Abstract
More than 30 years ago, the Nordic Gene Bank established a long-term experiment on seeds stored under permafrost conditions in an abandoned mine corridor in Svalbard, as a tool to monitor storage life under these conditions. The study included seeds from 16 Nordic agricultural and horticultural crops, each represented by two or three cultivars (altogether 38 accessions). All seeds were ultra-dried to 3–5% moisture before being sealed in glass tubes. Germination tests were performed in accordance with the International Seed Testing Association (ISTA) protocols. At the initiation of the experiment, the samples showed good germination with the median value at 92%. The overall picture remained stable over the first twenty to twenty-five years. However, the variation became larger over time and at 30 years, the median value had dropped to 80%. At the lower end, with a high drop in germination, we found rye, wheat, and English ryegrass. At the upper end, we found Kentucky bluegrass and cucumber. The lowest germination was found in samples with the highest initial seed moisture levels. Pre-storage conditions are likely to be of major importance for longevity.
Keywords: ex situ conservation, germination, longevity, plant genetic resources, seed storage
1. Introduction
Most food plants produce seeds that can be stored under low temperature and moisture conditions. Much of our knowledge on seed longevity is based on artificial ageing experiments, where seeds are exposed to suboptimal conditions of elevated temperature and moisture for some weeks, and storage life is predicted based on the seed moisture content, storage temperature, and seed lot characters [1,2]. Such calculations have predicted that high-quality seeds could survive ideal conditions for hundreds of years or more [3,4], which was good news for many gene banks, but might be unrealistic as such studies have rarely been confirmed in long-term storage studies. Long-term seed storage is crucial for ex situ gene bank conservation [5,6,7]. Gene banks maintain crop diversity and facilitate the utilization of seeds for breeding, research, education, and other purposes [8,9,10,11]. Safety back-ups are kept, ideally at a second location, to spread the risks [12,13]. The Global Seed Vault at the Arctic Archipelago of Svalbard was opened in 2008 and is facilitating such back-up collections with a world outreach [14]. However, more than twenty years before this, the Nordic Gene Bank (NGB) started a small seed storage facility in an abandoned coalmine corridor in Svalbard. Different options had been considered, such as inland ice caves in Greenland or mountain caves in Jotunheimen, Norway, but in the end, a coalmine in the permafrost in Svalbard was chosen due to its good logistics, despite its remote location [15]. The temperature of the Global Seed Vault in Svalbard was −18 °C compared to the −3.5 °C present in the abandoned coalmine corridor. The rock temperature was stable, which meant that it was independent of an external energy supply. As a tool to monitor storage life under these permafrost conditions, a long-term seed storage experiment was initiated. The experiment started in 1986 and included samples for germination monitoring until 2086, thus it was termed “the 100 year experiment”. Important crops for Nordic agriculture and horticulture were included. The investigation is still ongoing, and in this paper, we summarize the results after the first 30 years.
2. Results and Discussion
2.1. Overall Patterns
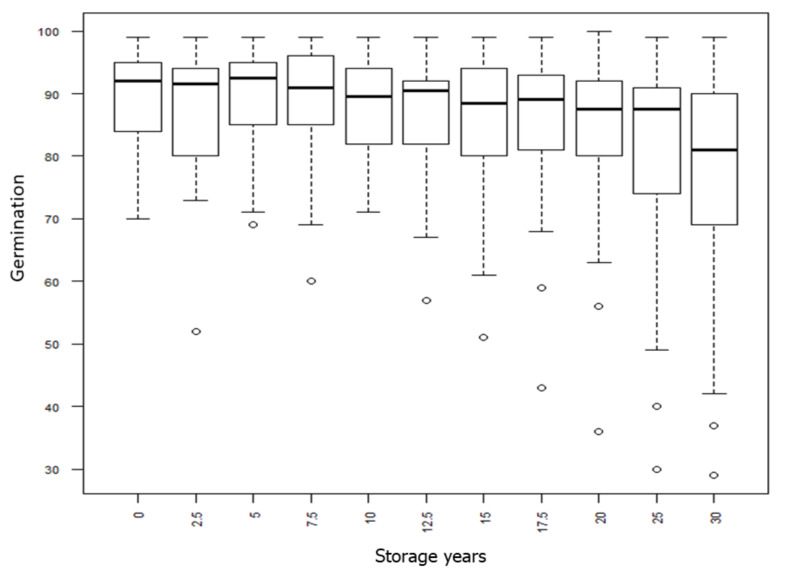
The overall picture of seed germination development over storage time across all accessions (species and cultivars) is illustrated by boxplots (Figure 1). A lower and upper percentile defines the box, in which 75% of the observations were found. A marked line denotes the median germination value, and the whiskers and small circles show observations away from the box. At the initiation of the experiment in 1986 (year 0 = y0), most of the seed lots showed an excellent germination ability. The box-range was from 83% to 95%, and the median was at 92% germination. The overall picture was relatively stable over the first twenty to twenty-five years, but the variation increased over time as some seed lots showed a reduced germination. After 30 years, the median value across all the lots was 80%, but with outlier samples below 40%, and some of the lots in the 50–70% range.
