Abstract
Background
Cyanoacrylate alone or in combination with other interventions, can be used to achieve variable rates of success in preventing rebleeding. Our study aims to assess the pooled risk of gastric and esophageal varices rebleeding after an initial treatment with cyanoacrylate alone and/or in combination with other treatments, by a systematic review of the literature and pooled analysis.
Methods
PubMed, EMBASE, SCOPUS, and the Cochrane library were searched for studies that reported the risk of rebleeding during the follow-up period after treatment of gastric or esophageal varices with either cyanoacrylate alone or in combination with other treatments. Standard error, upper and lower confidence intervals at 95% confidence interval for the risk were obtained using STATA Version 15 which was also used to generate forest plots for pooled analysis. The random or fixed effect model was applied depending on the heterogeneity (I2).
Results
A total of 39 studies were found to report treatment of either gastric or esophageal varices with either cyanoacrylate alone or in combination with other treatments. When gastric varices are treated with cyanoacrylate alone, the risk of rebleeding during the follow-up period is 0.15(Confidence Interval: 0.11–0.18). When combined with lipiodol; polidocanol or sclerotherapy the rebleeding risks are 0.13 (CI:0.03–0.22), 0.10(CI:0.02–0.19), and 0.10(CI:0.05–0.18), respectively. When combined with percutaneous transhepatic variceal embolization; percutaneous transhepatic variceal embolization; endoscopic ultrasound guided coils; or with ethanolamine, the rebleeding risk are 0.10(CI:0.03–0.17), 0.10(CI:0.03–0.17), 0.07(CI:0.03–0.11) and 0.08(CI:0.02–0.14), respectively.
When esophageal varices are treated with cyanoacrylate alone, the risk of rebleeding is 0.29(CI:0.11–0.47). When combined with percutaneous transhepatic variceal embolization; sclerotherapy; or band ligation, the risks of rebleeding are 0.16(CI:0.10–0.22), 0.12(CI:0.04–0.20) and 0.10(CI:0.04–0.24), respectively. When combined with a transjugular intrahepatic portosystemic shunt; or ethanolamine, the risks of rebleeding are 0.06(CI: − 0.01-0.12) and 0.02 (CI: − 0.02-0.05), respectively.
Conclusion
In treating both gastric and esophageal varices, cyanoacrylate produces better results in terms of lower risk of rebleeding when combined with other treatments than when used alone. The combination of cyanoacrylate with ethanolamine or with endoscopic ultrasound guided coils produces the lowest risk of rebleeding in esophageal and gastric varices, respectively. We call upon randomized trials to test these hypotheses.
Keywords: Cyanoacrylate, Tissue adhesive, Endoscopic hemostasis, Esophageal varices, Gastric varices, Rebleeding
Background
Liver cirrhosis is the leading cause of portal hypertension which in turn, leads to portal hypertension and gastrointestinal varices. Up to 17% of liver cirrhosis patients will develop esophageal varices, while 15% will develop gastric varices. Up to 30%, gastroesophageal varices will bleed within 2 years [1]. Bleeding from varices is one among gastrointestinal emergencies that account for the majority of mortalities and morbidities among portal hypertension patients despite the cause [2]. About 50 to 80% of patients who survive the first episode of variceal hemorrhage will have a recurrent early or late rebleeding episode [3]. Up to 20% of patients with a rebleeding episode will not survive [4].
From older literature, half of the variceal hemorrhages would stop spontaneously however, the risk of rebleeding and mortality increases significantly [5]. Current studies, however, report that in patients with cirrhosis Child-Pugh of class C or with hepatic venous pressure of higher than 20 mm Hg are less likely to spontaneous stoppage of bleeding. These patients would require interventional hemostatic measures with pharmacological drugs such as octreotide, somatostatin and beta blockers; endoscopic sclerotherapy, band ligation, or tissue adhesives injection; and/or shunting by surgery or by transjugular intrahepatic portosystemic shunt to achieve hemostasis. A selective combination of these approaches has also been reported [1]. Different hemostatic approaches differ in terms of their success rates in achieving hemostasis, preventing rebleeding, and reducing mortality and morbidity. With advancing technology, each approach has evolved, and tissue adhesives have increasingly being used as the first line of therapy during the last decades [6, 7].
Also known as “tissue glue”, tissue adhesives were approved by the United States of America’s Food and Drug Authority in 1998, however, there have been previous studies reporting their use as back as the year 1981 [8]. Primarily containing n-butyl-2 cyanoacrylate or 2-octyl cyanoacrylate, tissue adhesives are liquid monomers that undergo chemical reactions upon contact with moisture, to form polymers that can strongly attach to tissue [9]. Despite a number of reported complications associated with their use such as embolism and needle impaction [10], cyanoacrylate has been reported to have higher hemostasis and lower rebleeding rates than traditional band ligation and sclerotherapy in gastroesophageal varices [2]. Moreover, they have been reported to have antibiotic activity towards gram-positive bacteria [11].
Cyanoacrylate can be used alone or in combination with other interventions, to achieve variable rates of successes in hemostasis, reducing mortality and prevention of rebleeding. Our study was aimed at assessing the overall risk of gastroesophageal rebleeding after an initial treatment with cyanoacrylate alone and/or in combination with other treatments, by a systematic review of literature and pooled analysis.
Methods
Eligibility criteria
The current study involved participants with bleeding gastroesophageal varices who underwent hemostasis by cyanoacrylate injection alone or in combination with other treatments. Observational and interventional studies reporting the risk of rebleeding after hemostasis treatment were included. Extending the external validity, eligible English published literature from across the world were included.
Information sources
Four online databases, namely PubMed, EMBASE, SCOPUS, and the Cochrane library were systematically searched with no time range specified. Secondary referencing of eligible studies extended the search scope. The last search was conducted on 4th March 2020.
The search
Advanced search tools employing MeSH and keywords, were utilized in all three online databases. Using PubMed, advanced search was done as; (cyanoacrylate [MeSH Terms]) AND endoscopic hemostasis [MeSH Terms]) AND esophageal varices [MeSH Terms]) OR gastric varices [MeSH Terms]) AND reble*. The search was repeated as; (adhes*) AND endosc*) AND varic*) AND reble*. The searches were independently performed by two authors; ZH and JS. Results were exported to EndNote X9 (Builld 12,062) which kept track of references.
Study selection process
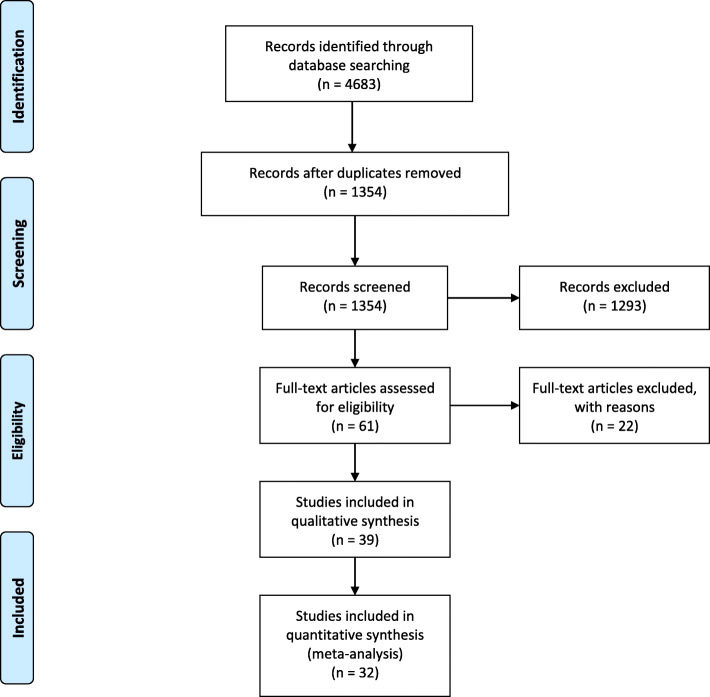
Two authors screened titles and abstracts of all articles from online database searches to identify the most relevant articles in line with our study question. The relevant articles were sought for full texts and finally included studies were identified after thorough reading full text articles to assess inclusion and exclusion criteria. This process was done by two authors; ZH and JS with the third author, TL assisting to resolve discrepancies. The search, screening, and study identification process are summarized in Fig. 1.
