Abstract
Background:
Between 2010 and 2014, the percentage of 13–17 year-old girls administered ≥3 doses of the human papilloma virus (HPV) vaccine (“fully vaccinated”) increased by 7.7 percentage points to 39.7%, and the percentage not administered any doses of the HPV vaccine (“not immunized”) decreased by 11.3 percentage points to 40.0%.
Objective:
To evaluate the complex interactions between parents’ vaccine-related beliefs, demographic factors, and HPV immunization status.
Methods:
Vaccine-related parental beliefs and sociodemographic data collected by the 2010 National Immunization Survey-Teen among teen girls (n = 8490) were analyzed. HPV vaccination status was determined from teens’ health care provider (HCP) records.
Results:
Among teen girls either unvaccinated or fully vaccinated against HPV, teen girls whose parent was positively influenced to vaccinate their teen daughter against HPV were 48.2 percentage points more likely to be fully vaccinated. Parents who reported being positively influenced to vaccinate against HPV were 28.9 percentage points more likely to report that their daughter’s HCP talked about the HPV vaccine, 27.2 percentage points more likely to report that their daughter’s HCP gave enough time to discuss the HPV shot, and 43.4 percentage points more likely to report that their daughter’s HCP recommended the HPV vaccine (p < 0.05). Among teen girls administered 1–2 doses of the HPV vaccine, 87.0% had missed opportunities for HPV vaccine administration.
Conclusion:
Results suggest that an important pathway to achieving higher ≥3 dose HPV vaccine coverage is by increasing HPV vaccination series initiation though HCP talking to parents about the HPV vaccine, giving parents time to discuss the vaccine, and by making a strong recommendation for the HPV. Also, HPV vaccination series completion rates may be increased by eliminating missed opportunities to vaccinate against HPV and scheduling additional follow-up visits to administer missing HPV vaccine doses.
Keywords: HPV, Parental concerns, Provider influence, Attributable risk
1. Background
In 2007, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) recommended routine administration of 3 doses of the human papilloma virus (HPV) vaccine be administered for girls between 11 and 12 years of age [1]. In 2006, the ACIP recommended 1 dose of tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap) for routine administration for all teens between 11 and 12 years of age and, in 2005,1 dose of the meningococcal vaccine (MenACWY) was recommended for routine administration to all teens between 11 and 12 years of age [2,3]. Compared to uptake of the recommended single doses of MenACWY and Tdap vaccines, uptake of ≥1 dose of HPV vaccine for 13–17 year-old teen girls (“teen girls”) has been more slow [4]. In 2007, estimated national coverage for Tdap and MenACWY vaccines exceeded 30% [5] and, in the subsequent 4 years, coverage increased by 47.8 and 38.1 percentage points, respectively [6]. In comparison, the percentage of teen girls administered ≥3 doses of HPV vaccine did not exceed 30% until 2010 [7] and, in the subsequent 4 years, increased by only 7.7 percentage points [8]. In 2014, 39.7% (±1.9%) of all 13–17 year-old teen girls were administered ≥3 doses of HPV vaccine (“fully vaccinated”), 20.3% (±1.6%) were administered 1–2 doses, and 40.0% (±1.9%) of all teen girls were not administered any HPV vaccine doses [9].
Increasing coverage of the HPV vaccine requires understanding the dynamics of parents’ decision-making around acceptance of the HPV vaccine and developing interventions based on these insights. The purpose of this manuscript is to explore how complex interactions between parents’ vaccine-related beliefs and demographic characteristics are associated with whether teen girls are fully vaccinated against HPV, and to explore how the influence of health care providers affects parents’ decision to vaccinate their teen daughter against HPV.
2. Methods
We analyzed the most recent psychosocial data on vaccine-related parental beliefs data collected from the parents of 4437 parents sampled in the 2010 National Immunization Survey-Teen (NIS-Teen), a survey of 13–17 year-old teens in the United States. Data from 2010 continue to be relevant because the percentage of teen girls who were fully vaccinated changed by only 7.7 percentage points between 2010 and 2014, and because data from the 2010 NIS-Teen include the most recent information on vaccine-related parental beliefs collected by the NIS-Teen that cover the 4 original domains of the Health Belief Model [9,10], a behavioral conceptual framework for understanding the psychosocial determinants of parents’ failure to vaccinate their children.
In 2010, the NIS-Teen used a list-assisted random-digit-dial survey of households with landline-phone numbers to identify households with age-eligible teens. Parental reports of teen, maternal, and household characteristics were collected during the telephone interviews. If consent was obtained to contact teens’ vaccination providers, a mail survey was sent to providers to collect provider-recorded vaccination histories. In this paper, provider-recorded vaccination histories are used to evaluate vaccination status. Teen girls are “fully vaccinated” if provider records show that they were administered ≥3 doses of the HPV vaccine and “not vaccinated against HPV” if the records show that no doses of the HPV vaccine were administered. We define teen girls sampled by the NIS-Teen to have “missed opportunities” for HPV vaccine administration if
they were not fully vaccinated against HPV by the time of the NIS-Teen telephone interview; and
between their 11th birthday and the NIS-Teen telephone interview date, were administered at least 1 dose of either the Tdap, MenACWY, influenza, H1N1, Hepatitis A, Hepatitis B, measles containing vaccine, pneumococcal polysaccharide, or varicella vaccine on any calendar date when an HPV vaccine dose was not recorded as having been administered.