Figure 1.
Boxplots showing the germination percentages across species and cultivars throughout the first thirty years (year 0, year 2.5, year 5, year 7.5, year 10, year 12.5, year 15, year 17.5, year 20, year 25, and year 30).
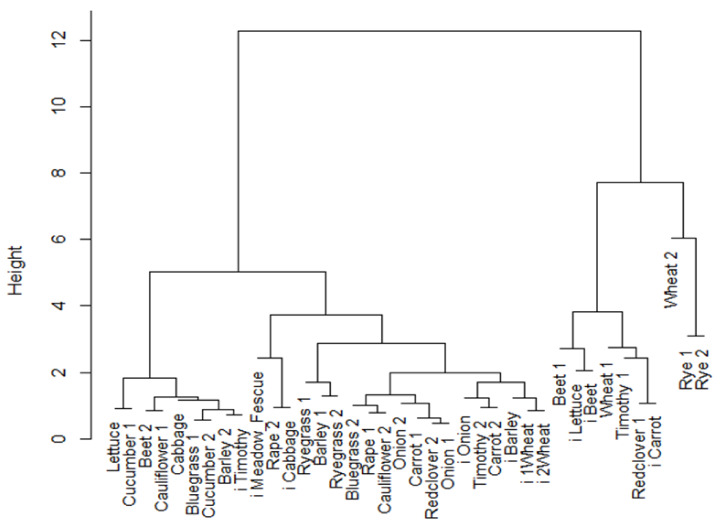
Figure 2 shows a dendrogram of a cluster analysis of the germination results of the accessions. The dissimilarity values of the fusion level values of the dendrogram indicated the cutting level six clusters to be correct. The largest cluster, cluster 1, contained 19 lots. It contained both normal and lots with seed-borne pathogens (i-prefix lots). Also, cluster 2 contained both normal and seed-borne pathogens, as did cluster 6. In cluster 1 we found Barley 1, Ryegrass 1, Ryegrass 2, Timothy 2, Bluegrass 2, Redclover 2, Rape 1, Rape 2, Onion 1, Onion 2, Carrot 1, Carrot 2, Cauliflower 2, i1 Wheat, i2 Wheat, i Barley, i Meadow_fescue, i Onion, and i Cabbage. In cluster two, we found Barley 2, Bluegrass 1, Beet 2, Lettuce, Cabbage, Cucumber 1, Cucumber 2, Cauliflower 1, and i-Timothy. In cluster three, we found Wheat 1, Timothy 1, Redclover 1, and i Carrot. In cluster 4, we found Wheat 2. In cluster 5, we found Rye 1 and Rye 2. In cluster 6, we found Beet 1, i Lettuce, and i Beet. Lots with seed-borne pathogens (i-prefix lots) were spread over the clusters, showing that such pathogens do not explain much of the seed longevity. Furthermore, seed lots of the same species were only partly in the same clusters; for example, the two cucumber (Cucumis sativus L.) lots and the two rye (Secale cereale L.) lots. This also shows that interspecies differences are not of importance for explaining seed longevity.
Figure 2.
The dendrogram of a cluster analysis of the germination results of the seed lots.
The seeds of all the species remained viable after 30 years in permafrost under the given conditions (dried to 3–5% moisture content and stored in sealed glass ampoules). Crops, but also cultivars, within the same crops showed different results. Similar patterns have also been observed in other long-term experiments, both under ambient [16,17] or −18 °C conditions [18,19,20,21]. The cold storage of seeds should provide improved longevity compared to ambient storage [22]. Another factor of importance is seed maturation [23,24].
For instance, the potential seed longevity of barley was found to be best if it was harvested a week or so after grain filling was completed [25]. The same has been found in tomatos (Solanum lycopersicum L. var. lycopersicum) [26]. Furthermore, weather, seed coat damages, diseases, and pests may influence seed storage life [24,27]. In our experiment, the samples were all from newly harvested seeds, but we do not have data on the weather or other pre-harvest conditions. We only assume that the seed lots selected for the experiment were of a high quality.