Fig. 1.
PRISMA 2009 flow diagram
Data extraction
Before the data extraction process from full-text articles meeting eligibility criteria for inclusion, assessment for methodological biases was done by using the Joanna Briggs institute meta-analysis of statistics assessment and review instrument. PRISMA [12](preferred reporting items for systematic reviews and meta-analyses) tool was used to minimize reporting bias upon the write-up of this study.
Data collected included author name, year of publication, country of study, study design, comparison groups involved, varicose lesion location, study sample size, definitive diagnoses, number/ proportion of rebleeding events among followed up patients, the name of tissue adhesive utilized, treatment urgency, extent of live damage prior to treatment and follow-up duration. This was independently performed by two authors, namely; ZH and DZ with SL to resolve discrepancies. The current study had one outcome, the risk of rebleeding.
Analysis
The risk of rebleeding among gastric and esophageal varices patients were analyzed separately. Moreover, the risk of rebleeding in gastric or esophageal varices groups was analyzed separately depending on whether the cyanoacrylate was utilized alone or in combination with other treatments. This gave rise to five separate analyses on which quantitative analysis was conducted: [1] Analyzing the pooled risk of rebleeding in gastric varices treated with cyanoacrylate alone [2]; analyzing the pooled risk of rebleeding in esophageal varices treated with cyanoacrylate alone [3]; analyzing the pooled risk of rebleeding in gastric varices treated with cyanoacrylate with ethanolamine [4]; analyzing the pooled risk of rebleeding in gastric varices treated with cyanoacrylate with endoscopic ultrasound guided coils; and [5] analyzing a pooled risk of rebleeding in esophageal varices treated with cyanoacrylate with percutaneous transhepatic variceal embolization. A qualitative narrative (i.e. descriptive) approach was utilized in assessing the risk of rebleeding in gastroesophageal varices treated with cyanoacrylate with sclerotherapy as the eligible studies involved different participants.
The risk of rebleeding was calculated dividing the number of patients rebleeding during the follow-up period after endoscopic hemostasis by the total number of patients that initially underwent the endoscopic hemostasis procedure. The denominator did not include patients lost during the follow up. Standard error, upper and lower confidence intervals (at 95% confidence interval) for the risk, were obtained from the “generate command” in computer software STATA Version 15 which was also used to generate forest plots for pooled analysis. The software was customized to a random or fixed effect model depending on the heterogeneity (I2) of the studies when analyzing the outcomes. The fixed effect model was used when I2 was less than 50% and the random effect model was used when I2 was more than 50% indicating significant heterogeneity.
Assumptions
Participants were considered to have been correctly diagnosed with upper gastrointestinal bleeding due to gastric or esophageal varices, and not due to other causes such as Mallory-Weiss tear or gastritis. Despite the country under which treatment was given, all patients were considered to have received standard care.
Results
A total of sixty (60) studies that seemed to be relevant to our study basing on screening titles and abstract, were sought for full texts. Five of these were eliminated after thorough full-text reading. Webb et al. (1981) [8] did not report our outcome of interest; Datta et al. (2003) [13] and Smith et al. (2014) [14] utilized fibrin glue; Noh et al. (2004) [15] and Zhang et al. (2007) [16] used Korean and Chinese language, respectively. A total of 39 studies were included in the systematic review while 32 studies were pooled for statistical analysis.
Characteristics of included studies
Table 1 illustrates the characteristics of all included studies in our pooled analysis. These were published between the years 1989 and 2019 from countries in Africa, Europe, Asia, and North America. Eleven studies were retrospective observational; 16 were prospective observational; two were case series, and ten were randomized clinical trials. Thirteen studies were comparative, one arm of which was cyanoacrylate. Eleven studies were non-comparative involving only cyanoacrylate outcome assessment while of the two studies, one involved comparing different doses of cyanoacrylate (i.e. 0.5mls versus 1.0mls) while another compared diluted versus undiluted cyanoacrylate. Follow-up duration after treatment with cyanoacrylate ranged from 6 weeks to 15 years in another study. One study did not report the duration of follow-up.
Table 1.
Study characteristics
| Author (Year) | Country of study | Study design | Comparison groups | Lesion; precise location | Diagnoses | Liver function/ extent of cirrhosis before treatment - number of patients | Sample size; number of participants rebled (%) | Treatment urgency | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|
| Ramond (1989) [17] | France | Case series | Butyl cyanoacrylate versus Sclerosant | Gastric; Unspecified | Cirrhosis; Portal vein thrombosis | Child-Pugh: A-12; B-11; C-4 | 27; 10 (37%) | Emergency and elective | 1–38 Months (Mean: 14.7 ± 11.0) |
| Oho 1995 [18] | Japan | Randomized trial | Ethanolamine oleate or butyl cyanoacrylate | Gastric; cardiac and fundal. | Gastric varices | Child-Pugh: A-0; B-17; C-12 | 29; 9 (31%) | Emergency | 14 months |
| D’Imperio 1996 [19] | Italy | Prospective | N-butyl-2- cyanoacrylate |
Esophageal; Gastric; fundal Duodenal; |
Upper gastrointestinal tract varices | Child-Pugh: A-17; B-37; C-23 |
24; 2 (3.7%) from gastric varices; 54; 0 (0%) from duodenal varices; Esophageal not reported |
Emergency and elective | 6 Months |
| Omar 1998 [20] | Egypt | Prospective trial | Polidocanol, ethanolamine, cyanoacrylate | Esophageal; | Schistosoma hepatic fibrosis | Data not accessed | 60; not reported | Emergency | Not accessed |
