Abstract
It has been thought that caloric restriction favors longevity and healthy aging where autophagy plays a vital role. However, autophagy decreases during aging and that can lead to the development of aging-associated diseases such as cancer, diabetes, neurodegeneration, etc. It was shown that autophagy can be induced by mechanical or chemical stress. In this regard, various pharmacological compounds were proposed, including natural polyphenols. Apart from the ability to induce autophagy, polyphenols, such as resveratrol, are capable of modulating the expression of pro- and anti-apoptotic factors, neutralizing free radical species, affecting mitochondrial functions, chelating redox-active transition metal ions, and preventing protein aggregation. Moreover, polyphenols have advantages compared to chemical inducers of autophagy due to their intrinsic natural bio-compatibility and safety. In this context, polyphenols can be considered as a potential therapeutic tool for healthy aging either as a part of a diet or as separate compounds (supplements). This review discusses the epigenetic aspect and the underlying molecular mechanism of polyphenols as an anti-aging remedy. In addition, the recent advances of studies on NAD-dependent deacetylase sirtuin-1 (SIRT1) regulation of autophagy, the role of senescence-associated secretory phenotype (SASP) in cells senescence and their regulation by polyphenols have been highlighted as well. Apart from that, the review also revised the latest information on how polyphenols can help to improve mitochondrial function and modulate apoptosis (programmed cell death).
Keywords: polyphenols, caloric-restriction mimetics, autophagy, aging, apoptosis
1. Introduction
According to Kirkwood, aging can be considered as the result of a continuous interaction between the genetic composition of the body and environmental factors. This interaction is characterized by a lifelong accumulation of damage to genetic components and a progressive loss of tissue and organ functionality [1]. The environmental impact comprises of many factors, including but not limited to quality and availability of food, level of pollution, health care, etc. In fact, increasingly favorable living conditions such as food availability and medical care, contribute to increased life expectancy in developed countries. The result is the growth of the proportion of older people in the general population structure [2]. However, aging has been associated with an increased risk of developing age-related neurodegenerative diseases, cardiovascular diseases, diabetes, osteoarthritis or cancer [3].
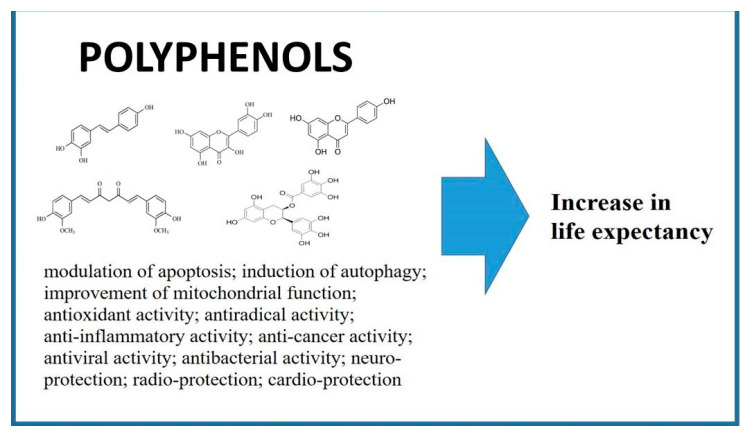
The trend towards an increase in the prevalence of age-related diseases is obvious of course, but it is also apparent that people who reach old age are evidence of the possibility of successful healthy aging [4]. In general, these observations have prompted studies on how aging pathways can be slowed down or blocked in relation to the development of age-related pathology [5]. The idea is that the most effective strategies in this area should focus on molecular mechanisms of age-associated disorders and malfunctions [6,7]. To date, many natural and synthetic agents have been investigated for anti-aging properties at the cellular level in animal models as well as in humans [8,9]. In this context, polyphenols play an important role owing to the fact that they are natural compounds with proven antioxidant and anti-inflammatory activity [10,11,12]. These features could be harnessed to counteract signaling pathways at the molecular level that are responsible for the cascade reactions leading to aging [13,14,15] (Figure 1). Polyphenols are a unique family of secondary metabolites present in leaves, bark, vegetables, fruits, herbs and many higher plants [16,17,18]. They are the most common bioactive natural products and are involved in the chemical protection of plants, and also play an important role in plant reproduction and growth.
Figure 1.
Biological activities of polyphenols. Modulation of apoptosis [23,24]; induction of autophagy [25,26]; improvement of mitochondrial function [27]; antioxidant activity [28,29,30]; antiradical activity [31,32,33]; anti-inflammatory activity [34]; anti-cancer activity [35]; antiviral and antibacterial activity [36,37]; neuro-protection [38]; radio-protection [39,40]; cardio-protection [41,42].
In vitro studies have shown that cell aging can occur as a result of replicative and non-replicative stress [19]. A study of replicative aging on cell models demonstrated that this type of aging is associated with a limitation of proliferative ability [20]. Non-replicative aging can be associated with various stressors, including chemical and physical damage, such as exposure to X-rays, oxidative stress, DNA breaks and chromatin, mitochondrial dysfunction. In addition, various endogenous processes, such as transcriptional stress, which is evident in the excessive expression of activated oncogenes [21,22], could also contribute to the aging.
Even if the existing programmed aging pathways and non-specificity of cell aging markers are recognized by the organism, a number of phenotypic cell aging features have been observed both in in vitro and in vivo studies [43]. Aging cells usually have distinctive morphological features, such as an increase in volume, a flattened shape and, in general, “irregular morphology” with a larger than usual nucleus, a large nucleolus and an increased number of cytoplasmic vacuoles [44].
The term ‘aging’ can be defined as the irreversible proliferative deterioration of the physiological processes of the organism that are responsible for its survival and fertility [45,46]. The aging encompasses changes occurring without any reference to death, resulting in the progressive loss of physiological integrity and impaired function of tissues and organs [46]. At the same time, the term ‘senescence’ mainly refers to the phase when irreversible symptoms of death appear, i.e., irreversible cell arrest that limits the further proliferation of damaged cells [46]. Senescent cells are characterized by enlarged cell morphology, increased senescence-associated β-galactosidase (SA-β-gal) activity, p16 upregulation, reduced lamin B1 expression, translocation of nuclear HMGB1 into the cytoplasm and extracellular space and secretion of senescence-associated secretory phenotype (SASP) factors such as inflammatory cytokines and metallo-proteinases [47].
The aging phenotype is characterized by an increase in β-galactosidase (β-gal) activity, a typical lysosomal enzyme associated with cell aging. β-gal l activity (measured at pH 6.0) is often used as a marker of cell aging both in vitro and in vivo [48] although according to some researchers, β-gal data is more reliable when accompanied by analysis of other markers such as p16 [49,50,51]. Cellular aging is associated mainly with a loss of proliferation ability. The inhibition of cell cycle progression during aging is mediated by over-expression of inhibitory proteins such as p53, mp21 and p16InK4a, as well as inhibition of cell replication-promoting proteins such as cyclins, c-Fos and pCNA [52]. Therefore, p16 and p21 markers can be used as age-related biomarkers.
Since genetic modification is not yet fully controllable, the best anti-aging strategy currently is to intervene in environmental factors aimed at reducing the severity of risk factors. Calorie restriction (CR) is the only non-genetic intervention that has evidence of prolonging the lifespan of model organisms from yeast to mammals. It protects against inhibition of biological functions by delaying or reducing the risk of many age-related diseases. The biological mechanisms of the beneficial effects of CR include modifying energy metabolism, reducing oxidative stress, increasing insulin sensitivity, suppressing chronic inflammation, stimulating autophagy, neuroendocrine function, and inducing hormesis. Molecular signaling pathways that mediate the anti-aging effect of CR include sirtuins, G-coactivator-1α, activated AMP protein kinase, insulin/insulin-1 growth factor and the target of rapamycin mTOR, all of which form a rather active interacting network.
However, most people would not adhere to such a strict CR diet program, therefore there is no doubt that it is advisable to search for natural and/or pharmacological molecules that mimic the effect of CR without reducing food intake, especially between the mid-age and old age. Such substances have become known as CR mimetics (CRM).
Potential candidates known to date (resveratrol and other polyphenols, rapamycin, 2-deoxy-D-glucose and other glycolytic inhibitors) act on the same signaling pathways as CR, the insulin pathway and activated AMP protein kinase activators, autophagy stimulants, alpha-lipoic acid, and other antioxidants [53].
Nutrient depletion, which is one of the physiological triggers of autophagy, leads to the exhaustion of intracellular acetyl coenzyme A (AcCoA) associated with deacetylation of cellular proteins. Three possible triggers of these effects are suggested: (i) depletion of the cytosolic AcCoA, preventing its biosynthesis; (ii) inhibition of acetyltransferase by enzymes that transfer acetyl groups from AcCoA to other molecules, and (iii) stimulation of deacetylases that catalyze the removal of acetyl groups from leucine residues [25,54,55]. There are several examples of fairly nontoxic natural compounds that act as AcCoA depleting substances (e.g., hydroxycitrate), acetyltransferase inhibitors (e.g., anacardic acid, curcumin, epigallocatechin-3-gallate, garcinol, spermidine) [24,56,57,58,59] or deacetylase activators (e.g., nicotinamide, resveratrol) [60,61] which are highly effective inducers of in vitro and in vivo autophagy. Another common feature of these agents is their ability to reduce the risk of age-related diseases.
Therefore, we classify them as “caloric-restriction mimetics” (CRMs). Here, we suggest that CRMs can impact the same molecular pathways that are usually triggered by long-term calorie restriction or short-term fasting, and this implies the impending induction of autophagy [25].
2. Polyphenols as Epigenetic Modulators
The term “epigenetics” was first proposed by Conrad Hal Waddington (1905–1975) in 1942. He defined epigenetics as a branch of biology that studies causal interactions between genes and their products, which are responsible for the phenotype of an organism [62]. Later, in 1987, Robin Holliday revised epigenetics as a variant of nuclear inheritance not based on differences in DNA sequence [63,64]. It is generally accepted that epigenetics is a study of hereditary changes excluding DNA sequence modifications that control gene expression. In contrast to genetic changes, epigenetic modifications are reversible. Epigenetic inheritance is now recognized as a critical mechanism in the regulation of genes involved in all biological processes and cellular memory. The molecular mechanisms of epigenetic modifications, including DNA methylation, histone modification, and miRNAs, are described fairly well [65,66,67,68]. In fact, the epigenetic modifications play a decisive role in the patterns of physiological and pathophysiological processes.
Epigenetic changes are considered the earliest and most reversible, thus they present new interesting targets for therapeutic intervention. There is growing evidence that epigenetic processes are modulated by diet components [69,70,71,72], in particular polyphenols and alkaloids presented in food [73,74,75]. Polyphenols are water-soluble compounds that have several phenolic groups (12 to 16 phenolic hydroxyl groups). Polyphenols have molecular weights in the range of 500–5000 Da and from five to seven aromatic rings [76,77]. Based on their general structure, polyphenols can be divided into at least ten different classes, such as phenolic acids and derivatives, flavonoids, stilbens, coumarins, tannins, etc. [76,78,79]. In fact, there are many varieties of polyphenols, and thousands of plant polyphenols have been identified up to date. They demonstrate a wide range of biological activity, including antioxidant activity, antiradical, anti-inflammatory, anti-cancer, antiviral, antibacterial, anti-thrombogenic, anti-atherogenic activity [34,80,81,82,83,84,85]. However, most importantly, plant polyphenols are capable to cause epigenetic changes. Here, we summarize and briefly discuss the role of the main groups of polyphenols and the possibility of epigenetic modulations with role in age-associated diseases and aging.
It is generally accepted that epigenetics is a study of hereditary changes excluding DNA sequence modifications that control gene expression. DNA methylation, non-coding RNA and histone modifications are the main mechanisms in epigenetics [86,87]. Epigenetic switches establish links between low-level chronic inflammation and tumor cell transformation, and include complex regulatory loops: pro-inflammatory cytokines, transcription factors NF-κB and STAT3, and miRNAs such as let-7 and Lin28 [35,88].
The polyphenols demonstrated the ability to modulate the inflammatory cascade [89]. In fact, the anti-inflammatory effect induced by polyphenols may diminish inflammaging, intestinal barrier disruption, and neuro-inflammation, thus contributing to the resilience to aging and predisposition for age-related diseases [89]. For example, anti-inflammatory properties of resveratrol, well-known CRM, has been intensively studied and reported recent decades [90,91,92,93,94]. Josifovska et al. investigated the effect of resveratrol on autophagy, survival, and inflammation for the treatment of age-related macular degeneration [90]. The results of the study showed that resveratrol induced autophagy in ARPE-19 cells as determined by the augmented presence of autophagic vacuoles, increased LC3II/I ratio and decreased p62 expression. In addition, resveratrol induced a specific anti-inflammatory response in ARPE-19 cells. In recent studies using tumor cell lines, quercetin and resveratrol suppressed the NF-κB inflammatory response pathway and reduced miRNA155 expression [95,96,97].
The results of recent research on CRMs also suggest that they are capable to modulate epigenetic signals responsible for the cancer progression or other age-related epigenetic modifications that may be reversible. It has been also shown that histone complexes could be transcriptionally activated, and therefore, epigenetically modified genes might be silenced [88].
It is not ideal situation if random or daily intake of these natural nutrients results in long-term epigenetic control of gene expression, consistent chemo-protective effects, or both. It is now well known that the ability of resveratrol to improve mitochondrial function requiring SIRT1 is dose dependent [98,99]. Activation of AMPK in the absence of SIRT1 does not activate mitochondrial function. In experimental studies on mice that received a low dose of resveratrol, mitochondrial function and biogenesis were increased, AMPK activated, and also NAD + levels in skeletal muscle increased. Conversely, a high dose of resveratrol activated AMPK independent of SIRT [100].
There is a number of studies indicating that polyphenols are capable to suppress the suppression of cancer by the modulation of epigenetic machinery, including the regulation of DNA-methyltransferase (DNMT) and HDACs activities [101,102]. In this context, resveratrol demonstrated an ability to suppress enzymatic activity of DNMT and also mRNA levels of DNMT1, DNMT3A and DNMT3B in HCC1806 breast cancer cells [103]. Moreover, no any significant alterations in DNMT activity were found out in MCF10A control cells even after 72 h of the treatment.
Medina-Aguilar and co-workers carried out a genome-wide survey of DNA methylation signatures in triple negative breast cancer cells exposed to resveratrol [104]. The results indicated that resveratrol treatment for 24 h and 48 h decreased gene promoter hypermethylation and increased DNA hypomethylation. The conducted integrative analysis of methylome and transcriptome profiles in response to resveratrol demonstrated that methylation alterations were concordant with changes in mRNA expression in several oncogenes (AURKA, CCNB1, DDIT4, DLGAP5, EYS, FAM83D, IL24, LPXN, NFIL3, PFKFB3, SLC14A1, STC1, GPR110, HK2, MMP9, NFIL3, PSMD11, RUNX2, SH3KBP1) and tumor suppressor genes (AMY2A, IL18, SLIT3, MPHOSPH9, SLC27A2, TMOD2, TTI1, and XYLB).
Dhar et al. showed that resveratrol promotes acetylation and reactivation of PTEN via inhibition of the MTA1/HDAC complex leading to the suppression of the Akt pathway [105]. The study also demonstrated that MTA1 knockdown is sufficient to augment acetylation of PTEN indicating a crucial role of MTA1 itself in the regulation of PTEN acetylation contributing to its lipid phosphatase activity. In addition, using prostate cancer xenografts, Dhar and co-workers showed that both resveratrol treatment and MTA1 knockdown is able to increase the PTEN levels resulting in a decrease of p-Akt expression and proliferation index [105].
Another well-studied polyphenol is epigallocatechin-gallate (polyphenolic catechin) with proven anti-bacterial, anti-inflammatory and anti-cancer activities [38,106,107]. It was found out that epigallocatechin-gallate can trigger apoptosis and inhibition of cell proliferation through epigenetic mechanisms [108]. Moreover, the data of recent studies indicate epigallocatechin-gallate not only acts as an epigenetic modulator, but can also modify miRNA expression, thus contributing to the inhibition of carcinogenesis, including prostate cancer [109]. Thakur et al. demonstrated that epigallocatechin-gallate epigenetically reactivated p21/waf1, Bax and PUMA in prostate cancer cells, resulting in cell cycle arrest and apoptosis mediated by proteasomal degradation of class I HDACs [23]. It was demonstrated that epigallocatechin-gallate is able to regulate the progression of hepatocellular carcinoma by modulating key molecular targets such as NFκB and p53; ERK1/2 and PI3K–Akt–mTOR; and FGF and VEGF. In fact, the transition from hepatocellular carcinoma tumor initiation to its proliferation has been effectively suppressed by epigallocatechin-gallate through downregulation of expression levels of p53, NFκB, EGFR, cyclins, and upregulation of BAX [110].
