Abstract
Vitamin D (VD) levels have been gaining growing attention in Oral Health. During growth and adulthood, VD deficiency (VDD) is associated with a wide variety of oral health disorders, and impaired VD synthesis may expedite some of these conditions. In children, severe VDD can induce defective tooth mineralization, resulting in dentin and enamel defects. As a consequence, these defects may increase the risk of the onset and progression of dental caries. Further, VDD has been associated with higher prevalence of periodontitis and gingival inflammation, and several recent preclinical and clinical studies have unveiled potential pathways through which Vitamin D may interact with the periodontium. VDD correction through supplementation may contribute to a successful treatment of periodontitis; however, alveolar bone regeneration procedures performed in baseline VDD patients seem more prone to failure. Vitamin D may also be linked with some oral pathology entities such as certain oral cancers and events of osteonecrosis of the jaw. This review aims to provide comprehensive evidence of how VD levels should be considered to promote good oral health, and to summarize how VDD may hamper oral development and its role in certain oral conditions.
Keywords: vitamins, vitamin D deficiency, oral health, periodontal health, periodontal disease, periodontitis, caries, orthodontics, oral cancer
1. Introduction
Vitamin D is a steroid hormone obtained mainly from exposure to sunlight, but also from diet and dietary supplements [1,2,3,4,5]. Foods naturally containing vitamin D are rare, and it can be found in oily fish (such as salmon, mackerel, and herring) and oils from fish (e.g., cod liver oil) [2]. Vitamin D is a generic name comprising Vitamin D2 and D3. While Vitamin D2 is manufactured through ultraviolet irradiation of ergosterol from yeast, Vitamin D3 results from ultraviolet irradiation of 7-dehydrocholesterol from lanolin [4,6,7] (Figure 1) exhibiting the biological activity of cholecalciferol (vitamin D3), and it is synthesized in the human skin. Measurement of serum 25-hydroxyvitamin D (25[OH]D) is a widely accepted biomarker analysis for vitamin D status [3].
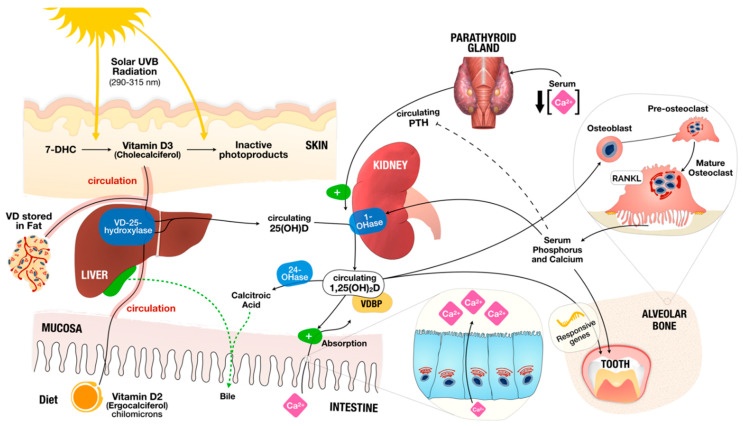
Figure 1.
Vitamin D and Calcium, Phosphorus and Bone Metabolism. Vitamin D is obtained mostly from sunlight exposure (Vitamin D3) and from diet (Vitamin D2). In contact to solar ultraviolet B (UVB) radiation, 7-dehydrocholesterol (7-DHC) present in the skin is immediately converted to vitamin D3 in a heat-dependent process. Excessive sunlight exposure can destroy Vitamin D3 and convert it into inactive photoproducts. Vitamin D2 from diet is absorbed in the form of chylomicrons when they spread into the bloodstream as Vitamin D (encompassing Vitamin D2 or D3). Vitamin D in the circulation is bound to the vitamin D–binding protein (VDBP), which transports it to the liver. There, Vitamin D is converted by vitamin D-25-hydroxylase (VD-25-hydroxylase) to 25-hydroxyvitamin D (25(OH)D) (used as standard surrogate to determine vitamin D status). Then, 25(OH)D circulates, reaching the kidneys where it is activated by 25-hydroxyvitamin D-1αhydroxylase (1-OHase) to 1,25-dihydroxyvitamin D (1,25(OH)2D). Serum phosphorus or calcium can impact renal production of 1,25(OH)2D. 1,25(OH)2D promotes negative feedback of parathyroid hormone (PTH) by the parathyroid glands. 1,25(OH)2D increases the expression of 25-hydroxyvitamin D-24-hydroxylase (24-OHase), upholding its excretion in the bile. 1,25(OH)2D is recognized in the osteoblasts, causing the induction of mature osteoclast through expression of the receptor activator of nuclear factor-κB ligand (RANKL). Mature osteoclasts remove calcium and phosphorus from the bone, maintaining the serum levels of calcium and phosphorus.
Vitamin D acts primordially as a hormone, and its endocrine activity promotes serum calcium and phosphate homeostasis through intestinal absorption regulation [2,6,8] (Figure 1). Vitamin D also acts like an autocrine and paracrine agent, regulating cell differentiation, cell maturation and innate immune system [9,10,11]. In detail, the cellular actions of Vitamin D are mediated through the Vitamin D receptor (VDR), which is a receptor molecule that binds to the active form of Vitamin D (Figure 2) [9,11,12,13]. As such, Vitamin D actions depend on the regulation of VDR for its genomic effects and on a membrane associated proteins to nongenomic effects (such as signaling pathways) [14] (Figure 2). This vast action is due to the fact that this vitamin modulates the expression of a considerable number of genes, and it is estimated to do so for 5–10% of the entire genome [9].
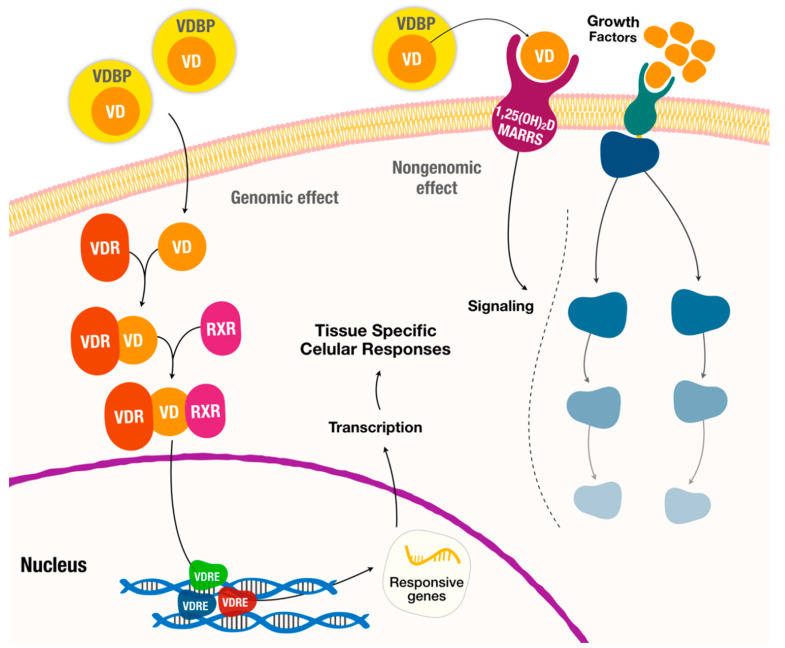
Figure 2.
Hormonal actions of Vitamin D with genomic and nongenomic effects. MARRS-membrane-associated, rapid response steroid-binding protein; RDR—retinoid-X receptor; VD—Vitamin D; VDR—Vitamin D Receptor; VDRE—Vitamin D response elements; VDBP–Vitamin D-binding protein.
Public awareness of vitamin D has increased exponentially due to a worldwide prevalence of vitamin D deficiency (VDD) [2,3,7,15,16,17,18]. This prevalence is worrisome and is of the utmost importance for general health, with special focus on children, pregnancy, some forms of cancer and infection prevention [19,20,21,22].