| Kind 2000 [21] | Italy | Retrospective | One arm study: Bucrylate | Gastric; cardia and fundus | Gastric varices | Child-Pugh: A-8; B-64; C-101 | 174; 27 (15.52%) | Emergency | 12 years |
| Evrad 2003 [22] | Belgium | Retrospective | N-butyl-2- cyanoacrylate versus Propranolol |
Esophageal; unspecified Gastric; |
Esophagogastric varices | Unspecified |
16; 4 (25%); Esophageal 5; 2 (40%) Gastric |
Emergency | 6 weeks |
| Noophun 2005 [23] | Thailand | Prospective | One arm study: cyanoacrylate | Gastric; fundus | Gastric varices | Child-Pugh: A-6; B-11; C-7 | 24; 10 (41.67%) | Emergency and elective | Minimum of 4 weeks |
| Tan 2006 [24] | Taiwan | Prospective | Band ligation Versus N-butyl-2- cyanoacrylate | Gastric; fundus, antrum and isolated | Liver cirrhosis | Child-Pugh: A-13; B-26; C-10 | 49; 11 (22.45%) from cyanoacrylate group | Emergency | 680.67 ± 710.54 days |
| Cheng 2007 [25] | China | Retrospective | One arm study: N-butyl-2- cyanoacrylate | Gastric; cardia and fundus | Gastric varices | Child-Pugh: A-194; B-254; C-134 | 635; 44 (8%) | Emergency | Up to 10 years |
| Kuo 2007 [26] | China | Randomized trial | Histoacryl versus Histoacryl + hypertonic glucose solution | Gastric; | Gastric varices | Child-Pugh: A-20; B-36; C-11 | 67; 2 (5.9%) from histoacryl-alone group | Emergent and elective | 37.9 ± 18.5 months |
| Hong 2009 [27] | Korea | Randomized trial | Endoscopic N-butyl-2-cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration | Gastric; unspecified | Gastric variceal hemorrhage | Child-Pugh: A-3; B-8; C-3 | 27; 10 (71.43%) from the N-butyl-2-cyanoacrylate group | Emergent and elective | Up to 17 Months |
| Hou 2009 [28] | Taiwan | Randomized trial | 0.5 mL Versus 1.0 mL of cyanoacrylate | Gastric; cardiac, fundal and undetermined | Gastric variceal hemorrhage |
Mean ± standard deviation of Child-Pugh scores: 0.5 mL group 7.61 ± 1.82 1.0 mL group 7.79 ± 2.27 |
46; 14 (29.79%) from the 0.5mls group; 44; 17 (38.64%) from the 1 ml group |
Emergent and elective | Up to 2 years |
| Procaccini 2009 [29] | USA | Retrospective | Cyanoacrylate versus TIPS | Gastric; unspecified | Gastric variceal hemorrhage |
Model for End-Stage Liver Disease (MELD), Mean ± standard deviation score: Cyanoacrylate, 15.4 ± 6.3; TIPS, 15.7 ± 8.3 |
105; 13 (21.31%) from the Cyanoacrylate group | Emergent and elective | Up to 1 year |
| Rivet 2009 [30] | France | Prospective | Cyanoacrylate versus Band ligation | Esophageal; | Portal hypertension due to portal vein thrombosis, biliary atresia and antitrypsin deficiency | Pediatric End-stage Liver Disease (PELD) model, mean ± 20.1 ± 9.9 | 8; 3 (37.5%) from cyanoacrylate group | Emergent and elective | 10.6 weeks |
| Cheng 2010 [31] | China | Retrospective | Butyl cyanoacrylate | Gastric; cardiac and fundus | Gastric varices due to viral hepatitis and others | Child-Pugh: A-244; B-297; C-179; unspecified-33 | 753; 33 (4.38%) | Emergent and elective | Up to 6 months after initial endoscopy |
| Choudhuri 2010 [32] | India | Prospective | N-butyl-2-cyanoacrylate | Gastric; unspecified | Gastric variceal hemorrhage | Child-Pugh: A-40; B-62; C-40 | 170; 23 (14.56%) | Emergent and elective | 30.7 + 17.2 months |
| Mishra 2010 [33] | India | Prospective | Cyanoacrylate versus beta blocker |
Gastric; cardiac, fundus and isolated Esophageal; |
Gastric varices | Child-Pugh: A-4; B-12; C-17 | 33; 3 (9.09%) from the cyanoacrylate group | Emergency | 26 Months |
| Soga 2010 [34] | Japan | Case report | N-butyl-2-cyanoacrylate |
Gastric; Duodenal; |
Gastroduodenal varices | Unspecified | 1; 0 (0%) | Elective | 53 days |
| Binmoellar 2011 [35] | USA | Retrospective | N-butyl-2-cyanoacrylate | Gastric; fundus | Gastric varices; Non variceal lesion |
MELD score < 10 mean ± standard deviation, 11 ± 40.7; MELD score 11–18 mean ± standard deviation, 12 ± 44.4; MELD score 19–24, mean ± standard deviation, 3 ± 11.1 |
24; 0 (0%) from gastric variceal group | Emergent and elective | 193 (24–589) days |
| Kang 2011 [36] | Korea | Retrospective |
N-butyl-2-cyanoacrylate cyanoacrylate |
Gastric; cardiac, fundal and isolated | Gastric varices | Child-Pugh: A-42; B-59; C-26 | 127; 29 (22.83%) | Emergent and elective | 1 year |
| Liao 2013 [37] | Taiwan | Prospective | Cyanoacrylate | Gastric; unspecified | Gastric varices | Child-Pugh: A-16; B-13; C-6 | 69; 10 (14.49%) | Emergency and elective | More than 30 months |
| Tantau 2013 [38] | Romania | Prospective | Cyanoacrylate versus Band ligation | Gastric; cardiac and fundus | Gastric varices | Child-Pugh: A-11; B-18; C-8 | 37; 6 (31.58%) from the Cyanoacrylate group | Emergency and elective | 27.26 ± 214.16 days |
| Al-Bawardy 2016 [39] | USA | Retrospective | 2-octyl cyanoacrylate | Gastric; fundal |
Gastric Variceal Hemorrhage |
MELD score median value, 11 | 95; 8 (8.42%) | Emergency | Up to 15 years |
| Singh 2016 [10] | India | Prospective | Diluted versus undiluted cyanoacrylate | Gastric; cardiac, fundus and isolated |
Gastric Variceal Hemorrhage |
Child-Pugh: A-9; B-15; C-6 | 30; 5 (16.67%) | Emergency and elective | Up to one year |
| Liu 2019 [40] | China | Prospective | Cyanoacrylate with versus without antibiotic | Gastric; unspecified | Gastric varices | Child-Pugh: A-76; B-31; C-0 | 107; 106 (99.07%) | Emergency | 4.59 ± 1.63; 4.30 ± 1.48 Days |
| Xiaoqing 2019 [2] | China | Prospective | Cyanoacrylate versus cyanoacrylate + lauromacrogol | Gastric; cardiac, fundus and isolated | Gastric varices | Child-Pugh: A-27; B-74; C-9 | 130; 8 (12.90%) from the Cyanoacrylate group | Emergency | 38.8 months for Cyanoacrylate group |
| Thakeb 1995 [41] | Egypt | Randomized trial | N-butyl-2-cyanoacrylate plus ethanolamine oleate 5% versus ethanolamine alone |
Gastric; Unspecified Esophageal; |
Gastroesophageal varices | Child-Pugh: A-16; B-33; C-9 |
57; 3 (5.26%) from the gastric varices; 59; 1 (1.69%) from the esophageal varices group. |
Emergency and elective | Up to 32 months |
| Maruyama 2010 [42] | Japan | Retrospective | Cyanoacrylate plus ethanolamine | Gastric; fundus | Gastric varices | Child-Pugh: A-2; B-4; C-4 | 20; 10 (50%) | Emergency | 28.1 months |
