Abstract
Klippel-Feil syndrome (KFS) is an exceerlingly rare constitutional disorder in which a paucity of knowledge exists about the disease and its associated morbidity and mortality. We present a 4-year-old male with KFS, who notably was also diagnosed with large-cell anaplastic medulloblastoma. We evaluated the genetic basis of co-occurring KFS and medulloblastoma and the role of MYO18B as related to medulloblastoma. Constitutional and somatic variant and copy number analyses were performed from DNA-based exome studies, along with RNA-sequencing of tumor tissue, to elucidate the genetic etiology of the co-existing disease states. We identified novel constitiitional compound heterozygous frameshift variants (NM_032608.5: p.Leu2257SerfsTerl6 and p.Arg2220SerfsTer74) each encoding a premature stop of translation in MYO18B, consistent with a diagnosis of KFS. We did not identify any somatic variants of known relevance or disease-relevant therapeutic targets in the tumor. The somatic copy number profile was suggestive of Group 3γ medulloblastoma. Relative to pediatric brain tremors, medulloblastoma, particularly, Group 3, had increased gene expression of MYO18B. In summary, coexisting constitutional and somatic diagnoses in this patient enabled the elucidation of the genetic etiology of KFS and provided support for the role of MYO18B in tumor suppression.
Keywords: Exome sequencing, Constitutional disease, Pediatric, Brain cancer, MYO18B
1. Introduction
Klippel-Feil syndrome (KFS) is a congenital disorder characterized by fusion of the cervical spine and risk for sequelae, including congenital scoliosis (Tracy et al., 2004). It is caused by a failure in the normal segmentation of the cervical vertebrae during the early weeks of fetal development. The most common signs of the disorder are short neck, low hairline at the back of the head, and restricted mobility of the upper spine (Thomsen et al. 1997). KFS occurs in approximately 1 in 40,000—42,000 newborns worldwide and most cases occur sporadically (Thomsen et al., 1997). In addition to fusion of the cervical vertebrae, individuals with KFS may be afflicted with hearing impairment, cardiac structure malformations, renal defects, and neurological complication due to spinal cord injury associated with vertebral instability (Thomsen et al., 1997). Uncommonly, KFS is associated with intracranial tumors, the most frequent being benign dermoid tumors usually located in the posterior fossa, for which approximately 23 cases are reported (Adorno et al., 2015). Dermoid tumors are typically benign; however, rare reports of malignancy are described in the setting of KFS (Adorno et al., 2015). Though the precise mechanism of dermoid tumor formation in KFS patients has yet to be established, several hypotheses related to abnormal embryogenesis are described (Adorno et al., 2015). There are no known associations of KFS with intracranial malignancies in pediatric patients.
KFS is associated with mutations in PAX1 (8 unrelated patients) (McGaughran et al., 2003), MEOX1 (MIM #214300, 2 unrelated patients and 1 family) (Bayrakli et al., 2013; Mohamed et al., 2013), GDFS (MIM #613702, 2 related patients) (Ye et al., 2010), and GDF6 (MIM #118100, 2 unrelated patients and 1 family) (Tassabehji et al., 2008). Alterations in MYO18B (MIM #616549 (Alazami et al., 2015; Malfatti et al., 2015) are described in three unrelated patients. MYO18B (myosin XVIIIB) is an unconventional class XVIII myosin preferentially expressed in skeletal muscle and cardiac tissue with a putative role in transcriptional regulation of muscle-specific genes and intracellular trafficking (Salamon et al., 2003). Zebrafish models of myo18b loss-of-function mutants revealed that myo18b is necessary for proper sarcomere assembly, whereas Myo18b deficiency in mice and zebrafish demonstrated embryonic lethality (Ajima et al., 2008; Berger et al., 2017). Emerging evidence in the literature suggests a phenotypic impact on cardiac and muscle development in the setting of constitutional bi-allelic MYO18B alteration (Alazami et al., 2015; Malfatti et al., 2015).
Here, we present a unique case of medulloblastoma in a pediatric patient with a previous diagnosis of KFS harboring a MYO18B constitutional alteration. Medulloblastoma is a highly malignant tumor of neuroepithelial origin, often arising in the posterior fossa. Coexisting constitutional and somatic disease states in this patient allowed for elucidation of the genetic underpinnings of disease.