The response rate for the 2010 NIS-Teen landline phone survey is a product of the resolution rate, the screening completion rate, and the completion rate. The resolution rate is the number of telephone number determined to be a residence divided by the number of telephone numbers randomly sampled from a list of telephone numbers that are potentially residences. For the 2010 NIS-Teen survey, the resolution rate is 2,707,821/3,275,206 = 82.6%. The screening completion rate is the number of sampled households determined to have a 13–17 year-old teen from the sampled telephone numbers in the list divided by the number of telephone numbers sampled that were resolved to be households. For the 2010 NIS-Teen survey, the screening completion rate is 485,138/571,039 = 85.0%. The completion rate is the number of sampled households with a 13–17 year-old teen that completed the NIS-Teen telephone interview among households determined to have a 13–17 year-old teen. For the 2010 NIS-Teen survey, the completion rate is 35,004/42,414 = 82.5%. The product of these three rates is 58%. Among all households sampled by the 2010 NIS-Teen that completed the phone interview, the percentage with an adequate provider-reported vaccination history was 19,488/32,933 = 59.2%.
In the 3rd and 4th quarters of 2010, the NIS-Teen collected data on parents’ vaccine-related beliefs for 4016 teen girls who had an adequate provider-reported vaccination history. To assess those beliefs, parents were read 15 statements and asked whether they agreed or disagreed with each statement on a scale of 0 (strongly disagree) to 10 (strongly agree). Numeric responses ≥7 were interpreted as in agreement. Also, parents were asked whether their teen daughter’s health care provider (HCP) made their decision to vaccinate their daughter against HPV more likely or not, and we dichotomized parents’ responses as either being “positively influenced” by their daughters’ HCP or as being “not positively influenced.” Vaccine-related parental beliefs were organized using the Health Belief Model [11,12]. To explore how complex interactions between parents’ vaccine-related beliefs and demographic characteristics are associated with whether teen girls are fully vaccinated or not, we conducted 2 analyses. The first analysis compares teen girls who were not vaccinated against HPV to teen girls who were fully vaccinated, and the second analysis compares teen girls who were administered either 1 or 2 doses of the HPV vaccine to teen girls who were fully vaccinated. We used multivariable recursive partitioning analysis [13,14], and attributable risk analysis [15] to evaluate the extent to which being undervaccinated was attributable to HCPs not being a positive influence on parents’ decision to vaccinate their daughter.
Statistical analyses used the survey library [16] in the R statistical software package [17]. All estimates account for the surveys’ sampling weights and sampling design of the NIS and NIS-Teen and are reported with 95% confidence intervals. Differences between estimated percentages were evaluated using z-tests and are declared to be statistically significant if p < 0.05. The NIS-Teen has been approved annually by Ethics and Research Review Board of the National Center for Health Statistics since 2005.
3. Results
Statistical analyses comparing teen girls who were fully vaccinated to teen girls not vaccinated against HPV.
In 2010, 50.1% (±2.7%) of all 13–17 year-old teen girls were not vaccinated against HPV. Among teen girls not vaccinated against HPV, 60.5% (±2.5%) had missed opportunities for HPV vaccine administration.
Bivariable analyses found that compared to teen girls who were fully vaccinated, teen girls not vaccinated against HPV (zero doses of HPV vaccine administered) were significantly less likely to have been vaccinated by a pediatrician; more likely to be entitled to publically purchased vaccines from the Vaccines for Children program (VFC) [18]; less likely to have a mother with less than a high school education; more likely to live in a household with an annual income in the third income quintile, and less likely to live in a central city metropolitan statistical area (Table 1). Also, compared to parents of teen girls who were fully vaccinated, parents of teen girls unvaccinated against HPV had many significant differences across all 4 domains of the Health Belief Model and had significantly lower assessments (1) of their teens’s risk of getting a vaccine preventable disease (VPD), (2) of VPDs as a concern that make vaccinations relevant, (3) of vaccines’ efficacy to reduce the threat of a VPD; and (4) were significantly less likely to report that their decision to vaccinate their child was favorably influenced by a health care provider, and significantly less likely to believe that vaccines are safe (Table 2).
Table 1.
Estimated distribution across selected teen, maternal, and household demographic factors for 13–17 year-old girls by number of HPV doses administered. Q3 and Q4 2010 NIS-Teen.
