Abstract
Posttraumatic stress disorder (PTSD) and tobacco use are prevalent conditions that co-occur at striking rates in the US. Previous reviews examined prevalence and factors associated with cigarette smoking among individuals with PTSD but have not been summarized since 2007. Moreover, none explored rates and factors associated with the use of other tobacco products. This study aimed to systematically review the most recent literature examining the comorbidity of PTSD and tobacco use to provide prevalence rates, as well as summarize the literature exploring other factors associated with tobacco use among individuals with PTSD. Studies were identified using a systematic search of keywords related to tobacco use and PTSD within the following databases: PubMed, PsycINFO, Web of Knowledge, CINAHL, PsycARTICLES, and Cochrane Clinical Trials Library. The studies included in this review (N = 66) showed that the prevalence of current use of tobacco products in individuals with PTSD was 24.0% and the rate of PTSD among users of tobacco products was 20.2%. Additionally, results demonstrated that individuals with PTSD present with high levels of nicotine dependence and heavy use of tobacco products, as well as underscore the importance of negative emotional states as a contributing factor to tobacco use among individuals with PTSD. It is imperative that future studies continue monitoring tobacco use among individuals with PTSD while also assessing factors identified as having a prominent role in tobacco use among individuals with PTSD. These findings also demonstrate the need for more innovative approaches to reduce the pervasive tobacco use among individuals with PTSD.
Keywords: Posttraumatic stress disorder, Tobacco use, Smoking, Electronic cigarette, Cigar, Smokeless tobacco, Comorbidity
1. Introduction
Posttraumatic stress disorder (PTSD) and cigarette smoking are prevalent conditions that co-occur at high rates in the US (Fu, McFall, Saxon, et al., 2007). The prevalence of cigarette smoking among individuals with PTSD has been estimated as high as 45% (Feldner, Babson, & Zvolensky, 2007; Fu et al., 2007; Kelly, Jensen, & Sofuoglu, 2015), approximately three times the rate of the general population (Jamal et al., 2016). PTSD can also further increase rates of cigarette smoking when co-occurring with other risk factors. For example, among veterans with PTSD, rates of cigarette smoking are as high as 66% (Fu et al., 2007). Although both PTSD and tobacco dependence are independently associated with high rates of mortality (Chesney, Goodwin, & Fazel, 2014; Walker, McGee, & Druss, 2015), there is evidence that smoking is a primary contributor to reduced life expectancy among individuals with PTSD (Boscarino, 2008; Tam, Warner, & Meza, 2016). Thus, it is critical to implement effective strategies to reduce the consumption of cigarettes among those with PTSD.
Prevalence of cigarette smoking in the general population has steadily reduced over the last decade, whereas rates of smoking have remained relatively stable among individuals with mental illness generally, as well as among individuals with PTSD specifically (Kelly, Jensen, & Sofuoglu, 2015; Stanton, Keith, Gaalema, et al., 2016). Various hypotheses have been proposed to explain the high co-occurrence of smoking and PTSD. The self-medication hypothesis, perhaps the most widely accepted explanation (Feldner, Babson, & Zvolensky, 2007), posits that individuals with PTSD smoke cigarettes as a way to lessen or cope with symptoms associated with their diagnosis (Khantzian, 1997). Another explanation is that there is a bidirectional relationship between PTSD and cigarette smoking (Fu et al., 2007), whereby individuals with PTSD symptoms initially smoke as a means to alleviate its symptomology, but as tobacco use increases it heightens PTSD symptoms (Leventhal & Zvolensky, 2015). The high co-occurrence of PTSD and smoking could also be due to shared vulnerabilities, such as genetic factors (Amstadter, Nugent, Koenen, et al., 2009), neurobiological structures (Enman, Zhang, & Unterwald, 2014), personality traits (Morisano, Bacher, Audrain-McGovern, & George, 2009), psychological mechanisms (Mathew, Cook, Japuntich, & Leventhal, 2015), or prior exposure to traumatic events (Feldner, Babson, & Zvolensky, 2007). Finally, other underlying factors that may explain continued smoking are the high levels of nicotine dependence or the severity of withdrawal symptoms that they experience (Fu et al., 2007; Jamal et al., 2016). In addition, individuals with mental illness have lower cessation rates in comparison with the general population (Prochaska, Das, & Young-Wolff, 2017). Many smokers with a mental illness report that they would like to quit, and in fact there is evidence that motivation to cease smoking among them is similar to those without a mental condition (Young-Wolff et al., 2014). Unfortunately, smokers with mental illness typically experience greater difficulty quitting smoking and poorer cessation outcomes due to their heavier smoking, greater nicotine dependence, and greater withdrawal symptoms (Lawrence, Mitrou, & Zubrick, 2009; Smith, Mazure, & McKee, 2014). Among individuals with PTSD in particular, the inability to tolerate elevated levels of negative emotional states, especially when confronted to trauma- and stress-related situations, has been suggested as one the leading factors for their lower quit rates (Cook, McFall, Calhoun, & Beckham, 2007). There is therefore an urgent need to understand the reasons for the high prevalence of cigarette smoking in smokers with PTSD and to identify the barriers that limit their capability to quitting smoking.
However, cigarette smoking is only one area of concern. The use of alternative tobacco products, such as combustible products (e.g., cigars) and non-combustible products (e.g., e-cigarettes) is rising, especially among smokers with mental illness (Cummins, Zhu, Tedeschi, Gamst, & Myers, 2014; Sawchuk, Roy-Byrne, Noonan, et al., 2012). For example, two recent epidemiological studies reported that e-cigarette use was twice as likely in individuals with a mental condition compared to those without (Cummins et al., 2014; Spears, Jones, Weaver, Pechacek, & Eriksen, 2016). Given the high unemployment rates among people with PTSD, (Spears et al., 2016) it is likely that the lower prices of little cigars and cigarillos may contribute to elevated rates of use of these products among people with PTSD (Miller, Tidey, Bunn, et al., n.d.). Given their low quit rates, people with PTSD may be particularly likely to use non-combusted tobacco products to reduce their smoking rate (Cummins et al., 2014; Hefner et al., 2016). However, actual rates and factors that underlie alternative tobacco product use in this population are unknown.
Prior reviews have examined co-ocurrence and factors associated with cigarette smoking among individuals with PTSD (Feldner, Babson, & Zvolensky, 2007; Fu et al., 2007; Kelly, Jensen, & Sofuoglu, 2015; Tidey & Miller, 2015). Two of these reviews provided a systematic summary, but included data from studies published before 2007. Also, none used a meta-analytic approach to summarize and statistically integrate results of independent studies. Instead, these reviews provided largely divergent prevalence estimates or combined prevalence estimates using simple pooling techniques which have limited utility as metrics of the actual rates of PTSD and cigarette smoking. Moreover, while these reviews focused on the rates of cigarette smoking among individuals with PTSD, they did not examine the prevalence of PTSD among smokers, mainly due to the lack of studies providing these estimates. It is also noteworthy that no review has explored rates and factors associated with the use of other tobacco products, despite existing evidence indicating that individuals with PTSD frequently use novel/alternative tobacco products (Hefner et al., 2016; Sawchuk et al., 2012). Thus, understanding the co-occurrence of tobacco use and PTSD is both necessary and timely since this information may guide policy making, identify research priorities, and show changes and trends over time. The overall purpose of this study was to conduct a systematic review of the most recent literature examining the comorbidity of PTSD and tobacco use. The specific aims were to quantify the co-occurrence of PTSD and tobacco use, and summarize the literature exploring factors associated with tobacco use among individuals with PTSD.
2. Methods
2.1. Search strategy
This systematic review was conducted on following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). A study protocol was designed prior to the formal search process based on prior published protocols and the PRISMA-P checklist for systematic reviews and meta-analysis protocols. PubMed, PsycINFO, Web of Knowledge, CINAHL, PsycARTICLES, and Cochrane Clinical Trials Library databases were searched for literature related to PTSD and tobacco use published between January 2007 and December 2016 using the search strategy outlined in Supplementary Appendix A. Searches yielded 10,541 references. After removing duplicates, abstracts and titles of potential articles were screened, and relevant articles were selected for further investigation. Fig. 1 shows a flow diagram of study selection.
Fig. 1.
