Abstract
Cytomegaloviruses (CMVs) infect many mammals, including humans and non–human primates (NHPs). Human cytomegalovirus (HCMV) is an important opportunistic pathogen among immunocompromised patients and represents the most common infectious cause of birth defects. HCMV possesses a large genome and very high genetic diversity. NHP–infecting CMVs share with HCMV a similar genomic organization and coding content, as well as the course of viral infection. Recent technological advances have allowed the sequencing of several HCMV strains from clinical samples and provided insight into the diversity of NHP–infecting CMVs. The emerging picture indicates that, with the exclusion of core genes (genes that have orthologs in all herpesviruses), CMV genomes are relatively plastic and diverse in terms of gene content, both at the inter– and at the intra–species level. Such variability most likely underlies the strict species–specificity of these viruses, as well as their ability to persist lifelong and with relatively little damage to their hosts. However, core genes, despite their strong conservation, also represented a target of adaptive evolution and subtle changes in their coding sequence contributed to CMV adaptation to different hosts. Indubitably, important knowledge gaps remain, the most relevant of which concerns the role of viral genetics in HCMV–associated human disease.
Keywords: cytomegalovirus, non–human primates, genome organization, species–specificity, positive selection
1. Introduction
The family Herpesviridae includes a variety of enveloped double–stranded DNA viruses that infect many vertebrates, including small mammals, humans, and non–human primates (NHPs) [1,2]. The family is divided into three subfamilies (Alpha–, Beta–, and Gammaherpesvirinae), which differ in genomic structure, cell tropism, and in their host–switching capacity. Indeed, cross–species transmission is repeatedly reported for members of the Alphaherpesvirinae and the Gammaherpesvirinae subfamilies [3,4,5,6]. Conversely, in betaherpesviruses, inter–species transmissions are rare events, indicating that, in natura, these viruses are restricted to their natural host [7,8,9,10] (see below).
In this review, we focus our attention on the evolution and virus–host relationships of primate–infecting cytomegaloviruses (CMVs). These viruses are classified in the genus Cytomegalovirus in the Betaherpesvirinae subfamily [1]. Up to date, 11 CMVs have been recognized as species by the International Committee on Taxonomy of Viruses (ICTV, https://talk.ictvonline.org/, 2018b release, MSL #34), although the complete genome is only available for nine of them (Table 1).
Table 1.
List of known primate–infecting CMVs.
| Primate species | CMV species | Reference | |
|---|---|---|---|
| Scientific name | Common Name | Official name /Common Name (Abbreviation) | |
| Great apes | |||
| Homo sapiens | Human | Human betaherpesvirus 5 * | [11] |
| Pan paniscus | Bonobo | Pan paniscus cytomegalovirus (PpanCMV1, PpanCMV2) | [33] |
| Pan troglodytes | Chimpanzee | Panine betaherpesvirus 2 (CCMV) * | [34] |
| Pan troglodytes ellioti | Nigeria–Cameroon chimpanzee | Panine betaherpesvirus 2 (CCMV1, CCMV2) | [33] |
| Pan troglodytes schweinfurthii | Eastern chimpanzee | Panine betaherpesvirus 2 (CCMV1, CCMV2) | [33] |
| Pan troglodytes troglodytes | Central chimpanzee | Panine betaherpesvirus 2 (CCMV1, CCMV2) | [33] |
| Pan troglodytes verus | West African chimpanzee | Panine betaherpesvirus 2 (CCMV1, CCMV2) | [35] |
| Pongo pygmaeus pygmaeus | Orang–utan | Pongo pygmaeus cytomegalovirus (PpygCMV1) | [35] |
| Gorilla beringei beringei | Mountain gorilla | Gorilla beringei cytomegalovirus (GberCMV1, GberCMV2) | [33,36] |
| Gorilla beringei graueri | Eastern lowland gorilla | Gorilla graueri cytomegalovirus (GgraCMV1, GgraCMV2) | [33] |
| Gorilla gorilla gorilla | Western lowland gorilla | Gorilla gorilla cytomegalovirus (GgorCMV1, GgorCMV2) | [35] |
| Old World Monkeys | |||
| Colobus guereza | Mantled guereza | Colobus guereza cytomegalovirus (CgueCMV) | [37] |
| Colobus polykomos | Black–and–white colobus | Colobus polykomos cytomegalovirus (CpolCMV) | [7] |
| Pilocolobus badius | Western red colobus | Piliocolobus badius cytomegalovirus (PbadCMV) | [9] |
| Cercopithecus aethiops | Grivet | Cercopithecine betaherpesvirus 5 (SCMV) * | [38] |
| Cercopithecus campbelli | Campbell’s monkey | Cercopithecus campbelli cytomegalovirus (CcamCMV) | [9] |
| Cercopithecus cephus | Moustached monkey | Cercopithecus cephus cytomegalovirus (CcepCMV) | AY728178.1 |
| Cercopithecus diana | Diana monkey | Cercopithecus diana cytomegalovirus (CdiaCMV) | [9] |
| Cercopithecus kandti | Golden monkey | Cercopithecus kandti cytomegalovirus (CkanCMV) | [36] |
| Cercocebus agilis | Agile mangabey | Cercocebus agilis cytomegalovirus (CagiCMV) | AY608713.1 |
| Cercocebus atys | Sooty mangabey | Cercocebus atys cytomegalovirus (CatyCMV) | [9] |
| Macaca fascicularis | Crab–eating macaque | Macacine betaherpesvirus 8 (CyCMV) * | [8] |
| Macaca mulatta | Rhesus macaque | Macacine betaherpesvirus 3 (RhCMV) * | [39] |
| Papio anubis | Olive baboon | Papiine betaherpesvirus 3 (BaCMV) | [40] |
| Papio cynocephalus | Yellow baboon | Papio cynocephalus cytomegalovirus (PcynCMV) | [40] |
| Papio ursinus | Chacma baboon | Papiine betaherpesvirus 4 (BaCMV) * | [43] |
| Mandrillus leucophaeus | Drill | Mandrilline betaherpesvirus 1 (MleuCMV) * | [43] |
| Mandrillus sphinx | Mandrill | Mandrillus sphinx cytomegalovirus (MsphCMV) | [35] |
| New World Monkeys | |||
| Sapajus apella | Tufted capuchin | Sapajus apella cytomegalovirus (SapeCMV) | [10] |
| Cebus albifrons | White–fronted capuchin | Cebine betaherpesvirus 1 (CalbCMV) | [10] |
| Cebus capucinus | White–headed capuchin | Cebine betaherpesvirus 1 (CcapCMV) | [10] |
| Saimiri boliviensis boliviensis | Black–capped squirrel monkey | Saimiri boliviensi cytomegalovirus (SbolCMV) | [10] |
| Saimiri sciureus | Squirrel monkey | Saimiriine betaherpesvirus 4 (SMCMV) * | [38] |
| Saimiri sciureus albigena | Colombian common squirrel monkey | Saimiri albigena cytomegalovirus (SalbCMV) | [10] |
| Aotus trivirgatus | Three–striped night monkey | Aotine betaherpesvirus 1 (OMCMV) * | [38] |
| Aotus vociferans | Spix’s night monkey | Aotus vociferans cytomegalovirus (AvocCMV) | [10] |
| Aotus nancimaae | Nancy Ma’s night monkey | Aotus nancymaae cytomegalovirus (AnanCMV) | [10] |
| Pithecia pithecia | White–faced saki | Pithecia pithecia cytomegalovirus (PpitCMV) | [10] |
| Alouatta macconelli | Guyanan red howler | Alouatta macconelli cytomegalovirus (AmacCMV) | [10] |
| Alouatta palliata | Mantled howler | Alouatta palliata cytomegalovirus (ApalCMV) | [10] |
| Alouatta seniculus | Venezuelan red howler | Alouatta seniculus cytomegalovirus (AsenCMV) | [10] |
| Ateles paniscus | Red–facied spider monkey | Ateles paniscus cytomegalovirus (ApanCMV) | [10] |
Note: ICTV–recognized viral species are in italic; bold and asterisks denote complete genome sequences.
