Abstract
Studies in Asian Indians have examined the association of metabolic traits with vitamin D status. However, findings have been quite inconsistent. Hence, we aimed to explore the relationship between metabolic traits and 25-hydroxyvitamin D [25(OH)D] concentrations. We investigate whether this relationship was modified by lifestyle factors using a nutrigenetic approach in 545 Asian Indians randomly selected from the Chennai Urban Rural Epidemiology Study (219 normal glucose tolerant individuals, 151 with pre-diabetes and 175 individuals with type 2 diabetes). A metabolic genetic risk score (GRS) was developed using five common metabolic disease-related genetic variants. There was a significant interaction between metabolic GRS and carbohydrate intake (energy%) on 25(OH)D (Pinteraction = 0.047). Individuals consuming a low carbohydrate diet (≤62%) and those having lesser number of metabolic risk alleles (GRS ≤ 1) had significantly higher levels of 25(OH)D (p = 0.033). Conversely, individuals consuming a high carbohydrate diet despite having lesser number of risk alleles did not show a significant increase in 25(OH)D (p = 0.662). In summary, our findings show that individuals carrying a smaller number of metabolic risk alleles are likely to have higher 25(OH)D levels if they consume a low carbohydrate diet. These data support the current dietary carbohydrate recommendations of 50%–60% energy suggesting that reduced metabolic genetic risk increases 25(OH)D.
Keywords: GRS, SNP, metabolic traits, vitamin D, 25(OH)D, carbohydrate intake, Asian Indian, CURES
1. Introduction
Interaction between genetic and lifestyle factors have been shown to contribute to the development of metabolic disorders such as obesity and type 2 diabetes (T2D) [1,2]. The prevalence of metabolic diseases is increasing worldwide, and Asian Indians have a greater predisposition [3,4]. The Asian Indian population have a unique clinical phenotype characterized by increased visceral fat and waist circumference (WC), increased susceptibility to type 2 diabetes at a younger age, hyperinsulinemia, insulin resistance and dyslipidemia with raised triglycerides and low high density lipoprotein–cholesterol (HDL-c) levels at normal ranges of body mass index (BMI) collectively known as “Asian Indian Phenotype” [5,6]. Furthermore, several studies have demonstrated that metabolic diseases are associated with micronutrient deficiencies, such as vitamin D deficiency [7,8,9,10].
Vitamin D is a fat-soluble vitamin, known for its impact on skeletal and extra-skeletal physiological processes. Vitamin D deficiency exists in endemic proportions all over India, with a prevalence ranging from 80%–90% [11]. Adequate levels of vitamin D are important for calcium absorption, bone mineralization and skeletal growth as well as a multitude of biologic functions at the cellular level such as cell growth, proliferation, differentiation, inflammation and apoptosis. Additionally, vitamin D has been linked to cancer, cardiovascular diseases, inflammation and autoimmune diseases [12,13,14]. Several observational studies have associated vitamin D deficiency with increased obesity and reported inverse relationship between 25(OH)D concentration and BMI, WC and total body fat; however, the causal effect was not established [15,16]. Nevertheless, a Mendelian Randomization analysis in 42,024 participants of European ancestry concluded that increased BMI leads to reduced 25(OH)D concentrations while there was no causal association between lower 25(OH)D concentrations and higher BMI [17]. Given that observational studies are often prone to bias and confounding, a genetic approach to explain the relationship between metabolic diseases and vitamin D deficiency may be a better option to reduce any influence from unmeasured confounding factors.
Association of several genetic variants with metabolic diseases has been identified by candidate gene and genome-wide association studies (GWAS) [2,18,19,20]. Currently, the fat mass and obesity-associated (FTO) gene is the strongest risk loci for obesity [1,21]. The FTO gene is the first obesity susceptibility gene to be identified by two GWAS in European populations [22,23]. A study in an Asian Indian population has shown that lifestyle factors can influence the association of FTO gene with obesity traits [21]. Besides the FTO, Melanocortin 4 Receptor (MC4R) and Transcription Factor 7-Like 2 (TCF7L2) genes are the two commonly studied candidate genes for obesity and T2D [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. In the present study, we examined the association of a metabolic-genetic risk score (GRS) developed from five single-nucleotide polymorphisms (SNPs) [FTO (rs8050136 and rs2388405), MC4R (rs17782313) and TCF7L2 (rs12255372 and rs7903146)] with metabolic traits and vitamin D concentrations. In addition, we investigated the link between metabolic traits and vitamin D status by exploring the interactions between the metabolic GRS and lifestyle factors such as diet and physical activity on metabolic traits and vitamin D concentrations in an Asian Indian population.
2. Methods
2.1. Study Population
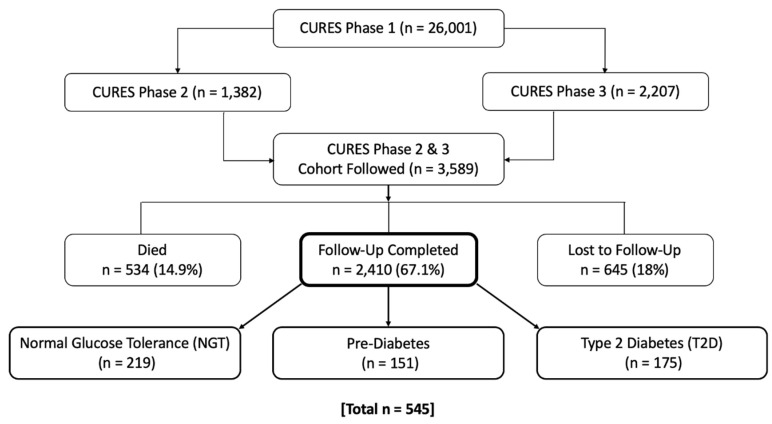
Five hundred forty-five study participants were recruited randomly from the Chennai Urban Rural Epidemiology Study (CURES) follow-up study (Figure 1), aged 29–85 years old [3]. CURES is an epidemiological cross-sectional study conducted on participants from Chennai city population in southern India, which is the fourth largest city in India. Details of the methodology have been previously published [39]. In brief, the CURES study was conducted in three phases. Phase 1: 26,001 adult participants (>20 years of age) were recruited using a systematic random sampling method covering the whole Chennai city and all participants were screened for diabetes. Phase 2: all 1382 diabetic participants were invited for further investigation (90.4% compliance). Phase 3: 2207 adult participants designated by way of every tenth participant from Phase 1, excluding diabetics, underwent further detailed investigations. Phases 2 & 3 constitutes the CURES follow-up cohort (n = 3589) [40]. For present study, 545 individuals were randomly selected from the follow-up cohort, which included: 219 normal glucose tolerant (NGT), 151 prediabetic and 175 T2D individuals. Three exclusion criteria were applied in this study: known cases of type 1 diabetes, diabetes secondary to other causes, and intake vitamin D supplements. The Madras Diabetes Research Foundation Institutional Ethics Committee granted ethical approval, and informed consent was obtained from the study participants. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki (ICH GCP).
Figure 1.
Selection of study participants from the Chennai Urban Rural Epidemiological Study (CURES follow-up study).