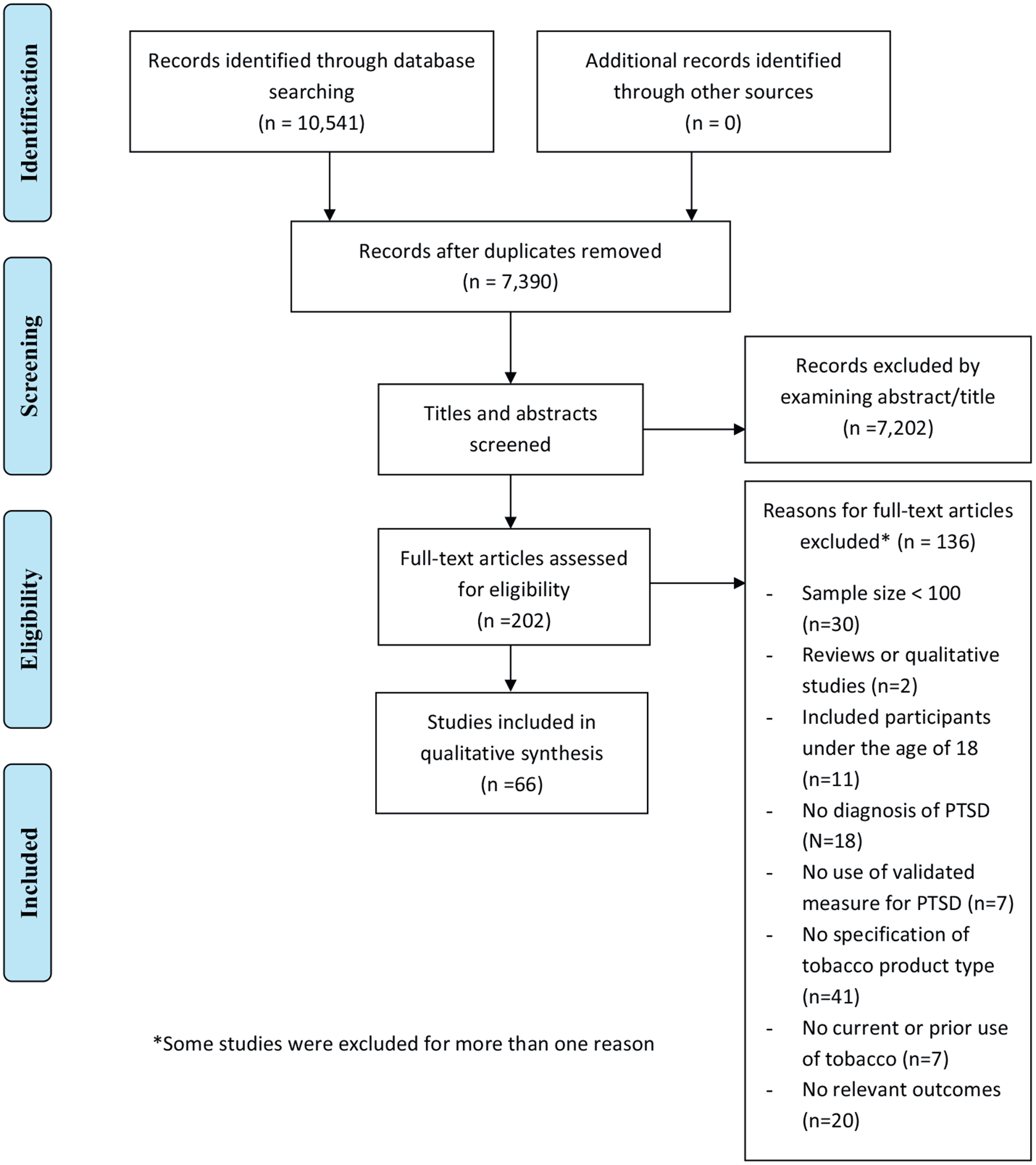
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection.
2.2. Inclusion and exclusion criteria
Studies were considered for inclusion if they (Fu et al., 2007) had an adequate sample size (n ≥ 100), based on the Consensus-based Standards for the Selection of Health Measurement Instruments guidelines (Terwee et al., 2012) (Feldner, Babson, & Zvolensky, 2007) enrolled adults aged ≥18, (Kelly, Jensen, & Sofuoglu, 2015) included those with current or lifetime diagnosis of PTSD, assessed using a standardized/validated instrument or a clinical assessment, and (Jamal et al., 2016) assessed current or prior use of tobacco products. Articles were excluded if they (Fu et al., 2007) were reviews/qualitative studies, (Feldner, Babson, & Zvolensky, 2007) used a single-case design, (Kelly, Jensen, & Sofuoglu, 2015) were not published in a peer-reviewed journal, (Jamal et al., 2016) were not written in English, (Jamal et al., 2016) included trauma-exposed individuals without meeting criteria for PTSD, or (Chesney et al., 2014) included individuals with no current or prior use of tobacco products. If a potentially eligible study had any relevant missing data, authors were contacted in order to obtain the information.
2.3. Data extraction and quality assessment
Two authors (I.P.V. and R.J.E.) independently reviewed all articles and extracted data from each study including first author, publication year, population sampled, sociodemographic characteristics, percentage of participants with diagnosis of PTSD, percentage of individuals with current or prior use of tobacco, instrument used to assess PTSD, and the main outcome. Extraction sheets were compared, and discrepancies between reviewers were discussed until reconciled. Very high agreement between reviewers was observed (Cohen’s k = 0.92). Quality assessments were conducted using the Newcastle Ottawa Scale (NOS) for cohort studies (Wells et al., 2017), the Physiotherapy Evidence Database (PEDro) scale for randomized controlled trials (Campos, Beckenkamp, & Moseley, 2013), and the 11-item questionnaire recommended by the Agency for Healthcare Research and Quality for cross-sectional studies (AHRQ) (Rostom et al., 2004). Possible scores range from 0 to 9 for the NOS and from 0 to 11 for PEDro and AHRQ with higher scores indicating higher quality.
2.4. Statistical analyses
Meta-analyses were performed using Comprehensive Meta-Analysis (Borenstein & Rothstein, 1999). Prevalence estimates of comorbid tobacco use and PTSD were calculated using a random effects approach due to the diversity of methodologies used across studies. These estimates were based on the proportion of participants within each study who presented the outcome of interest (e.g. proportion of current smokers with PTSD).
Heterogeneity between studies was assessed using the Q test with p values lower than 0.05 indicating significant, and the I2 statistic which quantifies the percentage of the total variation across studies due to between-study heterogeneity (i.e. not sampling error or chance), with larger values indicating greater variability (low 25%, moderate 50%, and high 75%) (Higgins, Thompson, Deeks, & Altman, 2003).
Publication bias was explored graphically using funnel plot and empirically using the Begg and & Mazumdar’s adjusted-rank correlation test and the Egger’s regression asymmetry test. The funnel plot is a scatter plot of the studies included in the meta-analysis. In the presence of bias, the graph resembles an asymmetrical inverted funnel. Asymmetry on the funnel plot could be due to other causes besides publication bias (Sterne, Sutton, Ioannidis, et al., 2011). Thus, we additionally computed significance tests of the presence of publication bias. More specifically, we conducted the Begg & Mazumdar’s test (Begg & Mazumdar, 1994) and the Egger’s test (Egger, Smith, Schneider, & Minder, 1997) which are analogues of the funnel plot approach but avoid the subjectivity associated with visual inspection of publication bias (Lau, Ioannidis, Terrin, Schmid, & Olkin, 2006).
3. Results
Sixty-six studies met the inclusion criteria and therefore were included. Across studies, most participants were White (70.6%), half were female (50.8%) and were on average 45.7 years old. The majority of the studies included samples with current or prior use of conventional cigarettes (N = 60). Only 6 studies (Crum-Cianflone, Powell, LeardMann, Russell, & Boyko, 2016; Fullerton, McKibben, Reissman, et al., 2013; Hermes, Wells, Smith, et al., 2012; Roberts, Roberts, Jones, & Bisson, 2015; Sawchuk et al., 2012; Van der Velden, Grievink, Olff, Gersons, & Kleber, 2007) included users of other tobacco products, such as smokeless tobacco (i.e., chew, dip, snuff) and other combustible tobacco products (i.e., cigars, pipe, papirossi (filterless cigarettes)). None of the studies of other combustible tobacco products provide rates of use by type of product (Fullerton et al., 2013; Roberts et al., 2015; Van der Velden et al., 2007). Among the studies examining smokeless tobacco product use (Crum-Cianflone et al., 2016; Fullerton et al., 2013; Hermes et al., 2012; Sawchuk et al., 2012), two studies did not specify type of product (Crum-Cianflone et al., 2016), two assessed use of chewing tobacco (Fullerton et al., 2013; Sawchuk et al., 2012), and one assessed jointly use of chew, dip, and snuff (Hermes et al., 2012). Quality rantings ranged from 3 to 9 among cross-sectional studies and from 5 to 7 among cohort studies. Global ratings for all randomized controlled trials was 7. Details of included studies are provided in Supplementary Appendix B.
3.1. Prevalence of tobacco use among individuals with PTSD
Thirteen studies examined prevalence of prior or current use of tobacco products among individuals with a history of or current PTSD (Baschnagel, Coffey, Schumache, Drobes, & Saladin, 2008; Boscarino, 2008; Bromet et al., 2016; Cook, Jakupcak, Rosenheck, Fontana, & McFall, 2009; Cougle, Zvolensky, Fitch, & Sachs-Ericsson, 2010; Crum-Cianflone et al., 2016; de la Hoz, Jeon, Miller, Wisnivesky, & Celedón, 2016; Fullerton et al., 2013; Gabert-Quillen, Selya, & Delahanty, 2015; Lawrence et al., 2009; Welch, Jasek, Caramanica, Chiles, & Johns, 2015; Witteveen et al., 2010; Zen, Whooley, Zhao, & Cohen, 2012), of which 11 provided rates of cigarette smoking while only two assessed the use of other tobacco products (smokeless, pipe, cigars, chewing tobacco) (Crum-Cianflone et al., 2016; Fullerton et al., 2013). Eleven studies provided rates of current tobacco use among individuals with PTSD (Baschnagel et al., 2008; Bromet et al., 2016; Cook et al., 2009; Crum-Cianflone et al., 2016; de la Hoz et al., 2016; Fullerton et al., 2013; Gabert-Quillen et al., 2015; Lawrence et al., 2009; Welch et al., 2015; Witteveen et al., 2010; Zen et al., 2012), which when combined yield a prevalence of 24% (95% CI, 18.9%–29.9%; Q (11) = 242.631, I2 = 95.466, p < 0.0001). The lowest prevalence was from a study assessing smokeless tobacco use among military members (7%) (Crum-Cianflone et al., 2016) while the highest was from a study examining SUD and PTSD comorbidity in a sample of adults exposed to a crime-related trauma (53%). Results are graphically displayed in Fig. 2. The funnel plot was asymmetrical (See Supplementary Appendix C). However, neither the results of the Begg and & Mazumdar’s adjusted-rank correlation test (p = 0.73) nor and the Egger’s regression asymmetry test were significant (p = 0.31) which suggests absence of publication bias. When analyses were conducted including only studies exploring cigarette smoking (Fig. 3), the prevalence increased to 27.1% (95% CI, 21.3%–33.8%; Q (9) = 206.262, I2 = 95.637, p < 0.0001). Visual examination of the forest plot evidenced an asymmetric feature (see Supplementary Appendix C) which raises the possibility of publication bias. However, the p values for the Begg & Mazumbar and Egger test were 0.28 and 0.11 respectively, which suggest an absence of publication bias.
