Abstract
Background
Consumption of high-fat diet (HF) leads to hyperphagia and increased body weight in male rodents. Female rodents are relatively resistant to hyperphagia and weight gain in response to HF, in part via effects of estrogen that suppresses food intake and increases energy expenditure. However, sex differences in energy expenditure and activity levels with HF challenge have not been systemically described. We hypothesized that, in response to short-term HF feeding, female mice will have a higher energy expenditure and be more resistant to HF-induced hyperphagia than male mice.
Methods
Six-week-old male and female C57BL/6J mice were fed either low fat (LF, 10 % fat) or moderate HF (45% fat) for 5 weeks, and energy expenditure, activity and meal pattern measured using comprehensive laboratory animal monitoring system (CLAMS).
Results
After 5 weeks, HF-fed male mice had a significant increase in body weight and fat mass, compared with LF-fed male mice. HF-fed female had a significant increase in body weight compared with LF-fed female mice, but there was no significant difference in fat mass. HF-fed male mice had lower energy expenditure compared to HF-fed female mice, likely due in part to reduced physical activity in the light phase. HF-fed male mice also had increased energy intake in the dark phase compared to LF-fed male mice and a reduced response to exogenous cholecystokinin-induced inhibition of food intake. In contrast, there was no difference in energy intake between LF-fed and HF-fed female mice.
Conclusions
The data show that female mice are generally protected from short-term HF-induced alterations in energy balance, possibly by maintaining higher energy expenditure and an absence of hyperphagia. However, HF-feeding in male mice induced weight and fat mass gain and hyperphagia. These findings suggest that there is a sex difference in the response to short-term HF-feeding in terms of both energy expenditure and control of food intake.
Keywords: meal patterns, energy intake, energy expenditure, high-fat diet, physical activity, sex differences
INTRODUCTION
Changes of lifestyle and food choices, over-nutrition and decreased physical activity have increased risk and incidence of obesity. In the United States, more than one-third of adults are obese which increases the risk of developing diabetes, cardiovascular diseases, cancers and infertility [1]. Accumulation of fat in adipose tissues in individuals with obesity increases plasma leptin, a hormone primarily secreted from adipocytes and involved in the regulation of energy homeostasis. There is evidence to suggest that high fat feeding induces chronic inflammation that in turn leads to leptin resistance in the peripheral and central nervous system, which impairs regulation of energy balance [2,3]. In addition to increased energy intake, longitudinal studies in humans and rodents show that long-term HF feeding (14 weeks) leads to a decrease in energy expenditure and an increased risk of weight gain [4,5]. However, 4-week consumption of HF in humans and rodents had no effect on energy expenditure [6,7]. Thus, despite decreases in energy expenditure contributing to weight gain, the onset of reduced energy expenditure in obesity and its role in pathogenesis remains unclear. Whether reduced energy expenditure is a cause of obesity or an outcome of weight gain is not clear.
While the majority of studies in HF diet-induced obesity in rodents have, until recently, largely focused on males, it has been reported that in female mice there is a delay in weight gain, a decrease in adiposity and overall resistance to the obesigenic effects of HF-feeding compared with male mice [8–13]. On average, female C57BL/6 mice do not show significant weight gain and increased fat mass until after 10 to 12 weeks of HF feeding. The sexual dimorphism in response to HF and metabolic protection in females is thought to be due to estradiol and estrogen receptor α (ER-α) signaling [14,15]. Energy intake and energy expenditure in women vary with the menstrual cycle [16–19]. Ovariectomized rodents and postmenopausal women have increased energy intake and reduced energy expenditure [20–22]. Although ovarian hormones may protect women from developing obesity, the prevalence of obesity in the United States is higher in women than in men (41.1% vs. 37.9%) [1]. Most studies of HF diet-induced obesity focus on long-term or end-point phenotypic changes and associated impairment in other systems, which lead to a lack of understanding of sex differences in the onset of changes in energy balance.
To understand sex differences in the alterations in energy balance at the onset of obesity, we used male and female C57BL/6J mice to study the change in energy intake and energy expenditure after 5 weeks of feeding a moderately HF diet (45% calories from fat), which more closely resembles the fat intake in overweight and obese humans [23]. We hypothesized that in response to short-term HF feeding, female mice will have higher energy expenditure and be more resistant to HF-induced hyperphagia than male mice. The experimental approach used was to monitor body weight and energy intake weekly for four weeks, after which we monitored 24-hour energy expenditure, physical activity, meal patterns and body composition. In a separate study, we determined whether the increase in energy intake observed in HF-fed male mice was due to alteration in intestinal satiety, we determined the effects of exogenous cholecystokinin (CCK) on food intake [24].