2.2. Crop-Wise Results
Our results show good performance for many of the vegetables, while the picture is more varied for cereals and forage species (Figure S1). Table 1 gives the germination result for each seed lot. According to the FAO’s gene bank standard [13], “regeneration shall be carried out when the viability drops below 85 percent of the initial viability or when the remaining seed quantity is less than what is required for three sowings of a representative population of the accession.” Seed lots with the highest loss in germination, here defined as a loss in 15% or more over the 30 years, were found in both of the rye lots, two of the three English ryegrass (Lolium perenne L.) lots (Ryegrass 1, Ryegrass 2), two out of four wheat (Triticum aestivum L.) lots (Wheat 1, Wheat 2), one of the three timothy (Phleum pratense L.) lots (Timothy 1), one of the three barley (Hordeum vulgare L.) lots (Barley 1), and in the one seed lot of meadow fescue (Schedonorus pratensis (Huds.) P. Beauv.). The literature shows that especially rye, but also some forage grasses, can be relatively short-lived [28,29,30,31]. Intermediate storage performance, with a 5–15% loss in germination over the 30-year period, was found in two of the three lettuce (Lactuca sativa L.) lots (i Lettuce, Lettuce 2), two of the three carrot (Daucus carota subsp. sativus (Hoffm.) Schübl & G. Martens) lots (Carrot 2, i-Carrot), one of the four wheat lots (i1-Wheat), one of the three barley lots (Barley 2), one of the three timothy lots (Timothy 2), one of the two cauliflower (Brassica oleracea L. var. botrytis) lots (Cauliflower 2), one of the two oilseed rape (Brassica napus L.) lots (Rape 1), and one out of the three red clover (Trifolium pratense L.) lots (Redclover 1). The most long-lived, with a loss in germination less than 5% after 30 years of storage, were found in all of the three beet (Beta vulgaris L.) lots, all of the three onion (Allium cepa L.) lots, both of the cucumber lots, both of the Kentucky bluegrass (Poa pratensis L.) seed lots, the only cabbage (Brassica oleracea L.) seed lot, and in the last of the red clover, cauliflower, barley, wheat, oilseed rape, carrot, and lettuce seed lots. Other studies have also shown that beet seeds as well as cucumber seeds have retained a high germination level over time, but, in contrast to our study, onions are generally found to be short-lived [17,18,19,29,30,31]. Our results are a little surprising as we found that onion, and to some extent lettuce, showed no decline in germination over the 30-year period.
Table 1.
Germination percentages for all the seed lots (values below 70% in bold), and loss in germination (Δ germ) over the first 30 years, calculated by averaging the germination percentage of the three first test occasions (year 0, year 2.5, and year 5) minus the last test occasion (year 30).
| Storage Years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed Lot ID | 0 | 2.5 | 5 | 7.5 | 10 | 12.5 | 15 | 17.5 | 20 | 25 | 30 | Δ Germ |
| Barley 1 | 95 | 96 | 96 | 96 | 95 | 91 | 95 | 95 | 79 | 90 | 76 | −20% |
| Barley 2 | 95 | 94 | 96 | 97 | 94 | 91 | 97 | 94 | 93 | 92 | 86 | −9% |
| i Barley | 90 | 89 | 94 | 94 | 92 | 82 | 89 | 88 | 85 | 85 | 88 | −3% |
| Wheat 1 | 75 | 75 | 83 | 83 | 80 | 85 | 77 | 76 | 71 | 58 | 57 | −21% |
| Wheat 2 | 70 | 52 | 89 | 89 | 82 | 86 | 79 | 76 | 64 | 40 | 37 | −33% |
| i1 Wheat | 89 | 85 | 89 | 89 | 88 | 84 | 87 | 87 | 87 | 82 | 80 | −8% |