| Unweighted sample size, n | Number of HPV vaccine doses administered | |||
|---|---|---|---|---|
| 0 % (95%CI) |
1–2 % (95%CI) |
≥3 (95%CI) |
||
| Teen characteristics | ||||
| Race/ethnicity | ||||
| Hispanic | 516 | 16.9 (±3.6) | 23.6 (±7.8) | 18.9 (±4.4) |
| White non-Hispanic | 2812 | 59.5 (±3.8) | 56.4 (±7.5) | 58.6 (±4.6) |
| Black non-Hispanic | 475 | 16.4 (±2.7) | 15.1 (±5.1) | 13.7 (±3.3) |
| Othera | 329 | 7.2 (±2.2) | 4.9 (±1.9) | 8.7 (±2.5) |
| Number of vaccination providers | ||||
| 1 provider | 2196 | 52.8 (±3.7) | 58.2 (±6.7) | 58.4 (±4.3) |
| ≥2 providers | 1936 | 47.2 (±3.7) | 41.8 (±6.7) | 41.6 (±4.3) |
| Specialty types of teens’ vaccination providers | ||||
| Pediatrician | 2828 | 67.7 (±3.3)b | 69.8 (±6.9) | 73.5 (±3.9) |
| Family practitioner | 1519 | 37.5 (±3.7) | 38.3 (±7.5) | 36.9 (±4.3) |
| General practitioner | 518 | 14.6 (±3.2) | 15.5 (±6.5) | 15.7 (±4.0) |
| Internal medicine | 627 | 17.6 (±3.4) | 15.6 (±6.0) | 18.5 (±3.7) |
| OB/GYN | 556 | 16.0 (±3.4) | 15.2 (±6.0) | 15.6 (±3.5) |
| Other specialty | 1158 | 27.5 (±2.9)b | 23.3 (±5.2) | 21.9 (±3.7) |
| Foreign born | ||||
| Yes | 138 | 3.0 (±1.5) | 5.3 (±3.8) | 4.6 (±2.4) |
| No | 8183 | 97.0 (±1.5) | 94.7 (±3.8) | 95.4 (±2.4) |
| Entitled to VFCc vaccines | ||||
| Yes | 1169 | 34.4 (±3.8)b | 39.4 (±7.3)b | 27.5 (±3.9) |
| No | 6007 | 65.6 (±3.8)b | 60.6 (±7.2) | 72.5 (±3.9) |
| Maternal characteristics | ||||
| Maternal age group | ||||
| ≤34 years of age | 295 | 7.5 (±2.2) | 10.0 (±3.5) | 7.9 (±2.7) |
| 35 to 44 years of age | 1646 | 43.5 (±3.8) | 46.3 (±7.2) | 41.4 (±4.5) |
| ≥45 years of age | 2191 | 49.0 (±3.7) | 43.8 (±7.3) | 50.8 (±4.5) |
| Marital status | ||||
| Married | 3105 | 74.3 (±3.3) | 69.3 (±6.7) | 72.2 (±4.2) |
| Widowed | 80 | 1.2 (±0.6)b | 1.0 (±0.8) | 3.5 (±1.9) |
| Divorced | 469 | 11.5 (±2.3) | 11.4 (±3.7) | 9.8 (±2.1) |
| Separated | 120 | 2.9(±1.1) | 5.7 (±3.1) | 4.4 (±2.3) |
| Never married | 322 | 9.2 (±2.5) | 11.9 (±5.7) | 8.4 (±2.7) |
| Deceased | 36 | 0.9 (±0.5) | 0.7 (±0.6) | 1.7 (±1.7) |
| Preferred language | ||||
| English | 3930 | 93.8 (±2.7) | 87.6 (±7.7) | 90.8 (±3.6) |
| Spanish | 165 | 4.4 (±2.3) | 11.6 (±7.7) | 7.7 (±3.5) |
| Other | 37 | 1.8 (±1.5) | 0.8 (±0.7) | 1.5 (±1.1) |
| Maternal education | ||||
| Non-high school graduate | 341 | 8.4 (±2.6)b | 24.6 (±8.1)b | 13.7 (±3.6) |
| High school graduate | 835 | 27.8 (±3.4) | 24.1 (±5.6) | 25.0 (±4.4) |
| Some college training | 1238 | 28.3 (±3.3) | 24.4 (±5.3) | 24.5 (±3.7) |
| College graduate | 1718 | 35.6 (±3.5) | 26.9 (±5.5)b | 36.8 (±4.0) |
| FPL (Federal Poverty Level) | ||||
| 1st quintile (lowest income) | 775 | 17.1 (±2.9) | 30.3 (±7.8)b | 19.4 (±3.5) |
| 2nd quintile | 661 | 20.7 (±3.2) | 16.1 (±4.9) | 20.9 (±4.4) |
| 3rd quintile | 807 | 23.0 (±3.1)b | 17.8 (±4.4) | 17.1 (±3.1) |
| 4th quintile | 875 | 20.9 (±3.2) | 16.6 (±4.8) | 20.5 (±3.4) |
| 5th quintile (highest income) | 1014 | 18.3 (±2.5) | 19.1 (±5.0) | 22.1 (±3.3) |
| Number of children ≤18years of age in the household | ||||
| 1 | 1630 | 32.4 (±3.3) | 22.4 (±4.5)b | 33.5 (±4.0) |
| 2–3 | 2097 | 55.8 (±3.7) | 58.6 (±7.1) | 55.7 (±4.4) |
| ≥4 | 405 | 11.8 (±2.8) | 19.0 (±6.9)b | 10.8 (±3.2) |
| Location of the household | ||||
| Central city metropolitan statistical area | 1550 | 34.1 (±3.7)b | 37.4 (±6.9) | 39.9 (±4.5) |
| Non-central city metropolitan statistical area | 1539 | 49.0 (±3.7) | 47.9 (±7.2) | 45.9 (±4.4) |
| Non-metropolitan statistical area | 964 | 16.9 (±2.1) | 14.7 (±3.9) | 14.2 (±2.4) |
Includes Asian non-Hispanic, American Indian/Alaska Native non-Hispanic, other non-Hispanic, and other mixed race children.
Estimated percentage is significantly different from the estimated percentage in the same row among teen girls administered ≥3 doses of HPV vaccine.
VFC, Vaccines for Children Program. Children and teens 0–18 years of age are entitled to publically purchased vaccines from providers who are enrolled in their state’s VFC program if they are eligible for Medicaid, not covered by health insurance, American Indian/Alaska Native, or covered by private health insurance that does not pay all of the costs vaccines and administered vaccine doses at a Federally Qualified Health Center or Rural Health Center. See http://www.cdc.gov/vaccines/programs/vfc/index.html.
Table 2.