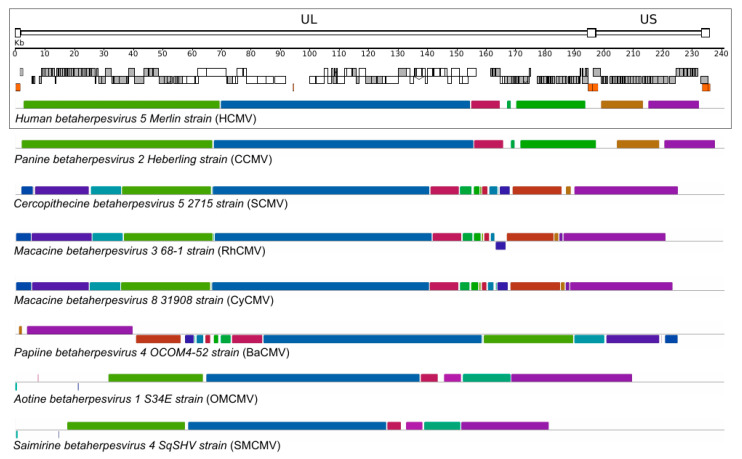
Typically, CMVs display large genomes, exceeding 200 Kb in length, with more than 160 protein–coding genes [2]. Human cytomegalovirus (HCMV, species Human herpesvirus 5) has a genome about 235 Kb in size, one of the largest among herpesviruses and, generally, among human–infecting viruses [11]. CMV genomes consist of unique long (UL) and unique short (US) segments, each of which is flanked by inverted repeats (RL and RS) (Figure 1). Protein–coding genes occupy the great majority of CMV genomes. As detailed below, genes located in the central portion of the UL region constitute a cluster of core genes that have homologs in other herpesviruses and perform core functions. The other genes, referred to as non–core, are primarily identified in betaherpesviruses or are species–specific. These genes are mainly involved in viral persistence and latency, cellular tropism, and host immune response modulation [2,11,12,13,14]. Moreover, CMVs encode a number of miRNAs that regulate host cellular and viral transcriptions to favor viral infection and inhibit the host’s immune response [15].
Figure 1.
Primate CMV genome organization. A schematic representation of the HCMV genome is shown in the upper panel. Annotated coding regions are represented as boxes (white: core genes, gray: non–core genes); repetitive elements are shown as orange boxes. Colored similarity plots with primate CMV genomes are also shown. The whole–genome CMV alignment was obtained with progressive MAUVE [16,17]. Each genome is laid out in a horizontal track and each colored block delimits a genome region that aligns to part of another genome (presumably homologous and free from internal rearrangements) and thus represents a locally collinear block. When the similarity plot points downward it indicates an alignment to the reverse strand of the genome. Genome IDs: HCMV, NC_006273; CCMV, NC_003521; SMCV, NC_012783; RhCMV, NC_006150; CyCMV, NC_033176; BaCMV, NC_027016; OMCMV, NC_016447; SMCMV, NC_016448.
HCMV is a ubiquitous human pathogen, which infects 56–94% of the global population [18]. The seroprevalence increases with age and varies with socioeconomic status and geographical location, with developing countries having the highest prevalence [18]. In most immunocompetent individuals, HCMV infections are asymptomatic, as viral replication is controlled by the host immune system; very often, such infections are undiagnosed, as symptoms are ambiguous and relatively mild [19]. However, in analogy to other herpesviruses, HCMV establishes lifelong persistence as a latent infection. Virus latency is characterized by absent or low–level virus replication [20], with the viral genome persisting in a dormant form predominantly in the CD34+ hematopoietic progenitor cell population in the bone marrow. Virus reactivation, as well as reinfection, is common, especially for susceptible groups, such as immunocompromised patients, pregnant women, newborns, and elderly people [19,21]. In immunocompromised individuals, including transplant recipients and AIDS patients, HCMV primary infection, reinfection, or reactivation can cause a life–threatening disease that affects many organs and results in considerable morbidity and mortality. HCMV is also the most common infectious cause of birth defects. In high–income countries, where maternal seroprevalence is relatively low, rates of congenital HCMV infection are around 0.6 to 0.7% of live births. However, in resource–limiting settings, the prevalence can be as high as 1.5% [22]. Congenital HCMV infection can result in mild to severe clinical outcomes in the developing immune naive fetus. Lifelong sequelae requiring long–term rehabilitation are common in congenitally infected infants and include sensorineural hearing loss, cognitive impairment, and/or vision defects [22]. Furthermore, some lines of evidence suggest that HCMV plays a role as an oncomodulator in the development of different types of cancer [20,23,24] and that it is involved in the pathogenesis of atherosclerosis [25], as well as of inflammatory bowel disease [26]. Finally, HCMV is thought to cause or accelerate immunosenescence in the elderly population, a condition also known as immune aging [27,28].
Thus, some aspects of HCMV pathogenesis are closely linked to the ability of the virus to persist latently in the host. Lifelong persistence is achieved by HCMV through a number of mechanisms that ensure escape from the humoral and cellular host immune responses [29]. Specifically, during their evolutionary history, CMVs (and herpesviruses in general) have acquired new coding genes, which, on the basis of sequence or structure similarity, were most likely captured from their hosts via lateral transfer [30]. These include homologs of host cytokines and their G coupled receptors [30,31,32] which are frequently involved in duplications and deletions in CMV genomes so as to generate species–specific arrays of immunomodulatory proteins. These undermine the host antiviral immunity, eventually reducing immune recognition of the pathogen through molecular mimicry and other mechanisms [29].
As anticipated above, CMVs infect a wide variety of NHPs (Table 1) [7,8,9,10,11,33,34,35,36,37,38,39,40]. As detailed in the following section, NHP–infecting CMVs share with HCMV a similar genomic organization and coding content, as well as the course of viral infection (lifelong persistence). As in humans, CMVs that infect other primates are generally widespread, reaching high seroprevalence levels. For example, in breeding colonies of rhesus macaques, almost the totality of animals are seropositive for RhCMV (rhesus macaque CMV) before the first year of age. High rates of infection were also reported for other NHPs in the wild [10,33,41]. Overlaps between HCMV and NHP CMVs are also observed in terms of infection outcome and clinical presentation. Thus, adult macaques manifest symptoms only when immunocompromised, as a result of primary infection or viral reactivation. In simian AIDS, CMV can produce end–organ disease in different tissues, reflecting HCMV pathogenesis in AIDS subjects [42]. Finally, different studies indicated that congenital RhCMV transmission recapitulates congenital HCMV pathogenesis, with spontaneous abortions or fetuses with brain and neurological defects [42].
2. Diversity and Host–Specificity of Primate CMVs
The first CMV identified in a NHP was isolated in 1962 from an African green monkey (Cercopithecus aethiops) [44]. Thereafter, natural CMV infection was reported in a multitude of NHPs [9,10,33,35,37]. Initially, NHP CMVs were detected mostly in captive animals, but in the last few years surveys were extended to animals living in the wild, so as to generate a more accurate picture of the genetic diversity and distribution of these viruses in nature [9,10,33,35]. To date, CMVs have been detected in over 40 different primates, including great apes, as well as Old World and New World primate species, although complete genome sequences are available for a minority of these viruses (see Table 1).
Each primate species is infected by one or few species–specific CMVs. Indeed, cross–species CMV transmission has never been reported in natural settings, either between closely related species sharing the same habitat, or in NHP predator–prey systems in the wild [7,9,33]. The only limited evidence of cross–species transmission in vivo relates to very peculiar experimental settings or anecdotal reports [8,45,46,47,48]. This marked species–specificity, together with the broad distribution and the almost asymptomatic condition of infected individuals [41], suggests that co–divergence and long–standing adaptation to their hosts largely shaped the evolution of CMVs. This view is also supported by the congruence between the viral and host phylogenies, a general feature for members of the Herpesvirales order [49,50]. These observations do not imply, however, that host–switches did not contribute to the shaping of CMV diversity and host associations. For instance, two CMVs (referred to as CMV1 and CMV2) were described in Western chimpanzees (Pan troglodytes verus) and Western lowland gorillas (Gorilla gorilla gorilla) [33,35]. Phylogenetic analyses showed that the sequences of these viruses cluster into two different clades, both including CMVs from chimpanzees and gorillas [33,35]. These data clearly suggest a more complex evolutionary scenario of CMVs in hominines, whereby two bidirectional ancestral cross–species transmission events between hosts belonging to chimpanzee and gorilla lineages most likely occurred. Recent estimates indicated that the transmission of CMV1 from gorillas to panines occurred around 2 million years ago, whereas CMV2 crossed the species barrier from panines to gorillas approximately 1.2 million years ago [33]. Conversely, the divergence of bonobo and chimpanzee CMV1 and CMV2 were synchronous with the divergence of their hosts (0.87 million years ago), indicating subsequent virus–host co–divergence [33].