| i2 Wheat | 90 | 83 | 91 | 93 | 89 | 87 | 85 | 89 | 84 | 83 | 87 | −1% |
| Rye 1 | 76 | 74 | 84 | 84 | 74 | 57 | 51 | 43 | 36 | 30 | 29 | −49% |
| Rye 2 | 81 | 78 | 87 | 83 | 74 | 67 | 61 | 59 | 56 | 49 | 48 | −34% |
| Ryegrass 1 | 99 | 96 | 96 | 96 | 95 | 93 | 93 | 88 | 85 | 74 | 59 | −38% |
| Ryegrass 2 | 96 | 95 | 97 | 95 | 96 | 93 | 94 | 92 | 94 | 87 | 69 | −27% |
| Timothy 1 | 73 | 77 | 77 | 69 | 79 | 71 | 73 | 68 | 63 | 63 | 42 | −34% |
| Timothy 2 | 90 | 91 | 93 | 89 | 90 | 92 | 88 | 92 | 85 | 81 | 78 | −13% |
| i Timothy | 94 | 95 | 92 | 96 | 92 | 94 | 95 | 92 | 94 | 91 | 92 | −2% |
| Bluegrass 1 | 92 | 95 | 95 | 97 | 97 | 96 | 95 | 94 | 92 | 93 | 92 | −2% |
| Bluegrass 2 | 94 | 91 | 89 | 96 | 93 | 91 | 94 | 90 | 92 | 89 | 88 | −3% |
| iMeadow fescue | 92 | 90 | 89 | 86 | 88 | 90 | 87 | 81 | 81 | 69 | 54 | −36% |
| Red clover 1 | 75 | 74 | 76 | 80 | 75 | 74 | 68 | 76 | 68 | 73 | 68 | −7% |
| Red clover 2 | 93 | 94 | 94 | 92 | 89 | 91 | 89 | 89 | 90 | 91 | 90 | −4% |
| Beet 1 | 79 | 79 | 71 | 60 | 76 | 72 | 75 | 70 | 80 | 70 | 78 | 0% |
| Beet 2 | 97 | 97 | 97 | 97 | 97 | 98 | 94 | 95 | 95 | 98 | 97 | 0% |
| i Beet | 78 | 78 | 69 | 77 | 81 | 81 | 80 | 82 | 82 | 79 | 77 | 0% |
| Rape 1 | 95 | 94 | 95 | 96 | 90 | 91 | 90 | 90 | 92 | 91 | 87 | −8% |
| Rape 2 | 84 | 84 | 83 | 87 | 85 | 84 | 84 | 82 | 88 | 82 | 81 | −3% |
| Onion 1 | 92 | 92 | 92 | 93 | 90 | 89 | 89 | 88 | 91 | 90 | 91 | −1% |
| Onion 2 | 89 | 94 | 92 | 92 | 91 | 94 | 92 | 94 | 90 | 92 | 89 | −3% |
| i Onion | 92 | 93 | 87 | 87 | 88 | 90 | 85 | 91 | 87 | 88 | 88 | −3% |
| Lettuce 1 | 98 | 95 | 98 | 98 | 98 | 92 | 99 | 98 | 98 | 99 | 99 | 0% |
| Lettuce 2 | 97 | 93 | 96 | 90 | 94 | 97 | 93 | 93 | 92 | 92 | 90 | −5% |
| i Lettuce | 84 | 80 | 78 | 80 | 76 | 71 | 78 | 77 | 73 | 75 | 74 | −7% |
| Cucumber 1 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 100 | 98 | 98 | −1% |
| Cucumber 2 | 93 | 94 | 94 | 95 | 97 | 92 | 96 | 95 | 92 | 92 | 90 | −4% |
| Carrot 1 | 92 | 90 | 93 | 90 | 89 | 91 | 86 | 89 | 88 | 90 | 91 | −1% |
| Carrot 2 | 91 | 93 | 94 | 86 | 88 | 90 | 85 | 89 | 90 | 89 | 81 | −12% |
| i Carrot | 76 | 73 | 72 | 74 | 71 | 75 | 67 | 78 | 65 | 71 | 68 | −6% |
| Cauliflower 1 | 95 | 93 | 94 | 98 | 98 | 98 | 98 | 94 | 96 | 94 | 91 | −3% |
| Cauliflower 2 | 95 | 92 | 94 | 95 | 94 | 92 | 90 | 92 | 93 | 90 | 79 | −15% |
| i Cabbage | 87 | 82 | 85 | 85 | 86 | 82 | 86 | 82 | 80 | 76 | 80 | −5% |
2.3. Moisture Measurements
For all samples, the variation between the highest and lowest moisture content over the ten years was between 0.3% and 0.8% (Table 2). The data showed that two lots had a higher moisture content exceeding 5% in the initial test; these were the samples Wheat 2 ‘Solid’, with 6.3% humidity, and Rye 1 ‘Petkus II’, with 5.3% humidity. For wheat, one seed lot showed a low decline, and two seed lots showed a steep decline in germination. Different pre-harvest conditions or genetic factors may explain a steep decline in germination. We saw an effect of drying the seeds to lower than 5% internal moisture content before packing. The two samples with the highest internal humidity (Wheat 2 and Rye 1) were among the ones that showed the most significant drop in germination in our experiment. The current FAO standards [13], which are used by most gene banks, recommend drying for three months at 15 °C and 15% RH. According to our experience, this would give a seed moisture content exceeding 5%, thus decreasing the longevity.
Table 2.