Percentage of parents agreeing to psychosocial statements by Group, among parents with a 13–17 year-old daughter either fully vaccinated or administered 0 doses of HPV vaccine. Q3 and Q4 2010 NIS-Teen, n = 4132.
| Domain of the health belief model | Psychosocial statement | Number of HPV doses administered | ||
|---|---|---|---|---|
| 0 % (95% CI) |
1–2 % (95% CI) |
≥3 % (95% CI) |
||
| Parents’ assessment of their teen’s risk of getting a VPD | Vaccines are necessary to protect the health of teenagers | 84.4 (±2.8)a | 95.5 (±2.2) | 96.2 (±1.4) |
| Parents’ assessment of whether VPDs are a sufficient health concern to make vaccinations relevant | If I do not vaccinate my teenager he/she may get a disease such as meningitis and cause other teenagers or adults also to get the disease | 75.0 (±3.3)a | 85.7 (±4.0) | 89.1 (±2.7) |
| I make a point to read and watch stories about health | 79.2 (±3.0) | 81.6 (±5.1) | 82.9 (±3.2) | |
| Parents’ assessment of whether vaccinating their teen can reduce the threat of a VPD | Vaccines do a good job in preventing the disease they are intended to prevent | 88.1 (±2.6)a | 91.5 (±3.8)a | 95.1 (±1.5) |
| Teenagers receive too many vaccines | 17.9 (±3.0) | 19.3 (±7.0) | 15.4 (±3.8) | |
| Health care provider talked about HPV shot | 68.4 (±3.7)a | 85.1 (±6.1)a | 91.7 (±2.6) | |
| Health care provider recommended the HPV shot | 54.4 (±3.8)a | 83.6 (±6.4) | 89.7 (±3.4) | |
| Health care provider gave enough time to discuss the HPV shot | 72.6 (±3.8)a | 84.6 (±6.8) | 91.1 (±3.9) | |
| Influences | Health care provider made parent’s decision to vaccinate their teenager more likely | 23.9 (±3.2)a | 71.0 (±5.8) | 73.1 (±4.2) |
| My teenager helps to make the decisions about whether she will receive a vaccine | 29.8 (±3.1)a | 35.8 (±7.1) | 40.3 (±4.6) | |
| I have a good relationship with my teenager’s health care provider | 92.0 (±2.5)a | 94.4 (±3.1) | 96.6 (±1.5) | |
| In general, medical professionals in charge of vaccinations have my teenager’s best interest at heart | 89.1 (±2.1)a | 95.5 (±2.3) | 95.7 (±1.5) | |
| Vaccines are safe | 70.4 (±3.4)a | 82.2 (±4.8) | 86.0 (±2.7) | |
| At the time that your teen was vaccinated, did you have concerns about safety? | 46.9 (±3.7)a | 45.5 (±7.1) | 40.6 (±4.4) | |
| If I vaccinate my teenager, she may have serious side effects | 32.7 (±3.7)a | 25.3 (±6.9) | 24.5 (±3.9) | |
| Vaccination should be delayed if a teenager has a minor illness | 65.7 (±3.7)a | 59.0 (±7.5) | 60.4 (±4.4) | |
Percentage is significantly different from the percentage in the same row among teen girls administered ≥3 HPV doses.
Multivariable analysis found that none of the 12 demographic factors and only 3 of the 16 vaccine-related belief factors were independent predictors associated with whether teen girls are fully vaccinated against HPV (Fig. 1). The recursive partitioning analysis used the independent predictors to segment the U.S. population of teen girls into 4 groups (Fig. 1), across which the percentage of teen girls who were fully vaccinated against HPV decreases:
Fig. 1.
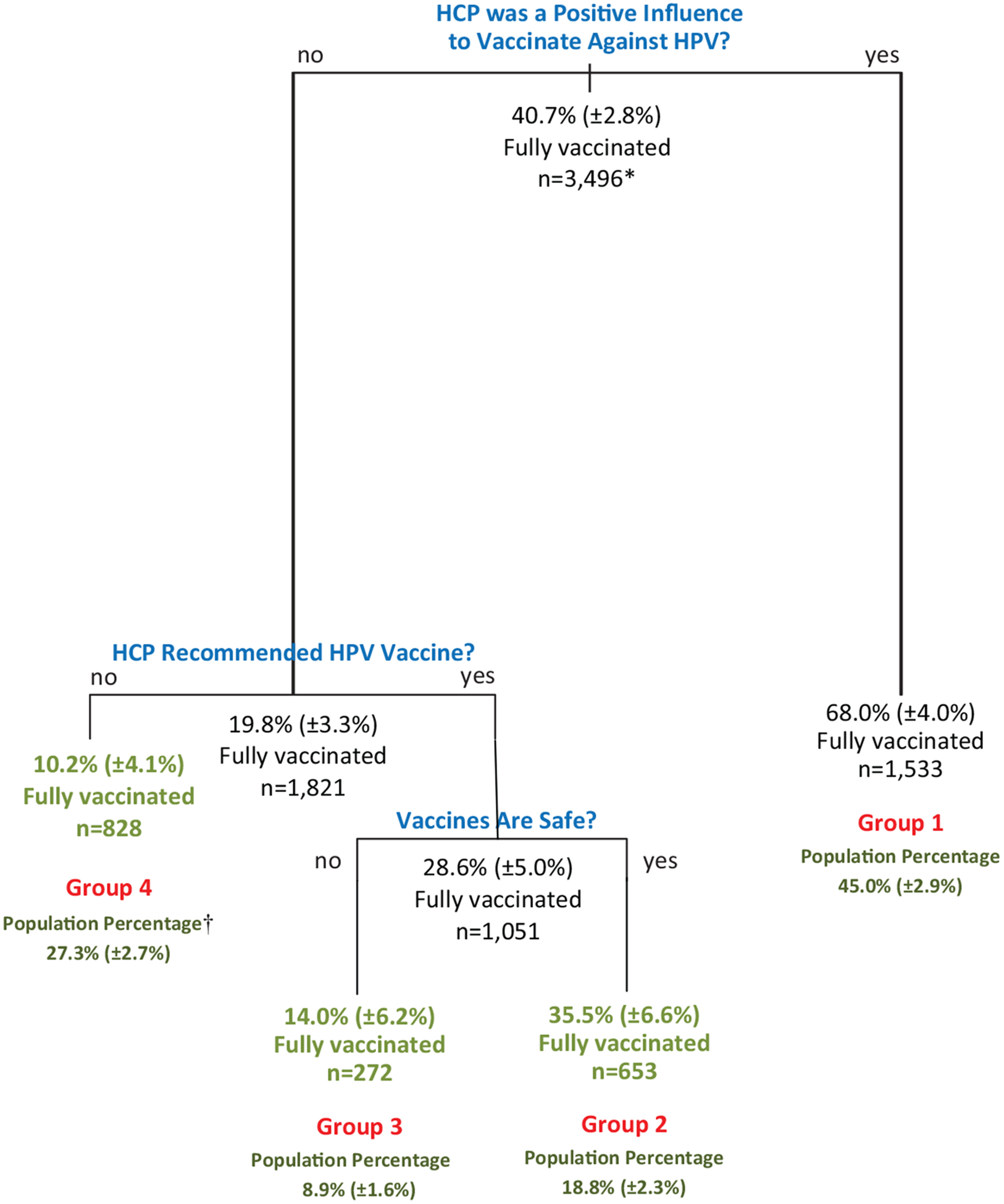
Estimated ≥3 dose HPV vaccination coverage from recursive partitioning analysis of statistically independent factors associated with being fully vaccinated vs. 0 doses. Q3 and Q4 2010 NIS-Teen. *Leaf sample sizes may not add to node sample size because of missing values. †Population percentage among teen girls in the terminal node among girls either fully vaccinated or unvaccinated against HPV.
Teen girls in group 1 (Fig. 1) had a parent who reported being positively influenced by their daughters’ HCP to vaccinate against HPV (Fig. 1). Among teen girls in group 1, 68.0% were fully vaccinated against HPV (≥3 doses of HPV vaccine administered).
