Abstract
Seedling rot disease in rice leads to significant loss in the production of seedlings. This research was conducted to explore yeasts that could be used as biological control agents against rice seedling rot disease caused by Curvularia lunata and Helminthosporium oryzae. In total, 167 epiphytic yeast strains were evaluated, revealing that 13 of these yeast strains demonstrated antagonistic activities against fungal pathogens and either C. lunata DOAC 2313 or H. oryzae DOAC 2293. The volatile organic compounds (VOCs) and biofilm produced were possible antagonistic mechanisms in vitro for all the antagonistic yeast strains. Using nursery trays in a greenhouse, this study evaluated the control of rice seedling rot disease caused by these two fungal pathogens using antagonistic yeasts, identified in the present study and from our previous study. Torulaspora indica DMKU-RP31 and Wickerhamomyces anomalus YE-42 were found to completely control rice seedling rot disease caused by both of these fungal pathogens. Furthermore, W. anomalus DMKU-RP04 revealed 100% disease control when the disease was caused by H. oryzae. This is the first report on using antagonistic yeasts to control rice seedling rot disease caused by C. lunata and H. oryzae. These three antagonistic yeasts also showed promising potential for development as biocontrol agents against rice seedling rot disease caused by fungi.
Keywords: epiphytic yeasts, rice seedling rot, biological control, Curvularia lunata, Helminthosporium oryzae, Wickerhamomyces anomalus, Torulaspora indica
1. Introduction
Rice (Oryza sativar) is the most widely produced and consumed staple food in Asian countries [1]. In 2018, the Rice Department in Thailand’s Ministry of Agriculture and Cooperatives reported that Thailand had approximately 59.2 million hectares used for rice cultivation with rice production of 24.2 million tons [2].
In rice cultivation, seeds are germinated in nursery trays and seedlings are transplanted to fields. During rice seed germination and the growth of seedlings, various fungal pathogens may cause diseases in seeds and seedlings [3]. One of the significant diseases in rice seeds and seedlings is seedling rot, caused by various seed-borne and soil-borne fungal pathogens, such as Curvularia lunata, Fusarium oxysporum, Helminthosporium oryzae, Pyricularia oryzae, Pythium spp., and Rhizoctonia solani [2,4,5,6,7]. In Thailand, two of the significant causal agents of rice seedling rot in nursery trays are C. lunata and H. oryzae, as reported by the Rice Department, Ministry of Agriculture and Cooperatives of Thailand [2]. Rice seedling rot disease affects seeds and seedlings of rice plants, with disease symptoms often appearing within a few days of sowing the seeds. Infected seeds become soft and pulpy, and may be surrounded by white mold growth, while some cannot germinate. From the infected seeds that manage to germinate, seedlings usually turn yellow or pale green in color, are weak, grow slowly, and then die. Seedlings may also turn dark brown at the roots and base of the plant. Rice seedling rot disease leads to significant loss of seedling production [6].
The management of rice seedling rot disease caused by fungi is mainly based on the use of chemical fungicide solution in which the seeds are soaked [4,8]. However, the use of synthetic chemical fungicides is not only harmful to humans and the environment, but is also costly [8,9]. Biological control, developed by firstly exploring antagonistic microorganisms as biological control agents, is an effective and sustainable strategy for controlling plant and post-harvest diseases [10,11,12,13,14,15,16]. Microorganisms associated with plants, on both epiphytes (plant surfaces) and endophytes (inside plant tissues), are good sources of potential biocontrol agents [7,11,12,13,14,15]. Microbial antagonists may use both direct and indirect antagonistic mechanisms to control pathogens [13,14,15,16,17,18,19]. Antagonistic mechanisms include emission of antifungal volatile organic compounds (VOCs); secretion of antibiotics; production of fungal cell wall lytic enzymes; competition for nutrients and space; parasitism; and induction of localized and systemic resistance in host plants [13,14,15,16,17]. Antagonistic yeasts, using a variety of biocontrol mechanisms, have been reported to carry out biological control activities against various fungal pathogens that cause plant and post-harvest diseases [13,15,18,19]. However, only one study in literature was reported to have used antagonistic yeasts for biological control disease in rice seeds: in that study, the antagonistic yeasts, Metschnikowia pulcherrima and Pichia guilliermondii, were used to control Fusarium fujikuroi which causes bakanae disease in rice seeds [20].
The objective of the present research was to explore antagonistic yeasts that could be used as biological control agents against rice seedling rot disease caused by two fungal pathogens, C. lunata and H. oryzae. In this research, we evaluated the antagonistic activities of yeasts (that had been isolated from the surfaces of plant leaves) against C. lunata DOAC 2313 and H. oryzae DOAC 2293. The antagonistic yeasts obtained were tested for their antagonistic mechanisms in vivo. Lastly, using nursery trays in a greenhouse, the antagonistic yeasts, obtained from this study and our previous study, were evaluated for their efficacy in controlling rice seedling rot disease caused by these two fungal pathogens.
2. Materials and Methods
2.1. Epiphytic Yeasts
In total, 167 epiphytic yeast strains were isolated from the surfaces of rice, corn, and sugarcane leaves in Thailand and maintained in the Yeast Research Laboratory, Department of Microbiology, Faculty of Science, Kasetsart University, Thailand (Table S1). These yeast strains were grown on yeast extract malt extract (YM) (comprising 3 g/L yeast extract, 3 g/L malt extract, 5 g/L peptone, and 10 g/L glucose) agar at 25 °C.
2.2. Fungal Pathogens
The fungal pathogens, C. lunata DOAC 2313 and Helminthosporium oryzae DOAC 2293, that cause rice seedling rot disease were obtained from the Department of Agriculture, Ministry of Agriculture and Cooperatives, Bangkok, Thailand.
2.3. Evaluation In Vitro of Antagonistic Activities of Epiphytic Yeasts against Fungal Pathogens Causing Rice Seedling Rot Disease
Using dual cultivation [21] with slight modification, the 167 epiphytic yeast strains were evaluated for their antagonistic activities against C. lunata DOAC 2313 and H. oryzae DOAC 2293. For each strain, an active yeast culture obtained by cultivation on YM agar at 25 °C for two days was inoculated on potato dextrose agar (PDA) [Difco™–BBL, Sparks, MD, USA] in a Petri dish by linear streaking 3 cm from the dish edge and incubated at 25 °C for two days. A fungal mycelial plug (5 mm in diameter), obtained from an active fungal pathogen colony seven days at 25 °C on PDA agar) and cut by a cork borer, was placed on the opposite edge of the Petri dish to that previously inoculated with yeast. A PDA dish inoculated only with the fungal pathogen was used as a control. The inoculated dishes were incubated at 25 °C for seven days. Three replicates were performed for each treatment, and the experiment was repeated three times. The percentage of fungal pathogen growth inhibition was calculated as follows:
Growth inhibition (%) = (radius of fungal pathogen colony cultured alone—radius of fungal pathogen colony culture with yeast)/radius of fungal pathogen colony cultured alone × 100.
2.4. Evaluation of Antagonistic Mechanisms of Antagonistic Yeasts
2.4.1. Production of Antifungal Volatile Organic Compounds (VOCs)
The volatile organic compound (VOC) production of the antagonistic yeasts was determined by a double dish assay [22] with slight modification, as described by Into et al. [13]. In brief, an aliquot (100 µL) of yeast cell suspension (108 colony forming units [CFU]/mL) was spread on a PDA dish and incubated at 25°C for two days, while another PDA dish, inoculated with a fungal mycelial plug, was placed upside down instead of the dish cover. The control was a PDA dish inoculated only with fungal pathogens. Three replicates were performed for each treatment and control. The percentage of fungal pathogen growth inhibition was calculated as follows:
Growth inhibition (%) (diameter of fungal pathogen colony cultured alone—diameter of fungal pathogen colony culture with yeast)/diameter of fungal pathogen colony cultured alone × 100.
2.4.2. Competition for Nutrients
Using the dual cultivation method described by Zhang et al. [23], the competition for nutrients was estimated by the amount of fungal pathogen growth inhibition by each antagonistic yeast in a PDA medium containing different nutrient concentrations. The evaluation was performed in the same way as in Section 2.3, but PDA media with four different nutrient concentrations were used. These consisted of: standard nutrient concentration; half of standard nutrient concentration; one-quarter of standard nutrient concentration; and one-tenth of standard nutrient concentration prepared from 39 g/L, 19.5 g/L, 9.7 g/L, and 3.9 g/L Difco™–BBL PDA powder, respectively. Three replicates for each treatment and control were carried out.
2.4.3. Production of β-Glucanase and Chitinase
Production of β-glucanase and chitinase by the antagonistic yeasts was carried out by their cultivation in potato dextrose broth (PDB) [Difco™–BBL, Sparks, MD, USA] at 150 rpm and 25 °C for five days, as described by Into et al. [13]. The culture broth was collected and centrifuged at 10,000× g for 5 min. The supernatant was analyzed for β-glucanase and chitinase activities.
The activities of β-glucanase and chitinase were determined by the colorimetric quantification of reducing sugar and N-acetyl glucosamine (NAG) released from laminarin and colloidal chitin, respectively, as described by Into et al. [13]. The concentrations of reducing sugar and N-acetyl glucosamine were determined by Miller’s [24] method, with β-glucanase and chitinase activities expressed as units (U) per mL. One unit (U) of β-glucanase was defined as 1 µg of reducing sugar released from laminarin per minute under the assay conditions, whereas one unit (U) of chitinase was defined as 1 µg of NAG released from colloidal chitin per minute also under the assay conditions.