MATERIALS AND METHODS
Animals
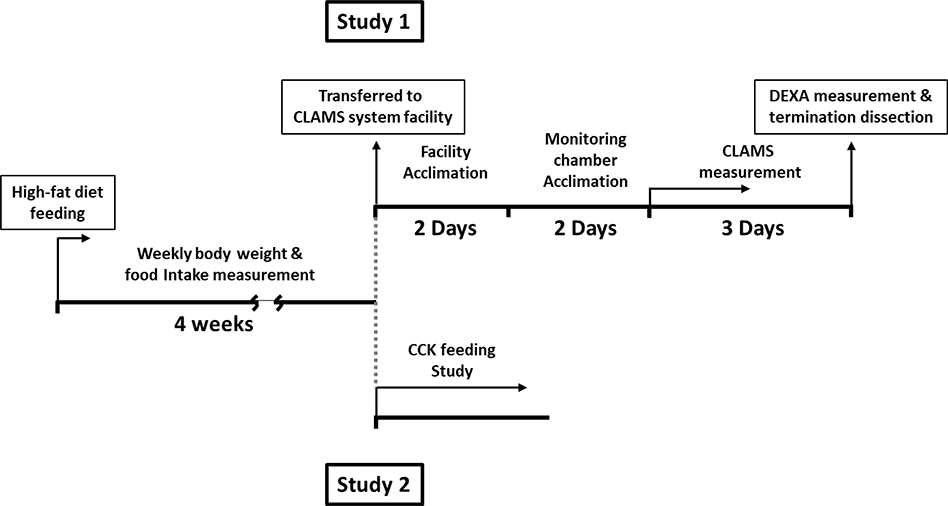
All studies were approved by UC Davis Institutional Animal Care and Use Committee. Six-week-old male and female C57BL/6J mice (000664, Jackson Laboratory, CA) were used for the study and mice were individually housed in a facility that had ventilated cage system (1285L, TECNIPLAST, Italy) and maintained at 22°C under a 12–12 hr light-dark schedule (lights on at 7am to 7pm). Mice were acclimated to the housing for 7 days and fed laboratory rodent chow (PicoLab® Mouse Diet 20, 5058, TestDiet, MO), then randomized into two groups and fed either 10% low fat control diet (LF, 10% calories from fat, D14110101, Research Diets Inc, NJ) or 45% high fat diet (HF, 45% calories from fat, D14110103) for 5 weeks (total N = 12–13 for each group). Mice were allowed ad libitum access to food and water unless specifically stated otherwise. Body weight and food intake were measured weekly from week 1 to week 4 in home cages. The experimental design is shown in Figure 1.
Figure 1. Schematic representation of the experimental design.
The study 1: mice were fed with semisynthetic diet for 4 weeks and transferred to CLAMS system facility. Prior to the data collection by CLAMS system, mice were allowed to acclimate to the facility and CLAMS chamber. After 3 days of data collection, body composition was measured and mice were terminated for tissue dissection. The study 2: mice were fed with semisynthetic diet for 4 weeks and the feeding study was performed on male mice.
Study 1: Measurement of Meal Patterns, Physical Activity and Metabolic Phenotype
At the start of the fifth week, to measure body composition, meal patterns and metabolic phenotype, mice were transferred to a core facility maintained at 22°C under a 12–12 hr light-dark schedule (lights on at 6am to 6pm). Male and female mice fed with either powdered LF or HF (N = 7–8 for each group) were individually housed in monitoring chambers for a 2-day acclimation period prior to the data collection and fed. Food intake, X-axis/Z-axis movement, oxygen consumption and carbon dioxide production were monitored by Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments, OH; UC Davis MMPC D4007) for three days. The data from the second and third day were averaged and used for analysis. The chamber air was analyzed for oxygen and carbon dioxide content every 26 min by using Oxymax system [25]. Energy expenditure was calculated by equation, [26]. The parameters for the determination of meal patterns were defined previously [27]. Briefly, meal size was measured for any food bout that was larger than 0.02g. The termination of a meal was determined as 10 min with no measurable feeding. After measurement of metabolic phenotype, body composition was measured using X-ray absorptiometry under isoflurane anesthesia (3–4%) (DEXA; UC Davis MMPC dx.doi.org/10.17504/protocols.io.ykffutn). Mice were euthanized by CO2, and individual fat pads, including subcutaneous, epididymal/gonadal, mesenteric and retroperitoneal, were measured at necropsy.
Study 2: Measurement of Exogenous CCK-induced Satiation
To determine the anorexigenic action of CCK, during the fifth week of feeding semi-synthetic diets, male mice fed with either LF or HF (N = 5 for each group) were fasted for 12 hr during the light phase. Mice received intraperitoneal (IP) injections of either saline (0.01 ml per gram body weight) or CCK octapeptide (1 μg/kg; Bachem, CA) just before dark onset using a crossover design, and food was immediately placed in the cage. Due to the short biological half-life of CCK, food intake was measured for every 20 min for 1 hr. Mice were allowed to recover for 2 days between the trials.
Statistics
Comparisons were calculated using two-way ANOVA with Bonferroni post-hoc test unless specifically stated otherwise. The seffect of diet on body weight, respiratory quotient (respiratory exchange, RER), food intake, and meal patterns were compared within each sex. The effect of sex on physical activity was compared within each diet. Two-tail unpaired t-test was used to analyze the data of fat pad mass within each sex. Two-tailed paired t-test was used to analyze the effect of diet on data of weekly food intake and CCK feeding study within each sex. Above statistical analyses were performed with GraphPad Prism 8.0 (GraphPad software, La Jolla, CA). Energy expenditure was analyzed by ANCOVA normalized by lean mass with comparison between the sex or the diet [28]. All results are presented as mean ± SEM, with the following significance levels: *p < 0.05, **p < 0.01, ***p < 0.001.