1.1. Klippel-Feil syndrome diagnosis and therapy
The male patient was born at 38 weeks 6/7 days’ gestation via vaginal delivery to non-consanguineous parents of European ancestry. Prenatal history was only significant for relative oligohydramnios and breech presentation. At delivery, micrognathia and mild congenital hip dysplasia was noted. He experienced feeding difficulties in the neonatal period. At 30 days of age, he was evaluated by a medical geneticist due to the micrognathia, mild congenital hip dysplasia, and feeding difficulties, in addition to mild hypotonia. At that time, his length was 56 cm (50th percentile), weight was 3150 g (10th percentile), and head circumference was 36.6 cm (25th percentile). At his 6-month visit, hypotonia was more pronounced and developmental delay was observed. He first rolled over at 5 months of age. Chromosome analysis was consistent with a normal male karyotype (46, XY) and methylation-specific PCR to assess for Prader-Willi syndrome (associated with a 15q imprinted gene region) was normal. At this visit, he was referred for evaluation of torticollis, and magnetic resonance imaging (MRI) of the cervical spine was performed, revealing partial fused posterior elements of several cervical vertebrae (C2–C3) suggestive of KFS. On physical exam, blue sclera, a mild high-arched palate, bilateral single palmar creases, and small hands were observed. Array-based comparative genomic hybridization was performed revealing a 106 kb interstitial loss on chromosome 1p36.12 (arr[hg18]1p36.12(21,477,800–21,583,610)x1), including the ECE1 (endothelin converting enzyme 1) gene involved in endothelin proteolytic processing. This was classified as a variant of uncertain significance. The patient was subsequently lost to genetic specialty follow-up.
The patient began occupational therapy at 8 months of age, with goals to improve upper extremity strength and fine motor skills. The patient had consistent occupational, physical, and later, speech therapy with some improvement in fine and gross motor skills. He was referred to Orthopedics at age 2 years 4 months due to severe scoliosis, and initiated treatment with nighttime Providence bracing. His scoliosis remained significant and supports were still required for walking at 3 years of age (Fig. 1A).
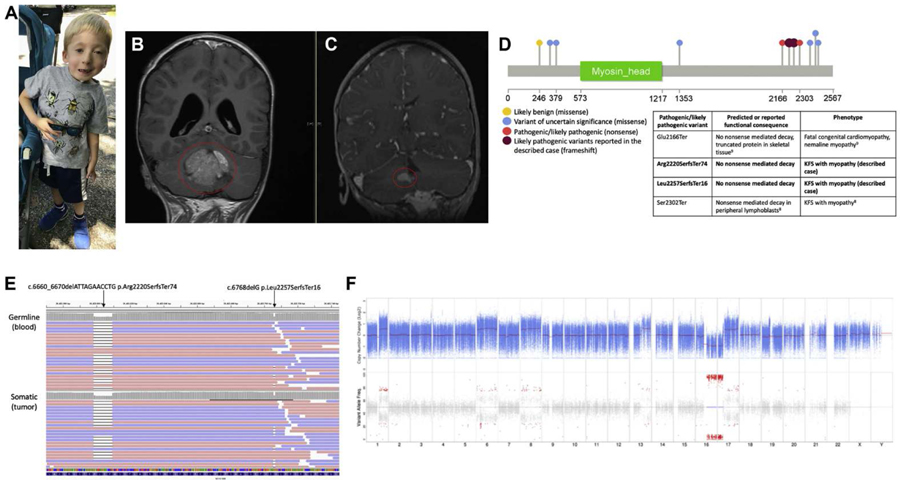
Fig. 1. Clinical presentation and molecular genomic findings.
(A) Full body image of the patient demonstrating clinical features of KFS and facial dysmorphism. (B) Magnetic resonance imaging of the brain with contrast, coronal T1 FLAIR at diagnosis demonstrating large homogenous cerebellar mass (circled). (C) Magnetic resonance imaging of the brain with contrast, coronal fast spoiled gradient-echo at recurrence demonstrating new nodules along the surface of the right cerebellum (circled). (D) Schematic of MYO18B variants reported in ClinVar with colors corresponding to variant interpretation: likely benign (yellow), blue (variant of uncertain significance), red tpathogenic/likely pathogenic). Likely pathogenic variants reported in the described patient are shown in dark red. A table describes the reported or predicted functional consequence of pathogenic/likely pathogenic variants and the associated patient phenotype. (E) Visualization of the MYO18B compound heterozygous variants suggestive of an in trans inheritance patterns. Top: Constitutional MYO18B reads from the peripheral blood aligned to GRCh37. Bottom: Somatic MYO18B reads from the tumor aligned to GRCh37. Reads are colored by read strand, red for positive strand and blue for negative strand. (F) Somatic copy member variation (CNV) analysis. Top: Tumor copy number relative to matched normal in 1og2 scale. Blue points represent log2 values based on sequence depth in 100-bp windows. Red lines indicate segmented CNV calls. Bottom: Tumor variant allele frequency for variants that are heterozygous in the normal. Points in red indicate significant loss of heterozygosity (LOH). The x-axis denotes the chromosome number.