2.2. Anthropometric and Biochemical Measurements
Standardized methods were used to measure weight, height and WC. BMI was calculated based on the body weight in kilograms divided by the square of body height in meters. Generalized obesity was defined according to the World Health Organization Asia Pacific Guidelines for Asians (The Asia Pacific perspective 2000) as non-obese (BMI < 25 kg/m2) and obese (BMI ≥ 25 kg/m2) [41]. The following biochemical measurements were performed using kits supplied by Roche Diagnostics (Mannheim) on a Hitachi-912 Auto Analyzer (Hitachi, Mannheim, Germany): fasting plasma glucose (glucose oxidase-peroxidase), serum total cholesterol (cholesterol oxidase-phenol-4-amino-antipyrene peroxidase), serum triglycerides (glycerol phosphatase oxidase-phenol-4-amino-antipyrene peroxidase) and HDL-c (direct method; polyethylene glycol-pretreated enzymes) [42]. The Friedewald formula was used to calculate low-density lipoprotein cholesterol (LDL-c). Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography using a Variant™ machine (Bio-Rad, Hercules, CA, USA). Serum insulin and 25(OH)D vitamin D concentrations were estimated using the electrochemiluminescence (ECLIA) using a Roche e601Cobas immunoassay analyzer (Roche Diagnostics, Indianapolis, IN, USA) [21]. The intra- and inter-assay coefficients of variation for vitamin D assay was 3.62% and 6.38%, respectively.
2.3. Assessment of Dietary Intake and Physical Activity
A validated, interviewer administered semi-quantitative food frequency questionnaire (FFQ) consisting of 222 different foods was used to assess dietary intake for the previous year [43]. In brief, participant had an interview ranging from 20 and 30 min where they had to estimate their usual portion size and usual frequencies (per day, week, month, year, never) with the help of visual aids of measurement equipment and food sizes. Description of the development of FFQ and the data on reproducibility and validity was previously published [43]. Daily average food and nutrient intake including macronutrient and total energy intake were analyzed and estimated by the EpiNu database system. A validated self-report questionnaire was used to assess physical activity levels of the participants [44]. Individuals were classified into three groups: 1. Vigorously active: where the participants exercised and engaged in demanding work activities; 2. Moderately active: where the participants either exercised or performed heavy physical work; 3. Sedentary: those participants who did not exercise or have physically demanding work.
2.4. SNP Selection and Genotyping
For the present study, five SNPs were chosen from three different genes based on their previous associations with obesity and T2D in several populations: FTO (rs8050136 & rs2388405) [21,25,26,38,45,46,47,48,49,50], TCF7L2 (rs12255372 & rs7903146) [24,28,51,52,53,54,55] and MC4R (rs17782313) [25,27,34,37,38]. FTO gene variants are known to be the strongest genetic predictors of obesity to date [56,57]. The FTO SNP rs8050136 has shown a strong association with obesity and T2D [27,49,50,57,58]. Furthermore, the FTO SNPs, rs8050136 and rs2388405, have also been reported as intronic enhancers, as they may have an influence on the gene expression [45,59,60]. MC4R SNP rs17782313 was shown to be associated with obesity in European populations [34,38] and this finding then replicated in other populations including South Asians [27,37,61]. TCF7L2 SNPs, rs12255372 and rs7903146, were shown to be associated with increased susceptibility to T2D in two large multiethnic meta-analyses [52,55]. Some studies have reported that the TCF7L2 SNPs are involved in modulating and reducing adiposity through changes in the lifestyle [62,63,64]. Based on the previous studies, the above mentioned five SNPs were chosen for the present study.
Phenol–chloroform method of DNA extraction from whole blood was performed. The genotyping methodology for the five SNPs have been previously published [4,24,28]. Direct sequencing by an ABI 310 genetic analyzer (Applied Biosystems, Foster City, CA) was performed to confirm the efficiency of the genotyping; there was 99% concordance based on random duplicates of 20% of the samples.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS statistical software (version 24; SPSS, Inc., Chicago, IL, USA). Allele frequencies were calculated by gene counting and chi-squared test was carried out to compare the proportions of genotypes/alleles. The genotypic frequencies of the five SNPs were in the Hardy–Weinberg Equilibrium (p > 0.05) (Table S1). To obtain normal distribution, all metabolic outcomes and vitamin D values were log transformed. The difference in the means of continuous variables between the participants with NGT vs. pre-diabetes and NGT vs. T2D was analyzed by independent sample t-test. Descriptive statistics for continuous variables are presented as means and standard deviation (SD). The chi-squared test was used to analyze and compare physical activity levels (vigorously active, moderately active and sedentary) between individuals with NGT vs. those with pre-diabetes and individuals with NGT vs. those with T2D. Unweighted metabolic GRS was calculated for each participant by adding the number of risk alleles for metabolic diseases. The SNPs, rs8050136, rs2388405, rs12255372, rs7903146 and rs17782313, were used to generate the GRS. A value of zero, one and two was assigned to each SNP, which indicates the number of metabolic disease-related risk alleles. These values were then calculated by adding the number of metabolic disease-related risk alleles across each SNP. The risk allele score was then divided by the median into those carrying ≤1 risk allele vs. those with >1 risk allele.
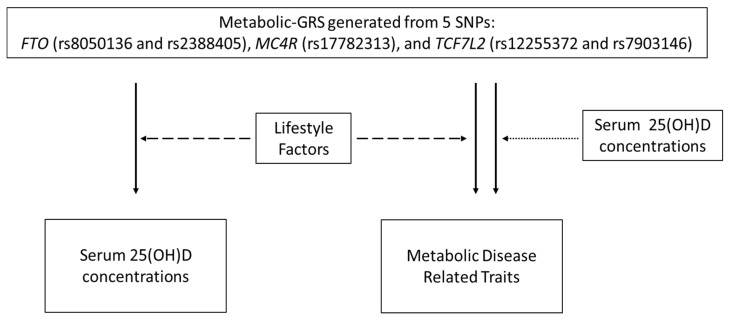
A schematic representation of the study objectives is presented in Figure 2. Association analysis between the GRS and continuous and categorical variables were carried out using general linear and binary logistic regression models, respectively, adjusting for age, BMI, T2D and month of sample collection, wherever appropriate. The variable ‘month of sample collection’ was created based on the three seasons in India: summer (March to June), autumn/monsoon (July to October) and winter (November to February) [65]. Linear and logistic regression analyses were also used for investigating the interaction between SNPs and lifestyle factors (dietary intake and physical activity), where the interaction terms were incorporated into the models and adjusted for age, gender, BMI, T2D, total energy intake and month of sample collection wherever appropriate. Further tertile stratification of the lifestyle factor (diet/physical activity) was performed when there was a significant interaction between metabolic GRS and lifestyle factors on 25(OH)D concentrations and metabolic traits. Power calculation was not performed, given that there are no studies on metabolic GRS and no previously reported effect sizes for South Asians.
Figure 2.
Study objectives. The unbroken one-sided arrows indicate the associations that were tested between the metabolic GRS and vitamin D concentrations and metabolic disease related traits. The broken one-sided arrows represent the interactions that were investigated between the GRS and lifestyle factors (diet and physical activity levels) on serum vitamin D and metabolic disease related traits. The one-sided dotted arrow indicates the interaction that was examined between metabolic GRS and 25(OH)D concentrations on metabolic disease -related traits.
3. Results
3.1. Characteristics of Study Participants
The anthropometric, biochemical and lifestyle characteristics of the CURES participants are presented in Table 1. Significant differences were found between individuals with NGT, pre-diabetes and T2D, where individuals with T2D were older (p < 0.001), had higher WC (p < 0.001), fasting plasma insulin (p < 0.001), systolic and diastolic blood pressure (p < 0.001) and serum triglycerides (p < 0.001). However, individuals with pre-diabetes had higher BMI (p = 0.001) and LDL-c (p = 0.004) than individuals with NGT and T2D. No significant differences were observed in the levels of vitamin D, diastolic blood pressure, total cholesterol, HDL-c and dietary intakes across the three groups (p > 0.05).
Table 1.