| Bhat 2016 [43] | United States of America | Retrospective | Cyanoacrylate and coils guided by endoscopic ultrasound | Gastric; fundal | Gastric varices |
MELD score < 10, 31; MELD score 11–18, 70; MELD score 19–24, 10; MELD score > 24, 3 |
125; 10 (8%) | Elective | Median: 436 days; |
| Robles-Medranda 2019 [44] | Ecuador | Prospective | Cyanoacrylate and coils guided by endoscopic ultrasound | Gastric; cardiac, fundus and isolated | Gastric varices | Child-Pugh: A-28; B-2; C-17 | 30: 1 (3.7%) | Emergency and elective | Up to 12 months |
| Zhang 2007 [16] | China | Randomized trial | Cyanoacrylate with percutaneous transhepatic variceal embolization | Esophageal; | Esophageal varices | Data not accessed | 92; 14 (16%) | Emergency and elective | Mean: 31.5 months |
| Zhang 2008 [45] | China | Randomized trial | Cyanoacrylate with percutaneous transhepatic variceal embolization | Esophageal; cardiac, fundus and isolated | Esophageal varices | Child-Pugh: A-10; B-25; C-17 | 52; 8 (15.38%) | Emergency and elective | Median: 25 months |
| Tian 2011 [46] | China | Prospective | Cyanoacrylate with percutaneous transhepatic variceal embolization | Gastric; cardiac, fundus, isolated | Gastric varices | Child-Pugh: A-24; B-31; C-17 | 71; 7 (9.86%) | Emergency and elective | Mean; 24.2 ± 12.4 months |
| Feritis 1995 [47] | Greece | Randomized trial | Cyanoacrylate with sclerotherapy | Esophageal; | Esophageal varices | Child-Pugh: A-12; B-83; C-35 | 126; 8 (11.94%) | Emergency | 30 days |
| Dhiman 2002 [48] | India | Prospective | Cyanoacrylate with sclerotherapy | Gastric; fundal | Gastric varices | Child-Pugh: A-5; B-5; C-3 | 29; 3 (10.34%) | Emergency and elective | Up to 6 months |
| Shi 2014 [49] | China | Retrospective | Transjugular intrahepatic portosystemic shunt alone versus combined with cyanoacrylate | Esophageal; | Esophageal Variceal Bleeding | Child-Pugh: A-27; B-57; C-17 | 53; 3 (5.66%) | Emergency | 35.8 months |
| Ma 2018 [50] | China | Prospective | Combined cyanoacrylate with balloon-occluded retrograde transvenous obliteration | Gastroesophageal; | Gastroesophageal varices | Child-Pugh: A-16; B-10; C-2 | 28; 8 (31%) | Elective | 90 days |
| Dai 2017 [51] | China | Randomized trial | Band ligation alone versus in combination with cyanoacrylate | Gastroesophageal; | Gastroesophageal varices | Data not accessed | 97; 7 (14.29%) | Emergency and elective | 20 months |
| Zeng 2017 [52] | China | Randomized trial | Cyanoacrylate plus polidocanol versus cyanoacrylate plus lipiodol in | Gastric; cardiac, fundus and isolated | Gastric varices | Child-Pugh: A-50; B-44; C-4 | 96; 11 (11.70%) | Emergency and elective | 6 months |
A total of 39 studies reported 3630 who had either gastric or esophageal variceal and underwent hemostasis with cyanoacrylate alone or in combination with other treatments. A total of 497 had gastric or esophageal recurrent bleeding episodes during the follow-up period.
Pooled risk of rebleeding in gastric varices treated with cyanoacrylate alone
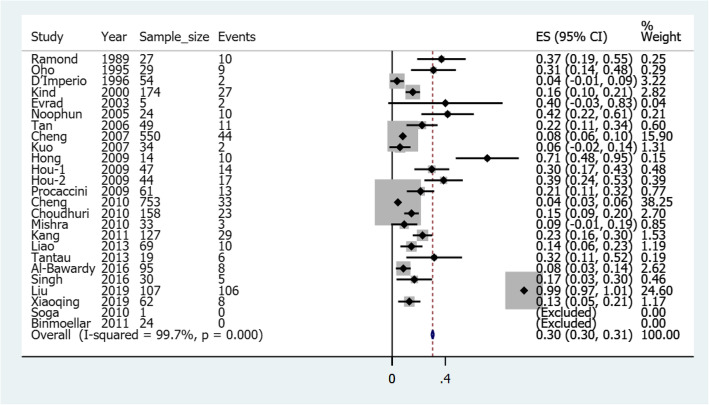
Figure 2 illustrates a forest plot of the pooled risk of rebleeding for gastric varices after cyanoacrylate treatment. A total of 25 studies reported 2590 gastric variceal patients, of whom 402 had had rebleeding after initial treatment with cyanoacrylate hemostasis. The risk ranged from a minimum of 0.04 (4%) to a maximum of 0.99 (99%) in another study. Two studies were excluded for not having rebleeding incidences during the follow up period. The pooled overall risk of rebleeding was 0.30 (confidence interval: 0.30–0.31).
Fig. 2.
A forest plot of the pooled risk of rebleeding for gastric varices after cyanoacrylate treatment
There was a significant heterogeneity observed with I2 of 99.7%, p-Value< 0.05. This led us to conduct sensitivity analysis, eliminating peculiar studies from the analysis. Figure 3 illustrates a sensitivity analysis forest plot of the pooled risk of rebleeding for gastric varices after the elimination of peculiar studies. Ramond et al. (1989) [17] and Soga et al. (2010) [34] were case series and case report respectively; D’Imperio et al. (1996) [19], Omar et al. (1998) [20], Noophun et al. (2005) [23], Rivet et al. (2009) [30], Cheng et al. (2010) [31], Binmoellar et al. (2011) [35] and Tantau et al. (2013) [38] had less than one-year of follow-up; while Kind et al. (2000) [21], Tan et al. (2006) [24], Procaccini et al. (2009) [29], Choudhuri et al. (2010) [32], Mishra et al. (2010) [33], Liao et al. (2013) [37], Singh et al. (2016) [10], Cheng et al. (2007) [25], Kuo et al. (2007) [26], Huo et al. (2009) [28], Kang et al. (2011) [36], Al-Baward et al. (2016) [39] and Xiaoqing et al. (2019) [2] were excluded by meta-regression. Evrad et al. (2003) [22], Hong et al. (2009) [27], Soga et al. (2010) [34] and Liu et al. (2019) [40] were excluded because their findings did not fulfill normality test criteria for calculation of confidence interval (i.e.N(1-Pe) ≥10). Moreover, regarding follow-up time, Sigh et al. (2016), Procaccini et al. (2009), and Kind et al. (2000) were excluded for distinct follow-up times. Each of the 4 remaining studies had an estimate of 2 years of follow-up. The resulting overall pooled risk was 0.15 (Confidence interval: 0.11–0.18) with no significant heterogeneity (i.e. I2 = 0.0%, p-Value = 0.4).
Fig. 3.
Sensitivity analysis-forest plot of the pooled risk of rebleeding for gastric varices after the elimination of peculiar studies
Pooled risk of rebleeding in esophageal varices treated with cyanoacrylate alone
Figure 4 illustrates a forest plot of the pooled risk of rebleeding for esophageal varices after cyanoacrylate treatment. A total of five studies reported 134 esophageal variceal patients, 7 of whom had had rebleeding after initial treatment with cyanoacrylate hemostasis. The risk of rebleeding ranged from a minimum of 0.25 (25%) to a maximum of 0.38 (99%) in another study. Three studies were excluded for not having rebleeding incidences during the follow up period. The pooled overall risk of rebleeding was 0.29 (confidence interval: 0.11–0.47). There was no significant heterogeneity observed; I2 of 0.0%, p-Value = 0.53.7).