Teen girls in group 2 had a parent who reported not being positively influenced by their daughter’s HCP to vaccinate against HPV, reported that their daughters’ HCP recommended the HPV vaccine, and reported that they believed that vaccines are safe. Among teen girls in group 2, 35.5% were fully vaccinated against HPV.
Teen girls in group 3 had a parent who reported not being positively influenced by their daughter’s HCP to vaccinate against HPV, reported that their daughters’ HCP recommended the HPV vaccine, and reported that they believed that vaccines are not safe. Among teen girls in group 3, 14.0% were fully vaccinated against HPV (Fig. 1).
Teen girls in group 4 had a parent who reported not being positively influenced by their daughter’s HCP to vaccinate against HPV, and reported that their daughters’ HCP did not recommend the HPV vaccine. Among teen girls in group 3, 10.2% of the girls in group 4 were fully vaccinated against HPV (Fig. 1).
Compared to teen girls whose parent (i) reported not being positively influenced by their daughter’s HCP to vaccinate their daughter against HPV and (ii) reported receiving a recommendation by their daughter’s HCP to vaccinate against HPV, teen girls whose parent was positively influenced to vaccinate their daughter against HPV were 39.4 percentage points more likely to be fully vaccinated against HPV (68.0% vs. 28.6%, p < 0.05) (Fig. 1).
Overall, compared to teen girls whose parent was not positively influenced to vaccinate against HPV, teen girls whose parent was positively influenced to vaccinate against HPV were 48.2 percentage points more likely to be fully vaccinated against HPV (68.0% vs. 19.8%, p < 0.05) (Fig. 1). Of all teen girls who were unvaccinated against HPV, the reason for not being fully vaccinated was attributed to parents not being positively influenced to vaccinate against HPV for 60.1% of the unvaccinated girls (95% confidence interval: 54.6%, 65.0%).
Compared to parents who did not report being positively influenced to vaccinate against HPV, parents who reported being positively influenced to vaccinate against HPV were
28.9 percentage points more likely to report that their daughter’s health care provider (HCP) talked about the HPV vaccine (94.8% vs. 65.9%, p < 0.05),
27.2 percentage points more likely to report that their daughter’s HCP gave enough time to discuss the HPV shot (95.9% vs. 68.7%, p < 0.05), and
43.4 percentage points more likely to report that their daughter’s HCP recommended the HPV shot (93.8% vs. 50.4%, p < 0.05)
Statistical analyses comparing teen girls who were fully vaccinated to teen girls administered 1–2 doses of HPV vaccine.
In 2010, 15.6% (±2.0%) of all 13–17 year-old teen girls were administered 1–2 doses of HPV vaccine and, among those, 87.0% (±3.5%) had missed opportunities for HPV vaccine administration.
Bivariable analysis found that compared to teen girls who were fully vaccinated, teen girls administered 1–2 doses were significantly more likely to be entitled to publicly purchased vaccines from the Vaccines for Children Program (VFC) [19]; more likely to have a mother who had less than a high school education and less likely to have a college degree; more likely to live in a household with an annual income in the lowest income quintile, more likely to have 4 or more children 18 years of age or younger in their household, and less likely to have only one child 18 years of age or younger in their household (Table 1). Also, compared to teen girls who were fully vaccinated, teen girls administered 1–2 HPV vaccine doses had parents who were significantly less likely to report that vaccines do a good job in preventing the disease they are intended to prevent, and were significantly less likely to report that their daughter’s health care provider (HCP) talked about the HPV vaccine (Table 2).