Baseline characteristics of the study participants.
| Characteristics of Study Participants | n | Normal Glucose Tolerance | n | Pre-Diabetes | n | Type 2 Diabetes | p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 219 | 46.82 ± 10.54 | 151 | 47.79 ± 11.5 | 175 | 54.19 ± 11.04 | <0.001 α γ |
| BMI (kg/m2) | 219 | 26.10 ± 5.15 | 151 | 27.95 ± 5.22 | 174 | 26.56 ± 4.58 | 0.001 β |
| WC (cm) | 219 | 86.04 ± 11.73 | 151 | 89.54 ± 11.2 | 173 | 90.11 ± 10.27 | <0.001 α β |
| Vitamin D (ng/mL) | 219 | 19.55 ± 13.5 | 151 | 19.14 ± 10.47 | 175 | 17.8 ± 10.03 | 0.381 |
| Fasting plasma glucose (mg/dL) | 201 | 89.74 ± 6.54 | 144 | 103.43 ± 11.59 | 172 | 156.28 ± 64.43 | <0.001 α β γ |
| HbA1c (%) | 219 | 5.61 ± 0.47 | 151 | 5.91 ± 0.59 | 175 | 8.19 ± 2.07 | <0.001 α β γ |
| Fasting plasma insulin (µLU/mL) | 216 | 7.76 ± 5.13 | 139 | 8.13 ± 4.73 | 132 | 11.48 ± 7.69 | <0.001 α γ |
| Systolic BP (mmHg) | 219 | 125.77 ± 20.97 | 151 | 126.54 ± 17.77 | 175 | 134.53 ± 19.6 | <0.001α γ |
| Diastolic BP (mmHg) | 219 | 79.17 ± 12.84 | 151 | 79.79 ± 10.78 | 175 | 80.67 ± 10.95 | 0.320 |
| Total cholesterol (mg/dL) | 219 | 181.07 ± 35.81 | 151 | 187.72 ± 35.28 | 175 | 181.2 ± 38.77 | 0.126 |
| LDL cholesterol (mg/dL) | 219 | 114.58 ± 31.58 | 151 | 119.17 ± 31.43 | 175 | 107.74 ± 34.63 | 0.004 α γ |
| HDL cholesterol (mg/dL) | 219 | 42.06 ± 9.57 | 151 | 40.10 ± 7.82 | 175 | 40.32 ± 8.58 | 0.093 |
| Serum triglycerides (mg/dL) | 219 | 122.15 ± 63.7 | 151 | 142.25 ± 83.08 | 175 | 165.71 ± 95.93 | <0.001 α β γ |
| Total energy intake (kcal) | 185 | 2620.04 ± 752.02 | 83 | 2535.29 ± 803.78 | 93 | 2585.85 ± 787.79 | 0.609 |
| Protein energy% | 185 | 11.28 ± 1.19 | 83 | 11.31 ± 0.89 | 93 | 11.38 ± 1.2 | 0.758 |
| Fat energy% | 185 | 23.91 ± 4.76 | 83 | 23.33 ± 4.51 | 93 | 24 ± 4.72 | 0.582 |
| Carbohydrate energy% | 185 | 64.09 ± 6.69 | 83 | 64.89 ± 5.51 | 93 | 64.36 ± 5.97 | 0.556 |
| Protein (g) | 185 | 73.47 ± 21.39 | 83 | 71.59 ± 23.74 | 93 | 72.78 ± 21.2 | 0.704 |
| Fat (g) | 185 | 69.62 ± 25.15 | 83 | 65.92 ± 26.97 | 93 | 67.94 ± 22.15 | 0.407 |
| Carbohydrate (g) | 185 | 417.24 ± 115.73 | 83 | 409.91 ± 125.98 | 93 | 418.82 ± 142.37 | 0.847 |
| Dietary fiber (g) | 185 | 32.18 ± 10.91 | 83 | 30.77 ± 11.4 | 93 | 33.01 ± 11.85 | 0.235 |
| Physical activity level | 171 | Sedentary (80.1%) | 73 | Sedentary (83.6%) | 81 | Sedentary (84.0%) | 0.676 δ |
| Moderate (18.7%) | Moderate (13.7%) | Moderate (13.6%) | |||||
| Vigorous (1.2%) | Vigorous (2.7%) | Vigorous (2.5%) |
Data shown are represented as means ± SD; p values were calculated using one-way ANOVA; δ p values were calculated using the chi-squared test. α indicates significance between non-diabetics and T2D individuals, β indicates significance between normal glucose tolerance and pre-diabetics, γ indicates significance between pre-diabetes and Type 2 diabetes. Abbreviations: CURES: Chennai Urban Rural Epidemiological Study, BMI: body mass index, WC: waist circumference, BP: blood pressure, LDL: low-density lipoprotein, HDL: high-density lipoprotein.
3.2. Association of 25(OH)D Concentrations with Obesity and Type 2 Diabetes
There was a significant association of 25(OH)D concentrations with BMI (p = 0.017) and WC (p = 0.047) after adjusting for age, gender, month of sample collection and T2D. However, there was no association of 25(OH)D concentrations with fasting plasma glucose (p = 0.739), HbA1c (p = 0.823) and fasting plasma insulin (p = 0.387) after adjusting for age, gender, month of sample collection and BMI.
3.3. Association of the Metabolic GRS with 25(OH)D Level and Metabolic-Related Traits
No significant associations were observed between metabolic GRS and 25(OH)D concentrations (p = 0.34). None of the clinical and biochemical parameters such as BMI, WC, fasting plasma glucose and insulin, HbA1c, systolic and diastolic blood pressure, total cholesterol, HDL-c, LDL-c, and triglycerides, showed a significant association with metabolic GRS (p > 0.19 for all comparisons) (Table S2).
3.4. Interaction between Metabolic GRS and 25(OH)D Concentrations on Metabolic Traits
There was a borderline interaction between the metabolic GRS and 25(OH)D concentrations on HbA1c level (p = 0.048) after adjusting for age, gender, BMI, T2D and month of sample collection. However, no association was detected between the metabolic GRS and HbA1c when participants were grouped in tertiles of 25(OH)D concentrations (tertile 1, p = 0.471; tertile 2, p = 0.870; tertile 3, p = 0.486).
3.5. Interaction between Metabolic GRS and Lifestyle Factors on 25(OH)D Concentrations
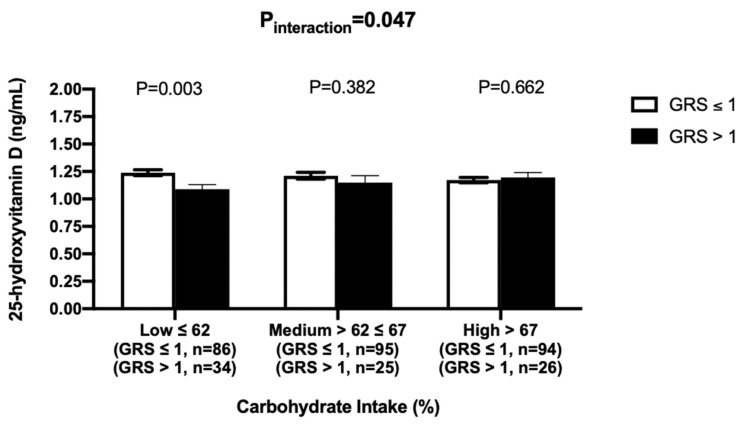
After adjusting for age, gender, BMI, T2D and month of sample collection, there was a significant interaction between the GRS and dietary carbohydrate intake on 25(OH)D concentrations (P-interaction = 0.047). Tertile analysis was performed where individuals were grouped based on the tertiles of carbohydrate intake (energy%) [low ≤62%, medium = 62%–67% and high >67%)]. There were significant differences between the two GRS groups only among those who were in the first tertile of carbohydrate intake (p = 0.003), where individuals with lesser number of risk alleles (GRS ≤ 1) had greater 25(OH)D concentrations compared to those with higher number of risk alleles (GRS > 1) (Figure 3). Among individuals who had a higher carbohydrate intake (>67.28%), despite having lesser number of metabolic risk alleles, did not show a significant higher 25(OH)D concentrations (p = 0.66) compared to those with higher number of risk alleles (GRS > 1) (Figure 3).