RESULTS
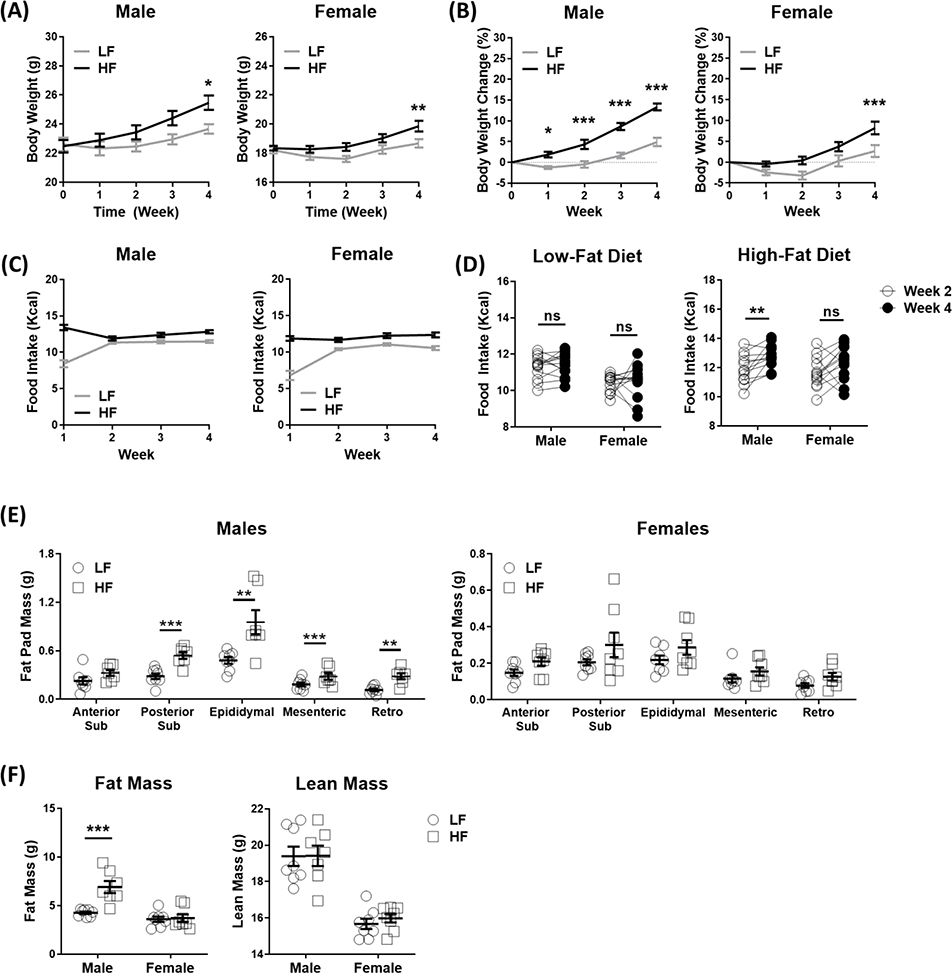
Compared with LF-fed, HF-fed male mice gained significantly more weight by the end of the first week, and weight gain rapidly increased over the following weeks (Figure 2A–B). In contrast, HF-fed female mice had no significant weight gain until week 4. In the first week, food intake is significantly higher in both male and female HF-fed compared to LF-fed mice (Figure 2C). The reduced food intake in LF-fed mice might be due to the food neophobia to LF. To avoid the confounder of adaptation to the new diets in the first week, weekly energy intake was compared between the 2nd and 4th week. There was no significant difference in energy intake between week 2 and 4 in either LF male or female mice. However, there was a significant increase in energy intake in HF-fed male but not female mice (Figure 2D), suggesting that the faster weight gain in HF-fed male mice was, at least in part, due to increased energy intake.
Figure 2. High-fat diet led to an increase in body weight gain, fat mass and hyperphagia in male mice.
Male and female C57BL/6J mice were fed with either high-fat diet (HF) or low-fat control diet (LF) for 5 weeks. (A) Weekly body weight, (B) weekly body weight change, statistical differences determined using two-way ANOVA, and (C) weekly food intake were measured (N = 12–13 per group, combined data from mice in Study 1 and Study 2). (D) Comparison of food intake between 2nd week and 4th week, statistical differences were determined using paired t-test. (E) Fat pad mass and (F) body composition at 5th week of HF, statistical differences were determined using unpaired t-test for fat pad mass and two-way ANOVA for body composition (N = 7–8 per group; Study 1). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Body composition was measured by DEXA in the 5th week of HF and fat pad weight determined at necropsy (Figure 2E–F). HF feeding in male mice induced a significant increase in total fat mass as measured by DEXA (Figure 2F) and in posterior subcutaneous, epididymal, mesenteric, and retroperitoneal fat pad mass measured at necropsy (Figure 2E). However, there was no significant effect of HF feeding on total fat mass or fat pad mass in female mice.