Fig. 2.
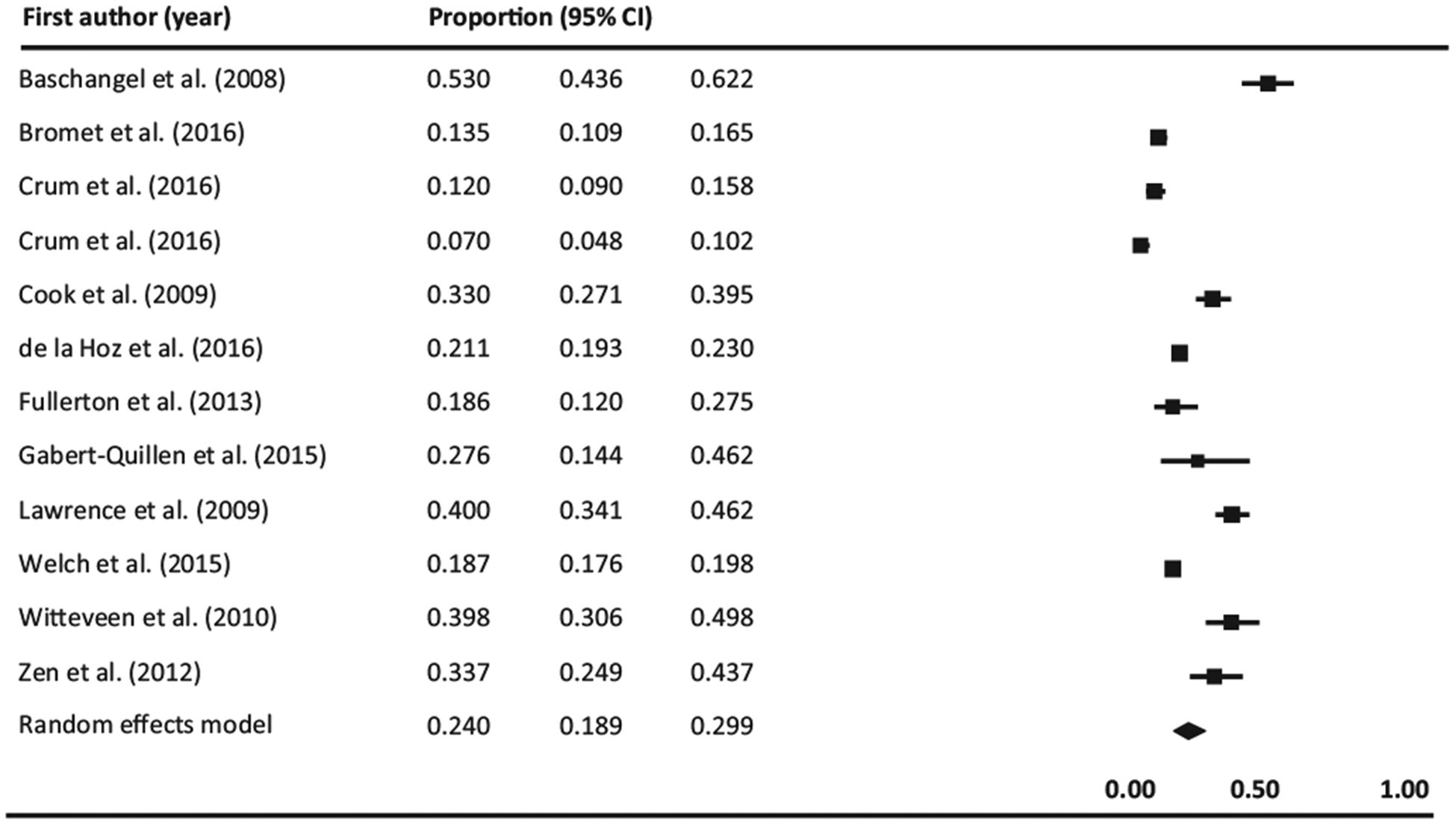
Forest plot of prevalence of current tobacco use among individuals with PTSD. Black squares represent the study’s weight in the meta-analysis and horizontal lines represent 95% confidence intervals. Black diamonds represent estimated prevalences.
Fig. 3.
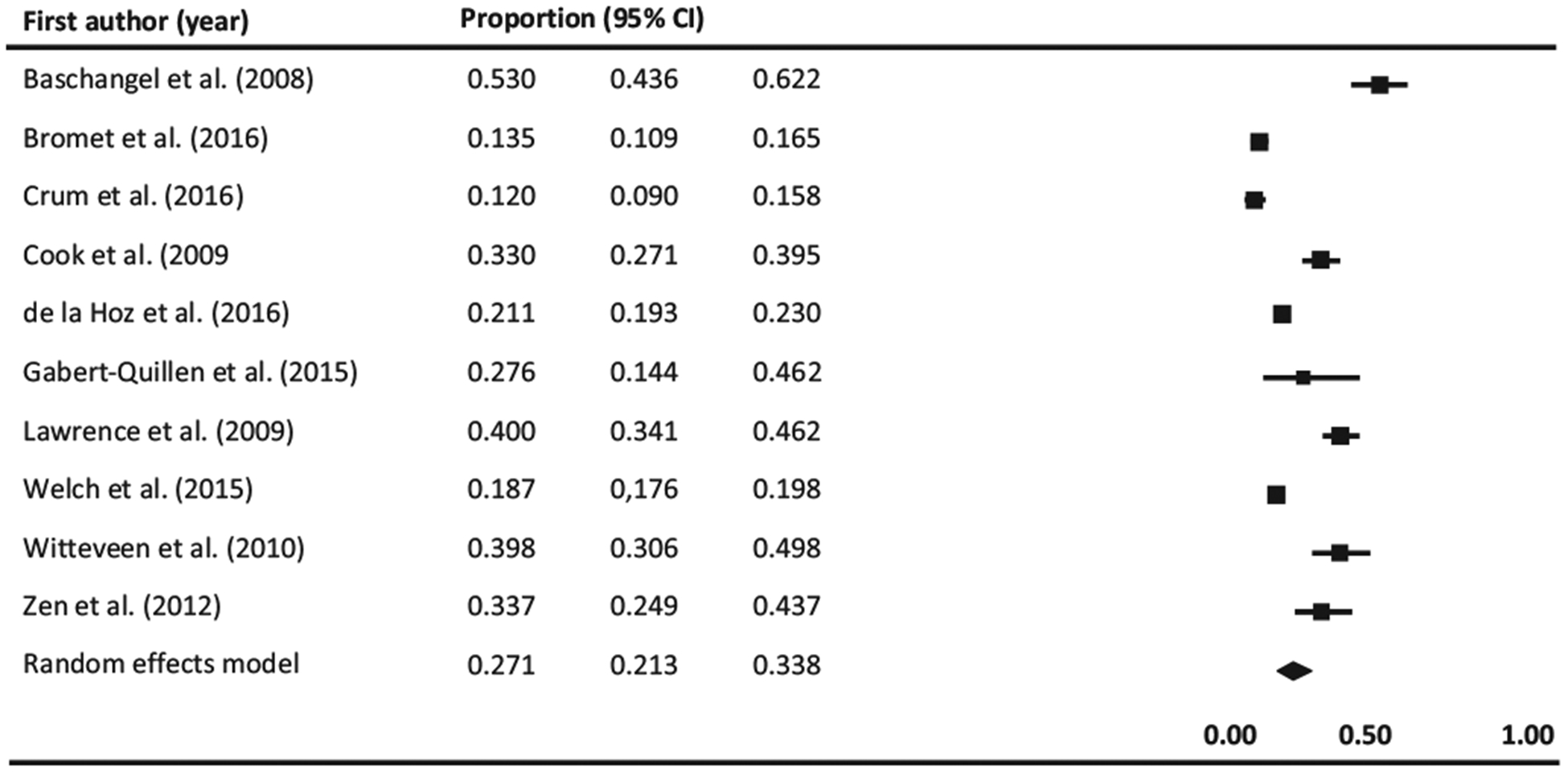
Forest plot of prevalence of current cigarette smoking among individuals with PTSD. Black squares represent the study’s weight in the meta-analysis and horizontal lines represent 95% confidence intervals. Black diamonds represent estimated prevalences.
Three studies reported rates of prior use of tobacco products among individuals with PTSD where the mean prevalence was 32.2% (95% CI, 20.1%–47.4%; Q (2) = 25.548, I2 = 92.172, p < 0.0001) (de la Hoz et al., 2016; Witteveen et al., 2010; Zen et al., 2012). The funnel plot was asymmetrical (See Supplementary Appendix C). However, the Begg and & Mazumdar’s adjusted-rank correlation test (p = 0.50) and the Egger’s regression asymmetry test (p = 0.27) did not suggest any significant risk for publication bias among these studies.
Finally, one study provided an average lifetime smoking total of 36.7 pack-years of cigarette smoking (i.e., calculated using packs smoked per day and number of years smoked) (Boscarino, 2008) and another study reported lifetime and 12-month rates of cigarette smoking of 64.5% and 37.9%, respectively (Cougle et al., 2010). Results are displayed graphically in Supplementary Appendix D.