Figure 3.
Interaction between metabolic GRS and log carbohydrate intake (%) on log 25 hydroxyvitamin D. White bars indicate individuals with GRS ≤ 1 risk allele; Black bars indicate individuals with GRS > 1 risk allele. Among individuals with low carbohydrates intake, those with < 1 risk allele had significantly higher 25 hydroxyvitamin D concentrations compared to those with > 1 risk allele (p = 0.003).
3.6. Interaction between the GRS and Lifestyle Factors on Clinical and Biochemical Parameters Traits
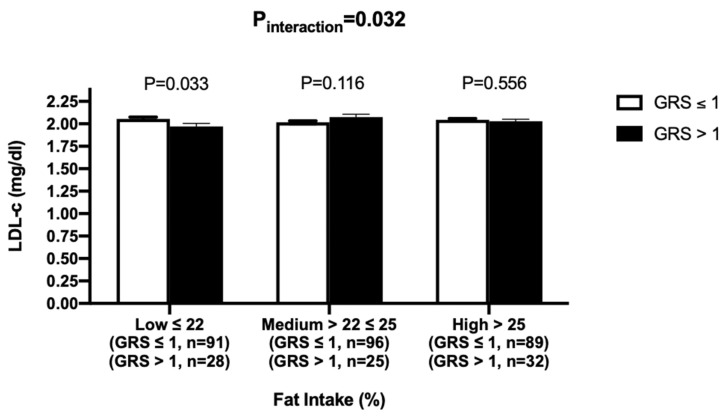
There were significant interactions of metabolic GRS with dietary total fat intake and carbohydrates intake on LDL-c concentrations (Pinteraction = 0.032 and Pinteraction = 0.028, respectively) after adjusting for age, gender, BMI and T2D. However, no significant interaction was found between GRS and saturated, polyunsaturated and monounsaturated fat intake on LDL-c concentrations (p > 0.05, for all comparisons). After individuals were split into tertiles based on their fat intake (energy%) (low ≤22%, medium = 22%–25% and high >25%), there was a significant association of metabolic GRS with LDL-c in the low fat intake group (p = 0.033), where individuals despite having a higher genetic risk (>1 risk allele) had significantly lower LDL-c concentrations (Figure 4).
Figure 4.
Interaction between metabolic GRS and fat intake (%) on log LDL-c. White bars indicate individuals with GRS ≤ 1 risk allele; Black bars indicate individuals with GRS > 1 risk allele. Among individuals consuming a low-fat diet, despite having a higher genetic risk (>1 risk allele), they had significantly lower LDL-c concentrations (p = 0.033).
On the other hand, the tertile analysis of carbohydrate intake did not show any significant association between GRS and LDL-c concentrations (tertile 1, p = 0.453; tertile 2, p = 0.146; tertile 3, p = 0.460). None of the other lifestyle factors including physical activity showed a significant interaction with metabolic GRS on metabolic traits (p > 0.11 for all comparisons) (Table 2).
Table 2.
Interaction between genetic risk score and lifestyle factors on clinical and biochemical parameters.
| Outcome Measures | Physical Activity Levels | Protein% | Fat% | Carbohydrates% | Saturated Fatty Acids g/d * | Polyunsaturated Fatty Acids g/d * | Monounsaturated Fatty Acids g/d * |
|---|---|---|---|---|---|---|---|
| Body Mass Index | 0.89 | 0.94 | 0.16 | 0.20 | - | - | - |
| Waist Circumference | 0.45 | 0.70 | 0.54 | 0.47 | - | - | - |
| 25(OH)D ** | 0.90 | 0.69 | 0.32 | 0.047 | - | - | - |
| Fasting Plasma Glucose | 0.12 | 0.90 | 0.16 | 0.09 | - | - | - |
| Glycated Hemoglobin | 0.13 | 0.52 | 0.44 | 0.32 | - | - | - |
| Fasting Plasma Insulin | 0.84 | 0.41 | 0.14 | 0.76 | |||
| Systolic Blood Pressure | 0.72 | 0.19 | 0.96 | 0.62 | - | - | - |
| Diastolic Blood Pressure | 0.93 | 0.93 | 0.22 | 0.54 | - | - | - |
| Total Cholesterol | 0.80 | 0.40 | 0.47 | 0.55 | - | - | - |
| Low density lipoprotein Cholesterol | 0.90 | 0.12 | 0.032 | 0.028 | 0.21 | 0.28 | 0.27 |
| High density lipoprotein cholesterol | 0.68 | 0.55 | 0.72 | 0.80 | - | - | - |
| Fasting serum triglycerides | 0.87 | 0.11 | 0.26 | 0.11 | - | - | - |
p value for interactions obtained by general linear univariate analysis. All interactions were adjusted for age, gender, type 2 diabetes and BMI (except BMI) * Adjusted for log-total energy intake; ** Adjusted for month of sample collection.
4. Discussion
Our study is the first to investigate gene-lifestyle interactions on 25(OH)D concentrations in Asian Indians. The main finding of our study is the interaction between carbohydrate intake and metabolic GRS, generated from five common metabolic-disease-related genetic variants, on 25(OH)D concentrations, where individuals who had less number of risk alleles (GRS ≤1) and consumed lower amounts of carbohydrates (≤62%) had significantly higher levels of 25(OH)D. Achieving and maintaining adequate levels of vitamin D is a desirable outcome as vitamin D deficiency is linked to several chronic diseases [66]. Given that previous studies have reported that Asian Indians have lower 25(OH)D concentrations [11,67], our findings suggest that, even if the genetic risk is lower, following the dietary carbohydrate recommendations (50%–60%) is required to improve the vitamin D status in this Asian Indian population.
Epidemiological studies have demonstrated a link between metabolic diseases such as obesity and T2D and vitamin D deficiency; however, it remains uncertain whether improving the metabolic status would reduce the risk of vitamin D deficiency [68,69,70,71]. The association between obesity and vitamin D status was consistent across different populations in several meta-analyses [70,71]. A large meta-analysis of 23 studies (n = 65,445) of mixed races reported that 35% of obese individuals suffer from vitamin D deficiency [70]. Several longitudinal studies have shown an inverse association between 25(OH)D status and T2D [66]. A meta-analysis in 2320 Caucasians showed that participants with adequate 25(OH)D concentrations had a 43% reduced risk of T2D [68]. However, unlike observational studies, vitamin D supplementation (4000 IU/day intake of vitamin D3) did not show beneficial effects on glycemic measures in two randomized, controlled trials (RCTs). In the first trial, well-controlled patients with T2D (n = 127) did not show any improvement in β-cell function, insulin secretion rate, nor in HbA1c levels after 48 weeks of supplementation [72]. In the second trial, 2423 prediabetic adults were evaluated for the development of diabetes for an average of 2.5 years; at the end of the study, 293 out of 1211 participants (24.2%) in the vitamin D supplementation group developed diabetes compared to 323 out of 1212 (26.7%) in the placebo group [73]. Furthermore, majority of RCTs also did not show an effect of vitamin D supplementation on weight loss [74].