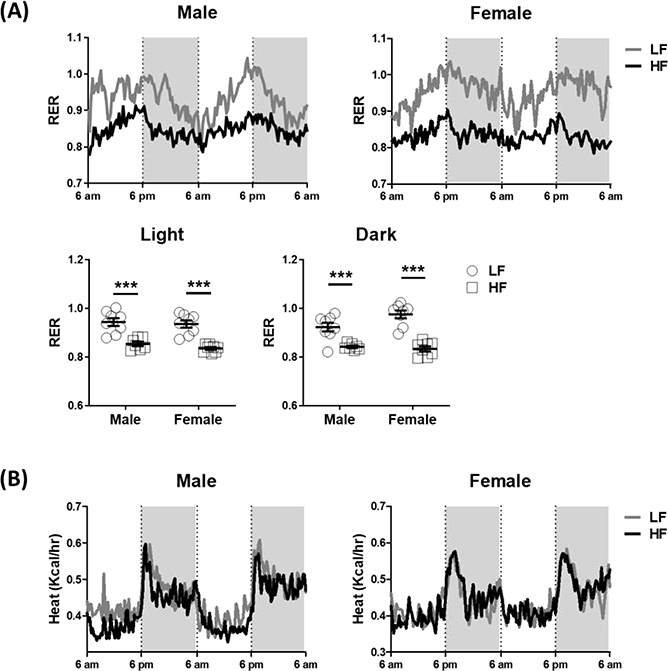
Both male and female mice fed a HF had a decrease in the RER, compared with LF mice (Figure 3A), consistent with ingestion of a lipid-rich diet increasing the utilization of fat as an energy source. There was no significant diet effect on energy expenditure (expressed as heat production) in either male or female mice (Figure 3B and Table 1). However, HF-fed male mice had significantly lower energy expenditure in the light phase compared with HF-fed female mice, suggesting there was a sex effect on energy expenditure in HF-fed mice. In the dark phase, HF-fed male mice had a trend of lower energy expenditure than HF-fed female mice although this was not significant (p = 0.07).
Figure 3. Male mice ingesting a high-fat diet had decreased energy expenditure.
Metabolic phenotype was measured continuously for 48 hours in the 5th week of feeding either the LF or HF diet in male and female mice. (A) Respiratory exchange ratio (RER) and (B) energy expenditure, statistical differences were determined by using two-way ANOVA (N = 7–8 per group, Study 1). Data are presented as mean ± SEM. ***p < 0.001.
Table 1.
ANCOVA analysis adjusted by lean mass for energy expenditure
| Sex Effect | Male | Female | P-value | |
| Light Phase | Low Fat | 0.41 ± 0.02 | 0.41 ± 0.02 | 0.93 |
| High Fat | 0.35 ± 0.02 | 0.42 ± 0.01 | 0.02 | |
| Dark Phase | Low Fat | 0.47 ± 0.01 | 0.49 ± 0.01 | 0.33 |
| High Fat | 0.45 ± 0.02 | 0.51 ± 0.02 | 0.07 | |
| Diet Effect | Low Fat | High Fat | P-value | |
| Light Phase | Male | 0.40 ± 0.01 | 0.37 ± 0.01 | 0.10 |
| Female | 0.41 ± 0.01 | 0.40 ± 0.01 | 0.33 | |
| Dark Phase | Male | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.13 |
| Female | 0.48 ± 0.01 | 0.48 ± 0.01 | 0.91 | |
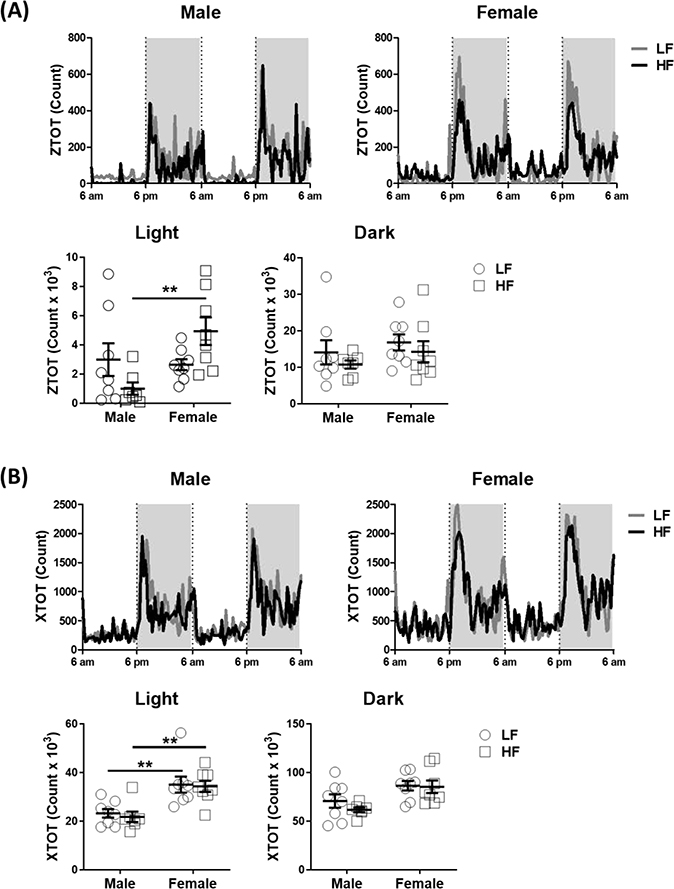
Physical activity was measured by the number of interceptions of the laser beam in both the X-axis (horizontal movement) and Z-axis (vertical movement). There was no significant difference in Z-axis activity between LF-fed male and female mice in either the light or the dark phase. However, HF-fed male mice had lower vertical activity than HF-fed female mice during the light phase (Figure 4A). Two-way ANOVA analysis showed that there was a sex effect on vertical movement during the light phase (p = 0.03); furthermore, the interaction between sex effect and diet effect is statistically significant (p = 0.01), suggesting the sex effect on vertical movement is dependent on the diet. The difference in Z-axis physical activity during the light phase between male and female mice might result in the observed lower energy expenditure in HF-fed male mice (Table 1). Male mice fed with either LF or HF had lower X-axis activity than female mice in the light phase (Figure 4B). Two-way ANOVA analysis showed that there was a sex effect on horizontal movement during the light phase (p < 0.001) and no statistical interaction between sex effect and diet effect. This suggests male mice have lower horizontal activity than female mice that is independent on the diet.