1.2. Medulloblastoma diagnosis and treatment
At 3 years 8 months, the patient presented with recent onset ataxia, and 4-days of early morning vomiting and progressive somnolence. A brain MRI revealed a 4.7 x 4.2 × 3.4 cm predominantly solid mass with homogenous enhancement and restricted diffusion centered in the fourth ventricle resulting in obstructive hydrocephalus (Fig. 1B). The patient underwent gross total resection of the mass. Pathology was consistent with large-cell anaplastic medulloblastoma with no immunoreactivity for GAB1 or YAP1 and cytoplasmic beta-catenin immunostaining, suggestive of a non-WNT, non-SHH medulloblastoma phenotype. Weak p53 nuclear staining was reported. Metastatic workup was negative. Treatment was initiated as per the CCG 99703 protocol with induction chemotherapy followed by consolidation with marrow ablative chemotherapy, and autologous stem cell rescue. Three months off therapy, and 11 months following initial diagnosis, the patient began experiencing headaches and vomiting. A brain MRI revealed multiple new lesions in the cerebellum and leptomeningeal disease in the parietal lobe consistent with recurrent and metastatic medulloblastoma (Fig. 1C). The patient was enrolled in our Nationwide Children’s Hospital Institute for Genomic Medicine translational genomic profiling study at this time, along with treatment on a phase I trial. He died 31 days after starting treatment due to tumor progression with subsequent brainstem compression at the age of 4 years 9 months.
1.3. Genomic analysis
The patient was enrolled as part of an Institutional Review Board (IRB) approved study (IRB17–00206) within the Institute for Genomic Medicine at Nationwide Children’s Hospital. Informed consent was provided by the parents for molecular genetic analysis, including enhanced exome sequencing and total RNA-sequencing. Comprehensive genomic and transcriptomic analysis was performed from DNA and RNA extracted from the patient’s peripheral blood and tumor (additional information provided in the Supplemental Methods). Exome sequencing of the patient’s peripheral blood-derived DNA revealed variants consistent with a genetic diagnosis of KFS (MIM #616549). We identified novel compound heterozygous frameshift variants in MYO18B, each encoding a premature stop of translation in the penultimate exon of the gene (NM_032608.5:c.6768delG:p.Leu2257SerfsTerl6 occurring 88% through the translated sequence (ClinVar: SCV000844982) and NM_032608.5:c.6660_6670delATTAGAACCTG:p.Arg2220SerfsTer74 occurring 86% through the translated sequence (ClinVar: SCV000844943)) (Fig. 1D, Table 1). These frameshift variants are located in a region of previously reported disease-associated variation (Alazami et al., 2015; Malfatti et al., 2015). Database and literature evidence support that pathogenic and likely pathogenic variants are described as nonsense and frameshift in nature. The 11-bp deletion (rs756408696) is rare in the general population (gnomAD population frequency = 0.0001076); however, the l-bp deletion is novel to online genomic databases (ClinVar, gnomAD, and dbSNP). Using the Association for Molecular Pathology and American College of Medical Genetics and Genomics joint recommendation for the interpretation of sequence variants (Richards et al., 2015), each MYO18B variant was classified as likely pathogenic (Table 1). Due to the close proximity of the two alterations, spanning reads were reviewed in the Integrative Genomics Viewer (Broad Institute, Cambridge, MA) and it was determined that the variant alleles occurred on separate reads of DNA (in trans), thus associated with compound heterozygosiry (Fig. 1E). The variants were clinically confirmed using targeted Sanger sequencing in the proband, and parental samples were studied to determine variant etiology, with the NM_032608.5:p.Arg2220SerfsTer74 alteration observed to be maternally-inherited and the NM_032608.5:p.Leu2257SerfsTerl6 alteration observed to be paternally-inherited. The variants were reviewed in RNA sequencing data, in libraries constructed from cDNA capture. The 11-bp and 1-bp deletions were found at a variant allele frequency of 50.0% and 48.5%, respectively, suggesting that these alleles do not undergo nonsense-mediated decay in the brain (Table 1).