2.4.4. Biofilm Formation
The biofilm formation of the antagonistic yeast strains was investigated using Růžička et al.’s [25] method with slight modification, as described by Into et al. [13]. In brief, an aliquot (20 µL) of yeast cell suspension (cells grown on PDA at 25 °C for two days suspended in sterile water and adjusted to an optical density (OD) measured at 600 nm of 0.5) was inoculated into each well of a 96-well microtiter plate containing 180 µL of PDB. The microtiter plate was incubated at 25 °C for 48 h (h). A well containing only PDB was used as a negative control. Three replicates were performed for each treatment. After 48 h, the wells were emptied, rinsed with water, and air-dried at room temperature. The adherent biofilm layer was stained with an aqueous solution of 1% (w/v) crystal violet for 20 min, rinsed with water, and air-dried. The stained biofilm layer was eluted from each well with 200 µL of ethanol, and the OD of each well was measured at 620 nm. Biofilm formation in a well was regarded as positive when the mean OD of the treatment was higher than the mean OD of the negative control (ODc). The following classification was applied in the determination of biofilm formation: weak biofilm producer (ODc < ODt ≤ 2 ODc); moderate biofilm producer (2 ODc < ODt ≤ 4 ODc); and strong biofilm producer (4 ODc < ODt) [26].
2.4.5. Siderophore Production
Siderophore production by the antagonistic yeasts was investigated by their cultivation on chrome azurol S (CAS) [Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany] blue agar in a Petri dish [27]. An aliquot of yeast cell suspension was prepared by suspending a loopful of culture grown on YM agar at 25 °C for two days in 3 mL of 0.85% sterile normal saline, adjusted to an OD600 of 0.10. An aliquot (10 µL) of yeast cell suspension was dropped onto the surface of CAS blue agar and incubated in the dark at 25 °C for 10 days. Three replicates were performed for each treatment. Siderophore production was confirmed by a change in the color of the medium from blue to purple or yellow around the yeast colony.
2.4.6. Phosphate and Zinc Oxide Solubilization
Phosphate and zinc oxide solubilization by the antagonistic yeasts was determined by their cultivation on Pikovskaya’s agar [28] and zinc oxide agar [29], respectively. An aliquot (5 µL) of yeast cell suspension (OD600 of 0.10), prepared in the same way as in Section 2.4.5, was dropped onto the surface of Pikovskaya’s agar or zinc oxide agar in a Petri dish and incubated at 25 °C for five days. Three replicates were performed for each treatment. The solubilization efficiency (SE) of the phosphate and zinc oxide was designed as a ratio of the halo zone diameter and the colony diameter, with both these diameters measured.
2.5. Evaluation of the Efficacy of Antagonistic Yeasts in Controlling Rice Seedling Rot Disease in a Greenhouse
In this study, yeast strains, which showed antagonistic activities against C. lunata DOAC 2313 and/or H. oryzae DOAC 2293, were evaluated for their efficacy in the control of rice seedling rot disease caused by C. lunata or H. oryzae. In addition, from the previous study, the other nine yeast strains were subjected for the same evaluation. They consisted of four strains (Torulaspora indica DMKU-RP31, T. indica DMKU-RP35, Wickerhamomyces anomalus DMKU-RP04 and W. anomalus DMKU-RP25), which revealed antagonistic activities against both fungal pathogens, five strains (Kodamaea ohmeri DMKU-RP06, K. ohmeri DMKU-RP57, K. ohmeri DMKU-RP233 and Meyerozyma caribbica DMKU-RP07) that inhibited only C. lunata DOAC 2313, and one strain (M. caribica DMKU-RP55), which inhibited H. oryzae DOAC 2293 [13].
In the present study, the Thai Jasmine rice cultivar, Khao Dawk Mali 105, was used. Rice seeds (15 g) were surface sterilized by 100 mL of 10% Clorox solution for one minute, rinsed with sterile distilled water, and air dried at room temperature.
Each antagonistic yeast was cultivated in yeast extract peptone dextrose (YPD) broth (comprised of 20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract) on a rotary shaker at 150 rpm, at 25 °C for 48 h (h). Yeast cell suspension was prepared by suspending cells collected from the culture broth by centrifugation at 5000× g for 10 min in the sterile Ringer solution. Cells were quantified with a hemacytometer to reach a concentration of 108 cells/mL.
Fungal pathogens were grown on PDA at 25 °C for seven days, with the spore suspension (105 spores/mL) prepared in sterile 0.05% Tween 20. Soil was put in a plastic bag and sterilized in an autoclave at 121 °C for 30 min. The sterilization was performed twice.
Infected rice seed was prepared, as described by Khalili et al. [12]. Sterilized rice seeds were soaked in the spore suspension (105 spores/mL) of each fungal pathogen and then shaken on a rotary shaker (TAITAC BR-300LF, Saitama, Japan), at 100 rpm, at room temperature (28–32 °C) for 24 h and then air-dried. The infected seeds were soaked in the yeast cell suspension (108 cells/mL) for two hours; 100 treated seeds were then transplanted into a plastic tray (20 cm wide × 35 cm long × 10 cm high) containing sterile soil (4 kg). The seeds were sown in four rows: in each row, 16–17 seeds were placed, with each seed 2–3 cm apart from the other seeds. The tray was incubated in greenhouse conditions (11 h light/13 h dark, and at 30–34 °C day/20–24 °C night) for 45 days. Three replicates were performed for each treatment (3 × 100 seeds). The number of germinated seeds, and seedlings’ stem height, root length and dry weight were recorded.
The percentages of seed germination, seedling vigor index (SVI), disease incidence and disease control were calculated as follows:
Seed germination (%) = (number of germinated seeds/total number of seeds planted) × 100
Seedling vigor index (SVI) (%) = (stem height + root length) × seed germination (%)
Disease incidence (%) = [(SVI of negative control − SVI of positive control or treatment)/SVI of negative control] × 100
Disease control (%) = [(disease incidence of positive control − disease incidence of each treatment)/disease incidence in positive control] × 100
2.6. Statistical Analysis
Data were analyzed with statistical analysis software IBM® SPSS Statistics version 22 (Armonk, NY, USA). All data were first subjected to analysis of variance (ANOVA). Means were compared using Duncan’s multiple range test and a significance level of p ≤ 0.05 was considered as being significantly different.
3. Results
3.1. Evaluation in Vitro of Antagonistic Activities of Epiphytic Yeasts against Fungal Pathogens Causing Rice Seedling Rot Disease
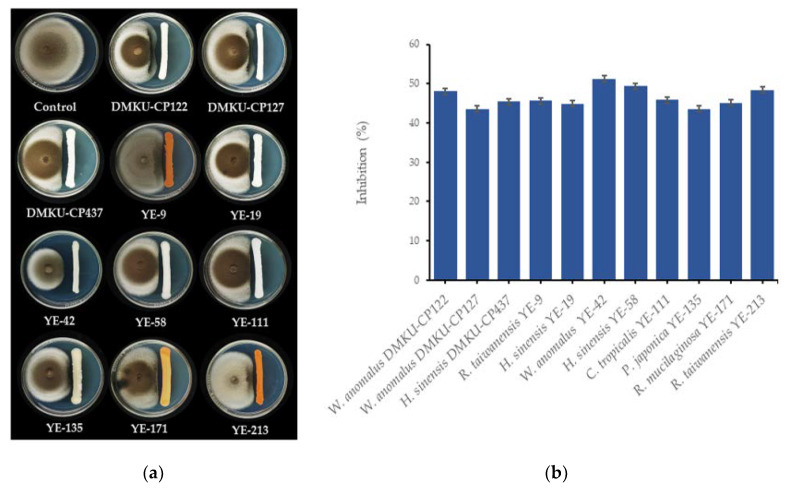
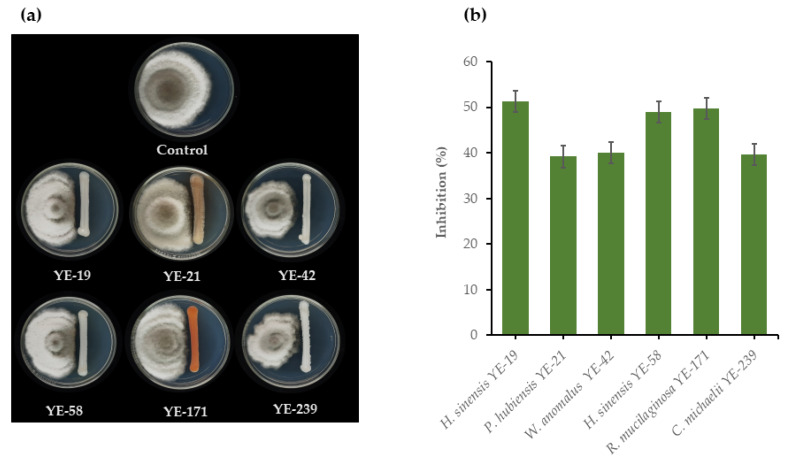
Evaluation of the antagonistic activities of 167 epiphytic yeasts against two rice seedling rot fungal pathogens, C. lunata DOAC 2313 and H. oryzae DOAC 2293, revealed that only 13 yeast strains from eight species possessed these antagonistic activities. These species were Candida michaelii (one strain), Candida tropicalis (one strain), Hannaella sinensis (three strains), Papiliotrema japonica (one strain), Pseudozyma hubiensis (one strain), Rhodotorula mucilaginosa (one strain), Rhodotorula taiwanensis (two strains), and W. anomalus (three strains). Of the antagonistic yeast strains, 11 inhibited the growth of C. lunata DOAC 2313 by 43.5%–51.2%, while W. anomalus YE-42 was the antagonist with the strongest inhibitory effect (Figure 1). Six antagonistic yeasts inhibited the growth of H. oryzae DOAC 2293 by 39.2%–51.2%, with H. sinensis YE-19 the antagonist with the strongest inhibitory effect (Figure 2). Moreover, four yeast strains, namely, H. sinensis YE-19, H. sinensis YE-58, R. mucilaginosa YE-171, and W. anomalus YE-42, inhibited both fungal pathogens that caused rice seedling rot disease (Figure 1 and Figure 2).