Seed moisture content (in %) in the different lots over the first ten years of the experiment.
| Storage Years | ||||||
|---|---|---|---|---|---|---|
| Seed Lot | 0 | 2.5 | 5.0 | 7.5 | 10 | Max/Min |
| Barley 1 | 5.0 | 4.7 | 4.6 | 4.7 | 4.8 | 5.0/4.6 |
| Barley 2 | 4.6 | 4.6 | 4.3 | 4.3 | 4.7 | 4.7/4.3 |
| Wheat 1 | 4.0 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3/4.0 |
| Wheat 2 | 6.3 | 5.8 | 5.5 | 5.8 | 5.8 | 6.3/5.5 |
| Rye 1 | 5.3 | 5.1 | 4.9 | 5.1 | 5.2 | 5.3/4.9 |
| Rye 2 | 4.9 | 4.7 | 4.4 | 4.7 | 4.8 | 4.9/4.4 |
| Beet 1 | 3.7 | 4.3 | 4.3 | 3.7 | 4.1 | 4.3/3.7 |
| Beet 2 | 3.5 | 3.7 | 3.6 | 3.9 | 3.5 | 3.9/3.5 |
| Cucumber 1 | 3.0 | 3.5 | 3.6 | 2.8 | 2.9 | 3.6/2.8 |
| Cucumber 1 | 2.8 | 3.3 | 2.7 | 2.8 | 2.7 | 3.3/2.7 |
3. Conclusions
The study has so far revealed valuable results concerning the longevity of seeds after 30 years in permafrost. Nine out of the 38 seed lots showed a germination loss exceeding 15%, which is the level recommended by the FAO for carrying out regeneration. Rye and ryegrass in particular showed a rapid decline, while many of the vegetables showed a low decline in germination. The results are relevant for the seeds in the first Nordic back-up collection stored in the abandoned coalmine. A given storage condition is an essential characteristic for comparing results with other experiments. Here, seeds were stored in permafrost with a stable sub-zero temperature. We had no reference material at −18 °C conditions. Despite the limited number of samples and the lack of a −18 °C control, the observations add knowledge about the longevity of seeds.
4. Materials and Methods
4.1. Seed Samples and Seed Storage
In total, 38 seed lots covering 16 crops were included in the study (Table 3). Each species was represented by two to four seed lots, except for cabbage and meadow fescue, which had only one. The seed lot ID was given by crop name and code. A number after the crop name (1 or 2) refers to seed lots where we have no information on seed-borne diseases. A prefix “i” letter applies to seed lots where we know that seed-borne diseases were present.
Table 3.
Overview of the examined crops and sample information. A prefix number refers to the samples with no information on seed-borne diseases, while a prefix i-letter refers to the samples where we detected seed-borne diseases to be present at the start of the experiment.
| Crop/Species | Sample ID and Cultivars (Country of Origin) |
|---|---|
| Barley (Hordeum vulgare) | 1 = Inga Abed (DNK), 2 = Tunga (NOR), i = Bamse (NOR) |
| Wheat (Triticum aestivum) | 1 = Vakka (FIN), 2 = Solid (SWE), i1 = Runar (NOR), i2 = Line 79 CBW A72 (CAN) |
| Rye (Secale cereale) | 1 = Petkus II (DNK), 2 = Voima (DNK) |
| English ryegrass (Lolium perenne) | 1 = Pippin (DNK), 2 = Riikka (FIN) |
| Timothy (Phleum pratense) | 1 = Tammisto (FIN), 2 = Bodin (NOR), I = Forus (NOR) |
| Kentucky bluegrass (Poa pratensis) | 1 = Hankkijan Kyösti (FIN), 2 = Annika (DNK) |
| Meadow fescue (Schedonorus pratensis) | I = Salten (NOR) |
| Red clover (Trifolium pratense) | 1 = Jokioinen (FIN), 2 = Molstad (NOR) |
| Beet (Beta vulgaris) | 1 = 311 N typ (SWE), 2 = 70500 (DNK)2, i = Hilma (GBR) |
| Oilseed rape (Brassica napus) | 1 = Jupiter (SWE), 2 = Linrama (DNK) |
| Onion (Allium cepa) | 1 = Hamund (SWE), 2 = Owa (DNK), i = Laskala (NOR) |
| Lettuce (Lactuca sativa) | 1 = Attraktion Sana (DNK), 2 = Hilro (SWE), i = Attractie (NLD) |
| Cucumber (Cucumis sativus) | 1 = Langelands gigant (DNK), 2 = Rhensk Druv (SWE) |
| Carrot (Daucus carota) | 1 = Nantes Fancy (DNK), 2 = Regulus (SWE), I = Forto Nantes (NLD) |
| Cauliflower (B. oleracea v. botrytis) | 1 = Svavit (SWE), 2 = Pari (DNK) |
| Cabbage (Brassica oleracea) | I = Trønder Lunde (NOR) |
The lots were from different cultivars and origins. No cultivation details are available except that the seed lots should be of good quality. Initial germination tests were conducted (y0). Before storage, all the seeds were dried to 3–5% internal moisture content using a Munters (Kista, Sweden) dehumidifier adjusted to 10% RH in a room at 25 °C. After that, they were placed in a freezer overnight before they were sealed in glass ampoules. Each seed lot was divided into sub-samples of 2 × 500 seeds for each withdrawal. The sub-samples were boxed and labelled with the date for withdrawal, transported to Longyearbyen, Svalbard, and placed in the abandoned coalmine corridor. The temperature inside the coalmine was measured as −3.5 °C (± 0.2 °C).