In general, the major cause of VDD is the lack of exposure to sunlight with adequate ultraviolet B rays (exogenous factor) [6,17]. VDD can also arise from a nutritional deficit due to inadequate intakes of vitamin D, or hereditary disorders from absorption and metabolic conversion [6]. Additionally, drug related VDD is also possible due to iatrogenic increased clearance (for instance, with phenytoin, carbamazepine and oxcarbazepine regimens) [23].
The role of nutrition in oral diseases has gained popularity and recent investigations have uncovered increasingly relevant relationships between nutritional deficits and oral pathologies [24,25]. Concerning oral diseases, caries and periodontal diseases are complex multifactorial diseases and remain the two most prevalent diseases worldwide [26,27]. Both caries and periodontal disease are associated with VDD and its pathophysiologic processes [28,29,30,31]. The mechanisms by which vitamin D impacts oral health are not just based on bone metabolism. Nowadays, research has unveiled that VDD compromises odontogenesis, resulting in a hypomineralized dentition susceptible to fracture and caries lesions [29]. VDD was also linked to worse periodontal health and might be involved in the immune mechanism towards periodontal infection [32]. Furthermore, VDD in association with periodontitis has been recently linked to potential systemic repercussions during pregnancy, throughout orthodontic treatment, in post-menopausal women or in some oral pathologies [22,33,34].
The latest breakthroughs led us to elaborate a review aiming to comprehensively summarize the available evidence of the effect of vitamin D on oral health and its major complications. Furthermore, we discuss the impact of recent studies where VDD correction was implemented through supplementation which might underpin future clinical guidelines.
2. Vitamin D Deficiency Impact on Oral Health
2.1. Vitamin D Deficiency in Tooth Mineralization and Caries
Teeth are mineralized organs, surrounded by alveolar bone, and formed by three distinctive hard tissues: enamel, dentin, and cementum. The tooth mineralization process occurs parallel to skeletal mineralization, yet if mineral metabolism is disturbed then failures will occur similarly to those that occur in bone tissue. Vitamin D plays a key role in bone and tooth mineralization, and when levels are unregulated it can lead to the “rachitic tooth”, which is a defective and hypomineralized organ highly susceptible to fracture and decay [35,36].
The mechanisms by which VDD affects tooth mineralization are well debated elsewhere [35,36]. The main biological basis relies on the fact that severe VDD (<10 ng/mL) causes hypocalcemia and hypophosphatemia with secondary hyperparathyroidism (driven by hypocalcemia) [37,38]. This hyperparathyroidism promotes intestinal absorption of calcium (Ca2+), and renal production of 1 α,25-dihydroxyvitamin D (1,25[OH]2D), increasing bone turnover leading to elevated serum levels of Ca2+ and low serum levels of inorganic phosphate (Pi) [37,38]. The initial hypophosphatemia is then severely worsened. Ultimately, the loss of vitamin D signaling pathways in tooth cells with low concentrations of Ca2+ and phosphate ions inhibit proper mineralization of teeth and mineralization defects occur [35].
Apart from its mineralization homeostasis role, circulating vitamin D can initiate a signaling pathway through vitamin D receptors (VDR). VDR is a ligand-activated transcription factor that controls gene expression through vitamin D elements (VDRE) [39]. For instance, some of these responsive genes affect bone, mineral metabolism, immune response, cell life cycle and migration, skeletal muscle, detoxification, and energy metabolism [8,39,40,41,42,43]. Vitamin D upregulates VDR which, in turn, can induce structural gene products, including calcium-binding proteins and various extracellular matrix proteins (e.g., enamels, amelogenins, dentin sialoglycoproteins, and dentin phosphoproteins), resulting in the formation of dentin and enamel [35,44].
Beyond the typical VDD causes, nutritional deficiency or reduction of sunlight exposure, there are genetic deficiencies originating from mutations encoding elements of the vitamin D metabolic machinery. The main causes of VDD, second to genetic mutations, are abnormal enzyme secretion (i.e., vitamin D-dependent rickets type 1, VDDR-I) and anomalous VDR function or signaling (vitamin D-dependent rickets type 2, VDDR-IIa; hereditary defects in the vitamin D receptor-effector system, HDVDR) [35]. These genetic conditions cause defective mineralized tissues, despite normal vitamin D consumption or sunlight exposure and, ultimately, will increase the risk of mineralized tooth tissue hypoplasia (i.e., amelogenesis imperfecta, dentinogenesis imperfecta, enamel hypoplasia) or higher risk of caries [35].
Remarkably, deciduous dentition can be influenced by maternal 25(OH)D levels, despite the influence of inherited defects of the fetus [45,46]. Fetal serum-circulating levels of vitamin D follow the maternal concentration and can be used as a standard surrogate marker to the fetus [47]. Therefore, if maternal 25(OH)D levels turn unbalanced, this may have direct repercussions on the baby’s health [48] and, in particular, on tooth development [45,46,49,50,51]. The pattern of mineralization defect depends on the specific week of gestation when maternal VDD occurred. For example, approximately at the 13th week from conception, the human primary maxillary central incisor begins its calcification, and if there is a VDD status, there could be a hypoplasia/mineralization defect on the incisal third of the crown. Nowadays, it is known that maternal VDD at 12–16, 20–32 and 36–40 weeks results in defects at the incisal third, middle third and cervical third, respectively [51]. In a randomized clinical trial (RCT), vitamin D supplementation during pregnancy revealed that pregnant women with < 15 ng/mL of vitamin D had a 14% higher risk of deciduous dentition [51]. In contrast, high-dose vitamin D supplementation during pregnancy was associated with an approximately 50% reduced odds of enamel defects [49,52]. In another RCT, high-dose Vitamin D supplementation during pregnancy was linked to 50% lower risk of enamel defects in the newborn, underlying once more the likely preventive role of Vitamin D for enamel defects [49].
Furthermore, untreated caries in deciduous and permanent teeth were the most prevalent condition, affecting 9% and 35% of global population, respectively [53]. Moreover, according to WHO, caries is the fourth-most expensive chronic disease to treat [54]. This infectious disease has a complex and multifactorial etiology. Environmental factors, such as cariogenic diet with a high carbohydrate content, cariogenic bacteria, and poor oral hygiene were the most widely studied risk factors [55,56,57]. Nevertheless, when exposed to the same environmental risk factors, some patients are more susceptible or resistant to caries than others, so that environmental factors alone are insufficient to explain the prevalence and incidence of caries [58,59,60]. Currently the evidence highlights the association of low levels of vitamin D and the high prevalence of caries in both children and adults, although the mechanism remains unclear [61,62,63,64,65,66,67,68].
Additionally, vitamin D exerts several roles in the control of the human immune system, and an optimal vitamin D concentration (≥75 nmol/L) is associated with lower odds for dental caries in children [29,69,70]. However, the studies’ results are contradictory [45,62,71,72]. A recent systematic review of controlled clinical trials, with data from 2827 children, investigated the impact of vitamin D supplementation on dental caries prevention [28,73]. The results of this study show that vitamin D supplementation reduced the risk of caries in about 47%, but with low certainty [28]. Another research supports that caries-free children were twice as likely to have optimal vitamin D concentrations (≥75 nmol/L) and those with severe early childhood caries were at nearly three times the odds of having deficient levels (<35 nmol/L) [29]. On the one hand, it is important to clarify that serum vitamin D does not change the major structure of teeth since this structure remains constant until some extrinsic factor causes its wear. Notwithstanding, apparently vitamin D prevents caries lesions through immune regulation, promoting microbial eradication with peptide activity as discussed above.
The roles of both UVB and antimicrobial peptides (AMPs) in cariogenic bacteria reduction have been studied [74,75,76,77,78,79,80,81]. The mechanism through which UVB reduces the risk of dental caries is likely to be through the production of vitamin D and followed by the induction of AMPs, which have antimicrobial properties [74,76,77,78,79,80,81]. AMPs are host defense peptides, mostly cationic and amphiphilic molecules, that are essential elements of the innate immunity against several bacteria, fungi and viruses [75,78]. Investigations seem to point to a combination of AMPs rather than a specific role of a single AMP [79], and they have been proposed as potential application for the prevention and treatment of dental caries [80,81]. Remarkably, Streptococcus mutans, a primary etiological agent of dental caries, may resist host salivary AMPs explaining its virulence in dental caries pathophysiology [74,77,80].