Fig. 4.
A forest plot of the pooled risk of rebleeding of esophageal varices after cyanoacrylate treatment
Pooled risk of rebleeding in gastric varices treated with cyanoacrylate with ethanolamine
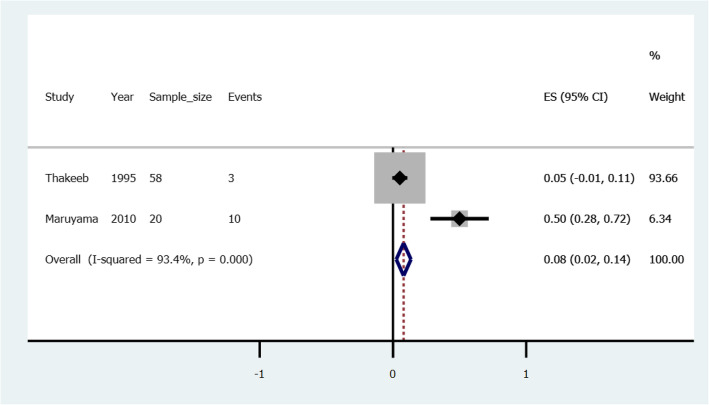
Two studies illustrated treatment with a combination of cyanoacrylate and ethanolamine; Thakeeb et al. (1995) [41] and Maruyama et al. (2010) [42]. Thakeeb reported 3 (i.e. risk = 0.052) rebleeding events among gastric variceal patients; and one (risk = 0.017) rebleeding events among esophageal varices patients. Maruayama reported 10 (i.e. risk =0.5) rebleeding events among gastric varices patients. Figure 5 illustrates a forest plot of pooled risk, 0.08(0.02–0.14) of rebleeding in gastric varices treated with a combination of cyanoacrylate with ethanolamine.
Fig. 5.
A forest plot of the pooled risk of rebleeding in gastric varices treated with a combination of cyanoacrylate with ethanolamine
Pooled risk of rebleeding in gastric varices treated with cyanoacrylate with endoscopic ultrasound guided coils
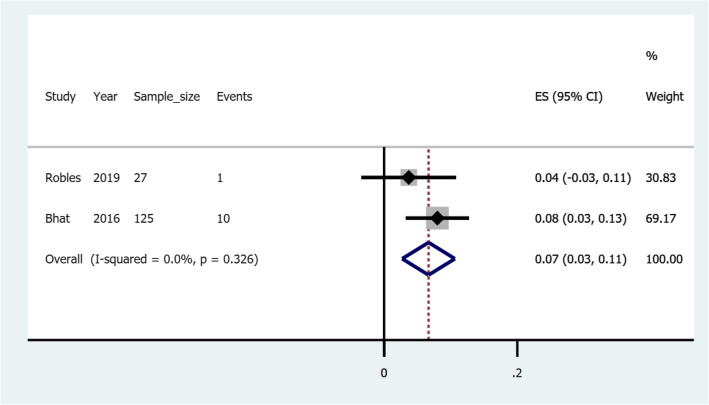
Two studies illustrated treatment with a combination of cyanoacrylate and coils guided by endoscopic ultrasound; Bhat et al. (2016) [43] and Robles-Medranda et al. (2019) [44]. Bhat et al. (2016) reported 10 rebleeding events out of 125 gastric varices patients who were followed-up. This corresponds to the risk of 0.08 (Confidence interval: 0.03–0.13). Robles-Medranda et al. (2019) reported 1 rebleeding event out of 27 gastric varices patients, which corresponds to the risk of 0.04 (Confidence interval: − 0.03-0.11). Figure 6 illustrates a forest plot of the pooled risk of rebleeding in gastric varices treated with cyanoacrylate with endoscopic ultrasound guided coils.
Fig. 6.
A forest plot of the pooled risk of rebleeding in gastric varices treated with cyanoacrylate with endoscopic ultrasound guided coils
Pooled risk of rebleeding in esophageal varices treated with cyanoacrylate with percutaneous transhepatic variceal embolization
Three studies illustrated treatment with a combination of cyanoacrylate and percutaneous transhepatic variceal embolization in gastroesophageal varices; Zhang et al. (2007) [16] and Zhang et al. (2008) [45] involved esophageal varices patients and reported rebleeding risks of 0.16 (confidence interval: 0.08–0.24) and 0.15 (confidence interval: 0.06–0.25), respectively. Tian et al. (2011) [46] involved gastric varices patients and reported a rebleeding risk of 0.10(confidence interval: 0.03–0.17). Figure 7 illustrates a forest plot of the pooled risk of rebleeding in esophageal varices treated with cyanoacrylate with percutaneous transhepatic variceal embolization.
Fig. 7.
A forest plot of the pooled risk of rebleeding in esophageal varices treated with cyanoacrylate with percutaneous transhepatic variceal embolization
Risk of rebleeding in gastroesophageal varices treated with cyanoacrylate with sclerotherapy
Two studies assessed the efficacy of a combination of cyanoacrylate and sclerotherapy in the treatment of gastroesophageal varices. In one study, Feretis et al. (1995) [47] compared the combination versus sclerotherapy alone in the treatment of esophageal varices and reported the risk for rebleeding in the combination group to be 0.12 (Confidence interval: 0.04–0.20). In another one arm study, Dhiman et al.(2002) [48] assessed the outcome of the combination therapy in the treatment of gastric varices and reported a risk of 0.10 (Confidence interval: 0.05–0.18). Forest plot was not constructed as the two studies involved different participants (i.e. gastric and esophageal varices).
Other combination treatments with cyanoacrylate
In their study, Shi et al. (2014) [49] compared between transjugular intrahepatic portosystemic shunt alone versus combined with Cyanoacrylate for esophageal variceal bleeding. The combination therapy reduced the rebleeding risk to a third of one observed in transjugular intrahepatic portosystemic shunt alone. That is from 0.19 to 0.06, p-Value of 0.04. In another study, Ma et al. (2018) [50] combined cyanoacrylate with balloon-occluded retrograde transvenous obliteration in 28 patients with gastroesophageal varices and reported a rebleeding risk of 0.31 (confidence interval: 0.13–0.49).
Dai et al. (2017) [51] compared band ligation alone versus in combination with cyanoacrylate in the treatment of gastroesophageal varices. The risk of rebleeding in the combination therapy was reduced to a quarter that recorded in band ligation alone. That is from 0.56 to 0.14, p-Value< 0.01. Zeng et al. (2017) [52] compared two combinations; cyanoacrylate plus polidocanol versus cyanoacrylate plus lipiodol in the treatment of gastric varices. The later showed the risk of rebleeding of 0.13 (Confidence Interval: 0.03–0.22) as compared to 0.10 (Confidence interval: 0.02–0.19) in the polidocanol combination.
Table 2 summarizes risks of rebleeding in gastric and esophageal varices when treated with cyanoacrylate alone or in combination with other treatments as discussed earlier.
Table 2.