4.2. Germination Studies
The first set of samples was tested in December 1986 (year 0), with a plan to have the last tests in 2086 (year 100). Since the start of the experiment, 11 boxes have been retrieved. The moisture content of the seed was monitored from year 0 to year 10 (Table 4). The germination tests and the moisture content tests were carried out at the Kimen Seed Laboratory (Ås, Norway). The testing followed the International Seed Testing Association (ISTA) rules [32,33]. The details of the germination conditions for the different species in the study are shown in Table 5. In the case of oilseed rape and onion, the number of days to final count was, however, larger than the number of days in the ISTA protocols. In accordance with the protocols, we germinated 4x100 or 8x50 seeds per sample and each replicate was compared to the mean. If a large variation was detected, the samples were re-tested. Different types of filter paper (Seedburo Equipment Co, Des Plaines, IL, USA) were used as substrates for the germination tests.
Table 4.
Dates of the retrieval of the samples over the first 30 years, and types of tests conducted.
| Year | Date | Germination | Moisture |
|---|---|---|---|
| 0 | 1986, December | X | X |
| 2.5 | 1989, June | X | X |
| 5 | 1991, December | X | X |
| 7.5 | 1994, June | X | X |
| 10 | 1996, December | X | X |
| 12.5 | 1999, June | X | |
| 15 | 2001, December | X | |
| 17.5 | 2004, June | X | |
| 20 | 2006, December | X | |
| 25 | 2011, December | X | |
| 30 | 2016, December | X |
Table 5.
Germination conditions for the different crops included in the experiment.
| Crop/Species | Germination Substrate |
Temperature (°C) |
Final Count (Day Number) |
|---|---|---|---|
| Barley | BP | 20 | 7 |
| Wheat | BP | 20 | 8 |
| Rye | BP | 20 | 7 |
| English ryegrass | TP | 20<=>30 | 14 |
| Timothy | TP | 15<=>25 | 10 |
| Kentucky bluegrass | TP | 15<=>25 | 28 |
| Meadow fescue | TP | 20<=>30 | 14 |
| Red clover | TP | 20 | 10 |
| Beet | PP | 20 | 14 |
| Oilseed rape | TP | 20 | 103 |
| Onion | TP | 15 | 144 |
| Lettuce | TP | 20-light | 7 |
| Cucumber | TP | 20<=>30 | 8 |
| Carrot | TP | 20 | 14 |
| Cauliflower, cabbage | TP | 20<=>30 | 10 |
For cereals, in-between paper methods (BP) were used; the seeds were placed on one moist paper with a second paper on top, and thereafter rolled and placed vertically in plastic. Inside the roll, the seeds germinated, and the seedlings developed. For grasses, red clover, and vegetables (except for beet), a Jacobsen apparatus was used [34], which is a plate where circular pieces of wet filter paper and seeds are placed and kept moist by a wick (TP). The seeds of beet were germinated between humidified pleated filter paper. The germination temperature varied from alternating between +20 °C for 16 h and +30 °C for 8 h to constant ambient temperatures. Lettuce required light for germination, but for the other species light was not applied. Tests of actual moisture content were made along with seed germination tests in year 0 and the first 10 years (Table 2).