Hence, we can conclude that vitamin D control levels prior to conception may be important to reduce the risk of enamel defects in deciduous teeth and should be controlled throughout pregnancy and after delivery.
2.2. Vitamin D Deficiency and Periodontitis
Periodontitis is a complex polymicrobial disease induced by plaque and with persistent chronic inflammation [82]. Periodontitis is one of the two most prevalent diseases worldwide and its severe stage is the sixth most prevalent, with strong socioeconomic and systemic repercussions [26,27,83,84,85]. It has great impact on quality of life and is recovered after periodontal therapy [86,87]. The systemic link between periodontitis and other diseases and conditions has escalated, such as diabetes [88], ischemic stroke [89], cardiovascular disease (CVD) [90], rheumatoid arthritis [91], inflammatory bowel disease [92], stress [93], solid-organ transplanted individuals [94], or preterm birth [95]. Furthermore, the impact of nutrition on periodontal health, and in particular VDD, has been intensively investigated [96,97,98,99,100] and a recent European consensus stated that an inadequate vitamin D status impacts periodontal health and oral functions [24].
Several cross-sectional studies have compared the levels of Vitamin D between individuals with periodontitis and without periodontitis; however, the results remain diverse. While most reports show that periodontitis was associated with lower levels of Vitamin D compared to non-periodontitis [101,102,103,104,105], another has reported no differences [106]. Further, vitamin D concentrations were associated with higher periodontal destruction, severe periodontitis stages and higher tooth loss [30,107,108,109]. In otherwise healthy patients (CVD and diabetes mellitus), lower levels of Vitamin D were also associated when periodontitis was diagnosed [102,104].
Data from the NHANES III study, performed in the USA, showed that individuals with the highest levels of vitamin D experienced 20% less bleeding on probing than those with the lowest levels [30]. Other investigations also demonstrated that lower levels of gingival inflammation are associated with people without periodontitis [101,104].
Comprehensively, the inflammatory and immune actions against periodontal pathogens are triggered by the host immune system. As previously mentioned, salivary low levels of vitamin D were associated with higher levels of inflammation biomarkers in periodontitis patients when compared to periodontally healthy patients (namely IL-35, IL-17A and transforming growth factor), supporting the presence of an inflammatory microenvironment [106]. Remarkably, vitamin D supplementation was linked to a decrease of salivary cytokines before nonsurgical periodontal treatment [110]. In addition, a cross-sectional study showed through gingival samples that periodontitis patients exhibited lower levels of VDR and fewer fibroblast cells with higher inflammatory cell infiltration compared with healthy periodontal individuals [111].
Although not fully understood, Vitamin D has apparent fine-tuning, anti-inflammatory and mineralization effects on the periodontium according to the latest in vitro evidence. A study showed that vitamin D may decrease the number of live porphyromonas gingivalis through active autophagy [112] and might alleviate the inflammatory burden of periodontitis in rodent models: decreasing inflammatory levels (RANKL, TNF-α, IL-1, MMP-9) [113,114,115,116]; inhibiting IL-6 overexpression [117]; and suppressing alveolar damage via inhibition of bone loss, apparently through systemic T-helper cells [118]. In cultured human periodontal cells, Vitamin D induced a comparable mineralization effect to vitamin C [119].
Thus both preclinical and clinical studies support the idea that vitamin D, through its metabolic pathway, might be involved in the pathogenesis of periodontitis, by impacting tooth mineral density and being reversely correlated with disease severity of periodontitis [25,97,120] (Figure 3).
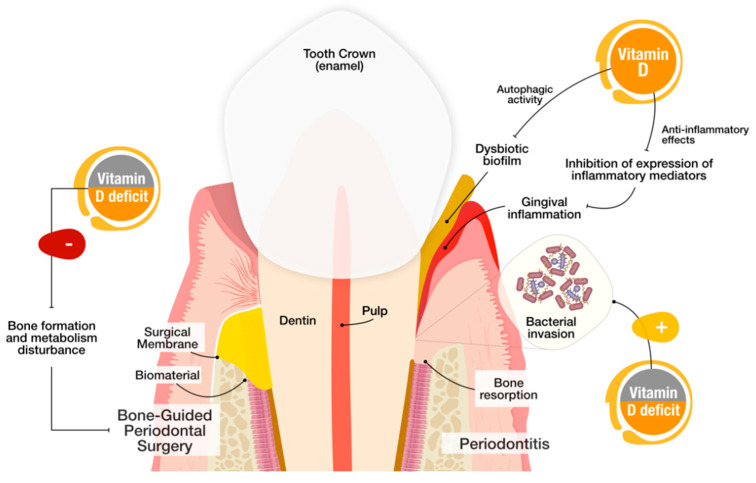
Figure 3.
Suggested mechanisms of Vitamin D and Vitamin D deficiency (VDD) impact on periodontitis and periodontal regenerative surgery. (Left) Baseline VDD patients who undergo periodontal surgeries may be more prone to treatment failure due to the disturbance of bone formation and metabolism. (Right) In periodontitis, Vitamin D may promote P. gingivalis autophagy and anti-inflammatory effects via inhibition of expression of inflammatory mediators, decreasing gingival inflammation.
From a genetic perspective, the role of VDR variants in periodontitis has been the subject of great consideration. In two recent evidence-based studies, a number of VDR polymorphisms were correlated with higher risk of developing periodontitis [121,122]. Notwithstanding, the VDR variants’ impact on periodontitis still remains to be consolidated since it depends on the number of studies, and is expected to increase considerably in the future.
On the other hand, the influence of vitamin D supplementation was studied in both nonsurgical and surgical periodontal treatments. Vitamin D and calcium supplementation showed moderate positive effects on periodontal health after nonsurgical periodontal treatment [98,110,111,123,124]. Further, baseline VDD negatively influenced periodontal surgery outcomes, even when supplementation was used to compensate for low levels [125] (Figure 3). Despite that these studies show vitamin D as a hypothetical hallmark for the success of patients’ treatment, more studies are warranted to infer scientific fallouts and permit definite conclusions.
In addition, a number of reports have explored the association between periodontitis and maternal VDD and found that pregnant women with moderate to severe periodontitis were linked to lower serum levels of vitamin D, compared to women with periodontal health [99,126,127,128,129]. Importantly, non-surgical periodontal treatment during pregnancy was proved to be successful in reducing adverse pregnancy outcomes; however, concomitant vitamin D supplementation showed mild clinical improvements in birthweight [128,130,131,132,133]. As such, future studies should assess this relationship in greater detail considering the amount of evidence that Vitamin D impacts periodontal health and maternal health.
2.3. Vitamin D Deficiency and Orthodontics
With the increase in importance of aesthetic requirements, facial micro- and macro-aesthetics and smile have become a priority for adolescents and adults [134,135]. Consequently, orthodontic treatments have become increasingly prevalent.
Tooth movement relies on the application of predetermined forces that cause mechanical stimuli with two simultaneous processes: (1) bone resorption on the pressure site, through osteoclastic activity; and (2) bone formation on the tension site, by osteoblastic action [136,137,138]. These two processes in combination with mechanical, chemical or electrical stimuli, might result in faster teeth movement [139,140,141,142]. Regarding the chemical factors, vitamin D might play a key role in tooth movement during orthodontic treatment and has shown promising results [33]. Albeit animal observational in nature, there is an increasing body of evidence showing that local application of vitamin D results in a faster tooth movement [136,143,144,145]. Notwithstanding, VDD in animal models causes a slower rate of tooth movement, and consequent treatment delay or complications [136,143,144]. In this sense, future studies in humans are needed to determine if VDD has a clinically significant effect on tooth movement and if vitamin D supplementation during orthodontic treatment, for instance in hypovitaminosis cases, improves the coupling of formation and resorption in alveolar bone remodeling during orthodontic tooth movement.