Table 1.
Constitutional MYO18B variants identified by exome sequencing.
| Gene (Transcript ID; GRCh37) | Genomic Change (GRCh37) | Nucleotide change | Zygosity/Inheritance | Predicted protein change | Associated Disease | dbSNP ID | Variant Interpretation (ACMG/AMP Evidence) | Constitutional DNA VAF (Read depth at variant) | Tumor DNA VAF (Read depth at variant) | Tumor RNA VAF (Read depth at variant) |
|---|---|---|---|---|---|---|---|---|---|---|
| MYO18B (NM_032608.5) | chr22:26422600_26422640del | c.6768delG | Heterozygous/Maternal | p.Leu2257SerfsTer16 | (AR) Klippel-Feil syndrome 4, with nemaline myopathy and facial dysmorphism (MIM #616549) | Not reported | Likely pathogenic (PVS1, PM2) | 43% (225x) | 46% (225x) | 49% (35x) |
| MYO18B (NM_032608.5) | chr22:26422708delG | c.6660_6670del ATTAGAACCTG | Heterozygous/Paternal | p.Arg2220SerfsTer74 | (AR) Klippel-Feil syndrome 4, with nemaline myopathy and facial dysmorphism (MIM #616549) | rs756408696 | Likely pathogenic (PVS1, PM2) | 49% (213x) | 46% (231x) | 50% (32x) |
VAF: variant allele frequency; AR: autosomal recessive; MIM: Online Mendelian Inheritance in Man; ACMG: American College of Medical Genetics and Genomics; AMP: Association for Molecular Pathology; PVS1: very strong evidence for pathogenicity defined as null variation in gene with loss-of-function as an established disease mechanism; PM2: moderate evidence for pathogenicity defined as exceedingly rare or absent from control populations.
Somatic alterations were identified via exome sequencing of DNA extracted from tumor tissue and the comparator normal peripheral blood. We found 29 somatic rare coding variants; however, none were found to be damaging or in well-described cancer-associated genes. Prior cytogenetic analysis demonstrated a complex karyotype: 48, XY, +6, der (7)t(1; 7) (p22; q21), +8, i(17)(q10) [cp8]. Most notable was the presence of an isochromosome 17q (i(17)(q10)), and relative gain of 1q. Copy number analysis derived from exome sequencing data identified the i(17)(q10), in addition to a bi-allelic loss of 16q, gains of chromosome arms 1q and 13q, and whole chromosome gains of 6 and 8 (Fig. 1F). No focal amplifications or deletions were seen via the exome copy number variation data, consistent with previous interphase fluorescence in situ hybridization (FISH) results in which no amplification of MYC, MYCN, or the C19MC miRNA cluster on 19q13 was detected. This genomic profile was suggestive of Group 3γ medulloblastoma (Cavalli et al., 2017).
To assess the role of MYO18B in medulloblastoma, we evaluated MYO18B gene expression amongst pediatric central nervous system (CNS) tumors within the University of California Santa Cruz Treehouse Initiative dataset, including medulloblastoma (n = 101), dysembryoplastic neuroepithelial tumor (n = 16), glioma (n = 76), glioblastoma (n = 12), ependymoma (n = 29), and atypical teratoid rhabdoid tumor (n = 4). Additionally, medulloblastoma patients enrolled at our institution were included (n = 4). Publicly available data representing 42 brain specimens from the Allen BrainSpan Developmental Transcriptome found very low expression of MYO18B from the embryonic stage through adulthood (Miller et al., 2014). In agreement, our analysis revealed low levels of MYO18B in pediatric CNS tumors; however, medulloblastoma demonstrated higher mRNA expression compared to other CNS tumors, with our described patient showing relatively high expression within the medulloblastoma cohort (Supplemental Fig. lA). Within the medulloblastoma cohort, patients with Group 3 medulloblastoma (n = 33, P = 0.001) had significantly higher expression of MYO18B relative to Group 4 medulloblastoma (n = 38) (Supplemental Fig. lB). The described case demonstrated a gene expression around the median value of the Group 3 medulloblastoma cohort. Using cDNA capture-based RNA-sequencing, we determined that the alleles harboring the mutations do not undergo nonsense-mediated decay (RNA variant allele frequency NM_032608.5:p.Arg2220SerfsTer74 = 50%, RNA variant allele frequency NM_032608.5:p.Leu2257SerfsTer16 = 48.5%), thus consistent with observed levels of expression. The higher levels of MYO18B found in medulloblastoma relative to other pediatric CNS tumors, as well as in comparison to varying developmental stages of the brain, suggests that there may be a role for this gene in tumorigenesis in this collectively common pediatric malignancy.