4.3. Data Analyses
R software was used for statistical examination. One value was missing for year 5 for red clover (cultivar Jokioinen). It was replaced with the average of the four nearest neighbor values: 76.0%. The short descriptor names of the eleven test results are year 0, year 2.5, year 5, year 7.5, year 10, year 12.5, year 15, year 17.5, year 20, year 25, and year 30, where year 0 represents the results of the trial that started in 1986. Summary statistics and boxplots were used to overview the data. The R function ‘time series’ was used to illustrate the fluctuation of the results over the 11 test occasions over the first 30 years. We calculated the loss in germination (Δ germ) by averaging the germination percentage of the three first test occasions (y0, y2.5, and y5) minus the test occasion at y30. We then categorized the samples into three: the most short-lived group (with Δ germ of 15% or more, which is at a level where regeneration should have been carried out), the intermediate group (Δ germ 5% to 15%), and the most long-lived (Δ germ less than 5%, or with less than 95% germination loss over the 30 years).
Acknowledgments
We would like to thank Store Norske Spitsbergen Kulkompani for the seed storage and for organizing transport. A special thanks to the technical staff at the Kimen Seed Laboratory/Norwegian State Seed Testing Station for the exact and laborious germination and moisture tests, and to H. Tangerås who administered, and was responsible for, the laboratory work for most of the 30 years. We would also like to honor the Norwegian Ministry of Agriculture and Food and the Nordic Gene Bank/NordGen staff involved in this long-term seed trial.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/5/579/s1, Figure S1: Illustration of germination for each seed lot included in the trial.
Author Contributions
Conceptualization, F.Y.; Methodology, G.B., F.Y., and E.M.; Formal analysis, F.Y.; Investigation, S.Ø.S. and F.Y.; Resources, Å.A.; Data curation, F.Y.; Writing—Original draft preparation, S.Ø.S.; Writing—Review and editing, C.A., Å.A., G.B., and R.v.B.; Visualization, F.Y.; Supervision, Å.A. and R.v.B.; Project administration, Å.A.; Funding acquisition, S.Ø.S. and Å.A. All authors have read and agreed to the published version of the manuscript.
Funding
We also thank the Nordic Joint Committee for Food and Agricultural Research for network funds to publish the results from the first 30 years of the long-term experiment (Grant number SLU 202100-2817).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ellis R.H., Roberts E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980;45:13–30. doi: 10.1093/oxfordjournals.aob.a085797. [DOI] [Google Scholar]
- 2.Priestley D.A. Seed Aging: Implications for Seed Storage and Persistence in the Soil. Cornell University Press; Ithaca, NY, USA: 1986. [Google Scholar]
- 3.Roos E.E. Precepts of successful seed storage. In: McDonald M.B., Nelson C., editors. Physiology of Seed Deterioration, Special Publication no. 11. Crop Science Society of America; Madison, WI, USA: 1986. pp. 1–25. [Google Scholar]
- 4.Ellis R.H. The longevity of seeds. HortScience. 1991;26:1119–1125. doi: 10.21273/HORTSCI.26.9.1119. [DOI] [Google Scholar]
- 5.Frankel O., Hawkes J. Crop Genetic Resources for Today and Tomorrow. Cambridge University Press; Cambridge, UK: New York, NY, USA: 1975. 492p [Google Scholar]
- 6.Pistorius R. Scientists, Plants and Politics – A History of the Plant Genetic Resources Movement. International Board for Plant Genetic Resources; Rome, Italy: 1997. 134p [Google Scholar]
- 7.FAO . The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture. Food and Agriculture Organization of the United Nations; Rome, Italy: 2010. [Google Scholar]
- 8.Plucknett D.L., Smith N.J.H., Williams J.T., Anishetty N.M. Gene Banks and the World’s Food. Princeton University Press; Princeton, NJ, USA: 1987. [Google Scholar]
- 9.Harlan J.R. The Living Fields. Our Agricultural Heritage. Cambridge University Press; Cambridge, UK: New York, NY, USA: 1995. 273p [Google Scholar]
- 10.Walters C. Principles for preserving germplasm in genebanks. In: Guerrant E., Havens K., Maunder M., editors. Ex Situ Plant Conservation: Supporting Species Survival in the Wild. Island Press; Covelo, CA, USA: 2004. pp. 442–453. [Google Scholar]
- 11.Fowler C., Hodgkin T. Plant genetic resources for food and agriculture: Assessing global availability. Annu. Rev. Environ. Resour. 2004;29:143–179. doi: 10.1146/annurev.energy.29.062403.102203. [DOI] [Google Scholar]