Figure 4. Female mice ingesting a high-fat diet had increased physical activity in the light phase.
Physical activity was measured continually for 48 hours in male and female mice in 5th week of feeding either the LF or HF diets. (A) Z-axis movement and (B) X-axis movement, statistical differences were determined by using two-way ANOVA (N = 7–8 per group, Study 1). Data are presented as mean ± SEM. ** < 0.01.
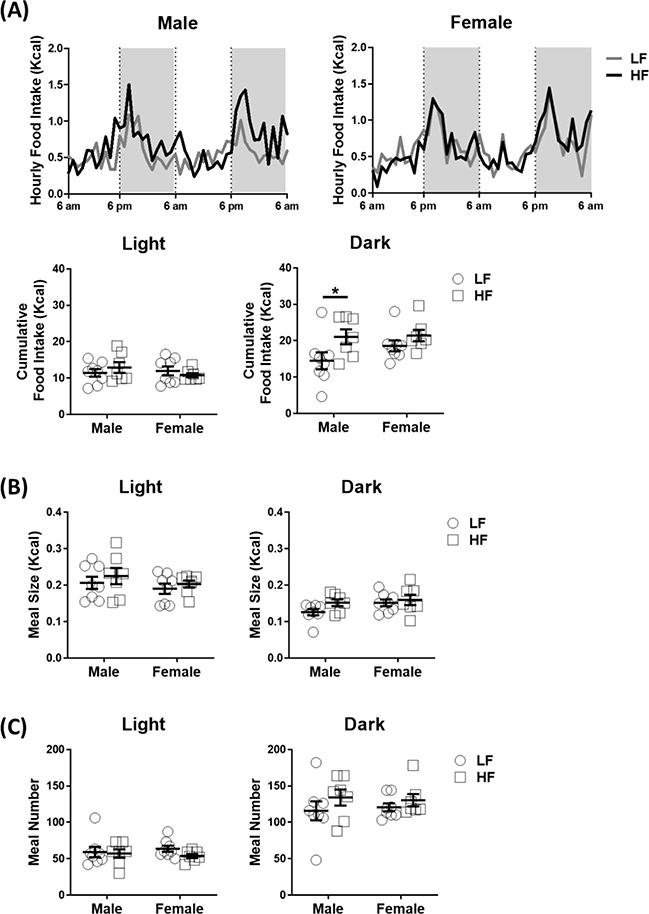
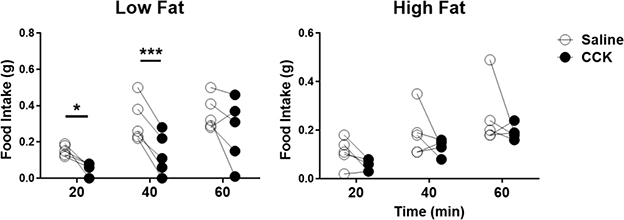
To determine the effect of sex and diet on feeding behavior, feeding behavior was recorded for three days; food intake and meal patterns were analyzed from measurements during the second and third day. There was a significant increase in cumulative food intake in the dark phase in HF-fed male compared to LF-fed male mice (Figure 5A). In contrast, there was no difference in food intake in the dark phase between LF-fed and HF-fed female mice. Two-way ANOVA analysis showed that there was a diet effect on dark-phase food intake (p = 0.02), suggesting feeding of HF could affect energy intake in the dark phase. In the analysis of meal pattern, there was no significant difference in either meal size (p = 0.17) or meal number (p = 0.41) between LF-fed and HF-fed male mice in the dark phase (Figure 5B–C). The hyperphagia observed in HF-fed male mice is consistent with attenuation of intestinal satiety in HF-fed rats as previously described [24]. CCK is a well-known satiety signal released from the gut that acts to terminate feeding. To determine whether hyperphagia in HF-fed male mice is due to impairment of CCK-induced satiety, during the fifth week of HF, male mice fasted for 12 h were injected with CCK (1μg/kg intraperitoneally) at the onset of the dark phase and food intake measured for 1 h. Exogenous CCK inhibited food intake in LF-fed male mice at 20 and 40 min post injection, but had no significant effect in HF-fed male mice (Figure 6).
Figure 5. Male mice ingesting a high-fat diet had an increase in food intake during the dark phase.