In another study, Li et al. showed that epigallocatechin-gallate can reactivate ERα expression in ERα-negative MDA-MB-231 breast cancer cells [111]. It was also revealed that the combination of epigallocatechin-gallate with the histone deacetylase (HDAC) inhibitor, trichostatin A (TSA), led to a synergistic reactivation of ERα expression in ERα-negative breast cancer cells. This reactivation, in turn, induced sensitization of ERα-dependent cellular responses to activator 17β-estradiol (E2) and antagonist tamoxifen in ERα-negative breast cancer cells. Apart from that, it also demonstrated that epigallocatechin-gallate triggered re-modelling of the chromatin structure of the ERα promoter by altering histone acetylation and methylation status thereby resulting in ERα reactivation. These studies showed that epigallocatechin-gallate is able to restore ERα expression by regulating epigenetic mechanisms [111].
Quercetin has been also intensively investigated in context of its anti-viral, anti-aging, neuroprotective, and anti-cancer activities, including the potential for epigenetic modulation [112,113,114,115]. In the recent study conducted by Sundaram and co-workers, it was found out that quercetin is capable to trigger the modulation of chromatin modifiers such as DNMTs, HDACs, histone acetyltransferases (HAT) and HMTs [112]. In addition, it was shown that quercetin modulates the expression of various chromatin modifiers and decreases the activity of DNMTs, HDACs, and HMTs in a dose-dependent manner. Moreover, quercetin downregulated total DNA methylation levels as well. Alvarez et al. studied the molecular mechanisms underlying the pro-apoptotic effects of quercetin on DNA methylation and posttranslational histone modifications of genes related to the apoptosis pathways [113]. The results of the study showed that quercetin possesses DNA demethylating activity along with HDAC inhibition resulting in apoptosis enhancement. In another study, Sharme et al. demonstrated that a combination of quercetin and curcumin was effective in suppression of DNMT, resulting in global hypomethylation, restoring Androgen Receptor mRNA and protein levels, and inducing apoptosis via mitochondrial depolarization in cancer cells [116].
3. The Potential of Polyphenols for Neuro-Protection
Neurogenesis, the complex process by which stem cells in the hippocampus region of the brain differentiate and multiply into new neurons and other resident brain cells, is known to be affected by many internal and external factors, including diet [117,118,119,120]. Neurogenesis plays a critical role in neural plasticity, brain homeostasis and maintenance in the central nervous system and is a decisive factor in maintaining cognitive function and repairing damaged brain cells affected by aging and brain damage. Internal factors such as aging, neuro-inflammation, oxidative stress and traumatic brain injury, as well as lifestyle factors such as high fat and high sugar diets, alcohol and opioid addiction, can adversely affect neurogenesis in adults. On the contrary, it has been shown that many dietary components such as curcumin, resveratrol, blueberry polyphenols, sulforaphane, savionic acid, polyunsaturated fatty acids (PUFAs) and diets enriched with polyphenols and PUFAs, as well as calorie restriction, exercise and training, induce neurogenesis in adult brains [121,122,123,124,125]. Although many of the mechanisms by which nutrients and dietary factors influence neurogenesis in adults have not yet been determined, nutritional approaches provide promising perspectives for stimulating neurogenesis in adults, combating neurodegenerative diseases and improving cognitive ability.
The question of whether cognitive impairment is an integral part of aging or should be considered as a pathological pre-section of dementia is currently under discussion [126,127,128,129]. This field requires further intensive research, since accelerated brain aging, as well as a further decrease in cognition, can be prevented in the early stages of cognitive impairment. Here we discuss the evidence from clinical and experimental studies on the role of polyphenols in maintaining cognitive performance throughout life.
In recent years, there has been an increase in the number of studies on the possible beneficial effects of plant polyphenols on the cognitive pathways. There is growing evidence of the ability of polyphenols to protect neurons from trauma caused by oxidative stress, suppressing neuro-inflammation and neurotoxicity, and improving cardiovascular function [130,131,132,133,134,135]. In most of these studies, it is concluded that dietary polyphenols, in particular flavonoids, can have a beneficial effect on the central nervous system, which is a potential tool for maintaining cognitive performance in aging [136].
In fact, the neuroprotective capacity of natural polyphenols has been extensively studied and reported during recent decades [137,138,139,140,141,142,143,144]. One of the most studied polyphenols in terms of its neuroprotective activity is resveratrol [145,146,147,148,149,150,151]. In the recent study, Lin and co-workers scrutinized the neuroprotective potential of the resveratrol on the rotenone-induced oxidative stress cellular model [150]. The data of the study indicated that the treatment with resveratrol suppressed the formation of reactive oxygen species (ROS), apoptosis induction, and at the same time, increased survival rate. Resveratrol administration led to an increase in autophagic induction and autophagic flux. The study showed that the neuroprotective effect of resveratrol was a result of the modulation of mitochondria dynamics and upregulating autophagic flux via the MEK/extracellular signal-regulated kinase (ERK) signaling pathway.
The effect of resveratrol on the animal brain affected by the combination of Alzheimer’s disease and diabetes mellitus was reported by Ma et al. [145]. The results demonstrated that resveratrol significantly elevated the Sirt1 expression, suppressed the memory impairment, increased the levels of acetylcholinesterase, malondialdehyde, interleukin-1β and interleukin 6, and at the same time, it decreased levels of choline acetyltransferase, superoxide dismutase, and glutathione. Lin et al. investigated the association between the resveratrol treatment, reduction reactive oxygen species (ROS) and cognitive impairment in rats with angiotensin II (Ang-II)-induced by early Alzheimer’s disease [152]. The results of the study demonstrated a decrease of blood pressure, increase of hippocampal brain-derived neurotrophic factor (BDNF) level, and decrease of nucleus tractus solitarius (NTS) ROS production in the Ang-II groups with losartan (10 mg/kg), or resveratrol (10 mg/kg/day) treatment. The findings indicated that losartan (drug) and resveratrol exert neuroprotective effects against memory impairment and hippocampal damage by oxidative stress reduction in the early stage of Alzheimer’s disease.
There is accumulating evidence of an association between the development of Alzheimer’s disease and disrupting autophagy mechanism in the affected brain cells [153]. In this regard, autophagy has been considered as a therapeutic target for the prevention and treatment of Alzheimer’s disease [154]. Taking into account the ability of polyphenols, and particularly resveratrol, modulate autophagy processes it has been proposed to modulate cellular processes by activating key metabolic sensors/effectors, including AMP-activated protein kinase (AMPK), sirtuin 1 (SIRT1), and peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) [154,155]. Moreover, it is well-known that resveratrol is able to modulate the function of mitochondria and induce the activity of SIRT1 and the clearance of mutant proteins associated with neurodegenerative diseases (such as AD) through the mTOR-dependent or independent manner to promote neuronal survival [154,156]. It has been suggested that the neuroprotective effect of resveratrol obliged to activation of AMPK by increasing intracellular Ca2+ and promoting AMPK phosphorylation at Thr172 site, leading to the suppression of mTOR activity and enhancement of autophagic and lysosomal clearance of Aβ-amyloid [154]. These findings indicate that resveratrol possesses a therapeutic potential for the treatment of Alzheimer’s disease via induction of autophagy and modulating of SIRT1-mediated transcriptional regulation or mTOR-dependent signal pathway [154].
There is a range of reports on the effect of resveratrol on neuro-inflammation [151,157,158,159,160,161]. Simao et al. studied the effect of resveratrol on NF-κB inflammatory cascade, COX-2, iNOS and JNK levels in rats with global cerebral ischemia [158]. Resveratrol significantly reduced NF-κB and JNK activation by decreasing COX-2 and iNOS production. The authors suggest that neuro-protective effect of resveratrol can be explained by the suppression of the inflammatory response via regulation of NF-κB, COX-2 and iNOS induced by brain ischemia. In fact, resveratrol suppresses activity of pro-inflammatory enzyme COX-1/2, decreases activation of NF-κB, PGE2, NO, and TNFα production, cytokine release and increases HO1 expression and activity in brain cells [151]. Moreover, resveratrol is able to modulate various signaling pathways involved in cell survival (AMPK, PI3-k, AkT), apoptosis (caspase-3/12, Bax, MMP-3/9, AIF, cytochrome c) and synaptic plasticity (PKC, ERK1/2) [151]. In addition, the activation of the deacetylase sirtuins by resveratrol has also been demonstrated.
Apart from resveratrol, other natural polyphenols have also been intensively explored for neuro-protective activity the last decades, including curcumin [162,163,164,165], apigenin [166,167,168], quercetin [169,170,171] and epigallocatechin gallate [172,173]. However, it must be noted that natural polyphenols undergo significant chemical transformations after oral consumption such as deglycosylation, dehydroxylation, demethylation and oxidation [143]. Moreover, the bioavailability of dietary polyphenols depends on many factors and physiological features the organism, including level of metabolism, liver’s conditions, concomitant diseases, specifics of the microbiome, etc. [174]. In fact, bioavailability of any natural polyphenol differs; and there is no a direct relation between the quantity of polyphenols in food and their bioavailability in the organism after oral intake [175].
4. The Role of Autophagy and SASP in Senescence and Their Regulation by Polyphenols
The multicomponent secretion of a huge variety of proteins, including soluble signaling factors, proteases, insoluble extracellular matrix proteins, and non-protein components, collectively referred to as senescence-associated secretory phenotype (SASP) is a key feature of cell senescence induced by replicative exhaustion or stressors [176,177,178,179]. Recent data from several laboratories have revealed that senescent cells accumulate with age resulting in an increase in SASP activity in tissues and organs [180,181,182,183]. SASP mediates the negative impact of senescent cells on other cells via chronic inflammation and accelerates aging in tissues [177,184]. It was established that SASP could promote cardiovascular aging [185], osteoarthritis [186,187] musculoskeletal senescence [188], atherosclerosis [189], increased blood clotting [190], kidney dysfunction [191], and cancer [192]. However, it must be noted that the senescence mainly occurs on replicative cells and not all cells undergo senescence during aging.
The mechanisms of SASP regulation are based on both transcriptional and post transcriptional control of different gene expression [193]. For example, research has provided evidence for the nuclear factor-κB (NF-κB) is major transcription factor of the regulation of SASP components expression [194,195]. There are DDR (DNA damage response)-dependent and independent mechanism of post transcriptional control of SASP regulation [196]. As has been previously reported in the literature that one of the DDR-dependent mechanisms of SASP regulation is dependent on the autophagy [197,198]. It should be noted that two types of autophagy (basal autophagy versus oncogene-induced one) regulate SASP differently [199]. Kang and coauthors have reported that GATA4, an important regulator of SASP, is degraded by p62-mediated autophagy [197]. In this context, one important observation is that, a reduction in autophagic capacity contributes to the accumulation of GATA4 in senescent cells and SASP activation. The key point of GATA4 regulation is the requirement of ‘general’ autophagy activity [197,199].
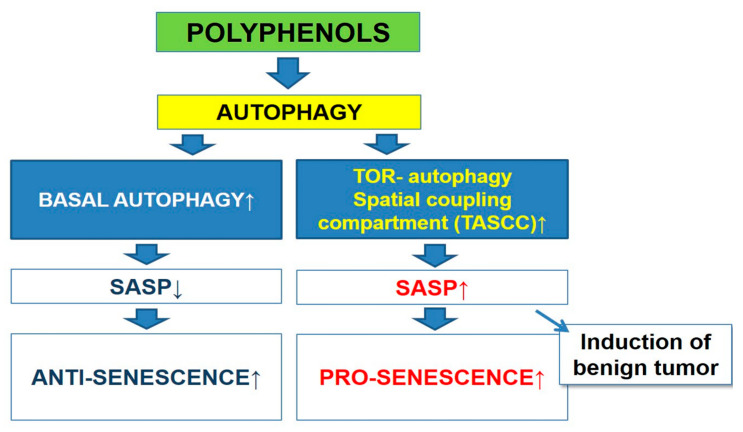
On the other hand, according to studies carried out by the Narita’s group, oncogene-induced senescence triggered activation of autophagy leading to the upregulation of SASP components [198,200]. It was also shown that a specialized type of autophagy called the TOR-autophagy spatial coupling compartment (TASCC), has been involved in this process. It is important to highlight the fact that mTOR accumulation was induced by TASCC which in turn leads to acceleration of the synthesis of SASP factors (Figure 2).
Figure 2.
The scheme of relationship between autophagy and senescence-associated secretory phenotype (SASP).
As mentioned above there is multilevel control of both autophagy and SASP in cellular senescence [177,201,202]. These control mechanisms may interplay and overlap to some extent [199,203]. Crosstalk between major signaling pathways of these processes is an important element for a better understanding of the regulation of cell senescence by polyphenols. There are some crucial elements of interconnections between signaling pathways, such as SIRT1, mTOR, NF-κB [201].
Polyphenols are known to be able to activate autophagy through various mechanisms [204], one of which is the activation of SIRT1 protein by polyphenols based on by stabilizing SIRT1/peptide interactions in a substrate-specific manner [205,206,207]. SIRT1 can trigger autophagy by activating autophagy-related proteins such as (Atg) 5 and 7 and LC3 [49,208]. The indirect mechanism is based on activation of FOXO1 which induces the expression of Rab7 leading to the maturation of autophagosomes and endosomes [209]. In addition, SIRT1 may induce Bnip3-mediated autophagy via activation FOXO3 [208,210]. One of the most important results of SIRT1 activation caused by exposure to polyphenols is the negative regulation of the mTOR signaling pathway, which also leads to activation of autophagy via ULK1/2-ATG13-FIP200 complex [211,212].
On the other hand, SIRT1 also plays an important role in the regulation of SASP by epigenetic mechanisms [213,214,215]. It was established that SIRT1 suppressed the expression of IL-8 and IL-6, major components of SASP, via deacetylation of histones in their promoter regions [213]. As noted above, polyphenols activate SIRT1, which leads to the downregulation of mTOR [211,212]. It is important to highlight the fact that mTOR is one of the key mechanisms of initiation of the SASP [216,217,218,219]. The underlying mechanism that causes SASP activation via mTOR is regulation the translation of the MK2/MAPKAPK2 kinase, which inhibits the ability of protein ZFP36L1 to degrade the transcripts of numerous SASP components [216]. Other mechanisms of SASP control are based on inhibition of cytokine IL1A which causes suppression NF-κB transcriptional activity [219] or via mTOR-mediated regulation of TRPC6 channel [218].
Several studies have found out that the autophagy induction by multiple stimuli is dependent on activation of the inhibitor of NF-κB (IκBα) kinase (IKK) complex [220,221]. It well established that NF-κB signaling pathway is involved in repression of autophagy [222,223,224,225]. NF-κB is considered as a molecular target for polyphenols, for instance, it was reported that polyphenols could reduce the activity of NFκB, which in turn leads to the activation of autophagy [201,226,227]. Several mechanisms for the interaction of polyphenols and NF-κB have been proposed [227]. It has been shown that the effects of polyphenols on NF-κB can be functionally diverse [228]. Some polyphenol inhibits the degradation of IkB via phosphorylation or ubiquitination of kinases [228]. It was demonstrated that inhibition of NF-κB in cells exposed by a polyphenol-rich pomegranate fruit extract is based on the phosphorylation of multiple upstream kinases including NF-κB-inducing kinase (NIK) and IKKβ [229]. Resveratrol reduces IκB phosphorylation and phosphorylation, acetylation and translocation of NF-κB p65 [230]. In addition, there is another interesting regulatory mechanism that is based on the downregulation of NF-κB-mediated miRNAs (mir-21, miR-30a-5p, miR-19) [231,232]. Moreover, it was reported that SIRT1 suppresses NF-κB signaling pathway directly by deacetylating the p65 subunit of NF-κB complex [233] and the upregulation of SIRT1 through resveratrol inhibit activation of NF-κB signaling pathway [234]
On the other hand, NF-κB signaling pathway is the main regulator of SASP [194,195]. Kolesnichenko and coauthors demonstrated that DNA damage consistently induces two phases of NF-κB activation, where first is IKK and proteasome-dependent phase, which activates anti-apoptotic gene expression, while the second (independent from IKK and proteasome) initiate expression of SASP genes [235]. Therefore, the regulation by polyphenols of this protein is significant for cell senescence. It was established that resveratrol decreased SASP by SIRT1/NF-κB pathway in the gut of the annual fish Nothobranchius guentheri [236]. Therefore, it should be noted that the use of polyphenols could activate processes that can trigger both the induction of autophagy and inhibition of SASP. Alternatively, targeting SASP for promoting an anti-tumor microenvironment can be employed for cancer prevention [201].