Whereas CMV host–specificity is very strict in vivo, replication in different host cells in vitro seems to be slightly more permissive, with reported cases of cell infection for closely related host species only [51,52,53] but not for phylogenetically distant hosts [54]. For instance, HCMV can replicate at low levels in chimpanzee primary fibroblasts [53], but other NHP cells do not support the production of infectious virions [55,56,57,58].
The molecular mechanisms responsible for this strict host–specificity are not fully understood. As mentioned above, CMVs display very large genomes, organized into two covalently linked segments [34]. As shown in Figure 1, the overall genome architecture is conserved among primate CMVs. The majority of core genes, which encode essential proteins involved in DNA replication, virion assembly and maturation, are confined to the central shared collinear block (Figure 1). Clearly, core genes are highly conserved in sequence among CMVs. For example, HCMV sequence identity ranges from 60 to 90% with chimpanzee CMV (CCMV), and from 50 to 82% with RhCMV [41]. Conversely, non–core genes are less conserved in sequence and number, with variability observed between closely–related CMV species and, sometimes, even among isolates of the same species (see below). These genes are often dispensable for viral growth in cell culture [13,14], but play important roles during infection in vivo [13,41,59]. In particular, a significant proportion of non–core genes have evolved to elude and subvert the host immune response [41,59]. It is worth mentioning here that adaptation to cell culture is a common feature of CMVs. This is due to the fact that viral mutants are selected during isolation and passage in fibroblasts or other cell types, where no pressure is exerted by the host adaptive immune response [12]. Thus, mutations (including relatively large deletions and rearrangements) occur in genomic regions that are not essential for viral replication and the process originates lab–adapted strains, which differ in gene content from clinical isolates [12].
Both commonalities and differences among primate CMV genomes most likely represent key elements to understand the adaptation of these viruses to different, but closely related, primate hosts. One of the best examples of host specificity of primate CMVs is the relationship between RhCMV, CyCMV (cynomolgus macaque cytomegalovirus), and their respective hosts. The two viruses are closely related, they possess collinear genomes and show a high level of genetic identity, mirroring the close phylogenetic relationship among their hosts (i.e., Indian–origin rhesus macaques and Mauritian–origin cynomolgus macaques) (Figure 1) [8]. Despite these similarities, in vivo cross–infection has not been documented yet, although several lines of evidence suggest that CyCMV can infect different hosts and different cell types in vitro [52,53,60,61]. At the same time, different lab–adapted CyCMV and RhCMV strains have shown the ability to infect different host cells [8,51,61]. This peculiar characteristic of lab–adapted strains has allowed the identification of specific genes that may have a role in cross–species infection [8,60]. In particular, an engineered strain of RhCMV, with restored expression of UL36 or UL128, together with UL130, can infect cynomolgus macaques both in vitro and in vivo [8]. The specific function of these genes underlies their role in cross–species infections. pUL36 plays a role in the inhibition of apoptosis by binding to caspase–8 and preventing its activation [62] pUL128 and pUL130 interact with each other and form a complex with other viral envelope glycoproteins to facilitate entry in non–fibroblast cell types [63]. Overall, these results indicate that different mechanisms contribute to species–specificity and that cell culture passages can have an impact in studying cross–species transmission.
Other interesting candidates as genetic determinants of host–specificity in vivo are the members of gene families encoding viral CXC chemokine ligand–like proteins (vCXCL) and genes encoding viral G protein–coupled receptor–like proteins (vGPR). These genes are found to be tandemly repeated in primate CMVs, and each viral genome encodes a different number of them [64]. Although their function is not fully understood, it has been proposed that their diversity in terms of number and sequence is associated with viral infection in vivo, likely by a different modulation of the host immune response [64,65].
However, as mentioned above, some CMVs such as HCMV display species–specificity even in cell culture i.e., in the absence of the host adaptive immune response. Such specificity is mainly driven by post–entry events [56,57,58,66,67], indicating that, in order to become one of the most successful human pathogens, HCMV must have adapted to efficiently complete its infectious cycle in human cells. As expected, the closest relative of HCMV is CCMV, which displays a strong conservation in terms of genome organization and sequence (Figure 1) [34]. Thus, comparison of the genomes of these two viruses can provide relevant information on the events underlying HCMV adaptation to our species. Adaptation to a new host is often accompanied by episodes of positive selection, a situation whereby amino acid replacements accumulate at a faster rate than expected based on the rate observed at synonymous sites [68]. A recent genome–wide scan of positive selection identified changes that most likely arose during HCMV adaptation to the human host. Several signals of positive selection were found to be located within core genes [69]. In particular, evidence of selection was found in genes encoding capsid components and tegument proteins that drive important steps in virion maturation and assembly. Overall, these results suggest polygenic adaptation of HCMV to its human host. However, analysis of selected sites in a core viral enzyme, the primase (pUL70), indicated that two amino acid changes, which most likely arose during the emergence of HCMV as a species, decrease rather than increase replication in human cells. In human fibroblasts, mutant viruses that recapitulate the amino acid state observed in non–human infecting CMVs had higher fitness than the wild–type HCMV strain [69]. Although this finding may seem counter–intuitive, the evolution of mechanisms that reduce or control viral replication in the host might be necessary to decrease virulence and ultimately promote long–standing host–pathogen associations. A similar hypothesis had previously been put forward by Dunn and co–workers, when a functional assessment of HCMV coding potential indicated that the deletion of some genes (e.g., UL10, UL23, and US16) increased replication in specific cell types. The authors thus suggested that a temperance effect is necessary to promote the long–term co–existence of the virus with its host [70].
3. High Population–Level Diversity of Circulating HCMV Strains
The first complete HCMV genome sequence, that of the high–passage strain AD169, was obtained in 1989, from a plasmid library [71]. More than a decade later, other laboratory strains and clinical isolates were grown in cell culture and sequenced [11,12,70]. These and other early efforts started to reveal that the genetic diversity of HCMV is high and that mutations frequently arise in cell culture [72]. More recently, the development of high–throughput technologies has allowed the sequencing of a sizable number of HCMV strains, either directly from clinical specimens or after a small number of passages [73,74,75,76,77,78]. To date, more than 300 complete HCMV genomes are available in public databases. However, the geographic origin of these sequences is highly biased, with the majority of samples coming from Europe and USA, a few from Israel, and one from Asia. As for Sub–Saharan Africa, where the virus is highly prevalent [18], the first four complete HCMV genome sequences were obtained in 2019 [74].
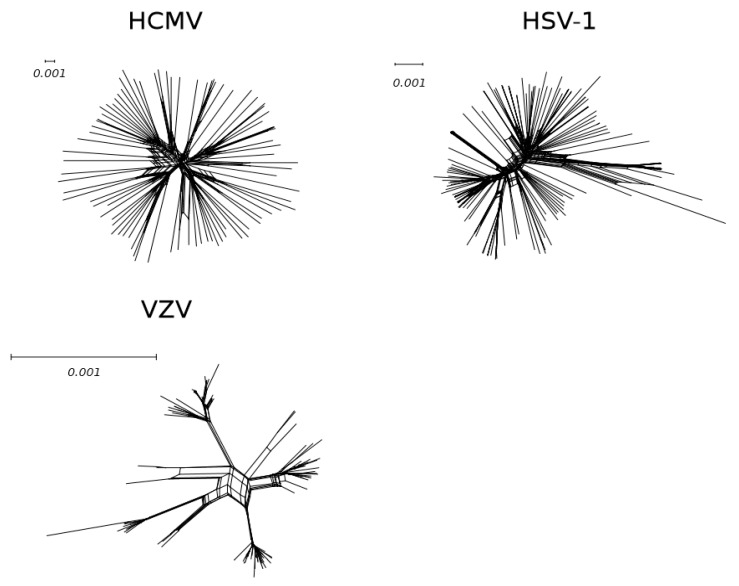
Parallel advances in the sequencing of other human herpesviruses allowed comparison among these common human pathogens. It was thus shown that, at the population level, HCMV is significantly more genetically diverse than HSV–1 (Herpes simplex virus), EBV (Epstein–Barr virus), and VZV (Varicella–zoster virus) [75]. If the scale is considered, the difference between the HCMV and HSV–1 trees is impressive, and even more so if HCMV is compared to VZV (Figure 2).