2. Discussion
KFS associated with constitutional variation in MYO18B is rarely described. Two KFS patients born of unrelated consanguineous Saudi Arabian families were observed to harbor a constitutional homozygous recessive nonsense variant in MYO18B (p.Ser2302Ter), experimentally determined to undergo nonsense-mediated decay (Alazami et al., 2015; Malfatti et a l., 2015). In one of these patients, a muscle biopsy revealed disorganization of the normal myofibrillar architecture, consistent with a myopathy phenotype (Alazami et al., 2015). A second study reported a child born to consanguineous Portuguese parents with a constitutional homozygous recessive nonsense variant in MYO18B (p.Glu2l66Ter), the effect of which was consistent with loss of the C-terminus of the protein (Malfatti et al., 2015). In this latter study, the child did not present with KFS, but with congenital fatal cardiomyopathy and nemaline myopathy (Malfatti et al., 2015). Here, we present a patient with KFS resulting from compound heterozygous frameshift deletions in MYO18B born to a non-consanguineous family of European ancestry. Nonsense-mediated decay was not observed in tumor-derived brain tissue from our described patient, and expression values are at the median for group 3 medulloblastoma (Supplemental Fig. 1). Our patient was not assessed for myopathy by electromyography or muscle biopsy; however, the clinical findings, including congenital hip dysplasia, feeding difficulties, and congenital hypotonia, are suggestive of myopathy on the basis of expert consensus. Furthermore, comparable phenotypes were seen between our patient and the aforementioned reported cases, including the presence of facial dysmorphism, feeding difficulties, and hypotonia in all four cases (Supplemental Table 1).
Both previously reported variants (Alazami et al., 2015; Malfatti et al., 2015) and the compound heterozygous frameshift variants found in our patient lie within an uncharacterized protein domain of MYO18B that harbors a nuclear localization signal and a Sug1 binding site, which targets the protein for degradation by the ubiquitin-proteasome pathway (Inoue et al., 2006; Salamon et al., 2003). Alterations that affect ubiquitin-proteasome activity, resulting in escape from protein degradation, is reported as a potential mechanism of oncogenic activation for the receptor tyrosine kinases (RTK), MET and PDGF receptors (Demoulin and Montano-Almendras, 2012; Peschard et al., 2001). The E3 ubiquitin ligase, c-Cbl targets these RTKs for ubiquitination (Demoulin and Montano-Almendras, 2012; Peschard et al., 2001). Various malignancies have reported mutations within the RTK c-Cb1 tyrosine kinase binding domain that impair degradation and increase receptor signaling (Demoulin and Montano-Almendras, 2012; Peschard et al., 2001). A possible cause of MYO18B overexpression could involve loss of the Sug1 binding site which may act through a similar mechanism as MET and PDGFR.
Notably, MYO18B is proposed as a tumor suppressor gene, since molecular events resulting in gene inactivation were found to contribute to tumor progression in multiple solid tumors (Bhatla et al., 2016; Bleeker et al., 2009; Nakano et al., 2005; Nishioka et al., 2002; Yanaihara et al., 2004). In particular, MYO18B promoter hypermethylation, chromosomal deletion, or somatic mutation were identified in about 50% of lung cancer cell lines and tissues obtained from surgical resection, suggesting that inactivation of MYO18B has a role in lung carcinogenesis (Nishioka et al., 2002). Similar findings were also reported in ovarian cancer (Yanaihara et al., 2004). In colorectal cancer cell lines and surgically resected tumor, MYO18B silencing occurred through histone H3 and H4 hypoacetylation (Nakano et al., 2005). A role for MYO18B as a tumor suppressor in the brain is not reported nor is there an association between mutations in MYO18B and increased brain cancer risk.