2.4. Vitamin D Deficiency and Oral Pathology
Vitamin D might play an important function in the onset and progression of certain oral cancers, though there is still much to research. Remarkably, VDD is more common in patients with oral neoplastic lesions [22]. In a case-control study, VDD was associated with increased risk of squamous cell carcinoma of the esophagus, oral, and pharyngeal cancers, which were more prevalent in heavy smokers and severe alcoholism [146]. Another study has revealed that VDR expression was increased in premalignant lesions and oral cancer, and vitamin D supplementation significantly diminished therapy-related toxicities in late-stage oral cancers, with less morbidity and better quality of life [120]. Therefore, future studies should make efforts to clarify how VDD relates to oral cancer development and its therapeutic efficacy towards anticancer toxicity medications.
The role of VDD in osteonecrosis of the jaw (ONJ) is an increasingly discussed topic; however, Vitamin D status in ONJ individuals remains unestablished [147,148,149]. ONJ is characterized by a progressive death of the exposed jawbone in a patient chronically exposed to anti-absorption or anti-angiogenic medications, and no history of mandibular radiotherapy or metastatic disease of the jaw (Patel et al., 2018). Due to its role in bone mineralization, several studies have investigated the levels of Vitamin D in ONJ cases, mainly derived from osteoporosis and cancer patients [148,149,150,151,152,153,154]. The most recent evidence suggests that VDD is not a risk factor for the onset of ONJ events [148,154], while other studies underline a potential role [149,153]. Nonetheless, future studies should focus on the influence of baseline VDD and of Vitamin D supplementation towards ONJ, as well as the establishment of 25(OH)D level standards for patients at high risk of ONJ events.
3. Conclusions
VDD is highly implicated with oral diseases and has been linked with a higher risk of tooth defects, caries, periodontitis and oral treatments failure. The maintenance of appropriate 25(OH)D levels has shown to be associated with better oral development and health throughout life, though the impact of VDD correction through supplementation demands more evidence to allow definitive conclusions and potential clinical guidelines. Nevertheless, 25(OH)D levels should be considered to ensure a balanced oral health, and these levels need to be verified before the treatment of any oral conditions to warrant fruitful treatment outcomes.
Author Contributions
Conceptualization, Resources, Data Curation, J.B. and V.M.; Writing—original draft preparation, writing—review and editing, J.B., V.M., L.P., A.S.D., J.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Borel P., Caillaud D., Cano N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015;55:1193–1205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F., Chen T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87:1080–1086. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 3.Turck D., Bresson J.L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A., et al. Update of the tolerable upper intake level for vitamin D for infants. EFSA J. 2018;16:1–118. doi: 10.2903/j.efsa.2018.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson L.R., Tripkovic L., Hart K.H., Lanham-New S.A. Vitamin D deficiency as a public health issue: Using Vitamin D2 or Vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017;76:1–8. doi: 10.1017/S0029665117000349. [DOI] [PubMed] [Google Scholar]
- 5.Jones G. The discovery and synthesis of the nutritional factor vitamin D. Int. J. Paleopathol. 2018;23:96–99. doi: 10.1016/j.ijpp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Lanham-New S.A., Wilson L.R. Vitamin D—Has the new dawn for dietary recommendations arrived? Nutr. Bull. 2016;41:2–5. doi: 10.1111/nbu.12185. [DOI] [PubMed] [Google Scholar]
- 8.Girgis C.M., Clifton-Bligh R.J., Hamrick M.W., Holick M.F., Gunton J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013;34:33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 9.Morris H.A., Anderson P.H. Autocrine and paracrine actions of vitamin d. Clin. Biochem. Rev. 2010;31:129–138. [PMC free article] [PubMed] [Google Scholar]
- 10.Bikle D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008;17:348–352. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]
- 11.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon S.M., Shin E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kongsbak M., Levring T.B., Geisler C., von Essen M.R. The vitamin D receptor and T cell function. Front. Immunol. 2013;4:1–10. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard C.L., Farach-Carson M.C., Rohe B., Nemere I., Meckling K.A. Involvement of 1,25D3-MARRS (membrane associated, rapid response steroid-binding), a novel vitamin D receptor, in growth inhibition of breast cancer cells. Exp. Cell Res. 2010;316:695–703. doi: 10.1016/j.yexcr.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 15.McKenna M.J., Murray B. Vitamin D Deficiency. Springer; New York, NY, USA: 2014. [Google Scholar]
- 16.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A., Pierroz D.D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 17.de Boer I.H. Chronic Kidney Disease, Dialysis, and Transplantation. Volume 357. Elsevier Saunders; Philadelphia, PA, USA: 2010. Vitamin D deficiency; pp. 115–127. [Google Scholar]
- 18.Mogire R.M., Mutua A., Kimita W., Kamau A., Bejon P., Pettifor J.M., Adeyemo A., Williams T.N., Atkinson S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health. 2020;8:e134–e142. doi: 10.1016/S2214-109X(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguiar M., Andronis L., Pallan M., Högler W., Frew E. The economic case for prevention of population vitamin D deficiency: A modelling study using data from England and Wales. Eur. J. Clin. Nutr. 2019;74:825–833. doi: 10.1038/s41430-019-0486-x. [DOI] [PubMed] [Google Scholar]
- 20.Alonso M.A., Mantecón L., Santos F. Vitamin D deficiency in children: A challenging diagnosis! Pediatr. Res. 2019;85:596–601. doi: 10.1038/s41390-019-0289-8. [DOI] [PubMed] [Google Scholar]
- 21.White J.H. Vitamin D and human health: More than just bone. Nat. Rev. Endocrinol. 2013;9:623. doi: 10.1038/nrendo.2013.75-c1. [DOI] [PubMed] [Google Scholar]
- 22.Fathi N., Ahmadian E., Shahi S., Roshangar L., Khan H., Kouhsoltani M., Maleki Dizaj S., Sharifi S. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed. Pharmacother. 2019;109:391–401. doi: 10.1016/j.biopha.2018.10.102. [DOI] [PubMed] [Google Scholar]
- 23.Gröber U., Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinology. 2012;4:158–166. doi: 10.4161/derm.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapple I.L.C., Bouchard P., Cagetti M.G., Campus G., Carra M.C., Cocco F., Nibali L., Hujoel P., Laine M.L., Lingstrom P., et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017;44:S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 25.Uwitonze A.M., Murererehe J., Ineza M.C., Harelimana E.I., Nsabimana U., Uwambaye P., Gatarayiha A., Haq A., Razzaque M.S. Effects of vitamin D status on oral health. J. Steroid Biochem. Mol. Biol. 2018;175:190–194. doi: 10.1016/j.jsbmb.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Peres M.A., Macpherson L.M.D., Weyant R.J., Daly B., Venturelli R., Mathur M.R., Listl S., Celeste R.K., Guarnizo-Herreño C.C., Kearns C., et al. Oral diseases: A global public health challenge. Lancet. 2019;394:249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 27.Watt R.G., Daly B., Allison P., Macpherson L.M.D., Venturelli R., Listl S., Weyant R.J., Mathur M.R., Guarnizo-Herreño C.C., Celeste R.K., et al. Ending the neglect of global oral health: Time for radical action. Lancet. 2019;394:261–272. doi: 10.1016/S0140-6736(19)31133-X. [DOI] [PubMed] [Google Scholar]