Figure 2.
Genetic diversity of human herpesviruses. Neighbor–net split networks of 84 unpassaged HCMV (Supplementary Table S1), 140 HSV–1 [79], and 110 VZV [80] genomes. A phylogenetic network is a graph to visualize the evolutionary relationships (edges) among taxa (nodes). In particular, a split network represents incompatibilities within a data set by combining the results of different phylogenetic trees. Neighbor–net split networks of all whole genome sequences were constructed with SplitsTree v4.13.1 [81] using uncorrected p–distances and all polymorphic sites, after removing gap sites.
Another notable difference among human herpesviruses is the degree of geographic structuring of viral populations. At one extreme stand VZV and EBV, which display a clear spatial distribution of genetic diversity [82,83,84,85]. For instance, VZV clades 1, 3, and 6 are mainly transmitted in Europe, North America, and Australia, whereas clade 2 is primarily Asian [83]. Other human herpesviruses such as HSV–1 and HSV–2 display limited geographic stratification of genetic diversity, and this is also the case for HCMV [74,75,79,86,87]. On one hand, the absence of geographic clustering might be partially due to the skewed origin of available HCMV sequences. For instance, in the case of HSV–2, a viral lineage that is mainly transmitted in Africa was discovered only recently [88], suggesting that, likewise, a portion of HCMV diversity remains unknown, either in Africa or in other continents. On the other hand, different factors related to distinct aspects of viral biology most likely account for the geographic structuring of genetic diversity or lack thereof. In the case of VZV, recent data indicated that viral lineage extinction may be common for this virus, most likely as a consequence of its epidemiology, which is mainly characterized by large chickenpox outbreaks among children (before the introduction of a vaccine) [80]. This feature may contribute to the local emergence of viral lineages and favor geographic segregation. Human herpesviruses other than VZV are not characterized by epidemic spread, though. Recent analyses, however, indicated that HCMV and HSV–1 have a different recombination pattern compared to EBV. In particular, the two former viruses are essentially freely recombining, with only limited regions of linkage disequilibrium (LD) throughout their genomes [85,87]. Conversely, despite abundant recombination, EBV shows extensive genome–wide LD [85]. Because sites in LD are enriched in EBV genes that encode immunogenic proteins, Wegner and coworkers suggested that viral population structure is determined by local adaptation to human populations [85]. In summary, these data suggest that, although a wider sampling of HCMV diversity may in the future reveal some level of geographic clustering, most HCMV stains derive from a highly recombining panmictic population distributed worldwide.
Indeed, recombination stands out as a major driver of HCMV genetic diversity [73,75,87,89]. Recombination clearly requires that the same individual is infected by more than one strain and, as mentioned above, mixed HCMV infections are very common. Based on these observations, as well on their data showing that a minority of genes involved in immune evasion or cell entry are highly divergent and non–recombining, Lassalle and coworkers [87] suggested that HCMV has evolved strategies to promote super–infection and, consequently, recombination. This would serve the purpose of reassorting such highly diverse regions and to ensure efficient immunoevasion.
Clearly, recombination is not the only force shaping HCMV diversity, and genetic polymorphism is not evenly distributed across the genome [75]. Genes encoding viral glycoproteins have long been recognized among the most variable in HCMV strains [90,91]. More recent studies that systematically analyzed sequence diversity along HCMV genomes indicated that glycoproteins and proteins involved in immunomodulation tend to be less constrained and to display the strongest signals of positive selection [69,75,76,86,92]. Also, selection is stronger in protein regions exposed on the virion surface and, for viral proteins expressed at the host cell membrane, in the extracellular domains [69]. Overall, these observations suggest that the selective pressure exerted by the host immune system has likely played a major role in the shaping of genetic diversity among circulating HCMV strains. In line with this view, several sites targeted by positive selection are located within epitopes recognized by human antibodies or in protein regions that directly interact with host molecules involved in immune response [69,75]. These features are consistent with an ongoing genetic conflict between HCMV and the human immune system.
However, recent analyses also detected hotspots of diversity and positive selection that are more difficult to reconcile with an underlying selective pressure. One of these is the furin cleavage site of glycoprotein B (gB) [69,93]. Positively selected sites in this region affect the kinetic of cleavage by furin and were suggested to modulate viral spread in different tissues, although evidence for this is presently lacking [69,93]. Another unusual selection signature was reported within the signal peptides of several HCMV proteins [69]. Functional characterization of one of these indicated that the sequence of the signal peptide of the pUL144 glycoprotein determines the timing of cleavage by the signal peptidase and the dynamics of pUL144 intracellular trafficking. The phenotypic effect of such changes in sorting dynamics is presently unclear but, by analogy with the HIV–1 envelope protein [94], we recently suggested that variation within the signal peptide might affect protein folding or glycosylation, ultimately modulating antigenic properties [69]. If this were the case, HCMV signal peptide diversity might represent an additional layer of control over host immune surveillance.
4. Frequency and Origin of Gene–Disrupting Mutations in HCMV Strains
Another unexpected feature that emerged from the analysis of several HCMV genomes is the frequency with which disruptive mutations occur in different genes. These mutations, which include single nucleotide substitutions, small frameshifting insertions/deletions, and large genomic deletions, are predicted to have deleterious effects on gene expression and protein function. This phenomenon is well described in laboratory passaged HCMV strains [95,96], but several lines of evidence suggest that it also occurs in clinical isolates [75,77,96,97].
A peculiar feature of these mutations is that specific genes and gene families are usually affected. These include the UL11, RL1, US6, and US12 families [75] and most mutated genes are dispensable for viral growth in vitro [75], but possibly have a role in cell tropism [96]. Disrupting mutations have been found in other genomic regions, but they seem to be sporadic [96].
The emergence of deleterious mutations in laboratory strains can be explained by viral adaptation to several cell–cultured passages [95], but for clinical samples the explanation is likely to be more complex. In fact, some of these disruptive changes (both indels or point mutations) are shared among different strains, suggesting that they were already present in their common ancestor [96].