The multivariable analysis found that among all of the 12 demographic and 16 vaccine-related belief factors measured on parents of teen girls, there were no parental belief factors found to be independent predictors of being fully vaccinated against HPV, and only teen girls’ VFC eligibility status was found to be independently associated with whether teen girls are fully vaccinated against HPV or administered only 1–2 doses. Compared to teen girls who were not VFC-entitled, those who were VFC-entitled were 8.0 percentage points less likely to be fully vaccinated against HPV (60.5% vs. 72.5%, p < 0.05).
4. Discussion
Because the percentage of teen girls who were fully vaccinated against HPV increased by only 7.7 percentage points between 2010 and 2014 to 39.7%, and because the percentage not vaccinated against HPV over that period decreased by only 11.3 percentage points to 40.0%, psychosocial data from the 2010 NIS-Teen survey continues to be relevant because they provide the most recent nationally representative data that enables us to explore how both vaccine-related parental beliefs and demographic factors explain why teen girls are undervaccinated against HPV. We found that the factors associated with being unvaccinated against HPV are different from the factors associated with being administered 1–2 doses of HPV vaccine.
In comparing teen girls who were unvaccinated against HPV to girls who were fully vaccinated, we found (i) that the most important independent predictor associated with being fully vaccinated against HPV was having an HCP who is a positive influence on parents’ decision to vaccinate their teen daughter against HPV, (ii) that teen girls whose parent was positively influenced to vaccinate against HPV were 48.2 percentage points more likely to be fully vaccinated against HPV, and (iii) that among teens who were unvaccinated against HPV, 60.1% of the undervaccination was attributed to parents not being positively influenced to vaccinate against HPV by their daughters HCP. Also, we found that parents who reported being positively influenced to vaccinate their daughter against HPV reported significantly and considerably higher levels of provider communication, with nearly all reporting that their daughter’s HCPs talked to them about the HPV vaccine, gave them time to discuss the HPV vaccine, and recommended the HPV vaccination. Among parents reporting not being positively influenced to vaccinate, 34.1% reported that their daughter’s HCP did not talk to them about the HPV vaccine.
Previous research has shown that spending time, discussion, and exchange of information are hallmarks of ‘shared decision making’ between providers and patients that has been found to be associated with significantly greater patient satisfaction [19], knowledge, and higher levels of patient adherence to provider recommendations [20]. Parents’ report of lack of information about vaccines has been shown to be associated with negative attitudes about vaccines and vaccination providers [21], and concerns about vaccine safety have been shown to be associated with lower childhood vaccination coverage [22]. However, other literature has found vaccination coverage among children whose parent has concerns about vaccine safety can be as high as vaccination coverage among children whose parent does not have concerns, if parents with concerns are positively influenced by an HCP to vaccinate [23]. Advice for providers for talking with parents are available [24–32] that include vaccine fact sheets, schedules for parents and patients [33], and advice on time-savers for talking with parents about the HPV vaccine [34]. This advice includes listening to parents to understand and address their concerns [35,36]; and making a clear, strong, and unambiguous recommendation to vaccinate. Model encounters for showing providers how to talk to parents about the HPV vaccine are available [37,38].
In comparing teen girls who were administered 1–2 doses of HPV vaccine to girls who were fully vaccinated, we found that teen girls’ entitlement to VFC was significantly associated with being administered only 1–2 HPV vaccine doses. Although financial barriers attributable to the cost of vaccines are eliminated for adolescents entitled to publicly purchased vaccines at no cost from providers enrolled in their state’s Vaccines for Children Program, these adolescents live in lower socio-economic conditions and have lower vaccination coverage in general [39,40]. Other barriers to vaccination may remain among children and adolescent living in low-income households [41–43]. Also, we found that 87.0% of teen girls who were administered 1–2 doses of HPV vaccine had missed other opportunities for HPV vaccine administration, and among those only 7.8% reported refusing the HPV vaccine. These findings suggest that missed opportunities to vaccinate are a main factor associated with not being fully vaccinated against HPV, and support the use of standards of care for pediatric immunization practices that recommend that providers review teen’s vaccination records at every visit to assess whether catch-up doses of HPV and other vaccines need to be administered [44].
Strengths and limitations.
Strengths of our study include statistical analyses conducted on nationally representative data. Limitations of our study include the annual surveys of the NIS-Teen used in our study collected data from households with landline-phones, none of those surveys collected data from households with only cell-phone service. Other analyses have shown that the potential bias in our estimates of vaccination coverage resulting from not sampling households with cell phone service, only, in 2008–2010 is small [45].