In view of the above, the regulation of SASP via autophagy is an attractive strategy for healthy aging. In pursuit of a SASP modulation strategy, the anti-SASP activity of polyphenolic compounds is considered as a promising candidate. Despite the number of studies, there are no reports on the use of the detection of both SASP and autophagy in senescent cells treated by polyphenols.
Therefore, we decided to compare the studies conducted on the same types of polyphenols, where the regulation of SASP and autophagy were detected. Table 1 summarizes the existing evidence of anti-aging (based on SASP and autophagy regulation) activity of natural polyphenols.
Table 1.
Regulation of senescence-associated secretory phenotype (SASP) and autophagy by polyphenols.
| Polyphenols | Anti-SASP | Autophagy |
|---|---|---|
| Resveratrol | Mitigates the Inflammatory phenotype in senescent human fibroblast [147] | Upregulation of autophagic pathways in HUVECs treated by hydrogen peroxide [150] |
| Resveratrol | Down-regulation of SASP-associated proinflammatory cytokines IL-8 and TNFα, and up-regulation of anti-inflammatory cytokine IL-10 in gut of the fish Nothobranchius guentheri [149] | Restoration of autophagic flux in muscle cells after palmitate-induced cellular senescence [151] |
| Epigallocatechin gallate | The suppression of SASP in preadipocytes treated by hydrogen peroxide [152] | Activation of autophagy through a CaMKKβ/AMPK-dependent mechanism and support autophagic flux in BAEC [153] |
| Apigenin | Reduction of SASP in BJ cells treated with bleomycin and the kidney of aged rats [154] | Amentoflavone (dimer composed of apigenin) caused induction of autophagy in A549 and WI-38 cells trated by the treatment with insulin- like growth factor-1 (IGF-1) [155] |
| Quercetin | Decreasing of SASP components in BJ cells treated with bleomycin [154] | Upregulation of MST1-mediated autophagy in RAW264.7 macrophages treated by oxidized low-density lipoprotein [156] |
There is a growing body of evidence that polyphenols can inhibit SASP in senescent cells [8,201,237]. For instance, Pitozzi et al. have demonstrated that resveratrol treatment reduced SASP in senescent human fibroblasts [237]. Another study found out that resveratrol decreased senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri [236]. This polyphenol is also able to serve as an autophagy modulator in senescent cells. Thus, it was demonstrated that resveratrol reduces hydrogen peroxide-induced aging through the activation of autophagy in human umbilical vein endothelial cells (HUVECs) [26]. Another recent study showed that resveratrol restored autophagic flux in muscle cells after palmitate-induced cellular senescence [238].
SASP activity was also suppressed by epigallocatechin gallate in pre-adipocytes treated by hydrogen peroxide [239]. In addition, it was also shown that epigallocatechin gallate activates autophagy through a CaMKKβ/AMPK-dependent mechanism and support autophagic flux in primary bovine aortic endothelial cells (BAEC) [240]. Lim et al. demonstrated that apigenin strongly reduced SASP in BJ cells (human foreskin fibroblast) treated with bleomycin [8,241]. Similarly, amentoflavone (dimer composed of apigenin) caused induction of autophagy in A549 and WI-38 cells treated by the treatment with insulin- like growth factor-1 (IGF-1) [242].
Besides that, it was also established that quercetin decreased SASP components in BJ cells treated with bleomycin [8]. Furthermore, the upregulation of MST1-mediated autophagy was detected in RAW264.7 macrophages treated by oxidized low-density lipoprotein [243].
Collectively, these data support a hypothesis that there is an intimate relationship between the regulation of autophagy and SASP, as seen above. Moreover, it is possible that polyphenols targeting SASP via autophagy might have anti-aging effects.
However, the possible negative effects of accelerating autophagy in senescent cells should be considered. As mentioned before, oncogene-induced senescence causes the upregulation of SASP components via TOR-autophagy spatial coupling compartment [198,200]. In recent years, several reports have demonstrated that oncogene-induced senescence is usually observed in benign tumors where it does control tumor growth and transformation [244,245,246,247,248,249,250]. Doppler and Jansen-Durr stressed out that the transformation triggered by the Ras-oncogene can promote metastasis as well as induction of senescence via increased tissue remodeling such as matrix metalloproteases [245]. In another study, Volonte et al. showed that a lack of caveolin-1 expression can suppress oncogenic K-Ras (K-RasG12V)-induced premature senescence in mouse embryonic fibroblasts and normal human bronchial epithelial cells. At the same time, it was found out that oncogenic K-Ras is able to trigger the senescence by limiting the detoxification function of MTH1 [247].
In fact, SASP activation due to autophagy can disrupt benign tumor restriction mechanisms and lead to the cancer development [199,251]. Therefore, the use of polyphenols as activators of autophagy and SASP inhibitors in patients with benign tumors could be dangerous. In this regard, the carcinogenic potential of SASP activation by polyphenols should be thoroughly investigated. Moreover, it must be taken into account the fact that polyphenols in certain population groups may have the opposite effects that might affect the clinical applications.
5. Improved Mitochondrial Function
It has been reported that mitochondrial division can occur in an asymmetric manner [252,253,254]. This results in the formation of one functionally complete mitochondria (which undergoes successive rounds of fusion and division) and another dysfunctional one with a low membrane potential (DJm), which is intended for autophagic destruction [255]. This mechanism demonstrates the importance of autophagy-mitochondrial cell quality control. One prominent hypothesis of aging postulates the accumulation of mitochondrial lesions, leading to progressive bioenergy deficiency with increased production of reactive oxygen species (ROS) [256]. Inhibition of autophagy is known to induce a decrease in mitochondrial function in the cells. For example, in mitochondria isolated from ATG-deficient postmitotic cells (for example, skeletal muscle without Atg7 expression), a defective type of oxidative phosphorylation is detected with a shift in cellular metabolism from respiration to glycolysis [257].
Autophagy can be stimulated through different mechanism and chemical agents. In this case, natural polyphenols, such as resveratrol, can be successfully employed to induce autophagy, and thus, to improve mitochondrial function [258]. Vidoni et al. demonstrated that Similarly, resveratrol, can be employed to speed up the degradation of polyQ huntingtin protein aggregates through the modification of ROS-mediated ATG4 activity [259]. The study showed that resveratrol protects cells expressing mutant huntingtin from dopamine toxicity through recovering ATG4-mediated autophagosome formation [259].
Another polyphenol, epigallocatechin 3-gallate (EGCG), has been intensively studied in context of possible anti-oxidant activity and improvement of mitochondrial function. The reports indicate the EGCG is able to enhance mitochondrial fat utilization and reduce adipogenesis in fat tissue [260,261]. It was also found out that EGCG activates mitochondrial biogenesis and promotes oxidative phosphorylation through a cAMP/PKA- and sirtuin-dependent mechanism [27]. In another study, it was showed that low concentrations of EGCG (10 μM) is capable to stimulate autophagy that leads to the degradation of endotoxin-induced aggregation of high mobility group B-1 (HMGB1) resulting in anti-inflammatory activity [262]. Kim et al. demonstrated that EGCG (dosage 10 μM) can induce autophagy and autophagic flux in endothelial cells leading to the degradation of lipid droplets through a Ca2+/CaMKKβ/AMPK dependent mechanism, and as a result, to the decrease of lipo- toxicity [240].
Filomeni et al. investigated the neuroprotective ability of natural polyphenol kaempferol by autophagy in models of rotenone-mediated acute toxicity [263]. The study demonstrated that kaempferol treatment changed the shape of the mitochondria in the cells indicating that kempferol triggered mitochondrial fission and facilitated its removal by autophagy (‘mitophagy’). The data of the study showed that the formation of the autophago-lysosomes increased after 6 h of drug’s incubation in the neuronal cells.
6. Modulation of Autophagy and Apoptosis by Polyphenols
The first indication that autophagy stimulation may prolong life was obtained from observation of C. elegans, in which inhibition of an insulin-like growth factor accompanied by a growth of autophagy activity leads to an increase in life expectancy [264].
Autophagy can be triggered by different stimuli, including starvation, chemical and mechanical stresses [265]. In fact, calorie restriction (CR) has been considered as the main physiological inducer of autophagy [266,267,268]. It was found out that the inhibition of autophagy prevents the effect of anti-aging CR in all studied species [269,270,271,272]. CR is believed to induce autophagy through AMPK activation and Sirtuin 1 (SIRT1), which are conjugated and involved in the so-called positive mutual activation loop [273]. In addition, it was revealed that CR can trigger the autophagy by inhibiting signaling along the insulin/insulin-like growth factor (IGF) pathway, in which case TOR inhibition also occurs in parallel [274].
How SIRT1 triggers autophagy is not entirely clear yet. SIRT1 is a NAD + -dependent deacetylase acting both in the nucleus and in the cytoplasm [275]. The cytoplasmic variant of SIRT1 is as effective as the SIRT1 nucleus, this explains, for example, the induction of autophagy using resveratrol under conditions of cell enucleation [276]. Accordingly, if SIRT1 deacetylates several protein products of the gene (ATG5, ATG7 and ATG8/LC3) [49], then resveratrol induces deacetylation of more than a dozen cytoplasmic proteins. SIRT1 also deacetylates transcription factors p53, NF-kB, HSF1, FOXO1, 3, −4, and PGC1a, for which effects on life span control are known [277].
Autophagy plays an important role in organelle protein homeostasis. This role is especially important in non-proliferating cells, because unlike cells in the mitosis phase, there is no “dilution” of intracellular debris during division. In addition, the anti-aging effects of cyto-protection are especially important for the cells that are not targets of stem cells. Aggregation of intracellular proteins are distinctive features of many neurodegenerative diseases called ‘proteopathies’ [278,279,280], including Alzheimer’s and Parkinson’s diseases. It is known that a number of experimental autophagy inducers, such as rapamycin, rapalogs, valproate and lithium, can weaken the accumulation of mutated protein and reduce the possibility of cell death [281,282,283,284].
In fact, autophagy can increase the body’s viability by inhibiting cell death, reducing the risk of oncogenic transformation, or increasing hormesis, both in dormant and dividing cells. In addition, autophagy can contribute to an increase in longevity through various mechanisms in post-mitotic and proliferating cells [285,286,287].
One of the well-studied natural polyphenol is quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone). There is a number of reports on the modulation of autophagy by quercetin [288,289,290,291]. It has been shown that quercetin is capable to inhibit of formation of reactive oxygen species (ROS) [292,293,294], modulating sirtuins [295,296,297,298], JNK/P38 MAPK signaling activation [299,300], and modulation of PI3K/Akt pathway [301,302,303]. Tsai et al. demonstrated that quercetin is capable of triggering autophagy, proved by the increased processing of specific marker protein of autophagy (LC3-II) [290]. Moreover, it was also found out that the pretreatment of autophagy inhibitors, Baf A1 and chloroquine, significantly induced apoptosis in 5637 and T24 cells. In another in vivo study conducted by Cao et al., the protective effect of quercetin on atherosclerosis was demonstrated [288]. The study demonstrated that quercetin triggered autophagy leading to the alleviation of atherosclerosis lesions, reduction of lipid accumulation in aortic roots and levels of TC and LDL-C, as well as the expression levels of TNF-α, IL-1β and IL-18.
The modulation of autophagy by resveratrol has been intensively investigated in the last decades [150,304,305,306,307,308]. In the recent study, Gong and Xia showed that treatment melanoma cells with resveratrol led to the upregulation of proteins associated with autophagy (Beclin 1 and microtubule-associated protein 1A/1B-light chain 3 (LC3)-II/I), while the p62 expression was downregulated [309]. The results of the study indicate resveratrol can suppress the viability and migration of melanoma cells through inhibiting the AKT/mTOR pathway by triggering the autophagy. Park et al. found out that resveratrol induces autophagy by directly inhibiting the mTOR-ULK1 pathway [310]. The researchers showed that the inhibition of mTOR activity and presence of ULK1 are required for autophagy induction by resveratrol. It was revealed that resveratrol suppresses mTOR by docking onto the ATP-binding pocket of mTOR.
Yang and co-workers studied the role of SIRT1 in autophagy in osteoblasts through PI3K/Akt signaling pathway in osteoporotic rats treated by resveratrol [311]. The data if the study demonstrated that treatment with resveratrol led to an increase in the expression of SIRT1, LC3, and Beclin-1, whilst p-AKT and p-mTOR were downregulated. Moreover, resveratrol treatment elevated the SIRT1 activity, LC3 and Beclin-1 mRNA expression in the dexamethasone (DEX)-treated osteoblasts. The results indicated that resveratrol is able to protect osteoblasts through the enhancement of autophagy by modulating SIRT1 and PI3K/AKT/mTOR signaling pathway.
Du et al. scrutinized the ability of resveratrol to delay cellular aging through the upregulation of autophagy by using cell model (human umbilical endothelial vein cells) [26]. It was showed that resveratrol treatment led to the suppression of the high rate of senescence-associated β-galactosidase and increased intracellular ROS levels induced by H2O2. It was also found out that resveratrol was able to upregulate autophagy via the regulation of p-Rb, LC3, and p62 levels. Moreover, the anti-aging activity of resveratrol through an autophagy regulation mechanism was confirmed by the suppression of these effects with 3-MA treatment.
Apoptosis, ‘programmed cell death’, plays a vital role in a range of important processes during fetal development and physiological processes [312,313]. Moreover, it was found out that the breakdown of apoptotic machinery contributes to various disorders that are associated with cell accumulation (cancer) or cell loss (ischemia, neurodegeneration, AIDS) [312]. The specific features of apoptosis are chromatin condensation, nuclear shrinkage and production of apoptosis ‘body’. It has been thought that the manifestation of apoptosis occurs through two pathways: intrinsic (mitochondrial) and extrinsic [314,315]. The intrinsic pathway is characterized by the interplay between anti-apoptotic proteins (Bcl-2, Bcl-XL, Mcl-1, Bcl-W, Bfl-1) and pro-apoptotic proteins Bax and Bak [316,317,318]. The extrinsic apoptosis pathway has been associated with the activation of the death receptors that bind to the ligands (tumor necrosis factor, TNF) on the cell surface, and thus, initiating apoptosis signaling cascade.
The links between autophagy and apoptosis are complex and still poorly understood [319]. It was revealed that the main proteins involved apoptosis cascades play a crucial role in autophagy reactions as well. For instance, Bcl-2 family proteins are responsible for intrinsic apoptotic pathway through releasing cytochrome c from the mitochondria. At the same time, Bcl-2 binds to Bcl-1 leading to the inhibition of autophagic response [320,321].
It has been revealed that sufficient nutrition suppresses the activation both autophagy and apoptosis [315,322]. Inversely, starvation leads to triggering of autophagy, through the activation of C-Jun N-terminal protein kinase 1 (JNK1) and phosphorylation of Bcl-2 family [323]. As a result, phosphorylated Bcl-2 combines with Bax protein, thus suppressing apoptosis and preserving the mitochondrial membrane completeness [315]. But in the situation of extreme starvation, JNK1 promotes hyper-phosphorylation of Bcl-2 leading to the its detachment from Bax proteins and triggering apoptosis and cell death [324].
It must be noted that both induction of autophagy and apoptosis lead to cell death, but through different and interrelated molecular mechanisms. From therapeutic point of view, the triggering of cell death via autophagy can be considered as an alternative to programmed cell death (apoptosis), particularly for destroying malignant cells that intrinsically resistant to apoptosis [325]. To address this problem, the modulation of autophagy by chemical and natural compounds was a subject of numerous studies [326,327,328]. It has been showed that polyphenols such as quercetin, curcumin, and resveratrol are capable of inducing autophagy-associated cell death through the canonical (Bcl-1 dependent) and non-canonical (Bcl-1 independent) pathways of autophagy [329].
Interestingly, it has been shown that resveratrol is able to modulate both autophagy and apoptosis [330,331,332,333,334]. For instance, Xu et al. demonstrated that resveratrol can protect cardiac cells through the modulation of the switch between autophagy and apoptotic processes under diabetic conditions associated with AMPK-mediated phosphorylation of mTORC1/p70S6K1/4EBP1 and JNK-mediated dissociation of Beclin1-Bcl-2 [332]. The authors of the study proposed that autophagy can be a target for resveratrol in the treatment of diabetic cardiomyopathy. In another study of resveratrol activity, Fan et al. showed that resveratrol-mediated apoptosis manifests via both the intrinsic and extrinsic apoptotic pathways [333]. It was revealed that resveratrol induced an increase of mitochondrial membrane potential and apoptosis-related markers (Bax/Bcl-2). In addition, it was found out that resveratrol increases the levels of microtubule-associated protein 1 light chain 3-II and the number of autophagosomes. Moreover, it was also demonstrated that resveratrol-induced autophagy depends on the LKB1-AMPK-mTOR pathway. The findings indicated that resveratrol-induced programmed cell death of HL-60 cells depends on the autophagy activated via both the LKB1-AMPK and PI3K/AKT-regulated mTOR signaling pathways [333].