Figure 1.
(a) Growth on potato dextrose agar (PDA) at 25 °C for seven days of Curvularia lunata DOAC 2313 alone, and dual cultured with Wickerhamomyces anomalus DMKU-CP122, W. anomalus DMKU-CP127, Hannaella sinensis DMKU-CP437, Rhodotorula taiwanensis YE-9, H. sinensis YE-19, W. anomalus YE-42, H. sinensis YE-58, Candida tropicalis YE-111, Papiliotrema japonica YE-135, Rhodotorula mucilaginosa YE-171, or R. taiwanensis YE-213; and (b) inhibition of growth of C. lunata DOAC 2313 by 11 antagonistic yeast strains determined by dual cultivation on PDA at 25 °C for seven days.
Figure 2.
(a) Growth on PDA at 25 °C for seven days of Helminthosporium oryzae DOAC 2293 alone, and dual cultured with Hannaella sinensis YE-19, Pseudozyma hubiensis YE-21, Wickerhamomyces anomalus YE-42, H. sinensis YE-58, Rhodotorula mucilaginosa YE-171 and Candida michaelii YE-239; and (b) Inhibition of H. oryzae DOAC 2293 growth by six antagonistic yeast strains determined by dual cultivation on PDA at 25 °C for seven days.
3.2. Evaluation of Antagonistic Mechanisms of Antagonistic Yeasts
3.2.1. Production of Antifungal Volatile Organic Compounds (VOCs)
The present study evaluated the antagonistic activities against C. lunata DOAC 2313 and H. oryzae DOAC 2293 by VOCs production form 11 and six antagonistic yeast strains, respectively. The results revealed that all antagonistic yeast strains produced VOCs inhibited growth of their related fungal pathogens but with lower inhibition percentages than using yeast cultures (Table 1). The 10 antagonistic yeast strains tested inhibited C. lunata DOAC 2313 growth by 4.2–32.1% and W. anomalus YE-42 produced VOCs that showed the strongest inhibitory effect on this fungal pathogen. On the other hand, the six antagonistic yeast strains tested with H. oryzae DOAC 2293 produced VOCs inhibited this fungal pathogen in the range of 0.7% to 25.1% and W. anomalus YE-42 revealed the strongest antifungal VOCs activity. However, H. sinensis YE-19, which showed the strongest inhibitory effect when tested by dual cultivation with H. oryzae DOAC 2293 produced VOCs inhibited this fungal pathogen only by 1%.
Table 1.
Growth inhibition of Curvularia lunata DOAC 2313 and Helminthosporium oryzae DOAC 2293 by antifungal volatile organic compounds (VOCs) produced by antagonistic yeasts, on PDA containing different nutrient concentrations.
| Fungal Pathogen and Yeast | Growth Inhibition by VOCs (%) 1 | Growth Inhibition on PDA with Different Nutrient Concentration 2 | |||
|---|---|---|---|---|---|
| A 3 | B 4 | C 5 | D 6 | ||
| Curvularia lunata DOAC 2313 with | |||||
| Candida tropicalis YE-111 | 20.1 ± 1.4c | 45.9 ± 2.4a | 25.2 ± 2.0b | 0c | 0c |
| Hannaella sinensis DMKU-CP437 | 14.3 ± 1.6f | 45.4 ± 1.3a | 17.1 ± 1.3b | 0c | 0c |
| Hannaella sinensis YE-19 | 17.4 ± 0.8de | 44.8 ± 0.6a | 17.9 ± 0.7b | 0c | 0c |
| Hannaella sinensis YE-58 | 5.5 ± 2.2h | 49.3 ± 0.2a | 21.8 ± 1.6b | 0c | 0c |
| Papiliotrema japonica YE-135 | 4.2 ± 0.8h | 43.6 ± 1.9a | 20.1 ± 1.6 | 0c | 0c |
| Rhodotorula mucilaginosa YE-171 | 10.4 ± 0.9g | 45.1 ± 2.0a | 19.4 ± 1.0b | 0c | 0c |
| Rhodotorula taiwanensis YE-9 | 32.1 ± 1.0b | 45.6 ± 1.9a | 17.4 ± 0.8b | 0c | 0c |
| Rhodotorula taiwanensis YE-213 | 31.8 ± 1.0b | 48.4 ± 0.5a | 19.0 ± 5.6 | 0c | 0c |
| Wickerhamomyces anomalus DMKU-CP122 | 19.8 ± 0.9cd | 48.1 ± 1.4a | 21.2 ± 1.1b | 6.1 ± 1.2c | 0d |
| Wickerhamomyces anomalus DMKU-CP127 | 15.3 ± 1.4ef | 43.5 ± 3.0a | 22.0 ± 1.2b | 3.0 ± 1.8c | 0d |
| Wickerhamomyces anomalus YE-42 | 48.9 ± 0.8a | 51.2 ± 1.6a | 35.2 ± 1.3b | 0c | 0c |
| Helminthosporium oryzae DOAC 2293 with | |||||
| Candida michaelii YE-239 | 12.6 ± 0.8b | 39.6 ± 1.9a | 20.9 ± 0.8b | 5.7 ± 1.4c | 0d |
| Hannaella sinensis YE-19 | 1.0 ± 1.2d | 51.2 ± 1.4a | 39.1 ± 1.1b | 18.2 ± 0.9c | 2.3 ± 2.4d |
| Hannaella sinensis YE-58 | 0.7 ± 1.3d | 49.0 ± 3.5a | 23.7 ± 0.7b | 7.5 ± 2.1c | 0d |
| Hannaella hubeiensis YE-21 | 11.0 ± 0.2bc | 39.2 ± 1.8a | 21.1 ± 1.1b | 11.9 ± 1.8c | 0d |
| Rhodotorula mucilaginosa YE-171 | 9.3 ± 1.0c | 49.7 ± 2.5a | 26.8 ± 1.8b | 7.0 ± 1.4c | 0.5 ± 1.4d |
| Wickerhamomyces anomalus YE-42 | 25.1 ± 1.0a | 40.0 ± 1.2a | 22.0 ± 1.8b | 17.1 ± 0.7c | 0d |
1 Inhibition (%) = (Diameter of fungal colony grow alone—Diameter of fungal colony grow with yeast/Diameter of colony grow alone) ×100; Each value represents a mean “ ± ” standard deviation (SD). In the same column for each rice pathogenic fungus tested data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. 2 Inhibition (%) = (Radius of control fungal colony—Radius of fungal colony grow with yeast/Radius of control fungal colony) ×100; Each value represents a mean “ ± ” standard deviation (SD). In the same row data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. 3 Standard nutrient concentration (39 g/L PDA powder). 4 Half of standard nutrient concentration (19.5 g/L PDA powder). 5 One-fourth of standard nutrient concentration (9.7 g/L PDA powder). 6 One-tenth of standard nutrient concentration (3.9 g/L PDA powder).
3.2.2. Production of β-Glucanase and Chitinase
The production of β-glucanase and chitinase by the 13 antagonistic yeast strains was determined by their cultivation in PDB at 25 °C for five days with observation of the enzymatic activities of cell-free culture broth. The results revealed that β-glucanase was produced by 11 strains: however, only a small quantity (0.2–116.5 mU/mL) was produced, with R. mucilaginosa YE-171 producing the highest amount (Table 2). Eight antagonistic yeast strains produced small amounts (1.0–504.5 mU/mL) of chitinase, while P. japonica YE-135 produced the largest amount.
Table 2.