5. Conclusion
Our findings suggest that a different intervention is required to transition teen girls from being unvaccinated against HPV to fully vaccinated, compared to the intervention that is required to transition teen girls administered 1–2 doses of the HPV vaccine to being fully vaccinated. Specifically, our results suggest that an important pathway to achieving higher ≥3 dose HPV vaccine coverage is by HCPs positively influencing parents’ decision to initiate the 3 dose HPV vaccination series, by talking to parents about the HPV vaccine, giving parents enough time to discuss the HPV vaccine, and by making a strong recommendation for administration of the HPV vaccine. Also, our results suggest that a further pathway to achieving higher ≥3 dose HPV vaccine coverage is by increasing HPV vaccination series completion rates among teen girls administered 1–2 doses of HPV vaccine by eliminating missed opportunities to vaccinate against HPV. Our results support the use of standards of care for pediatric immunization practices that recommend that providers review teen’s vaccination records at every visit to assess whether catch-up doses of the HPV and other vaccines recommended for teens need to be administered [44]. Client reminder and recall systems [46,47] have been shown to be effective as a part of a strategy to administer missed doses of all recommended childhood vaccines, and a systematic review of the literature has confirmed that client reminder and recall systems are effective for increasing HPV vaccination coverage, also [48]. Another systematic review found that educational interventions to increase HPV vaccination acceptance found that those interventions generally did not demonstrate effectiveness, however [49]. The results of our paper suggest that interventions focused on cultivating vaccination provider skill at being a positive influence on parents’ decision to vaccinate against HPV will be important in increasing 3-dose HPV vaccination coverage rates. Finally, although we found that 58.6% of teen girls who were administered 1–2 doses of HPV vaccine had missed other opportunities for HPV vaccine administration, 41.4% did not have missed opportunities to vaccinate, but were eligible to receive HPV vaccine doses if visits were made. This suggests that in addition to taking advantage of every opportunity, HCPs need to create opportunities to vaccinate teen girls who are undervaccinated against HPV or who have not completed the 3 dose HPV vaccination series. This can happen by scheduling follow-up visits to administer the next HPV vaccine dose in the series before the teen leaves the office and sending reminder notices to parents and teens when vaccines are due.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this paper are those of the author(s), and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
None of the authors have any conflicts of interest.
References
- [1].Centers for Disease Control and Prevention. Quadravalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morib Mortal Wkly Rep 2007;56(No. RR-2). http://www.cdc.gov/Mmwr/preview/mmwrhtml/rr56e312a1.htm (accessed on 26.08.14). [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morib Mortal Wkly Rep 2006;55(RR03):1–34. [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morib Mortal Wkly Rep 2005;54(RR07):1–2. [Google Scholar]
- [4].Centers for Disease Control and Prevention. National, Regional, State, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2013. Morib Mortal Wkly Rep 2014;63(29):625–33. [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. National vaccination coverage among adolescents aged 13–17 years — United States, 2007. Morbid Mortal Wkly Rep 2008;57(40):1100–3. [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13–17 years — United States. 2012. Morib Mortal Wkly Rep 2011;34:671–7. [Google Scholar]
- [7].Centers for Disease Control and Prevention. National and State vaccination coverage among adolescents aged 13 through 17 years — United States, 2010. Morib Mortal Wkly Rep 2011;60(33):1117–23. [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention. National and State vaccination coverage among adolescents aged 13 through 17 years — United States, 2014. Morib Mortal Wkly Rep 2015;64(29):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosenstock IM, Derryberry M, Carriger BK. Why people fail to seek poliomyelitis vaccination. Pub Health Rep 1959;74:98–103. [PMC free article] [PubMed] [Google Scholar]
- [10].Armstrong K, Berlin M, Schwartz JS, Propert K, Ubel PA. Barriers to influenza immunization in a low-income urban population. Am J Prev Med 2001;20(10):21–5. [DOI] [PubMed] [Google Scholar]
- [11].Smith PJ, Humiston SG, Marcuse E, Zhao Dorell CG, Howes C, Hibbs B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Pub Health Rep 2011;126(Suppl. 2):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith PJ, Marcuse EK, Seward JF, Zhao Z, Orenstein WA. Children & adolescents unvaccinated 1 against measles: 2 geographic clustering, parents’ beliefs, and missed opportunities. Pub Health Rep 2015;130:1–20. http://www.publichealthreports.org/documents/Measles_130_5.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Breiman L Classification Regression Trees. Boca Raton: Chapman, & Hall/CRC; 1984. [Google Scholar]
- [14].Ripley B Classification and Regression Trees: Package ‘tree’; 2015. http://cran.r-project.org/web/packages/tree/tree.pdf.
- [15].Cole P, MacMahon B. Attributable risk percent in case-control studies. Brit J Prev Soc Med 1971;25:242–4. http://jech.bmj.com/content/25/4/242.full.pdf (accessed on 06.08.14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lumley T Analysis of complex survey samples. J Stat Softw 2004;9(April (8)). http://www.jstatsoit.org/v09/i08 (accessed on 29.11.08). [Google Scholar]
- [17].Venables WN, Smith DM, R Development Core Teafm. An Introduction to R. Notes on R: A Programming Environment for Data Analysis and Graphics. Version 2.8.0 (2008-10-20); 2008. http://www.r-project.org/ (accessed on 29.11.08). [Google Scholar]
- [18].Centers for Disease Control and Prevention. Vacines for Children Program (VFC); 2014. http://www.cdc.gov/vaccines/programs/vfc/index.html (accessed on 05.08.14). [Google Scholar]
- [19].Smith CK, Polis E, Hadac RR. Characteristics of the initial medical interview associated with patient satisfaction and understanding. J Fam Pract 1981; 12:283. [PubMed] [Google Scholar]
- [20].Joosten EAG, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CPF, de Jong CAJ. Systematic review of shared decision-making on partient statfacton, treatment adherence and health status. Psychother Psychosom 2008;77:219–26. [DOI] [PubMed] [Google Scholar]
- [21].Gust DA, Kennedy A, Shui I, Smith PJ, Nowak G, Pickering LK. Parent attitudes toward immunizations and healthcare providers the role of information. Am J Prev Med 2005;29(2):105–12. [DOI] [PubMed] [Google Scholar]
- [22].Gust D, Strine TW, Maurice E, Smith PJ, Yusuf H, Wilkinson M, Battaglia MP, Wright RA, Schwartz B. Under-immunization in children: impact of vaccine safety concerns on immunization status. Pediatrics 2004;114(1):e16–22. http://www.pediatrics.org/cgi/content/full/114/1/e16. [DOI] [PubMed] [Google Scholar]
- [23].Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. The association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics 2006;118(5):e1287–92. [DOI] [PubMed] [Google Scholar]
- [24].American Academy of Pediatrics. Immunization; 2012. http://www2.aap.org/immunization/illnesses/hpv/hpv.html (accessed on 08.07.14).