3.2. Prevalence of PTSD among individuals using tobacco products and comorbid PTSD and tobacco use
Twenty-nine studies reported the prevalence of prior or current PTSD among current or lifetime users of tobacco products (Alexander, Ali, McDevitt-Murphy, et al., 2017; Baggett, Campbell, Chang, Magid, & Rigotti, 2016; Farris, 2015; Farris, 2016; Farris, Vujanovic, Hogan, Schmidt, & Zvolensky, 2014; Farris, Zvolensky, Beckham, Vujanovic, & Schmidt, 2014; Feldner, Babson, Zvolensky, et al., 2007; Flanagan, Hakes, McClure, et al., 2016; Garey, Cheema, Otal, et al., 2016; Greenberg, Ameringer, Trujillo, et al., 2012; Hermes et al., 2012; Hruska, Bernier, Kenner, et al., 2014; Jessup, Dibble, & Cooper, 2012; Lawrence et al., 2009; Lombardero et al., 2014; Lopez, Konrath, & Seng, 2011; Lopez & Seng, 2014; Mahaffey, Gonzalez, Farris, et al., 2016; Mathew et al., 2015; McClernon, Calhoun, Hertzberg, et al., 2013; Roberts et al., 2015; Sawchuk et al., 2012; Sawchuk, Roy-Byrne, Noonan, et al., 2016; Van der Velden et al., 2007; Weber, Boggero, Carlson, et al., 2016; Young-Wolff et al., 2014; Zvolensky, 2014; Zvolensky, Farris, Schmidt, & Smits, 2014; Zvolensky, Vujanovic, Miller, et al., 2007). Of these studies, twenty-five explored rates of PTSD among cigarette smokers, two among smokeless tobacco users (Hermes et al., 2012; Sawchuk et al., 2012), and the other two among users of other combustible tobacco products (Roberts et al., 2015; Van der Velden et al., 2007). Twenty-four studies reported a prevalence rate of current PTSD among current users of tobacco products, which when combined yield a prevalence of 20.2% (95% CI, 13.7%–28.8%; Q (23) = 2100.137, I2 = 98.908, p < 0.0001). The lowest rate of PTSD among current users of tobacco products was found in a study involving cigarette smokers from the general population (3.0%) (Zvolensky et al., 2014) whereas the highest rate of PTSD among current users of tobacco products was reported in a study of homeless cigarette smokers (66.3%) (Baggett et al., 2016). Results are graphically displayed in Fig 4. While the funnel plot was asymmetrical (See Supplementary Appendix C), the p values for the Egger test and Begg & Mazumbar test were 0.24 and 0.35, respectively, which is evidence of the absence of publication bias.
Fig. 4.
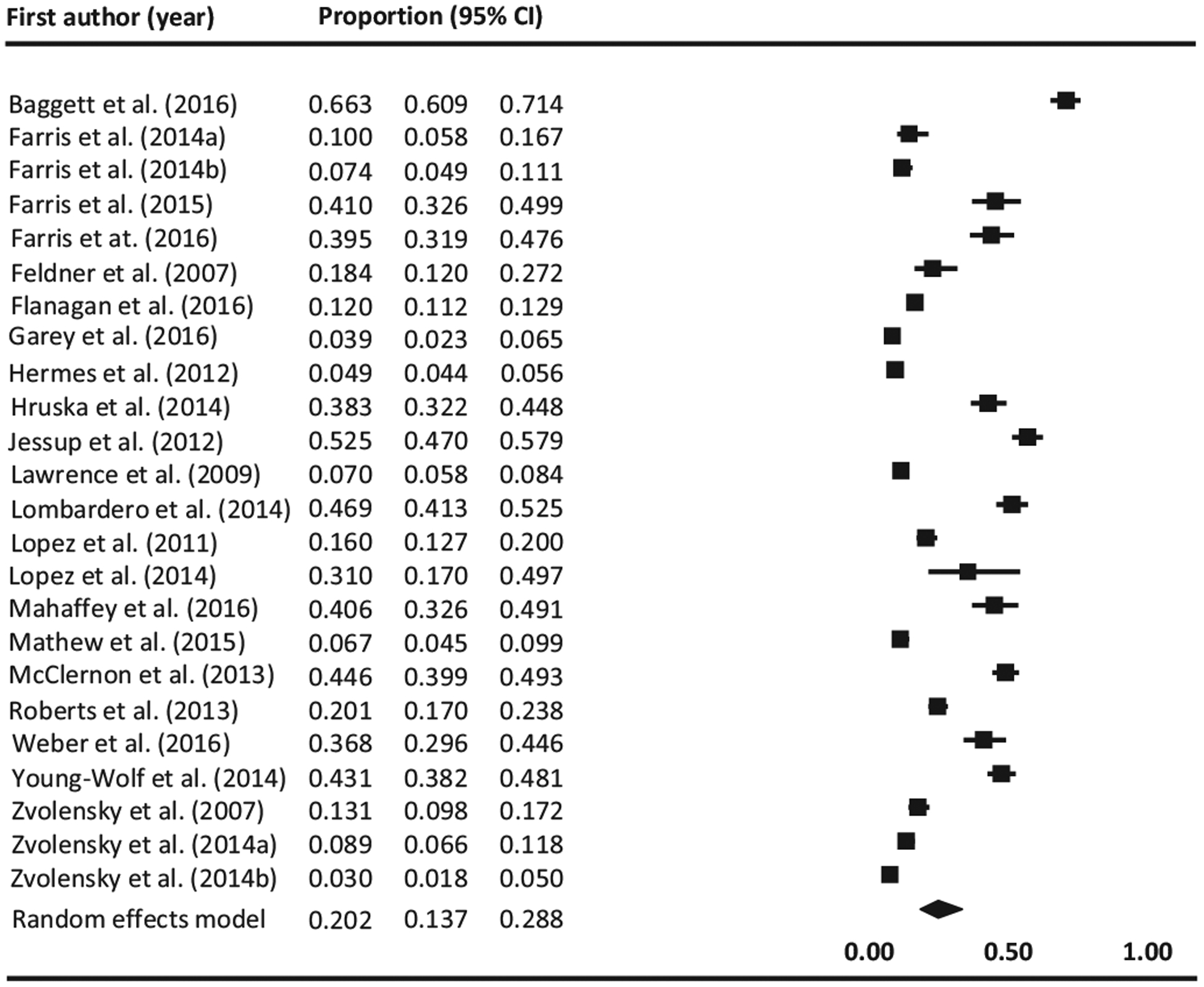
Forest plot of prevalence of PTSD among current tobacco users. Black squares represent the study’s weight in the meta-analysis and horizontal lines represent 95% confidence intervals. Black diamonds represent estimated prevalences.
Other studies explored rates of current or history of PTSD among current or former users of tobacco products. Four studies (Hermes et al., 2012; Lopez et al., 2011; Lopez & Seng, 2014; McClernon et al., 2013) reported prevalence of current PTSD among former users of tobacco(12.3% 95% CI 4.4%–30.2%; Q (3) = 130.787, I2 = 97.706, p < 0.0001). The funnel plot was asymmetrical (See Supplementary Appendix C). However, the publication bias tests, Egger test (p = 0.95) and Begg & Mazumbar (p = 0.73), did not suggest any significant risk for publication bias. Two studies reported rates of lifetime PTSD among current and former users (Lopez et al., 2011; Lopez & Seng, 2014) which yield prevalence estimates of 43.9% (95% CI 21.6%–68.9% Q (1) = 7.422, I2 = 86.527, p = 0.0006) and 34.7% (95% CI 20.6%–52.0%; Q (1) = 4.638, I2 = 78.437, p < 0.0031). Neither the funnel plot nor publication bias tests were computed due to the number of studies being fewer than 3.
Five studies reported prevalence of current (Alexander et al., 2017; Greenberg et al., 2012; Van der Velden et al., 2007) (23.6%, 95% CI 12.6%–39.7%; Q (1) = 85.743, I2 = 97.667, p < 0.0001) or lifetime (16.4%, 95% CI 14.9%–18.0%; Q (1) = 0.845, I2 = 0.00, p = 0.358) PTSD among lifetime users of tobacco products (Sawchuk et al., 2012; Sawchuk et al., 2016). Results are displayed graphically in Supplementary Material D. For the first estimate of the publication bias, Egger test (p = 0.59) and Begg & Mazumbar (p = 0.60), did not suggest any significant risk for publication bias. For the second estimate, tests were not computed due to the number of studies being fewer than 3. However, the funnel plot was asymmetrical for both estimates (See Supplementary Appendix C).
Finally, only one study reported the comorbidity of PTSD and tobacco use. More specifically, this study reported a prevalence rate of cigarette smoking among a sample of war veterans of 21.7% (Hasanović & Pajević, 2010).
3.3. Nicotine dependence
A total of seventeen studies investigated the association between PTSD and nicotine dependence. Of these studies, nine studies found significant associations between PTSD diagnosis and nicotine dependence. In particular, six found higher levels of nicotine dependence among individuals with PTSD compared to those without PTSD (Mitchell, Van Voorhees, Dennis, et al., 2012; Vrana et al., 2013; Vrana, Calhoun, Dennis, Kirby, & Beckham, 2015) or any other psychiatric condition (Baggett et al., 2016; Beckham, Calhoun, Dennis, Wilson, & Dedert, 2013; Wilson, Dedert, Dennis, et al., 2014), and three found that diagnosis of PTSD was associated with increased risk for nicotine dependence(Cougle et al., 2010; Greenberg et al., 2012; Roberts, Chikovani, Makhashvili, Patel, & McKee, 2013). It should be noted that the proportion of individuals with other psychiatric conditions in these studies was extremely low (≤6%).
Seven studies found that individuals with PTSD do not have higher levels of nicotine dependence compared to those without PTSD. Specifically, five studies found that individuals with PTSD do not have higher levels of dependence than individuals with no psychiatric condition (Marshall et al., 2008; Marshall et al., 2009) or trauma exposed adults (Baschnagel et al., 2008; Beckham et al., 2007; Farris, 2015). Four studies found no difference in levels of dependence between individuals with PTSD and those with other conditions, such as anxiety or depression (Marshall et al., 2008; Marshall et al., 2009; Young-Wolff et al., 2014; Zvolensky, Gibson, Vujanovic, et al., 2008). Finally, one study found higher levels of nicotine dependence among individuals with PTSD compared to those with no psychiatric condition, but there no differences compared to individuals with major depressive disorder (Cook, Baker, Beckham, & McFall, 2017).