Activities of cell wall lytic enzymes produced by antagonistic yeasts.
| Yeast | Enzyme Activities (mU/mL) | |
|---|---|---|
| Glucanase | Chitinase | |
| Candida michaelii YE-239 | 62.6 ± 0.8 | 6.0 ± 18.3 |
| Candida tropicalis YE-111 | 102.4 ± 3.0 | 16.9 ± 29.5 |
| Hannaella sinensis DMKU-CP437 | 0.2 ± 0.0 | 35.2 ± 3.5 |
| Hannaella sinensis YE-19 | 8.5 ± 3.1 | 3.8 ± 3.8 |
| Hannaella sinensis YE-58 | 0.2 ± 0.5 | 0 |
| Papiliotrema japonica YE-135 | 112.9 ± 0.8 | 504.5 ± 148.4 |
| Pseudozyma hubiensis YE-21 | 27.5 ± 1.8 | 0 |
| Rhodotorula mucilaginosa YE-171 | 116.5 ± 1.9 | 173.4 ± 17.7 |
| Rhodotorula taiwanensis YE-9 | 58.9 ± 1.5 | 1.0 ± 3.2 |
| Rhodotorula taiwanensis YE-213 | 0 | 0 |
| Wickerhamomyces anomalus DMKU-CP122 | 30.3 ± 4.5 | 45.2 ± 1.8 |
| Wickerhamomyces anomalus DMKU-CP127 | 0 | 0 |
| Wickerhamomyces anomalus YE-42 | 48.9 ± 3.5 | 0 |
3.2.3. Biofilm Formation
The biofilm formation of the 13 antagonistic yeast strains was determined, with the results indicating that all 13 strains formed biofilm. Of the 13 strains, eight revealed moderate biofilm formation, while four showed weak biofilm formation. The only strain with strong biofilm formation was C. tropicalis YE-111 (Table 3).
Table 3.
Biofilm formation, siderophore production, and solubilization of zinc oxide and phosphate by antagonistic yeasts.
| Yeast | Biofilm formation | Siderophore Production 4 |
SE e | |||
|---|---|---|---|---|---|---|
| ODT 1 | OD Value 2 | Sum 3 | Ca3(PO)4 | ZnO | ||
| Candida michaelii YE-239 | 0.0973 ± 0.0023 | 1.68 | weak | 0 | 0 | 0 |
| Candida tropicalis YE-111 | 0.3025 ± 0.0085 | 5.21 | strong | 0 | 0 | 0 |
| Hannaella sinensis DMKU-CP437 | 0.2296 ± 0.0141 | 3.96 | moderate | 0 | 0 | 0 |
| Hannaella sinensis YE-19 | 0.2054 ± 0.0096 | 3.54 | moderate | 0 | 0 | 0 |
| Hannaella sinensis YE-58 | 0.2130 ± 0.0133 | 3.67 | moderate | 0 | 0 | 0 |
| Papiliotrema japonica YE-135 | 0.1263 ± 0.0026 | 2.18 | moderate | 0 | 0 | 0 |
| Pseudozyma hubiensis YE-21 | 0.1155 ± 0.0065 | 1.99 | weak | 46.5 ± 1.2 | 0 | 0 |
| Rhodotorula mucilaginosa YE-171 | 0.3586 ± 0.0194 | 6.18 | weak | 9.1 ± 0.2 | 0 | 0 |
| Rhodotorula taiwanensis YE-9 | 0.2389 ± 0.0060 | 4.12 | weak | 15.5 ± 0.5 | 0 | 0 |
| Rhodotorula taiwanensis YE-213 | 0.2062 ± 0.0130 | 3.56 | moderate | 14.1 ± 0.1 | 0 | 0 |
| Wickerhamomyces anomalus DMKU-CP122 | 0.1831 ± 0.0156 | 3.16 | moderate | 32.8 ± 1.1 | 1.3 ± 0.1 | 1.3 ± 0.0 |
| Wickerhamomyces anomalus DMKU-CP127 | 0.1331 ± 0.0057 | 2.29 | moderate | 32.4 ± 1.6 | 1.4 ± 0.0 | 1.3 ± 0.0 |
| Wickerhamomyces anomalus YE-42 | 0.2143 ± 0.0058 | 3.69 | moderate | 34.5 ± 0.6 | 1.5 ± 0.1 | 1.4 ± 0.1 |
1 The optical density at 620 nm of biofilm layer stained with crystal violet (ODT), data express as average ODT ± SD). 2 Average optical density of biofilm layer (ODT) as a portion of optical density of control (ODC). 3 Interpretation of biofilm formation: ODT ≤ Ac, no biofilm formation; weak biofilm producer (ODC < ODT ≤ 2 ODC), moderate biofilm producer (2 ODC < ODT ≤ 4 ODC) and strong biofilm producer (4 ODC < ODT), when ODC = 0.0580). 4 Diameter of holo zone (cm). e Solubilization efficiency (SE) = Diameter of the halo zone (cm)/Diameter of the colony (cm).
3.2.4. Siderophore Production
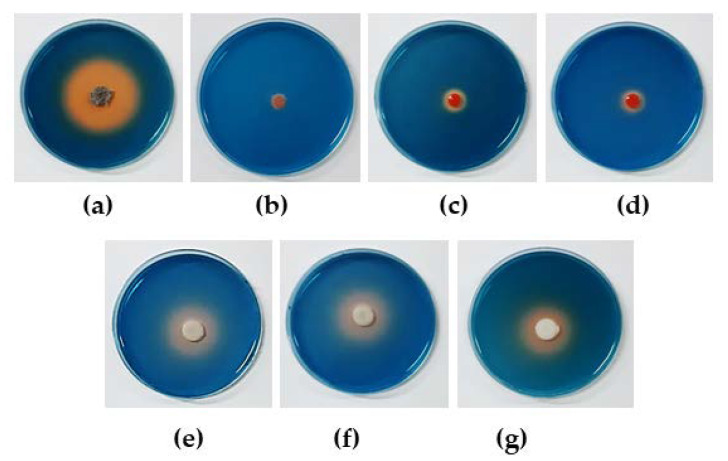
Determination of the siderophore production of the antagonistic yeast strains on chrome azurol sulfonate (CAS) agar dishes revealed that seven strains, namely P. hubiensis YE-21, R. mucilaginosa YE-71, R. taiwanensis YE-9, R. taiwanensis YE-213, W. anomalus DMKU-CP122, W. anomalus DMKU-CP127, and W. anomalus YE-42, formed orange halo zones (9.1–46.5 mm) around the colony (Table 3, Figure 3). This result indicated that these seven antagonistic yeast strains produced siderophores.
Figure 3.
Siderophore production by antagonistic yeasts on chrome azurol S blue agar incubated for 10 days; (a) Pseudozyma hubiensis YE-21; (b) Rhodotorula mucilaginosa YE-171; (c) Rhodotorula taiwanensis YE-9; (d) R. taiwanensis YE-213; (e) Wickerhamomyces anomalus DMKU-CP122; (f) W. anomalus DMKU-CP127; and (g) W. anomalus YE-42.
3.2.5. Phosphate and Zinc Oxide Solubilization
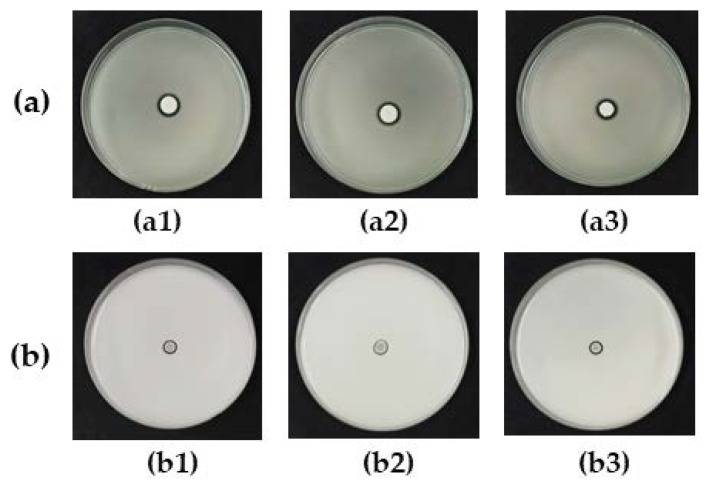
The phosphate and zinc oxide solubilizing activity of all antagonistic yeasts were determined on Pikovskayaʹs agar and zinc oxide agar, respectively, showing that only three strains of W. anomalus grew and produced halo zones around colonies. The phosphate and zinc oxide solubilization efficiency (SE) units were in the range of 1.3–1.5 (Table 3, Figure 4).
Figure 4.
Solubilization of (a) phosphate on Pikovskaya’s agar and (b) zinc oxide on zinc oxide agar incubated for 5 days by antagonistic yeasts (a1,b1) Wickerhamomyces anomalus DMKU-CP122; (a2,b2) W. anomalus DMKU-CP127; and (a3,b3) W. anomalus YE-42.
3.3. Evaluation of the Control of Rice Seedling Rot Disease in a Greenhouse
The efficacy of antagonistic yeasts, obtained from the present study and the previous study, in the control of rice seedling rot disease caused by C. lunata DOAC 2313 and H. oryzae DOAC 2293 were evaluated in nursery trays in a greenhouse. The two chemical fungicides, carbendazim® and mancozeb®, were used for comparison.