Medulloblastoma, a posterior fossa neuroepithelial tumor, is a common malignant pediatric brain tumor. To date, medulloblastoma in a patient with KFS is not reported in the literature. Given the increased expression of MYO18B found in Group 3 medulloblastoma patients (Supplemental Fig. 1), further evaluation of this gene and its potential role in medulloblastoma is warranted. As our patient harbored compound heterozygous frameshift variants both predicted to encode a premature stop of translation, a possible mechanism associated with MYO18B is impaired function due to bi-allelic mutation. However, a dominant negative effect or gain of function may also be at play, similar to that described with tumor suppressor gene TPS3 (Harvey et al., 1995; Wijnhoven et al., 2007). Notably, although a dominant negative effect or gain of function was not reported for skin tumor development in association with TPS3 alteration, these effects were demonstrated for mammary and lung epithelium, suggesting these mechanisms may be tissue-specific (Wijnhoven et al. 2007).
In summary, we describe the first case of a pediatric patient with KFS co-occurring with Group 3γ medulloblastoma. Our transcriptomic analysis indicated that medulloblastoma expressed increased levels of MYO18B compared to other pediatric CNS tumors, thus further evaluation of the role for this gene in disease pathogenesis is warranted. Moreover, within our patient’s tumor, we identified transcripts harboring each mutant allele. Thus, this patient is inferred to express transcripts predicted to encode bi-allelic premature stops of translation, and likely a dysfunctional protein. The constitutional variants identified in our described patient are in close proximity to previously reported pathogenic MYO18B variants (Fig. 1D) (Alazami et al., 2015; Malfatti et al., 2015). To date, loss of function is hypothesized as the mechanism of action in the setting of KFS phenotype. Through enrollment on our translational research protocol, coexisting constitutional and somatic diagnoses in this patient enabled the elucidation of the genetic etiology of KFS, expanded our understanding of the phenotype, morbidity and mortality associated with KFS, and provided further support for the role of MYO18B in tumor suppression.
Supplementary Material
Acknowledgements
We thank the patient and their family for participating in our translational research protocol and the University of California Santa Cruz Treehouse Childhood Cancer Initiative for generating and providing a publicly available pediatric cancer dataset for comparative analyses. We thank the Nationwide Foundation Pediatric Innovation Fund for generously supporting sequencing, data production and analysis.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmg.2019.103701.
ClinVar accession numbers
SCV000844943.
SCV000844982.
References
- Adorno A, Alafaci C, Sanfilippo F, Cafarella D, Scordino M, Granata F, Grasso G, Salpietro FM, 2015. Malignant teratoma in Klippel-Feil syndrome: a case report and review of the literature. J. Med. Case Rep 9 (1). 10.1186/s13256-015-0700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajima R, Akazawa H, Kodama M, Takeshita F, Otsuka A, Kohno T, Komuro I, Ochiya T, Yokota J, 2008. Deficiency of Myo18B in mice results in embryonic lethality with cardiac myofibrillar aberrations. Genes Cells 13 (10), 987–999. 10.1111/j1365-2443.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Alazami AM, Kentab AY, Faqeih E, Mohamed JY, Alkhalidi H, Hijazi H, Alktiraya FS, 2015. A novel syndrome of Klippel-Feil anomaly, myopathy, and characteristic facies is linked to a null mutation in MYO18B. J. Med. Genet 52 (6), 400–404. 10.1136/jinedgenet-2014-102964. [DOI] [PubMed] [Google Scholar]