Across these studies, the Fagerström Test for Nicotine Dependence was the instrument most commonly (82% or 14/17) used to assess dependence. Other instruments used were the Revised Tolerance Questionnaire, the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV), and the WMH-CIDI (World Mental Health Survey Initiative version of the World Health Organization Composite International Diagnostic Interview). Regarding the quality of the studies, one was given a strong methodological rating, ten were scored as moderate, and one was scored as weak.
3.4. Heaviness of tobacco use
Twenty-one studies explored the relationship between PTSD and heaviness of tobacco use, none of which explored products other than conventional cigarettes. Nine studies found PTSD was associated with heavier use of cigarettes (Amstadter et al., 2009; Cougle et al., 2010; de la Hoz et al., 2016; Dennis, Watkins, Calhoun, et al., 2014; Feldner, Babson, Zvolensky, et al., 2007; Flanagan et al., 2016; Fullerton et al., 2013; Greenberg et al., 2012; Zen et al., 2012). Specifically, two studies (2/9) found individuals with PTSD compared to those without this condition showed higher increases in cigarette use after disaster exposure (Amstadter et al., 2009; Fullerton et al., 2013). Four studies (4/9) found that individuals with PTSD smoke more heavily, either as number of cigarettes per day (Dennis et al., 2014; Feldner, Babson, Zvolensky, et al., 2007) or number of packs smoked lifetime (de la Hoz et al., 2016; Zen et al., 2012), and three studies (3/9) found that PTSD diagnosis increases the risk of smoking more cigarettes per day (Cougle et al., 2010; Flanagan et al., 2016; Greenberg et al., 2012).
Eleven studies found PTSD was not associated with heavier use of tobacco products (Baggett et al., 2016; Baschnagel et al., 2008; Beaudoin, 2011; Beckham et al., 2007; Cook et al., 2017; Farris, 2015; Marshall et al., 2008; Marshall et al., 2009; Mitchell et al., 2012; Ritter, McCauley, Amstadter, et al., 2011; Wilson et al., 2014). Of those studies, ten found that individuals with PTSD do not smoke a higher number of cigarettes than those without this condition, and one study reported that a diagnosis of PTSD was not associated with cigarettes smoked per day. Again, when exploring the comparison groups we found that most were vulnerable populations including individuals who are homeless (Baggett et al., 2016), individuals exposed to a traumatic event (Baschnagel et al., 2008; Beaudoin, 2011; Farris, 2015; Ritter et al., 2011), and individuals with current or prior diagnosis of a psychiatric disorder (Cook et al., 2017; Marshall et al., 2008; Marshall et al., 2009; Mitchell et al., 2012; Wilson et al., 2014). Finally, one study found mixed results wherein individuals with PTSD smoked more heavily than those without this condition (Zvolensky et al., 2008), but not more than individuals with another anxiety disorder.
Regarding the methodological quality of the studies included, nine studies were given a strong rating, eleven a moderate rating, and two were scored as weak.
3.5. Withdrawal symptoms
Nine studies explored withdrawal symptoms among individuals with PTSD (Asnaani, Alpert, McLean, & Foa, 2015; Asnaani, Farris, Carpenter, Zandberg, & Foa, 2015; Baggett et al., 2016; Beckham et al., 2013; Carmody, McFall, Saxon, et al., 2012; Farris, 2015; Feldner, Babson, Zvolensky, et al., 2007;Marshall et al., 2008; Zvolensky et al., 2008). Three studies found smokers with PTSD, compared to those without, experience higher levels of withdrawal symptoms (Farris, 2015; Marshall et al., 2008; Zvolensky et al., 2008) of which two found evidence that individuals with PTSD suffer more severe symptoms when attempting to quit smoking (Marshall et al., 2008; Zvolensky et al., 2008) and one study found that individuals with PTSD are more likely than those without to attribute smoking lapse to experiencing withdrawal symptoms (Beckham et al., 2013). In addition, three studies found that individuals with PTSD, compared to those without, have greater expectancies that smoking a cigarette would reduce their withdrawal symptoms (Baggett et al., 2016;Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008). Finally, two studies showed higher levels of withdrawal symptoms are associated with greater PTSD symptom severity (Asnaani, Alpert, et al., 2015; Asnaani, Farris, et al., 2015).
A total of six different instruments were used in these studies to assess withdrawal symptoms. More specifically, two used the Withdrawal Symptoms Checklist, two used the Smoking Outcomes Expectancies questionnaire, and two used the Reasons for Smoking questionnaire, while the remaining three studies used items extracted from DSM-IV criteria for nicotine withdrawal, the Smoking Abstinence Expectancies Questionnaire, or the Minnesota Nicotine Withdrawal Scale. With regard to the quality of the studies, four studies were coded as having strong methodological quality, another four as moderate, and one was considered weak.
3.6. Craving
Five studies examined craving among individuals with PTSD (Beckham et al., 2007; Beckham et al., 2013;Carmody et al., 2012; Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008). Three of these studies demonstrated that craving drives cigarette smoking behavior among individuals with PTSD (Carmody et al., 2012; Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008), and two indicated that smokers with PTSD compared to individuals without this condition reported greater motivation to smoke to reduce craving (Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008). One study, exploring the effect of trauma- and stress-related scripts versus neutral scripts on cigarette craving, demonstrated that smokers with PTSD experience greater levels of cigarette craving across script types compared to smokers without PTSD, and smoking a cigarette results in reductions in craving, but with no differences between individuals with or without PTSD (Beckham et al., 2007). Additionally, one study reported that craving is an antecedent of lapse for individuals with and without PTSD (Beckham et al., 2013).
The instrument used to assess craving varied widely across studies. More specifically, two studies used the Reasons for Smoking questionnaire, and the other three studies used a single item to assess craving, the 11-item Questionnaire on Smoking Urges, or the Smoking Abstinence Self-Efficacy Scale. With regard to the quality of the studies, four were given a moderate methodological ranting and one was scored as weak.
3.7. Quit attempts and relapse
A considerable number of studies examined rates and factors associated with quitting smoking among individuals with PTSD. Four of these studies found that smokers with PTSD exhibit faster (Beckham et al., 2013; Vrana et al., 2015; Wilson et al., 2014) and higher lapse rate (Zvolensky et al., 2008) compared to smokers without PTSD. Two studies also reported that individuals with PTSD are less likely to quit (Lopez et al., 2011; Welch et al., 2015) while one study found that PTSD diagnosis was not associated with more difficulty with quitting (Young-Wolff et al., 2014).
With regard to the number of quit attempts, three studies reported a PTSD diagnosis is associated with higher numbers of quit attempts (Cougle et al., 2010; Marshall et al., 2008; Marshall et al., 2009), and a study carried out among smokers exposed to the 9/11 disaster found no evidence of an association (Farris, 2015).
Four studies that attempted to identify barriers for quitting smoking among individuals with PTSD found that poor mental health, negative affect, and trauma reminders are factors associated with unsuccessful quit attempts (Beckham et al., 2013; Carmody et al., 2012; Malte, Dennis, Saxon, et al., 2015; McFall, Saxon, Malte, et al., 2010). Additionally, one study exploring patterns of quitting among smokers with PTSD found that not having quit smoking for at least one year, lower confidence to quit, and both poorer mental health and quality of life were associated with the worst patterns of quitting smoking (fewer quit attempts and faster relapse) (Malte et al., 2015).
Two studies examined reasons and motivation for quitting smoking among individuals with PTSD, of which one found that individuals with PTSD report greater desire to prove their self-control and more immediate positive consequences (saving money/time) for quitting than individuals without PTSD (Marshall et al., 2009), and the other finding that individuals with PTSD report greater desire and intentions to quit smoking than individuals without PTSD (Young-Wolff et al., 2014). Finally, one study found that quitting smoking is associated with improvements in quality of life among individuals with PTSD (Aversa, Stoddard, Doran, et al., 2012; Aversa, Stoddard, Doran, et al., 2013).
With regard to the methodological quality of these studies. Four were given a strong methodological ranting and eleven were scored as moderate.
3.8. Negative affect, distress, and anxiety sensitivity
Sixteen studies explored the relationship between tobacco use and negative affect, distress, or anxiety sensitivity among individuals with PTSD (Alexander et al., 2017; Asnaani, Alpert, et al., 2015; Asnaani, Farris, et al., 2015; Baggett et al., 2016; Beckham et al., 2007; Beckham et al., 2013; Carmody et al., 2012; Cook et al., 2017; Farris, 2015; Feldner, Babson, Zvolensky, et al., 2007; Malte et al., 2015; Marshall et al., 2008; Vrana et al., 2013; Vrana et al., 2015; Wilson et al., 2014; Zvolensky et al., 2008). Seven studies found that individuals with PTSD compared to individuals without PTSD experience higher levels of negative affect (Cook et al., 2017; Vrana et al., 2013; Vrana et al., 2015; Wilson et al., 2014; Zvolensky et al., 2008) or anxiety sensitivity (Farris, 2015) in neutral conditions or after the exposure to a stressor (Beckham et al., 2007; Cook et al., 2017) while one study did not find differences (Beckham et al., 2013).