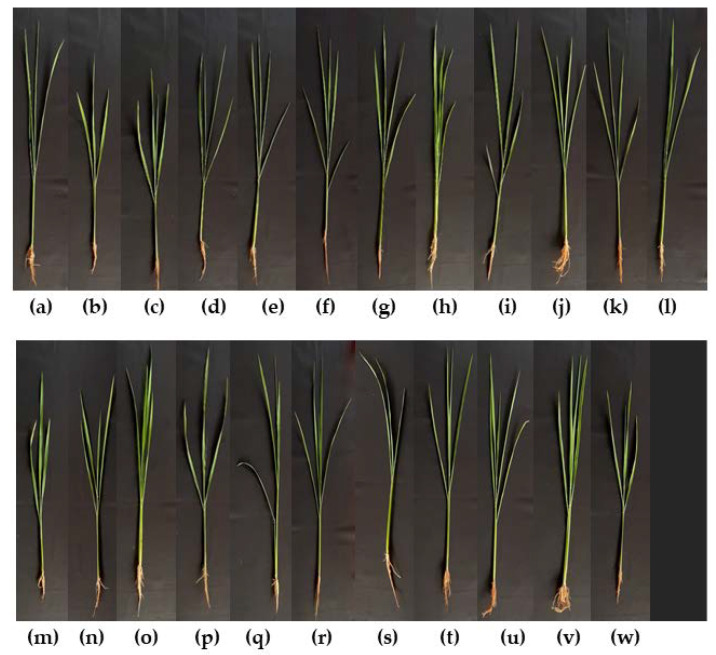
To test the control of rice seedling rot disease caused by C. lunata DOAC, 19 antagonistic yeast strains were tested. T. indica DMKU-RP31 and W. anomalus YE-42 were found to show higher seedling vigor index (SVI) percentages than the negative control and carbendazim®: these two strains resulted in no disease incidence and, consequently, 100% disease control (Table 4, Figure 5). Fourteen of the 19 antagonistic yeast strains showed no significantly different SVI percentages when compared with the negative control and carbendazim®: they resulted in disease incidence ranging from 2.17%–16.57% and, consequently, disease control of 45.70%–92.88%. The remaining four yeast strains revealed lower SVI percentages than the negative control and carbendazim®: they resulted in low disease control ranging from 33.19%–42.50%. When using the two chemical fungicides, carbendazim® and mancozeb®, the study revealed 6.27% and 30.48% disease incidence and, consequently, 79.47% and 0.15% disease control, respectively.
Table 4.
Control of rice seedling rot disease caused by Curvularia lunata DOAC 2313 by antagonistic yeasts and chemical fungicides.
| Treatment | Stem (cm) | Root (cm) | Dry Weight (g) 2 | Seed Germination (%) | Seedling Vigor Index | Disease Incidence (%) | Disease Control (%) |
|---|---|---|---|---|---|---|---|
| Negative control | 51.87 ± 6.75abc | 10.70 ± 4.22cd | 120.09 ± 6.63abc | 89.00 ± 2.45a | 5569.73 ± 218.38a | - | - |
| Positive control: Curvularia lunata DOAC 2313 alone | 46.02 ± 7.17i | 8.48 ± 3.49e | 71.73 ± 7.25d | 71.00 ± 4.90gh | 3869.67 ± 177.95g | 30.52 | - |
| Curvularia lunata DOAC 2313 with | |||||||
| Candida tropicalis YE-111 | 47.86 ± 6.88h | 11.01 ± 3.92bcd | 86.19 ± 11.34d | 77.67 ± 4.19cdefg | 4572.33 ± 325.00ef | 17.91 | 41.33 |
| Hannaella sinensis YE-19 | 49.27 ± 7.18efg | 12.01 ± 4.52a | 110.55 ± 7.88abc | 83.00 ± 3.27abcde | 5085.67 ± 226.99abcde | 8.69 | 71.52 |
| Hannaella sinensis YE-58 | 49.65 ± 6.87ef | 11.31 ± 4.08abcd | 105.28 ± 8.00bc | 75.33 ± 3.40efg | 4592.33 ± 242.28def | 17.55 | 42.50 |
| Hannaella sinensis DMKU-CP437 | 50.91 ± 5.85cd | 11.32 ± 4.15abcd | 84.68 ± 10.29d | 74.67 ± 3.68fg | 4646.67 ± 289.27cdef | 16.57 | 45.70 |
| Kodamaea ohmeri DMKU-RP06 1 | 50.37 ± 6.23de | 11.54 ± 3.98abc | 104.89 ± 6.62bc | 81.67 ± 3.40abcdef | 5055.67 ± 166.01abcde | 9.23 | 69.76 |
| Kodamaea ohmeri DMKU-RP57 1 | 51.65 ± 6.04abc | 11.00 ± 4.03bcd | 102.30 ± 5.70c | 82.33 ± 3.68abcdef | 5158.33 ± 233.27abcde | 7.39 | 75.80 |
| Kodamaea ohmeri DMKU-RP233 1 | 51.44 ± 5.86abcd | 11.34 ± 3.83abc | 116.72 ± 4.35abc | 86.33 ± 2.87ab | 5419.33 ± 172.56a | 2.70 | 91.15 |
| Meyerozyma caribbica DMKU-RP07 1 | 52.23 ± 6.34ab | 11.15 ± 4.27abcd | 114.81 ± 2.93abc | 83.00 ± 2.16abcdef | 5259.37 ± 147.95abc | 5.57 | 81.74 |
| Papiliotrema japonica YE-135 | 52.10 ± 5.21abc | 10.99 ± 3.77bcd | 109.54 ± 3.67abc | 81.00 ± 1.63abcdef | 5110.00 ± 25.66abcde | 8.25 | 72.96 |
| Rhodotorula mucilaginosa YE-171 | 51.20 ± 6.82bcd | 10.85 ± 3.68cd | 116.82 ± 11.73abc | 85.67 ± 3.68abc | 5316.00 ± 250.50ab | 4.56 | 85.07 |
| Rhodotorula taiwanensis YE-9 | 48.16 ± 7.25gh | 10.44 ± 4.16d | 84.76 ± 5.02d | 75.67 ± 4.92defg | 4434.00 ± 326.70f | 20.39 | 33.19 |
| Rhodotorula taiwanensis YE-213 | 48.58 ± 6.81fgh | 11.58 ± 4.02abc | 82.95 ± 7.59d | 78.67 ± 3.30bcdefg | 4733.70 ± 232.82bcdef | 15.02 | 50.78 |
| Torulaspora indica DMKU-RP31 1 | 52.18 ± 4.87abc | 11.16 ± 4.05abcd | 122.91 ± 8.80ab | 88.33 ± 2.49a | 5595.00 ± 302.03a | 0 | 100 |
| Torulaspora indica DMKU-RP35 1 | 52.24 ± 5.25ab | 10.84 ± 3.75cd | 114.63 ± 3.07abc | 83.67 ± 2.49abcde | 5277.67 ± 179.00ab | 5.24 | 82.82 |
| Wickerhamomyces anomalus DMKU-CP122 | 51.98 ± 5.44abc | 11.43 ± 3.93abc | 105.23 ± 8.43bc | 82.00 ± 4.55abcdef | 5199.67 ± 472.67abcd | 6.64 | 78.23 |
| Wickerhamomyces anomalus DMKU-CP127 | 52.52 ± 5.00a | 11.43 ± 4.42abc | 115.15 ± 3.52abc | 83.67 ± 3.30abcde | 5350.33 ± 358.39ab | 3.94 | 87.09 |
| Wickerhamomyces anomalus DMKU-RP04 1 | 52.48 ± 4.90ab | 11.12 ± 3.91bcd | 115.81 ± 2.15abc | 85.67 ± 4.99abc | 5448.67 ± 349.44a | 2.17 | 92.88 |
| Wickerhamomyces anomalus DMKU-RP25 1 | 51.47 ± 5.98abcd | 11.20 ± 3.99abcd | 112.51 ± 11.4abc | 84.00 ± 2.16abcd | 5264.33 ± 172.62abc | 5.48 | 82.03 |
| Wickerhamomyces anomalus YE-42 | 52.45 ± 4.60ab | 11.26 ± 3.84abcd | 121.52 ± 10.59ab | 88.67 ± 2.87a | 5649.00 ± 338.01a | 0 | 100 |
| Carbendazim® | 52.61 ± 5.55a | 11.84 ± 4.13ab | 127.73 ± 4.54a | 81.00 ± 2.94abcdef | 5220.67 ± 239.98abc | 6.27 | 79.47 |
| Mancozeb® | 48.78 ± 6.55fgh | 11.10 ± 4.67bcd | 79.94 ± 12.34d | 64.67 ± 3.68h | 3872.33 ± 183.03g | 30.48 | 0.15 |
Each value represents a mean “ ± ” standard deviation (SD). In the same column, data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. 1 Antagonistic yeasts from previous investigation [13]. 2 Dry weight of 100 rice plants nd = not determined.
Figure 5.
Rice seedlings (45-day-old) obtained from (a) seed treated with sterile water (negative control); (b) seed inoculated with C. lunata DOAC 2313 alone (positive control); and seed inoculated with C. lunata DOAC 2313 (c) with C. tropicalis YE-111; (d) with H. sinensis YE-19; (e) with H. sinensis YE-58; (f) with H. sinensis DMKU-CP437; (g) with K. ohmeri DMKU-RP06; (h) with K. ohmeri DMKU-RP57; (i) with K. ohmeri DMKU-RP233; (j) with M. caribica DMKU-RP07; (k) with P. japonica YE-135; (l) with R. mucilaginosa YE-171; (m) with R. taiwanensis YE-9; (n) with R. taiwanensis YE-213; (o) with T. indica DMKU-RP31; (p) with T. indica DMKU-RP35; (q) with W. anomalus DMKU-CP122; (r) with W. anomalus DMKU-CP127; (s) with W. anomalus DMKU-RP04; (t) with W. anomalus DMKU-RP25; (u) with W. anomalus YE-42; (v) with carbendazim®; or (w) with mancozeb®.