- Bayrakli F, Guclu B, Yakicier C, Balaban H, Kartal U, Erguner B, Sagiroglu MS, Yuksel S, Ozturk AR, Kazanci B, Ozum U, Kens HZ, 2013. Mutation in MEOX1 gene causes a recessive Klippel-Feil syndrome subtype. BMC Genet 14, 95 10.1186/1471-2156-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Berger S, Li M, Currie PD, 2017. Myo18b is essential for sarcomere assembly in fast skeletal muscle. Hum. Mol. Genet 26 (6), 1146–1156. 10.1093/hing/ddx025. [DOI] [PubMed] [Google Scholar]
- Bhatla T, Dandekar S, Lu BY, Wang J, Han E, Bitterman D, Jones CL, Evensen NA, Magid M, Meyer JA, Carroll WL, 2016. Genomic characterization of poorly differentiated neuroendocrine carcinoma in a pediatric patient. J. Pediatr. Hematol. Oncol 38 (1), e21–25. 10.1097/MPH.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker FE, Lamba S, Rodolfo M, Scarpa A, Leenstra S, Vandertop WP, Bardelli A, 2009. Mutational profiling of cancer candidate genes in glioblastorna, melanoma and pancreatic carcinoma reveals a snapshot of their genomic landscapes. Hum. Mutat 30 (2), E451–E459. 10.1002/humti.20927. [DOI] [PubMed] [Google Scholar]
- Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, Garzia L, Torchia J, Nor C, Morrissy AS, Agnihotri S, Thompson YY, Kuzaii-Fischer CM, Farooq H, Isaev K, Daniels C, Cho BK, Kim SK, Wang KC, Lee JY, Grajkowska WA, Perek-Polnik M, Vasiljevic A, Faure-Conter C, Jouvet A, Giannini C, Nageswara Rao AA, Li KKW, Ng HK, Eberhart CG, Pollock IF, Hamilton RL, Gillespie GY, Olson JM, Leary S, Weiss WA, Lach B, Chambless LB, Thompson RC, Cooper MK, Vibhakar R, Hauser P, van Veeleii MC, Kros JM, French PJ, Ra YS, Kumabe T, Lopez-Aguilar E, Zitterbart K, Sterba J, Finocchiaro G, Massimino M, Van Meir EG, Ostika S, Shofuda T, Klekner A, Zollo M, Leonard JR, Rubin JB, Jabado N, Albrecht S, Mora J, Van Meter TE, Jung S, Moore AS, Hallahan AR, Chan JA, Tirapelli DPC, Carlotti CG, Fouladi M, Pimeiitel J, Faria CC, Saad AG, Massimi L, Liam LM, Wheeler H, Nakamura H, Elbabma SK, Perezpeiia-Diazcoiiti M, Chico Ponce de Leon F, Robinson S, Zapotocky M, Lassaletta A, Huang A, Hawkins CE, Tabori U, Botiffet E, Bartels U, Dirks PB, Rtitka JT, Bader GD, Reimaiid J, Goldenberg A, Ramaswamy V, Taylor MD, 2017. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 31 (6), 737–754. e736. 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin JB, Montano-Alinendras CP, 2012. Platelet-derived growth factors and their receptors in normal and malignant lieniatopoiesis. ln J Blood Res 2 (1), 44–56. [PMC free article] [PubMed] [Google Scholar]
- Harvey M, Vogel H, Morris D, Bradley A, Bernstein A, Donehower LA, 1995. A mutant p53 transgene accelerates tumour development in heterozygous but not iiullizygous p53-deficient mice. Nat. Genet 9 (3), 305–311. 10.1038/ng0395-305. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kon T, Ajima R, Ohkura R, Tani M, Yokota J, Sutoh K, 2006. MYO18B interacts with the proteasomal subunit Sug1 and is degraded by the ubiquitin-proteasome pathway. Biochem. Biophys. Res. Commtin 342 (3), 829–834. 10.1016/j.bbrc.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Malfatti E, Bohm J, Lacene E, Beuvin M, Romero NB, Laporte J, 2015. A premature stop codon in MYO18B is associated with severe nemaline myopathy with cardiomyopathy. J. Neuromuscul. Dis 2 (3), 219–227. 10.3233/JND-150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughran JM, Oates A, Donnai D, Read AP, Tassabehji M, 2003. Mutations in PAX1 may be associated with Klippel-Feil syndrome. Eur. J. Hum. Genet 11 (6), 468–474. 10.1038/sj.ejlig.5200987. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, Bennet C, Bertagnolli D, Browner K, Butler S, Caldejon S, Carey A, Cuhaciyan C, Dalley RA, Dee N, Dolbeare TA, Facer BA, Feng D, Fliss TP, Gee G, Goldy J, Gourley L, Gregor BW, Gu G, Howard RE, Jochim JM, Kuan CL, Lau C, Lee CK, Lee F, Lemon TA, Lesnar P, McMurray B, Master N, Mosqueda N, Naluai-Cecchini T, Ngo NK, Nyhus J, Oldre A, Olson E, Parente J, Parker PD, Parry SE, Stevens A, Pletikos M, Reding M, Roll K, Sandmaii D, Sarreal M, Shapotiri S, Shapovalova NV, Shen EH, Sjoquist N, Slaughterbeck CR, Smith M, Sodt AJ, Williams D, Zollei L, Fischl B, Gerstein MB, Geschwind DH, Glass IA, Hawrylycz MJ, Hevner RF, Huang H, Jones AR, Knowles JA, Levitt P, Phillips JW, Sestan N, Wohnoutka P, Dang C, Bernard A, Hohmann JG, Lein ES, 2014. Transcriptional landscape of the prenatal human brain. Nature 508 (7495), 199–206. 