- [25].American Academy of Pediatrics. AAP Immunization Resources Adolescent Immunizations: Strategies for Increasing Coverage Rates; 2011. http://www2.aap.org/immunization/pediatricians/pdf/TopStrategiesforIncreasingCoverage.pdf (accessed on 08.07.14).
- [26].Centers for Disease Control and Prevention. Vaccine safety: Frequently Asked Questions about HPV Vaccine Safety; 2013. http://www.cdc.gov/vaccinesafety/Vaccines/HPV/hpv_faqs.html (accessed on 08.07.14).
- [27].Centers for Disease Control and Prevention. Human Papillomavirus (HPV): HPV Vaccine Information for Clinicians - Fact Sheet; 2014. http://www.cdc.gov/std/HPV/STDFact-HPV-vaccine-hcp.htm (accessed on 08.07.14).
- [28].Centers for Disease Control and Prevention. Vaccines and Immunizations. Education and Training: Patient Education; 2011. http://www.cdc.gov/vaccines/ed/patient-ed.htm (accessed on 28.02.12).
- [29].Centers for Disease Control and Prevention. Vaccines and Immunizations. Specific Groups of People: Parents; 2012. http://www.cdc.gov/vaccines/spec-grps/parents.htm (accessed on 28.02.12).
- [30].Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents; 2013. http://www.cdc.gov/vaccines/hcp/patient-ed/conversations/ (accessed on 29.05.13).
- [31].Opel DJ, Diekema DS, Lee NR, Marcuse EK. Social marketing as a strategy to increase immunization rates. Arch Pediatr Adolesc Med 2009;163(5):432–7. [DOI] [PubMed] [Google Scholar]
- [32].Gowda C, Schaffer SE, Kopec K, Markel A, Dempsey AF. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum Vaccin Immunother 2013;9(2):43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Centers for Disease Control and Prevention. HPV Vaccine Resources for Healthcare Professionals; 2015. http://www.cdc.gov/vaccines/who/teens/for-hcp/hpv-resources.html (accessed on 17.07.15).
- [34].Centers for Diease Control and Prevention. Tips and time-Savers for Talking with Parents About HPV Vaccine; 2015. http://www.cdc.gov/vaccines/who/teens/for-hcp-tipsheet-hpv.html (accessed 17.07.15).
- [35].Diekema DS American Academy of Pediatrics Committee on Bioethics. Responding to parental refusals of immunization of children. Pediatrics 2005;115:1428–31. [DOI] [PubMed] [Google Scholar]
- [36].Diekema DS. Improving childhood vaccination rates. N Engl J Med 2012;366:391–3. [DOI] [PubMed] [Google Scholar]
- [37].Minnesota Department of Health . HPV Vaccine Video for Health Care Providers; 2015. http://www.health.state.mn.us/divs/idepc/immunize/hcp/adol/hpvvideos.html (accessed 21.07.15).
- [38].National Foundation for Infectious Diseases. Five key steps to improve HPV vaccination rates in your practice. Last access July 21, 2015.
- [39].Smith PJ, Lindley MC, Rodewald LE. Vaccination coverage among U.S. children aged 19–35 months entitled by the Vaccines for Children Program, 2009. Pub Health Rep 2011;126(Suppl. 2):109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lindley MC, Smith PJ, Rodewald LE. Vaccination coverage among U.S. adolescents aged 13–17 years entitled by the Vaccines for Children Program, 2009. Pub Health Rep 2011;126(Suppl. 2):124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lannon C, Brack V, Stuart J, Caplow M, McNeill A, Bordley WC, Margolis P. What mothers say about why poor children fall behind on immunizations. Arch Pediatr Adolesc Med 1995;149:1070–5. [DOI] [PubMed] [Google Scholar]
- [42].Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med 2011;41(4):428–33. [DOI] [PubMed] [Google Scholar]
- [43].Srivastav A, Zhai Y, Santibanez TA, Kahn KE, Smith PJ, Singleton JA. Influenza vaccination coverage of Vaccine for Children (VFC)-entitled versus privately insured children, United States, 2011–2013. Vaccine 2015;33:3114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].National Vaccine Advisory Committee. Standards for Pediatric Immunization Practices. Morbid Mortal Wkly Rep 1993;42(RR-5). [PubMed] [Google Scholar]
- [45].Molinari N-AM, Wolter K, Skalland B, Montgomery R, Smith PJ, Khare M, Barron M, Copeland K, Santos K, Singleton JA. Quantifyin bias in a health survey: modeling total survey error in the national immunization survey. Stat Med 2011;30(5):505–14. [DOI] [PubMed] [Google Scholar]
- [46].Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA, Rodewald LE. Effect of patien reminder/recall interventions on immunization rates: a review. J Am Med Assoc 2000;284(October(14)):1820–7. [DOI] [PubMed] [Google Scholar]
- [47].Saville AW, Beaty B, Dickinson LM, Lockhart S, Kempe A. Novel immunization reminder/recall approaches: rural and urban differences in parent perceptions. Acad Pediatr 2014;14(3):249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Niccolai LM, Hansen CE. Practice- and community-based interventions to increase Human Papilomavirus vaccine coverage - a systematic review. JAMA Pediatr 2015;169(July (7)):686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fu LY, Bonhomme L-A, Chenoa Cooper S, Joseph JG, Zimet GD. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine 2014;32:1901–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