Evaluation of 11 antagonistic yeast strains was conducted to investigate if they had an inhibitory effect on H. oryzae DOAC 2293 to control rice seedling rot disease which it caused. T. indica DMKU-RP31, W. anomalus DMKU-RP04, and W. anomalus YE-42 showed higher SVI percentages than the negative control and carbendazim®; these strains resulted in no disease incidence and, consequently, 100% disease control (Table 5, Figure 6). Six antagonistic strains revealed no SVI percentages that were significantly different and, consequently, disease control ranged from 75.27%–97.45%. The remaining three strains showed lower SVI percentages than the negative control and carbendazim® and, consequently, 44.95%–63.37% disease control. Mancozeb® revealed the lowest disease control (of 3.05%) whereas carbendazim® showed a high level of disease control at 94.04%.
Table 5.
Control of rice seedling rot disease caused by Helminthosporium oryzae DOAC 2293 by antagonistic yeasts and chemical fungicides.
| Treatment | Stem (cm) | Root (cm) | Dry Weight (g) 2 | Seed Germination (%) | Seedling Vigor Index | Disease Incidence (%) | Disease Control (%) |
|---|---|---|---|---|---|---|---|
| Negative control | 51.87 ± 6.75a | 10.70 ± 4.22d | 120.09 ± 6.63ab | 89.00 ± 2.45a | 5544.00 ± 209.93ab | nd | nd |
| Positive control: Helminthosporium oryzae DOAC 2293 alone | 45.84 ± 6.95d | 8.53 ± 3.56e | 82.57 ± 3.31e | 76.00 ± 1.63e | 4132.00 ± 120.78e | 25.47 | nd |
| Helminthosporium oryzae oryzae DOAC 2293 with | |||||||
| Candida michaelii YE-239 | 52.44 ± 5.47a | 12.15 ± 8.12a | 103.56 ± 1.40cd | 81.33 ± 1.70bcde | 5253.67 ± 97.04bc | 5.24 | 79.44 |
| Hannaella sinensis YE-19 | 49.07 ± 6.17c | 10.52 ± 3.95d | 95.27 ± 2.14d | 80.00 ± 2.16cde | 4766.67 ± 88.21d | 14.02 | 44.95 |
| Hannaella sinensis YE-58 | 52.38 ± 4.46a | 11.25 ± 4.07bcd | 94.47 ± 1.41d | 79.00 ± 2.16de | 5027.00 ± 92.61cd | 9.33 | 63.37 |
| Meyerozyma caribbica DMKU-RP55 1 | 52.06 ± 6.15a | 11.03 ± 4.30bcd | 101.86 ± 3.84d | 82.33 ± 2.87bcd | 5195.00 ± 194.37bc | 6.30 | 75.27 |
| Pseudozyma hubiensis YE-21 | 50.72 ± 6.05b | 10.78 ± 4.03cd | 105.60 ± 0.84cd | 86.67 ± 2.49ab | 5330.33 ± 209.48abc | 3.85 | 84.88 |
| Rhodotorula mucilaginosa YE-171 | 52.35 ± 5.27a | 10.92 ± 3.68bcd | 94.97 ± 2.12d | 78.67 ± 2.87de | 4977.33 ± 142.64cd | 10.22 | 59.87 |
| Torulaspora indica DMKU-RP31 1 | 52.24 ± 4.95a | 11.08 ± 4.06bcd | 123.22 ± 0.58ab | 90.33 ± 0.94a | 5719.33 ± 120.63a | 0 | 100 |
| Torulaspora indica DMKU-RP35 1 | 52.37 ± 5.17a | 10.94 ± 3.83bcd | 117.15 ± 3.36ab | 87.00 ± 2.94ab | 5508.00 ± 247.92ab | 0.65 | 97.45 |
| Wickerhamomyces anomalus DMKU-RP04 1 | 52.26 ± 4.94a | 10.77 ± 3.92cd | 118.11 ± 3.99ab | 88.33 ± 2.05a | 5567.33 ± 200.73ab | 0 | 100 |
| Wickerhamomyces anomalus DMKU-RP25 1 | 51.75 ± 5.62a | 11.27 ± 4.14bcd | 109.58 ± 3.27bc | 85.00 ± 1.63abc | 5357.33 ± 121.26abc | 3.37 | 86.77 |
| Wickerhamomyces anomalus YE-42 | 52.39 ± 4.82a | 11.65 ± 4.24abc | 121.85 ± 7.20ab | 89.00 ± 1.41a | 5699.33 ± 189.84a | 0 | 100 |
| Carbendazim® | 52.59 ± 5.57a | 11.85 ± 4.06ab | 127.73 ± 4.54a | 84.67 ± 3.68abc | 5445.67 ± 238.94ab | 1.77 | 94.04 |
| Mancozeb® | 48.83 ± 6.51c | 11.22 ± 4.61bcd | 79.94 ± 8.34e | 69.67 ± 4.11f | 4175.92 ± 265.19e | 24.69 | 3.05 |
Each value represents a mean “ ± ” standard deviation (SD). In the same column, data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. 1 Antagonistic yeasts from previous investigation [13]. 2 Dry weight of 100 rice plants. nd = not determined.
Figure 6.
Rice seedlings (45-day-old) obtained from (a) seed treated with sterile water (negative control); (b) seed inoculated with H. oryzae DOAC 2293 alone (positive control); and seed inoculated with H. oryzae DOAC 2293 (c) with C. michaelii YE-239; (d) with H. sinensis YE-19; (e) with H. sinensis YE-58; (f) with M. caribica DMKU-RP55; (g) with P. hubiensis YE-21; (h) with K. ohmeri DMKU-RP57; (i) with R. mucilaginosa YE-171; (j) with T. indica DMKU-RP31; (k) with P. japonica YE-135; (l) with W. anomalus DMKU-RP04; (m) with W. anomalus YE-42; (n) with carbendazim®; or (o) with mancozeb®.
4. Discussion
The results of this study revealed that the yeast species showing antagonistic activity against the two fungal pathogens, C. lunata DOAC 2313 and H. oryzae DOAC 2293, the causes of rice seedling rot disease, were from both yeast phyla, namely, phyla Ascomycota (C. michaelii, C. tropicalis, and W. anomalus) and Basidiomycota (H. sinensis, P. japonica, P. hubeiensis, R. mucilaginosa, and R. taiwanensis). Some antagonistic yeast species found in the present study have previously been reported to possess antagonistic activities. W. anomalus was reported for its ability to antagonize various fungal pathogens, the causes of plant and post-harvest diseases, such as Alternaria alternata, Aspergillus carbonarius, C. lunata, Botrytis cinerea, Fusarium moniliforme, Helmintosporium oryzae, Monilinia fructicola, Penicillium expansum, Rhizoctonia solani, Cladosporium spp., and Colletotrichum spp. [14,30,31]. C. tropicalis revealed the ability to control black rot in pineapple caused by Chalara paradoxa [32]. Cryptococcus albidus was found to be the effective antagonistic yeast against P. expansum and apple blue mold [30]. H. sinensis and R. taiwanensis were reported for their antagonistic activities against Aspergilus flavus [33,34]. R. mucilaginosa demonstrated the ability to antagonize Botrytis cinerea, the cause of gray mold spoilage in strawberries [35] and Penicillium expansum, the cause of post-harvest apple disease [36]. P. hubeiensis could control Lasiodiplodia theobromae [15].
In the present study, the antagonistic mechanisms of 13 yeast strains from eight species were evaluated in vitro, with the results revealing that all strains possessed the ability to produce antifungal VOCs and biofilm to antagonize C. lunata and H. oryzae. Some strains produced both of the cell wall lytic enzymes, β-glucanase and chitinase, some strains produced only β-glucanase, whereas some did not produce either enzyme. The production of cell wall lytic enzymes appeared to be a strain-specific characteristic, not a species-specific characteristic. The antagonistic yeast strains of four species, namely, P. hubiensis, R. mucilaginosa, R. taiwanensis, and W. anomalus produced siderophores, with W. anomalus also capable of phosphate and zinc oxide solubilization. Therefore, the antagonistic mechanisms of all the yeast strains were found to emit antagonistic antifungal VOCs and to form biofilm. Some yeast strains could possibly have additional mechanisms.
As shown in the present study, the production of antifungal VOCs, which are low molecular weight chemical compounds with high vapor pressure, played a role in the antagonistic activity of C. michaelii, C. tropicalis, H. sinensis, P. japonica, P. hubeiensis, R. mucilaginosa, R. taiwanensis, and W. anomalus. This result was in agreement with other investigators’ results which showed that the emission of VOCs by antagonistic yeasts has proven to be one of the important direct antagonistic mechanisms against various fungal pathogens of antagonistic yeasts [14,33,37,38,39]. Debaryomyces nepalensis produced VOCs to control the pathogen Colletotrichum gloeosporioides in mango fruit [40]. Metschnikowia pulcherrima was found to produce VOCs that could suppress the growth of C. gloesporioides [41]. T. indica and P. hubeiensis were reported to produce VOCs that inhibited the growth of L. theobromae, whereas P. aspenensis produced VOCs that could inhibit the growth of C. gloeosporioides [15]. W. anomalus, M. pulcherrima, and S. cerevisiae produced VOCs capable of decreasing the mycelial growth of B. cinerea, M. fructicola, A. alternata, A. carbonarius, P. digitatum, Cladosporium spp., and Colletotrichum spp. [31]. W. anomalus produced VOCs which inhibited the growth of C. lunata, F. moniliforme, and R. solani, whereas K. ohmeri produced VOCs which inhibited only the growth of R. solani [14]. T. indica produced VOCs to strongly inhibit the growth of R. solani and Pyricularia oryza [13].