- 28.Hujoel P.P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr. Rev. 2013;71:88–97. doi: 10.1111/j.1753-4887.2012.00544.x. [DOI] [PubMed] [Google Scholar]
- 29.Schroth R.J., Levi J.A., Sellers E.A., Friel J., Kliewer E., Moffatt M.E.K. Vitamin D status of children with severe early childhood caries: A case-control study. BMC Pediatr. 2013;13:174. doi: 10.1186/1471-2431-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich T., Joshipura K.J., Dawson-hughes B., Bischoff-ferrari H.A. Association between serum concentrations of 25-hydroxyvitamin D 3 and periodontal disease in the US population 1–3. Am. J. Clin. Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 31.Scardina G.A., Messina P. Good oral health and diet. J. Biomed. Biotechnol. 2012;2012:1–8. doi: 10.1155/2012/720692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012;13:21–29. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 33.Ganesh M.L., Saravana Pandian K. Acceleration of tooth movement during orthodontic treatment-A frontier in orthodontics. J. Pharm. Sci. Res. 2017;9:741–744. [Google Scholar]
- 34.Martínez-Maestre M.A., González-Cejudo C., MacHuca G., Torrejón R., Castelo-Branco C. Periodontitis and osteoporosis: A systematic review. Climacteric. 2010;13:523–529. doi: 10.3109/13697137.2010.500749. [DOI] [PubMed] [Google Scholar]
- 35.Foster B.L., Nociti F.H., Somerman M.J. The rachitic tooth. Endocr. Rev. 2014;35:1–34. doi: 10.1210/er.2013-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Ortenzio L., Kahlon B., Peacock T., Salahuddin H., Brickley M. The rachitic tooth: Refining the use of interglobular dentine in diagnosing vitamin D deficiency. Int. J. Paleopathol. 2018;22:101–108. doi: 10.1016/j.ijpp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Allgrove J. Physiology of calcium, phosphate and magnesium. Endocr. Dev. 2009;16:8–31. doi: 10.1159/000223685. [DOI] [PubMed] [Google Scholar]
- 38.Bergwitz C., Jüppner H. Regulation of phosphate homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike J.W., Meyer M.B. The Vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-Dihydroxyvitamin D 3. Rheum. Dis. Clin. N. Am. 2012;38:13–27. doi: 10.1016/j.rdc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH) 2 vitamin D 3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Pike J.W., Meyer M.B., Bishop K.A. Regulation of target gene expression by the vitamin D receptor—An update on mechanisms. Rev. Endocr. Metab. Disord. 2012;13:45–55. doi: 10.1007/s11154-011-9198-9. [DOI] [PubMed] [Google Scholar]
- 42.Rosen C.J., Adams J.S., Bikle D.D., Black D.M., Demay M.B., Manson J.A.E., Murad M.H., Kovacs C.S. The nonskeletal effects of vitamin D: An endocrine society scientific statement. Endocr. Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haussler M.R., Haussler C.A., Whitfield G.K., Hsieh J.C., Thompson P.D., Barthel T.K., Bartik L., Egan J.B., Wu Y., Kubicek J.L., et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “ Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010;121:88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slavkin H.C., Hu C.C., Sakakura Y., Diekwisch T., Chai Y., Mayo M., Bringas P., Simmer J., Mak G., Sasano Y., et al. Gene expression, signal transduction and tissue-specific biomineralization during mammalian tooth development. Crit. Rev. Eukaryot. Gene Expr. 1992;2:315–329. [PubMed] [Google Scholar]
- 45.Schroth R.J., Lavelle C., Tate R., Bruce S., Billings R.J., Moffatt M.E.K. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:133. doi: 10.1542/peds.2013-2215. [DOI] [PubMed] [Google Scholar]
- 46.Singleton R., Day G., Thomas T., Schroth R., Klejka J., Lenaker D., Berner J. Association of maternal Vitamin D deficiency with early childhood caries. J. Dent. Res. 2019;98:549–555. doi: 10.1177/0022034519834518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollist B.W., Pittard W.B. Evaluation of the total fetomaternal vitamin d relationships at term: Evidence for racial differences. J. Clin. Endocrinol. Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 48.Karras S.N., Fakhoury H., Muscogiuri G., Grant W.B., van den Ouweland J.M., Colao A.M., Kotsa K. Maternal vitamin D levels during pregnancy and neonatal health: Evidence to date and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016;8:124–135. doi: 10.1177/1759720X16656810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nørrisgaard P.E., Haubek D., Kühnisch J., Chawes B.L., Stokholm J., Bønnelykke K., Bisgaard H. Association of high-dose vitamin d supplementation during pregnancy with the risk of enamel defects in offspring: A 6-year follow-up of a randomized clinical trial. JAMA Pediatr. 2019;173:924–930. doi: 10.1001/jamapediatrics.2019.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed S.G., Miller C.S., Wagner C.L., Hollis B.W., Lawson A.B. Toward preventing enamel hypoplasia: Modeling maternal and neonatal biomarkers of human calcium homeostasis. Caries Res. 2020;54:55–67. doi: 10.1159/000502793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed S.G., Voronca D., Wingate J.S., Murali M., Lawson A.B., Hulsey T.C., Ebeling M.D., Hollis B.W., Wagner C.L. Prenatal vitamin D and enamel hypoplasia in human primary maxillary central incisors: A pilot study. Pediatr. Dent. J. 2017;27:21–28. doi: 10.1016/j.pdj.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka K., Hitsumoto S., Miyake Y., Okubo H., Sasaki S., Miyatake N., Arakawa M. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann. Epidemiol. 2015;25:620–625. doi: 10.1016/j.annepidem.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015;94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 54.Petersen P.E. World Health Organization global policy for improvement of oral health—World Health Assembly 2007. Int. Dent. J. 2008;58:342–348. doi: 10.1111/j.1875-595X.2008.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 55.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 56.Conrads G., About I. Pathophysiology of dental caries. Monogr. Oral Sci. 2018;27:1–10. doi: 10.1159/000487826. [DOI] [PubMed] [Google Scholar]
- 57.Hemadi A.S., Huang R., Zhou Y., Zou J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017;9:e1. doi: 10.1038/ijos.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yildiz G., Ermis R.B., Calapoglu N.S., Celik E.U., Türel G.Y. Gene-environment interactions in the etiology of dental caries. J. Dent. Res. 2016;95:74–79. doi: 10.1177/0022034515605281. [DOI] [PubMed] [Google Scholar]
- 59.Rosier B.T., Marsh P.D., Mira A. Resilience of the oral microbiota in health: Mechanisms that prevent dysbiosis. J. Dent. Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- 60.Jágr M., Eckhardt A., Pataridis S., Foltán R., Myšák J., Mikšík I. Proteomic analysis of human tooth pulp proteomes—Comparison of caries-resistant and caries-susceptible persons. J. Proteom. 2016;145:127–136. doi: 10.1016/j.jprot.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Zhou F., Zhou Y., Shi J. The association between serum 25-hydroxyvitamin D levels and dental caries in US adults. Oral Dis. 2020 doi: 10.1111/odi.13360. [DOI] [PubMed] [Google Scholar]