Food intake and meal patterns were measured continually for 48 hours in male and female mice after in the 5th week of ingesting either the LF or HF diet. (A) Food intake, (B) meal size and (C) meal number, statistical differences were determined by using two-way ANOVA (N = 7–8 per group, Study 1). Data are presented as mean ± SEM. *p < 0.05.
Figure 6. Male mice ingesting a high-fat diet had a decrease in cholecystokinin-induced satiation.
Cholecystokinin (CCK) feeding study in male mice after 5 weeks of HF and LF diet, statistical differences were determined by using paired t-test (N = 5 per group, Study 2). Mice were fasted for 12 hours and injected intraperitoneally either saline or CCK (1 μg/kg) at the onset of the dark phase. Food intake was measured every 20 min for 1 hour. Data are presented as mean ± SEM. *p < 0.05, ***p < 0.001.
DISCUSSION
Protection against the obesigenic effects of HF feeding in female mice has been reported in other studies [8–13]. However, the underlying mechanism is not clear. In the current study, we studied the effect of five-weeks feeding of a moderately HF on energy expenditure and energy intake in both male and female mice. The data show that female mice gain little weight with no additional fat accumulation compared to male mice that had significantly increased body weight gain and fat accumulation. The increase in body weight gain in male mice was accompanied by an increase in energy intake and, consistent with the body weight data, there was no difference in energy intake in HF fed female mice. In addition, HF-fed male mice showed less physical activity in the light phase than HF-fed female mice, contributing to the observed decrease in energy expenditure that likely further promotes weight gain. The data also show that HF-fed male mice develop hyperphagia, possibly due to the observed reduced CCK-induced suppression on food intake. Overall, these data suggest that female mice have higher energy expenditure and an absence of hyperphagia during short-term feeding of HF, which slows weight gain and fat accumulation.
HF-fed female mice had higher physical activity during the light phase than HF-fed male mice thus maintaining energy expenditure, which may slow down the progression in development of an obese phenotype. The higher physical activity in female mice was not accompanied with increased food intake, suggesting that most of these movements were not associated with feeding. Other than the energy demand, physical activity is positively regulated by the mesolimbic pathway, also known as the dopamine reward pathway, in the central nervous system [29,30]. Attenuation of the dopaminergic pathway leads to decreased physical activity and energy expenditure, which can lead to an obese phenotype [29,30]. Estrogens have been shown to stimulate the release of dopamine in nucleus accumbens through either genomic or non-genomic pathways [31]. Mice with global deletion of aromatase, a key enzyme to produce estrogens, have decreased physical activity [32]. Taken together, this evidence suggests that female mice have higher physical activity that is not related to feeding behavior than male mice. The high physical activity leads to higher energy expenditure in female than male mice; coincidentally, the high energy expenditure possibly prevents the diet-induced weight gain in female mice.
After 5 weeks of HF, male mice had increased energy intake with reduced CCK-induced satiation, resulting in weight gain. The phenotype of hyperphagia has been shown in rats with short-term HF, suggesting that short-term HF leads to leptin resistance in the peripheral nervous system and impairs the responsiveness to gut hormones [24,33]. However, there was no increased energy intake in HF-fed female mice. There are two potential mechanisms that could be involved in the delay in development of hyperphagia in HF-fed female mice. First, leptin signaling is modulated through cross-talk with estradiol signaling in the hypothalamus [34]. High systemic estradiol, rodents have increases in central leptin signaling and leptin-induced anorexigenic action [35]. In addition, estradiol has been showed to prevent HF-induced leptin resistance and thus weight gain [36]. Second, estradiol directly modulates the responsiveness to gut hormones, such as CCK, glucagon-like peptide-1, and amylin, which increases its anorexigenic action and reduces energy intake in the female [14,37].
Short term feeding of male mice with a HF diet results in peripheral insensitivity to exogenous leptin, but sensitive to central leptin-induced anorexigenic action [38]. To study leptin resistance in the peripheral nervous system, we have previously reported the mice (Nav1.8-Cre X LepRfl/fl, VANLepRΔ) with deletion of leptin receptor in sensory neurons, primarily vagal afferent neurons (VAN) [27]. Chow diet-fed male VANLepRΔ mice had the same feeding phenotype, increased total food intake and meal size, as HF-fed male mice. In this study, we showed sex dimorphism in energy intake and energy expenditure after short-term consumption of HF. Overall, female mice were protected from HF-induced obese phenotype. Interestingly, the metabolic protection is not fully shown in female VANLepRΔ mice due to disruption of estradiol signaling in VAN [39]. Female VANLepRΔ mice have increased fat pads mass and hyperphagia with increased meal number and altered responsiveness to gut hormones. This suggests that peripheral leptin signaling works with estradiol, and this may delay the development of HF-induced obesity in female mice.
As discussed above, estrogens increase energy expenditure and decrease energy intake [14,15]. Therefore, it is likely that the metabolic phenotype and meal patterns might vary across the estrous cycle in female mice. In this study, we were not able to identify the phase of the estrous cycle during measurement of the metabolic phenotype. We did not observe significant differences in energy expenditure, physical activity, or meal patterns between the first and second day of measurement, we cannot exclude the effect of estrous cycle in metabolic phenotype and meal patterns. In addition, all female mice were housed in the same room, including during acclimation and measurements in metabolic cages, and it is possible that the estrous cycle might have synchronized in the mice. In any case, the variability in the measurements obtained using the CLAMS system between each female mouse in either group was small.