Risks of rebleeding in gastric and esophageal varices after treatment with cyanoacrylate alone or in combination with other treatments
| Hemostasis treatment type | Pooled risk of gastric varices rebleeding (confidence interval) | Pooled risk of esophageal varices rebleeding (confidence interval) |
|---|---|---|
| Cyanoacrylate alone | 0.15 (0.11–0.18) | 0.29 (0.11–0.47) |
| Cyanoacrylate combined with ethanolamine | 0.08 (0.02–0.14) | 0.02 (− 0.02–0.05). |
| Cyanoacrylate combined with endoscopic ultrasound guided coils | 0.07 (0.03–0.11) | – |
| Cyanoacrylate combined with percutaneous transhepatic variceal embolization | 0.10 (0.03–0.17) a | 0.16 (0.10–0.22) |
| Cyanoacrylate combined with transjugular intrahepatic portosystemic shunt | – | 0.06(−0.01–0.12) a |
| Cyanoacrylate combined with sclerotherapy | 0.10 (0.05–0.18) a | 0.12 (0.04–0.20) a. |
| Cyanoacrylate combined with band ligation | – | 0.10 (0.04–0.24) a |
| Cyanoacrylate combined with polidocanol | 0.10 (0.02–0.19) a | – |
| Cyanoacrylate combined with lipiodol | 0.13 (0.03–0.22) a | – |
| Cyanoacrylate combined with balloon-occluded retrograde transvenous obliteration | 0.31 (0.13–0.49) b | |
Note: The values in the table are independently calculated and the table does not mean statistical comparison between them
Key: a Calculated from a single study (Not pooled); b Gastric or esophageal varices not specified (Gastroesophageal)
Discussion
Through decades-long progressive improvements in the treatment of gastroesophageal varices, cyanoacrylate has evolved to be one of the favored first lines of treatment. The current study was aimed at utilizing a systematic review of literature and pooled analysis to assess the overall risk of gastroesophageal rebleeding after an initial treatment with cyanoacrylate alone and/or in combination with other treatments.
Following the treatment of gastric varices with cyanoacrylate alone, 25 studies demonstrated different risks of rebleeding from the minimum of 0.04 to a maximum of 0.99 in another study, with the overall pooled risk of 0.30 (confidence interval: 0.30–0.31). However, after getting rid of peculiar studies that increased heterogeneity, the resulting overall pooled risk was 0.15 (Confidence interval: 0.11–0.18). This risk of rebleeding coincides with that previously reported by Hou et al. (2009) [28] but differed from the majority of other studies. Authors believe that the reason for the differences among studies to be technological advancement with time. This can be demonstrated the majority of studies from the year 2010 forward having a lower risk of rebleeding than studies before 2010. Different sample sizes and different study methodologies could also explain the differences.
Esophageal varices treated with cyanoacrylate alone showed the risk of rebleeding ranging from 0.25 to 0.38 in different studies with the pooled overall risk of 0.29 (confidence interval: 0.11–0.47). Following a fewer number of studies, a meta regression could not be conducted. However, authors believe that the reason for the differences between studies to be due to different methodological approaches between the studies as Rivet et al. (2009) [30] followed up their patients for twice the duration used by Evrad et al. (2003) [22]. The authors of this study hypothesize that gastric varices respond better to cyanoacrylate as compared to esophageal varices in terms of lower risk of rebleeding. We call upon randomized clinical trials comparing the risk of rebleeding between gastric varices and esophageal varices treated with cyanoacrylate alone.
When cyanoacrylate is combined with ethanolamine in the treatment of gastric varices the pooled risk of rebleeding after treatment is 0.08 (Confidence interval: 0.02–0.14). The result aligns with that reported by Thakeb et al. (1995) but differs from Maruyama who reported a higher risk of 0.5. The difference is accounted for by fewer sample size by Maruyama. On the other hand, when the combination is used to treat esophageal varices the risk of rebleeding is 0.017(confidence interval: − 0.02-0.05). From an otherwise weak basis, we hypothesis that esophageal varices in contrast to gastric varices, respond better to the combination of cyanoacrylate and ethanolamine, in terms of lower risk of rebleeding. We call upon clinical randomized clinical trials to test this hypothesis.
From our findings, when cyanoacrylate is combined with endoscopic ultrasound guided coils to treat gastric varices the pooled risk of rebleeding is 0.07(confidence interval: 0.03–0.11). This finding is more or less similar to that reported by Bhat et al. (2016) [43] but is higher than that reported by Robles-Medranda et al. (2019) [44]. The reason for the differences could be explained by different sample sizes among studies pooled. One study had nearly five times the sample size used by the other.
When esophageal varices are treated with a combination of cyanoacrylate and percutaneous transhepatic variceal embolization the pooled risk of rebleeding is 0.16(confidence interval: 0.10–0.22). This is coinciding with findings previously reported by Zhang et al. (2007) [16]. In another study by Tian et al. (2011) [46] when the combination is used to treat gastric varices, the risk of rebleeding is 0.10(confidence interval: 0.03–0.17). We hypothesize that esophageal varices in contrast to gastric varices, respond better to the combination of cyanoacrylate and percutaneous transhepatic variceal embolization in terms of lower risk of rebleeding. The authors call upon randomized clinical trials to test this hypothesis.
The risk of rebleeding in gastric varices treated with cyanoacrylate with sclerotherapy was lower by 0.02 from that of esophageal varices treated with the same combination. The difference could partly be due to more or less the same number of sample sizes among the two studies descriptively analyzed. In combination with other treatments such as transjugular intrahepatic portosystemic shunt and balloon-occluded retrograde transvenous obliteration, it is evident that cyanoacrylate improves the efficacy of the treatment of gastroesophageal varices in terms of lowering rebleeding risk.
Study limitations, measures taken, and recommendations
Our study search was limited to English published literature; involved pooling of studies with different sample sizes, different study designs, and different follow-up durations. As demonstrated by Child-Pugh or the model for end-stage liver disease (MELD) classifications, different studies involved participants with different extents of liver damage/cirrhosis. Despite a few studies involving either emergent [49] or elective [50] participants only, the majority of studies combined the two [16, 48]. Moreover, from older literature by Sarin et al. (1992) [53], the risk of rebleeding varied with lesion’s location on the gastric wall. From the study, isolated varices bled more often as compared to cardia and fundal varices.
These were thought to introduce heterogeneity in the pooled analysis. However, authors appraised eligible studies; performed sensitivity analyses, meta-regression, study exclusion, and used random effect models to deal with high heterogeneity among pooled studies. We also utilized PRISMA tools to minimize reporting biases.
We call upon robust randomized studies taking into account biases encountered in our study and adequately matching participants by the extent of liver damage/cirrhosis; treatment urgency whether elective or emergency; lesion location and follow-up duration.
Conclusion
In treating both gastric and esophageal varices, cyanoacrylate produces better results in terms of lower risk of rebleeding when combined with other treatments than when used alone. The combination of cyanoacrylate with ethanolamine or with endoscopic ultrasound guided coils produces the lowest risk of rebleeding in esophageal and gastric varices, respectively. We call upon randomized trials to test these hypotheses.
Acknowledgements
Not applicable.
Abbreviations
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- MeSH
Medical Subject Headings
- ZH
Zixuan Hu (Author)
- DZ
Decai Zhang (Author)
- JS
Joel Swai (Author)
- TL
Tao Liu (Author)
- SL
Shaojun Liu (Author)
Authors’ contributions
Study designing: JS; data search ZH, JS and TL; data extraction: ZH, DZ, and SL; data analysis and interpretation: JS and ZH; Manuscript drafting: JS; manuscript critical intellectual content revision: SL, TL, and DZ. All authors read and approved the final version of the manuscript.