- 62.Herzog K., Scott J.M., Hujoel P., Seminario A.L. Association of Vitamin D and dental caries in children Findings from the National Health and Nutrition Examination Survey, 2005–2006. J. Am. Dent. Assoc. 2016;147:413–420. doi: 10.1016/j.adaj.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 63.Kim I.J., Lee H.S., Ju H.J., Na J.Y., Oh H.W. A cross-sectional study on the association between vitamin D levels and caries in the permanent dentition of Korean children. BMC Oral Health. 2018;18:43. doi: 10.1186/s12903-018-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta A., Chhonkar A., Arya V. Comparison of Vitamin D level of children with severe early childhood caries and children with no caries. Int. J. Clin. Pediatr. Dent. 2018;11:199–204. doi: 10.5005/jp-journals-10005-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deane S., Schroth R.J., Sharma A., Rodd C. Combined deficiencies of 25-hydroxyvitamin D and anemia in preschool children with severe early childhood caries: A case-control study. Paediatr. Child Health. 2018;23:e40–e45. doi: 10.1093/pch/pxx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guizar J.M., Muñoz N., Amador N., Garcia G. Association of alimentary factors and nutritional status with caries in children of leon, Mexico. Oral Health Prev. Dent. 2016;14:563–569. doi: 10.3290/j.ohpd.a37141. [DOI] [PubMed] [Google Scholar]
- 67.Wagner Y., Heinrich-Weltzien R. Evaluation of an interdisciplinary preventive programme for early childhood caries: Findings of a regional German birth cohort study. Clin. Oral Investig. 2016;20:1943–1952. doi: 10.1007/s00784-015-1685-z. [DOI] [PubMed] [Google Scholar]
- 68.Schroth R.J., Rabbani R., Loewen G., Moffatt M.E. Vitamin D and dental caries in children. J. Dent. Res. 2016;95:173–179. doi: 10.1177/0022034515616335. [DOI] [PubMed] [Google Scholar]
- 69.Wójcik D., Krzewska A., Szalewski L., Pietryka-Michałowska E., Szalewska M., Krzewski S., Pels E., Beń-Skowronek I. Dental caries and Vitamin D 3 in children with growth hormone deficiency. Medicine. 2018;97:e9811. doi: 10.1097/MD.0000000000009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akinkugbe A.A., Moreno O., Brickhouse T.H. Serum cotinine, vitamin D exposure levels and dental caries experience in U.S. adolescents. Community Dent. Oral Epidemiol. 2019;47:185–192. doi: 10.1111/cdoe.12442. [DOI] [PubMed] [Google Scholar]
- 71.Gyll J., Ridell K., Öhlund I., Karlsland Åkeson P., Johansson I., Lif Holgerson P. Vitamin D status and dental caries in healthy Swedish children. Nutr. J. 2018;17:11. doi: 10.1186/s12937-018-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudding T., Thomas S.J., Duncan K., Lawlor D.A., Timpson N.J. Re-examining the association between Vitamin D and childhood caries. PLoS ONE. 2015;10:e0143769. doi: 10.1371/journal.pone.0143769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kühnisch J., Thiering E., Heinrich-Weltzien R., Hellwig E., Hickel R., Heinrich J. Fluoride/vitamin D tablet supplementation in infants—Effects on dental health after 10 years. Clin. Oral Investig. 2017;21:2283–2290. doi: 10.1007/s00784-016-2021-y. [DOI] [PubMed] [Google Scholar]
- 74.Altman H., Steinberg D., Porat Y., Mor A., Fridman D., Friedman M., Bachrach G. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 2006;58:198–201. doi: 10.1093/jac/dkl181. [DOI] [PubMed] [Google Scholar]
- 75.Grant W.B. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinology. 2011;3:193–198. doi: 10.4161/derm.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidopoulou S., Diza E., Menexes G., Kalfas S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012;57:865–869. doi: 10.1016/j.archoralbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Phattarataratip E., Olson B., Broffitt B., Qian F., Brogden K.A., Drake D.R., Levy S.M., Banas J.A. Streptococcus mutans strains recovered from caries-active or caries-free individuals differ in sensitivity to host antimicrobial peptides. Mol. Oral Microbiol. 2011;26:187–199. doi: 10.1111/j.2041-1014.2011.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong J.H., Ye X.J., Ng T.B. Cathelicidins: Peptides with antimicrobial, immunomodulatory, anti-inflammatory, angiogenic, anticancer and procancer activities. Curr. Protein Pept. Sci. 2013;14:504–514. doi: 10.2174/13892037113149990067. [DOI] [PubMed] [Google Scholar]
- 79.Colombo N.H., Ribas L.F.F., Pereira J.A., Kreling P.F., Kressirer C.A., Tanner A.C.R., Duque C. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch. Oral Biol. 2016;69:40–46. doi: 10.1016/j.archoralbio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Goeke J.E., Kist S., Schubert S., Hickel R., Huth K.C., Kollmuss M. Sensitivity of caries pathogens to antimicrobial peptides related to caries risk. Clin. Oral Investig. 2018;22:2519–2525. doi: 10.1007/s00784-018-2348-7. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z., Yang G., Lu S., Chen D., Fan S., Xu J., Wu B., He J. Design and antimicrobial activities of LL-37 derivatives inhibiting the formation of Streptococcus mutans biofilm. Chem. Biol. Drug Des. 2019;93:1175–1185. doi: 10.1111/cbdd.13419. [DOI] [PubMed] [Google Scholar]
- 82.Darveau R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 83.Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 84.Machado V., Botelho J., Amaral A., Proença L., Alves R., Rua J., Cavacas M.A., Delgado A.S., Mendes J.J. Prevalence and extent of chronic periodontitis and its risk factors in a Portuguese subpopulation: A retrospective cross-sectional study and analysis of clinical attachment loss. PeerJ. 2018;6:e5258. doi: 10.7717/peerj.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Botelho J., Machado V., Proença L., Alves R., Cavacas M.A., Amaro L., Mendes J.J. Study of Periodontal Health in Almada-Seixal (SoPHiAS): A cross-sectional study in the Lisbon Metropolitan Area. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-52116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buset S.L., Walter C., Friedmann A., Weiger R., Borgnakke W.S., Zitzmann N.U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J. Clin. Periodontol. 2016;43:333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 87.Botelho J., Machado V., Proença L., Bellini D.H., Chambrone L., Alcoforado G., Mendes J.J. The impact of nonsurgical periodontal treatment on oral health-related quality of life: A systematic review and meta-analysis. Clin. Oral Investig. 2020;24:585–596. doi: 10.1007/s00784-019-03188-1. [DOI] [PubMed] [Google Scholar]
- 88.Preshaw P.M., Alba A.L., Herrera D., Jepsen S., Konstantinidis A., Makrilakis K., Taylor R. Periodontitis and diabetes: A two-way relationship Matrix metalloproteinase NHANES National Health and Nutrition Examination Survey. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leira Y., Seoane J., Blanco M., Rodríguez-Yáñez M., Takkouche B., Blanco J., Castillo J. Association between periodontitis and ischemic stroke: A systematic review and meta-analysis. Eur. J. Epidemiol. 2017;32:43–53. doi: 10.1007/s10654-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 90.Muñoz Aguilera E., Suvan J., Buti J., Czesnikiewicz-Guzik M., Barbosa Ribeiro A., Orlandi M., Guzik T.J., Hingorani A.D., Nart J., D’Aiuto F., et al. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 91.Hussain S.B., Botelho J., Machado V., Zehra S.A., Mendes J.J., Ciurtin C., Orlandi M., Aiuto F.D. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020 doi: 10.1016/j.semarthrit.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Papageorgiou S.N., Hagner M., Nogueira A.V.B., Franke A., Jäger A., Deschner J. Inflammatory bowel disease and oral health: Systematic review and a meta-analysis. J. Clin. Periodontol. 2017;44:382–393. doi: 10.1111/jcpe.12698. [DOI] [PubMed] [Google Scholar]