Two studies found negative affect is positively associated with PTSD symptoms in a sample of adults with PTSD (Asnaani, Alpert, et al., 2015; Asnaani, Farris, et al., 2015), and one study found that mental distress is associated with a higher likelihood of having PTSD among lifetime smokers (Alexander et al., 2017). Three studies found individuals with PTSD compared to those without report greater motivation to smoke to reduce negative affect (Baggett et al., 2016; Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008) and two studies showed that smoking a cigarette, independent of the nicotine content (nicotinized, with very low nicotine content, or denicotinized), reduces negative affect among individuals with PTSD (Beckham et al., 2007; Cook et al., 2017). Moreover, one study found that among individuals with PTSD, expectancies that smoking would reduce negative affect mediates the relationship between PTSD symptom severity and both level of tobacco dependence and smoking behavior (Carmody et al., 2012). However, in a sample of veterans with PTSD there were no differences in distress levels among individuals able and not able to quit (Malte et al., 2015). Finally, one study showed that individuals with PTSD are more likely to attribute lapses to negative affect compared to individuals without psychiatric diagnoses (Beckham et al., 2013) whereas the other found negative affect was not associated with higher likelihood of relapse among those with PTSD (Zvolensky et al., 2008).
Eleven studies evaluated negative affect. More specifically, seven of these studies directly measured negative affect and the other four evaluated whether negative affect is a precipitant of tobacco use. Of the studies assessing negative affect, three used the Positive and Negative Affect Scale, two used the Positive and Negative Affect Schedule, and the another two did not specify the type of instrument used. With regard to the studies exploring whether negative affect is a precipitant of smoking, two used the Reasons for Smoking questionnaire, and the remaining two used the Smoking Effects questionnaire or the Smoking Outcome Expectancies. Three studies assessed mental distress of which two used the Short Form Health Survey (SF-36) and the another used a single item. Two studies evaluated anxiety sensitivity, one with the Anxiety Sensitivity Index-3 and the other with the Anxiety Sensitivity Index. Regarding the quality of the studies, seven were given a strong methodological rating, eight studies were of moderate quality, and one was considered weak.
4. Discussion
This systematic review and meta-analysis was conducted to estimate the prevalence of prior or current use of tobacco products among individuals with a history of or current PTSD, and the prevalence of prior or current PTSD among current or lifetime users of tobacco products. Additionally, this study summarized the most recent literature that has explored mechanisms underlying tobacco use among individuals with PTSD.
This is the first time that a systematic review and meta-analysis have been conducted to estimate prevalence of use of any form of tobacco among individuals with PTSD (24%). However, the limited number of studies assessing use of tobacco products other than cigarettes (n = 5) hindered our ability to estimate the comorbidity of PTSD and use of novel/alternative products. It should be highlighted that the finding that the prevalence of overall tobacco product use among individuals with PTSD was lower (24%) than the prevalence of cigarette smoking (27.1%). Differing definitions used to determine current cigarette smoking and current use of other tobacco products or distinct populations included in these studies might lead to an under estimation of overall tobacco use or an overestimation of cigarette smoking. It is also noteworthy that rate of current cigarette smoking among individuals with PTSD (27.1%) was remarkably lower than that found in prior reviews (45.0%). This discrepancy may be explained by the different methodology used. Unlike prior reviews, this study used statistical techniques to pool prevalence estimates from individual studies into a single quantitative result. It is also important to consider that this review included only studies published in the last decade whereas previous reviews included studies conducted as far back as thirty years ago. Thus, this disparity could also be due to the decline in cigarette smoking observed among individuals with mental illness over the last decade (Lasser et al., 2000). One finding not reported in prior reviews was the large proportion of users of tobacco products who have PTSD (20.2%), which is higher than the prevalence of PTSD among non-smokers (6.2%) (Flanagan et al., 2016). A possible explanation is that tobacco use could have an additive effect on the development of PTSD when other shared risk factors are present (Gurillo, Jauhar, Murray, & MacCabe, 2015). For example, prior research demonstrated that continued use of tobacco sensitizes neurobiological stress response systems, resulting in an increased vulnerability to developing PTSD after the exposure to a life-threatening situation (Brady & Sinha, 2007). Tobacco use has also been associated with impaired decision-making processes (Xiao, Koritzky, Johnson, & Bechara, 2013) and such alterations may cause a person to expose themselves to high-risk situations which may put them at a higher risk of PTSD. Given the high comorbidity of PTSD and tobacco use found, it is imperative that major public health efforts are conducted to reduce tobacco use among individuals with PTSD, especially the most harmful products (cigarettes).
A noteworthy finding was that individuals with PTSD were generally more dependent and heavier tobacco users than those without a psychiatric condition, but no differences were observed between individuals with PTSD and those with other mental health conditions. Results were similar regarding craving and withdrawal symptoms, however, they should be interpreted cautiously due to the small number of studies conducted. It should also be noted that none of these studies included users of tobacco products other than conventional cigarettes. Thus, the generalizability of these findings is limited to cigarette smokers. While this review did not offer insight into levels of dependence, heaviness of use, craving, or withdrawal symptoms among users of other tobacco products, its findings regarding these factors among cigarette smokers may partially explain the high rates of smoking and low quit rates observed among individuals with PTSD compared to individuals without any mental condition. Population-interventions, such as increases in price or smoking bans, have been postulated as a solution to reduce the burden of cigarette smoking (Lawrence et al., 2009; Smith et al., 2014). However, increased difficulty quitting among individuals with PTSD may require the implementation of other tobacco control measures, such as a multi-faceted approach or intense targeted interventions to reduce cigarette smoking (Hill, Amos, Clifford, & Platt, 2014).
Another finding that merits further comment is that smokers with PTSD compared to those without report greater motivation to smoke to reduce negative emotional states (Baggett et al., 2016; Feldner, Babson, Zvolensky, et al., 2007; Marshall et al., 2008). This finding supports the self-medication hypothesis as a plausible explanation to understand the frequent comorbidity of PTSD and tobacco use. Alternatively, our results may also support an array of competing theories that posit co-occurrence of tobacco use and PTSD is due to other common underlying risk factors, such exposure to a trauma (Feldner, Babson, & Zvolensky, 2007) or genetic/familiar factors (Koenen, Hitsman, Lyons, et al., 2005). Most recently, anxiety sensitivity (AS) (i.e., tendency to believe that anxiety-related bodily sensations have harmful consequences) is one possible explanatory factors that has received considerable attention (Asnaani, Farris, et al., 2015). AS has been identified as a risk factor for the development of PTSD and an amplifier of negative affect, as well as a factor associated with the initiation and maintenance of cigarette smoking (Leventhal & Zvolensky, 2015). Collectively, the research reviewed suggests mechanisms underlying the association between PTSD and tobacco use are multifaceted and complex with considerable potential for interactions.
Clarifying the mechanisms contributing to smoking relapse among smokers with PTSD has been the focus of extensive research. This study demonstrated that smokers with PTSD are more likely to smoke heavily and are more nicotine-dependent than those without this condition which may partially explain their poor outcomes during a quit attempt (McFall et al., 2010). This study also identified factors that should be targeted when treatment or prevention programs are conducted including negative affect, anxiety sensitivity, and traumatic memories (Roberts et al., 2015). Thus, a potential solution to reduce rates of cigarette smoking among individuals with PTSD could be the implementation of interventions directed to alleviate negative emotional states, such as coping skills training or exposure-based strategies (Short, Oglesby, Raines, Zvolensky, & Schmidt, 2015). Despite the potential of this approach, studies conducted reported poor smoking cessation outcomes (Feldner, Smith, Monson, & Zvolensky, 2013; Kelly et al., 2015). Therefore, the need for other effective ways to reduce smoking prevalence among individuals with PTSD is indisputable.
Nicotine reduction policy could be a way to address both over-arching challenges. The rationale of this approach is that reducing the nicotine content of cigarettes to a non-addictive level could result in a lower nicotine intake and nicotine dependence among current smokers which may facilitate quitting smoking (Gaalema, Miller, & Tidey, 2015). In support of this perspective, results from experimental studies demonstrated that smoking low-nicotine content cigarettes reduces smoking rates among smokers with mental illness without worsening psychiatric symptoms (Higgins, Heil, Sigmon, et al., 2017; Tidey, Pacek, Koopmeiners, et al., 2016). It is also important to underscore the evidence, found in two studies, that smoking a cigarette independent of nicotine content produces similar reductions on negative affect (Beckham et al., 2007; Cook et al., 2017). These findings suggest that cigarettes with low nicotine content can be effective at relieving negative affect among individuals with PTSD, making them an adequate substitute for conventional cigarettes.
An alternative proposed is to encourage smokers to switch from cigarettes to products with potentially lower toxicity and abuse liability, such as e-cigarettes (Hefner et al., 2016). Given the difficulties individuals with PTSD have quitting smoking, harm reduction strategies may be a more reasonable approach (Tidey & Miller, 2015). Moreover, the substitution of less harmful products for cigarettes or reducing the nicotine content of cigarettes has the potential to reduce the health burden of cigarette smoking (Gaalema et al., 2015), if switching to these products leads to eventual quitting.
This systematic review and meta-analysis has some limitations. First, studies included varied in terms of sample characteristics, designs used, or outcomes reported, which limited our capability to conduct a meta-analysis to only prevalence estimates. Second, the majority of the studies were cross-sectional thereby precluding causal inferences. Third, most studies were conducted in the US, which makes generalization of this review’s findings to other countries difficult. Finally, this research was limited to studies that were published in peer review journals, so potentially relevant studies on the topic may not have been included.
Overall, this systematic review demonstrates high rates of comorbidity of PTSD and tobacco use. This study also demonstrated that individuals with PTSD have higher levels of nicotine dependence and heavier use of tobacco products, while underscoring the importance of negative emotional states as a contributing factor for tobacco use among individuals with PTSD.
Supplementary Material
HIGHLIGHTS.
This systematic review quantified the prevalence of comorbid PTSD and tobacco use.
This study summarized factors associated with tobacco use in individuals with PTSD.
Overall, high rates of comorbidity of PTSD and tobacco use were found.
Negative affect was identified as a major contributing factor for tobacco use.
Policies to reduce the burden of tobacco use among individuals with PTSD are proposed.
Role of funding sources
This research was supported in part by Tobacco Centers of Regulatory Science awardsP50DA036114 and U54DA031659 from the National Institute on Drug Abuse (NIDA) and US Food and Drug Administration (FDA), and by Center of Biomedical Research Excellence awardP20GM103644 from the National Institute of General Medical Sciences (NIGMS). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, FDA, or NIGMS.