Funding
No funds were given.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declared no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joel Swai, Email: joel.swai@hotmail.com, http://orcid.org/0000-0001-5363-3977.
Shaojun Liu, Email: shaojun.liu.99@hotmail.com.
References
- 1.Habib A, Sanyal AJ. Acute variceal hemorrhage. Gastrointest Endosc Clin N Am. 2007;17(2):223–252. doi: 10.1016/j.giec.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Xiaoqing Z, Na L, Lili M, Jie C, Tiancheng L, Jian W, et al. Endoscopic cyanoacrylate injection with Lauromacrogol for gastric Varices: long-term outcomes and predictors in a retrospective cohort study. J Laparoendoscopic Advanced Surgical Techniques Part A. 2019;29(9):1135–1143. doi: 10.1089/lap.2019.0360. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs AK, McCormick PA. Prevention of variceal rebleeding. Gastroenterol Clin N Am. 1992;21(1):119–147. [PubMed] [Google Scholar]
- 4.Mehmood T, Zia MQ, Latif A, Ansar S. Mortality related factors in patients with Variceal bleeding with MELD score >/= 18. J College Physicians Surgeons--Pakistan. 2019;29(12):1199–1202. doi: 10.29271/jcpsp.2019.12.1199. [DOI] [PubMed] [Google Scholar]
- 5.Prandi D, Rueff B, Roche-Sicot J, Sicot C, Maillard JN, Benhamou JP, et al. Life-threatening hemorrhage of the digestive tract in cirrhotic patients. An assessment of the postoperative mortality after emergency portacaval shunt. Am J Surg. 1976;131(2):204–209. doi: 10.1016/0002-9610(76)90098-2. [DOI] [PubMed] [Google Scholar]
- 6.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology (Baltimore, Md) 2007;46(3):922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 8.Webb WA, McDaniel L, Johnson RC, Haynes CD. Endoscopic evaluation of 125 cases of upper gastrointestinal bleeding. Ann Surg. 1981;193(5):624–627. doi: 10.1097/00000658-198105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26(4):490–496. doi: 10.1016/j.ajem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Singh R, Bhalla A, Sharma N. Cyanoacrylate therapy for the treatment of gastric varices: a new method. J Dig Dis. 2016;17(6):392–398. doi: 10.1111/1751-2980.12351. [DOI] [PubMed] [Google Scholar]
- 11.Rushbrook JL, White G, Kidger L, Marsh P, Taggart TF. The antibacterial effect of 2-octyl cyanoacrylate (Dermabond(R)) skin adhesive. J Infect Prev. 2014;15(6):236–239. doi: 10.1177/1757177414551562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and Meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta D, Vlavianos P, Alisa A, Westaby D. Use of fibrin glue (beriplast) in the management of bleeding gastric varices. Endoscopy. 2003;35(8):675–678. doi: 10.1055/s-2003-41517. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, Tidswell R, Tripathi D. Outcomes of endoscopic human thrombin injection in the management of gastric varices. Eur J Gastroenterol Hepatol. 2014;26(8):846–852. doi: 10.1097/MEG.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 15.Noh DY, Park SY, Joo SY, Park CH, Lee WS, Joo YE, et al. Therapeutic effect of the endoscopic N-butyl-2-cyanoacrylate injection for acute esophagogastric variceal bleeding: comparison with transjugular intrahepatic portosystemic shunt. Korean J Gastroenterol. 2004;43(3):186–195. [PubMed] [Google Scholar]
- 16.Zhang CQ, Liu FL, Xu HW, Feng K, Zhu Q, Zhang JY, et al. Percutaneous transhepatic varices embolization with cyanoacrylate in the treatment of varices. Zhonghua Yi Xue Za Zhi. 2007;87(48):3389–3393. [PubMed] [Google Scholar]
- 17.Ramond MJ, Valla D, Mosnier JF, Degott C, Bernuau J, Rueff B, et al. Successful endoscopic obturation of gastric varices with butyl cyanoacrylate. Hepatology (Baltimore, Md) 1989;10(4):488–493. doi: 10.1002/hep.1840100415. [DOI] [PubMed] [Google Scholar]
- 18.Oho K, Iwao T, Sumino M, Toyonaga A, Tanikawa K. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: a nonrandomized study. Endoscopy. 1995;27(5):349–354. doi: 10.1055/s-2007-1005712. [DOI] [PubMed] [Google Scholar]
- 19.D'Imperio N, Piemontese A, Baroncini D, Billi P, Borioni D, Dal Monte PP, et al. Evaluation of undiluted N-butyl-2-cyanoacrylate in the endoscopic treatment of upper gastrointestinal tract varices. Endoscopy. 1996;28(2):239–243. doi: 10.1055/s-2007-1005435. [DOI] [PubMed] [Google Scholar]
- 20.Omar MM, Fakhry SM, Mostafa I. Immediate endoscopic injection therapy of bleeding oesophageal varices: a prospective comparative evaluation of injecting materials in Egyptian patients with portal hypertension. J Egypt Soc Parasitol. 1998;28(1):159–168. [PubMed] [Google Scholar]
- 21.Kind R, Guglielmi A, Rodella L, Lombardo F, Catalano F, Ruzzenente A, et al. Bucrylate treatment of bleeding gastric varices: 12 years' experience. Endoscopy. 2000;32(7):512–519. doi: 10.1055/s-2000-3817. [DOI] [PubMed] [Google Scholar]
- 22.Evrard S, Dumonceau JM, Delhaye M, Golstein P, Deviere J, Le Moine O. Endoscopic histoacryl obliteration vs. propranolol in the prevention of esophagogastric variceal rebleeding: a randomized trial. Endoscopy. 2003;35(9):729–735. doi: 10.1055/s-2003-41581. [DOI] [PubMed] [Google Scholar]
- 23.Noophun P, Kongkam P, Gonlachanvit S, Rerknimitr R. Bleeding gastric varices: results of endoscopic injection with cyanoacrylate at King Chulalongkorn Memorial Hospital. World J Gastroenterol. 2005;11(47):7531–7535. doi: 10.3748/wjg.v11.i47.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology (Baltimore, Md) 2006;43(4):690–697. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]
- 25.Cheng LF, Wang ZQ, Li CZ, Cai FC, Huang QY, Linghu EQ, et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years' experience of 635 cases. Chin Med J. 2007;120(23):2081–2085. [PubMed] [Google Scholar]
- 26.Kuo MJ, Yeh HZ, Chen GH, Poon SK, Yang SS, Lien HC, et al. Improvement of tissue-adhesive obliteration of bleeding gastric varices using adjuvant hypertonic glucose injection: a prospective randomized trial. Endoscopy. 2007;39(6):487–491. doi: 10.1055/s-2007-966267. [DOI] [PubMed] [Google Scholar]
- 27.Hong CH, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Treatment of patients with gastric variceal hemorrhage: endoscopic N-butyl-2-cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 2009;24(3):372–378. doi: 10.1111/j.1440-1746.2008.05651.x. [DOI] [PubMed] [Google Scholar]
- 28.Hou MC, Lin HC, Lee HS, Liao WC, Lee FY, Lee SD. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 1.0 mL. Gastrointest Endosc. 2009;70(4):668–675. doi: 10.1016/j.gie.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Procaccini NJ, Al-Osaimi AM, Northup P, Argo C, Caldwell SH. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70(5):881–887. doi: 10.1016/j.gie.2009.03.1169. [DOI] [PubMed] [Google Scholar]