Genetic studies are considered to be an effective approach in investigating the relationship between vitamin D and metabolic outcomes [75], as they are free from confounding and bias, which have been shown to affect the association between vitamin D levels and metabolic diseases. To our knowledge, there are no previous studies that have investigated the effect of metabolic disease-related genetic variants on vitamin D status. However, there are genetic association studies that have investigated the effect of vitamin D-related genetic variants on metabolic disease outcomes; but, the findings have been quite inconsistent [76,77,78,79]. A large bidirectional meta-analysis of 42,024 Europeans reported that there was no association between vitamin D-related genetic score and higher BMI; however, there was a significant association between genetically instrumented BMI and low vitamin D status [17]. In our study, the phenotypic associations of 25(OH)D concentrations with BMI and WC were statistically significant; but, the metabolic GRS did not show any association with 25(OH)D concentrations suggesting that the phenotypic associations are highly confounded.
To understand whether the genetic risk of metabolic diseases was influenced by vitamin D status, we tested for the interaction between the metabolic GRS and 25(OH)D concentrations on metabolic-disease related traits. None of the interactions were significant, except for a borderline interaction between the metabolic GRS and 25(OH)D concentrations on HbA1c level (p = 0.048); however, there was no association between metabolic GRS and HbA1c levels when participants were grouped into tertiles of 25(OH)D concentrations suggesting that there was no evidence for metabolic genetic risk acting as effect modifiers of the association between vitamin D status and metabolic traits. A study in 5160 participants of European ancestry also provided no evidence of vitamin D-related genetic variants acting as major modifiers of the association between 25(OH)D levels and cardio-metabolic risk [80]. Hence, these findings including the results from the present study indicate that vitamin D status is unlikely to have a significant impact on metabolic disease risk.
In the present study we found an interaction between the metabolic GRS and carbohydrate intake on vitamin D levels where lower consumption of carbohydrates was shown to be associated with higher 25(OH)D concentrations in the presence of reduced genetic risk. In the CURES, the carbohydrate intake included cereal grains, pulses, legumes, tubers, fruits, sweets, sweet beverages, carbonated beverages, junk food and added sugar, where consumption of refined cereals (i.e., mainly white rice) accounted for 78.1% of total calories [81]. This is a high intake compared to the recommended carbohydrate intake 50%–60% of total calories for Asian Indians [82], and the WHO (2002) recommendations of total carbohydrate intake at 55%–75% of total dietary energy [83]. The lowest tertile of the carbohydrate intake (≤62%), where we observed the positive association with vitamin D status, was close to the recommended dietary intake for Asian Indians (50%–60%), which supports the benefits of the current carbohydrate recommendations for Asian Indians. Our findings are also in line with a five-week intervention study [84], which used a reduced carbohydrate diet (43% carbohydrate; 27% fat) in 28 obese African American girls (9–14 years). The study showed that 25(OH)D concentrations were inversely associated with fasting glucose levels providing evidence that vitamin D may exert alterations in the biologic response to macronutrients such as dietary carbohydrates.
A further interesting finding in our study is the significant interaction between metabolic GRS and fat intake (%) on LDL-c concentrations, where despite having higher metabolic risk alleles, individuals who consumed a low-fat diet (≤21.89%) had significantly lower LDL-c levels. This suggests that lower dietary fat intake may influence the genetic risk of higher serum LDC-c concentrations, although mechanisms of action are unclear. This finding is in accordance with a GWAS on lipids in 541 individuals from the Quebec Family Study which reported an interaction between GRS (29 SNPs) and total fat intake on LDL-c concentrations (p << 10−5) [85]. The recommended dietary fat intake for Asian Indians is <30% [82]; however, in our study, only those individuals consuming total fat <21.8% demonstrated a significantly lower serum LDL-c concentrations, despite higher genetic susceptibility. Hence, our findings, if replicated using larger cohorts and dietary intervention studies, may have significant implications in providing dietary recommendations for those with higher metabolic-risk alleles.
The present study has several strengths, which include the use of a representative sample of Chennai [39] and an extensive and a validated semi-quantitative FFQ for dietary assessment [43]. In addition, the semi-quantitative FFQ has demonstrated high reproducibility and validity for macronutrient intakes such as dietary carbohydrate and fiber intake. Furthermore, the use of a metabolic GRS, which combines the effect of multiple SNPs, has been shown to increase the statistical power and an effective approach to study metabolic diseases [86,87]. However, there are some underlying limitations that need to be acknowledged. The measurement bias that is associated with self-reported FFQ and physical activity questionnaire cannot be ruled out. The study used a cross-sectional design and hence, no cause and effect conclusions can be established. Even though potential confounders were adjusted in all our statistical analyses, confounding factors such as sun exposure cannot be ruled out; however, we have adjusted for month of sample collection to overcome this limitation [65]. Finally, small sample size could be considered as another limitation in our study; nevertheless, we have identified significant findings, which suggest that the study is statistically powered to identify gene-diet interactions.
5. Conclusions
The present study has identified a novel interaction between metabolic GRS and carbohydrate intake on 25(OH)D levels in an Asian Indian population where individuals carrying a lesser number of metabolic risk alleles are likely to have higher 25(OH)D concentrations, only if they have a carbohydrate intake <62% energy. This is broadly in line with current dietary recommendations in India (50%–60% energy). This finding needs to be replicated in a larger cohort before these data can be confirmed. Mechanistic links also need to be identified.
Acknowledgments
We thank all study participants for their cooperation. Karani S Vimaleswaran acknowledges support from the British Nutrition Foundation. The CURES was supported by Lady Tata Memorial Trust, Mumbai. The Chennai Wellingdon Corporate Foundation supported the CURES field studies and this is the 155th study from CURES (CURES-155). We thank the Public Authority for Applied Education and Training of Kuwait for the scholarship given to Ms. Buthaina AlAthari.
Abbreviations
| T2D | type 2 diabetes |
| 25(OH)D | 25-hydroxyvitamin D |
| WC | waist circumference |
| BMI | body mass index |
| LDL-c | low density lipoprotein cholesterol |
| HDL-c | high density lipoprotein cholesterol |
| GWAS | genome wide association studies |
| FTO | fat mass and obesity-associated gene |
| MC4R | melanocortin 4 receptor gene |
| TCF7L2 | transcription factor 7-like 2 gene |
| GRS | genetic risk score |
| SNP | single nucleotide polymorphism |
| CURES | Chennai Urban Rural Epidemiological Study |
| NGT | normal glucose tolerant |
| HbA1c | glycated hemoglobin |
| ECLIA | electrochemiluminescence |
| FFQ | food frequency questionnaire |
| SD | standard deviation |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/5/1357/s1, Table S1: Association Between Genetic Risk Score and Metabolic Traits, Table S2: Genotypic and allelic frequencies of the Single Nucleotide Polymorphisms that were used to create the genetic risk score.