Energy imbalance, excess caloric intake and reduced use of calories, results in fat accumulation and obesity. The present study showed that female mice had higher physical activity and energy expenditure than male mice during short-term consumption of HF. Furthermore, we provide evidence that short-term HF impairs the intestinal satiety signal to terminate feeding in male mice, which in turn leads to increased food intake. Taken together, the energy imbalance results in significant weight gain and fat accumulation in HF-fed male mice. Overall, female mice are metabolically protected from short-term HF led energy imbalance. Although the present study does not provide a mechanism by which this occurs, it does identify both food intake and physical activity as potential targets to investigate further. The sexual dimorphism in response to short-term high fat diet suggests that the prevention and treatment for obesity should be considered in a sex-dependent manner. Further studies of the mechanisms contributing to metabolic protection in female are needed to help develop better approaches to treat obesity.
Highlights.
Male mice are more susceptible to short-term HF-induced weight gain than female mice.
HF-fed male mice have less physical activity, accompanied with lower energy expenditure, than female mice in the light phase.
HF-fed male mice have increased food intake and reduced CCK-induced satiation.
Ackowledgements
The study was supported by NIH grant DK41004 (HER). Additional support was provided by the UC Davis MMPC Energy Balance, Exercise, & Behavior Core (NIH grant U24-DK092993, RRID: SCR_015357 and SCR_015364). We acknowledge Dr Lihong Qi, Department of Public Health Sciences and Mouse Metabolic Phenotyping Center, UC Davis for the statistical consulting. The EE ANCOVA analysis done for this work was provided by the NIDDK Mouse Metabolic Phenotyping Centers (MMPC, www.mmpc.org) using their Energy Expenditure Analysis page (http://www.mmpc.org/shared/regression.aspx) and supported by grants DK076169 and DK115255.”
Funding: This work was funded by grant NIHDDK 41004 (HER).
List of Abbreviations
- BAT
brown adipose tissue
- CCK
cholecystokinin
- CLAMS
comprehensive laboratory animal monitoring system
- CNS
central nervous system
- DEXA
X-ray absorptiometry
- ERα
estrogen receptor α
- HF
high fat diet
- LepR
leptin receptor
- LF
low fat diet
- PNS
peripheral nervous system
- RER
respiratory echange ratio
- SNS
sympathetic nervous system
- VAN
vagal afferent neurons
Footnotes
Competing interests: The authors have no financial or non-financial competing interests to declare.
Declarations
Ethics approval: not applicable
Consent for publication: not applicable
Availability of data: All data generated or analyzed in this study and included in the current article are available Figshare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hales CM, Carroll MD, Fryar CD, Ogden CL, Prevalence of Obesity Among Adults and Youth: United States, 2015–2016., NCHS Data Brief. (2017) 1–8. [PubMed] [Google Scholar]
- [2].Pan WW, Myers MG Jr, Leptin and the maintenance of elevated body weight, Nat. Rev. Neurosci. 19 (2018)95. [DOI] [PubMed] [Google Scholar]
- [3].deLartigue G, Role of the vagus nerve in the development and treatment of diet-induced obesity., J. Physiol. 594 (2016) 5791–5815. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, Boyce V, VHoward B, Bogardus C, Reduced Rate of Energy Expenditure as a Risk Factor for Body-Weight Gain, N. Engl. J. Med. 318 (1988) 467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- [5].Choi M-S, Kim Y-J, Kwon E-Y, Ryoo JY, Kim SR, Jung UJ, High-fat diet decreases energy expenditure and expression of genes controlling lipid metabolism, mitochondrial function and skeletal system development in the adipose tissue, along with increased expression of extracellular matrix remodelling- and inflamm, Br. J. Nutr. 113 (2015) 867–877. doi: 10.1017/S0007114515000100. [DOI] [PubMed] [Google Scholar]
- [6].Abbott WG, VHoward B, Ruotolo G, Ravussin E, Energy expenditure in humans: effects of dietary fat and carbohydrate, Am. J. Physiol. Endocrinol. Metab. 258 (1990) E347–E351. doi: 10.1152/ajpendo.1990.258.2.E347. [DOI] [PubMed] [Google Scholar]
- [7].Assaad H, Yao K, Tekwe CD, Feng S, Bazer FW, Zhou L, Carroll RJ, Meininger CJ, Wu G, Analysis of energy expenditure in diet-induced obese rats., Front. Biosci. 19 (2014) 967–985. doi: 10.2741/4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hwang L-L, Wang C-H, Li T-L, Chang S-D, Lin L-C, Chen C-P, Chen C-T, Liang K-C, Ho I-K, Yang W-S, Chiou L-C, Sex Differences in High-fat Diet-induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice, Obesity. 18 (2010) 463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- [9].Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M, Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue, PLoS One. 7 (2012) e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP, Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice, Exp. Clin. Psychopharmacol. 23 (2015) 228–237. doi: 10.1037/pha0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bruder-Nascimento T, Ekeledo OJ, Anderson R, Le HB, Belin de Chantemèle EJ, Long Term High Fat Diet Treatment: An Appropriate Approach to Study the Sex-Specificity of the Autonomic and Cardiovascular Responses to Obesity in Mice, Front. Physiol. 8 (2017) 32. doi: 10.3389/fphys.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ingvorsen C, Karp NA, Lelliott CJ, The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice., Nutr. Diabetes. 7 (2017) e261. doi: 10.1038/nutd.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paruthiyil S, Hagiwara S-I, Kundassery K, Bhargava A, Sexually dimorphic metabolic responses mediated by CRF2 receptor during nutritional stress in mice, Biol. Sex Differ. 9 (2018) 49. doi: 10.1186/s13293-018-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asarian L, Geary N, Sex differences in the physiology of eating., Am. J. Physiol. Regul. Integr. Comp. Physiol. 305 (2013) R1215–67. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leeners B, Tobler PN, Asarian L, Geary N, Ovarian hormones and obesity, Hum. Reprod. Update. 23 (2017) 300–321. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fong AK, Kretsch MJ, Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle., Am. J. Clin. Nutr. 57 (1993) 43–46. doi: 10.1093/ajcn/57.1.43. [DOI] [PubMed] [Google Scholar]