Footnotes
Conflict of interest
The authors have nothing to declare other than the federal research support acknowledged above.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.addbeh.2018.04.024.
References
- Alexander AC, Ali J, McDevitt-Murphy ME, et al. (2017). Racial differences in posttraumatic stress disorder vulnerability following Hurricane Katrina among a sample of adult cigarette smokers from New Orleans. Journal of Racial and Ethnic Health Disparities, 4(1), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC, et al. (2009). Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry, 72(4), 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaani A, Alpert E, McLean CP, & Foa EB (2015). Resilient but addicted: The impact of resilience on the relationship between smoking withdrawal and PTSD. Journal of Psychiatric Research, 65, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaani A, Farris SG, Carpenter JK, Zandberg LJ, & Foa EB (2015). The relationship between anxiety sensitivity and posttraumatic stress disorder: What is the impact of nicotine withdrawal? Cognitive Therapy and Research, 39(5), 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa LH, Stoddard JA, Doran NM, et al. (2012). PTSD and depression as predictors of physical health-related quality of life in tobacco-dependent veterans. Journal of Psychosomatic Research, 73(3), 185–190. [DOI] [PubMed] [Google Scholar]
- Aversa LH, Stoddard JA, Doran NM, et al. (2013). Longitudinal analysis of the relationship between PTSD symptom clusters, cigarette use, and physical health-related quality of life. Quality of Life Research, 22(6), 1381–1389. [DOI] [PubMed] [Google Scholar]
- Baggett TP, Campbell EG, Chang Y, Magid LM, & Rigotti NA (2016). Posttraumatic stress symptoms and their association with smoking outcome expectancies among homeless smokers in Boston. Nicotine & Tobacco Research, 18(6), 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschnagel JS, Coffey SF, Schumache JA, Drobes DJ, & Saladin ME (2008). Relationship between PTSD symptomatology and nicotine dependence severity in crime victims. Addictive Behaviors, 33(11), 1441–1447. [DOI] [PubMed] [Google Scholar]
- Beaudoin CE (2011). Hurricane Katrina: Addictive behavior trends and predictors. Public Health Reports, 126(3), 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, & Dedert EA (2013). Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine & Tobacco Research, 15(6), 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, & Vrana SR (2007). The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addictive Behaviors, 32(12), 2900–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. [PubMed] [Google Scholar]
- Borenstein M, & Rothstein H (1999). Comprehensive meta-analysis: A computer program for research synthesis. Englewood, NJ: Biostat, Inc. [Google Scholar]
- Boscarino JA (2008). A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosomatic Medicine, 70(6), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, & Sinha R (2007). Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. The American Journal of Psychiatry, 162(8), 1483–1493. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Hobbs MJ, Clouston SAP, Gonzalez A, Kotov R, & Luft BJ (2016). DSM-IV post-traumatic stress disorder among world trade center responders 11–13 years after the disaster of 11 September 2001 (9/11). Psychological Medicine, 46(4), 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos TF, Beckenkamp PR, & Moseley AM (2013). Usage evaluation of a resource to support evidence-based physiotherapy: The physiotherapy evidence database (PEDro). Physiotherapy, 99(3), 252–257. [DOI] [PubMed] [Google Scholar]
- Carmody TP, McFall M, Saxon AJ, et al. (2012). Smoking outcome expectancies in military veteran smokers with posttraumatic stress disorder. Nicotine & Tobacco Research, 14(8), 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, & Fazel S (2014). Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry, 13(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Jakupcak M, Rosenheck R, Fontana A, & McFall M (2009). Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine & Tobacco Research, 11(10), 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Baker TB, Beckham JC, & McFall M (2017). Smoking-induced affect modulation in nonwithdrawn smokers with posttraumatic stress disorder, depression, and in those with no psychiatric disorder. Journal of Abnormal Psychology, 162(2), 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, McFall MM, Calhoun PS, & Beckham JC (2007). Posttraumatic stress disorder and smoking relapse: A theoretical model. Journal of Traumatic Stress, 20(6), 989–998. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Zvolensky MJ, Fitch KE, & Sachs-Ericsson N (2010). The role of comorbidity in explaining the associations between anxiety disorders and smoking. Nicotine & Tobacco Research, 12(4), 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone NF, Powell TM, LeardMann CA, Russell DW, & Boyko EJ (2016). Mental health and comorbidities in US military members. Military Medicine, 181(6), 537–545. [DOI] [PubMed] [Google Scholar]
- Cummins SE, Zhu SH, Tedeschi GJ, Gamst AC, & Myers MG (2014). Use of e-cigarettes by individuals with mental health conditions. Tobacco Control, (Suppl 3iii), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Watkins L, Calhoun PS, et al. (2014). Posttraumatic stress, heart-rate variability, and the mediating role of behavioral health risks. Psychosomatic Medicine, 76(8), 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enman NM, Zhang Y, & Unterwald EM (2014). Connecting the pathology of posttraumatic stress and substance use disorders: Monoamines and neuropeptides. Pharmacology, Biochemistry, and Behavior, 117, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG (2015). Anxiety sensitivity mediates the association between post-traumatic stress sympthom severity and interioceptive threat-related smoking abstinence expectancies among world trade center disaster-exposed smokers. Addictive Behaviors, 51, 204–210. [DOI] [PubMed] [Google Scholar]
- Farris SG (2016). Posttraumatic stress sympthoms and body mass index among world trade center disaster-exposed smokers: A preliminary examination of the role of anxiety sensitivity. Psychiatry Research, 241 (135–14). [DOI] [PubMed] [Google Scholar]
- Farris SG, Vujanovic AA, Hogan J, Schmidt NB, & Zvolensky MJ (2014). Main and interactive effects of anxiety sensitivity and physical distress intolerance with regard to PTSD symptoms among trauma-exposed smokers. Journal of Trauma & Dissociation, 15(3), 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Beckham JC, Vujanovic AA, & Schmidt NB (2014). Trauma exposure and cigarette smoking. Journal of Addictive Diseases, 33(4), 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, & Zvolensky MJ (2007). Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clinical Psychology Review, 27(1), 14–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ, et al. (2007). Posttraumatic stress symptoms and smoking to reduce negative affect: An investigation of trauma-exposed daily smokers. Addictive Behaviors, 32(2), 214–227. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Smith RC, Monson CM, & Zvolensky MJ (2013). Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent, multiple-baseline design. Behavior Therapy, 44(3), 514–528. [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Hakes JK, McClure EA, et al. (2016). Effects of intimate partner violence, PTSD, and alcohol use on cigarette smoking in a nationally representative sample. The American Journal of Addictions, 25(4), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, et al. (2007). Post-traumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research, 9(11), 1071–1084. [DOI] [PubMed] [Google Scholar]
- Fullerton CS, McKibben JB, Reissman DB, et al. (2013). Posttraumatic stress disorder, depression, and alcohol and tobacco use in public health workers after the 2004 Florida hurricanes. Disaster Medicine and Public Health Preparedness, 7(1), 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaalema DE, Miller ME, & Tidey JW (2015). Predicted impact of nicotine reduction on smokers with affective disorders. Tobacco Regulatory Science, 1(2), 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabert-Quillen CA, Selya A, & Delahanty DL (2015). Post-traumatic stress disorder symptoms mediate the relationship between trauma exposure and smoking status in college students. Stress and Health, 31(1), 78–82. [DOI] [PubMed] [Google Scholar]
- Garey L, Cheema MK, Otal TK, et al. (2016). The sequential pathway between trauma-related symptm severity and cognitive-based smoking processes through perceived stress and negative affect reduction expectancies among trauma exposed smokers. The American Journal on Addictions, 25(7), 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JB, Ameringer KJ, Trujillo MA, et al. (2012). Associations between posttraumatic stress disorder symptom clusters and cigarette smoking. Psychology of Addictive Behaviors, 26(1), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurillo P, Jauhar S, Murray RM, & MacCabe JH (2015). Does tobacco use cause psychosis? Systematic review and meta-analysis. The Lancet Psychiatry, 2(8), 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanović M, & Pajević I (2010). Religious moral beliefs as mental health protective factor of war veterans suffering from PTSD, depressiveness, anxiety, tobacco and alcohol abuse in comorbidity. Psychiatria Danubina, 22(2), 203–210. [PubMed] [Google Scholar]
- Hefner K, Rosenheck R, Merrel J, Coffman M, Valentine G, & Sofuoglu M (2016). E-cigarette use in veterans seeking mental health and/or substance use services. Journal of Dual Diagnosis, 12(2), 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ED, Wells TS, Smith B, et al. (2012). Smokeless tobacco use related to military deployment, cigarettes and mental health symptoms in a large, prospective cohort study among US service members. Addiction, 107(5), 983–994. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, et al. (2017). Response to varying the nicotine content of cigarettes in vulnerable populations: An initial experimental examination of acute effects. Psychopharmacology, 234(1), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Amos A, Clifford D, & Platt S (2014). Impact of tobacco control interventions on socioeconomic inequalities in smoking: Review of the evidence. Tobacco Control, 23(e2), e89–e97. [DOI] [PubMed] [Google Scholar]
- Hruska B, Bernier J, Kenner F, et al. (2014). Examining the relationships between posttraumatic stress disorder sympthoms, positive smoking outcome expectancies, and cigarette smoking in people with substance use disorders: A multiple mediator model. Addictive Behaviors, 39(1), 273–281. [DOI] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, & Graffunder CM (2016). Current cigarette smoking among adults-United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report, 65(44), 1205–1211. [DOI] [PubMed] [Google Scholar]
- Jessup MA, Dibble SL, & Cooper BA (2012). Smoking and behavioral health of women. Journal of Womens Health, 21(7), 783–791. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Jensen KP, & Sofuoglu M (2015). Co-occurring tobacco use and post-traumatic stress disorder: Smoking cessation treatment implications. The American Journal on Addictions, 24(8), 695–704. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Sido H, Forsyth JP, Ziedonis DM, Kalman D, & Cooney JL (2015). Acceptance and commitment therapy smoking cessation treatment for veterans with posttraumatic stress disorder: A pilot study. Journal of Dual Diagnosis, 11(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4(5), 231–244. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, et al. (2005). A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of General Psychiatry, 62(11), 1258–1265. [DOI] [PubMed] [Google Scholar]