Recently, Wang et al. showed that resveratrol is able to trigger SIRT1-dependent autophagy to prevent H2O2-induced oxidative stress and apoptosis in HTR8/SVneo cells [305]. The results demonstrated that resveratrol treatment significantly neutralized H2O2-induced cytotoxicity, morphological damage, oxidative stress and apoptosis. It was revealed that resveratrol restored the levels of SIRT1 and autophagy-related proteins including LC3-II, Beclin-1 and p62 that were dysregulated by hydrogen peroxide. In another work, Guo and co-workers reported on the ability of resveratrol of activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway [335]. The results of Western blot analysis demonstrated that the expression of Beclin-1, LC3-II, LC3-II/LC3-I, and Bcl-2 was increased in resveratrol-treated rats, whilst the expression of p-Akt, p-mTOR, p62, cleaved caspase-3, caspase-9, and Bcl-2-associated X protein was decreased.
7. Conclusions
In the range of studies, it has been demonstrated that caloric restriction improves the longevity and adjourn the development of age-related disorders [336,337]. It was also shown that caloric restriction triggers many molecular processes in the cell, including autophagy [25,338,339]. Autophagy is a vital part of cell activity by being responsible for providing energy, utilization of redundant products of cell metabolism, and modulation of the response on oxidative stress [340]. It is thought that autophagy decreases during the aging and that can lead to the development of aging-associated diseases such as cancer, diabetes, neurodegeneration, etc. Taking into account the role of autophagy in the prevention of age-related conditions, caloric restriction as an inducer of autophagy has been considered as a possible therapeutic approach during the last decades [341,342,343].
Apart from caloric restriction, autophagy can be induced by mechanical or chemical stress. In this regard, various pharmacological compounds have been proposed and studied. This approach facilitates the control and safety of autophagy induction and minimizes the suffering of the patients from the strict diet and fasting. In fact, natural polyphenols demonstrated a low toxicity and the absence of severe adverse effects [340,344]. In addition to the ability to induce autophagy, it was also shown that polyphenols, such as resveratrol, are capable to modulate the expression of pro- and anti-apoptotic factors, neutralizing free radical species, affecting mitochondrial functions, chelating redox-active transition metal ions, and preventing protein aggregation [345].
Nevertheless, it must be also noted that the polyphenols are largely metabolized so the results of in vitro studies may not reflect the real biological situations [346,347]. The main critical point is polyphenols absorption in the gastrointestinal tract and liver metabolism that affects their bioavailability [348,349]. In fact, the current knowledge about polyphenols plasma concentration and the half-life is incomplete and requires further intensive studies [346]. Moreover, the individual differences in gut microbiome and pharmacokinetics must be also taken into consideration. All the above-mentioned factors may affect the therapeutic impact and anti-aging potential of natural polyphenols in humans.
To summarize, polyphenols have some advantages compared to chemical inducers of autophagy due to their intrinsic natural bio-compatibility and safety. In this context, polyphenols can be considered as a potential therapeutic tool for healthy aging either as a part of a diet or as separate compounds (supplements). However, the clinical effectiveness and potential toxicity of high-dose polyphenol intake need to be thoroughly investigated and validated in the close future.
Author Contributions
Conceptualization, A.Y. and T.S.; writing—original draft preparation, A.Y., T.S., M.Z., B.U. and S.S., writing—review and editing, T.S., A.-R.M., E.K., A.G., and T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant of the Ministry of Education and Science of the Republic of Kazakhstan № BR05236508-OT-18. The work was also supported through the grant of the Ministry of Education and Science of the Republic of Kazakhstan № 0118РК01029.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Kirkwood T.B.L. Why and how are we living longer? Exp. Physiol. 2017;102:1067–1074. doi: 10.1113/EP086205. [DOI] [PubMed] [Google Scholar]
- 2.Menotti A., Puddu P.E., Lanti M., Maiani G., Catasta G., Fidanza A.A. Lifestyle habits and mortality from all and specific causes of death: 40-year follow-up in the italian rural areas of the seven countries study. J. Nutr. Health Aging. 2014;18:314–321. doi: 10.1007/s12603-013-0392-1. [DOI] [PubMed] [Google Scholar]
- 3.St Sauver J.L., Boyd C.M., Grossardt B.R., Bobo W.V., Rutten L.J.F., Roger V.L., Ebbert J.O., Therneau T.M., Yawn B.P., Rocca W.A. Risk of developing multimorbidity across all ages in an historical cohort study: Differences by sex and ethnicity. BMJ Open. 2015;5:e006413. doi: 10.1136/bmjopen-2014-006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi C., Bonafè M. Centenarians as a model for healthy aging. Biochem. Soc. Trans. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy B.K., Berger S.L., Brunet A., Campisi J., Cuervo A.M., Epel E.S., Franceschi C., Lithgow G.J., Morimoto R.I., Pessin J.E., et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seals D.R., Melov S. Translational Geroscience: Emphasizing function to achieve optimal longevity. Aging. 2014;6:718–730. doi: 10.18632/aging.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana L., Partridge L., Longo V.D. Extending healthy life span-from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mária J., Ingrid Ž. Effects of bioactive compounds on senescence and components of senescence associated secretory phenotypes in vitro. Food Funct. 2017;8:2394–2418. doi: 10.1039/C7FO00161D. [DOI] [PubMed] [Google Scholar]
- 9.Vaiserman A.M., Lushchak O.V., Koliada A.K. Anti-aging pharmacology: Promises and pitfalls. Ageing Res. Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Santarelli V., Neri L., Sacchetti G., Di Mattia C.D., Mastrocola D., Pittia P. Response of organic and conventional apples to freezing and freezing pre-treatments: Focus on polyphenols content and antioxidant activity. Food Chem. 2020;308:125570. doi: 10.1016/j.foodchem.2019.125570. [DOI] [PubMed] [Google Scholar]
- 11.Kourouma V., Mu T.H., Zhang M., Sun H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020;55:369–378. doi: 10.1111/ijfs.14336. [DOI] [Google Scholar]
- 12.Bermudez-Oria A., Rodriguez-Gutierrez G., Alaiz M., Vioque J., Giron-Calle J., Fernandez-Bolanos J. Polyphenols associated to pectic polysaccharides account for most of the antiproliferative and antioxidant activities in olive extracts. J. Funct. Foods. 2019;62:103530. doi: 10.1016/j.jff.2019.103530. [DOI] [Google Scholar]
- 13.Liu W.F., Wang Z.Y., Xia Y., Kuang H.Y., Liu S.P., Li L., Tang C.F., Yin D.Z. The balance of apoptosis and autophagy via regulation of the AMPK signal pathway in aging rat striatum during regular aerobic exercise. Exp. Gerontol. 2019;124:110647. doi: 10.1016/j.exger.2019.110647. [DOI] [PubMed] [Google Scholar]
- 14.Erdman V.V., Nasibullin T.R., Tuktarova I.A., Somova R.S., Mustafina O.E. Association Analysis of Polymorphic Gene Variants in the JAK/STAT Signaling Pathway with Aging and Longevity. Russ. J. Genet. 2019;55:728–737. doi: 10.1134/S1022795419050077. [DOI] [Google Scholar]
- 15.Brown B.A., Connolly G.M., Mill C.E.J., Williams H., Angelini G.D., Johnson J.L., George S.J. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell. 2019;18:e12844. doi: 10.1111/acel.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwierello W., Maruszewska A., Skorka-Majewicz M., Goschorska M., Baranowska-Bosiacka I., Dec K., Styburski D., Nowakowska A., Gutowska I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere. 2020;240:124901. doi: 10.1016/j.chemosphere.2019.124901. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y.H., Zhang T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules. 2019;24:816. doi: 10.3390/molecules24040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Bo’ C., Bernardi S., Marino M., Porrini M., Tucci M., Guglielmetti S., Cherubini A., Carrieri B., Kirkup B., Kroon P., et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients. 2019;11:1355. doi: 10.3390/nu11061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S., Sharpless N.E. Senescence in Health and Disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristofalo V.J., Lorenzini A., Allen R.G., Torres C., Tresini M. Replicative senescence: A critical review. Mech. Ageing Dev. 2004;125:827–848. doi: 10.1016/j.mad.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toussaint O., Medrano E.E., Von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 23.Thakur V.S., Gupta K., Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis. 2012;33:377–384. doi: 10.1093/carcin/bgr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azami S.H., Nazarian H., Abdollahifar M.A., Allahbakhshian-Farsani M., Banihosseini S.Z., Novin M.G. Curcumin Delays Oocyte Apoptosis Through Overexpression of BCL-2 Gene in Young and Middle-Aged Mouse Models. Int. J. Womens Health. 2020;8:53–60. [Google Scholar]
- 25.Mariño G., Pietrocola F., Madeo F., Kroemer G. Caloric restriction mimetics: Natural/physiological pharmacological autophagy inducers. Autophagy. 2014;10:1879–1882. doi: 10.4161/auto.36413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du L.G., Chen E.P., Wu T., Ruan Y.J., Wu S.Z. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Dev. Ther. 2019;13:747–755. doi: 10.2147/DDDT.S179894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenti D., De Rasmo D., Signorile A., Rossi L., de Bari L., Scala I., Granese B., Papa S., Vacca R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta. 2013;1832:542–552. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Deseo M.A., Elkins A., Rochfort S., Kitchen B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020;314:126180. doi: 10.1016/j.foodchem.2020.126180. [DOI] [PubMed] [Google Scholar]
- 29.Curti V., Zaccaria V., Tsetegho Sokeng A.J., Dacrema M., Masiello I., Mascaro A., D’Antona G., Daglia M. Bioavailability and In Vivo Antioxidant Activity of a Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019;20:1250. doi: 10.3390/ijms20051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffredo L., Perri L., Nocella C., Violi F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharmacol. 2017;83:96–102. doi: 10.1111/bcp.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskandari M., Rembiesa J., Startaite L., Holefors A., Valanciute A., Faridbod F., Ganjali M.R., Engblom J., Ruzgas T. Polyphenol-hydrogen peroxide reactions in skin: In vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta. 2019;1075:91–97. doi: 10.1016/j.aca.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Grigorakis S., Makris D.P. Characterisation of Polyphenol-Containing Extracts from Stachys mucronata and Evaluation of Their Antiradical Activity. Medicines. 2018;5:14. doi: 10.3390/medicines5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.San Miguel S.M., Opperman L.A., Allen E.P., Zielinski J., Svoboda K.K. Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch. Oral Biol. 2012;57:1657–1667. doi: 10.1016/j.archoralbio.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Yahfoufi N., Alsadi N., Jambi M., Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Musarra-Pizzo M., Ginestra G., Smeriglio A., Pennisi R., Sciortino M.T., Mandalari G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients. 2019;11:2355. doi: 10.3390/nu11102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catel-Ferreira M., Tnani H., Hellio C., Cosette P., Lebrun L. Antiviral effects of polyphenols: Development of bio-based cleaning wipes and filters. J. Virol. Methods. 2015;212:1–7. doi: 10.1016/j.jviromet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Mao L.M., Hochstetter D., Yao L.Y., Zhao Y.L., Zhou J.H., Wang Y.F., Xu P. Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019;20:5081. doi: 10.3390/ijms20205081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pons-Fuster Lopez E., Gomez Garcia F., Lopez Jornet P. Combination of 5-Florouracil and polyphenol EGCG exerts suppressive effects on oral cancer cells exposed to radiation. Arch. Oral Biol. 2019;101:8–12. doi: 10.1016/j.archoralbio.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Tiwari M., Dixit B., Parvez S., Agrawala P.K. EGCG, a tea polyphenol, as a potential mitigator of hematopoietic radiation injury in mice. Biomed. Pharmacother. 2017;88:203–209. doi: 10.1016/j.biopha.2016.12.129. [DOI] [PubMed] [Google Scholar]
- 41.Lee S.K., Kim J.H., Kim J.S., Jang Y., Kim J., Park Y.H., Chun K.J., Lee M.Y. Polyphenol (-)-epigallocatechin gallate-induced cardioprotection may attenuate ischemia-reperfusion injury through adenosine receptor activation: A preliminary study. Korean J. Anesthesiol. 2012;63:340–345. doi: 10.4097/kjae.2012.63.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagi S., Matsumura K., Marui A., Morishima M., Hyon S.H., Ikeda T., Sakata R. Oral pretreatment with a green tea polyphenol for cardioprotection against ischemia-reperfusion injury in an isolated rat heart model. J. Thorac. Cardiovasc. Surg. 2011;141:511–517. doi: 10.1016/j.jtcvs.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Campisi J., Andersen J.K., Kapahi P., Melov S. Cellular senescence: A link between cancer and age-related degenerative disease? Semin. Cancer Biol. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strehler B.L. Aging Methods and Protocols. Volume 38. Humana Press; Totowa, NJ, USA: 2000. Understanding Aging; pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 46.Dodig S., Čepelak I., Pavić I. Hallmarks of senescence and aging. Biochem. Medica. 2019;29:483–497. doi: 10.11613/BM.2019.030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang A.S., Dreesen O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018;9:247. doi: 10.3389/fgene.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.In H.L., Cao L., Mostoslavsky R., Lombard D.B., Liu J., Bruns N.E., Tsokos M., Alt F.W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Severino J., Allen R.G., Balin S., Balin A., Cristofalo V.J. Is β-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 51.Xu S., Cai Y., Wei Y. mTOR signaling from cellular senescence to organismal aging. Aging Dis. 2014;5:263–273. doi: 10.14336/AD.2014.0500263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narita M., Nũnez S., Heard E., Narita M., Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- 53.Chung K.W., Kim D.H., Park M.H., Choi Y.J., Kim N.D., Lee J., Yu B.P., Chung H.Y. Recent advances in calorie restriction research on aging. Exp. Gerontol. 2013;48:1049–1053. doi: 10.1016/j.exger.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Eisenberg T., Schroeder S., Andryushkova A., Pendl T., Kuttner V., Bhukel A., Marino G., Pietrocola F., Harger A., Zimmermann A., et al. Nucleocytosolic Depletion of the Energy Metabolite Acetyl-Coenzyme A Stimulates Autophagy and Prolongs Lifespan. Cell Metab. 2014;19:431–444. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aboalroub A.A., Bachman A.B., Zhang Z.M., Keramisanou D., Merkler D.J., Gelis I. Acetyl group coordinated progression through the catalytic cycle of an arylalkylamine N-acetyltransferase. PLoS ONE. 2017;12:e0177270. doi: 10.1371/journal.pone.0177270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sienkiewicz N., Czlonka S., Kairyte A., Vaitkus S. Curcumin as a natural compound in the synthesis of rigid polyurethane foams with enhanced mechanical, antibacterial and anti-ageing properties. Polym. Test. 2019;79:106046. doi: 10.1016/j.polymertesting.2019.106046. [DOI] [Google Scholar]
- 57.Yamanaka D., Kawano T., Nishigaki A., Aoyama B., Tateiwa H., Shigematsu-Locatelli M., Locatelli F.M., Yokoyama M. Effects of epigallocatechin-3-gallate on systemic inflammation-induced cognitive dysfunction in aged rats. J. Anesth. 2017;31:726–735. doi: 10.1007/s00540-017-2392-5. [DOI] [PubMed] [Google Scholar]