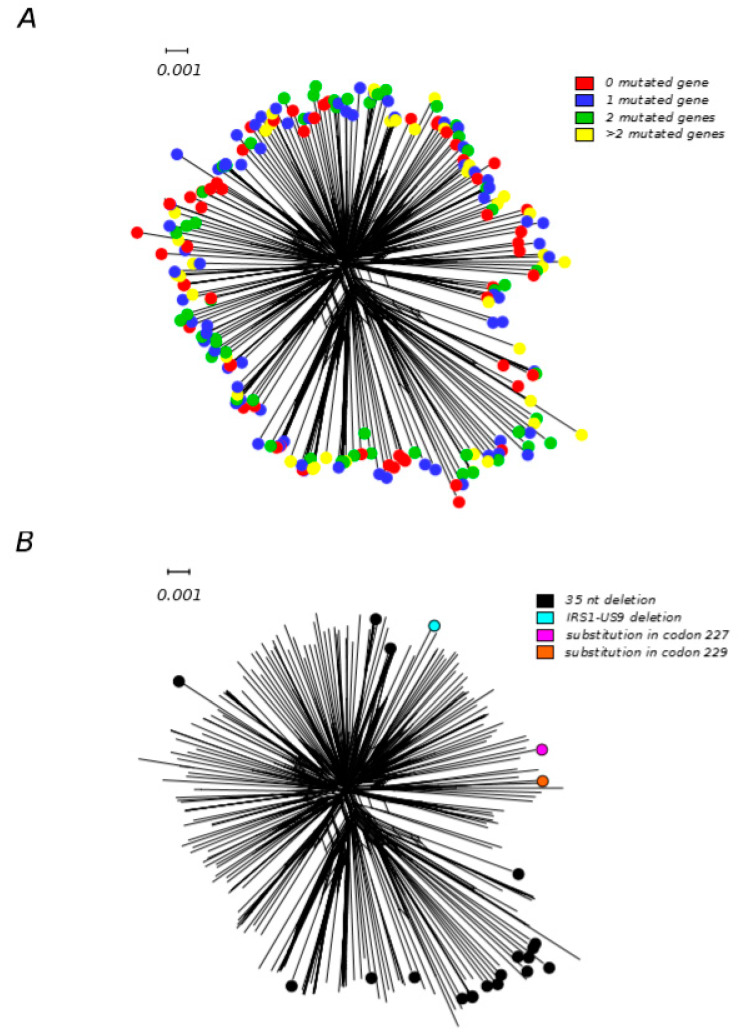
To further explore this scenario, we retrieved gene annotation for 233 HCMV genomes from the GenBank database (Supplementary Table S1) and we analyzed the disrupted gene frequency and the number of passages in cell culture. Among these strains, 84 were annotated as unpassaged, the remaining went through one to several passages. One–hundred–and–seventy out of 233 strains (58 unpassaged, 112 with at least one passage) had at least one disrupted gene, reinforcing the idea that this phenomenon is not dependent on laboratory adaptation, as previously suggested [73,75]. For many genes, different genomic variations (i.e., insertions, deletions, and single nucleotide mutations) were identified, indicating that disruptive mutations arose multiple times and are not only the result of common ancestry. We then analyzed the number of genes and which genes were mutated. Ninety–seven genomes carried more than one truncated coding sequence (Figure 3A), resulting in several different combinations of gene losses in HCMV strains. As previously shown [75], the most frequently mutated genes were UL9, RL5A, UL1, RL6, and US9. So we tested whether strains with the same mutated gene (regardless of the specific mutation they carry) shared a common ancestor and, consequently, a common evolutionary history. To that purpose, we applied two different methods that, by taking phylogenetic uncertainty into account, evaluate whether different viruses from a population follow or not independent evolutionary histories [98]. In particular, we applied one method based on reconstructing phylogenetic trees (Slatkin–Maddison test) and one genetic–distance–based method (Nearest–neighbor statistic) [98,99] on all 233 genome sequences. These sequences were divided between the ones carrying all different mutations in one of the mutated genes and all the other strains. This analysis was repeated for the five most frequent mutated genes. We found that genomes carrying four different US9 detrimental mutations shared a more similar evolutionary history compared to what would be expected by chance (p < 0.01 for both tests) (Figure 3), suggesting that the genetic background influences the ability to tolerate a non–functional US9 gene. The best characterized function of the pUS9 protein is to target MAVS and the STING–TBK1 signaling pathway to inhibit IFN–β expression and, consequently, the downstream antiviral responses [100]. This function is, however, partially redundant with those exerted by other viral proteins (i.e., pUL83, pUL82, and pUL122) [101]. pUS9 might also play antiapoptotic functions, by inhibiting the short form of MAVS. Antiapoptotic activity is also exerted by pUL37 (also known as vMIA), which binds to and recruits Bax to mitochondria [102]. It is thus possible that, depending on their genetic make–up, some HCMV strains are particularly efficient at inhibiting apoptosis and IFN–β expression, and that the action of pUS9 is therefore dispensable. However, the maintenance in the HCMV population of multiple genes with partially overlapping functions, together with sustained recombination rates, is likely to confer mutational robustness and important adaptive advantages.
Figure 3.
Gene disrupting mutations in HCMV strains. Neighbor–net split network of 233 HCMV genome sequences. (A) Strains are color–coded on the basis of the number of mutated genes. (B) Strains are color–coded depending on the type of US9 mutation (Supplementary Table S1). Neighbor–net split networks of genomic sequences were constructed with SplitsTree v4.13.1 [81] using uncorrected p–distances and all polymorphic sites, after removing gap sites.
5. Conclusions and Future Perspectives
For a long time, the large size of CMV genomes has posed challenges to their sequencing. The advent of high–throughput technologies has thus particularly benefited the field of CMV genomics. Such technologies not only allow the rapid sequencing of long genomic regions, but also offer the possibility to obtain complete genome sequences from little amounts of viral DNA. Thus, the direct sequencing from patients’ samples has bypassed the problems associated with the in vitro passaging of HCMV strains, so that hundreds of genomes of clinical isolates were obtained [73,74,75,77,78,97]. In parallel, field work has extended knowledge about CMVs hosted by NHPs living in the wild [9,10,33,35]. These advances have provided invaluable insight into the evolutionary history of primate–infecting CMVs. The emerging picture indicates that, with the exclusion of core genes, CMV genomes are relatively plastic and diverse in terms of gene content, both at the inter– and at the intra–species level. Such variability is most likely a key element underlying the strict species–specificity of these viruses, as well as their ability to persist lifelong and with relatively little damage to their hosts. Nonetheless, core genes, despite their strong conservation, also represent a target of adaptive evolution and subtle changes in their coding sequence contribute to the success of CMV infection.
However, large gaps of knowledge remain, first and foremost the role of HCMV genetic diversity in the development of human disease. Several studies have indicated that mixed HCMV infection is associated with poor clinical outcome in immunocompromised individuals [103,104,105]. Nonetheless, the role of specific viral variants in the progression and severity of disease in these individuals, as well as in congenitally infected infants, remains unknown. Congenital HCMV infection results in a wide variety of clinical outcomes [22], suggesting a complex interplay between viral and host factors. The increasing ability to sequence viral genomes from infected newborns, as well as the collection of related clinical data might in the future allow the identification of viral markers (if any) associated with short–term and long–term outcomes. Likewise, deeper knowledge of worldwide HCMV diversity will be instrumental to the development of a much needed effective vaccine.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/5/624/s1, Table S1: List of HCMV strains analyzed in this study.
Author Contributions
Conceptualization: A.M., R.C., D.F. and M.S.; Writing—Original Draft Preparation, A.M., R.C., D.F. and M.S.; Writing—Review & Editing, M.S.; Visualization, A.M., R.C., D.F.; Supervision, M.S.; Funding Acquisition, M.S. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Italian Ministry of Health (“Ricerca Corrente 2019–2020” to MS, “Ricerca Corrente 2018–2020” to DF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Davison A.J., Eberle R., Ehlers B., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. The Order Herpesvirales. Arch. Virol. 2008;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davison A.J. Comparative Analysis of the Genomes. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 3.Huff J.L., Barry P.A. B-Virus (Cercopithecine herpesvirus 1) Infection in Humans and Macaques: Potential for Zoonotic Disease. Emerg. Infect. Dis. 2003;9:246–250. doi: 10.3201/eid0902.020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrenzel M., Osborn K., Shima A., Klieforth R., Maalouf G. Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia) J. Med. Primatol. 2003;32:7–14. doi: 10.1034/j.1600-0684.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- 5.Oya C., Ochiai Y., Taniuchi Y., Takano T., Ueda F., Yoshikawa Y., Hondo R. Specific Detection and Identification of Herpes B Virus by a PCR-Microplate Hybridization Assay. J. Clin. Microbiol. 2004;42:1869–1874. doi: 10.1128/JCM.42.5.1869-1874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell G.C., Stewart J., Haig D.M. Malignant catarrhal fever: A review. Veter J. 2009;179:324–335. doi: 10.1016/j.tvjl.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Murthy S., Couacy-Hymann E., Metzger S., Nowak K., De Nys H.M., Boesch C., Wittig R., Jarvis M., Leendertz F.H., Ehlers B. Absence of Frequent Herpesvirus Transmission in a Nonhuman Primate Predator-Prey System in the Wild. J. Virol. 2013;87:10651–10659. doi: 10.1128/JVI.