In this study, no antagonistic yeasts had the characteristic of competition for nutrients when dual cultured with C. lunata DOAC 2313 and H. oryzae DOAC 2293. However, some yeast species were reported to compete for nutrients with the pathogens. For example, with P. hubeiensis and T. indica, it was revealed that competition for nutrients resulted in inhibition in the growth of L. theobromae [40]. M. pulcherrima was reported to control C. gloeosporioides through its ability to compete for nutrients [41].
All the antagonistic yeast strains tested could form biofilm to different degrees. This means that competition for space was one of the antagonistic mechanisms of these antagonistic yeasts. Competition for space is based on biofilm formation. Some yeasts form biofilm, allowing them to adhere to surfaces, colonize, and resist stresses [42]. W. anomalus and P. hubeiensis were previously reported to form biofilm [14,15,43]. In addition, some other yeast species were reported to form biofilm that played a role in biological control activities, such as K. ohmeri, Pichia fermentans, P. aspenensis, and T. indica [15,44].
The results of the present study revealed that β-glucanase and chitinase were produced by some strains of C. michaelii, C. tropicalis, H. sinensis, P. japonica, P. hubiensis, R. mucilaginosa, R. taiwanensis, and W. anomalus. This indicated that the fungal cell wall lytic enzymes of some strains of these yeast species could be one of the mechanisms for controlling rice seedling rot fungal pathogens. Some antagonistic yeasts were reported to produce cell wall lytic enzymes which could play a role in antagonistic activity, for example, W. anomalus, K. ohmeri, M. caribbica, Meyerozyma guilliermondii, M. pulcherrima, and T. indica [13,45,46,47].
Siderophores are involved in competition for the nutrient iron (Fe+3) [48]. When antagonistic microorganisms have this ability, they can use the iron; therefore, the iron in the substrate is depleted, resulting in limited growth of the pathogens. The result of this study showed that seven antagonistic yeast strains in four species, P. hubiensis, R. mucilaginosa, R. taiwanensis, and W. anomalus, produced siderophores. Therefore, producing siderophores could be one of the antagonistic mechanisms of these antagonistic yeasts. P. hubeiensis and W. anomalus have previously been reported to produce siderophores [13,14,15]. In addition, other yeast species were found to produce siderophores, such as P. aspenensis, Rhodotorula glutinis, and T. indica [15,49].
Solubilization of phosphate and zinc oxide can convert them to a soluble form, easily assimilated by plants, that promotes plant growth [50,51]. The results of the present study indicated that phosphate and zinc oxide solubilization was among the indirect mechanisms of W. anomalus, with strains of this species having previously been reported to have the ability to solubilize phosphate and zinc oxide [13,14].
The evaluation of the control of rice seedling rot disease caused by C. lunata DOAC 2313 and H. oryzae DOAC 2293 revealed that all the antagonistic yeast strains tested could control this disease. Of these antagonistic yeasts, T. indica DMKU-RP31, obtained from Into et al.’s [13] previous study, and W. anomalus YE-42, derived in the present study, could completely control the disease caused by both fungal pathogens. On the other hand, W. anomalus DMKU-RP04, obtained from the previous study, could completely control the disease only when infected with C. lunata DOAC 2313. These three yeast species demonstrated better control of rice seedling rot disease than the effective chemical fungicide, carbendazim®. Interestingly, T. indica DMKU-RP31 was recently reported to control fruit disease in post-harvest ripe mangos caused by Lasiodiplodia theobromae [15].
Reports on the management of diseases in rice seed and seedlings to date are limited. Chemical fungicides have been used to control rice seed and seedling diseases caused by different fungal pathogens, with these including carbendazim®, difolatan®, mancozeb®, propiconazole®, and validamycin® [52,53,54,55,56]. In the present study, two chemical fungicides, carbendazim® and mancozeb®, were used for comparison with antagonistic yeasts in the control of rice seedling rot disease caused by C. lunata and H. oryzae. Mancozeb® was found to have very weak controlling ability, 0.15% and 3.05% disease control, for C. lunata and H. oryzae, respectively. The reason may be that mancozeb® inhibited rice seed germination [57]. The management of rice seedling rot disease by using soil amended with tricin-releasing rice hulls was reported, with this showing suppression of the soil-borne fungal pathogens viz. F. oxysporum and R. solani that caused rice seedling rot disease [Kong et al., 2010]. The reason was that ricin was reported as being detected in rice hulls and that it had fungicidal activity. Few reports were found on using antagonistic microorganisms to control rice seedling rot disease. Research has explored the use of antagonistic bacteria, Pseudomonas fluorescens and Pseudomonas tolaasii, to control rice seedling rot disease caused by Achlya klebsiana and Pythium spinosum [11]. However, for yeasts, the only report found used Metschnikowia pulcherrima and Pichia guilliermondii to control Fusarium fujikuroi caused by bakanae disease in rice seeds [20]. To the best of our knowledge, the report on the present study is the first one on the use of yeasts for the control of rice seedling rot disease caused by C. lunata and H. oryzae.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/5/647/s1.
Author Contributions
S.L. provided conceptualization, funding acquisition, project administration, resource, supervision, and writing—original draft preparation. P.I. performed the data curation, formal analysis, investigation, methodology, and writing—original draft preparation. P.A. performed investigation and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Thailand Research Fund (TRF) through a Team Promotion Grant (RTA 6080004) for Professor Dr. Savitree Limtong.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- 1.Plodpai P., Chuenchitt S., Petcharat V., Chakthong S., Voravuthikunchai S.P. Anti-Rhizoctonia solani activity by Desmos chinensis extracts and its mechanism of action. Crop. Prot. 2012;43:65–71. [Google Scholar]
- 2.Rice Department, Ministry of Agriculture and Cooperatives of Thailand Seedling Rot Disease in Nursery Box. [(accessed on 20 January 2020)];2014 Available online: http://www.ricethailand.go.th/rkb3/title-index.php-file=content.php&id=131-1.htm.
- 3.Imolehin E. Rice seed borne fungi and their effect on seed germination. Plant Dis. 1983;67:1334–1336. [Google Scholar]
- 4.Ibaraki T. Diseases of rice seedlings growing in the nursery box. JARQ. 1988;21:251–256. [Google Scholar]
- 5.Kong C., Xu X., Zhang M., Zhang S. Allelochemical tricin in rice hull and its aurone isomer against rice seedling rot disease. Pest Manag. Sci. 2010;66:1018–1024. doi: 10.1002/ps.1976. [DOI] [PubMed] [Google Scholar]
- 6.Ito S., Tokunaga Y. Studies on the rot-disease of rice-seedlings caused by Pythium-species. J. Fac. Agric. Hokkaido Imp. Univ. 1933;32:201–228. [Google Scholar]
- 7.Verma S., Kingsley K., Bergen M., Kowalski K., White J. Fungal disease prevention in seedlings of rice (Oryza sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms. 2018;6:21. doi: 10.3390/microorganisms6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi N., Tsukamoto S., Inoue Y., Azegami K. Control of bacterial seedling rot and seedling blight of rice by bacteriophage. Plant Dis. 2012;96:1033–1036. doi: 10.1094/PDIS-03-11-0232-RE. [DOI] [PubMed] [Google Scholar]
- 9.Williamson M., Fokkema N. Phyllosphere yeasts antagonize penetration from appressoria and subsequent infection of maize leaves by Colletotrichum graminicola. Eur. J. Plant Pathol. 1985;91:265–276. [Google Scholar]
- 10.Adams P. The potential of mycoparasites for biological control of plant pathogens. Annu. Rev. Phytopathol. 1990;28:59–72. doi: 10.1146/annurev.py.28.090190.000423. [DOI] [PubMed] [Google Scholar]
- 11.Adhikari T., Joseph C., Yang G., Phillips D., Nelson L. Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling disease of rice. Can. J. Microbiol. 2001;47:916–924. doi: 10.1139/w01-097. [DOI] [PubMed] [Google Scholar]
- 12.Khalili E., Sadravi M., Naeimi S., Khosravi V. Biological control of rice brown spot with native isolates of three Trichoderma species. Braz. J. Microbiol. 2012;43:297–305. doi: 10.1590/S1517-838220120001000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Into P., Khunnamwong P., Jindamoragot S., Am-in S., Intanoo W., Limtong S. Yeast associated with rice phylloplane and their contribution to control of rice sheath blight disease. Microorganisms. 2020;8:362. doi: 10.3390/microorganisms8030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khunnamwong P., Lertwattanasakul N., Jindamorakot S., Suwannarach N., Matsui K., Limtong S. Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop diseases. Folia Microbiol. 2019:1–18. doi: 10.1007/s12223-019-00764-6. [DOI] [PubMed] [Google Scholar]