- 58.Maglione M., Kochlamazashvili G., Eisenberg T., Racz B., Michael E., Toppe D., Stumpf A., Wirth A., Zeug A., Muller F.E., et al. Spermidine protects from age-related synaptic alterations at hippocampal mossy fiber-CA3 synapses. Sci. Rep. 2019;9:19616. doi: 10.1038/s41598-019-56133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madeo F., Carmona-Gutierrez D., Kepp O., Kroemer G. Spermidine delays aging in humans. Aging. 2018;10:2209–2211. doi: 10.18632/aging.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou J.L., Xue Z.Y.Y., He H.N., Liu X., Yin S.Y., Wu D.Y., Zhang X., Schatten H., Miao Y.L. Resveratrol delays postovulatory aging of mouse oocytes through activating mitophagy. Aging. 2019;11:11504–11519. doi: 10.18632/aging.102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farhadnejad H., Emamat H., Zand H. The Effect of Resveratrol on Cellular Senescence in Normal and Cancer Cells: Focusing on Cancer and Age-Related Diseases. Nutr. Cancer. 2019;71:1175–1180. doi: 10.1080/01635581.2019.1597907. [DOI] [PubMed] [Google Scholar]
- 62.Goldberg A.D., Allis C.D., Bernstein E. Epigenetics: A landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Holliday R. Epigenetics comes of age in the twentyfirst century. J. Genet. 2002;81:1–4. doi: 10.1007/BF02715863. [DOI] [PubMed] [Google Scholar]
- 64.Holliday R. Mechanisms for the Control of Gene Activity during Development. Biol. Rev. 1990;65:431–471. doi: 10.1111/j.1469-185X.1990.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 65.Ueda M., Seki M. Histone Modifications Form Epigenetic Regulatory Networks to Regulate Abiotic Stress Response. Plant Physiol. 2020;182:15–26. doi: 10.1104/pp.19.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang Z., Riaz A., Chachar S., Ding Y.K., Du H., Gu X.F. Epigenetic Modifications of mRNA and DNA in Plants. Mol. Plant. 2020;13:14–30. doi: 10.1016/j.molp.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Dasinger J.H., Alsheikh A.J., Abais-Battad J.M., Pan X.Q., Fehrenbach D.J., Lund H., Roberts M.L., Cowley A.W., Kidambi S., Kotchen T.A., et al. Epigenetic Modifications in T Cells The Role of DNA Methylation in Salt-Sensitive Hypertension. Hypertension. 2020;75:372–382. doi: 10.1161/HYPERTENSIONAHA.119.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z.B., Shilatifard A. Epigenetic modifications of histones in cancer. Genome Biol. 2019;20:245. doi: 10.1186/s13059-019-1870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schellong K., Melchior K., Ziska T., Henrich W., Rancourt R.C., Plagemann A. Sex-specific epigenetic alterations of the hypothalamic Agrp-Pomc system do not explain ‘diabesity’ in the offspring of high-fat diet (HFD) overfed maternal rats. J. Nutr. Biochem. 2020;75:108257. doi: 10.1016/j.jnutbio.2019.108257. [DOI] [PubMed] [Google Scholar]
- 70.Contreras R.E., Schriever S.C., Pfluger P. Physiological and Epigenetic Features of Yoyo Dieting and Weight Control. Front. Genet. 2019;10:1015. doi: 10.3389/fgene.2019.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quach A., Levine M.E., Tanaka T., Lu A.T., Chen B.H., Ferrucci L., Ritz B., Bandinelli S., Neuhouser M.L., Beasley J.M., et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–446. doi: 10.18632/aging.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bultman S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017;61:1500902. doi: 10.1002/mnfr.201500902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyun T.K. Roles of polyphenols as dietary epigenetic modulators. Minerva Biotecnol. 2019;31:74–75. doi: 10.23736/S1120-4826.19.02522-9. [DOI] [Google Scholar]
- 74.Lewandowska P., Wozniak K. Effect of Natural Polyphenols on Epigenetic Mechanisms of Gene Expression. Postep. Biol. Komorki. 2017;44:213–225. [Google Scholar]
- 75.Yang P.L., He X.J., Malhotra A. Epigenetic Targets of Polyphenols in Cancer. J. Environ. Pathol. Toxicol. Oncol. 2014;33:159–165. doi: 10.1615/JEnvironPatholToxicolOncol.2014011094. [DOI] [PubMed] [Google Scholar]
- 76.Singla R.K., Dubey A.K., Garg A., Sharma R.K., Fiorino M., Ameen S.M., Haddad M.A., Al-Hiary M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019;102:1397–1400. doi: 10.1093/jaoac/102.5.1397. [DOI] [PubMed] [Google Scholar]
- 77.Scalbert A., Perez-Jimenez J., Rothwell J., Touvier M., Feuzeu L., Galan P. Abstracts of Papers of the American Chemical Society. Volume 240 American Chemical Society; Washington, DC, USA: 2010. Fine chemical structures of dietary polyphenols and their importance in understanding their role in the prevention of diseases. [Google Scholar]
- 78.Zhang C., Li Y.N., Liu L.L., Gong Y., Xie Y.X., Cao Y. Chemical Structures of Polyphenols That Critically Influence the Toxicity of ZnO Nanoparticles. J. Agric. Food Chem. 2018;66:1714–1722. doi: 10.1021/acs.jafc.8b00368. [DOI] [PubMed] [Google Scholar]
- 79.Cutrim C.S., Cortez M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018;71:564–578. doi: 10.1111/1471-0307.12515. [DOI] [Google Scholar]
- 80.Ruskovska T., Maksimova V., Milenkovic D. Polyphenols in human nutrition: From the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020;123:241–254. doi: 10.1017/S0007114519002733. [DOI] [PubMed] [Google Scholar]
- 81.Cherubim D.J.D., Martins C.V.B., Farina L.O., de Lucca R.A.D. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020;19:33–37. doi: 10.1111/jocd.13093. [DOI] [PubMed] [Google Scholar]
- 82.Quan T.H., Benjakul S., Sae-leaw T., Balange A.K., Maqsood S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019;91:507–517. doi: 10.1016/j.tifs.2019.07.049. [DOI] [Google Scholar]
- 83.Alcalde B., Granados M., Saurina J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants. 2019;8:523. doi: 10.3390/antiox8110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Lan M., Lu J.P., Li J.F., Zhang K.Y., Zhi H., Zhang H., Sun J.M. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus rutilus. Chem. Biodivers. 2020;17:e1900479. doi: 10.1002/cbdv.201900479. [DOI] [PubMed] [Google Scholar]
- 85.Oliviero F., Scanu A., Zamudio-Cuevas Y., Punzi L., Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018;98:1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- 86.Braicu C., Calin G., Berindan-Neagoe I. MicroRNAs and Cancer Therapy—From Bystanders to Major Players. Curr. Med. Chem. 2013;20:3561–3573. doi: 10.2174/0929867311320290002. [DOI] [PubMed] [Google Scholar]
- 87.Catana C.S., Pichler M., Giannelli G., Mader R.M., Berindan-Neagoe I. Non-coding RNAs, the Trojan horse in two-way communication between tumor and stroma in colorectal and hepatocellular carcinoma. Oncotarget. 2017;8:29519–29534. doi: 10.18632/oncotarget.15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braakhuis A.J., Campion P., Bishop K.S. Reducing breast cancer recurrence: The role of dietary polyphenolics. Nutrients. 2016;8:547. doi: 10.3390/nu8090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabande-Rodriguez E., de las M Gómez Heras M., Mittelbrunn M. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells. 2019;9:82. doi: 10.3390/cells9010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Josifovska N., Albert R., Nagymihaly R., Lytvynchuk L., Moe M.C., Kaarniranta K., Vereb Z.J., Petrovski G. Resveratrol as Inducer of Autophagy, Pro-Survival, and Anti-Inflammatory Stimuli in Cultured Human RPE Cells. Int. J. Mol. Sci. 2020;21:813. doi: 10.3390/ijms21030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serini S., Cassano R., Facchinetti E., Amendola G., Trombino S., Calviello G. Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients. 2019;11:1400. doi: 10.3390/nu11061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lomholt S., Mellemkjaer A., Iversen M.B., Pedersen S.B., Kragstrup T.W. Resveratrol displays anti-inflammatory properties in an ex vivo model of immune mediated inflammatory arthritis. BMC Rheumatol. 2018;2:27. doi: 10.1186/s41927-018-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jia R., Li Y., Cao L., Du J., Zheng T., Qian H., Gu Z., Jeney G., Xu P., Yin G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;215:56–66. doi: 10.1016/j.cbpc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 94.de Sa Coutinho D., Pacheco M.T., Frozza R.L., Bernardi A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018;19:1812. doi: 10.3390/ijms19061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tili E., Michaille J.J. Promiscuous Effects of Some Phenolic Natural Products on Inflammation at Least in Part Arise from Their Ability to Modulate the Expression of Global Regulators, Namely microRNAs. Molecules. 2016;21:1263. doi: 10.3390/molecules21091263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kocic H., Damiani G., Stamenkovic B., Tirant M., Jovic A., Tiodorovic D., Peris K. Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther. Adv. Chronic Dis. 2019;10:2040622319864805. doi: 10.1177/2040622319864805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lancon A., Michaille J.J., Latruffe N. Effects of dietary phytophenols on the expression of microRNAs involved in mammalian cell homeostasis. J. Sci. Food Agric. 2013;93:3155–3164. doi: 10.1002/jsfa.6228. [DOI] [PubMed] [Google Scholar]
- 98.Li Y.G., Zhu W., Tao J.P., Xin P., Liu M.Y., Li J.B., Wei M. Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem. Biophys. Res. Commun. 2013;438:270–276. doi: 10.1016/j.bbrc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 99.Denu J.M. Fortifying the Link between SIRT1, Resveratrol, and Mitochondrial Function. Cell Metab. 2012;15:566–567. doi: 10.1016/j.cmet.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sundaram M.K., Ansari M.Z., Al Mutery A., Ashraf M., Nasab R., Rai S., Rais N., Hussain A. Genistein Induces Alterations of Epigenetic Modulatory Signatures in Human Cervical Cancer Cells. Anti-Cancer Agents Med. Chem. 2018;18:412–421. doi: 10.2174/1871520617666170918142114. [DOI] [PubMed] [Google Scholar]
- 102.Carlos-Reyes A., Lopez-Gonzalez J.S., Meneses-Flores M., Gallardo-Rincon D., Ruiz-Garcia E., Marchat L.A., Astudillo-de la Vega H., de la Cruz O.N.H., Lopez-Camarillo C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019;10:79. doi: 10.3389/fgene.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kala R., Shah H.N., Martin S.L., Tollefsbol T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer. 2015;15:672. doi: 10.1186/s12885-015-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medina-Aguilar R., Perez-Plasencia C., Marchat L.A., Gariglio P., Mena J.G., Cuevas S.R., Ruiz-Garcia E., Astudillo-de la Vega H., Juarez J.H., Flores-Perez A., et al. Methylation Landscape of Human Breast Cancer Cells in Response to Dietary Compound Resveratrol. PLoS ONE. 2016;11:e0157866. doi: 10.1371/journal.pone.0157866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dhar S., Kumar A., Li K., Tzivion G., Levenson A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. BBA-Mol. Cell Res. 2015;1853:265–275. doi: 10.1016/j.bbamcr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Das S., Sarmah S., Hazarika Z., Rohman M.A., Sarkhel P., Jha A.N., Roy A.S. Targeting the heme protein hemoglobin by (-)-epigallocatechin gallate and the study of polyphenol-protein association using multi-spectroscopic and computational methods. Phys. Chem. Chem. Phys. 2020;22:2212–2228. doi: 10.1039/C9CP05301H. [DOI] [PubMed] [Google Scholar]
- 107.Krupkova O., Handa J., Hlavna M., Klasen J., Ospelt C., Ferguson S.J., Wuertz-Kozak K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid. Med. Cell. Longev. 2016;2016:7031397. doi: 10.1155/2016/7031397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shukla S., Meeran S.M., Katiyar S.K. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014;355:9–17. doi: 10.1016/j.canlet.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giudice A., Montella M., Boccellino M., Crispo A., D’Arena G., Bimonte S., Facchini G., Ciliberto G., Botti G., Quagliuolo L., et al. Epigenetic Changes Induced by Green Tea Catechins are Associated with Prostate Cancer. Curr. Mol. Med. 2017;17:405–420. doi: 10.2174/1566524018666171219101937. [DOI] [PubMed] [Google Scholar]
- 110.Bimonte S., Albino V., Piccirillo M., Nasto A., Molino C., Palaia R., Cascella M. Epigallocatechin-3-gallate in the prevention and treatment of hepatocellular carcinoma: Experimental findings and translational perspectives. Drug Des. Dev. Ther. 2019;13:611–621. doi: 10.2147/DDDT.S180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y., Yuan Y.-Y., Meeran S.M., Tollefsbol T.O. Synergistic epigenetic reactivation of estrogen receptor-alpha (ERalpha) by combined green tea polyphenol and histone deacetylase inhibitor in ERalpha-negative breast cancer cells. Mol. Cancer. 2010;9:274. doi: 10.1186/1476-4598-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sundaram M.K., Hussain A., Haque S., Raina R., Afroze N. Quercetin modifies 5 CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019;120:18357–18369. doi: 10.1002/jcb.29147. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez M.C., Maso V., Torello C.O., Ferro K.P., Saad S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenet. 2018;10:139. doi: 10.1186/s13148-018-0563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Babulogaiah S., Ponnusamy K., Kumar S.S., Naidu J.R. Neuroprotective Epigenetic and DNA-Repairing Molecular Mechanisms of L-Carnitine and Quercetin against Middle Cerebral Artery Occlusion in Aged Rats. Int. J. Stroke. 2016;11:190. [Google Scholar]
- 115.Jones A., Taylor E., Henagan T. Epigenetic Regulation of Pgc1 alpha Splice Variants in Response to Dietary Quercetin Supplementation. FASEB J. 2015;29:958. [Google Scholar]
- 116.Sharma V., Kumar L., Mohanty S.K., Maikhuri J.P., Rajender S., Gupta G. Sensitization of androgen refractory prostate cancer cells to anti androgens through re-expression of epigenetically repressed androgen receptor—Synergistic action of quercetin and curcumin. Mol. Cell. Endocrinol. 2016;431:12–23. doi: 10.1016/j.mce.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 117.Robison L.S., Albert N.M., Camargo L.A., Anderson B.M., Salinero A.E., Riccio D.A., Abi-Ghanem C., Gannon O.J., Zuloaga K.L. High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice. eNeuro. 2020;7 doi: 10.1523/ENEURO.0391-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klein C., Jonas W., Wiedmer P., Schreyer S., Akyuz L., Spranger J., Hellweg R., Steiner B. High-fat Diet and Physical Exercise Differentially Modulate Adult Neurogenesis in the Mouse Hypothalamus. Neuroscience. 2019;400:146–156. doi: 10.1016/j.neuroscience.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 119.Saha M., Wang Z.L., Kulkarni S., Pasricha P.J. The Effects of Diet-Induced Obesity on Myenteric Neurogenesis, Neural Differentiation and Motility in the Adult Small Intestine. Gastroenterology. 2018;154:S54. doi: 10.1016/S0016-5085(18)30643-7. [DOI] [Google Scholar]
- 120.Stankiewicz A.J., McGowan E.M., Yu L.L., Zhdanova I.V. Impaired Sleep, Circadian Rhythms and Neurogenesis in Diet-Induced Premature Aging. Int. J. Mol. Sci. 2017;18:2243. doi: 10.3390/ijms18112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tiwari S.K., Agarwal S., Tripathi A., Chaturvedi R.K. Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway. Mol. Neurobiol. 2016;53:3010–3029. doi: 10.1007/s12035-015-9197-z. [DOI] [PubMed] [Google Scholar]
- 122.Kodali M., Hattiangady B., Shetty G.A., Bates A., Shuai B., Shetty A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh S., Pant A.B. Biphasic responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. J. Neurochem. 2017;142:247. doi: 10.1038/srep28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Torres-Perez M., Tellez-Ballesteros R.I., Ortiz-Lopez L., Ichwan M., Vega-Rivera N.M., Castro-Garcia M., Gomez-Sanchez A., Kempermann G., Ramirez-Rodriguez G.B. Resveratrol Enhances Neuroplastic Changes, Including Hippocampal Neurogenesis, and Memory in Balb/C Mice at Six Months of Age. PLoS ONE. 2015;10:e0145687. doi: 10.1371/journal.pone.0145687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sarubbo F., Moranta D., Pani G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018;90:456–470. doi: 10.1016/j.neubiorev.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 126.Zaganas I.V., Simos P., Basta M., Kapetanaki S., Panagiotakis S., Koutentaki I., Fountoulakis N., Bertsias A., Duijker G., Tziraki C., et al. The Cretan Aging Cohort: Cohort Description and Burden of Dementia and Mild Cognitive Impairment. Am. J. Alzheimers Dis. 2019;34:23–33. doi: 10.1177/1533317518802414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Panza F., Lozupone M., Sardone R., Battista P., Piccininni M., Dibetlo V., La Montagna M., Stallone R., Venezia P., Liguori A., et al. Sensorial frailty: Age-related hearing loss and the risk of cognitive impairment and dementia in later life. Ther. Adv. Chronic Dis. 2019;10:2040622318811000. doi: 10.1177/2040622318811000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaneshwaran K., Olah M., Tasaki S., Yu L., Bradshaw E.M., Schneider J.A., Buchman A.S., Bennett D.A., De Jager P.L., Lim A.S.P. Sleep fragmentation, microglial aging, and cognitive impairment in adults with and without Alzheimer’s dementia. Sci. Adv. 2019;5:eaax7331. doi: 10.1126/sciadv.aax7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tortelli R., Piccininni M., Battista P., Di Lena L., Abbrescia D.I., Barulli M.R., Capozzo R., Coppola F., Lozupone M., Panza F., et al. Dementia and vascular risk scores in an aging population: An association with cognitive and sensory impairment. Neurology. 2018;90(Suppl. 15):194. [Google Scholar]