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwitz B.J., Malouli D., Bimber B.N., Reed J.S., Ventura A.B., Hancock M.H., Uebelhoer L.S., Bhusari A., Hammond K.B., Trethewy R.G.E., et al. Cross-Species Rhesus Cytomegalovirus Infection of Cynomolgus Macaques. PLoS Pathog. 2016;12:e1006014. doi: 10.1371/journal.ppat.1006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anoh A.E., Murthy S., Koffi C.A., Couacy-Hymann E., Leendertz F.H., Calvignac-Spencer S., Ehlers B. Cytomegaloviruses in a Community of Wild Nonhuman Primates in Taï National Park, Côte D’Ivoire. Viruses. 2017;10:11. doi: 10.3390/v10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James S., Donato D., Pouliquen J.-F., Ruiz-Garcia M., Lavergne A., Lacoste V. DNA Polymerase Sequences of New World Monkey Cytomegaloviruses: Another Molecular Marker with Which to Infer Platyrrhini Systematics. J. Virol. 2018;92:e00980-18. doi: 10.1128/JVI.00980-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan A., Cunningham C., Hector R., Hassan-Walker A.F., Lee L., Addison C., Dargan D.J., McGeoch D.J., Gatherer D., Emery V., et al. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E., Yu N., Grimwood J., Schmutz J., Dickson M., Jarvis M., Hahn G., Nelson J.A., Myers R.M., Shenk T.E. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGeoch D.J., Rixon F.J., Davison A.J. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Van Damme E., Van Loock M. Functional annotation of human cytomegalovirus gene products: An update. Front. Microbiol. 2014;5:218. doi: 10.3389/fmicb.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Yu J., Liu Z. MicroRNAs expressed by human cytomegalovirus. Virol. J. 2020;17:34. doi: 10.1186/s12985-020-1296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darling A.E., Mau B., Blattner F.R., Perna N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darling A.E., Mau B., Perna N.T. progressiveMauve: Multiple Genome Alignment with Gene Gain, Loss and Rearrangement. PLoS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuhair M., Smit G.S.A., Wallis G., Jabbar F., Smith C., Devleesschauwer B., Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019;29:e2034. doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 19.Boeckh M., Geballe A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Investig. 2011;121:1673–1680. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins-McMillen D., Buehler J.C., Peppenelli M., Goodrum F. Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation. Viruses. 2018;10:444. doi: 10.3390/v10080444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths P., Baraniak I., Reeves M.B. The pathogenesis of human cytomegalovirus. J. Pathol. 2014;235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 22.Manicklal S., Emery V., Lazzarotto T., Boppana S.B., Gupta R.K. The “Silent” Global Burden of Congenital Cytomegalovirus. Clin. Microbiol. Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisler J., Touma J., Rahbar A., Söderberg-Naucler C., Vetvik K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers. 2019;11:1842. doi: 10.3390/cancers11121842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nauclér C.S., Geisler J., Vetvik K. The emerging role of human cytomegalovirus infection in human carcinogenesis: A review of current evidence and potential therapeutic implications. Oncotarget. 2019;10:4333–4347. doi: 10.18632/oncotarget.27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caposio P., Orloff S.L., Streblow D.N. The role of cytomegalovirus in angiogenesis. Virus Res. 2010;157:204–211. doi: 10.1016/j.virusres.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor G., Moss A.C. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflamm. Bowel Dis. 2010;16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 27.Wistuba-Hamprecht K., Frasca D., Blomberg B.B., Pawelec G., Derhovanessian E. Age-associated alterations in γδ T-cells are present predominantly in individuals infected with Cytomegalovirus. Immun. Ageing. 2013;10:26. doi: 10.1186/1742-4933-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch S., Larbi A., Özcelik D., Solana R., Gouttefangeas C., Attig S., Wikby A., Strindhall J., Franceschi C., Pawelec G. Cytomegalovirus Infection: A Driving Force in Human T Cell Immunosenescence. Ann. N. Y. Acad. Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 29.Manandhar T., Hò G.-G.T., Pump W.C., Blasczyk R., Bade-Doeding C. Battle between Host Immune Cellular Responses and HCMV Immune Evasion. Int. J. Mol. Sci. 2019;20:3626. doi: 10.3390/ijms20153626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 2003;3:36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- 31.McSharry B.P., Avdic S., Slobedman B. Human Cytomegalovirus Encoded Homologs of Cytokines, Chemokines and their Receptors: Roles in Immunomodulation. Viruses. 2012;4:2448–2470. doi: 10.3390/v4112448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vink C., Beisser P.S., Bruggeman C.A. Molecular mimicry by cytomegaloviruses. Function of cytomegalovirus-encoded homologues of G protein-coupled receptors, MHC class I heavy chains and chemokines. Intervirology. 1999;42:342–349. doi: 10.1159/000053970. [DOI] [PubMed] [Google Scholar]
- 33.Murthy S., O’Brien K., Agbor A., Angedakin S., Arandjelovic M., Ayimisin E.A., Bailey E., Bergl R.A., Brazzola G., Dieguez P., et al. Cytomegalovirus distribution and evolution in hominines. Virus Evol. 2019;5:vez015. doi: 10.1093/ve/vez015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davison A., Dolan A., Akter P., Addison C., Dargan D.J., Alcendor D., McGeoch D.J., Hayward G.S. The human cytomegalovirus genome revisited: Comparison with the chimpanzee cytomegalovirus genome FN1. J. Gen. Virol. 2003;84:17–28. doi: 10.1099/vir.0.18606-0. [DOI] [PubMed] [Google Scholar]
- 35.Leendertz F.H., Deckers M., Schempp W., Lankester F., Boesch C., Mugisha L., Dolan A., Gatherer D., McGeoch D.J., Ehlers B. Novel cytomegaloviruses in free-ranging and captive great apes: Phylogenetic evidence for bidirectional horizontal transmission. J. Gen. Virol. 2009;90:2386–2394. doi: 10.1099/vir.0.011866-0. [DOI] [PubMed] [Google Scholar]
- 36.Evans T.S., Gilardi K.V., Barry P.A., Ssebide B.J., Kinani J.F., Nizeyimana F., Noheri J.B., Byarugaba D.K., Mudakikwa A., Cranfield M.R., et al. Detection of viruses using discarded plants from wild mountain gorillas and golden monkeys. Am. J. Primatol. 2016;78:1222–1234. doi: 10.1002/ajp.22576. [DOI] [PubMed] [Google Scholar]
- 37.Prepens S., Kreuzer K.-A., Leendertz F.H., Nitsche A., Ehlers B. Discovery of herpesviruses in multi-infected primates using locked nucleic acids (LNA) and a bigenic PCR approach. Virol. J. 2007;4:84. doi: 10.1186/1743-422X-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davison A.J., Holton M., Dolan A., Dargan D.J., Gatherer D., Hayward G.S. Comparative genomics of primate cytomegaloviruses. Cytomegaloviruses Mol. Pathog. Interv. 2013;1:1–22. [Google Scholar]
- 39.Hansen S.G., Strelow L.I., Franchi D.C., Anders D.G., Wong S.W. Complete Sequence and Genomic Analysis of Rhesus Cytomegalovirus. J. Virol. 2003;77:6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blewett E.L., White G., Saliki J.T., Eberle R. Isolation and characterization of an endogenous cytomegalovirus (BaCMV) from baboons. Arch. Virol. 2001;146:1723–1738. doi: 10.1007/s007050170059. [DOI] [PubMed] [Google Scholar]
- 41.Barry P.A., William Chang W. Primate betaherpesviruses. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 42.Itell H.L., Kaur A., Deere J.D., Barry P.A., Permar S.R. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr. Opin. Virol. 2017;25:126–133. doi: 10.1016/j.coviro.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blewett E.L., Sherrod C.J., Texier J.R., Conrad T.M., Dittmer D.P. Complete Genome Sequences of Mandrillus leucophaeus and Papio ursinus Cytomegaloviruses. Genome Announc. 2015;3:e00781-15. doi: 10.1128/genomeA.00781-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black P.H., Hartley J.W., Rowe W.P. Isolation of a Cytomegalovirus from African Green Monkey. Exp. Boil. Med. 1963;112:601–605. doi: 10.3181/00379727-112-28115. [DOI] [PubMed] [Google Scholar]