- 93.Botelho J., Machado V., Mascarenhas P., Rua J., Alves R., Cavacas M.A., Delgado A., João Mendes J. Stress, salivary cortisol and periodontitis: A systematic review and meta-analysis of observational studies. Arch. Oral Biol. 2018;96:58–65. doi: 10.1016/j.archoralbio.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Machado V., Botelho J., Lopes J., Patrão M., Alves R., Chambrone L., Alcoforado G., Mendes J.J. Periodontitis impact in interleukin-6 serum levels in solid organ transplanted patients: A systematic review and meta-analysis. Diagnostics. 2020;10:184. doi: 10.3390/diagnostics10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manrique-Corredor E.J., Orozco-Beltran D., Lopez-Pineda A., Quesada J.A., Gil-Guillen V.F., Carratala-Munuera C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019;47:243–251. doi: 10.1111/cdoe.12450. [DOI] [PubMed] [Google Scholar]
- 96.Najeeb S., Zafar M.S., Khurshid Z., Zohaib S., Almas K. The role of nutrition in periodontal health: An update. Nutrients. 2016;8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jagelavičienė E., Vaitkevičienė I., Šilingaitė D., Šinkūnaitė E., Daugėlaitė G. The relationship between vitamin D and periodontal pathology. Medicina. 2018;54:45. doi: 10.3390/medicina54030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia M.N., Hildebolt C.F., Miley D.D., Dixon D.A., Couture R.A., Anderson Spearie C.L., Langenwalter E.M., Shannon W.D., Deych E., Mueller C., et al. One-year effects of Vitamin D and calcium supplementation on chronic periodontitis. J. Periodontol. 2011;82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grant W.B., Boucher B.J. Are hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinology. 2010;2:30–36. doi: 10.4161/derm.2.1.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein S.H., Livada R., Tipton D.A. Re-evaluating the role of vitamin D in the periodontium. J. Periodontal Res. 2014;49:545–553. doi: 10.1111/jre.12149. [DOI] [PubMed] [Google Scholar]
- 101.Anbarcioglu E., Kirtiloglu T., Öztürk A., Kolbakir F., Acıkgöz G., Colak R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2019;25:242–249. doi: 10.1111/odi.12968. [DOI] [PubMed] [Google Scholar]
- 102.Agrawal A.A., Kolte A.P., Kolte R.A., Chari S., Gupta M., Pakhmode R. Evaluation and comparison of serum vitamin D and calcium levels in periodontally healthy, chronic gingivitis and chronic periodontitis in patients with and without diabetes mellitus–a cross-sectional study. Acta Odontol. Scand. 2019;77:592–599. doi: 10.1080/00016357.2019.1623910. [DOI] [PubMed] [Google Scholar]
- 103.Ebersole J.L., Lambert J., Bush H., Huja P.E., Basu A. Serum nutrient levels and aging effects on periodontitis. Nutrients. 2018;10:1986. doi: 10.3390/nu10121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Isola G., Alibrandi A., Rapisarda E., Matarese G., Williams R.C., Leonardi R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020:1–11. doi: 10.1111/jre.12746. [DOI] [PubMed] [Google Scholar]
- 105.Ketharanathan V., Torgersen G.R., Petrovski B.É., Preus H.R. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D 3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health. 2019;19:83. doi: 10.1186/s12903-019-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costantini E., Sinjari B., Piscopo F., Porreca A., Reale M., Caputi S., Murmura G. Evaluation of salivary cytokines and Vitamin D levels in periodontopathic patients. Int. J. Mol. Sci. 2020;21:2669. doi: 10.3390/ijms21082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhan Y., Samietz S., Holtfreter B., Hannemann A., Meisel P., Nauck M., Volzke H., Wallaschofski H., Dietrich T., Kocher T., et al. Prospective study of serum 25-hydroxy vitamin d and tooth loss. J. Dent. Res. 2014;93:639–644. doi: 10.1177/0022034514534985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Millen A.E., Hovey K.M., LaMonte M.J., Swanson M., Andrews C.A., Kluczynski M.A., Genco R.J., Wactawski-Wende J. Plasma 25-Hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. J. Periodontol. 2013;84:1243–1256. doi: 10.1902/jop.2012.120445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Antonoglou G.N., Knuuttila M., Niemelä O., Raunio T., Karttunen R., Vainio O., Hedberg P., Ylöstalo P., Tervonen T. Low serum level of 1,25(OH)2D is associated with chronic periodontitis. J. Periodontal Res. 2015;50:274–280. doi: 10.1111/jre.12207. [DOI] [PubMed] [Google Scholar]
- 110.Meghil M.M., Hutchens L., Raed A., Multani N.A., Rajendran M., Zhu H., Looney S., Elashiry M., Arce R.M., Peacock M.E., et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019;25:1403–1413. doi: 10.1111/odi.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taskan M., Gevrek F. PPAR-γ, RXR, VDR, and COX-2 expressions in gingival tissue samples of healthy individuals, periodontitis and peri-implantitis patients MM. Niger. J. Clin. Pract. 2019;22:46–53. doi: 10.4103/njcp.njcp_349_19. [DOI] [PubMed] [Google Scholar]
- 112.Hu X., Niu L., Ma C., Huang Y., Yang X., Shi Y., Pan C., Liu J., Wang H., Li Q., et al. Calcitriol decreases live Porphyromonas gingivalis internalized into epithelial cells and monocytes by promoting autophagy. J. Periodontol. 2019 doi: 10.1002/JPER.19-0510. [DOI] [PubMed] [Google Scholar]
- 113.Han J., Cheng C., Zhu Z., Lin M., Zhang D.X., Wang Z.M., Wang S. Vitamin D reduces the serum levels of inflammatory cytokines in rat models of periodontitis and chronic obstructive pulmonary disease. J. Oral Sci. 2019;61:53–60. doi: 10.2334/josnusd.17-0357. [DOI] [PubMed] [Google Scholar]
- 114.Li H., Zhong X., Li W., Wang Q. Effects of 1,25-dihydroxyvitamin on experimental periodontitis and ahr/nf-κb/nlrp3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019;27:1–10. doi: 10.1590/1678-7757-2018-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oh C., Kim H.J., Kim H.M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal Implant. Sci. 2019;49:270–286. doi: 10.5051/jpis.2019.49.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Q., Zhou X., Zhang P., Zhao P., Nie L., Ji N., Ding Y., Wang Q. 25-Hydroxyvitamin D3 positively regulates periodontal inflammaging via SOCS3/STAT signaling in diabetic mice. Steroids. 2020;156:108570. doi: 10.1016/j.steroids.2019.108570. [DOI] [PubMed] [Google Scholar]
- 117.Li H., Li W., Wang Q. 1,25-dihydroxyvitamin D3 suppresses lipopolysaccharide-induced interleukin-6 production through aryl hydrocarbon receptor/nuclear factor-κB signaling in oral epithelial cells. BMC oral health. 2019;19:1–9. doi: 10.1186/s12903-019-0935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bi C.S., Wang J., Qu H.L., Li X., Tian B.M., Ge S., Chen F.M. Calcitriol suppresses lipopolysaccharide-induced alveolar bone damage in rats by regulating T helper cell subset polarization. J. Periodontal Res. 2019;54:612–623. doi: 10.1111/jre.12661. [DOI] [PubMed] [Google Scholar]
- 119.Hong H., Hong A., Wang C., Huang E., Chiang C. Calcitriol exerts a mineralization-inductive effect comparable to that of vitamin C in cultured human periodontium cells. Am. J. Transl. Res. 2019;11:2304–2316. [PMC free article] [PubMed] [Google Scholar]
- 120.Anand A., Singh S., Sonkar A.A., Husain N., Singh K.R., Singh S., Kushwaha J.K. Expression of Vitamin D receptor and Vitamin D status in patients with oral neoplasms and effect of Vitamin D supplementation on quality of life in advanced cancer treatment. Wspolczesna Onkol. 2017;21:145–151. doi: 10.5114/wo.2017.68623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu X., Zong X., Pan Y. Associations between vitamin D receptor genetic variants and periodontitis: A meta-analysis. Acta Odontol. Scand. 2019;77:484–494. doi: 10.1080/00016357.2019.1597160. [DOI] [PubMed] [Google Scholar]
- 122.Wan Q.S., Li L., Yang S.K., Liu Z.L., Song N. Role of Vitamin D receptor gene polymorphisms on the susceptibility to periodontitis: A meta-analysis of a controversial issue. Genet. Test. Mol. Biomark. 2019;23:618–633. doi: 10.1089/gtmb.2019.0021. [DOI] [PubMed] [Google Scholar]
- 123.Gao W., Tang H., Wang D., Zhou X., Song Y., Wang Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020:1–9. doi: 10.1111/jre.12719. [DOI] [PubMed] [Google Scholar]