- 130.Zhao J.B., Fang S.Q., Yuan Y.J., Guo Z.P., Zeng J.H., Guo Y., Tang P.F., Mei X.F. Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress. Neural Regen. Res. 2014;9:1379–1385. doi: 10.4103/1673-5374.137591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan M.H., Sheng W., He Y., Zong Y., Chuang D., Simonyi A., Sun A.Y., Sun G.Y. Effects of Magnolia Polyphenols on Oxidative Stress and Inflammatory Responses in Neurons and Glia. J. Neurochem. 2011;118:42. [Google Scholar]
- 132.Ibarretxe G., Sanchez-Gomez M.V., Campos-Esparza M.R., Alberdi E., Matute C. Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia. 2006;53:201–211. doi: 10.1002/glia.20267. [DOI] [PubMed] [Google Scholar]
- 133.Zhang B., Yao R.J., Li L.H., Wang Y.N., Luo R.F., Yang L., Wang Y.B. Green Tea Polyphenol Induced Mg2+-rich Multilayer Conversion Coating: Toward Enhanced Corrosion Resistance and Promoted in Situ Endothelialization of AZ31 for Potential Cardiovascular Applications. ACS Appl. Mater. Interfaces. 2019;11:41165–41177. doi: 10.1021/acsami.9b17221. [DOI] [PubMed] [Google Scholar]
- 134.Yamagata K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019;25:2443–2458. doi: 10.2174/1381612825666190722100504. [DOI] [PubMed] [Google Scholar]
- 135.Speer H., D’Cunha N.M., Botek M., McKune A.J., Sergi D., Georgousopoulou E., Mellor D.D., Naumovski N. The Effects of Dietary Polyphenols on Circulating Cardiovascular Disease Biomarkers and Iron Status: A Systematic Review. Nutr. Metab. Insights. 2019;12:1178638819882739. doi: 10.1177/1178638819882739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Castelli V., Grassi D., Bocale R., d’Angelo M., Antonosante A., Cimini A., Ferri C., Desideri G. Diet and Brain Health: Which Role for Polyphenols? Curr. Pharm. Des. 2017;24:227–238. doi: 10.2174/1381612824666171213100449. [DOI] [PubMed] [Google Scholar]
- 137.Zhao D.Y., Simon J.E., Wu Q.L. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. 2020;60:597–625. doi: 10.1080/10408398.2018.1546668. [DOI] [PubMed] [Google Scholar]
- 138.Silva R.F.M., Pogacnik L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants. 2020;9:61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naoi M., Wu Y.Q., Shamoto-Nagai M., Maruyama W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019;20:2451. doi: 10.3390/ijms20102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi D.D., Dong C.M., Ho L.C., Lam C.T.W., Zhou X.D., Wu E.X., Zhou Z.J., Wang X.M., Zhang Z.J. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol. Dis. 2018;114:164–173. doi: 10.1016/j.nbd.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 141.Martinez-Huelamo M., Rodriguez-Morato J., Boronat A., de la Torre R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxidants. 2017;6:73. doi: 10.3390/antiox6040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reglodi D., Renaud J., Tamas A., Tizabi Y., Socias S.B., Del-Bel E., Raisman-Vozari R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2017;155:120–148. doi: 10.1016/j.pneurobio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 143.Silva R.F.M., Pogacnik L. Food, polyphenols and neuroprotection. Neural Regen. Res. 2017;12:582–583. doi: 10.4103/1673-5374.205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sheng J.Y., Yang X.Y., Liu Q.Y., Luo H., Yin X.Q., Liang M., Liu W., Lan X.L., Wan J.L., Yang X.L. Coadministration with Tea Polyphenols Enhances the Neuroprotective Effect of Defatted Walnut Meal Hydrolysate against Scopolamine-Induced Learning and Memory Deficits in Mice. J. Agric. Food Chem. 2020;68:751–758. doi: 10.1021/acs.jafc.9b05081. [DOI] [PubMed] [Google Scholar]
- 145.Ma X.R., Sun Z.K., Han X., Li S.J., Jiang X.F., Chen S., Zhang J.W., Lu H. Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer’s Disease. Front. Neurosci. 2020;13:1400. doi: 10.3389/fnins.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mehta J., Kaur B., Pandey K.K., Dhar P. The Possible Neuroprotective Role of Resveratrol Supplementation on Arsenic Trioxide-Induced Neurotoxicity in Female Mice Hippocampus. Int. J. Toxicol. 2020;39:51. [Google Scholar]
- 147.Lin C.H., Nicol C.J.B., Cheng Y.C., Yen C.H., Wang Y.S., Chiang M.C. Neuroprotective effects of resveratrol against oxygen glucose deprivation induced mitochondrial dysfunction by activation of AMPK in SH-SY5Y cells with 3D gelatin scaffold. Brain Res. 2020;1726:146492. doi: 10.1016/j.brainres.2019.146492. [DOI] [PubMed] [Google Scholar]
- 148.Rahman M.R., Kumar V.K. Improving neuroprotective effects of resveratrol by brain targeting through chitosan glutamate nanoparticles in MPTP induced parkinsonism. J. Neurol. Sci. 2019;405:279–280. doi: 10.1016/j.jns.2019.10.1343. [DOI] [Google Scholar]
- 149.Irnidayanti Y., Sutiono D.R. Tempeh & Soybean Seed Coat: The Alternative Sources of Trans-Resveratrol as Neuroprotective Agents. Int. J. Morphol. 2019;37:1164–1171. [Google Scholar]
- 150.Lin K.L., Lin K.J., Wang P.W., Chuang J.H., Lin H.Y., Chen S.D., Chuang Y.C., Huang S.T., Tiao M.M., Chen J.B., et al. Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic. Res. 2018;52:1371–1386. doi: 10.1080/10715762.2018.1489128. [DOI] [PubMed] [Google Scholar]
- 151.Bastianetto S., Menard C., Quirion R. Neuroprotective action of resveratrol. BBA-Mol. Basis Dis. 2015;1852:1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 152.Lin Y.T., Wu Y.C., Sun G.C., Ho C.Y., Wong T.Y., Lin C.H., Chen H.H., Yeh T.C., Li C.J., Tseng C.J., et al. Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Ind uced Early Alzheimer’s Disease. J. Clin. Med. 2018;7:329. doi: 10.3390/jcm7100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J., Li L. Targeting Autophagy for the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Front. Mol. Neurosci. 2019;12:203. doi: 10.3389/fnmol.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kou X.J., Chen N. Resveratrol as a Natural Autophagy Regulator for Prevention and Treatment of Alzheimer’s Disease. Nutrients. 2017;9:927. doi: 10.3390/nu9090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Um J.H., Park S.J., Kang H., Yang S.T., Foretz M., McBurney M.W., Kim M.K., Viollet B., Chung J.H. AMP-Activated Protein Kinase-Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang S.F., Wu M.Y., Cai C.Z., Li M., Lu J.H. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 2016;194:861–876. doi: 10.1016/j.jep.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 157.Moussa C., Hebron M., Huang X., Ahn J., Rissman R.A., Aisen P.S., Turner R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017;14:1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simao F., Matte A., Pagnussat A.S., Netto C.A., Salbego C.G. Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem. Int. 2012;61:659–665. doi: 10.1016/j.neuint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 159.Monti D.M., Rigano M.M., Monti S.M., Peixoto H.S. Role of Antioxidants in the Protection from Aging-Related Diseases. Oxid. Med. Cell. Longev. 2019;2019:7450693. doi: 10.1155/2019/7450693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kashyap S.S., Johnson J.R., McCue H.V., Chen X., Edmonds M.J., Ayala M., Graham M.E., Jenn R.C., Barclay J.W., Burgoyne R.D., et al. Caenorhabditis elegans dnj-14, the orthologue of the DNAJC5 gene mutated in adult onset neuronal ceroid lipofuscinosis, provides a new platform for neuroprotective drug screening and identifies a SIR-2.1-independent action of resveratrol. Hum. Mol. Genet. 2014;23:5916–5927. doi: 10.1093/hmg/ddu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bastianetto S., Quirion R. Heme oxygenase 1: Another possible target to explain the neuroprotective action of resveratrol, a multifaceted nutrient-based molecule. Exp. Neurol. 2010;225:237–239. doi: 10.1016/j.expneurol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 162.Venigalla M., Sonego S., Gyengesi E., Munch G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015;10:1181. doi: 10.4103/1673-5374.162686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heidari S., Mahdiani S., Hashemi M., Kalalinia F. Recent advances in neurogenic and neuroprotective effects of curcumin through the induction of neural stem cells. Biotechnol. Appl. Biochem. 2020 doi: 10.1002/bab.1891. [DOI] [PubMed] [Google Scholar]
- 164.Forouzanfar F., Read M.I., Barreto G.E., Sahebkar A. Neuroprotective effects of curcumin through autophagy modulation. IUBMB Life. 2020;72:652–664. doi: 10.1002/iub.2209. [DOI] [PubMed] [Google Scholar]
- 165.Ferreira N., Saraiva M.J., Almeida M.R. Uncovering the Neuroprotective Mechanisms of Curcumin on Transthyretin Amyloidosis. Int. J. Mol. Sci. 2019;20:1287. doi: 10.3390/ijms20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dourado N.S., Souza C.D.S., Carneiro M.M.A.D., dos Santos B.L., de Assis A.M., de Souza D.O., Costa M.D.F.D., da Silva V.D.A., Costa S.L. Neuroimmunomodulatory and neuroprotective effects of flavonoid apigenin in in vitro models of neuroinflammation associated with Alzheimer’s Disease. Glia. 2019;67:E231–E232. doi: 10.3389/fnagi.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hashemi P., Babaei J.F., Vazifekhah S., Nikbakht F. Evaluation of the neuroprotective, anticonvulsant, and cognition-improvement effects of apigenin in temporal lobe epilepsy: Involvement of the mitochondrial apoptotic pathway. Iran. J. Basic Med. Sci. 2019;22:752–758. doi: 10.22038/ijbms.2019.33892.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nabavi S.F., Khan H., D’onofrio G., Samec D., Shirooie S., Dehpour A.R., Arguelles S., Habtemariam S., Sobarzo-Sanchez E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 169.Khan H., Ullah H., Aschner M., Cheang W.S., Akkol E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules. 2020;10:59. doi: 10.3390/biom10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sharma S., Raj K., Singh S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement-Induced Parkinson’s Disease in Experimental Rats. Neurotox. Res. 2020;37:198–209. doi: 10.1007/s12640-019-00120-z. [DOI] [PubMed] [Google Scholar]
- 171.Khan A., Ali T., Rehman S.U., Khan M.S., Alam S.I., Ikram M., Muhammad T., Saeed K., Badshah H., Kim M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018;9:1383. doi: 10.3389/fphar.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kian K., Khalatbary A.R., Ahmadvand H., Malekshah A.K., Shams Z. Neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) against peripheral nerve transection-induced apoptosis. Nutr. Neurosci. 2019;22:578–586. doi: 10.1080/1028415X.2017.1419542. [DOI] [PubMed] [Google Scholar]
- 173.Singh N.A., Mandal A.K.A., Khan Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG) Nutr. J. 2016;15:60. doi: 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lewandowska U., Szewczyk K., Hrabec E., Janecka A., Gorlach S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013;61:12183–12199. doi: 10.1021/jf404439b. [DOI] [PubMed] [Google Scholar]
- 175.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., Holtz A., Shah S., Sharma V., Ferrucci L., et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Watanabe S., Kawamoto S., Ohtani N., Hara E. The impact of SASP and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–569. doi: 10.1111/cas.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Borodkina A.V., Deryabin P.I., Giukova A.A., Nikolsky N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Nat. 2018;10:4–14. doi: 10.32607/20758251-2018-10-1-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yousefzadeh M.J., Zhao J., Bukata C., Wade E.A., McGowan S.J., Angelini L.A., Bank M.P., Gurkar A.U., McGuckian C.A., Calubag M.F., et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19:e13094. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Biran A., Zada L., Abou Karam P., Vadai E., Roitman L., Ovadya Y., Porat Z., Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017;16:661–671. doi: 10.1111/acel.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Karin O., Agrawal A., Porat Z., Krizhanovsky V., Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 2019;10:5495. doi: 10.1038/s41467-019-13192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ovadya Y., Landsberger T., Leins H., Vadai E., Gal H., Biran A., Yosef R., Sagiv A., Agrawal A., Shapira A., et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Freund A., Orjalo A.V., Desprez P.-Y., Campisi J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Childs B.G., Li H., van Deursen J.M. Senescent cells: A therapeutic target for cardiovascular disease. J. Clin. Investig. 2018;128:1217–1228. doi: 10.1172/JCI95146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greene M.A., Loeser R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015;23:1966–1971. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jeon O., David N., Campisi J., Elisseeff J. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018;128:1229–1237. doi: 10.1172/JCI95147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baar M.P., Perdiguero E., Muñoz-Cánoves P., de Keizer P.L.J. Musculoskeletal senescence: A moving target ready to be eliminated. Curr. Opin. Pharmacol. 2018;40:147–155. doi: 10.1016/j.coph.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 189.Childs B.G., Baker D.J., Wijshake T., Conover C.A., Campisi J., van Deursen J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wiley C.D., Liu S., Limbad C., Zawadzka A.M., Beck J., Demaria M., Artwood R., Alimirah F., Lopez-Dominguez J.-A., Kuehnemann C., et al. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep. 2019;28:3329–3337. doi: 10.1016/j.celrep.2019.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Valentijn F.A., Falke L.L., Nguyen T.Q., Goldschmeding R. Cellular senescence in the aging and diseased kidney. J. Cell Commun. Signal. 2018;12:69–82. doi: 10.1007/s12079-017-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baar M., Brandt R., Putavet D., Klein J., Derks K., Bourgeois B., Stryeck S., Rijksen Y., van Willigenburg H., Feijtel D., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132–147. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malaquin N., Martinez A., Rodier F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Exp. Gerontol. 2016;82:39–49. doi: 10.1016/j.exger.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 194.Ohanna M., Giuliano S., Bonet C., Imbert V., Hofman V., Zangari J., Bille K., Robert C., Bressac-de Paillerets B., Hofman P., et al. Senescent cells develop a PARP-1 and nuclear factor-κB-associated secretome (PNAS) Genes Dev. 2011;25:1245–1261. doi: 10.1101/gad.625811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chien Y., Scuoppo C., Wang X., Fang X., Balgley B., Bolden J.E., Premsrirut P., Luo W., Chicas A., Lee C.S., et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lujambio A. To clear, or not to clear (senescent cells)? That is the question. BioEssays. 2016;38:S56–S64. doi: 10.1002/bies.201670910. [DOI] [PubMed] [Google Scholar]