- 45.Huang E.S., Kilpatrick B., Lakeman A., Alford C.A. Genetic analysis of a cytomegalovirus-like agent isolated from human brain. J. Virol. 1978;26:718–723. doi: 10.1128/JVI.26.3.718-723.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin W.J., Zeng L.C., Ahmed K., Roy M. Cytomegalovirus-related sequence in an atypical cytopathic virus repeatedly isolated from a patient with chronic fatigue syndrome. Am. J. Pathol. 1994;145:440–451. [PMC free article] [PubMed] [Google Scholar]
- 47.Martin J., Ahmed K.N., Zeng L.C., Olsen J.-C., Seward J.G., Seehrai J.S. African green monkey origin of the atypical cytopathic ‘stealth virus’ isolated from a patient with chronic fatigue syndrome. Clin. Diagn. Virol. 1995;4:93–103. doi: 10.1016/0928-0197(95)00020-9. [DOI] [PubMed] [Google Scholar]
- 48.Michaels M.G., Jenkins F.J., George K.S., Nalesnik M.A., Starzl T.E., Rinaldo C.R. Detection of Infectious Baboon Cytomegalovirus after Baboon-to-Human Liver Xenotransplantation. J. Virol. 2001;75:2825–2828. doi: 10.1128/JVI.75.6.2825-2828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGeoch D.J., Cook S., Dolan A., Jamieson F.E., Telford E.A. Molecular Phylogeny and Evolutionary Timescale for the Family of Mammalian Herpesviruses. J. Mol. Boil. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 50.Russell J., Marsh A.K., Willer D.O., Ambagala A.P.N., Dzamba M., Chan J.K., Pilon R., Fournier J., Brudno M., Antony J.M., et al. A novel strain of cynomolgus macaque cytomegalovirus: Implications for host-virus co-evolution. BMC Genom. 2016;17:277. doi: 10.1186/s12864-016-2588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsh A.K., Willer D.O., Ambagala A.P.N., Dzamba M., Chan J.K., Pilon R., Fournier J., Sandstrom P., Brudno M., Macdonald K.S. Genomic Sequencing and Characterization of Cynomolgus Macaque Cytomegalovirus. J. Virol. 2011;85:12995–13009. doi: 10.1128/JVI.05840-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lilja A.E., Shenk T. Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proc. Natl. Acad. Sci. USA. 2008;105:19950–19955. doi: 10.1073/pnas.0811063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perot K., Walker C.M., Spaete R.R. Primary chimpanzee skin fibroblast cells are fully permissive for human cytomegalovirus replication. J. Gen. Virol. 1992;73:3281–3284. doi: 10.1099/0022-1317-73-12-3281. [DOI] [PubMed] [Google Scholar]
- 54.Tang Q., Maul G.G. Mouse Cytomegalovirus Crosses the Species Barrier with Help from a Few Human Cytomegalovirus Proteins. J. Virol. 2006;80:7510–7521. doi: 10.1128/JVI.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Child S.J., Brennan G., Braggin J.E., Geballe A.P. Species Specificity of Protein Kinase R Antagonism by Cytomegalovirus TRS1 Genes. J. Virol. 2012;86:3880–3889. doi: 10.1128/JVI.06158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fioretti A., Furukawa T., Santoli D., Plotkin S.A. Nonproductive Infection of Guinea Pig Cells with Human Cytomegalovirus. J. Virol. 1973;11:998–1003. doi: 10.1128/JVI.11.6.998-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaFemina R.L., Hayward G.S. Differences in Cell Type-specific Blocks to Immediate Early Gene Expression and DNA Replication of Human, Simian and Murine Cytomegalovirus. J. Gen. Virol. 1988;69:355–374. doi: 10.1099/0022-1317-69-2-355. [DOI] [PubMed] [Google Scholar]
- 58.Ellsmore V., Reid G.G., Stow N.D. Detection of human cytomegalovirus DNA replication in non-permissive Vero and 293 cells. J. Gen. Virol. 2003;84:639–645. doi: 10.1099/vir.0.18812-0. [DOI] [PubMed] [Google Scholar]
- 59.Mocarski E., Jr. Betaherpes viral genes and their functions. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 60.Marsh A.K., Ambagala A.P., Perciani C.T., Russell J., Chan J.K., Janes M., Antony J.M., Pilon R., Sandstrom P., Willer D.O., et al. Examining the Species-Specificity of Rhesus Macaque Cytomegalovirus (RhCMV) in Cynomolgus Macaques. PLoS ONE. 2015;10:e0121339. doi: 10.1371/journal.pone.0121339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambagala A.P., Marsh A.K., Chan J.K., Mason R., Pilon R., Fournier J., Sandstrom P., Willer D.O., Macdonald K.S. Establishment of an immortal cynomolgus macaque fibroblast cell line for propagation of cynomolgus macaque cytomegalovirus (CyCMV) Arch. Virol. 2012;158:955–965. doi: 10.1007/s00705-012-1568-4. [DOI] [PubMed] [Google Scholar]
- 62.Skaletskaya A., Bartle L.M., Chittenden T., McCormick A.L., Mocarski E.S., Goldmacher V.S. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryckman B.J., Rainish B.L., Chase M.C., Borton J.A., Nelson J.A., Jarvis M., Johnson D.C. Characterization of the Human Cytomegalovirus gH/gL/UL128-131 Complex That Mediates Entry into Epithelial and Endothelial Cells. J. Virol. 2007;82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcendor D., Zong J., Dolan A., Gatherer D., Davison A.J., Hayward G.S. Patterns of divergence in the vCXCL and vGPCR gene clusters in primate cytomegalovirus genomes. Virology. 2009;395:21–32. doi: 10.1016/j.virol.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scarborough J.A., Paul J.R., Spencer J. Evolution of the ability to modulate host chemokine networks via gene duplication in human cytomegalovirus (HCMV) Infect. Genet. Evol. 2017;51:46–53. doi: 10.1016/j.meegid.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jurak I., Brune W. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 2006;25:2634–2642. doi: 10.1038/sj.emboj.7601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schumacher U., Handke W., Jurak I., Brune W. Mutations in the M112/M113-Coding Region Facilitate Murine Cytomegalovirus Replication in Human Cells. J. Virol. 2010;84:7994–8006. doi: 10.1128/JVI.02624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sironi M., Cagliani R., Forni D., Clerici M. Evolutionary insights into host–pathogen interactions from mammalian sequence data. Nat. Rev. Genet. 2015;16:224–236. doi: 10.1038/nrg3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mozzi A., Biolatti M., Cagliani R., Forni D., Dell’Oste V., Pontremoli C., Vantaggiato C., Pozzoli U., Clerici M., Landolfo S., et al. Past and ongoing adaptation of human cytomegalovirus to its host. PLoS Pathog. 2020 doi: 10.1371/journal.ppat.1008476. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn W., Chou C., Li H., Hai R., Patterson D., Stolc V., Zhu H., Liu F. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA. 2003;100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chee M.S., Bankier A.T., Beck S., Bohni R., Brown C.M., Černý R., Horsnell T., Hutchison C.A., Kouzarides T., Martignetti J.A., et al. Analysis of the Protein-Coding Content of the Sequence of Human Cytomegalovirus Strain AD169. Hantaviruses. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 72.Cha T.A., Tom E., Kemble G.W., Duke G.M., Mocarski E.S., Spaete R.R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 1996;70:78–83. doi: 10.1128/JVI.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suárez N.M., Wilkie G.S., Hage E., Camiolo S., Holton M., Hughes J., Maabar M., Vattipally S.B., Dhingra A., Gompels U.A., et al. Human Cytomegalovirus Genomes Sequenced Directly from Clinical Material: Variation, Multiple-Strain Infection, Recombination, and Gene Loss. J. Infect. Dis. 2019;220:781–791. doi: 10.1093/infdis/jiz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suárez N.M., Musonda K.G., Escriva E., Njenga M., Agbueze A., Camiolo S., Davison A.J., Gompels U.A. Multiple-Strain Infections of Human Cytomegalovirus with High Genomic Diversity Are Common in Breast Milk from Human Immunodeficiency Virus–Infected Women in Zambia. J. Infect. Dis. 2019;220:792–801. doi: 10.1093/infdis/jiz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sijmons S., Thys K., Ngwese M.M., Van Damme E., Dvorak J., Van Loock M., Li G., Tachezy R., Busson L., Aerssens J., et al. High-Throughput Analysis of Human Cytomegalovirus Genome Diversity Highlights the Widespread Occurrence of Gene-Disrupting Mutations and Pervasive Recombination. J. Virol. 2015;89:7673–7695. doi: 10.1128/JVI.00578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Renzette N., Pokalyuk C., Gibson L., Bhattacharjee B., Schleiss M.R., Hamprecht K., Yamamoto A.Y., Mussi-Pinhata M.M., Britt W.J., Jensen J.D., et al. Limits and patterns of cytomegalovirus genomic diversity in humans. Proc. Natl. Acad. Sci. USA. 2015;112:E4120–E4128. doi: 10.1073/pnas.1501880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sijmons S., Thys K., Corthout M., Van Damme E., Van Loock M., Bollen S., Baguet S., Aerssens J., Van Ranst M., Maes P. A Method Enabling High-Throughput Sequencing of Human Cytomegalovirus Complete Genomes from Clinical Isolates. PLoS ONE. 2014;9:e95501. doi: 10.1371/journal.pone.0095501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hage E., Wilkie G.S., Linnenweber-Held S., Dhingra A., Suárez N.M., Schmidt J.J., Kay-Fedorov P.C., Mischak-Weissinger E., Heim A., Schwarz A., et al. Characterization of Human Cytomegalovirus Genome Diversity in Immunocompromised Hosts by Whole-Genome Sequencing Directly from Clinical Specimens. J. Infect. Dis. 2017;215:1673–1683. doi: 10.1093/infdis/jix157. [DOI] [PubMed] [Google Scholar]