- 12.Frankel O. Genetic resources: The founding years. Part IV: After twenty years. Diversity. 1987;3:25–27. [Google Scholar]
- 13.FAO . Genebank Standards for Plant Genetic Resources for Food and Agriculture. Food and Agriculture Organization of the United Nations; Rome, Italy: 2014. [Google Scholar]
- 14.Westengen O.T., Jeppson S., Guarino L. Global ex-situ crop diversity conservation and the Svalbard Global Seed Vault: Assessing the current status. PLoS ONE. 2013;8:e64146. doi: 10.1371/journal.pone.0064146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yndgaard F. Genebank security storage in permafrost. Plant Genet. Resour. Newsl. 1985;62:2–7. [Google Scholar]
- 16.Priestley D.A., Cullinan V.I., Wolfe J. Differences in seed longevity at the species level. Plant Cell Environ. 1985;8:557–562. doi: 10.1111/j.1365-3040.1985.tb01693.x. [DOI] [Google Scholar]
- 17.Nagel M., Börner A. The longevity of crop seeds stored under ambient conditions. Seed Sci. Res. 2010;20:1–12. doi: 10.1017/S0960258509990213. [DOI] [Google Scholar]
- 18.Rincker C.M. Germination of forage crop seeds after 20 years of subfreezing storage. Crop Sci. 1983;23:229–231. doi: 10.2135/cropsci1983.0011183X002300020009x. [DOI] [Google Scholar]
- 19.Roos E.E., Davidson D.A. Record longevities of vegetable seeds in storage. HortScience. 1986;27:393–396. doi: 10.21273/HORTSCI.27.5.393. [DOI] [Google Scholar]
- 20.Walters C., Wheeler L., Grotenhuis J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 1992;15:1–20. doi: 10.1079/SSR2004195. [DOI] [Google Scholar]
- 21.Desheva G. The longevity of crop seeds stored under long-term condition in the national gene bank of Bulgaria. Agriculture (Polnohospodárstvo) 2016;62:90–100. doi: 10.1515/agri-2016-0010. [DOI] [Google Scholar]
- 22.Cromarty A.S., Ellis R.H., Roberts E.H. The Design of Seed Storage Facilities for Genetic Conservation. International Board for Plant Genetic Resources; Rome, Italy: 1982. 96p [Google Scholar]
- 23.Austin R.B. Effect of environment before harvesting on viability. In: Roberts E.H., editor. Viability of Seeds. Chapman and Hall; London, UK: 1972. pp. 114–149. [Google Scholar]
- 24.Justice O.L., Bass L.N. Principles and Practices of Seed Storage. USDA; Washington, DC, USA: 1978. 289p [Google Scholar]
- 25.Pieta Filho C., Ellis R.H. The development of seed quality in spring barley in four environments. I. Germination and longevity. Seed Sci. Res. 1991;1:163–177. doi: 10.1017/S0960258500000830. [DOI] [Google Scholar]
- 26.Dias D.C.F.S., Ribeiro F.P., Dias L.A.S., Silva D.J.H., Vidigal D.S. Tomato seed quality in relation to fruit maturation and post-harvest storage. Seed Sci. Technol. 2006;34:691–699. doi: 10.15258/sst.2006.34.3.15. [DOI] [Google Scholar]
- 27.Rao N.K., Dulloo M.E., Engels J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017;64:1061–1074. [Google Scholar]
- 28.Nagel M., Arif M.A.R., Rosenhauer M., Börner A. Longevity of Seeds—Intraspecific Differences in the Gatersleben Genebank Collections. Volume 60. Tagung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Lehr- und Forschungsanstalt für Landwirtschaft Raumberg-Gumpenstein; Irdning, Austria: 2010. pp. 179–181. [Google Scholar]
- 29.Steiner A., Ruckenbauer P. Germination of 110-year-old cereal and weed seeds, the Vienna Sample of 1877. Verification of effective ultra-dry storage at ambient temperature. Seed Sci. Res. 1995;5:195–199. doi: 10.1017/S0960258500002853. [DOI] [Google Scholar]
- 30.Probert R., Adams J., Coneybeer. J., Crawford A., Hay F. Seed quality for conservation is critically affected by pre-storage factors. Australian J. Bot. 2007;55:326–335. doi: 10.1071/BT06046. [DOI] [Google Scholar]
- 31.Nagel M., Vogel H., Landjeva S., Buck-Sorlin G., Lohwasser U., Scholz U., Börner A. Seed conservation in ex situ genebanks-genetic studies on longevity in barley. Euphytica. 2009;170:5–14. doi: 10.1007/s10681-009-9975-7. [DOI] [Google Scholar]
- 32.ISTA International rules for seed testing. Seed Sci. Technol. 1985;13:299–355. [Google Scholar]
- 33.ISTA . International Rules for Seed Testing. International Seed Testing Association; Basserdorf, Switzerland: 2017. [Google Scholar]
- 34.ISTA . The Germination Tests. International Seed Testing Association; Basserdorf, Switzerland: 2011. International rules for seed testing, Chapter 5; p. 16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.