10.1038/nattirel3l85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed JY, Faqeih E, Alsiddiky A, Alshammari MJ, Ibrahim NA, Alkuraya FS, 2013. Mutations in MEOX1, encoding mesenchyme homeobox 1, cause Klippel-Feil anomaly. Am. J. Hum. tenet 92 (1), 157–161. https://doi.orQ10.1016/j.ajlig.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Tani M, Nishioka M, Kohno T, Otsuka A, Ohwada S, Yokota J, 2005. Genetic and epigenetic alterations of the candidate tumor-suppressor gene MYO18B, on chromosome arm 22q, in colorectal cancer. Genes Chromosomes Cancer 43 (2), 162–171. 10.1002/gcc.20180. [DOI] [PubMed] [Google Scholar]
- Nishioka M, Kohno T, Tant M, Yanaihara N, Tomizawa Y, Otsuka A, Sasaki S, Kobayashi K, Niki T, Maeshima A, Sekido Y, Minna JD, Sone S, Yokota J, 2002. MYO18B, a candidate tumor suppressor gene at chromosome 22q12.1, deleted, mutated, and methylated in human lung cancer. Proc. Natl. Acad. Sci. U. S. A 99 (19), 12269–12274. 10.1073/pnas.192445899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschard P, Fournier TM, Laniorte L, Naujokas MA, Band H, Langdon WY, Park M, 2001. Mutation of the c-cbl TKB Lorna in binding site on the met receptor tyrosine kinase it into a transforming protein. Mol. Cell 8, 995–1004. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee, 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular Pathology. Genet. Med 17 (5), 405–424. https://doi.ord10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon M, Millino C, Raftaello A, Mongillo M, Sandri C, Beni C, Negrisolo E, Pallavicini A, Valle G, Zaccolo M, Scliiaffino S, Lanfranchi G, 2003. Human MYO18B, a novel unconventional myosin heavy chain expressed in striated muscles moves into the myorruclei upon differentiation. J. Mol. Biol 326 (1), 137–149. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, de Bock CE, Howard E, Malass M, Donnai D, Diwan A, Manson FD, Murrell D, Clarke RA, 2008. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum. Mutat 29 (8), 1017–1027. https://doi.org/10.1002NitHnu.20741. [DOI] [PubMed] [Google Scholar]
- Thomsen MN, Schneider U, Weber M, Johannisson R, Niethard FU, 1997. Scoliosis and anomalies associated with klippel-feil syndrome types I-III. Spine 22 (4), 396–401. [DOI] [PubMed] [Google Scholar]
- Tracy MR, Dormans JP, Kusumi K, 2004. Klippel-feil syndrome: clinical features and current understanding of etiology. Clin. Orthop. Relat. Res 424, 183–190. [PubMed] [Google Scholar]
- Wijnhoven SW, Speksnijder EN, Liu X, Zwart E, vaiiOostrom CT, Beems RB, Hoogervorst EM, Schaap MM, Attardi LD, Jacks T, van Steeg H, Jonkers J, de Vries A, 2007. Dominant-negative but not gain-of-function effects of a p53.R270H mutation in mouse epithelium tissue after DNA damage. Cancer Res 67 (10), 4648–4656. 10.1158/0008-5472.CAN-06-4681. [DOI] [PubMed] [Google Scholar]
- Yanaihara N, Nishioka M, Kohno T, Otsuka A, Okamoto A, Ochiai K, Tasks T, Yokota J, 2004. Reduced expression of MYO18B, a candidate tumor-suppressor gene on chromosome arm 22q, in ovarian cancer. Int. J. Cancer 112 (1), 150–154. 10.1002/ijc.20339. [DOI] [PubMed] [Google Scholar]
- Ye M, Berry Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, French CR, Abitbol M, Fleisch VC, Corbett N, Allison WT, Drummond G, Walter MA, Underhill TM, Waskiewicz AJ, Lehmann OJ, 2010. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum. Mol. Genet 19 (2), 287–298. https://doi.ord10.1093/hig/ddp496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.