- 79.Forni D., Pontremoli C., Clerici M., Pozzoli U., Cagliani R., Sironi M. Recent Out-of-Africa Migration of Human Herpes Simplex Viruses. Mol. Boil. Evol. 2020:msaa001. doi: 10.1093/molbev/msaa001. [DOI] [PubMed] [Google Scholar]
- 80.Pontremoli C., Forni D., Clerici M., Cagliani R., Sironi M. Possible European origin of circulating Varicella-zoster virus strains. J. Infect. Dis. 2019;221:1286–1294. doi: 10.1093/infdis/jiz227. [DOI] [PubMed] [Google Scholar]
- 81.Huson D.H., Bryant D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Boil. Evol. 2005;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 82.Zanella L., Riquelme I., Buchegger K., Abanto M., Ili C., Brebi P. A reliable Epstein-Barr Virus classification based on phylogenomic and population analyses. Sci. Rep. 2019;9:9829. doi: 10.1038/s41598-019-45986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt-Chanasit J., Sauerbrei A. Evolution and world-wide distribution of varicella–zoster virus clades. Infect. Genet. Evol. 2011;11:1–10. doi: 10.1016/j.meegid.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Chiara M., Manzari C., Lionetti C., Mechelli R., Anastasiadou E., Buscarinu M.C., Ristori G., Salvetti M., Picardi E., D’Erchia A.M., et al. Geographic Population Structure in Epstein-Barr Virus Revealed by Comparative Genomics. Genome Boil. Evol. 2016;8:3284–3291. doi: 10.1093/gbe/evw226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wegner F., Lassalle F., Depledge D.P., Balloux F., Breuer J. Co-evolution of sites under immune selection shapes Epstein-Barr Virus population structure. Mol. Boil. Evol. 2019;36:2512–2521. doi: 10.1093/molbev/msz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szpara M.L., Gatherer D., Ochoa A., Greenbaum B., Dolan A., Bowden R.J., Enquist L.W., Legendre M., Davison A.J. Evolution and Diversity in Human Herpes Simplex Virus Genomes. J. Virol. 2013;88:1209–1227. doi: 10.1128/JVI.01987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lassalle F., Depledge D.P., Reeves M.B., Brown A.C., Christiansen M.T., Tutill H.J., Williams R.J., Einer-Jensen K., Holdstock J., Atkinson C., et al. Islands of linkage in an ocean of pervasive recombination reveals two-speed evolution of human cytomegalovirus genomes. Virus Evol. 2016;2:vew017. doi: 10.1093/ve/vew017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burrel S., Désiré N., Marlet J., Dacheux L., Seang S., Caumes E., Bourhy H., Agut H., Boutolleau D. Genetic Diversity within Alphaherpesviruses: Characterization of a Novel Variant of Herpes Simplex Virus 2. J. Virol. 2015;89:12273–12283. doi: 10.1128/JVI.01959-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cudini J., Roy S., Houldcroft C.J., Bryant J.M., Depledge D.P., Tutill H., Veys P., Williams R., Worth A.J.J., Tamuri A.U., et al. Human cytomegalovirus haplotype reconstruction reveals high diversity due to superinfection and evidence of within-host recombination. Proc. Natl. Acad. Sci. USA. 2019;116:5693–5698. doi: 10.1073/pnas.1818130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pignatelli S., Monte P.D., Rossini G., Landini M.P. Genetic polymorphisms among human cytomegalovirus (HCMV) wild-type strains. Rev. Med. Virol. 2004;14:383–410. doi: 10.1002/rmv.438. [DOI] [PubMed] [Google Scholar]
- 91.Paterson D.A., Dyer A.P., Milne R.S., Sevilla-Reyes E., Gompels U.A. A Role for Human Cytomegalovirus Glycoprotein O (gO) in Cell Fusion and a New Hypervariable Locus. Virology. 2002;293:281–294. doi: 10.1006/viro.2001.1274. [DOI] [PubMed] [Google Scholar]
- 92.Renzette N., Bhattacharjee B., Jensen J.D., Gibson L., Kowalik T.F. Extensive Genome-Wide Variability of Human Cytomegalovirus in Congenitally Infected Infants. PLoS Pathog. 2011;7:e1001344. doi: 10.1371/journal.ppat.1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stangherlin L.M., De Paula F.N., Icimoto M., Ruiz L.G.P., Nogueira M.L., Braz A.S., Juliano L., Da Silva M.C.C. Positively Selected Sites at HCMV gB Furin Processing Region and Their Effects in Cleavage Efficiency. Front. Microbiol. 2017;8:934. doi: 10.3389/fmicb.2017.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yolitz J., Schwing C., Chang J., Van Ryk N., Nawaz F., Wei D., Cicala C., Arthos J., Fauci A.S. Signal peptide of HIV envelope protein impacts glycosylation and antigenicity of gp120. Proc. Natl. Acad. Sci. USA. 2018;115:2443–2448. doi: 10.1073/pnas.1722627115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dargan D.J., Douglas E., Cunningham C., Jamieson F., Stanton R.J., Baluchova K., McSharry B.P., Tomasec P., Emery V., Percivalle E., et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J. Gen. Virol. 2010;91:1535–1546. doi: 10.1099/vir.0.018994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sijmons S., Van Ranst M., Maes P. Genomic and Functional Characteristics of Human Cytomegalovirus Revealed by Next-Generation Sequencing. Viruses. 2014;6:1049–1072. doi: 10.3390/v6031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cunningham C., Gatherer D., Hilfrich B., Baluchova K., Dargan D.J., Thomson M., Griffiths P.D., Wilkinson G.W.G., Schulz T.F., Davison A.J. Sequences of complete human cytomegalovirus genomes from infected cell cultures and clinical specimens. J. Gen. Virol. 2009;91:605–615. doi: 10.1099/vir.0.015891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zárate S., Pond S.L.K., Shapshak P., Frost S.D.W. Comparative Study of Methods for Detecting Sequence Compartmentalization in Human Immunodeficiency Virus Type 1. J. Virol. 2007;81:6643–6651. doi: 10.1128/JVI.02268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pond S.L.K., Muse S.V. HyPhy: Hypothesis Testing Using Phylogenies. Mod. Infect. Dis. Epidemiol. 2005;21:125–181. doi: 10.1093/bioinformatics/bti079. [DOI] [PubMed] [Google Scholar]
- 100.Choi H.J., Park A., Kang S., Lee E., Lee T.A., Ra E.A., Lee J., Lee S., Park B. Human cytomegalovirus-encoded US9 targets MAVS and STING signaling to evade type I interferon immune responses. Nat. Commun. 2018;9:125. doi: 10.1038/s41467-017-02624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biolatti M., Gugliesi F., Dell’Oste V., Landolfo S. Modulation of the innate immune response by human cytomegalovirus. Infect. Genet. Evol. 2018;64:105–114. doi: 10.1016/j.meegid.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 102.Ma J., Edlich F., Bermejo G.A., Norris K.L., Youle R.J., Tjandra N. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl. Acad. Sci. USA. 2012;109:20901–20906. doi: 10.1073/pnas.1217094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coaquette A., Bourgeois A., Dirand C., Varin A., Chen W., Herbein G. Mixed Cytomegalovirus Glycoprotein B Genotypes in Immunocompromised Patients. Clin. Infect. Dis. 2004;39:155–161. doi: 10.1086/421496. [DOI] [PubMed] [Google Scholar]
- 104.Lisboa L., Tong Y., Kumar D., Pang X., Åsberg A., Hartmann A., Rollag H., Jardine A., Pescovitz M., Humar A. Analysis and clinical correlation of genetic variation in cytomegalovirus. Transpl. Infect. Dis. 2011;14:132–140. doi: 10.1111/j.1399-3062.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 105.Houldcroft C.J., Bryant J.M., Depledge D.P., Margetts B.K., Simmonds J., Nicolaou S., Tutill H.J., Williams R., Worth A.J.J., Marks S.D., et al. Detection of Low Frequency Multi-Drug Resistance and Novel Putative Maribavir Resistance in Immunocompromised Pediatric Patients with Cytomegalovirus. Front. Microbiol. 2016;7:1317. doi: 10.3389/fmicb.2016.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.