Author Contributions
Conceptualization, K.S.V.; methodology, K.S.V. and B.E.A.; software, B.E.A., D.B., R.J.; validation, B.E.A., R.J., D.B.; formal analysis, B.E.A.; investigation, K.S.V., B.E.A.; resources, K.S.V., V.R.; data curation, K.S.V., B.E.A., R.P.; writing—original draft preparation, B.E.A. and K.S.V.; writing—review and editing, K.S.V., B.E.A., J.A.L., R.P., R.M.A., D.B., N.L., V.S., C.S.S.R., R.J.; supervision, K.S.V.; project training and administration, K.S.V., V.R., V.M., R.P.; funding acquisition, K.S.V., V.M. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Research Society for the Study of Diabetes in India (RSSDI) for the financial support for the study through their research grant (Project No: RSSDI/HQ/Grants/2014/250). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vimaleswaran K.S., Li S., Zhao J.H., Luan J., Bingham S.A., Khaw K.T., Ekelund U., Wareham N.J., Loos R.J. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am. J. Clin. Nutr. 2009;90:425–428. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 2.Vimaleswaran K.S., Loos R.J. Progress in the genetics of common obesity and type 2 diabetes. Expert Rev. Mol. Med. 2010;12:e7. doi: 10.1017/S1462399410001389. [DOI] [PubMed] [Google Scholar]
- 3.Anjana R.M., Unnikrishnan R., Mugilan P., Jagdish P.S., Parthasarathy B., Deepa M., Loganathan G., Kumar R.A., Rahulashankiruthiyayan T., Uma Sankari G., et al. Causes and predictors of mortality in Asian Indians with and without diabetes-10 year follow-up of the Chennai Urban Rural Epidemiology Study (CURES—150) PLoS ONE. 2018;13:e0197376. doi: 10.1371/journal.pone.0197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surendran S., Jayashri R., Drysdale L., Bodhini D., Lakshmipriya N., Shanthi Rani C.S., Sudha V., Lovegrove J.A., Anjana R.M., Mohan V., et al. Evidence for the association between FTO gene variants and vitamin B12 concentrations in an Asian Indian population. Genes Nutr. 2019;14:26. doi: 10.1186/s12263-019-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayyappa K.A., Shatwan I., Bodhini D., Bramwell L.R., Ramya K., Sudha V., Anjana R.M., Lovegrove J.A., Mohan V., Radha V., et al. High fat diet modifies the association of lipoprotein lipase gene polymorphism with high density lipoprotein cholesterol in an Asian Indian population. Nutr. Metab. (Lond.) 2017;14:8. doi: 10.1186/s12986-016-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S.A., Shivashankar R., Ali M.K., Anjana R.M., Deepa M., Kapoor D., Kondal D., Rautela G., Mohan V., Narayan K.M., et al. Is the “South Asian Phenotype” Unique to South Asians? Comparing Cardiometabolic Risk Factors in the CARRS and NHANES Studies. Glob Heart. 2016;11:89–96.e3. doi: 10.1016/j.gheart.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astrup A., Bugel S. Micronutrient deficiency in the aetiology of obesity. Int. J. Obes. 2010;34:947–948. doi: 10.1038/ijo.2010.81. [DOI] [PubMed] [Google Scholar]
- 8.Damms-Machado A., Weser G., Bischoff S.C. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr. J. 2012;11:34. doi: 10.1186/1475-2891-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehlawat U., Singh P., Pande S. Current status of Vitamin-D deficiency in India. Innov. Pharm. Pharmacother. 2014;2:328–335. [Google Scholar]
- 10.Thomas-Valdes S., Tostes M., Anunciacao P.C., da Silva B.P., Sant’Ana H.M.P. Association between vitamin deficiency and metabolic disorders related to obesity. Crit. Rev. Food Sci. Nutr. 2017;57:3332–3343. doi: 10.1080/10408398.2015.1117413. [DOI] [PubMed] [Google Scholar]
- 11.Aparna P., Muthathal S., Nongkynrih B., Gupta S.K. Vitamin D deficiency in India. J. Fam. Med. Prim. Care. 2018;7:324–330. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017;165:369–381. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Hossein-nezhad A., Holick M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiaras W.G., Weinstock M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011;91:115–124. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu S.V., Fischer K., Dawson-Hughes B., Freystaetter G., Beuschlein F., Schietzel S., Egli A., Bischoff-Ferrari H.A. Association between 25-Hydroxyvitamin D Status and Components of Body Composition and Glucose Metabolism in Older Men and Women. Nutrients. 2018;10:1826. doi: 10.3390/nu10121826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantovic A., Zec M., Zekovic M., Obrenovic R., Stankovic S., Glibetic M. Vitamin D Is Inversely Related to Obesity: Cross-Sectional Study in a Small Cohort of Serbian Adults. J. Am. Coll. Nutr. 2019;38:405–414. doi: 10.1080/07315724.2018.1538828. [DOI] [PubMed] [Google Scholar]
- 17.Vimaleswaran K.S., Berry D.J., Lu C., Tikkanen E., Pilz S., Hiraki L.T., Cooper J.D., Dastani Z., Li R., Houston D.K., et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler E., Barroso I. Genome-wide association studies and type 2 diabetes. Brief. Funct. Genom. 2011;10:52–60. doi: 10.1093/bfgp/elr008. [DOI] [PubMed] [Google Scholar]
- 20.Vimaleswaran K.S., Tachmazidou I., Zhao J.H., Hirschhorn J.N., Dudbridge F., Loos R.J. Candidate genes for obesity-susceptibility show enriched association within a large genome-wide association study for BMI. Hum Mol Genet. 2012;21:4537–4542. doi: 10.1093/hmg/dds283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vimaleswaran K.S., Bodhini D., Lakshmipriya N., Ramya K., Anjana R.M., Sudha V., Lovegrove J.A., Kinra S., Mohan V., Radha V. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. (Lond.) 2016;13:39. doi: 10.1186/s12986-016-0098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scuteri A., Sanna S., Chen W.-M., Uda M., Albai G., Strait J., Najjar S., Nagaraja R., Orrú M., Usala G., et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodhini D., Gaal S., Shatwan I., Ramya K., Ellahi B., Surendran S., Sudha V., Anjana M.R., Mohan V., Lovegrove J.A., et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE. 2017;12:e0188382. doi: 10.1371/journal.pone.0188382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozafarizadeh M., Mohammadi M., Sadeghi S., Hadizadeh M., Talebzade T., Houshmand M. Evaluation of FTO rs9939609 and MC4R rs17782313 Polymorphisms as Prognostic Biomarkers of Obesity: A Population-based Cross-sectional Study. Oman Med. J. 2019;34:56–62. doi: 10.5001/omj.2019.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roswall N., Angquist L., Ahluwalia T.S., Romaguera D., Larsen S.C., Ostergaard J.N., Halkjaer J., Vimaleswaran K.S., Wareham N.J., Bendinelli B., et al. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: Influence of FTO and TCF7L2 loci. Am. J. Clin. Nutr. 2014;100:1188–1197. doi: 10.3945/ajcn.114.089706. [DOI] [PubMed] [Google Scholar]
- 27.Vasan S.K., Fall T., Neville M.J., Antonisamy B., Fall C.H., Geethanjali F.S., Gu H.F., Raghupathy P., Samuel P., Thomas N., et al. Associations of variants in FTO and near MC4R with obesity traits in South Asian Indians. Obes. (Silver Spring) 2012;20:2268–2277. doi: 10.1038/oby.2012.64. [DOI] [PubMed] [Google Scholar]
- 28.Bodhini D., Radha V., Dhar M., Narayani N., Mohan V. The rs12255372(G/T) and rs7903146(C/T) polymorphisms of the TCF7L2 gene are associated with type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:1174–1178. doi: 10.1016/j.metabol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Chandak G.R., Janipalli C.S., Bhaskar S., Kulkarni S.R., Mohankrishna P., Hattersley A.T., Frayling T.M., Yajnik C.S. Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia. 2007;50:63–67. doi: 10.1007/s00125-006-0502-2. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 31.Florez J.C. The new type 2 diabetes gene TCF7L2. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:391–396. doi: 10.1097/MCO.0b013e3281e2c9be. [DOI] [PubMed] [Google Scholar]