- de la Hoz RE, Jeon Y, Miller GE, Wisnivesky JP, & Celedón JC (2016). Post-traumatic stress disorder, bronchodilator response, and incident asthma in world trade center rescue and recovery workers. American Journal of Respiratory and Critical Care Medicine, 194(11), 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, & Bor DH (2000). Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association, 284(20), 2606–2610. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, & Olkin I (2006). Evidence based medicine: The case of the misleading funnel plot. BMJ, 333(7568), 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, & Zubrick DL (2009). Smoking and mental illness: Results from population surveys in Australia and the United States. The American Journal of Geriatric Psychiatry, 9(1), 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, & Zvolensky MJ (2015). Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin, 141(1), 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardero A, Campbell DG, Harris KJ, Chaney EF, Lanto AB, & Rubenstein LV (2014). Prevalence and correlates of smoking status among veterans affairs primary care patients with probable major depressive disorder. Addictive Behaviors, 39(3), 538–545. [DOI] [PubMed] [Google Scholar]
- Lopez WD, Konrath SH, & Seng JS (2011). Abuse-related post-traumatic stress, coping, and tobacco use in pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 40(4), 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez WD, & Seng JS (2014). Posttraumatic stress disorder, smoking, and cortisol in a community sample of pregnant women. Addictive Behaviors, 39(10) (1408–1013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey BL, Gonzalez A, Farris SG, et al. (2016). Smoking to regulate affect: Disentangling the relationships between posttraumatic stress and emotional disorder sympthoms, nicotine dependence, and cessation-related problems. Nicotine & Tobacco Research, 18(6), 1471–1478. [DOI] [PubMed] [Google Scholar]
- Malte CA, Dennis PA, Saxon AJ, et al. (2015). Tobacco use trajectories among a large cohort of treated smokers with posttraumatic stress disorder. Addictive Behaviors, 41 (238–24). [DOI] [PubMed] [Google Scholar]
- Marshall EC, Vujanovic AA, Kutz A, Gibson L, Leyro T, & Zvolensky MJ (2009). Reasons for quitting smoking prior to a self-quit attempt among smokers with and without posttraumatic stress disorder or other anxiety/mood psychopathology. The American Journal on Addictions, 18(4), 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, & Bernstein A (2008). Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. Journal of Anxiety Disorders, 22(7), 1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR, Cook JW, Japuntich SJ, & Leventhal AM (2015). Post-traumatic stress disorder symptoms, underlying affective vulnerabilities, and smoking for affect regulation. The American Journal on Addictions, 24(1), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Calhoun PS, Hertzberg JS, et al. (2013). Associations between smoking and psychiatric comorbidity in US Iraq-and Afghanistan-era veterans. Psychology of Addictive Behaviors, 27(4), 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte CA, et al. (2010). Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. Journal of the American Medical Association, 304(22), 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Tidey JW, Bunn J, et al. (2018). Mental health status and population-level disparities in alternative tobacco use. [DOI] [PMC free article] [PubMed]
- Mitchell JT, Van Voorhees EE, Dennis MF, et al. (2012). Assessing the role of attention-deficit/hyperactivity disorder symptoms in smokers with and without posttraumatic stress disorder. Nicotine & Tobacco Research, 14(8), 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ, 339(b2535). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisano D, Bacher I, Audrain-McGovern J, & George TP (2009). Mechanisms underlying the comorbidity of tobacco use in mental health and addictive disorders. Canadian Journal of Psychiatry, 54(6), 356–367. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Das S, & Young-Wolff KC (2017). Smoking, mental illness, and public health. Annual Review of Public Health, 38, 165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter JD, McCauley JL, Amstadter AB, et al. (2011). Mental health correlates of post disaster increases in alcohol and cigarette smoking: A vietnamese study. International Journal of Mental Health and Addiction, 9(1), 118–125. [Google Scholar]
- Roberts B, Chikovani I, Makhashvili N, Patel V, & McKee M (2013). Tobacco use and nicotine dependence among conflict-affected men in the Republic of Georgia. International Journal of Environmental Research and Public Health, 10(6), 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, & Bisson JI (2015). Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review, 38, 25–38. [DOI] [PubMed] [Google Scholar]
- Rostom A, Dubé C, Cranney A, et al. (2004. September). Celiac disease. Rockville (MD): Agency for Healthcare Research and Quality (US) (Evidence Reports/Technology Assessments, No. 104.) 1, Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35154/. [Google Scholar]
- Sawchuk CN, Roy-Byrne P, Noonan C, et al. (2012). Smokeless tobacco use and its relation to panic disorder, major depression, and posttraumatic stress disorder in American Indians. Nicotine & Tobacco Research, 14(9), 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk CN, Roy-Byrne P, Noonan C, et al. (2016). The association of panic disorder, posttraumatic stress disorder, and major depression with smoking in American Indians. Nicotine & Tobacco Research, 18(3), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NA, Oglesby ME, Raines AM, Zvolensky MJ, & Schmidt NB (2015). Posttraumatic stress and emotion dysregulation: Relationships with smoking to reduce negative affect and barriers to smoking cessation. Comprehensive Psychiatry, 61, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Mazure CM, & McKee SA (2014). Smoking and mental illness in the US population. Tobacco Control, 23(e2), e147–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears CA, Jones DM, Weaver SR, Pechacek TF, & Eriksen MP (2016). Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. International Journal of Environmental Research and Public Health, 14(1) (pii: E10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Keith DR, Gaalema DE, et al. (2016). Trends in tobacco use among US adults with chronic health conditions: National Survey on drug use and health 2005–2013. Preventive Medicine, 92, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ, 343, D4002. [DOI] [PubMed] [Google Scholar]
- Tam J, Warner KE, & Meza R (2016). Smoking and the reduced life expectancy of individuals with serious mental illness. American Journal of Preventive Medicine, 51(6), 958–966. [DOI] [PubMed] [Google Scholar]
- Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, & de Vet HC (2012). Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Quality of Life Research: an International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 21(4), 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, & Miller ME (2015). Smoking cessation and reduction in people with chronic mental illness. BMJ, 351, h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Pacek LR, Koopmeiners JS, et al. (2016). Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine & Tobacco Research, 19(1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Velden PG, Grievink L, Olff M, Gersons BP, & Kleber RJ (2007). Smoking as a risk factor for mental health disturbances after a disaster: A prospective comparative study. The Journal of Clinical Psychiatry, 68(1), 87–92. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Calhoun PS, Dennis MF, Kirby AC, & Beckham JC (2015). Acoustic startle and prepulse inhibition predict smoking lapse in posttraumatic stress disorder. Journal of Psychopharmacology, 29(10), 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Calhoun PS, McClernon FJ, Dennis MF, Lee ST, & Beckham JC (2013). Effects of smoking on the acoustic startle response and prepulse inhibition in smokers with and without posttraumatic stress disorder. Psychopharmacology, 230(3), 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ER, McGee RE, & Druss BG (2015). Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry, 72(4), 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Boggero IA, Carlson CR, et al. (2016). Smoking and posttraumatic stress disorder symptomatology in orofacial pain. Journal of Dental Research, 95(10), 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AE, Jasek JP, Caramanica K, Chiles MC, & Johns M (2015). Cigarette smoking and 9/11-related posttraumatic stress disorder among world trade center health registry enrollees, 2003–12. Preventive Medicine, 73, 94–99. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connel D, Peterson J, Welch V, Losos M, et al. (2017). The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, Accessed date: 10 December 2018. [Google Scholar]
- Wilson SM, Dedert EA, Dennis PA, et al. (2014). Do ethnicity and gender moderate the influence of posttraumatic stress disorder on time to smoking lapse? Addicive Behaviors, 39(7), 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen AB, Huizink AC, Slottje P, Bramsen I, Smid T, & van der Ploeg HM (2010). Associations of cortisol with posttraumatic stress symptoms and negative life events: A study of police officers and firefighters. Psychoneuroendocrinology, 35(7), 1113–1118. [DOI] [PubMed] [Google Scholar]
- Xiao L, Koritzky G, Johnson CA, & Bechara A (2013). The cognitive processes underlying affective decision-making predicting adolescent smoking behaviors in a longitudinal study. Frontiers in Psychology, 4(685). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Fromont SC, Delucchi K, Hall SE, Hall SM, & Prochaska JJ (2014). PTSD symptomatology and readiness to quit smoking among women with serious mental illness. Addictive Behaviors, 39(8), 1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen AL, Whooley MA, Zhao S, & Cohen BE (2012). Post-traumatic stress disorder is associated with poor health behaviors: Findings from the heart and soul study. Health Psychology, 31(2), 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ (2014). Anxiety sensitivity mediates relations between emotional disorders and smoking. Psychology of Addictive Behaviors, 28(3), 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Schmidt NB, & Smits JA (2014). The role of smoking inflexibility/avoidance in the relation between anxiety sensitivity and tobacco use and beliefs among treatment-seeking smokers. Experimental and Clinical Psychopharmacology, 22(3), 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, et al. (2008). Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research, 10(8), 1415–1427. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Miller MO, et al. (2007). Incremental validity of anxiety sensitivity in terms of motivation to quit, reasons for quitting, and barriers to quitting among community-recruited daily smokers. Nicotine & Tobacco Research, 9(9), 965–975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