- 124.Patil V., Mali R., Moghe A. Evaluation and comparison of Vitamin D receptors in periodontal ligament tissue of Vitamin D-deficient chronic periodontitis patients before and after supplementation of Vitamin D3. J. Indian Soc. Periodontol. 2019;23:100–105. doi: 10.4103/jisp.jisp_173_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bashutski J.D., Eber R.M., Kinney J.S., Benavides E., Maitra S., Braun T.M., Giannobile W.V., McCauley L.K. The impact of vitamin D status on periodontal surgery outcomes. J. Dent. Res. 2011;90:1007–1012. doi: 10.1177/0022034511407771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boggess K.A., Espinola J.A., Moss K., Beck J., Offenbacher S., Camargo C.A., Jr. Vitamin D status and periodontal disease among pregnant women. J. Periodontol. 2011;82:195–200. doi: 10.1902/jop.2010.100384. [DOI] [PubMed] [Google Scholar]
- 127.Sablok A., Batra A., Thariani K., Batra A., Bharti R., Aggarwal A.R., Kabi B.C., Chellani H. Supplementation of Vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin. Endocrinol. (Oxf.) 2015;83:536–541. doi: 10.1111/cen.12751. [DOI] [PubMed] [Google Scholar]
- 128.Khan F.R., Ahmad T., Hussain R., Bhutta Z.A. A randomized controlled trial of oral Vitamin D supplementation in pregnancy to improve maternal periodontal health and birth weight. J. Int. Oral Health. 2016;8:657–665. [Google Scholar]
- 129.Khan F., Ahmad T., Hussain R., Bhutta Z. Relationship among Hypovitaminosis D, maternal periodontal disease, and low birth weight. J. Coll. Physicians Surg. Pak. 2018;28:36–39. doi: 10.29271/jcpsp.2018.01.36. [DOI] [PubMed] [Google Scholar]
- 130.Sabharwal A., Gomes-Filho I.S., Stellrecht E., Scannapieco F.A. Role of periodontal therapy in management of common complex systemic diseases and conditions: An update. Periodontology 2000. 2018;78:212–226. doi: 10.1111/prd.12226. [DOI] [PubMed] [Google Scholar]
- 131.Iheozor-Ejiofor Z., Middleton P., Esposito M., Glenny A.M. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database Syst. Rev. 2017;2017:CD005297. doi: 10.1002/14651858.CD005297.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Albandar J.M., Susin C., Hughes F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018;45:S171–S189. doi: 10.1111/jcpe.12947. [DOI] [PubMed] [Google Scholar]
- 133.Wei S.Q., Qi H.P., Luo Z.C., Fraser W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2013;26:889–899. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 134.Ziuchkovski J.P., Fields H.W., Johnston W.M., Lindsey D.T. Assessment of perceived orthodontic appliance attractiveness. Am. J. Orthod. Dentofac. Orthop. 2008;133:68–78. doi: 10.1016/j.ajodo.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 135.Sarver D.M. Interactions of hard tissues, soft tissues, and growth over time, and their impact on orthodontic diagnosis and treatment planning. Am. J. Orthod. Dentofac. Orthop. 2015;148:380–386. doi: 10.1016/j.ajodo.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 136.Kawakami M., Takano-Yamamoto T. Local injection of 1,25-dihydroxyvitamin D3 enhanced bone formation for tooth stabilization after experimental tooth movement in rats. J. Bone Miner. Metab. 2004;22:541–546. doi: 10.1007/s00774-004-0521-3. [DOI] [PubMed] [Google Scholar]
- 137.Anderson P.H. Vitamin D activity and metabolism in bone. Curr. Osteoporos. Rep. 2017;15:443–449. doi: 10.1007/s11914-017-0394-8. [DOI] [PubMed] [Google Scholar]
- 138.van Driel M., van Leeuwen J.P.T.M. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017;453:46–51. doi: 10.1016/j.mce.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 139.Meikle M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after carl sandstedt. Eur. J. Orthod. 2006;28:221–240. doi: 10.1093/ejo/cjl001. [DOI] [PubMed] [Google Scholar]
- 140.Davidovitch Z., Finkelson M.D., Steigman S., Shanfeld J.L., Montgomery P.C., Korostoff E. Electric currents, bone remodeling, and orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1980;77:14–32. doi: 10.1016/0002-9416(80)90221-3. [DOI] [PubMed] [Google Scholar]
- 141.Yamasaki K., Shibata Y., Imai S., Tani Y., Shibasaki Y., Fukuhara T. Clinical application of prostaglandin E 1 (PGE 1 ) upon orthodontic tooth movement. Am. J. Orthod. 1984;85:508–518. doi: 10.1016/0002-9416(84)90091-5. [DOI] [PubMed] [Google Scholar]
- 142.Stark T.N.I., Sinclair P.M. Effect of pulsed electromagnetic fields on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1987;91:91–104. doi: 10.1016/0889-5406(87)90465-3. [DOI] [PubMed] [Google Scholar]
- 143.Boyce R.W., Weisbrode S.E. Histogenesis of hyperosteoidosis in 1,25(OH)2D3-treated rats fed high levels of dietary calcium. Bone. 1985;6:105–112. doi: 10.1016/8756-3282(85)90314-X. [DOI] [PubMed] [Google Scholar]
- 144.Kale S., Kocadereli I., Atilla P., Aşan E. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2004;125:607–614. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Collins M.K., Sinclair P.M. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1988;94:278–284. doi: 10.1016/0889-5406(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 146.Lipworth L., Rossi M., McLaughlin J.K., Negri E., Talamini R., Levi F., Franceschi S., La Vecchia C. Dietary vitamin D and cancers of the oral cavity and esophagus. Ann. Oncol. 2009;20:1576–1581. doi: 10.1093/annonc/mdp036. [DOI] [PubMed] [Google Scholar]
- 147.Lehrer S., Montazem A., Ramanathan L., Pessin-Minsley M., Pfail J., Stock R.G., Kogan R. Normal serum bone markers in bisphosphonate-induced osteonecrosis of the jaws. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008;106:389–391. doi: 10.1016/j.tripleo.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 148.Bedogni A., Bettini G., Bedogni G., Basso D., Gatti D., Valisena S., Brunello A., Sorio M., Berno T., Giannini S., et al. Is vitamin D deficiency a risk factor for osteonecrosis of the jaw in patients with cancer? A matched case–control study. J. Cranio-Maxillofac. Surg. 2019;47:1203–1208. doi: 10.1016/j.jcms.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Heim N., Warwas F.B., Wilms C.T., Reich R.H., Martini M. Vitamin D (25-OHD) deficiency may increase the prevalence of medication-related osteonecrosis of the jaw. J. Cranio-Maxillofac. Surg. 2017;45:2068–2074. doi: 10.1016/j.jcms.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 150.Lowe L.C., Guy M., Mansi J.L., Peckitt C., Bliss J., Wilson R.G., Colston K.W. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur. J. Cancer. 2005;41:1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 151.Badros A., Goloubeva O., Evangelos T., Todd M., Maria R.B., Elizabeth S. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br. J. Haematol. 2008;142:492–494. doi: 10.1111/j.1365-2141.2008.07214.x. [DOI] [PubMed] [Google Scholar]
- 152.Sugimoto T., Matsumoto T., Hosoi T., Miki T., Gorai I., Yoshikawa H., Tanaka Y., Tanaka S., Fukunaga M., Sone T., et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: Results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT) Osteoporos. Int. 2014;26:765–774. doi: 10.1007/s00198-014-2964-2. [DOI] [PubMed] [Google Scholar]
- 153.Demircan S., Isler S. Changes in serological bone turnover markers in bisphosphonate induced osteonecrosis of the jaws: A case control study. Niger. J. Clin. Pract. 2020;23:154–158. doi: 10.4103/njcp.njcp_374_19. [DOI] [PubMed] [Google Scholar]
- 154.Danila M.I., Outman R.C., Rahn E.J., Mudano A.S., Redden D.T., Li P., Allison J.J., Anderson F.A., Wyman A., Greenspan S.L., et al. Evaluation of a multimodal, direct-to-patient educational intervention targeting barriers to osteoporosis care: A randomized clinical trial. J. Bone Miner. Res. 2018;33:763–772. doi: 10.1002/jbmr.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]