- 32.Grant S.F., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J., Helgason A., Stefansson H., Emilsson V., Helgadottir A., et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 33.Kalantari S., Sharafshah A., Keshavarz P., Davoudi A., Habibipour R. Single and multi-locus association study of TCF7L2 gene variants with susceptibility to type 2 diabetes mellitus in an Iranian population. Gene. 2019;696:88–94. doi: 10.1016/j.gene.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Loos R.J., Lindgren C.M., Li S., Wheeler E., Zhao J.H., Prokopenko I., Inouye M., Freathy R.M., Attwood A.P., Beckmann J.S., et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake K., Horikawa Y., Hara K., Yasuda K., Osawa H., Furuta H., Hirota Y., Yamagata K., Hinokio Y., Oka Y., et al. Association of TCF7L2 polymorphisms with susceptibility to type 2 diabetes in 4,087 Japanese subjects. J. Hum. Genet. 2008;53:174–180. doi: 10.1007/s10038-007-0231-5. [DOI] [PubMed] [Google Scholar]
- 36.Ngwa E.N., Sobngwi E., Atogho-Tiedeu B., Noubiap J.J., Donfack O.S., Guewo-Fokeng M., Mofo E.P., Fosso P.P., Djahmeni E., Djokam-Dadjeu R., et al. Association between the rs12255372 variant of the TCF7L2 gene and obesity in a Cameroonian population. BMC Res. Notes. 2015;8:717. doi: 10.1186/s13104-015-1661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana S., Sultana A., Bhatti A.A. Association of BDNF rs6265 and MC4R rs17782313 with metabolic syndrome in Pakistanis. J. Biosci. 2019;44:95. doi: 10.1007/s12038-019-9915-1. [DOI] [PubMed] [Google Scholar]
- 38.Willer C.J., Speliotes E.K., Loos R.J., Li S., Lindgren C.M., Heid I.M., Berndt S.I., Elliott A.L., Jackson A.U., Lamina C., et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deepa M., Pradeepa R., Rema M., Mohan A., Deepa R., Shanthirani S., Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)--study design and methodology (urban component) (CURES-I) J. Assoc. Physicians India. 2003;51:863–870. [PubMed] [Google Scholar]
- 40.Anjana R.M., Shanthi Rani C.S., Deepa M., Pradeepa R., Sudha V., Divya Nair H., Lakshmipriya N., Subhashini S., Binu V.S., Unnikrishnan R., et al. Incidence of Diabetes and Prediabetes and Predictors of Progression Among Asian Indians: 10-Year Follow-up of the Chennai Urban Rural Epidemiology Study (CURES) Diabetes Care. 2015;38:1441–1448. doi: 10.2337/dc14-2814. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization . The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Health Communications Australia; Sydney, Australia: 2000. [Google Scholar]
- 42.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 43.Sudha V., Radhika G., Sathya R.M., Ganesan A., Mohan V. Reproducibility and validity of an interviewer-administered semi-quantitative food frequency questionnaire to assess dietary intake of urban adults in southern India. Int. J. Food Sci. Nutr. 2006;57:481–493. doi: 10.1080/09637480600969220. [DOI] [PubMed] [Google Scholar]
- 44.Mohan V., Sandeep S., Deepa M., Gokulakrishnan K., Datta M., Deepa R. A diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians—The Chennai Urban Rural Epidemiology Study (CURES-38) Diabetes Obes. Metab. 2007;9:337–343. doi: 10.1111/j.1463-1326.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang Y.C., Liu P.H., Lee W.J., Chang T.J., Jiang Y.D., Li H.Y., Kuo S.S., Lee K.C., Chuang L.M. Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes. 2008;57:2245–2252. doi: 10.2337/db08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersen E., Skorpen F., Kvaloy K., Midthjell K., Grill V. Genetic heterogeneity in latent autoimmune diabetes is linked to various degrees of autoimmune activity: Results from the Nord-Trondelag Health Study. Diabetes. 2010;59:302–310. doi: 10.2337/db09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T., Huang Y., Xiao X.H., Wang D.M., Diao C.M., Zhang F., Xu L.L., Zhang Y.B., Li W.H., Zhang L.L., et al. The association between common genetic variation in the FTO gene and metabolic syndrome in Han Chinese. Chin. Med. J. (Engl.) 2010;123:1852–1858. [PubMed] [Google Scholar]
- 48.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Allen H.L., Lindgren C.M., Luan J.A., Mägi R., et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramya K., Radha V., Ghosh S., Majumder P.P., Mohan V. Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79) Diabetes Technol. Ther. 2011;13:33–42. doi: 10.1089/dia.2010.0071. [DOI] [PubMed] [Google Scholar]
- 50.Rees S.D., Islam M., Hydrie M.Z., Chaudhary B., Bellary S., Hashmi S., O’Hare J.P., Kumar S., Sanghera D.K., Chaturvedi N., et al. An FTO variant is associated with Type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet. Med. 2011;28:673–680. doi: 10.1111/j.1464-5491.2011.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isakova J., Talaibekova E., Vinnikov D., Saadanov I., Aldasheva N. ADIPOQ, KCNJ11 and TCF7L2 polymorphisms in type 2 diabetes in Kyrgyz population: A case-control study. J. Cell. Mol. Med. 2019;23:1628–1631. doi: 10.1111/jcmm.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou L., Wang J., Wang J. Genetic associations between Transcription Factor 7 Like 2 rs7903146 polymorphism and type 2 diabetes mellitus: A meta-analysis of 115,809 subjects. Diabetol. Metab. Syndr. 2019;11:56. doi: 10.1186/s13098-019-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohan V., Goldhaber-Fiebert J.D., Radha V., Gokulakrishnan K. Screening with OGTT alone or in combination with the Indian diabetes risk score or genotyping of TCF7L2 to detect undiagnosed type 2 diabetes in Asian Indians. Indian J. Med. Res. 2011;133:294–299. [PMC free article] [PubMed] [Google Scholar]
- 54.Ip W., Chiang Y.T., Jin T. The involvement of the wnt signaling pathway and TCF7L2 in diabetes mellitus: The current understanding, dispute, and perspective. Cell Biosci. 2012;2:28. doi: 10.1186/2045-3701-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong Y., Lin Y., Zhang Y., Yang J., Zhang Y., Liu H., Zhang B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: A large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med. Genet. 2009;10:15. doi: 10.1186/1471-2350-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loos R.J., Yeo G.S. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vimaleswaran K.S., Angquist L., Hansen R.D., van der A.D., Bouatia-Naji N., Holst C., Tjonneland A., Overvad K., Jakobsen M.U., Boeing H., et al. Association between FTO variant and change in body weight and its interaction with dietary factors: The DiOGenes study. Obes. (Silver Spring) 2012;20:1669–1674. doi: 10.1038/oby.2012.49. [DOI] [PubMed] [Google Scholar]
- 58.Zhao N.N., Dong G.P., Wu W., Wang J.L., Ullah R., Fu J.F. FTO gene polymorphisms and obesity risk in Chinese population: A meta-analysis. World J. Pediatr. 2019;15:382–389. doi: 10.1007/s12519-019-00254-2. [DOI] [PubMed] [Google Scholar]