- 197.Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L., Lu T., Yankner B.A., Campisi J., Elledge S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Narita M., Young A.R.J., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., Hong S., Berry L.S., Reichelt S., Ferreira M., et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kwon Y., Kim J.W., Jeoung J.A., Kim M.-S., Kang C. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol. Cells. 2017;40:607–612. doi: 10.14348/molcells.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Young A.R.J., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J.F.J., Tavaré S., Arakawa S., Shimizu S., Watt F.M., et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bian Y., Wei J., Zhao C., Li G. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. Int. J. Mol. Sci. 2020;21:684. doi: 10.3390/ijms21020684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Doherty J., Baehrecke E. Life, death and autophagy. Nat. Cell Biol. 2018;20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang S., Wang X., Cheng Y., Ouyang W., Sang X., Liu J., Su Y., Liu Y., Li C., Yang L., et al. Autophagy Dysfunction, Cellular Senescence, and Abnormal Immune-Inflammatory Responses in AMD: From Mechanisms to Therapeutic Potential. Oxid. Med. Cell. Longev. 2019;2019:3632169. doi: 10.1155/2019/3632169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chung S., Yao H., Caito S., Hwang J.-W., Arunachalam G., Rahman I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hou X., Rooklin D., Fang H., Zhang Y. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci. Rep. 2016;6:38186. doi: 10.1038/srep38186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Borra M., Smith B., Denu J. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 207.Boer V., Goffau M., Arts I., Hollman P., Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 208.Kitada M., Ogura Y., Koya D. Chapter 3—Role of Sirt1 as a Regulator of Autophagy. In: Hayat M.A., editor. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Academic Press; San Diego, CA, USA: 2016. pp. 89–100. [Google Scholar]
- 209.Kuchitsu Y., Fukuda M. Revisiting Rab7 Functions in Mammalian Autophagy: Rab7 Knockout Studies. Cells. 2018;7:215. doi: 10.3390/cells7110215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou J., Liao W., Yang J., Ma K., Li X., Wang Y., Wang D., Wang L., Zhang Y., Yin Y., et al. FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy. 2012;8:1712–1723. doi: 10.4161/auto.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ghosh H.S., McBurney M., Robbins P.D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ou X., Lee M.R., Huang X., Messina-Graham S., Broxmeyer H.E. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183–1194. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hayakawa T., Iwai M., Aoki S., Takimoto K., Maruyama M., Maruyama W., Motoyama N. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS ONE. 2015;10:e0116480. doi: 10.1371/journal.pone.0116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hekmatimoghaddam S., Dehghani Firoozabadi A., Zare-Khormizi M.R., Pourrajab F. Sirt1 and Parp1 as epigenome safeguards and microRNAs as SASP-associated signals, in cellular senescence and aging. Ageing Res. Rev. 2017;40:120–141. doi: 10.1016/j.arr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 215.Lamichane S., Baek S., Kim Y.-J., Park J., Dahal Lamichane B., Jang W., Ji S., Lee N., Dehua L., Kim D., et al. MHY2233 Attenuates Replicative Cellular Senescence in Human Endothelial Progenitor Cells via SIRT1 Signaling. Oxid. Med. Cell. Longev. 2019;2019:6492029. doi: 10.1155/2019/6492029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta J.C., Innes A.J., Banito A., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tomimatsu K., Narita M. Translating the effects of mTOR on secretory senescence. Nat. Cell Biol. 2015;17:1230–1232. doi: 10.1038/ncb3244. [DOI] [PubMed] [Google Scholar]
- 218.Chandrasekaran A., Zhang X., Lee M.Y., Shapiro R., Trebak M., Melendez J.A. 62—H2O2 and mTOR control the senescence-associated secretory phenotype by coordinating Ca2+ transients through TRPC6 expression and activation. Free Radic. Biol. Med. 2017;112:55–56. doi: 10.1016/j.freeradbiomed.2017.10.075. [DOI] [Google Scholar]
- 219.Laberge R.-M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., Curran S.C., Davalos A.R., Wilson-Edell K.A., Liu S., et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Boil. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Criollo A., Chereau F., Malik S.A., Niso-Santano M., Mariño G., Galluzzi L., Maiuri M.C., Baud V., Kroemer G. Autophagy is required for the activation of NFκB. Cell Cycle. 2012;11:194–199. doi: 10.4161/cc.11.1.18669. [DOI] [PubMed] [Google Scholar]
- 221.Criollo A., Senovilla L., Authier H., Maiuri M.C., Morselli E., Vitale I., Kepp O., Tasdemir E., Galluzzi L., Shen S., et al. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Djavaheri-Mergny M., Amelotti M., Mathieu J., Besançon F., Bauvy C., Codogno P. Regulation of Autophagy by NF-kappaB Transcription Factor and Reactives Oxygen Species. Autophagy. 2007;3:390–392. doi: 10.4161/auto.4248. [DOI] [PubMed] [Google Scholar]
- 223.Su Y., Qu Y., Zhao F., Li H., Mu D., Li X. Regulation of autophagy by the nuclear factor κB signaling pathway in the hippocampus of rats with sepsis. J. Neuroinflamm. 2015;12:116. doi: 10.1186/s12974-015-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Trocoli A., Djavaheri-Mergny M. The complex interplay between autophagy and NF-κB signaling pathways in cancer cells. Am. J. Cancer Res. 2011;1:629–649. [PMC free article] [PubMed] [Google Scholar]
- 225.Salminen A., Hyttinen J., Kauppinen A., Kaarniranta K. Context-Dependent Regulation of Autophagy by IKK-NF-κB Signaling: Impact on the Aging Process. Int. J. Cell Biol. 2012;2012:849541. doi: 10.1155/2012/849541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Feng Y., Cui Y., Gao J.-L., Li M.-H., Li R., Jiang X.-H., Tian Y.-X., Wang K.-J., Cui C.-M., Cui J.-Z. Resveratrol attenuates neuronal autophagy and inflammatory injury by inhibiting the TLR4/NF-κB signaling pathway in experimental traumatic brain injury. Int. J. Mol. Med. 2016;37:921–930. doi: 10.3892/ijmm.2016.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xu L., Botchway B.O.A., Zhang S., Zhou J., Liu X. Inhibition of NF-κB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front. Neurosci. 2018;12:690. doi: 10.3389/fnins.2018.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ruiz P.A., Haller D. Functional Diversity of Flavonoids in the Inhibition of the Proinflammatory NF-κB, IRF, and Akt Signaling Pathways in Murine Intestinal Epithelial Cells. J. Nutr. 2006;136:664–671. doi: 10.1093/jn/136.3.664. [DOI] [PubMed] [Google Scholar]
- 229.Haseeb A., Khan N.M., Ashruf O.S., Haqqi T.M. A Polyphenol-rich Pomegranate Fruit Extract Suppresses NF-κB and IL-6 Expression by Blocking the Activation of IKKβ and NIK in Primary Human Chondrocytes. Phytother. Res. 2017;31:778–782. doi: 10.1002/ptr.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu C.-W., Sung H.-C., Lin S.-R., Wu C.-W., Lee C.-W., Lee I.T., Yang Y.-F., Yu I.S., Lin S.-W., Chiang M.-H., et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci. Rep. 2017;7:44689. doi: 10.1038/srep44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li H., Jia Z., Li A., Jenkins G., Yang X., Hu J., Guo W. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol. Cell. Biochem. 2013;382:137–143. doi: 10.1007/s11010-013-1728-1. [DOI] [PubMed] [Google Scholar]
- 232.Wang G., Dai F., Yu K., Jia Z., Zhang A., Huang Q., Kang C., Jiang H., Pu P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int. J. Oncol. 2015;46:1739–1747. doi: 10.3892/ijo.2015.2863. [DOI] [PubMed] [Google Scholar]
- 233.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 234.Buhrmann C., Busch F., Shayan P., Shakibaei M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014;289:22048–22062. doi: 10.1074/jbc.M114.568790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kolesnichenko M., Mikuda N., Höpken U., Milanovic M., Tufan A.B., Uyar B., Sun W., Schleich K., Hoff L., Willenbrock M., et al. A Novel IKK- and Proteasome-Independent Mechanism of RelA Activation Triggers Senescence Associated Secretome via Transcriptional Repression of NFKBIA. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2019. [DOI] [Google Scholar]
- 236.Liu S., Zheng Z., Ji S., Liu T., Hou Y., Li S., Li G. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018;80:473–479. doi: 10.1016/j.fsi.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 237.Pitozzi V., Mocali A., Laurenzana A., Giannoni E., Cifola I., Battaglia C., Chiarugi P., Dolara P., Giovannelli L. Chronic Resveratrol Treatment Ameliorates Cell Adhesion and Mitigates the Inflammatory Phenotype in Senescent Human Fibroblasts. J. Gerontol. Ser. A. 2012;68:371–381. doi: 10.1093/gerona/gls183. [DOI] [PubMed] [Google Scholar]
- 238.Chang Y.-C., Liu H.-W., Chen Y.-T., Chen Y.-A., Chen Y.-J., Chang S.-J. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. J. Food Drug Anal. 2018;26:1066–1074. doi: 10.1016/j.jfda.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kumar R., Sharma A., Kumari A., Gulati A., Padwad Y., Sharma R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology. 2019;20:171–189. doi: 10.1007/s10522-018-9785-1. [DOI] [PubMed] [Google Scholar]
- 240.Kim H.-S., Montana V., Jang H., Parpura V., Kim J.-A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013;288:22693–22705. doi: 10.1074/jbc.M113.477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lim H., Park H., Kim H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015;96:337–348. doi: 10.1016/j.bcp.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 242.Park H.-J., Kim M.-M. Amentoflavone Induces Autophagy and Modulates p53. Cell J. 2019;21:27–34. doi: 10.22074/cellj.2019.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cao H., Jia Q., Yan L., Chen C., Xing S., Shen D. Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in ox-LDL-Induced RAW264.7 Macrophage Foam Cells. Int. J. Mol. Sci. 2019;20:6093. doi: 10.3390/ijms20236093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Courtois-Cox S., Jones S.L., Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 245.Doppler W., Jansen-Durr P. Regulation of mitochondrial ROS production by HIC-5: A common feature of oncogene-induced senescence and tumor invasiveness? FEBS J. 2019;286:456–458. doi: 10.1111/febs.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Galluzzi L., Vitale I. Oncogene-induced senescence and tumour control in complex biological systems. Cell Death Differ. 2018;25:1005–1006. doi: 10.1038/s41418-018-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Volonte D., Vyas A.R., Chen C., Dacic S., Stabile L.P., Kurland B.F., Abberbock S.R., Burns T.F., Herman J.G., Di Y.P., et al. Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J. Biol. Chem. 2018;293:1794–1809. doi: 10.1074/jbc.M117.815902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim L., Kim Y.S., Lee J.S., Choi S.J., Park I.S., Han J.Y., Kim J.M., Chu Y.C. Ciliated muconodular papillary tumor of the lung harboring BRAF V600E mutation and p16(INK4a) overexpression without proliferative activity may represent an example of oncogene-induced senescence. J. Thorac. Dis. 2017;9:E1039–E1044. doi: 10.21037/jtd.2017.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Barnoud T., Schmidt M.L., Donninger H., Clark G.J. The role of the NORE1A tumor suppressor in Oncogene-Induced Senescence. Cancer Lett. 2017;400:30–36. doi: 10.1016/j.canlet.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Harajly M., Zalzali H., Ghamloush F., Saab R.H. Investigating the role of the retinoblastoma protein in induction and maintenance of oncogene-induced cellular senescence in tumor suppression. Cancer Res. 2017;77 doi: 10.1158/1538-7445.AM2017-5483. [DOI] [Google Scholar]
- 251.Rao S.G., Jackson J.G. SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer. 2016;2:676–687. doi: 10.1016/j.trecan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 252.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bockler S., Chelius X., Hock N., Klecker T., Wolter M., Weiss M., Braun R.J., Westermann B. Fusion, fission, and transport control asymmetric inheritance of mitochondria and protein aggregates. J. Cell Biol. 2017;216:2481–2498. doi: 10.1083/jcb.201611197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Murke F., Castro S.V.D., Giebel B., Gorgens A. Concise Review: Asymmetric Cell Divisions in Stem Cell Biology. Symmetry. 2015;7:2025–2037. doi: 10.3390/sym7042025. [DOI] [Google Scholar]
- 255.Twig G., Elorza A., Molina A.J.A., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vijg J., Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu J.J., Quijano C., Chen E., Liu H., Cao L., Fergusson M.M., Rovira I.I., Gutkind S., Daniels M.P., Komatsu M., et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Huang S.S., Ding D.F., Chen S., Dong C.L., Ye X.L., Yuan Y.G., Feng Y.M., You N., Xu J.R., Miao H., et al. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci. Rep. 2017;7:45692. doi: 10.1038/srep45692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vidoni C., Secomandi E., Castiglioni A., Melone M.A.B., Isidoro C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2018;117:174–187. doi: 10.1016/j.neuint.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 260.Klaus S., Pultz S., Thone-Reineke C., Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 261.Kim H.S., Quon M.J., Kim J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li W., Zhu S., Li J., Assa A., Jundoria A., Xu J., Fan S., Eissa N.T., Tracey K.J., Sama A.E., et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011;81:1152–1163. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Filomeni G., Graziani I., De Zio D., Dini L., Centonze D., Rotilio G., Ciriolo M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging. 2012;33:767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 264.Meléndez A., Tallóczy Z., Seaman M., Eskelinen E.L., Hall D.H., Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 265.King J.S., Veltman D.M., Insall R.H. The induction of autophagy by mechanical stress. Autophagy. 2011;7:1490–1499. doi: 10.4161/auto.7.12.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Levine B., Kroemer G. Autophagy in the Pathogenesis of Disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chung K.W., Chung H.Y. The Effects of Calorie Restriction on Autophagy: Role on Aging Intervention. Nutrients. 2019;11:2923. doi: 10.3390/nu11122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gutierrez-Casado E., Khraiwesh H., Lopez-Dominguez J.A., Montero-Guisado J., Lopez-Lluch G., Navas P., de Cabo R., Ramsey J.J., Gonzalez-Reyes J.A., Villalba J.M. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. J. Gerontol. Ser. A. 2019;74:760–769. doi: 10.1093/gerona/gly161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chang K., Kang P., Liu Y., Huang K.R., Miao T., Sagona A.P., Nezis I.P., Bodmer R., Ocorr K., Bai H. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy. 2019 doi: 10.1080/15548627.2019.1704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen X.D., Lin S.Y., Gu L., Zhu X.H., Zhang Y.N., Zhang H.X., Shao B., Zhuge Q.C., Jin K.L. Inhibition of miR-497 improves functional outcome after ischemic stroke by enhancing neuronal autophagy in young and aged rats. Neurochem. Int. 2019;127:64–72. doi: 10.1016/j.neuint.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 271.Xu L., Fan Q.L., Wang X., Zhao X., Wang L.N. Inhibition of autophagy increased AGE/ROS-mediated apoptosis in mesangial cells. Cell Death Dis. 2016;7:e2445. doi: 10.1038/cddis.2016.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bueno M., St Croix C.M., Rojas M., Mora A.L. Aging-Related Inhibition Of Autophagy Is A Critical Factor For The Increased Susceptibility To Apoptosis And Lung Fibrosis With Age. Am. J. Respir. Crit. Care. 2013;187:A5177. [Google Scholar]
- 273.Cantó C., Jiang L.Q., Deshmukh A.S., Mataki C., Coste A., Lagouge M., Zierath J.R., Auwerx J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 275.Haigis M.C., Sinclair D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Morselli E., Mariño G., Bennetzen M.V., Eisenberg T., Megalou E., Schroeder S., Cabrera S., Bénit P., Rustin P., Criollo A., et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Saunders L.R., Verdin E. Cell biology. Stress response and aging. Science. 2009;323:1021–1022. doi: 10.1126/science.1170007. [DOI] [PubMed] [Google Scholar]
- 278.Schneider K.L., Nystrom T., Widlund P.O. Studying Spatial Protein Quality Control, Proteopathies, and Aging Using Different Model Misfolding Proteins in S. cerevisiae. Front. Mol. Neurosci. 2018;11:249. doi: 10.3389/fnmol.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Adzhubei A.A., Anashkina A.A., Makarov A.A. Left-handed polyproline-II helix revisited: Proteins causing proteopathies. J. Biomol. Struct. Dyn. 2017;35:2701–2713. doi: 10.1080/07391102.2016.1229220. [DOI] [PubMed] [Google Scholar]