- 15.Konsue W., Dethoup T., Limtong S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms. 2020;8:317. doi: 10.3390/microorganisms8030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chincholkar S., Mukerji K., Chandrashekara C., Chakravarthi M. Biological Control of Plant Diseases. In: Singh V.K., Singh Y., Singh A., editors. Eco-Friendly Innovative Approaches in Plant Disease Management. International Book Distributors; Dehradun, India: 2012. pp. 147–166. [Google Scholar]
- 17.Spadaro D., Gullino M. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop. Prot. 2005;24:601–613. [Google Scholar]
- 18.Elsharkawya M.M., Nakatanib M., Nishimurab M., Arakawab T., Shimizub M., Hyakumachib M. Suppression of rice blast, cabbage black leaf spot, and tomato bacterial wilt diseases by Meyerozyma guilliermondii TA-2 and the nature of protection. Acta Agric. Scand. Sect. B Plant Soil Sci. 2015;65:629–636. [Google Scholar]
- 19.Kunyosying D., To-anun C., Cheewangkoon R. Control of rice blast disease using antagonistic yeasts. Int. J. Agric. Technol. 2018;14:83–98. [Google Scholar]
- 20.Matić S., Spadaro D., Garibaldi A., Gullino M. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control. 2014;73:59–67. [Google Scholar]
- 21.Rosa M., Tauk-Tornisielo S., Rampazzo P., Ceccato-Antonini S. Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J. Microbiol. Biotechnol. 2010;26:1491–1502. [Google Scholar]
- 22.Di Francesco A., Ugolini L., Lazzeri L., Mari M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control. 2015;81:8–14. [Google Scholar]
- 23.Zhang D., Spadaro D., Garibaldi A., Gullino M. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control. 2010;54:172–180. doi: 10.1016/j.biocontrol.2010.05.003. [DOI] [Google Scholar]
- 24.Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–429. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 25.Růžička F., Holá V., Votava M., Tekkalov R. Importance of biofilm in Candida parapsilosis and evaluation of its susceptibility to antifungal agents by colorimetric method. Folia Microbiol. 2007;52:209–214. doi: 10.1007/BF02931300. [DOI] [PubMed] [Google Scholar]
- 26.Stepanović S., Vuković D., Hola V., Bonaventura G., Djukić S., Ćirković I., Ruzicka F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwyn B., Neilands J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi S., Usmani S., Singh B., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 29.Rokhbakhsh-Zamin F., Sachdev D., Kazemi-Pour N., Engineer A., Pardesi K., Zinjarde S., Dhakephalkar P., Chopade B. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011;21:556–566. [PubMed] [Google Scholar]
- 30.Hashem M., Alamri S., Hesham A., Al-Qahtani F., Kilany M. Biocontrol of apple blue mould by new yeast strains: Cryptococcus albidus KKUY0017 and Wickerhamomyces anomalus KKUY0051 and their mode of action. Biocontrol Sci. Technol. 2014;24:1137–1152. doi: 10.1080/09583157.2014.926857. [DOI] [Google Scholar]
- 31.Oro L., Feliziani E., Ciania M., Romanazzib G., Comitinia F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018;265:18–22. doi: 10.1016/j.ijfoodmicro.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Ou C., Liu Y., Wang W., Dong D. Integration of UV-C with antagonistic yeast treatment for controlling post-harvest disease and maintaining fruit quality of Ananas comosus. BioControl. 2016;61:591–603. doi: 10.1007/s10526-016-9740-5. [DOI] [Google Scholar]
- 33.Jaibangyang S., Nasanit R., Limtong S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. BioControl. 2020:1–10. doi: 10.1007/s10526-020-09996-9. [DOI] [Google Scholar]
- 34.Sukmawati D., Andrianto M., Arman Z., Ratnaningtyas N., Al Husna S., El-Enshasy H., Dailin D., Kenawy A. Antagonistic activity of phylloplane yeasts from Moringa oleifera Lam. leaves against Aspergillus flavus UNJCC F-30 from chicken feed. Indian Phytopathol. 2020;73:79–88. doi: 10.1007/s42360-020-00194-2. [DOI] [Google Scholar]
- 35.Zhang H., Yang Q., Lin H., Ren X., Zhao L., Hou J. Phytic acid enhances biocontrol efficacy of Rhodotorula mucilaginosa against postharvest gray mold spoilage and natural spoilage of strawberries. LWT-Food Sci. Technol. 2013;52:110–115. doi: 10.1016/j.lwt.2012.01.027. [DOI] [Google Scholar]
- 36.Cheng Z., Mengshan C., Guangkun L., Huizhen C., Yuan S., Hanjv S., Michael W., Yongsheng L., Jia L. Heat shock improves stress tolerance and biocontrol performance of Rhodotorula mucilaginosa. Biol. Control. 2016;95:49–56. doi: 10.1016/j.biocontrol.2016.01.001. [DOI] [Google Scholar]
- 37.Ando H., Hatanaka K., Ohata I., Yamashita-Kitaguchi Y., Kurata A., Kishimoto N. Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control. 2012;26:472–478. doi: 10.1016/j.foodcont.2012.02.017. [DOI] [Google Scholar]
- 38.Hua S., Beck J., Sarreal S., Gee W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014;30:71–78. doi: 10.1007/s12550-014-0189-z. [DOI] [PubMed] [Google Scholar]
- 39.Huang R., Li G., Zhang J., Yang L., Che H., Jiang D., Huang H. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology. 2011;101:859–869. doi: 10.1094/PHYTO-09-10-0255. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y., Li W., Zeng J., Shao Y. Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biol. Control. 2018;123:111–119. doi: 10.1016/j.biocontrol.2018.05.014. [DOI] [Google Scholar]
- 41.Tian Y., Li W., Jiang Z., Jing M., Shao Y. The preservation effect of Metschnikowia pulcherrima yeast on anthracnose of postharvest mango fruits and the possible mechanism. Food Sci. Biotechnol. 2018;27:95–105. doi: 10.1007/s10068-017-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Droby S., Wisniewski M., Macarisin D., Wilson C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009;52:137–145. doi: 10.1016/j.postharvbio.2008.11.009. [DOI] [Google Scholar]
- 43.Parafati L., Vitale A., Restuccia C., Cirvilleri G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015;47:85–92. doi: 10.1016/j.fm.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Giobbe S., Marceddu S., Scherm B., Zara G., Mazzarello V., Budroni M., Migheli Q. The strange case of a biofilm-forming strain of Pichia fermentans, which controls Monilinia brown rot on apple but is pathogenic on peach fruit. FEMS Yeast Res. 2007;7:1389–1398. doi: 10.1111/j.1567-1364.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 45.Lima J., Gondim D., Oliveira J., Oliveira F., Gonçalves L., Viana F. Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 2013;83:58–64. doi: 10.1016/j.postharvbio.2013.03.014. [DOI] [Google Scholar]
- 46.Zhang D., Spadaro D., Garibaldi A., Gullino M.L. Potential biocontrol activity of a strain of Pichia guilliermondii against grey mold of apples and its possible modes of action. Biol. Control. 2011;57:193–201. doi: 10.1016/j.biocontrol.2011.02.011. [DOI] [Google Scholar]
- 47.Zepeda-Giraud L., Olicón-Hernandez D., Martínez-López C., Guerra-Sánchez G. Study of the action mode of Wickerhamomyces anomalus against Colletotrichum gloeosporioides. J. Agric. Sci. Technol. 2016;6:341–349. [Google Scholar]
- 48.Spadaro D., Droby S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016;47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 49.Sansone G., Rezza I., Calvente V., Benuzzi D., de Tosetti M. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 2005;35:245–251. doi: 10.1016/j.postharvbio.2004.09.005. [DOI] [Google Scholar]
- 50.Olanrewaju O., Glick B., Babalola O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nutaratat P., Srisuk N., Arunrattiyakorn P., Limtong S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014;118:683–694. doi: 10.1016/j.funbio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Boukaew S., Chanasirin K., Poonsuk P. Potential for the integration of biological and chemical control of sheath blight disease caused by Rhizoctonia solani on rice. World J. Microbiol. Biotechnol. 2013;29:1885–1893. doi: 10.1007/s11274-013-1353-x. [DOI] [PubMed] [Google Scholar]
- 53.Groth D., Bond J. Effects of cultivars and fungicides on rice sheath blight, yield, and quality. Plant Dis. 2007;91:1647–1650. doi: 10.1094/PDIS-91-12-1647. [DOI] [PubMed] [Google Scholar]
- 54.Nandakumar R., Babu S., Viswanathan R., Raguchander T., Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001;33:603–612. doi: 10.1016/S0038-0717(00)00202-9. [DOI] [Google Scholar]
- 55.Rush M.C., Schneider R.W. Chemical Control of Seedling Diseases of Rice in Louisiana. In: Grayson B.T., Green M.B., Copping L.G., editors. Pest Management in Rice. Springer; Dordrecht, The Netherlands: 1990. pp. 53–70. [Google Scholar]
- 56.Webster R.K., Hall D.H., Bostad J., Wick C.M., Brandon D.M., Baskett R., Williams J.M. Chemical seed treatment for the control of seedling disease of water-sown rice. Hilgardia. 1973;41:689–698. doi: 10.3733/hilg.v41n21p689. [DOI] [Google Scholar]
- 57.Anitha S., Savitha G. Impact of mancozeb stress on seedling growth, seed germination, chlorophyll and phenolic contents of rice cultivars. IJSR. 2013;4:292–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.