- 59.Cooper D.N. Functional intronic polymorphisms: Buried treasure awaiting discovery within our genes. Hum. Genom. 2010;4:284–288. doi: 10.1186/1479-7364-4-5-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jowett J.B., Curran J.E., Johnson M.P., Carless M.A., Goring H.H., Dyer T.D., Cole S.A., Comuzzie A.G., MacCluer J.W., Moses E.K., et al. Genetic variation at the FTO locus influences RBL2 gene expression. Diabetes. 2010;59:726–732. doi: 10.2337/db09-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velayutham K., Ramanathan B., Murugan J., Murugan A., Thavamani V., Gomathinayagam R. Carriers of the TCF7L2 rs7903146, rs12255372 Risk Alleles in the South Tamil Nadu T2DM Patients Present with Early Incidence and Insulin Dependence. Indian J. Endocrinol. Metab. 2019;23:563–569. doi: 10.4103/ijem.IJEM_540_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattei J., Qi Q., Hu F.B., Sacks F.M., Qi L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am. J. Clin. Nutr. 2012;96:1129–1136. doi: 10.3945/ajcn.112.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grau K., Cauchi S., Holst C., Astrup A., Martinez J.A., Saris W.H., Blaak E.E., Oppert J.M., Arner P., Rossner S., et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am. J. Clin. Nutr. 2010;91:472–479. doi: 10.3945/ajcn.2009.27947. [DOI] [PubMed] [Google Scholar]
- 64.Haupt A., Thamer C., Heni M., Ketterer C., Machann J., Schick F., Machicao F., Stefan N., Claussen C.D., Haring H.U., et al. Gene variants of TCF7L2 influence weight loss and body composition during lifestyle intervention in a population at risk for type 2 diabetes. Diabetes. 2010;59:747–750. doi: 10.2337/db09-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marwaha R.K., Yenamandra V.K., Sreenivas V., Sahay R., Baruah M.P., Desai A., Kurvilla S., Joseph S., Unnikrishnan A.G., Lakshmy R., et al. Regional and seasonal variations in ultraviolet B irradiation and vitamin D synthesis in India. Osteoporos. Int. 2016;27:1611–1617. doi: 10.1007/s00198-015-3427-0. [DOI] [PubMed] [Google Scholar]
- 66.Basit S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013;70:161–172. doi: 10.1080/09674845.2013.11669951. [DOI] [PubMed] [Google Scholar]
- 67.Ashinne B., Rajalakshmi R., Anjana R.M., Narayan K.M.V., Jayashri R., Mohan V., Hendrick A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pract. 2018;139:308–313. doi: 10.1016/j.diabres.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 68.Mitri J., Muraru M.D., Pittas A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griz L.H., Bandeira F., Gabbay M.A., Dib S.A., Carvalho E.F. Vitamin D and diabetes mellitus: An update 2013. Arq. Bras. Endocrinol. Metabol. 2014;58:1–8. doi: 10.1590/0004-2730000002535. [DOI] [PubMed] [Google Scholar]
- 70.Pereira-Santos M., Costa P.R., Assis A.M., Santos C.A., Santos D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015;16:341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 71.Saneei P., Salehi-Abargouei A., Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis: 25(OH) vitamin D and BMI. Obes. Rev. 2013;14:393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 72.Angellotti E., D’Alessio D., Dawson-Hughes B., Nelson J., Cohen R.M., Gastaldelli A., Pittas A.G. Vitamin D Supplementation in Patients with Type 2 Diabetes: The Vitamin D for Established Type 2 Diabetes (DDM2) Study. J. Endocr. Soc. 2018;2:310–321. doi: 10.1210/js.2018-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R., Brodsky I., Ceglia L., Chadha C., Chatterjee R., et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vranić L., Mikolašević I., Milić S. Vitamin D Deficiency: Consequence or Cause of Obesity? Med. (Kaunas Lith.) 2019;55:541. doi: 10.3390/medicina55090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berry D.J., Vimaleswaran K.S., Whittaker J.C., Hingorani A.D., Hypponen E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS ONE. 2012;7:e37465. doi: 10.1371/journal.pone.0037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Hazmi A.S., Al-Mehmadi M.M., Al-Bogami S.M., Shami A.A., Al-Askary A.A., Alomery A.M., Al-Shehri S.S., Dahlawi H., Abdulrazag K., Ali T., et al. Vitamin D receptor gene polymorphisms as a risk factor for obesity in Saudi men. Electron. Physician. 2017;9:5427–5433. doi: 10.19082/5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Correa-Rodriguez M., Carrillo-Avila J.A., Schmidt-RioValle J., Gonzalez-Jimenez E., Vargas S., Martin J., Rueda-Medina B. Genetic association analysis of vitamin D receptor gene polymorphisms and obesity-related phenotypes. Gene. 2018;640:51–56. doi: 10.1016/j.gene.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 78.Malik R., Farooq R., Mehta P., Ishaq S., Din I., Shah P., Majid S. Association of Vitamin D Receptor Gene Polymorphism in Adults with Type 2 Diabetes in the Kashmir Valley. Can. J. Diabetes. 2018;42:251–256. doi: 10.1016/j.jcjd.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Rivera-Leon E.A., Palmeros-Sanchez B., Llamas-Covarrubias I.M., Fernandez S., Armendariz-Borunda J., Gonzalez-Hita M., Bastidas-Ramirez B.E., Zepeda-Moreno A., Sanchez-Enriquez S. Vitamin-D receptor gene polymorphisms (TaqI and ApaI) and circulating osteocalcin in type 2 diabetic patients and healthy subjects. Endokrynol. Pol. 2015;66:329–333. doi: 10.5603/EP.2015.0042. [DOI] [PubMed] [Google Scholar]
- 80.Vimaleswaran K.S., Power C., Hypponen E. Interaction between vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D concentrations on metabolic and cardiovascular disease outcomes. Diabetes Metab. 2014;40:386–389. doi: 10.1016/j.diabet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Sowmya N., Lakshmipriya N., Arumugam K., Venkatachalam S., Vijayalakshmi P., Ruchi V., Geetha G., Anjana R.M., Mohan V., Krishnaswamy K., et al. Comparison of dietary profile of a rural south Indian population with the current dietary recommendations for prevention of non-communicable diseases (CURES 147) Indian J. Med. Res. 2016;144:112–119. doi: 10.4103/0971-5916.193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Misra A., Sharma R., Gulati S., Joshi S.R., Sharma V., Ghafoorunissa, Ibrahim A., Joshi S., Laxmaiah A., Kurpad A., et al. Consensus dietary guidelines for healthy living and prevention of obesity, the metabolic syndrome, diabetes, and related disorders in Asian Indians. Diabetes Technol. Ther. 2011;13:683–694. doi: 10.1089/dia.2010.0198. [DOI] [PubMed] [Google Scholar]
- 83.Mann J., Cummings J.H., Englyst H.N., Key T., Liu S., Riccardi G., Summerbell C., Uauy R., van Dam R.M., Venn B., et al. FAO/WHO Scientific Update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007;61:S132–S137. doi: 10.1038/sj.ejcn.1602943. [DOI] [PubMed] [Google Scholar]
- 84.Newton A.L., Hanks L.J., Ashraf A.P., Williams E., Davis M., Casazza K. Macronutrient intake influences the effect of 25-hydroxy-vitamin d status on metabolic syndrome outcomes in african american girls. Cholesterol. 2012;2012:581432. doi: 10.1155/2012/581432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudkowska I., Pérusse L., Bellis C., Blangero J., Després J.-P., Bouchard C., Vohl M.-C. Interaction between Common Genetic Variants and Total Fat Intake on Low-Density Lipoprotein Peak Particle Diameter: A Genome-Wide Association Study. J. Nutrigenet. Nutrigenom. 2015;8:44–53. doi: 10.1159/000431151. [DOI] [PubMed] [Google Scholar]
- 86.Hüls A., Krämer U., Carlsten C., Schikowski T., Ickstadt K., Schwender H. Comparison of weighting approaches for genetic risk scores in gene-environment interaction studies. BMC Genet. 2017;18:115. doi: 10.1186/s12863-017-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belsky D.W., Moffitt T.E., Sugden K., Williams B., Houts R., McCarthy J., Caspi A. Development and evaluation of a genetic risk score for obesity. Biodemogr. Soc. Biol. 2013;59:85–100. doi: 10.1080/19485565.2013.774628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.