- 280.Walker L.C., LeVine H. The cerebral proteopathies. Neurobiol. Aging. 2000;21:559–561. doi: 10.1016/S0197-4580(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 281.Ma Y., Zhang J. Embryotoxicity of Sodium Valproate is correlated to the dysregulation of autophagy. Toxicol. Lett. 2019;314:S272. [Google Scholar]
- 282.Zhang W., Xu W., Chen W.L., Zhou Q. Interplay of Autophagy Inducer Rapamycin and Proteasome Inhibitor MG 132 in Reduction of Foam Cell Formation and Inflammatory Cytokine Expression. Cell Transplant. 2018;27:1235–1248. doi: 10.1177/0963689718786229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Li X.Z., Chen X.P., Zhao K., Bai L.M., Zhang H., Zhou X.P. Therapeutic Effects of Valproate Combined With Lithium Carbonate on MPTP-Induced Parkinsonism in Mice: Possible Mediation Through Enhanced Autophagy. Int. J. Neurosci. 2013;123:73–79. doi: 10.3109/00207454.2012.729234. [DOI] [PubMed] [Google Scholar]
- 284.Sarkar S., Ravikumar B., Floto R.A., Rubinsztein D.C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 285.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W., Jimenez-Sanchez M., Korolchuk V.I., Lichtenberg M., Luo S., et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 286.Wang J.Y., Chen X.D., Osland J., Gerber S.J., Luan C., Delfino K., Goodwin L., Yuan R. Deletion of Nrip1 Extends Female Mice Longevity, Increases Autophagy, and Delays Cell Senescence. J. Gerontol. Ser. A. 2018;73:882–892. doi: 10.1093/gerona/glx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Emanuele E., Minoretti P., Sanchis-Gomar F., Pareja-Galeano H., Yilmaz Y., Garatachea N., Lucia A. Can Enhanced Autophagy Be Associated with Human Longevity? Serum Levels of the Autophagy Biomarker Beclin-1 Are Increased in Healthy Centenarians. Rejuvenation Res. 2014;17:518–524. doi: 10.1089/rej.2014.1607. [DOI] [PubMed] [Google Scholar]
- 288.Cao H., Jia Q.L., Shen D.Z., Yan L., Chen C., Xing S.L. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE(-/-) mice. Exp. Ther. Med. 2019;18:2451–2458. doi: 10.3892/etm.2019.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ashrafizadeh M., Ahmadi Z., Farkhondeh T., Samarghandia S. Autophagy as a molecular target of quercetin underlying its protective effects in human diseases. Arch. Physiol. Biochem. 2019 doi: 10.1080/13813455.2019.1671458. [DOI] [PubMed] [Google Scholar]
- 290.Tsai T.F., Hwang T.I.S., Lin J.F., Chen H.E., Yang S.C., Lin Y.C., Chou K.Y. Suppression of Quercetin-Induced Autophagy Enhances Cytotoxicity through Elevating Apoptotic Cell Death in Human Bladder Cancer Cells. Urol. Sci. 2019;30:58–66. [Google Scholar]
- 291.Jia L.J., Huang S., Yin X.R., Zan Y., Guo Y., Han L.L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018;208:123–130. doi: 10.1016/j.lfs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 292.Cui W.J., Hu G., Peng J., Mu L., Liu J., Qiao L.J. Quercetin Exerted Protective Effects in a Rat Model of Sepsis via Inhibition of Reactive Oxygen Species (ROS) and Downregulation of High Mobility Group Box 1 (HMGB1) Protein Expression. Med. Sci. Monit. 2019;25:5795–5800. doi: 10.12659/MSM.916044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Akkoyun D.C., Akyuz A., Dogan M., Erboga M., Aktas C., Caglar V., Uygur R., Topcu B., Yilmaz A., Gurel A. Quercetin Inhibits Heart Injury in Lipopolysaccharide-induced Endotoxemic Model by Suppressing the Effects of Reactive Oxygen Species. Anal. Quant. Cytopathol. 2016;38:183–188. [Google Scholar]
- 294.Kang J.T., Kwon D.K., Park S.J., Kim S.J., Moon J.H., Koo O.J., Jang G., Lee B.C. Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels. J. Vet. Sci. 2013;14:15–20. doi: 10.4142/jvs.2013.14.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.You W.J., Zheng W., Weiss S., Chua K.F., Steegborn C. Structural basis for the activation and inhibition of Sirtuin 6 by quercetin and its derivatives. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-55654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Heger V., Tyni J., Hunyadi A., Horakova L., Lahtela-Kakkonen M., Rahnasto-Rilla M. Quercetin based derivatives as sirtuin inhibitors. Biomed. Pharmacother. 2019;111:1326–1333. doi: 10.1016/j.biopha.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 297.Lazo-Gomez R., Tapia R. Quercetin prevents spinal motor neuron degeneration induced by chronic excitotoxic stimulus by a sirtuin 1-dependent mechanism. Transl. Neurodegener. 2017;6:31. doi: 10.1186/s40035-017-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kemelo M.K., Pierzynova A., Canova N.K., Kucera T., Farghali H. The involvement of sirtuin 1 and heme oxygenase 1 in the hepatoprotective effects of quercetin against carbon tetrachloride-induced sub-chronic liver toxicity in rats. Chem. Biol. Interact. 2017;269:1–8. doi: 10.1016/j.cbi.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 299.Kim S.H., Yoo E.S., Woo J.S., Han S.H., Lee J.H., Jung S.H., Kim H.J., Jung J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019;860:172568. doi: 10.1016/j.ejphar.2019.172568. [DOI] [PubMed] [Google Scholar]
- 300.Liu H.J., Zhou M. Antitumor effect of Quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways. BMC Complement. Altern. Med. 2017;17:531. doi: 10.1186/s12906-017-2023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lan C.Y., Chen S.Y., Kuo C.W., Lu C.C., Yen G.C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019;27:887–896. doi: 10.1016/j.jfda.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Liang H.D., Yu F. Quercetin promotes MC3T3-E1 cell growth via PI3K/Akt signaling pathway. Trop. J. Pharm. Res. 2018;17:2371–2374. doi: 10.4314/tjpr.v17i12.8. [DOI] [Google Scholar]
- 303.Li X.L., Zhou N., Wang J., Liu Z.J., Wang X.H., Zhang Q., Liu Q.Y., Gao L.F., Wang R. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018;196:56–62. doi: 10.1016/j.lfs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 304.Wang X.Y., Xing C.H., Yang F., Zhou S.H., Li G.Y., Zhang C.Y., Cao H.B., Hu G.L. Abnormal expression of liver autophagy and apoptosis-related mRNA in fatty liver haemorrhagic syndrome and improvement function of resveratrol in laying hens. Avian Pathol. 2020;49:171–178. doi: 10.1080/03079457.2019.1698712. [DOI] [PubMed] [Google Scholar]
- 305.Wang P., Huang C.X., Gao J.J., Shi Y., Li H., Yan H., Yan S.J., Zhang Z. Resveratrol induces SIRT1-Dependent autophagy to prevent H2O2-Induced oxidative stress and apoptosis in HTR8/SVneo cells. Placenta. 2020;91:11–18. doi: 10.1016/j.placenta.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 306.Qu X.F., Chen X., Shi Q.Q., Wang X.F., Wang D.G., Yang L. Resveratrol alleviates ischemia/reperfusion injury of diabetic myocardium via inducing autophagy. Exp. Ther. Med. 2019;18:2719–2725. doi: 10.3892/etm.2019.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang Q.B., He Y.L., Zhong X.W., Xie W.G., Zhou J.G. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients. Inflammopharmacology. 2019;27:47–56. doi: 10.1007/s10787-018-00555-4. [DOI] [PubMed] [Google Scholar]
- 308.Wang F.M., Hu Z., Liu X.H., Feng J.Q., Augsburger R.A., Gutmann J.L., Glickman G.N. Resveratrol represses tumor necrosis factor alpha/c-Jun N-terminal kinase signaling via autophagy in human dental pulp stem cells. Arch. Oral Biol. 2019;97:116–121. doi: 10.1016/j.archoralbio.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gong C.H., Xia H.L. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020;19:1878–1886. doi: 10.3892/etm.2019.8359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 310.Park D., Jeong H., Lee M.N., Koh A., Kwon O., Yang Y.R., Noh J., Suh P.G., Park H., Ryu S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016;6:21772. doi: 10.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yang X.H., Jiang T.L., Wang Y., Guo L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-44766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Reed J.C. Apoptosis mechanisms: Implications for cancer drug discovery. Oncology. 2004;18:11–20. [PubMed] [Google Scholar]
- 313.D’Arcy M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 314.Chen C.W., Wu M.S., Huang Y.J., Lin P.W., Shih C.J., Lin F.P., Chang C.Y. Iridovirus CARD Protein Inhibits Apoptosis through Intrinsic and Extrinsic Pathways. PLoS ONE. 2015;10:e0129071. doi: 10.1371/journal.pone.0129071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Li M., Gao P., Zhang J.P. Crosstalk between Autophagy and Apoptosis: Potential and Emerging Therapeutic Targets for Cardiac Diseases. Int. J. Mol. Sci. 2016;17:332. doi: 10.3390/ijms17030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Li H.N., Mu J.H., Sun J.A., Xu S.T., Liu W.W., Xu F.X., Li Z.L., Xu J.Y., Hua H.M., Li D.H. Hydrogen sulfide releasing oridonin derivatives induce apoptosis through extrinsic and intrinsic pathways. Eur. J. Med. Chem. 2020;187:111978. doi: 10.1016/j.ejmech.2019.111978. [DOI] [PubMed] [Google Scholar]
- 317.Cao X.B., Wen P.B., Fu Y.F., Gao Y., Qi X.J., Chen B., Tao Y.P., Wu L.J., Xu A., Lu H.Y., et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell. Signal. 2019;62:109337. doi: 10.1016/j.cellsig.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 318.Ji Y., Shen J., Li M., Zhu X.X., Wang Y.Y., Ding J.Z., Jiang S.Y., Chen L.Q., Wei W.X. RMP/URI inhibits both intrinsic and extrinsic apoptosis through different signaling pathways. Int. J. Biol. Sci. 2019;15:2692–2706. doi: 10.7150/ijbs.36829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gump J.M., Thorburn A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011;21:387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhu H.X., He L. Beclin 1 Biology and its Role in Heart Disease. Curr. Cardiol. Rev. 2015;11:229–237. doi: 10.2174/1573403X10666141106104606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Decuypere J.P., Parys J.B., Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. 2012;1:284–312. doi: 10.3390/cells1030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Maiuri M.C., Criollo A., Tasdemir E., Vicencio J.M., Tajeddine N., Hickman J.A., Geneste O., Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 323.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wei Y.J., Sinha S., Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4:949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pal S., Salunke-Gawali S., Konkimalla V.B. Induction of Autophagic Cell Death in Apoptosis-resistant Pancreatic Cancer Cells using Benzo[alpha]phenoxazines Derivatives, 10-methyl-benzo[alpha]phenoxazine-5-one and benzo[alpha]phenoxazine-5-one. Anti-Cancer Agents Med. Chem. 2017;17:115–125. [PubMed] [Google Scholar]
- 326.Mc Quaid K., Kornfeld H., Keane J., Sullivan M., Seonadh O. Enhancing Bacterial Killing in Mtb Infected Macrophages through Pharmacological Modulation of the Autophagy Pathway. Ir. J. Med. Sci. 2019;188:315. [Google Scholar]
- 327.Mc Quaid K., Kornfeld H., Keane J., Sullivan M., O’Leary S. Pharmacological Modulation of Autophagy Pathway in Mtb Infected Macrophages. Ir. J. Med. Sci. 2018;187:S301. [Google Scholar]
- 328.Sciarretta S., Zhai P.Y., Volpe M., Sadoshima J. Pharmacological Modulation of Autophagy during Cardiac Stress. J. Cardiovasc. Pharm. 2012;60:235–241. doi: 10.1097/FJC.0b013e3182575f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hasima N., Ozpolat B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014;5:e1509. doi: 10.1038/cddis.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang H.Y., Peng Y., Wang J., Gu A.X., Li Q., Mao D.W., Guo L.Y. Effect of autophagy on the resveratrol-induced apoptosis of ovarian cancer SKOV3 cells. J. Cell. Biochem. 2019;120:7788–7793. doi: 10.1002/jcb.28053. [DOI] [PubMed] [Google Scholar]
- 331.Pourhanifeh M.H., Shafabakhsh R., JReiter R., Asemi Z. The Effect of Resveratrol on Neurodegenerative Disorders: Possible Protective Actions against Autophagy, Apoptosis, Inflammation and Oxidative Stress. Curr. Pharm. Des. 2019;25:2178–2191. doi: 10.2174/1381612825666190717110932. [DOI] [PubMed] [Google Scholar]
- 332.Xu K., Liu X.F., Ke Z.Q., Yao Q., Guo S., Liu C. Resveratrol Modulates Apoptosis and Autophagy Induced by High Glucose and Palmitate in Cardiac Cells. Cell. Physiol. Biochem. 2018;46:2031–2040. doi: 10.1159/000489442. [DOI] [PubMed] [Google Scholar]
- 333.Fan Y.Y., Chiu J.F., Liu J., Deng Y., Xu C., Zhang J., Li G.W. Resveratrol induces autophagy-dependent apoptosis in HL-60 cells. BMC Cancer. 2018;18 doi: 10.1186/s12885-018-4504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cao W., Zhang J.Q., Wang G.Y., Lu J.S., Wang T.R., Chen X.Y. Reducing-Autophagy Derived Mitochondrial Dysfunction during Resveratrol Promotes Fibroblast-Like Synovial Cell Apoptosis. Anat. Rec. 2018;301:1179–1188. doi: 10.1002/ar.23798. [DOI] [PubMed] [Google Scholar]
- 335.Guo D., Xie J.T., Zhao J.J., Huang T.Q., Guo X.Y., Song J.N. Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. Neuroreport. 2018;29:368–379. doi: 10.1097/WNR.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pifferi F., Aujard F. Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;95:109702. doi: 10.1016/j.pnpbp.2019.109702. [DOI] [PubMed] [Google Scholar]
- 337.Diaz-Ruiz A., Di Francesco A., Carboneau B.A., Levan S.R., Pearson K.J., Price N.L., Ward T.M., Bernier M., de Cabo R., Mercken E.M. Benefits of Caloric Restriction in Longevity and Chemical-Induced Tumorigenesis Are Transmitted Independent of NQO1. J. Gerontol. Ser. A. 2019;74:155–162. doi: 10.1093/gerona/gly112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Escobar K.A., Cole N.H., Mermier C.M., VanDusseldorp T.A. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18:e12876. doi: 10.1111/acel.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Chen X.W., O’Flanagan C.H., Coleman M., Der C.J., Hursting S.D. Separate and combined effects of caloric restriction mimetics and autophagy inhibition on KRAS-driven pancreatic adenocarcinoma. Cancer Res. 2018;78 doi: 10.1158/1538-7445.AM2018-3508. [DOI] [Google Scholar]
- 340.Pallauf K., Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013;12:237–252. doi: 10.1016/j.arr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 341.Burge M.R., Aldrete K., Gu Y., VanDyke L., Zhu Y., Myla M., Mudd M., Deretic V. Pro-autophagy, anti-aging effects of metformin in patients with prediabetes. Diabetologia. 2019;62:S288. [Google Scholar]
- 342.Park S.K., Seong R.K., Kim J.A., Son S.J., Kim Y., Yokozawa T., Shin O.S. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway. Nutr. Res. Pract. 2016;10:3–10. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bergamini E., Cavallini G., Donati A., Gori Z. The role of autophagy in aging—Its essential part in the anti-aging mechanism of caloric restriction. Healthy Aging Longev. 2007;1114:69–78. doi: 10.1196/annals.1396.020. [DOI] [PubMed] [Google Scholar]
- 344.Laurent C., Chabi B., Fouret G., Py G., Sairafi B., Elong C., Gaillet S., Cristol J.P., Coudray C., Feillet-Coudray C. Polyphenols decreased liver NADPH oxidase activity, increased muscle mitochondrial biogenesis and decreased gastrocnemius age-dependent autophagy in aged rats. Free Radic. Res. 2012;46:1140–1149. doi: 10.3109/10715762.2012.694428. [DOI] [PubMed] [Google Scholar]
- 345.Nabavi S.F., Sureda A., Dehpour A.R., Shirooie S., Silva A.S., Devi K.P., Ahmed T., Ishaq N., Hashim R., Sobarzo-Sanchez E., et al. Regulation of autophagy by polyphenols: Paving the road for treatment of neurodegeneration. Biotechnol. Adv. 2018;36:1768–1778. doi: 10.1016/j.biotechadv.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 346.Najmanova I., Voprsalova M., Saso L., Mladenka P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. 2019 doi: 10.1080/10408398.2019.1679085. [DOI] [PubMed] [Google Scholar]
- 347.Kanaze F.I., Bounartzi M.I., Georgarakis M., Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- 348.Jian T.Y., Lu H., Ding X.Q., Wu Y.X., Zuo Y.Y., Li J.W., Chen J., Gu H. Polyphenol-rich Trapa quadrispinosa pericarp extract ameliorates high-fat diet induced non-alcoholic fatty liver disease by regulating lipid metabolism and insulin resistance in mice. Peer J. 2019;7:e8165. doi: 10.7717/peerj.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Huang J.B., Zhou Y.B., Wan B., Wang Q.S., Wan X.C. Green tea polyphenols alter lipid metabolism in the livers of broiler chickens through increased phosphorylation of AMP-activated protein kinase. PLoS ONE. 2017;12:e0187061. doi: 10.1371/journal.pone.0187061. [DOI] [PMC free article] [PubMed] [Google Scholar]