- 30.Rivet C, Robles-Medranda C, Dumortier J, Le Gall C, Ponchon T, Lachaux A. Endoscopic treatment of gastroesophageal varices in young infants with cyanoacrylate glue: a pilot study. Gastrointest Endosc. 2009;69(6):1034–1038. doi: 10.1016/j.gie.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Cheng LF, Wang ZQ, Li CZ, Lin W, Yeo AE, Jin B. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol. 2010;8(9):760–766. doi: 10.1016/j.cgh.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Choudhuri G, Chetri K, Bhat G, Alexander G, Das K, Ghoshal UC, et al. Long-term efficacy and safety of N-butylcyanoacrylate in endoscopic treatment of gastric varices. Tropical Gastroenterol. 2010;31(3):155–164. [PubMed] [Google Scholar]
- 33.Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010;59(6):729–735. doi: 10.1136/gut.2009.192039. [DOI] [PubMed] [Google Scholar]
- 34.Soga K, Tomikashi K, Fukumoto K, Miyawaki K, Okuda K, Konishi H, et al. Successful endoscopic hemostasis for ruptured duodenal varices after balloon-occluded retrograde transvenous obliteration. Digestive Endoscopy. 2010;22(4):329–333. doi: 10.1111/j.1443-1661.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- 35.Binmoeller KF, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos) Gastrointest Endosc. 2011;74(5):1019–1025. doi: 10.1016/j.gie.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Kang EJ, Jeong SW, Jang JY, Cho JY, Lee SH, Kim HG, et al. Long-term result of endoscopic Histoacryl (N-butyl-2-cyanoacrylate) injection for treatment of gastric varices. World J Gastroenterol. 2011;17(11):1494–1500. doi: 10.3748/wjg.v17.i11.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao SC, Yang SS, Ko CW, Lien HC, Tung CF, Peng YC, et al. A miniature ultrasound probe is useful in reducing rebleeding after endoscopic cyanoacrylate injection for hemorrhagic gastric varices. Scand J Gastroenterol. 2013;48(11):1347–1353. doi: 10.3109/00365521.2013.838995. [DOI] [PubMed] [Google Scholar]
- 38.Tantau M, Crisan D, Popa D, Vesa S, Tantau A. Band ligation vs. N-Butyl-2-cyanoacrylate injection in acute gastric variceal bleeding: a prospective follow-up study. Ann Hepatol. 2013;13(1):75–83. [PubMed] [Google Scholar]
- 39.Al-Bawardy B, Gorospe EC, Saleem A, Buttar NS. Wong Kee song LM. Outcomes and predictors of Rebleeding after 2-Octyl cyanoacrylate injection in acute gastric Variceal hemorrhage. J Clin Gastroenterol. 2016;50(6):458–463. doi: 10.1097/MCG.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Ma L, Wang J, Li F, Tseng Y, Luo T, et al. Prophylactic use of antibiotics in endoscopic injection of tissue adhesive for the elective treatment of gastric varices: a randomized controlled study. J Gastroenterol Hepatol. 2019;34(9):1486–1491. doi: 10.1111/jgh.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakeb F, Salama Z, Salama H, Abdel Raouf T, Abdel Kader S, Abdel HH. The value of combined use of N-butyl-2-cyanoacrylate and ethanolamine oleate in the management of bleeding esophagogastric varices. Endoscopy. 1995;27(5):358–364. doi: 10.1055/s-2007-1005714. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama H, Okabe S, Ishihara T, Tsuyuguchi T, Yoshikawa M, Matsutani S, et al. Long-term effect of endoscopic injection therapy with combined cyanoacrylate and ethanol for gastric fundal varices in relation to portal hemodynamics. Abdom Imaging. 2010;35(1):8–14. doi: 10.1007/s00261-008-9497-0. [DOI] [PubMed] [Google Scholar]
- 43.Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, et al. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video) Gastrointest Endosc. 2016;83(6):1164–1172. doi: 10.1016/j.gie.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 44.Robles-Medranda C, Valero M, Nebel JA, de Britto Junior SR, Puga-Tejada M, Ospina J, et al. Endoscopic-ultrasound-guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic Doppler and endosonographic varicealography in vascular targeting. Digestive Endoscopy. 2019;31(3):283–290. doi: 10.1111/den.13305. [DOI] [PubMed] [Google Scholar]
- 45.Zhang CQ, Liu FL, Liang B, Sun ZQ, Xu HW, Xu L, et al. A modified percutaneous transhepatic variceal embolization with 2-octyl cyanoacrylate versus endoscopic ligation in esophageal variceal bleeding management: randomized controlled trial. Dig Dis Sci. 2008;53(8):2258–2267. doi: 10.1007/s10620-007-0106-9. [DOI] [PubMed] [Google Scholar]
- 46.Tian X, Wang Q, Zhang C, Liu F, Cui Y, Liu F, et al. Modified percutaneous transhepatic variceal embolization with 2-octylcyanoacrylate for bleeding gastric varices: long-term follow-up outcomes. AJR Am J Roentgenol. 2011;197(2):502–509. doi: 10.2214/AJR.10.6005. [DOI] [PubMed] [Google Scholar]
- 47.Feretis C, Dimopoulos C, Benakis P, Kalliakmanis B, Apostolidis N. N-butyl-2-cyanoacrylate (Histoacryl) plus sclerotherapy versus sclerotherapy alone in the treatment of bleeding esophageal varices: a randomized prospective study. Endoscopy. 1995;27(5):355–357. doi: 10.1055/s-2007-1005713. [DOI] [PubMed] [Google Scholar]
- 48.Dhiman RK, Chawla Y, Taneja S, Biswas R, Sharma TR, Dilawari JB. Endoscopic sclerotherapy of gastric variceal bleeding with N-butyl-2-cyanoacrylate. J Clin Gastroenterol. 2002;35(3):222–227. doi: 10.1097/00004836-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Tian X, Hu J, Zhang J, Zhang C, Yang Y, et al. Efficacy of transjugular intrahepatic portosystemic shunt with adjunctive embolotherapy with cyanoacrylate for esophageal variceal bleeding. Dig Dis Sci. 2014;59(9):2325–2332. doi: 10.1007/s10620-014-3150-2. [DOI] [PubMed] [Google Scholar]
- 50.Ma LL, Luo TC, Tseng YJ, Huang XQ, Luo JJ, Zhang W, et al. Balloon-occluded retrograde Transvenous obliteration of Portovenous shunts during endoscopic therapy for the treatment of gastric Varices. Surg Laparoscopy Endoscopy Percutaneous Techniques. 2018;28(6):e113–e1e6. doi: 10.1097/SLE.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 51.Dai YP, Gao Q. A prognostic analysis of cirrhotic esophageal variceal bleeding treated with standardized endoscopic therapy. Zhonghua Gan Zang Bing Za Zhi. 2017;25(3):195–199. doi: 10.3760/cma.j.issn.1007-3418.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Zeng XQ, Ma LL, Tseng YJ, Chen J, Cui CX, Luo TC, et al. Endoscopic cyanoacrylate injection with or without lauromacrogol for gastric varices: a randomized pilot study. J Gastroenterol Hepatol. 2017;32(3):631–638. doi: 10.1111/jgh.13496. [DOI] [PubMed] [Google Scholar]
- 53.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology (Baltimore, Md) 1992;16(6):1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.