- [17].Gong EJ, Garrel D, Calloway DH, Menstrual cycle and voluntary food intake., Am. J. Clin. Nutr. 49 (1989) 252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- [18].Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF, Reduction of food intake in the ovulatory phase of the menstrual cycle., Am. J. Clin. Nutr. 49 (1989) 1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- [19].Day DS, Gozansky WS, VanPelt RE, Schwartz RS, Kohrt WM, Sex hormone suppression reduces resting energy expenditure and {beta}-adrenergic support of resting energy expenditure., J. Clin. Endocrinol. Metab. 90 (2005) 3312–3317. doi: 10.1210/jc.2004-1344. [DOI] [PubMed] [Google Scholar]
- [20].Lovejoy JC, Champagne CM, deJonge L, Xie H, Smith SR, Increased visceral fat and decreased energy expenditure during the menopausal transition., Int. J. Obes. 32 (2008) 949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS, Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity., Endocrinology. 150 (2009) 2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asarian L, Geary N, Cyclic estradiol treatment normalize: body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats., Horm. Behav. 42 (2002) 461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- [23].Speakman JR, Use of high-fat diets to study rodent obesity as a model of human obesity., Int. J. Obes. 43 (2019) 1491–1492. doi: 10.1038/s41366-019-0363-7. [DOI] [PubMed] [Google Scholar]
- [24].deLartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE, Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats., PLoS One. 7 (2012) e32967. doi: 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA, A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice, Cell Metab. 26 (2017) 539–546.e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lusk G, The Elements of the Science of Nutrition, ed. 4, Philadelphia, W, B: Saunders Co. (1928) 64–68. [Google Scholar]
- [27].deLartigue G, Ronveaux CC, Raybould HE, Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity, Mol. Metab. 3 (2014) 595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, VFarese R Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E, A guide to analysis of mouse energy metabolism, Nat. Methods. 9 (2011) 57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beeler JA, Faust RP, Turkson S, Ye H, Zhuang X, Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity, Not Increased Appetitive Motivation., Biol. Psychiatry. 79 (2016) 887–897. doi: 10.1016/j.biopsych.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ruegsegger GN, Booth FW, Running from Disease: Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes, Front. Endocrinol. 8 (2017) 109. doi: 10.3389/fendo.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thompson TL, Moss RL, Estrogen Regulation of Dopamine Release in the Nucleus Accumbens: Genomic-and Nongenomic-Mediated Effects, J. Neurochem. 62 (1994) 1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- [32].Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER, Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity, Proc. Natl. Acad. Sci. 97 (2000) 12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].deLartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE, Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons., Am. J. Physiol. Endocrinol. Metab. 301 (2011) E187–95. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gao Q, Horvath TL, Cross-talk between estrogen and leptin signaling in the hypothalamus, Am. J. Physiol. Endocrinol. Metab. 294 (2008) E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- [35].Clegg DJ, Brown LM, Woods SC, Benoit SC, Gonadal Hormones Determine Sensitivity to Central Leptin and Insulin, Diabetes. 55 (2006) 978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- [36].Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, Enriori PJ, Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice., Endocrinology. 155 (2014) 4447–4460. doi: 10.1210/en.2014-1342. [DOI] [PubMed] [Google Scholar]
- [37].Kim JS, Rizwan MZ, Clegg DJ, Anderson GM, Leptin Signaling Is Not Required for Anorexigenic Estradiol Effects in Female Mice., Endocrinology. 157 (2016) 1991–2001. doi: 10.1210/en.2015-1594. [DOI] [PubMed] [Google Scholar]
- [38].Lin S, Thomas TC, Storlien LH, Huang XF, Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice., Int. J. Obes Relat. Metab. Disord. 24 (2000) 639–646. [DOI] [PubMed] [Google Scholar]
- [39].Huang K-P, Ronveaux CC, deLartigue G, Geary N, Asarian L, Raybould HE, Deletion of leptin receptors in vagal afferent neurons is disrupts estrogen signaling, body weight, food intake and hormonal controls of feeding in female mice., Am. J. Physiol. Endocrinol. Metab. 316 (2019) E568–E577. doi: 10.1152/ajpendo.00296.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]