain, Behavior, and Immunity, 48, 336–349. 10.1016/J.BBI.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, & Chang SL (2007). Spatial Learning and Memory in HIV-1 Transgenic Rats. Journal of Neuroimmune Pharmacology, 2(4), 319–328. 10.1007/s11481-007-9078-y [DOI] [PubMed] [Google Scholar]
- Wayman WN, Chen L, Hu X-T, & Napier TC (2016). HIV-1 Transgenic Rat Prefrontal Cortex Hyper-Excitability is Enhanced by Cocaine Self-Administration. Neuropsychopharmacology, 41(8), 1965–1973. 10.1038/npp.2015.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bertrand BJ, Harrod SB, Mactutus CF, & Booze RM (2015). HIV-1 transgenic rats exhibit attenuated cocaine-mediated increase in synaptosomal [3H] dopamine uptake in striatum following cocaine self-administration. Drug and Alcohol Dependence, 146, e32 10.1016/j.drugalcdep.2014.09.767 [DOI] [Google Scholar]
- Zhu Jun, Ananthan S, Mactutus CF, & Booze RM (2011). Recombinant human immunodeficiency virus-1 transactivator of transcription1–86 allosterically modulates dopamine transporter activity. Synapse (New York, N.Y.), 65(11), 1251–1254. 10.1002/syn.20949 [DOI] [PMC free article] [PubMed] [Google Scholar]


